- 1Radiotherapy Department of Meizhou People’s Hospital (Huangtang Hospital), Meizhou, China
- 2School of Medical Sciences, Universiti Sains Malaysia, Kelantan, Malaysia
- 3National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Purpose: This study aims to evaluate the dosimetric impact of dual-layer-stacked multi-leaf collimators (MLCs) by comparing stereotactic radiotherapy (SRT) plans for multiple brain metastases (BMs) using the dual-layer MLC of the Halcyon 3.0 system against the single-layer MLC of the Versa HD system.
Methods: Eighteen patients with multiple BMs were retrospectively selected for this study. For each patient, five SRT plans were generated: two using the dual-layer MLC (Halcyon 3.0) and three using the single-layer MLC (Versa HD, comprising two coplanar and one non-coplanar plan). All plans were optimized under identical conditions, with dose normalization ensuring 95% of the target volume received 100% of the prescribed dose. Dosimetric parameters such as D2%, Dx (the dose of x percent volume), D98%, Dmean, conformity index (CI), homogeneity index (HI), and gradient index (GI) for the planning target volume, as well as Dmax, Dmean, and volume-specific doses (VX) for organs at risks (OARs), were analyzed. Additionally, treatment time, monitor units (MUs), and radiobiological indices, including equivalent uniform dose (EUD), tumor control probability (TCP), and normal tissue complication probability (NTCP), were assessed.
Results: The Halcyon 3.0 plans with dual-layer MLC demonstrated superior dosimetric outcomes compared to Versa HD coplanar plans, particularly in reducing V5, V10, V20, V24, and Dmean for normal brain tissue. The GI of Halcyon 3.0 plans was comparable to that of Versa HD noncoplanar plans. Furthermore, Halcyon 3.0 plans achieved lower EUD and NTCP values for both brainstem and normal brain tissue, matching the performance of Versa HD non-coplanar plans. These findings highlight the efficacy of dual-layer MLC in achieving dosimetric results similar to non-coplanar techniques while offering enhanced protection to OARs.
Conclusion: The dual-layer stacked MLC of the Halcyon 3.0 system provides comparable OAR sparing and dose gradients to non-coplanar Versa HD plans in single-isocenter hypofractionated SRT for multiple BMs, with significant improvements over coplanar Versa HD plans. This suggests that Halcyon 3.0 could be a preferred option for such treatments when non-coplanar setups are not feasible.
1 Introduction
Brain metastases (BMs) represent one of the most prevalent intracranial malignancies, affecting 20%–40% of cancer patients (1), with approximately 70% presenting with multiple lesions at diagnosis (2). While whole-brain radiation therapy (WBRT) has historically been a cornerstone for preventing disease progression, stereotactic radiosurgery (SRS) has emerged as a preferred modality due to its superior preservation of neurocognitive function, minimizing quality-of-life compromise in survivors (3–5). With technological advancements, hypofractionated stereotactic radiotherapy (SRT)—administered over 2–5 fractions—has gained clinical traction as a versatile alternative for treating primary and metastatic brain tumors, balancing biological efficacy with logistical feasibility (6).
The management of multiple BMs demands precision in dose delivery to target lesions while sparing critical normal tissues, a challenge addressed by various radiotherapy platforms (7). Systems like the CyberKnife and Gamma Knife offer high conformality but are limited by tumor geometry and accessibility, prompting widespread reliance on linac-based solutions. In contrast, linac-based platforms, which are commonly employed in institutions worldwide, provide practical insights applicable to similar clinical settings. The absence of restrictions on tumor size and location, along with the capability to perform SRS or SRT, makes these platforms the preferred choice for many cancer centers.
As one of the latest linear accelerator systems, the Halcyon 3.0 (Varian Medical Systems, Palo Alto, CA, USA) features a unique staggered dual-layer stacked multi-leaf collimator (MLC). This system comprises two MLCs, each with a width of 1 cm and an effective resolution of 0.5 cm at the isocenter. It can achieve a maximum MLC speed of 5.0 cm/s while maintaining an extremely low MLC transmission rate of only 0.5% of the primary beam (8, 9). In contrast, the Versa HD system (Elekta Oncology Systems, Crawley, UK) features a single-layer stacked multi-leaf collimator (MLC) with 80 pairs of leaves, each 5 mm wide at the isocenter. This system can achieve a maximum MLC speed of 6.5 cm/s and is capable of delivering flattening filter-free (FFF) photon beams using agility (10). Figure 1 illustrates some physical design and functionality differences between Halcyon 3.0 MLC and Versa HD MLC. Both devices can be used for SRT treatment based on their excellent dose accuracy control performance. However, the dosimetric difference of SRT plans for multiple BMs based on these two devices has been rarely studied so far.
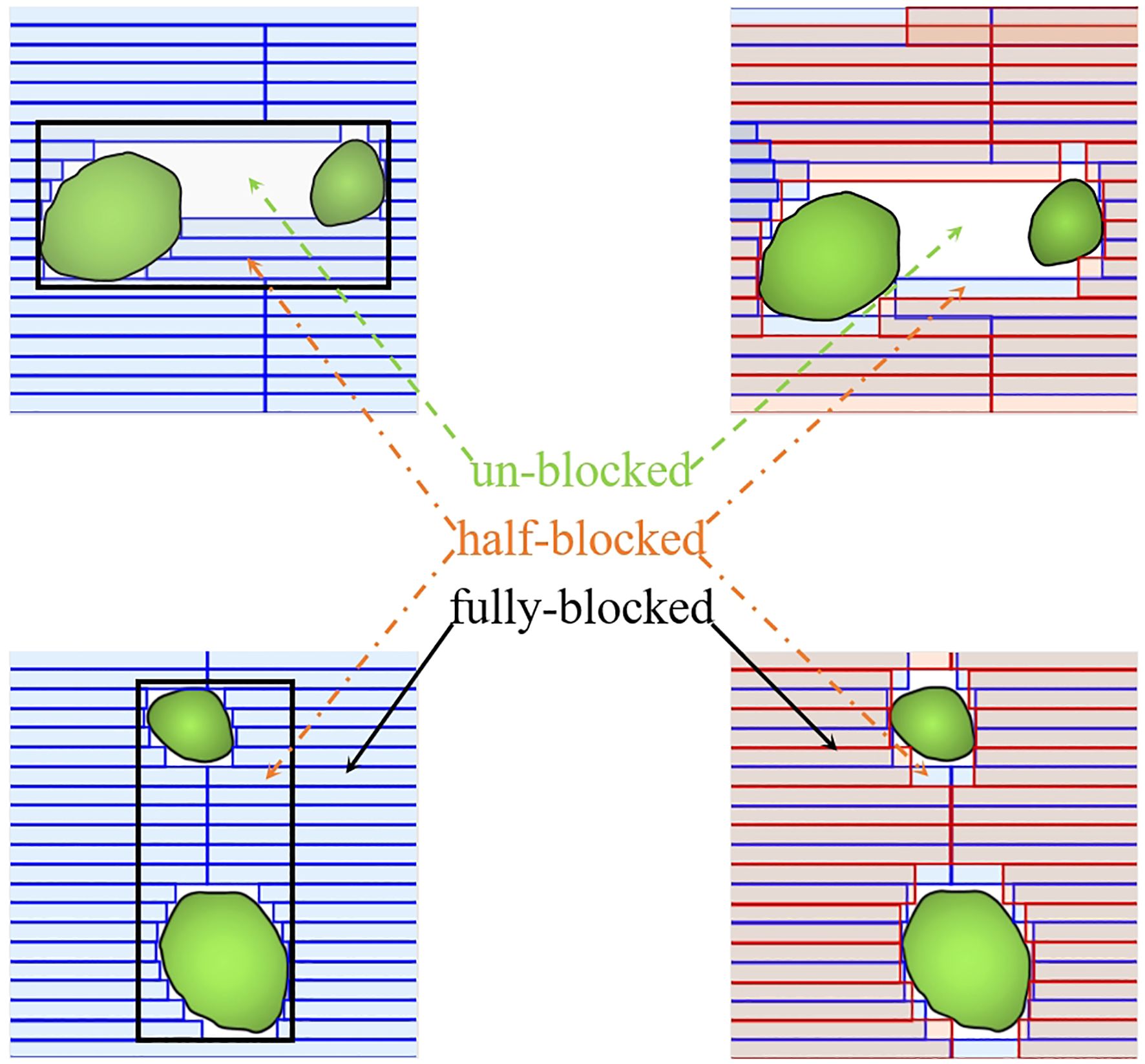
Figure 1. Comparison of the multi-leaf collimator (MLC) designs between the dual-layer Halcyon MLC (red) and the single-layer Versa HD MLC (blue). The dual-layer design of Halcyon MLC reduces leaf-to-leaf leakage and improves dose consistency, as shown by the overlapping areas.
In this study, we designed the SRT plans for multiple BM cases using the eclipse treatment planning system (TPS) based on the Halcyon (double-layer MLC, DLM) machine mode and then compared these plans with those using the Monaco TPS based on the Elekta Versa HD Agility (single-layer MLC, SLM) machine model.
2 Materials and methods
2.1 Patient selection
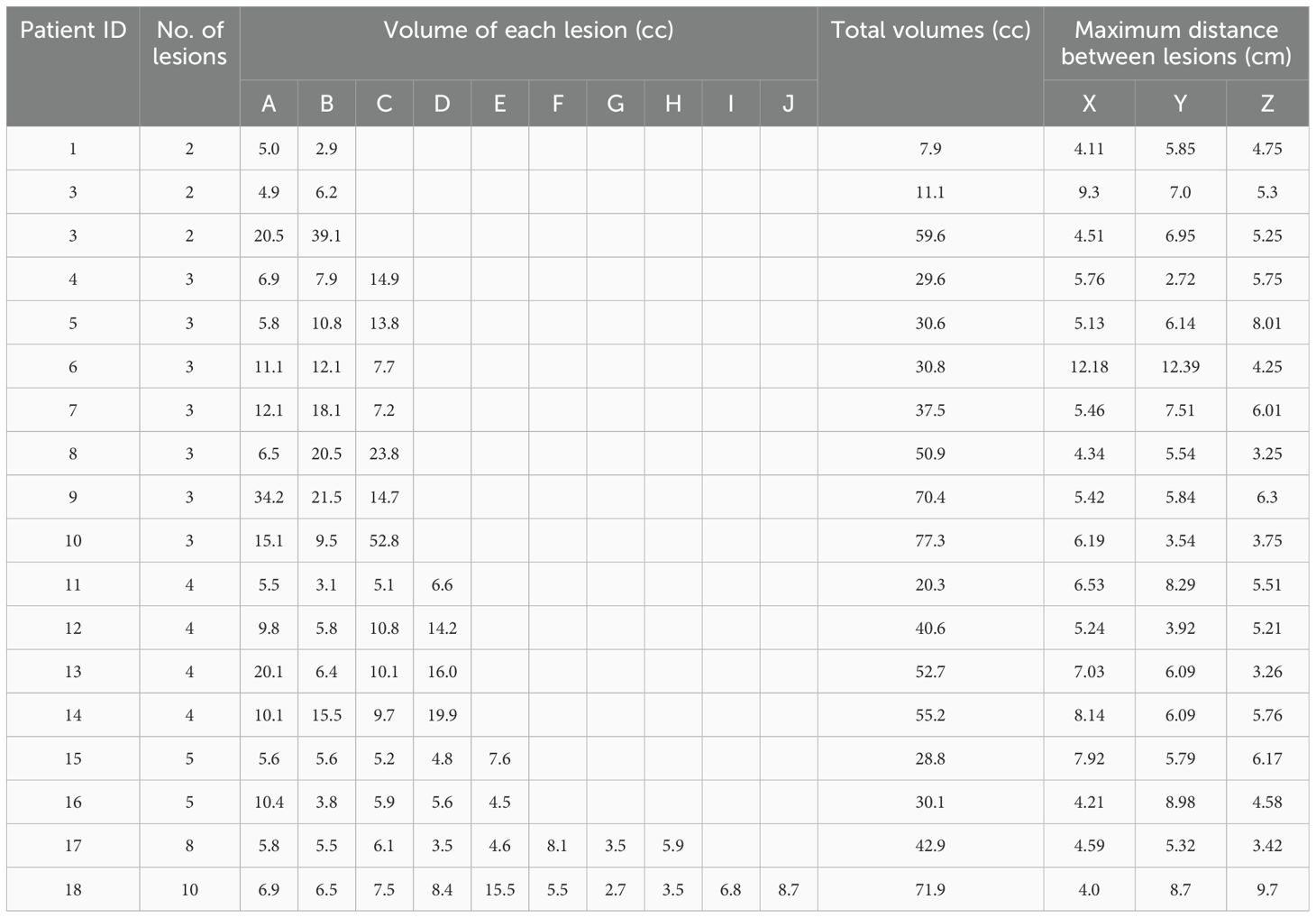
A retrospective analysis was conducted on eighteen patients with multiple BMs (2–10 lesions) treated between May 2020 and April 2023. Prior to treatment, computed tomography (CT) images were acquired on a Discovery RT590CT BigBore (GE Healthcare, Milwaukee, WI) with parameters set as 120 kV, 250 mAs, and 2.5 mm slice thickness. Patients were positioned supine and immobilized using a thermoplastic head mask to ensure reproducible setup. Gross tumor volumes (GTVs) were delineated on fused CT and magnetic resonance imaging (MRI) by two experienced radiation oncologists, incorporating all clinical information. The planning target volume (PTV) was generated by adding a 3-mm isotropic margin to the GTV to account for interfractional setup uncertainties. All contours were reviewed by a senior radiation oncologist to maintain consistency. Patient and target characteristics, including the number of lesions (2–10 per patient) and PTV volume (mean: 25.1 cc; range: 7.9–77.3 cc), are detailed in Table 1. The study was approved by the Ethics Committee of Meizhou People’s Hospital (IRB2023-C39), and written informed consent was waived due to its retrospective nature.
2.2 Treatment planning
For each patient, five treatment plans were developed: two using the Halcyon 3.0 platform (Varian Medical Systems, Palo Alto, CA, USA) with dual-layer stacked multi-leaf collimators (DLM) and three using the Versa HD platform (Elekta Oncology Systems, Crawley, UK) with single-layer stacked multi-leaf collimators (SLM). The Halcyon 3.0 plans included two coplanar 2-arc VMAT plans: DLM3/357 (360°arcs at 0°couch rotation, collimator angles 3°and 357°) and DLM3/90 (360°arcs at 0°couch rotation, collimator angles 3°and 90°). Dose calculations were performed using the analytical anisotropic algorithm (AAA) with 1-mm grid spacing, incorporating heterogeneity corrections.
The Versa HD plans comprised two coplanar 2-arc VMAT plans—SLM3/357 (collimator angles 3°and 357°) and SLM3/90 (collimator angles 3°and 90°), as well as one non-coplanar 4-arc VMAT plan, SLMnc (1 full 360°arc at 0°couch rotation and three 150°partial arcs at couch rotations of 45°, 90°, and 315°). These plans were optimized on the Monaco TPS using the Monte Carlo algorithm with a 1 mm grid. All plans utilized 6 MV FFF beams. Isocenters were automatically placed at the center of mass of the combined PTVs. The prescription dose was 30 Gy in 5 fractions, normalized to cover 95% of the PTV in each plan to ensure consistent target coverage.
Notably, all plans were designed by system-specific dosimetrists in a blinded manner, adhering to identical planning objectives and optimization strategies. The strategy prioritized steep dose fall-off over maximum dose constraints, with plan optimization terminated when further improvements in dose gradient were unattainable (4). This approach was uniformly applied to both platforms to isolate the effect of MLC design on dosimetric outcomes.
2.3 Plan comparison
Dose-volume histogram (DVH) data for PTVs and OARs were extracted for analysis. Target evaluation included near-maximum dose(D2%), near-minimum dose (D98%), and the mean dose(Dmean) of PTV, along with conformity index (CI, Equation 1), homogeneity index (HI, Equation 2), and gradient index (GI, Equation 3), which were utilized to evaluate the plan quality. CI, HI, and GI were calculated as follows (11, 12).
where Vref is the volume of PTV covered by reference isodose, VT is the PTV volume, and Dp is the prescription dose. V50% and V100% are volumes covered by 50% and 100% of prescription dose, respectively.
For OARs, parameters included maximum dose (Dmax), mean dose (Dmean), and dose-volume metrics (V5, V10, V20, V24) for normal brain tissue were recorded, with V20 and V24 highlighted as critical indicators of radiation-induced necrosis in hypofractionated SRT (6, 13). Brainstem, optic nerves, and optic chiasm were evaluated using structure-specific dose constraints referenced from the TG101 report (14) and Timmerman criteria (15).
2.4 TCP and NTCP evaluation
The evaluation of biological effects involved the equivalent uniform dose (EUD, Equation 4) and normal tissue complication probability (NTCP, Equation 5), calculated using Niemierko’s model (16, 17):
where vi is the percentage of voxels receiving dose di. The vi and di values are acquired from the DVHs, and the sum of vi over all voxels equals 1. a is a parameter that reflects the dose-response property of distinct organs, and in some literature, the parameter n is used with a = 1/n (18). TD50/5 is the dose for achieving a 50% probability of normal tissue complication in 5 years as the OAR is irradiated homogeneously, and γ50 is the slope of the sigmoidal dose response curve of the OAR. According to reference (17), for brainstem a = 7, TD50/5 = 65 Gy, γ50 = 3; for normal brain, a = 5, TD50/5 = 60 Gy, γ50 = 3.
2.5 Statistical analysis
Data were analyzed using SPSS 22.0 (SPSS Inc., Chicago, IL). Given the non-normal distribution of dosimetric parameters, paired comparisons between plans were performed using the Wilcoxon signed-rank test. A significance threshold of P < 0.05 was applied for all statistical tests.
3 Results
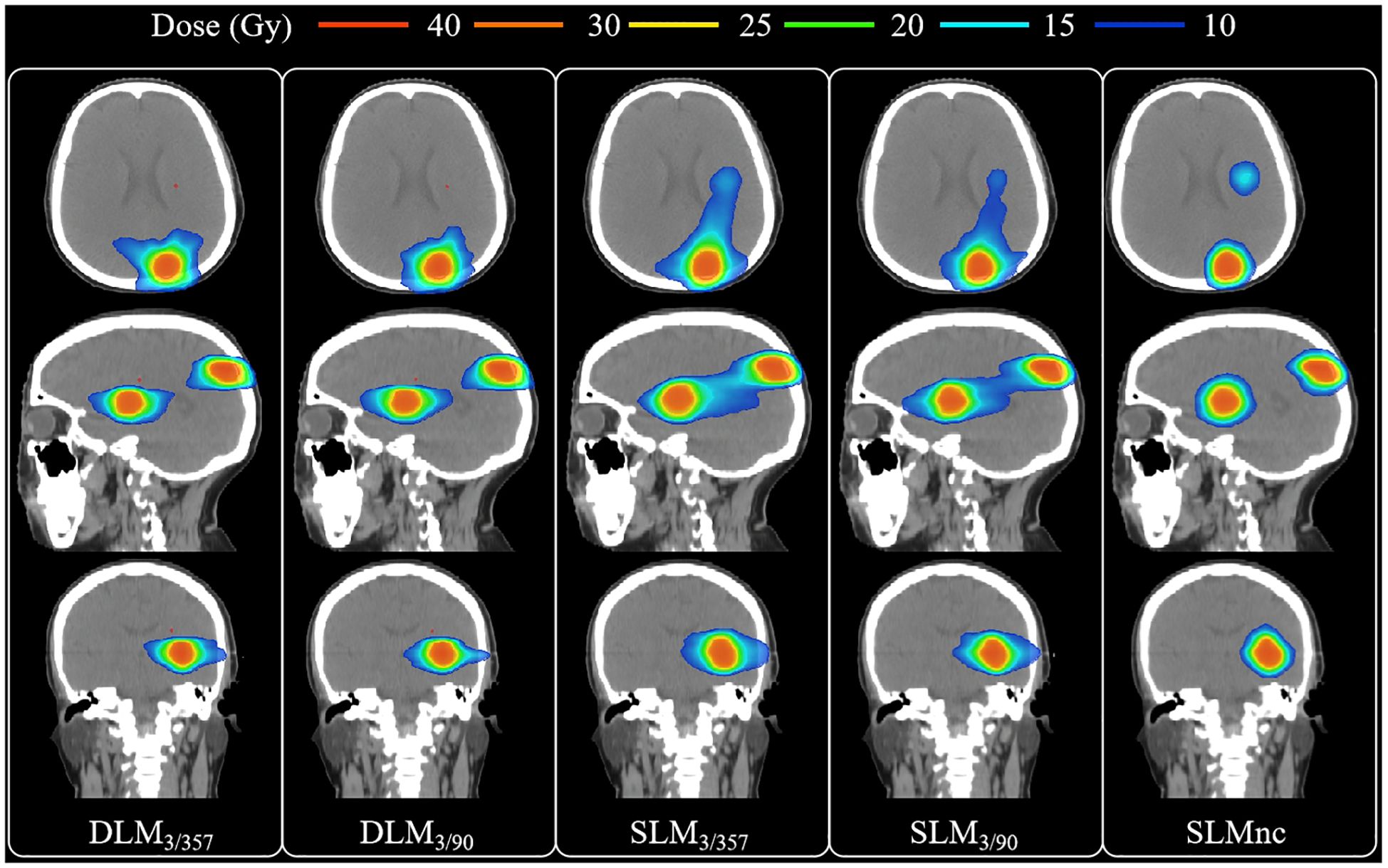
A total of 90 treatment plans from 18 patients were evaluated, all of which met clinical requirements with adequate target coverage and safe organ doses. Dosimetric parameters were summarized in Tables 2 and 3, with representative two-dimensional (2D) dose distributions and DVHs illustrated in Figures 2 and 3. Notably, the 10 Gy isodose line of Halcyon 3.0 plans was more conformal to the PTV contour compared to Versa HD plans, indicating reduced low-dose exposure to surrounding tissues.
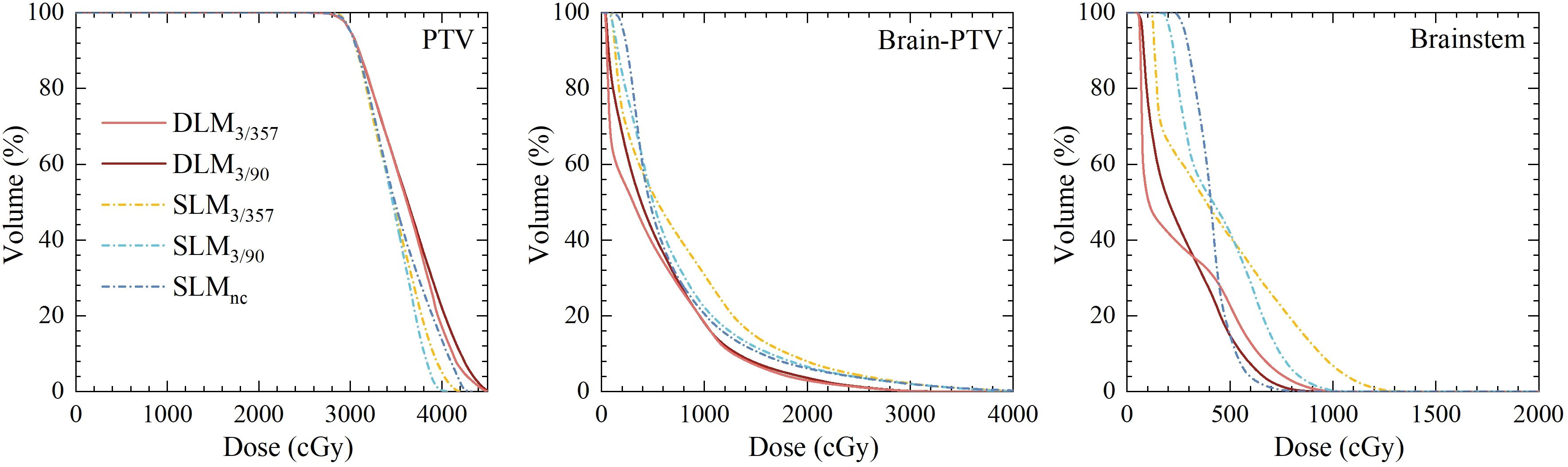
Figure 3. Comparison of dose volume histograms for PTV and OARs between five plans for the representative case.
3.1 Target volume (PTV) evaluation
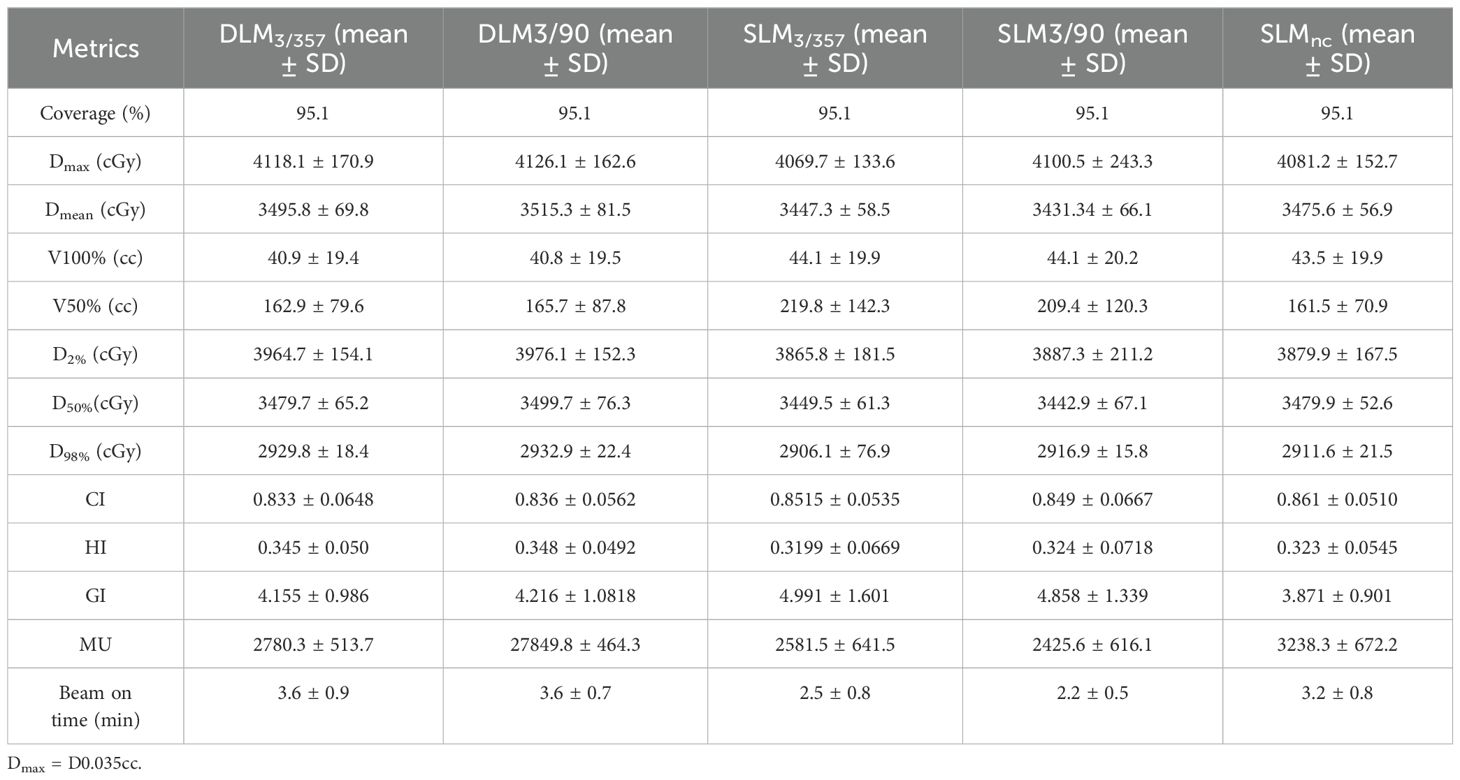
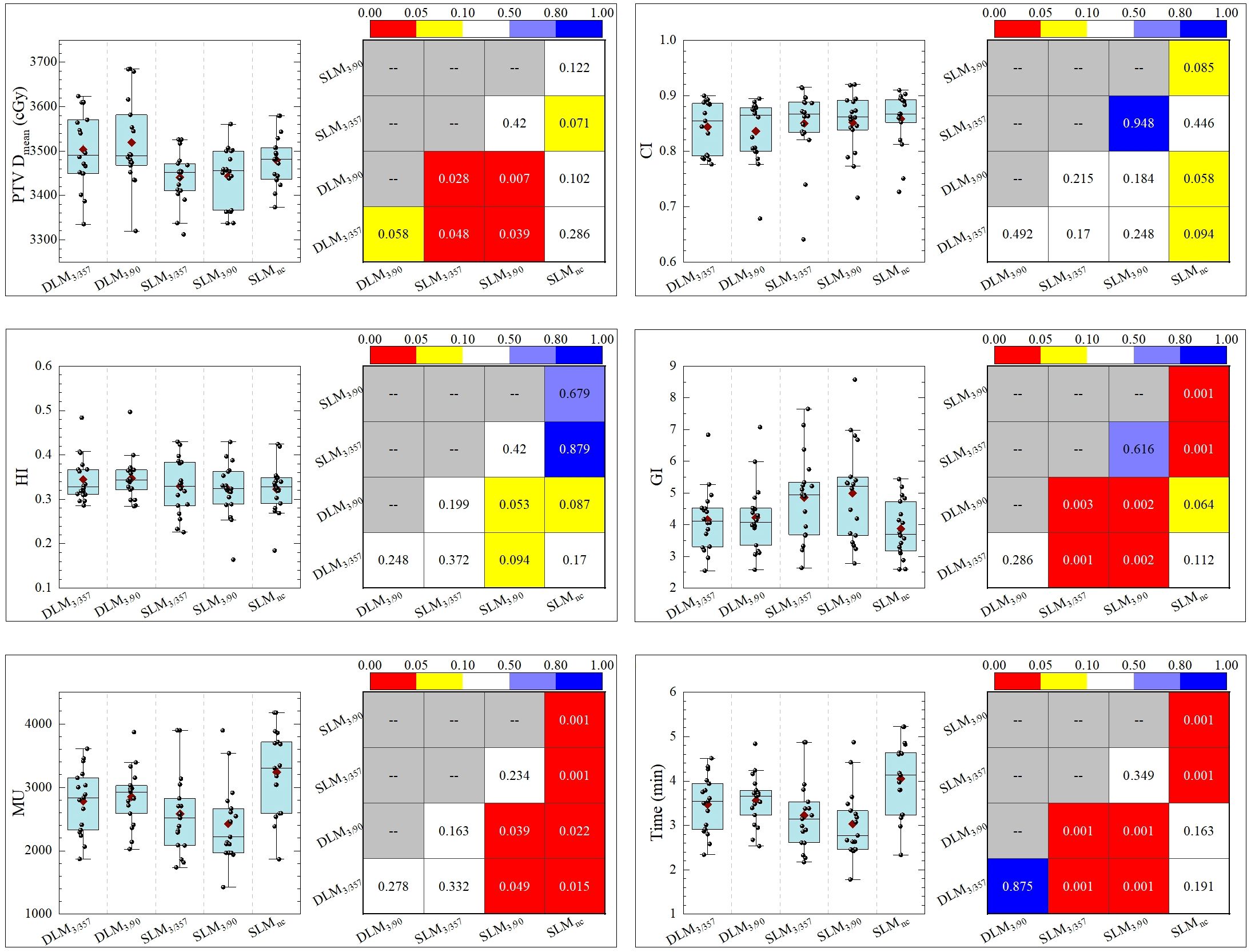
Double-layer MLC (DLM, Halcyon 3.0) plans exhibited comparable homogeneity index (HI) and CI to single-layer MLC (SLM, Versa HD) plans, with no significant differences across all five plans, as shown in Figure 4. The gradient index (GI), a measure of dose fall-off, was as follows: DLM3/357: 4.155 ± 0.986; DLM3/90: 4.216 ± 1.0818; SLM3/357: 4.991 ± 1.601; SLM3/90: 4.858 ± 1.339; and SLMnc: 3.871 ± 0.901. The DLM plans achieved a significantly smaller GI compared to coplanar plans of SLM DLM3/357 vs. SLM3/357: P < 0.001; DLM3/90 vs. SLM3/90: P = 0.002), indicating steeper dose gradients. However, GI values for DL-MLC plans were statistically comparable to the non-coplanar plan (DLM3/357 vs. SLMnc: P = 0.112; DLM3/90 vs. SLMnc: P = 0.064), demonstrating equivalent dose gradient quality to non-coplanar techniques. Mean dose (Dmean) for PTV was slightly higher in DLM plans than in coplanar SLM plans (DLM3/357 vs. SLM3/357: P = 0.048; DLM3/90 vs. SLM3/90: P = 0.007), but closely matched the non-coplanar (DLM3/357 vs. SLMnc: P = 0.286; DLM3/90 vs. SLMnc: P = 0.102). The results for the five plans are DLM3/357: 3495.8 ± 69.8 cGy; DLM3/90: 3515.3 ± 81.5 cGy; SLM3/90: 3431.34 ± 66.1 cGy; SLM3/357: 3447.3 ± 58.5 cGy; and SLMnc: 3475.6 ± 56.9 cGy, as detailed in Table 2.
3.2 Treatment efficiency (MU and delivery time)
As depicted in Table 2 and Figure 4, non-coplanar SLMncplans required significantly higher monitor units (MUs) than coplanar plans, reflecting the complexity of multi-arc non-coplanar delivery. Halcyon 3.0 plans had longer treatment times compared to Versa HD coplanar plans, primarily due to the lower maximum dose rate of the Halcyon platform (800 MU/min vs. 1,400 MU/min for Versa HD), though this difference did not impact clinical feasibility.
3.3 Organ-at-risk sparing
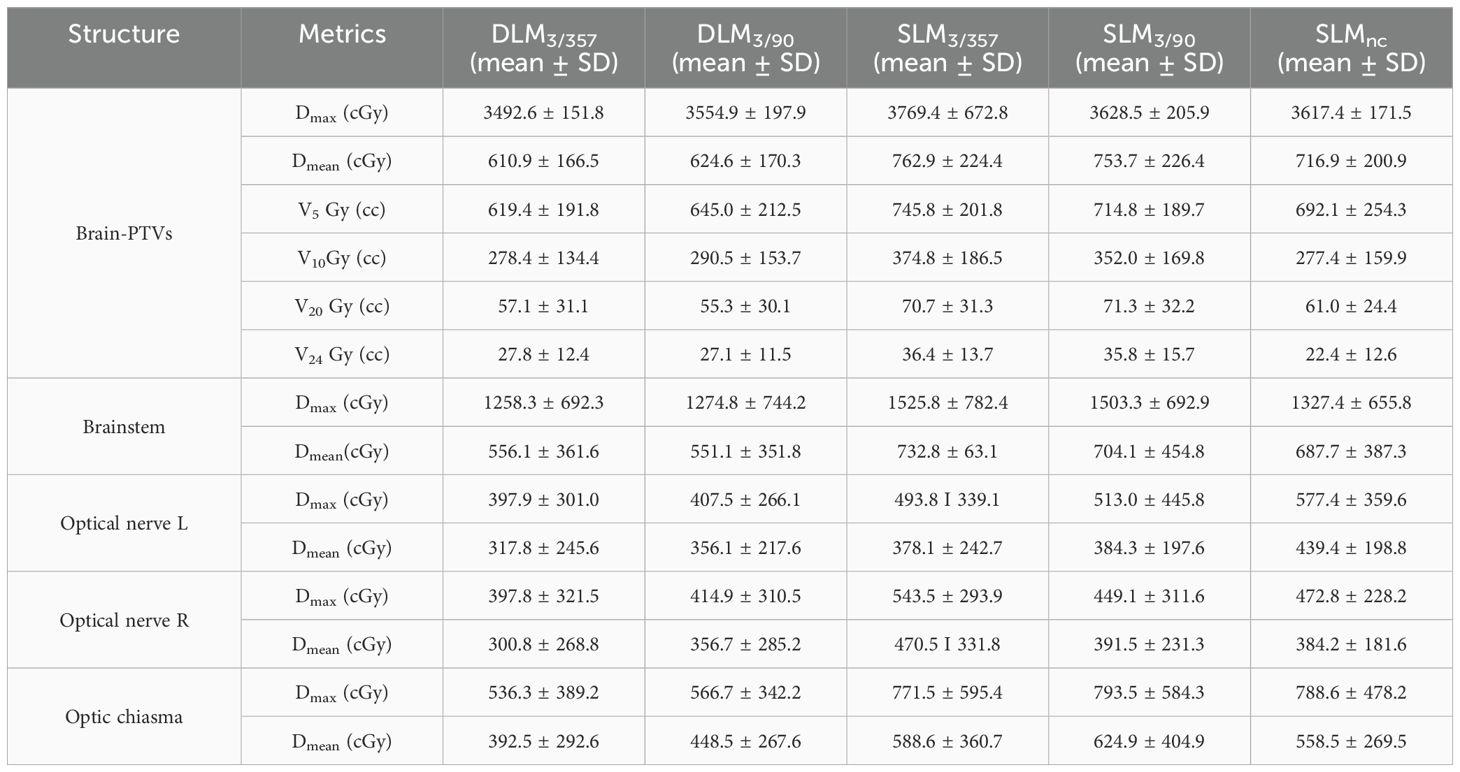
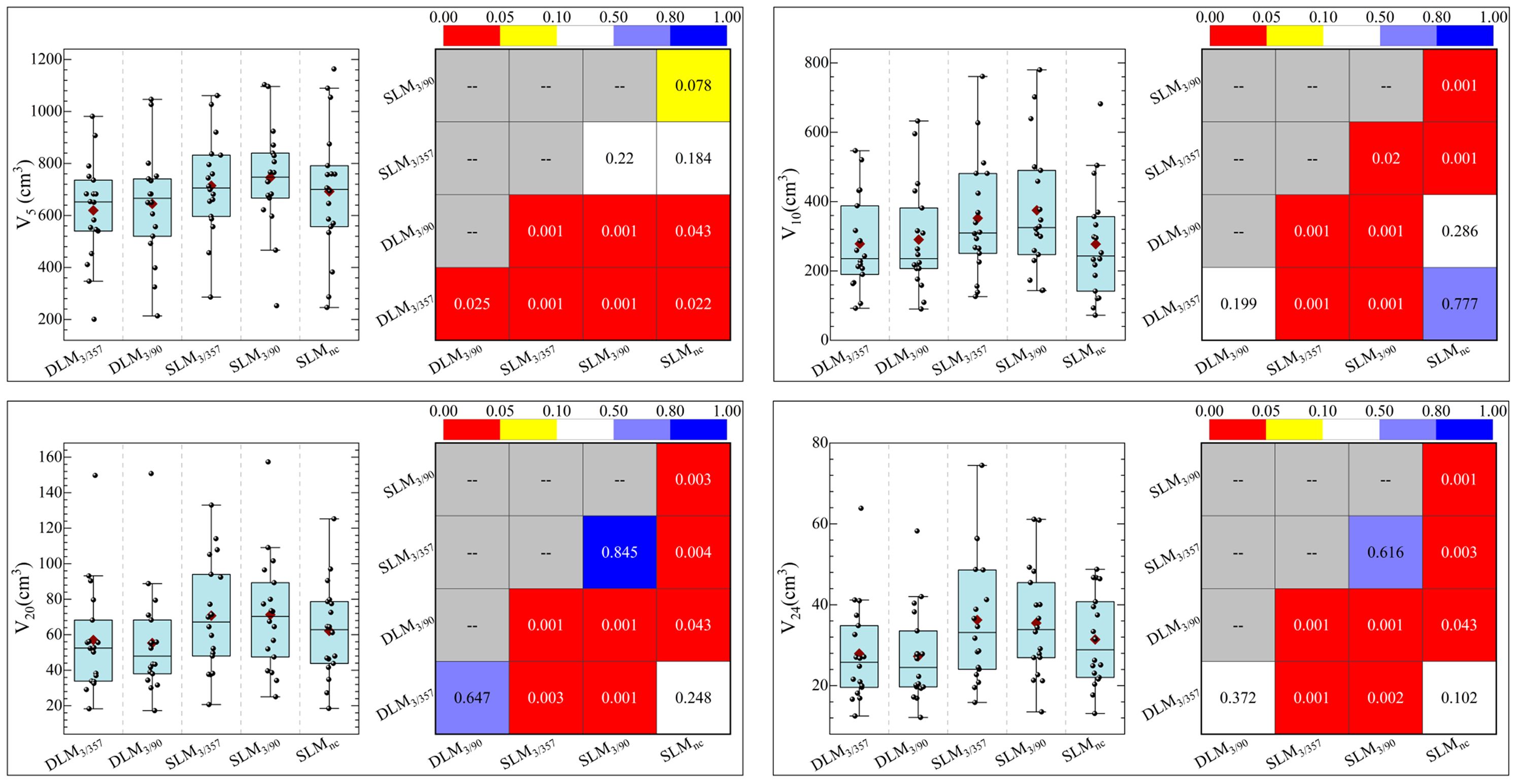
The statistical comparison of normal brain tissue was shown in Figure 5. DLM plans achieved superior sparing compared to all SLM coplanar plans with significantly lower mean dose (Dmean) for Brain-PTV compared to all other SLM groups (P < 0.001). The DLM3/357 plan had the lowest Dmean in the BrainPTV, with a value of 610.9± 166.5 cGy, followed by the DLM3/90, SLMnc, SLM3/357, and SLM3/90 with the values of 624.6± 170.3 cGy, 716.9± 200.9 cGy, 762.9± 224.4 cGy, and 753.7± 226.4 cGy, respectively. The values of V20 (cc) and V24 (cc) are particularly noteworthy due to their correlation with radionecrosis following SRT treatment. Our results indicate that DLM plans achieved superior V20(cc) and V24(cc) values compared to coplanar SLM plans, and were comparable to non-coplanar SLM plans. Additionally, the results for V10 (cc) were consistent with these findings. In addition, the low-dose V5 (cc) comparison indicated that DLM offered a reduced low-dose volume to normal brain tissue compared to both coplanar (P < 0.001) and non-coplanar plans (DLM3/357 vs. SLMnc: P = 0.02, DLM3/90 vs. SLMnc: P = 0.043).
For normal brain tissue (Brain-PTV), DLM plans achieved superior sparing compared to all SLM coplanar plans, with significantly lower mean dose (Dmean), V5, V10, V20, and V24 values (all P < 0.001, as shown in Figure 5 and Table 3). Specifically, DLM3/357 and DLM3/90 plans reduced V20 by 19.2% and 22.5%, respectively, compared to SLM3/357 and SLM3/90. While SLMnc plans showed comparable V20 and V24 to DLM plans, their V5 values were higher (P < 0.05), indicating greater low-dose exposure.
In the brainstem, DLM plans demonstrated lower maximum dose (Dmax) and mean dose (Dmean) than coplanar SLM plans (all P < 0.001) and were marginally better than the non-coplanar SLMnc plan (P = 0.001 and P = 0.002 for DLM3/357 and DLM3/90 vs. SLMnc, respectively), highlighting improved protection of this critical structure. Optic nerves and chiasma received low doses across all plans, with no significant differences between platforms (all P > 0.05, as shown in Table 3).
3.4 Radiobiological parameters for normal brain and brainstem
DLM plans yielded lower EUD for both normal brain tissue and the brainstem compared to coplanar SLM plans (P < 0.05), which correlated with reduced NTCP. Specifically, NTCP values for normal brain tissue were 0.35%–0.13% in DLM plans versus 0.41%–0.14% in coplanar SL-MLC plans (P < 0.05), and brainstem NTCP was 0.11%–0.12% in DLM versus 0.16%–0.17% in coplanar SLM plans (P < 0.05, as shown in Table 4 and Figure 6). These advantages were maintained when compared to non-coplanar SLMnc plans, with no significant differences in EUD or NTCP (P > 0.05), confirming equivalent radiobiological safety.
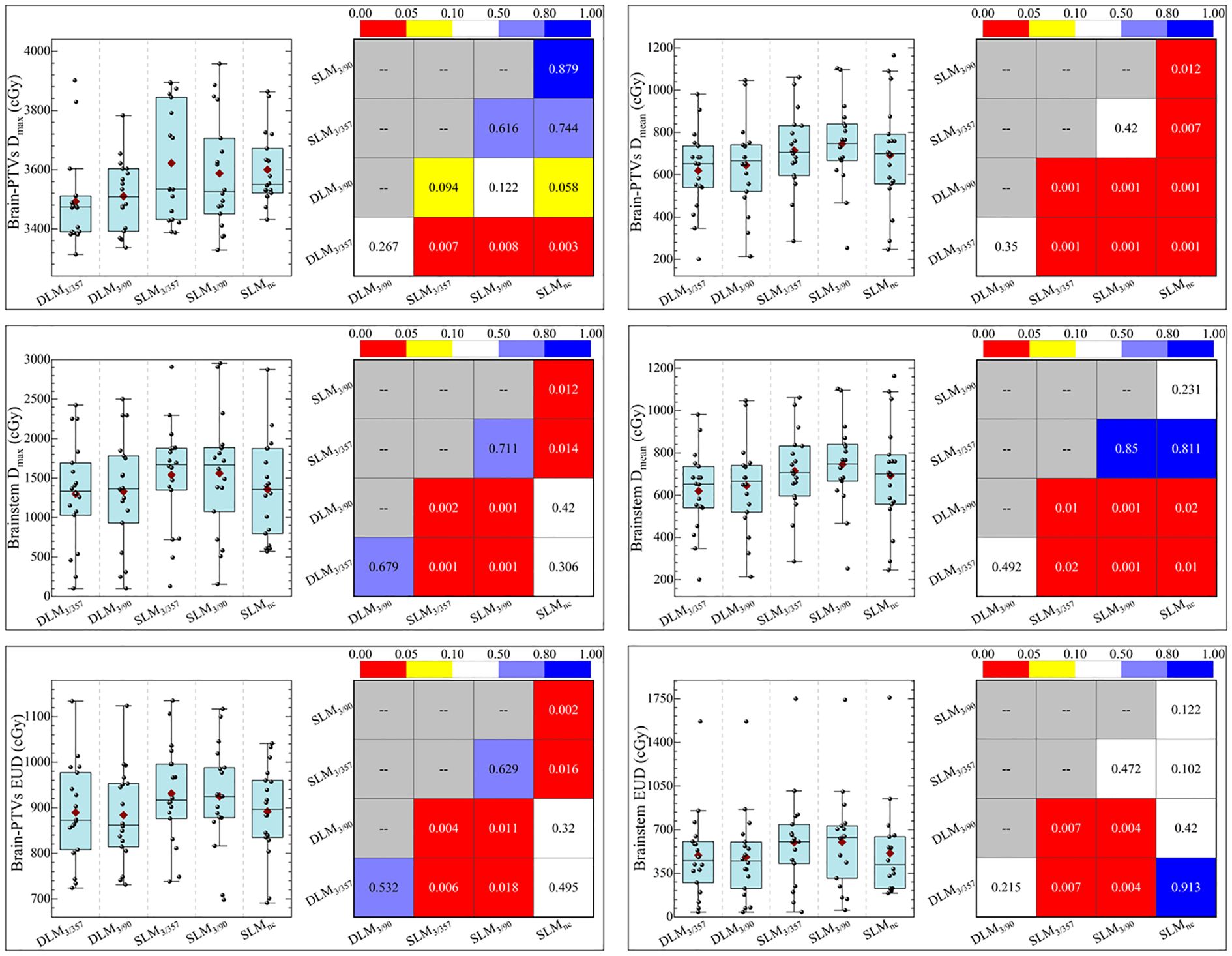
Figure 6. Statistical comparison of Dmax, Dmean, EUD, and NTCP of brainstem and normal brain between five plans.
4 Discussions
In this study, we evaluated the performance of the Halcyon 3.0 platform (double-layer MLC, DLM) and the Versa HD platform (single-layer MLC, SLM) in hypofractionated stereotactic radiation therapy (SRT) for multiple BMs. All five plans across both platforms met clinical criteria, demonstrating adequate target coverage, steep dose gradients, and safe organ-at-risk (OAR) doses. The DLM system demonstrated superior normal tissue sparing, particularly in terms of V5(cc) and D mean for normal brain tissue, while achieving dose conformity and gradient comparable to non-coplanar SLM plans. These findings highlight the potential of dual-layer collimation to balance precision and efficiency in metastatic brain radiotherapy.
The advantage of DLM in OAR protection can be attributed to its unique design: the Halcyon 3.0’s dual-layer MLC, featuring 77 mm leaf thickness and staggered interlayer positioning, effectively minimizes beam transmission (¡0.5%) compared to the Versa HD’s single-layer MLC (1.4%–1.5% transmission) (8–10, 19). This reduced leakage is critical during intensity-modulated optimization, as it limits unintended radiation exposure to normal tissues outside the target volume. Consequently, DLM plans showed significantly lower V20 and V24—key metrics for radiation-induced necrosis—than coplanar SLM plans and were comparable to non-coplanar SLM plans, which typically require more complex beam arrangements.
Notably, while non-coplanar SLM plans improve dose fall-off within the target plane, they increase low-dose spread in non-target axial planes, as evidenced by higher V5 values compared to DLM. This trade-off between dose conformity and low-dose volume highlights the clinical dilemma of noncoplanar techniques, which are associated with longer treatment times, potential setup errors from couch rotation (20), and incompatibility with cone-beam CT (CBCT) guidance (21). In contrast, DLM achieves steep dose gradients in coplanar arcs, offering a practical alternative when non-coplanar delivery is contraindicated due to logistical or technical constraints.
A critical challenge in multi-target SRT is the “island blocking” phenomenon, where overlapping MLC leaf usage between distant lesions exposes intervening brain tissue. Although collimator angles of 3°/357° and 3°/90° were tested to mitigate this issue, no significant differences were observed between these configurations, possibly due to heterogeneous lesion distributions. Future studies could explore subarc collimator angle optimization (SACAO), which dynamically adjusts leaf orientation to avoid shared MLC segments, potentially enhancing dose conformity for complex target geometries. The Halcyon 3.0’s lack of non-coplanar arc capability and 6D motion correction introduces setup uncertainty risks, particularly for lesions distant from the isocenter. Rotational errors, even minor ones, can compromise target coverage and OAR safety in single-isocenter plans, as previously reported (22–24). Clinicians using this platform must prioritize rigorous patient immobilization and daily image guidance to mitigate such risks, despite the system’s limitations in addressing rotational mismatches. Particularly, when using single-isocenter VMAT to treat multiple BMs with SRT, attention must be given to managing rotational uncertainties in patient setup (25–27). In multi-target single-isocenter treatment, rotation tolerances are more stringent because targets distant from the isocenter are highly sensitive to residual rotational mismatches between planned and actual treatment positions. Study (28) investigated the connection between rotational setup errors and dosimetric parameters in single-isocentric VMAT SRS for multiple target cases. Their findings indicated that D95% values deteriorated to 60% of the prescribed dose during uniform rotations of 2° around three axes. This study focused solely on the dosimetric effects on the planning target volume (PTV). However, if the rotational errors affecting the GTV were taken into account, the variations could be more pronounced, depending on the extent of the margin used for expansion. Undoubtedly, it can be predicted that when the rotation error reaches a certain degree, it will affect the dose coverage of GTV. Studies (29, 30) have addressed this issue by proposing methods to obtain a non-uniform clinical target volume (CTV) to PTV margin, accounting for both rotational and translational errors. 6D calibration, which couch correction is used to perform rotational corrections around the pitch, roll, and yaw axes, will significantly improve the SRT treatment accuracy (30). However, for reasons, such as safety and treatment complexity, non-coplanar has been constrained by the current machine design—the Halcyon 3.0, which does not support non-coplanar arcs. The daily CBCT online calibration in Halcyon 3.0 does not address rotational errors. Consequently, in single isocenter treatments for multiple BMs, the absence of 6D calibration in Halcyon presents a significant challenge, necessitating greater attention to rotational errors.
This study has several limitations. First, it was limited to plan comparisons without physical delivery validation, necessitating future in-vivo dosimetry verification. Second, the absence of direct comparisons with CyberKnife or HyperArc technologies restricts the generalizability of findings, though linac-based platforms remain the focus of most clinical settings. Third, only six MV FFF beams were evaluated, leaving energy-dependent dosimetric differences unaddressed. Last, SACAO was not incorporated into plan optimization, a technique shown to improve multi-target conformity and warranting further investigation.
5 Conclusion
Our results clearly indicate that the Halcyon 3.0 planning system, equipped with its dual-layer MLC, performs comparably to non-coplanar Versa HD plans in treating multiple BMs. It effectively delivers lower radiation doses to normal brain tissues and the brainstem compared to the coplanar Versa HD plans. These findings highlight the capability of the dual-layer MLC to enhance dose conformity and minimize exposure to organs at risk (OARs) without requiring complex non-coplanar beam arrangements. Consequently, the Halcyon 3.0 system emerges as a preferred choice for single-isocenter SRT in patients with multiple BMs, particularly in scenarios where non-coplanar setups are impractical or contraindicated. However, the absence of 6D correction capabilities in the Halcyon 3.0 system necessitates meticulous attention to rotational setup errors, underscoring the importance of robust patient immobilization and daily image guidance to ensure treatment precision and safety. Future studies should explore strategies to mitigate setup uncertainties and further validate these findings across larger patient cohorts.
Data availability statement
The datasets presented in this article are not readily available to keep data secure, private, and safe from breaches or damage. Requests to access the datasets should be directed to emN5X216QHNpbmEuY29t.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CZ: Writing – original draft. SB: Writing – review & editing. HY: Writing – review & editing. ZJ: Writing – review & editing. ZD: Writing – review & editing, Data curation, Formal analysis, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by Social Development Science and Technology Program of Meizhou city of Guangdong province (Grant No. 2023B23), Shenzhen Postdoctoral Research Funds (25005), Hospital Research Project (E010221008), Sanming Project of Medicine in Shenzhen (SZSM201612063) and Shenzhen Key Medical Discipline Construction Fund (SZXK013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsao MN, Mehta MP, Whelan TJ, Morris DE, Hayman JA, Mills JCFM, et al. The american society for therapeutic radiology and oncology (astro) evidence-based review of the role of radiosurgery for Malignant glioma. Int J Radiat Oncol Biol Phys. (2005) 63:47–55. doi: 10.1016/j.ijrobp.2005.05.024
2. Franchino F, Rudà R, and Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol. (2018) 8:161. doi: 10.3389/fonc.2018.00161
3. Khuntia D, Brown P, Li J, and Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. (2006) 24:1295–304. doi: 10.1200/JCO.2005.04.6185
4. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. J Am Med Assoc. (2006) 295:2483–91. doi: 10.1001/jama.295.21.2483
5. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. Jama. (2016) 316:401–9. doi: 10.1001/jama.2016.9839
6. Michael TM, Jimm G, Andrzej N, Scott GS, Vitali M, Kristin JR, et al. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. (2021) 1:68–86. doi: 10.1016/j.ijrobp.2020.08.013
7. Wang B and Yang J. New technologies and machines for stereotactic radiation therapy. Precis Radiat Oncol. (2022) 6:321–7. doi: 10.1002/pro6.1180
8. Yee T, Lim I, Dragojevic´ D, Hoffman EF, Martinez GY, and Kim. Experience in commissioning the halcyon linac. J Appl Clin Med Phys. (2019) 9:7. doi: 10.1002/acm2.12568
9. Tucker, Netherton Y, Li S, Gao A, Klopp 1P, Balter LE, et al. Characterization of the halcyontm multileaf collimator system. Med Phys. (2019) 46:4304–13. doi: 10.1002/mp.13723
10. Narayanasamy G, Saenz D, Cruz W, Ha CS, Papanikolaou N, and Stathakis S. Commissioning an elekta versa hd linear accelerator. J Appl Clin Med Phys. (2016) 17:179–91. doi: 10.1120/jacmp.v17i1.5799
11. Paddick I and Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J neurosurgery. (2006) 105:194–201. doi: 10.3171/sup.2006.105.7.194
12. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. J neurosurgery. (2000) 93:219–22. doi: 10.3171/jns.2000.93.supplement
13. Ernst SA, Ganslandt O, Lambrecht U, Sauer R, and Grabenbauer G. Phase ii trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiotherapy Oncol. (2006) 1:18–24. doi: 10.1016/j.radonc.2006.08.024
14. Stanley HB, Kamil MY, David F, James MG, William H, Brian K, et al. Stereotactic body radiation therapy: (1) highlights of the aapm task group report 101 on sbrt. Med Phys. (2006) 6:2176–2176. doi: 10.1118/1.2241475
15. Timmerman R. A story of hypofractionation and the table on the wall. Int J Radiat Oncol Biol Phys. (2022) 1:4–21. doi: 10.1016/j.ijrobp.2021.09.027
16. Wu Q, Mohan R, Niemierko A, and Schmidt-Ullrich R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. (2002) 52:224–35. doi: 10.1016/s0360-3016(01)02585-8
17. Gay HA and Niemierko A. A free program for calculating eud-based ntcp and tcp in external beam radiotherapy. Physica Med. (2007) 23:115–25. doi: 10.1016/j.ejmp.2007.07.001
18. Cao T, Yuan Q, and Dai Z. A new formula for calculating normal tissue complication probability. Precis Radiat Oncol. (2024) 8:126–31. doi: 10.1002/pro6.1240
19. Can S, Karaçetin D, and Meric¸ N. Beam modeling and commissioning for monte carlo photon beam on an elekta versa hd linac. Appl Radiat Isotopes. (2022) 180:110054. doi: 10.1016/j.apradiso.2021.110054
20. Lai J, Liu S, Liu J, Li X, Chen J, Jia Y, et al. Clinical feasibility of using single-isocentre non-coplanar volumetric modulated arc therapy combined with non-coplanar cone beam computed tomography in hypofractionated stereotactic radiotherapy for five or fewer multiple intracranial metastases. Clin Oncol (Royal Coll Radiologists (Great Britain)). (2023) 6:408–16. doi: 10.1016/j.clon.2023.03.011
21. Zhang S, Yang R, Shi C, Li J, Zhuang H, Tian S, et al. Noncoplanar vmat for brain metastases: A plan quality and delivery efficiency comparison with coplanar vmat, imrt, and cyberknife. Clin Oncol (Royal Coll Radiologists (Great Britain)). (2019) 18:1–8. doi: 10.1177/1533033819871621
22. Shen J, Dai Z, Yu J, Yuan Q, Kang K, Chen C, et al. Sub-arc collimator angle optimization based on the conformity index heatmap for vmat planning of multiple brain metastases srs treatments. Front Oncol. (2022) 12:987971. doi: 10.3389/fonc.2022.987971
23. Battinelli C, Fredriksson A, and Eriksson K. Collimator angle optimization for multiple brain metastases in dynamic conformal arc treatment planning. Med Phys. (2021) 48:5414–22. doi: 10.1002/mp.15078
24. Kuo L, Zhang P, Pham H, and Ballangrud ÅM. Implementation and validation of an in-house geometry optimization software for srs vmat planning of multiple cranial metastases. J Appl Clin Med Phys. (2020) 21:25–32. doi: 10.1002/acm2.12961
25. Nakano H, Shiinoki T, Tanabe S, Nakano T, Takizawa T, Utsunomiya S, et al. Multicomponent mathematical model for tumor volume calculation with setup error using single-isocenter stereotactic radiotherapy for multiple brain metastases. Phys Eng Sci Med. (2023) 46:945–53. doi: 10.1007/s13246-023-01241-8
26. Nakano H, Shiinoki T, Tanabe S, Utsunomiya S, Kaidu M, Nishio T, et al. Assessing tumor volumetric reduction with consideration for setup errors based on mathematical tumor model and microdosimetric kinetic model in single-isocenter vmat for brain metastases. Phys Eng Sci Med. (2024) 47(4):1385–1396. doi: 10.1007/s13246-024-01451-8
27. Kasirajan TS. Dosimetric effect of rotational setup errors in single-isocenter volumetric-modulated arc therapy of multiple brain metastases. J Of Med Phys. (2019) 44:84–90. doi: 10.4103/jmp.JMP_103_18
28. Roper J, Chanyavanich V, Betzel G, Switchenko J, and Dhabaan A. Single-isocenter multiple-target stereotactic radiosurgery: risk of compromised coverage. Int J Radiat Oncol Biol Phys. (2015) 93:540–6. doi: 10.1016/j.ijrobp.2015.07.2262
29. Miao J, Xu Y, Tian Y, Liu Z, and Dai J. A study of nonuniform ctv to ptv margin expansion incorporating both rotational and translational uncertainties. J Appl Clin Med Phys. (2019) 20:78–86. doi: 10.1002/pro6.1240
Keywords: stereotactic radiotherapy, multiple brain metastases, dual-layer MLC, non-coplanar radiotherapy, Halcyon 3.0
Citation: Zhong C, Bi S, Yu H, Jiang Z and Dai Z (2025) Dosimetric advantages of dual-layer MLC in hypofractionated stereotactic radiotherapy for multiple brain metastases: a comparative study with single-layer MLC. Front. Oncol. 15:1564801. doi: 10.3389/fonc.2025.1564801
Received: 22 January 2025; Accepted: 06 May 2025;
Published: 30 May 2025.
Edited by:
Sunil Dutt Sharma, Bhabha Atomic Research Centre (BARC), IndiaReviewed by:
Adams Hei Long Yuen, Tung Wah College, Hong Kong SAR, ChinaChristopher Morrison, University of Arizona, United States
Copyright © 2025 Zhong, Bi, Yu, Jiang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhitao Dai, ZGFpenRfc2luYXBAMTYzLmNvbQ==; Zhendong Jiang, amlhbmdjaGVueXUwNTEyQDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID: Haidong Yu, orcid.org/0009-0002-5543-129X
Zhengdong Jiang, orcid.org/0009-0005-5545-4805
 Changyou Zhong
Changyou Zhong Suyan Bi
Suyan Bi Haidong Yu1‡
Haidong Yu1‡ Zhitao Dai
Zhitao Dai