- Department of Thyroid-head & Neck Oncosurgery-1, Jilin Cancer Hospital, Changchun, China
Objective: To explore the efficacy of combination therapy of tyrosine kinase inhibitor (TKIs) and immune checkpoint inhibitor (ICIs) in patients with anaplastic thyroid carcinoma (ATC).
Method: This study enrolled 7 patients with ATC between June 2020 and June 2024, and conducted follow-up for a minimum of 6 months post-treatment, with the longest follow-up duration reaching 4 years. Patients received treatment with the TKI-Anlotinib and the ICI-Sintilimab, administered every 3 weeks in a treatment cycle.
Result: Following treatment, among the 7 patients, the primary lesion in 3 patients who had not undergone thyroidectomy exhibited significant shrinkage, the lung metastases in 5 patients with pulmonary involvement significantly regressed, and the lateral cervical lymph node metastases in all patients demonstrated notable reduction.
Conclusion: Combination therapy of TKIs and ICIs for the treatment of ATC patients can be highly effective and bringing better therapeutic outcomes and quality of life for patients.
Introduction
Anaplastic thyroid carcinoma (ATC) is rare and highly aggressive kind of thyroid decease which accounting for approximately 1%-2% of all thyroid cancers (1, 2). ATC typically occurs in the elderly, especially in individuals over the age 60, with a slightly higher incidence in female (1). Family history of thyroid carcinoma, exposure to high dose of radiation and history of thyroid nodules or thyroid carcinoma can be the risk factors of ATC. Most patients are found to have ATC with hoarseness, difficulty swallowing and breathing, or rapidly enlarging neck mass (3, 4). Treatment for ATC is often intractable, as ATC responses poorly to traditional treatment (1).
Anlotinib, a multikinase inhibitor, targets VEGFR/FGFR associated with angiogenesis, e-Kit kinase, and PDGFR-mediated downstream signaling pathways (5, 6). Since hypoxia-induced angiogenesis facilitates the advancement of ATC, anti-angiogenic treatment plays a crucial role in the management of ATC (5). The mechanism of action of Anlotinib involves the inhibition of tumor angiogenesis and the modulation of the immune microenvironment (7–9). By targeting multiple kinases, Anlotinib disrupts signaling pathways that are crucial for tumor growth and survival (6, 10).
Sintilimab is a human immunoglobulin G4 (IgG4) monoclonal antibody, and its principle is mainly to block the interaction between the PD-1 receptor expressed by T cells and its ligands PD-L1 and PD-L2, thereby relieving the immune suppression effect and enhancing T cells’ immune surveillance and killing ability against tumors (6, 11).
In this study, we used anlotinib and sintilimab to treat patients with ATC and achieved remarkable results. This study believes that the combination of targeted therapy and immunotherapy is very useful for the treatment of ATC and deserves further research and exploration.
Materials and methods
This is a single-center, retrospective study. Data were collected from 7 patients with ATC between June 2020 and June 2024. All 7 patients underwent combination therapy with TKI-Anlotinib and the ICI-Sintilimab and provided written informed consent for the clinical study. This retrospective investigation was approved by the Ethics Committee of Jilin Cancer Hospital.
Patients received oral administration of the TKI anlotinib at a daily dose of 12 mg for two consecutive weeks, followed by a one-week break. Every three weeks, they were administered an intravenous infusion of the PD-1 inhibitor sintilimab at a dose of 200 mg.
After 3 months of treatment with TKIs and PD-1 inhibitors, the neck lesions were evaluated by ultrasound and neck CT.
Results
All 7 patients received combination therapy of TKIs and ICIs
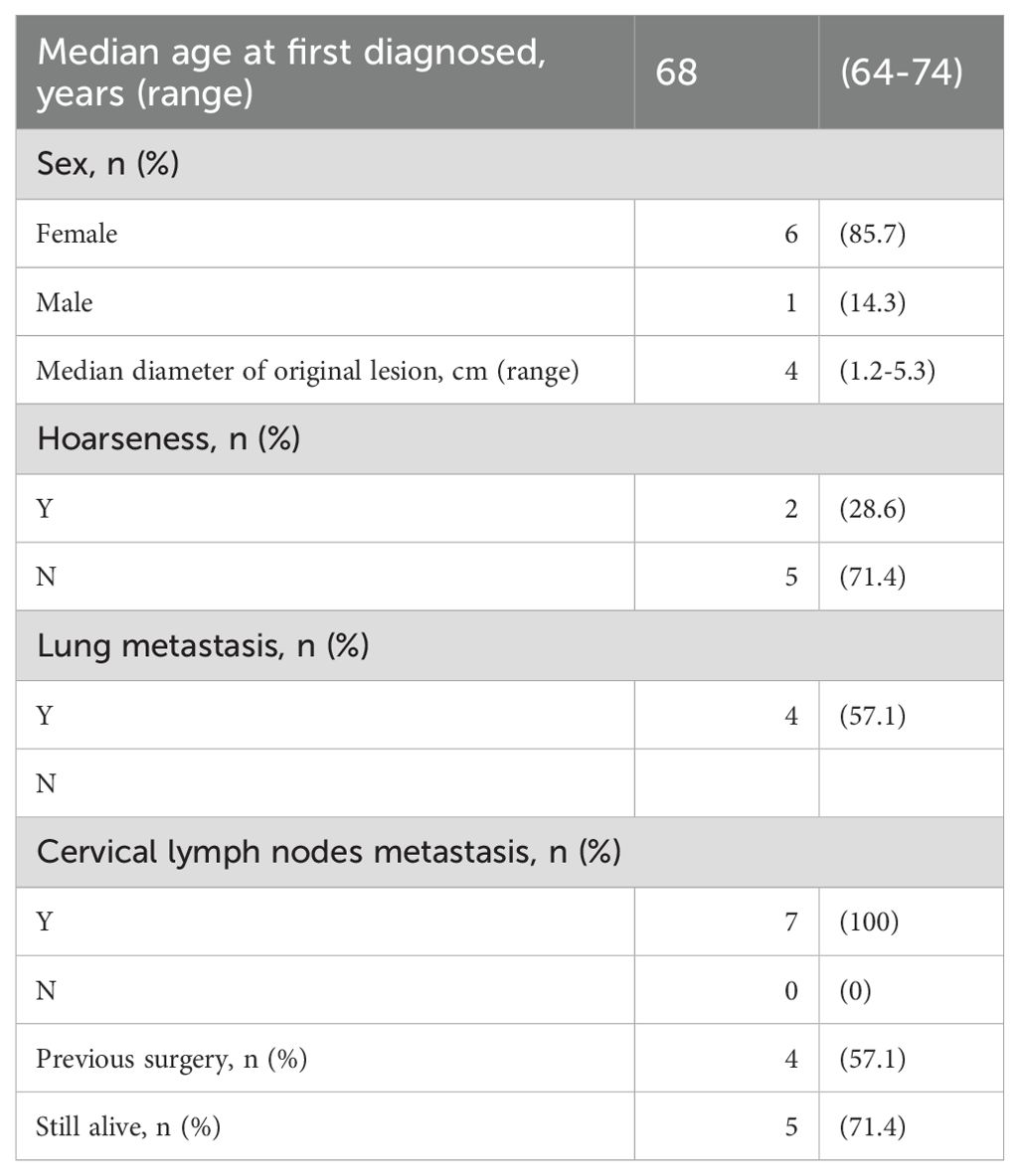
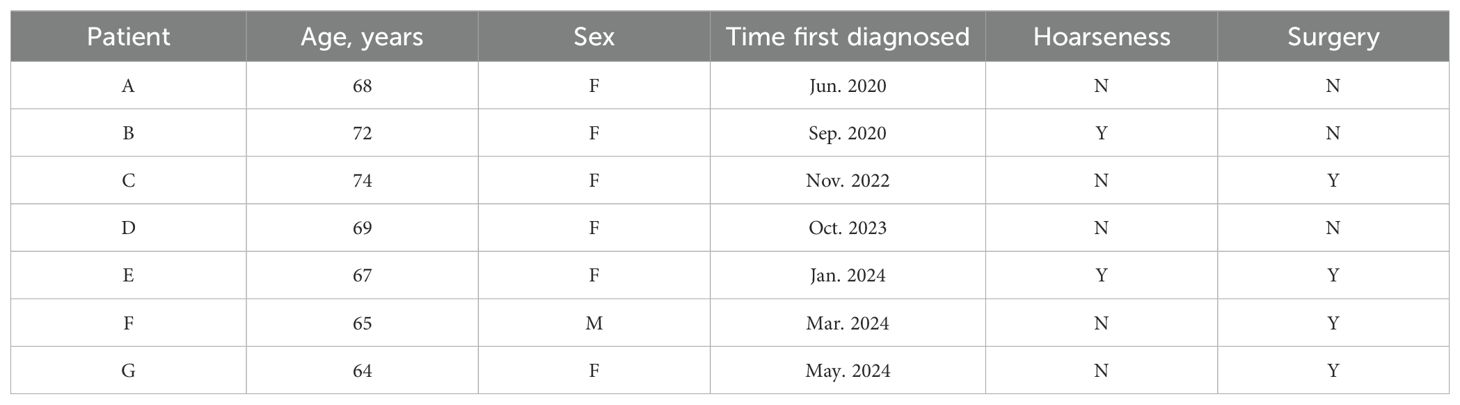
The median age of the cohort was 68 years, comprising 6 female and 1 male patient (Table 1). At the time of initial diagnosis, the median diameter of the primary thyroid lesion was measured at 4.0 cm. Clinically, 2 patients exhibited symptoms of hoarseness, while all 7 patients demonstrated cervical lymph node metastases, and 4 patients had developed lung metastases. Surgical intervention was pursued in 4 cases, with only 1 patient undergoing a total thyroidectomy (Table 2). All patients were classified as stage IVc. As of the latest follow-up, 5 patients remain alive.
Among the 7 patients enrolled in the study, 3 patients did not undergo thyroidectomy. Following combined therapy, the primary lesions of ATC in 3 patients exhibited significant regression, and the diameters of lateral neck metastatic lymph nodes and pulmonary metastatic nodules were notably diminished in all patients (Table 3).
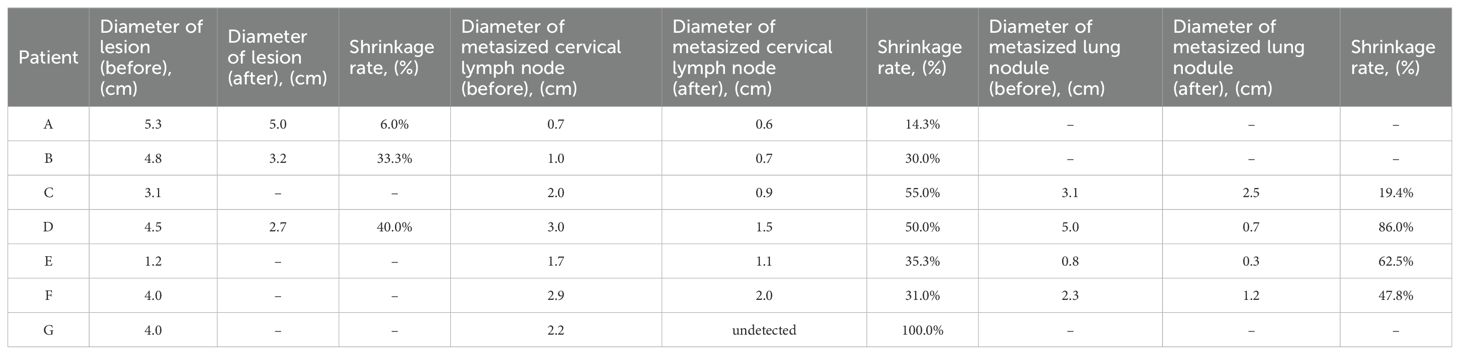
Table 3. Patients' diameter of thyroid lesion, metasized cervical lymph node and metasized lung nodule after 4 combine therapy cycle.
Notably, Patient G, devoid of pulmonary metastases, demonstrated bone metastases in the 12th thoracic vertebra and sacral bone S1 on PET-CT. After treatment, the follow-up PET-CT showed that the hypermetabolic foci were reduced compared with before.
Patient B, also without pulmonary metastases, was found to have a newly developed 6.5 cm malignant tumor in the pancreas on PET-CT 3 years and 3 months after initiating combined therapy, raising suspicion for a metastatic lesion, although the primary origin remained elusive. After 3 years and 7 months of combined therapy, the cervical lesion began to progressively enlarge, indicating suboptimal therapeutic response and potential development of resistance. Due to tumor-induced respiratory compromise, the patient underwent tracheostomy in July 2024. Postoperative radiotherapy was administered using 6MV-X rays with VMAT (Volumetric Modulated Arc Therapy) and IGRT (Image-Guided Radiation Therapy) techniques. A total of 7 radiation fractions were delivered within 10 days, accumulating a dose of 14 Gy. Given the poor radiosensitivity of ATC and the low radiation dose delivered to tissues, radiotherapy was discontinued, and palliative care was recommended.
As shown in Figure 1, Patient F exhibited a substantial mass on the anterior neck prior to the combined therapy. Following the combined therapeutic intervention, the reduction in the size of the mass was quite pronounced.
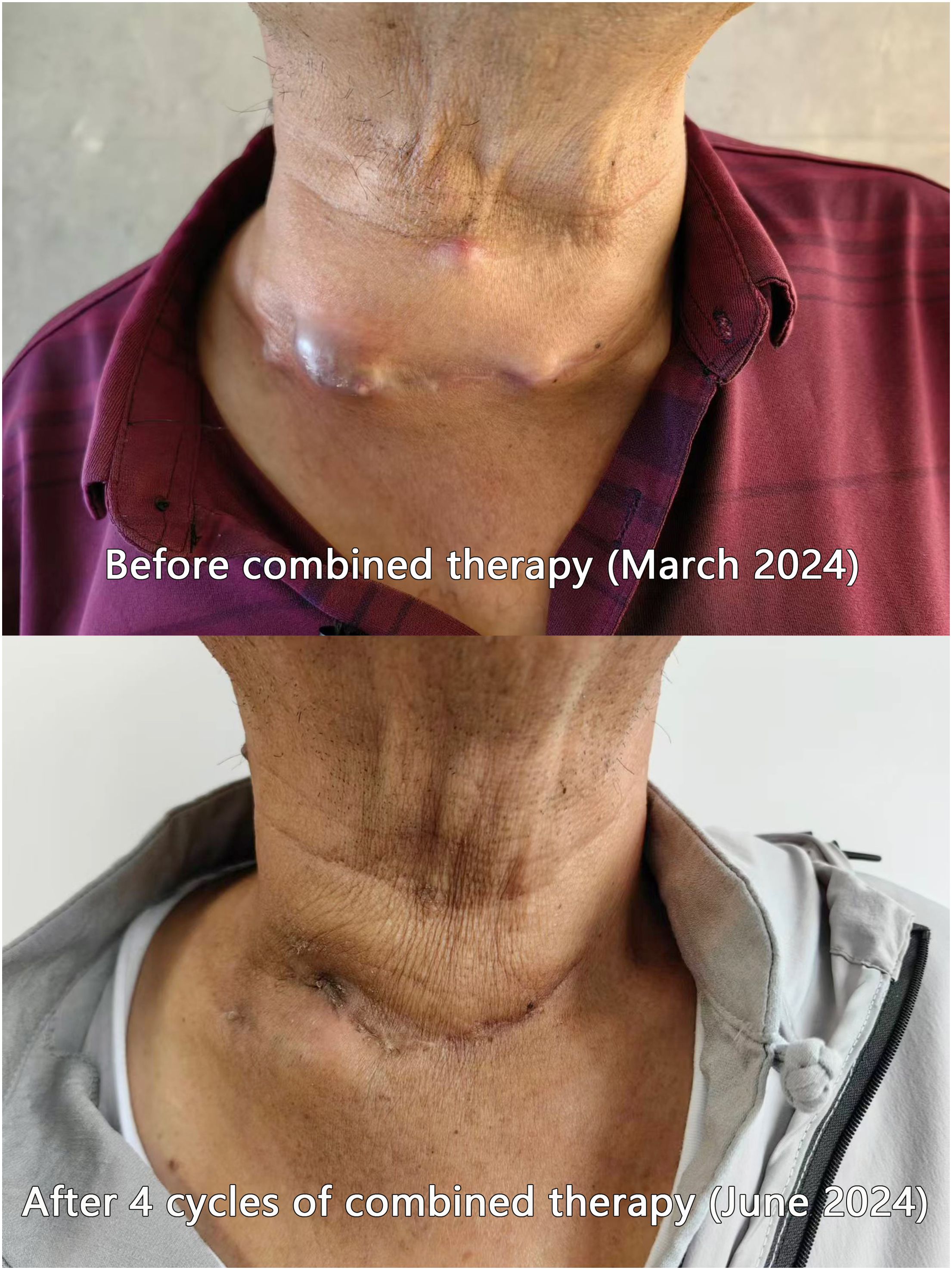
Figure 1. Comparison photos of Patient F before and after combined therapy. As is visible in the patient’s cervical photos, prior to the combined therapy, the tumor exhibited exophytic growth toward the anterior neck, nearly breaching the skin surface. Following the combined therapy, a marked reduction in the tumor size was observed.
The CT images of the cervical and pulmonary regions for Patient D after receiving 2 cycles and 7 cycles of combined therapy are shown in Figure 2. There is a marked reduction in the dimensions of both the primary thyroid lesion and metastatic pulmonary lesion.
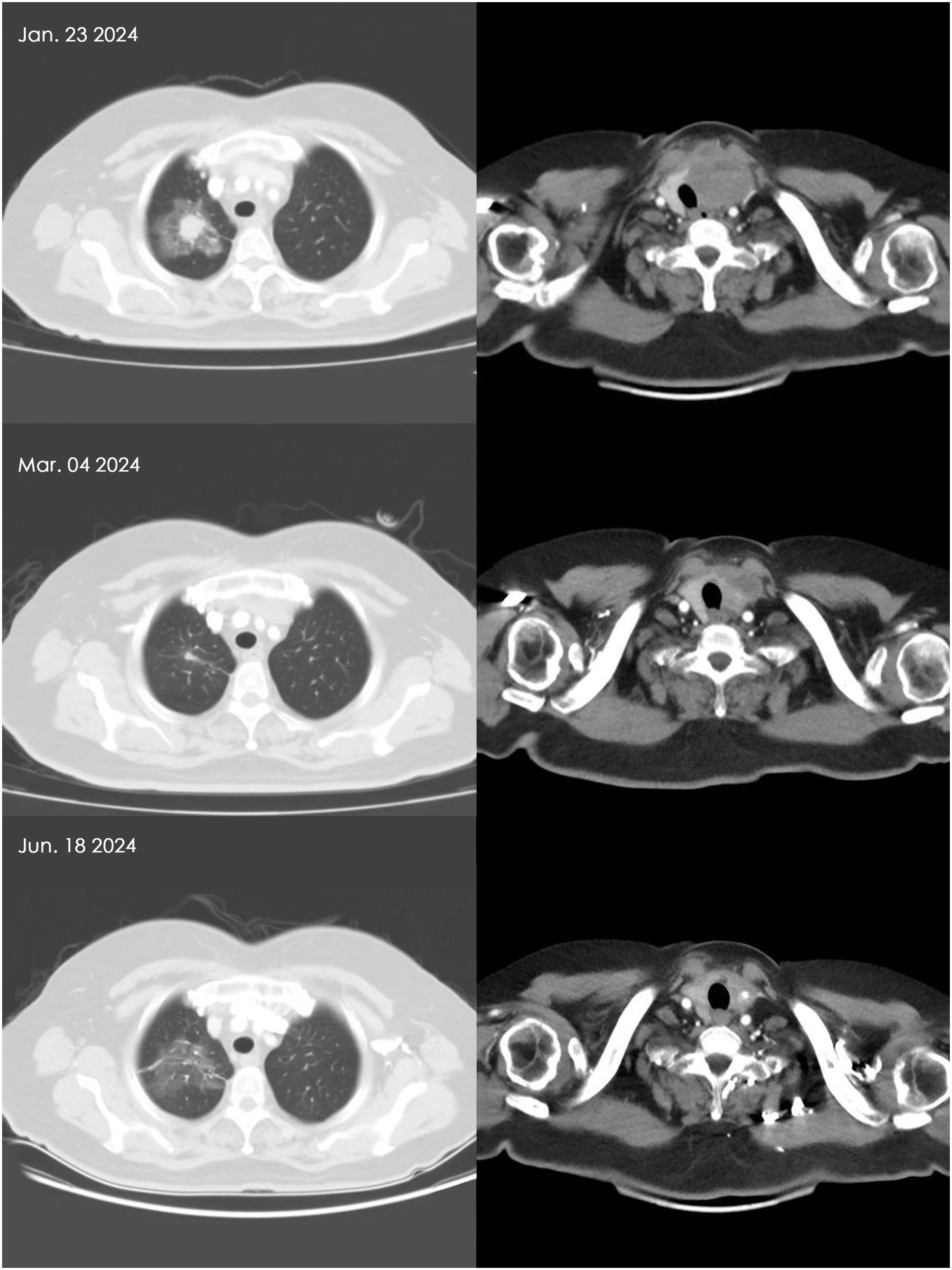
Figure 2. Comparison of thyroid and lung metastatic nodules in Patient D before and after combined therapy. Based on the patient’s CT scan findings, it is evident that the thyroid carcinoma and pulmonary metastatic lesions have appreciably regressed following 6 weeks and 21 weeks of combination therapy.
Conduct the initial efficacy evaluation of patients after 4 combination therapy cycles
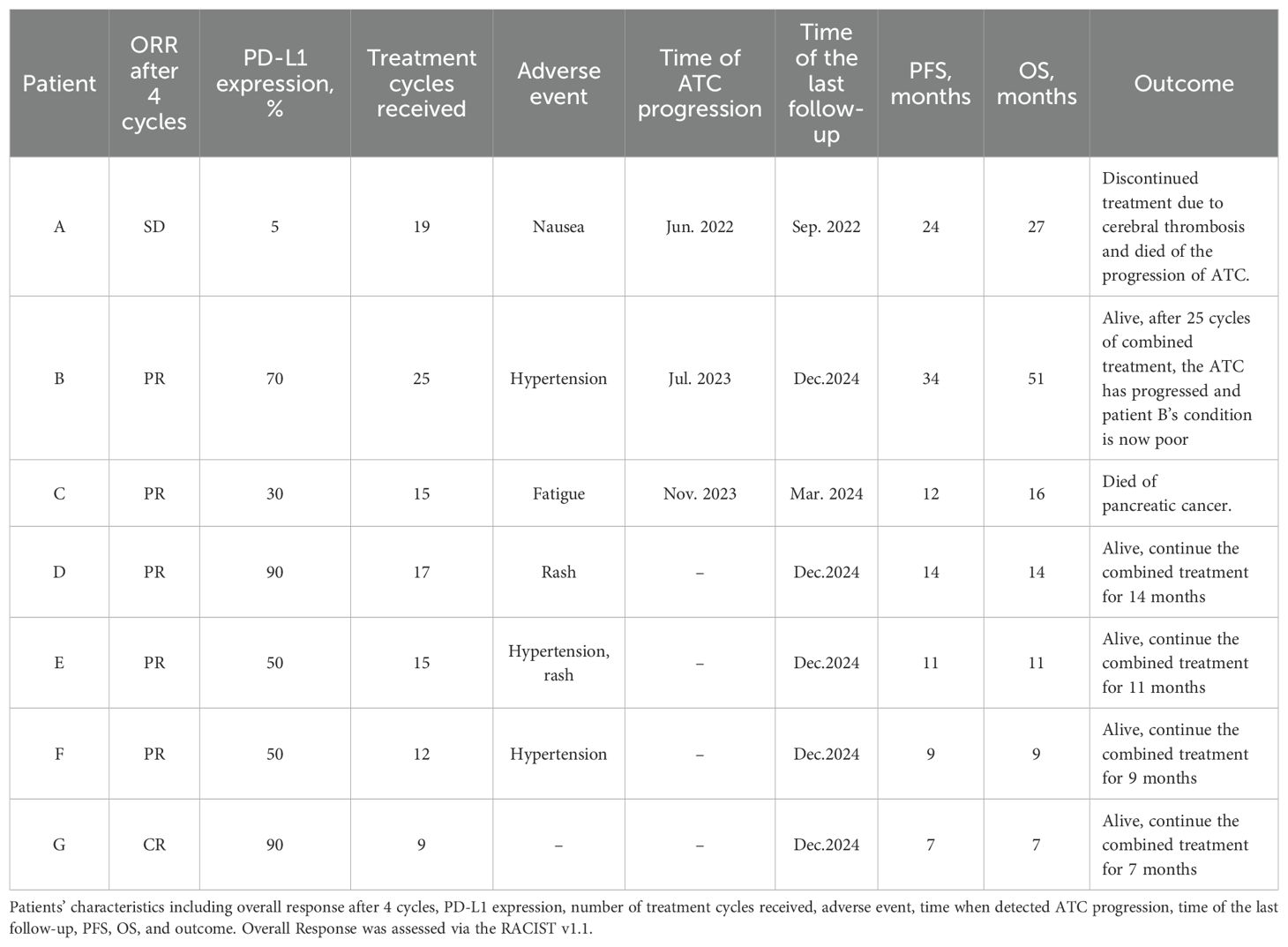
According to the RECIST v1.1 system, 1 patient achieved complete response (CR), 5 patients achieved partial response (PR), and 1 patient showed stable disease (SD). Prior to treatment, we assessed the PD-L1 expression levels of all patients. Patient A exhibited a relatively low PD-L1 expression, with insignificant reduction in the diameters of the lesion and metastatic cervical lymph nodes following treatment. Patient G had a PD-L1 expression level of 90% and attained CR after 4 combination therapy cycles.
As shown in Table 4, the total number of treatment cycles for patients can be observed. Initially, all patients were compliant with medical advice and adhered to regular treatment regimens. However, some patients, due to a subjective sense of well-being, were unable to maintain regular hospital visits for treatment after 10 cycles due to adverse reactions or other reasons. Among the 7 patients receiving treatment, 6 patients developed adverse reactions, with hypertension and rash being the most frequently reported.
Based on the progression free survival (PFS) and overall survival (OS) of the patients, the median PFS is 12 months, and the median OS is 14 months. This study reports 2 cases of patient deaths. Patient A discontinued combination therapy due to cerebral thrombosis and was found to have disease progression of ATC after discontinuation, ultimately succumbing to ATC. Patient C abandoned combination therapy after being diagnosed with pancreatic cancer and died of pancreatic cancer after discontinuation. The discontinuation of combination therapy by these 2 patients may have led to inaccuracies in the PFS and OS values. Notably, Patient B achieved an OS of 51 months.
Discussion
Most ATCs are found to be at IVc stage with distant metastasis and cannot be removed completely by surgical ways (12, 13). Despite several retrospective analyses indicating that a multimodal approach involving surgery along with radiotherapy and chemotherapy can positively influence the prognosis for ATC patients—such as administering postoperative adjuvant treatment with a combination of azithromycin and cisplatin, or implementing high-dose external radiotherapy after surgery—the overall prognosis remains unfavorable because of its high propensity to infiltrate adjacent tissues and metastasize distantly (14). With the development of personalized precision treatment, targeted therapies and immunotherapies have emerged as hot topic in recent years, offering renewed hope to individuals fighting with ATC.
The molecular mechanism of ATC involves various gene mutations and the imbalance of signaling pathways. The BRAF V600E mutation is the most common genetic alteration in ATC, along with mutations in TP53, PAX8, Ki-67, and other genes (15–17). BRAF V600E is the most common early driver in differentiated thyroid cancer, and 50% of ATC cases have a history or are associated with DTC, hence 10% to 50% of ATC cases harbor this mutation (18). Dabrafenib is a BRAF V600E inhibitor, while trametinib is a MEK kinase inhibitor downstream of BRAF (19, 20). The FDA approved the combination of dabrafenib and trametinib for use in BRAF V600E mutation-positive ATC in 2018.
The dysregulation of the MAPK and PI3K/AKT signaling pathways plays a crucial role in the development and progression of ATC (15, 21–23). DNA methylation exhibits high-frequency alterations in ATC, which are particularly associated with histology and correlate with gene mutations.
Anlotinib is a novel TKI developed in China that can inhibit tumor cell proliferation and angiogenesis by suppressing platelet-derived growth factor receptors, VEGFR, and others. The inhibitor acts on vascular endothelial cells through CXCL11-EGF-EGFR signaling, which can prevent the formation of microvessels even under hypoxia, interfere with the growth and proliferation of ATC cells, induce apoptosis, and inhibit migration (24).
Ruan et al. discovered through in vitro cell experiments that Anlotinib has anti-tumor effects on both ATC and papillary thyroid carcinoma cell lines (6). Recent studies have found that hypoxia promotes angiogenesis in ATC, and Anlotinib can effectively inhibit this phenomenon (7, 9). In addition, Anlotinib can block intercellular communication by dual inhibition of ATC and endothelial cells, and it can also reduce angiogenesis by inhibiting the epidermal growth factor receptor (EGFR) pathway. The aforementioned studies suggest that Anlotinib can exert anti-ATC effects through multiple targets, indicating its potential application prospects in ATC treatment.
As an immune checkpoint inhibitor, Sintilimab reactivates T cells’ anti-tumor activity by blocking the PD-1/PD-L1 pathway, thereby achieving the purpose of treating tumors. Currently, the main indications for Sintilimab are for the treatment of classical Hodgkin’s lymphoma, non-small cell lung cancer, and hepatocellular carcinoma (6, 11). A research indicates that the PD-L1’s combined positive scores ≥10, which demonstrated that using Sintilimab to treat patients with thyroid tumors may be effective (25).
Targeted therapy combined with immunotherapy can significantly improve the prognosis of ATC patients. It was reported that a 67-year-old ATC patient had a remarkable tumor shrinkage and an 18.3-month-sustained remission period after using Anlotinib combined with Sintilimab (26). Zichang’s report showed that TKIs combined with ICIs may be an effective way for primary squamous cell carcinoma of thyroid. The tumor volume significantly reduced and shrank after 5 courses of combined treatment of Anlotinib and Sintilimab (26). According to Laurys and Christine’s study, other types of TKIs and ICIs, such as the combination of lenvatinib and pembrolizumab, have also shown significant efficacy in ATC patients (27, 28).
The most common adverse effects of Anlotinib include hypertension, hand-foot skin reaction, oral mucositis, diarrhea, and fatigue. Adverse effects of Sintilimab include immune-mediated pneumonitis, immune-mediated hepatitis, anemia, neutropenia, hypoalbuminemia, hypertension, acute kidney injury, rash, etc. Patients are monitored for these effects, and dose adjustments may be necessary to manage toxicity.
Despite our belief in the efficacy of combination therapy, the emergence of drug resistance over time remains a formidable challenge. Once drug resistance occurs, the progression of the patient’s tumor tends to be extremely rapid, ultimately leading to mortality. Literatures indicate that the median overall survival(OS) is 3–5 months(3-4,34). In this study, Patient B, who has the longest follow-up period, has survived for four years since the detection of ATC following combination therapy. Recently, Patient B has exhibited signs of drug resistance, suggesting a poor prognosis and outcome for her disease. Although combination therapy has not completely eradicated the ATC lesion, it has at least extended the patient’s lifespan. Consequently, investigating strategies to mitigate tumor drug resistance represents a critical area for future research.
Conclusion
Combination therapy of TKIs and ICIs for the treatment of ATC patients can be highly effective. Anlotinib can inhibit tumor cell proliferation and angiogenesis. Sintilimab reactivates T cells’ anti-tumor activity by blocking the PD-1/PD-L1 pathway. The synergistic effect of the two is even more pronounced. We hypothesize that the application of other drugs of the same type may yield comparable effect and bringing better therapeutic outcomes and quality of life for patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Jilin Cancer Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. HL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. HG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing. HJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. (2017) 13:644–60. doi: 10.1038/nrendo.2017.76
2. Xu B and Ghossein R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr Pathol. (2016) 27:205–12. doi: 10.1007/s12022-016-9445-4
3. Smallridge RC and Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). (2010) 22:486–97. doi: 10.1016/j.clon.2010.03.013
4. Prasongsook N, Kumar A, Chintakuntlawar AV, Foote RL, Kasperbauer J, Molina J, et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab. (2017) 102:4506–14. doi: 10.1210/jc.2017-01180
5. Liang J, Jin Z, Kuang J, Feng H, Zhao Q, Yang Z, et al. The role of anlotinib-mediated EGFR blockade in a positive feedback loop of CXCL11-EGF-EGFR signalling in anaplastic thyroid cancer angiogenesis. Br J Cancer. (2021) 125:390–401. doi: 10.1038/s41416-021-01340-x
6. Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, et al. Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer. (2019) 26:153–64. doi: 10.1530/ERC-17-0558
7. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. (2016) 9:105. doi: 10.1186/s13045-016-0332-8
8. Wang G, Sun M, Jiang Y, Zhang T, Sun W, Wang H, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. (2019) 145:979–93. doi: 10.1002/ijc.32180
9. Rahma OE and Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
10. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:120. doi: 10.1186/s13045-018-0664-7
11. Hoy SM. Sintilimab: first global approval. Drugs. (2019) 79:341–6. doi: 10.1007/s40265-019-1066-z
12. O’Neill JP and Shaha AR. Anaplastic thyroid cancer. Oncol. (2013) 49:702–6. doi: 10.1016/j.oraloncology.2013.03.440
13. Onoda N, Sugitani I, Ito KI, Suzuki A, Higashiyama T, Fukumori T, et al. Evaluation of the 8th edition TNM classification for anaplastic thyroid carcinoma. Cancers (Basel). (2020) 12:552. doi: 10.3390/cancers12030552
14. Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, and Beriwal S. Anaplastic thyroid cancer: Prognostic factors, patterns of care, and overall survival. Head Neck. (2016) 38 Suppl 1:E2083–90. doi: 10.1002/hed.24384
15. Pozdeyev N, Rose MM, Bowles DW, and Schweppe RE. Molecular therapeutics for anaplastic thyroid cancer. Semin Cancer Biol. (2020) 61:23–9. doi: 10.1016/j.semcancer.2020.01.005
16. Cabanillas ME, Zafereo M, Gunn GB, and Ferrarotto R. Anaplastic thyroid carcinoma: treatment in the age of molecular targeted therapy. J Oncol Pract. (2016) 12:511–8. doi: 10.1200/JOP.2016.012013
17. Bonhomme B, Godbert Y, Perot G, Al Ghuzlan A, Bardet S, Belleannée G, et al. Molecular pathology of anaplastic thyroid carcinomas: A retrospective study of 144 cases. Thyroid. (2017) 27:682–92. doi: 10.1089/thy.2016.0254
18. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, et al. Correction to: 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer: American Thyroid Association Anaplastic Thyroid Cancer Guidelines Task Force. Thyroid. (2021) 31:337–86. doi: 10.1089/thy.2020.0944
19. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. (2018) 36:7–13. doi: 10.1200/JCO.2017.73.6785
20. Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid. (2019) 29:1036–43. doi: 10.1089/thy.2019.0133
21. Xing M, Haugen BR, and Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. (2013) 381:1058–69. doi: 10.1016/S0140-6736(13)60109-9
22. Yoo SK, Song YS, Lee EK, Hwang J, Kim HH, Jung G, et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun. (2019) 10:2764. doi: 10.1038/s41467-019-10680-5
23. Zhang GQ, Wei WJ, Song HJ, Sun ZK, Shen CT, Zhang XY, et al. Programmed cell death-ligand 1 overexpression in thyroid cancer. Endocr Pract. (2019) 25:279–86. doi: 10.4158/EP-2018-0342
24. Yuan J and Guo Y. Targeted therapy for anaplastic thyroid carcinoma: advances and management. Cancers (Basel). (2022) 15:179. doi: 10.3390/cancers15010179
25. Liu Z, Yu M, Zhao F, and Zhu C. Anlotinib combined with Sintilimab is win-win cooperation for primary squamous cell carcinoma of the thyroid: A case report and literature review. Front Oncol. (2023) 13:976415. doi: 10.3389/fonc.2023.976415
26. Gui L, Liu S, Zhang Y, and Shi Y. A remarkable and durable response to sintilimab and anlotinib in the first-line treatment of an anaplastic thyroid carcinoma without targetable genomic alterations: A case report. Onco Targets Ther. (2021) 14:2741–6. doi: 10.2147/OTT.S305196
27. Boudin L, Morvan JB, Thariat J, Métivier D, Marcy PY, and Delarbre D. Rationale efficacy and safety evidence of lenvatinib and pembrolizumab association in anaplastic thyroid carcinoma. Curr Oncol. (2022) 29:7718–31. doi: 10.3390/curroncol29100610
Keywords: anaplastic thyroid carcinoma, TKIs, anlotinib, ICIS, sintilimab, combination therapy
Citation: Zhang X, Lu H, Lv Y, Gao H and Jin H (2025) Combination therapy of TKIs and ICIs for the treatment of anaplastic thyroid carcinoma. Front. Oncol. 15:1564865. doi: 10.3389/fonc.2025.1564865
Received: 24 January 2025; Accepted: 06 May 2025;
Published: 23 May 2025.
Edited by:
Seema Kumari, Dr. B. R. Ambedkar University, IndiaReviewed by:
Yuan Cao, 960th Hospital of the PLA, ChinaHanqing Lin, First Affiliated Hospital of Fujian Medical University, China
Copyright © 2025 Zhang, Lu, Lv, Gao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Jin, amppbmh1aWlAMTYzLmNvbQ==
 Xi Zhang
Xi Zhang Hongzhi Lu
Hongzhi Lu