- Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de Recherche pour l'Agriculture, l'Alimentation et l'environnement (INRAE), Univ Rennes, Nutrition Metabolisms and Cancer, Rennes, France
Liver cancers show high interindividual and intratumor heterogeneity. Among them, hepatocellular carcinoma (HCCs) represents approximately 90% of liver cancers, followed by intrahepatic cholangiocarcinoma (iCCA; ~10 to 15%), childhood hepatoblastoma, angiosarcoma and hemangioendothelioma (< 1%). More than 80% of HCCs arise in a backdrop of chronic inflammatory liver diseases of diverse etiologies. These underlying liver diseases are major determinants of geographic diversity of HCCs. Across the world, substantial differences in the prevalence of chronic viral hepatitides, alcohol misuse, Metabolic Disfunction-Associated Steatotic Liver Disease (MASLD) and exposure to toxic substances are frequently related to social and economic inequalities. Vulnerable populations are more frequently exposed to infections such as hepatitis B and C viruses that, combined with other risk factors, lead to both vertical and horizontal transmission and, in turn, impact on age and sex-related diversity. In this review, we describe the global landscape of risk factors leading to HCC: MASLD, chronic hepatitis B and C infections, alcohol misuse, exposure to other toxic substances and genetic predispositions. We describe their combined effects on the clinical and epidemiological features of HCCs around the globe. Clinical presentation, incidence and mortality rates of HCCs show therefore great geographic heterogeneity, which is also related to the inequalities in the gross domestic product per capita, the socio-demographic index, the access to health care resources and to the implementation of policies for surveillance and screening of patients at risk. Awareness of the biological and geopolitical sources of HCC diversity will hopefully lead to more efficient international cooperation in the prevention and early management of chronic liver diseases and HCC.
Introduction
With approximately 800,000 deaths per year, liver cancer is the third deadliest cancer worldwide. According to the major cell type involved, liver cancers are classified as hepatocellular carcinoma (HCCs; ~90%), intrahepatic cholangiocarcinoma (iCCA, ~10 to 15%), childhood hepatoblastoma, angiosarcoma and hemangioendothelioma (< 1%). HCC occurs in over 80% of cases in the context of chronic fibroinflammatory liver diseases, and its heterogeneity complicates patient management (1). Despite advances in vaccination against hepatitis B virus and direct antivirals against hepatitis C virus, the incidence of HCC is increasing due to metabolic dysfunction-associated steatotic liver disease (MASLD), related to overweight, obesity, sedentary lifestyles and important changes in nutritional behaviors across the world (2, 3).
Therapeutic targeting of immune checkpoints has expanded treatment options for HCC; however, a response rate of 30% underscores the need to characterize tumor heterogeneity to better predict treatment response and target prescription (4). Surveillance for HCC emergence in patients with chronic liver diseases relies on biannual ultrasound or magnetic resonance imaging to detect small early-stage tumors that are candidates for potentially curative therapies (local ablation, resection or transplantation). However, high (up to 70%) 5-year recurrence rates after HCC resection or even higher after percutaneous ablation make liver transplantation the best possible treatment, with a recurrence rate of 10% (5). Approximately 80% of HCCs have metastases and/or local extension at diagnosis, limiting the access of patients to potentially curative therapies (4). Also, 30% to 40% of HCCs exhibiting clinical and molecular signs of good prognosis eventually recur within two years of resection (6, 7). HCC results from combined effects of comorbidities that lead to fibrogenesis, clinical signs of impaired liver function, and concomitantly, development of pre-cancerous and cancerous liver nodules (5). Some of the synergistically interacting comorbidities that contribute to HCC include social vulnerabilities and/or psychopathological conditions that result in alcohol and/or intravenous drug misuse. Also, as we will discuss in detail below, the epidemiology as viral hepatitides and MASLD is subject to significant geographic diversity that mirrors global social inequalities (8).
Therefore, the aim of this review is to raise awareness on the biological and socioeconomic sources of HCC diversity. This awareness will hopefully lead to more efficient international cooperation in the prevention and early management of chronic liver diseases and HCC.
To fulfill this aim, we first describe data sources and methods applied to construct this review, which are followed by a brief introductory overview on the epidemiology of liver cancer and on the pathophysiological features of HCC and iCCA. Then, we review the impact of socioeconomic diversity on liver cancer incidence and mortality and on the global landscape of risk factors. These concepts are further developed with an in-depth description of the clinical and epidemiological landscape of liver cancer in different world regions. Finally, we outline efforts across the world to improve surveillance of at-risk patients.
Data sources and methods
Data presented in this article are mainly based on the GLOBOCAN database compiled by the Global Cancer Observatory (GCO), coordinated by the International Agency for Research on Cancer (IARC). When appropriate, data are completed and/or compared with those issued from the Global Burden of Disease (GBD) study from the Institute for Health Metrics and Evaluation. In addition, when trends require to delve deeper on specific epidemiological or pathophysiological points, we refer to independent studies published in peer-reviewed scientific literature indexed in PubMed. Given that HCCs represent 90% of liver cancers, publicly available international registries like GLOBOCAN and the GBD study lump together different histological types of primary liver cancers. Thus, except when indicated otherwise, data on epidemiological trends, incidence and mortality rates apply to primary liver cancers, without distinction.
The GLOBOCAN database and the GBD study rely on different estimates. While both provide valuable information, being aware of their methodological specificities may help data interpretation and avoid pitfalls such as erroneous conclusions generated by sampling biases and/or reporting from inhomogeneous sources across the world.
GLOBOCAN uses population-based cancer registries, collecting data from specific geographic areas and providing cancer incidence and mortality. In countries where registries are absent or insufficient, GLOBOCAN uses statistical modeling estimates. These are based on IARC collaborations with national cancer registries worldwide. For example, when national data are not available, sub-national data with coverage > 50% are used. To accomplish this goal, IARC launched the Global Initiative for Cancer Registry Development, creating regional hubs with dedicated staff for cancer registration, which include the Caribbean, Latin America, Northern Africa, Central and Western Asia, Pacific Island, South East and South-Eastern Asia and Sub-Saharan Africa (9, 10).
While GLOBOCAN is primarily focused on cancer, the GBD study covers a wide range of health risks and outcomes. GBD uses a variety of data sources, including verbal autopsy (VA) reports, hospital records and surveys; hence integrating heterogeneous data from multiple sources. Thus, statistical modeling and standardization in the GBD study are designed to minimize the effect of biases and uncertainty in low- and middle-income countries, where data availability is often limited. VA reports are a key tool in the GBD study. Since many countries lack reliable registration systems, causes of death are missing. Thus, VA is used to determine causes of death and cause-specific mortality fractions in populations without complete registries. They are obtained by trained interviewers using a standardized questionnaire to collect information about signs, symptoms and demographic features of a recently deceased individual. The SmartVa open source algorithm has been developed to assign causes of death to VAs by applying clinical diagnostic gold standards (11).
While the GBD’s VA-based estimations offer valuable insights, it is important to interpret them with an understanding of their limitations. One significant limitation of this method is that VA data are less consistent than pathology reports. Consequently, VA reports might inadvertently combine data from primary liver cancers with liver metastases originating from extrahepatic cancers. A second caveat is the documented failure to reproduce analyses from VA data, which likely stems from discrepancies in data pre-processing and parameter weighting. This contingency highlights the importance of publishing in GitHub the necessary code to replicate the work (12). In view of these limitations, we emphasize the relevant caveats when appropriate. In some cases, these limitations are due to incomplete registries and sampling biases, which can arise from disparities in the material and human resources allocated to public health authorities worldwide. By drawing attention to these issues, we hope to highlight regions and risk factors that particularly require international collaboration.
Global overview on the epidemiology of liver cancer
As HCC is the most common form of liver cancer (13), both GLOBOCAN 2022 and the GDB study report data for primary liver cancers regardless of the histological type. Thus, unless specifically referring to data on one liver cancer type in particular, we report data on HCC and iCCA taken together.
In 2022, liver cancer was the sixth most common tumor and the third cause of cancer-associated death in the World, after lung and colorectal carcinomas, according to the International Agency for Research on Cancer (IARC) (8, 9, 14) with a global age-standardized incidence rate (ASIR) per 100,000 individuals of 12.7 in males; 4.8 in females and an age-standardized mortality rate (ASMR) per 100,000 individuals of 10.9 in males and 4.1 in females. Liver cancer remains the most common cause of cancer-related mortality in males in Mongolia; in many African countries, such as Egypt, Sudan, Mauritania, Senegal, Sierra Leone, Liberia, Ghana, Burkina Faso, Niger, Gabon, as well as in South-East Asian countries like Thailand, Laos, Cambodia, Vietnam and Papua New Guinea. Respectively, male/female ASIRs per 100,000 individuals are the highest in East Asia, in particular in Mongolia (22.4/7.2), in South-East Asia (21.2/6.8), followed by Australia-New Zealand (10.4/3.1), North America (10.0/3.6), Western Europe (8.5/2.8), Western Africa (9.7/5.7) and South America (5.4/3.5).
Figure 1 presents the estimated ASIRs and ASMRs of HCC and intrahepatic bile duct cancers worldwide per 100,000 individuals for men and women and Supplementary Table S1 lists age-standardized rates for 185 countries, according to GLOBOCAN 2022. The Asia-Pacific region, Southeast and sub-Saharan Africa account for approximately 85% of the total liver cancer cases worldwide. China represents approximately 50% of all cases.
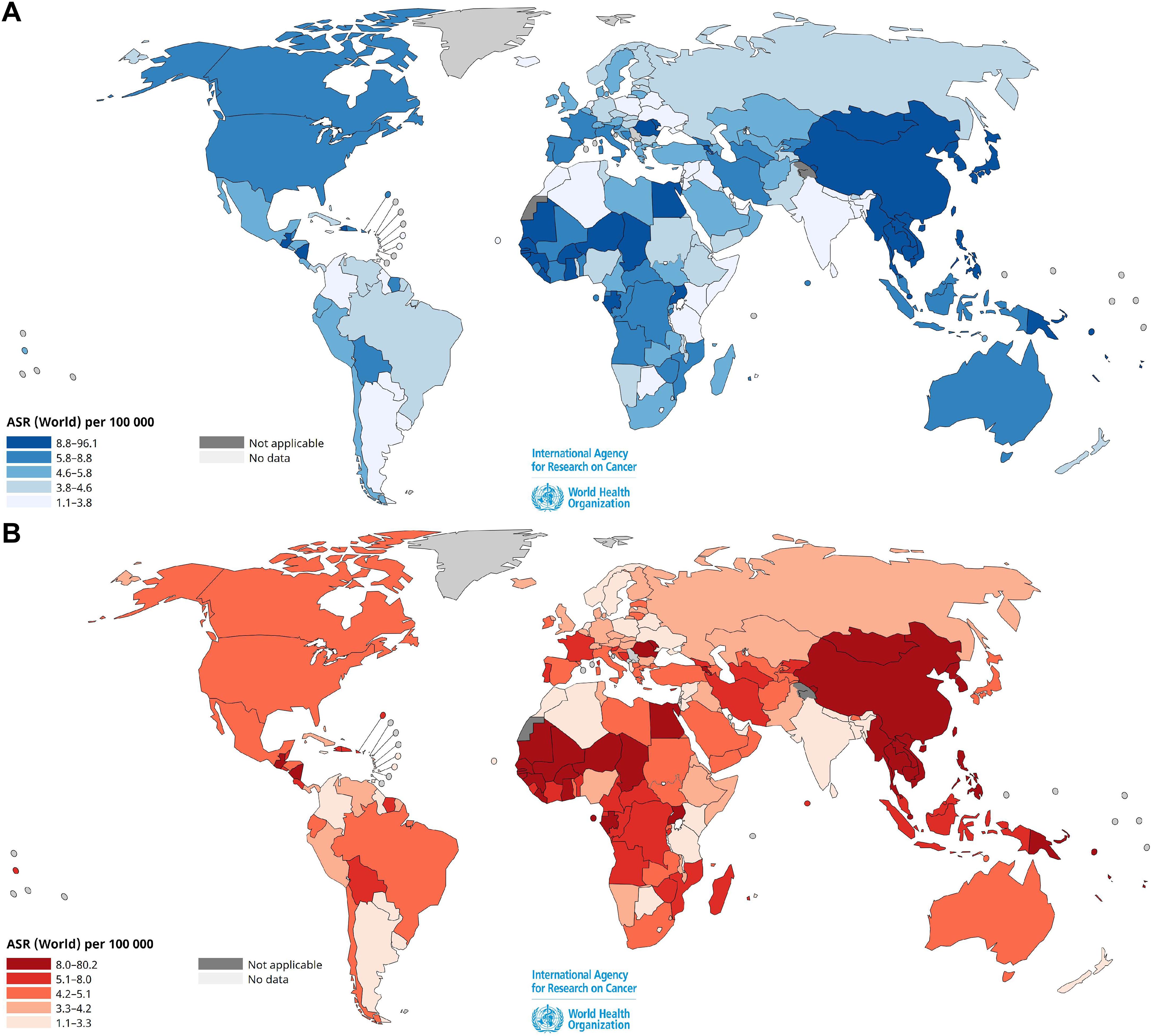
Figure 1. Estimated age-standardized incidence (A) and mortality (B) rates of liver and intrahepatic bile duct cancers worldwide per 105 individuals, for both sexes in 2022. Data source: GLOBOCAN 2022, Cancer Today, International Agency for Research on Cancer, World Health Organization (https://gco.iarc.fr/en). Age-standardized rates (ASR) for 185 countries are shown in Supplementary Table S1.
According to the GBD study, liver cancer is responsible for approximately one million new cases worldwide, with 800,000 deaths (15). In view of this global trend throughout the last three decades, the World Health Organization (WHO) estimates that more than one million patients will die with a diagnosis of liver cancer in 2030 (16). In consistency with these data, Figure 2A shows the GLOBOCAN’s estimated number of deaths from 2022 to 2050 for HCC and intrahepatic bile duct cancer impacting both men and women. The highest increases are seen in Africa (+149% for women and +145% for men) and in Latin America and the Caribbean (+106% for women and + 104% for men). Notably, in Asia, a higher increase is predicted for women (+108%) than for men (+80.4%). A closer look at the estimated timeline of new predicted cases of liver cancer-related deaths from 2022 through 2050 in 185 countries from five continents (Figure 2B, Supplementary Table S2), according to GLOBOCAN 2022 (9), anticipates alarmingly steeper slopes of the curves after 2025, with the Latin America/Caribbean region approaching the trends of Asia and Africa. This epidemiologic modeling was done assuming that the national rates, as estimated in 2022, and the national population projections, do not change throughout the 2022–2050 period. Thus, the expected number of new liver cancer-related deaths was calculated by multiplying the estimated age-specific mortality rates in 2022 by the expected population for a given year extracted from the United Nations, World Population Prospects (2019 revision) (9). In consistency with these data, an independent analysis of the global burden of primary liver cancer in 2020 predicts a rise of >55% in 2040 (17).
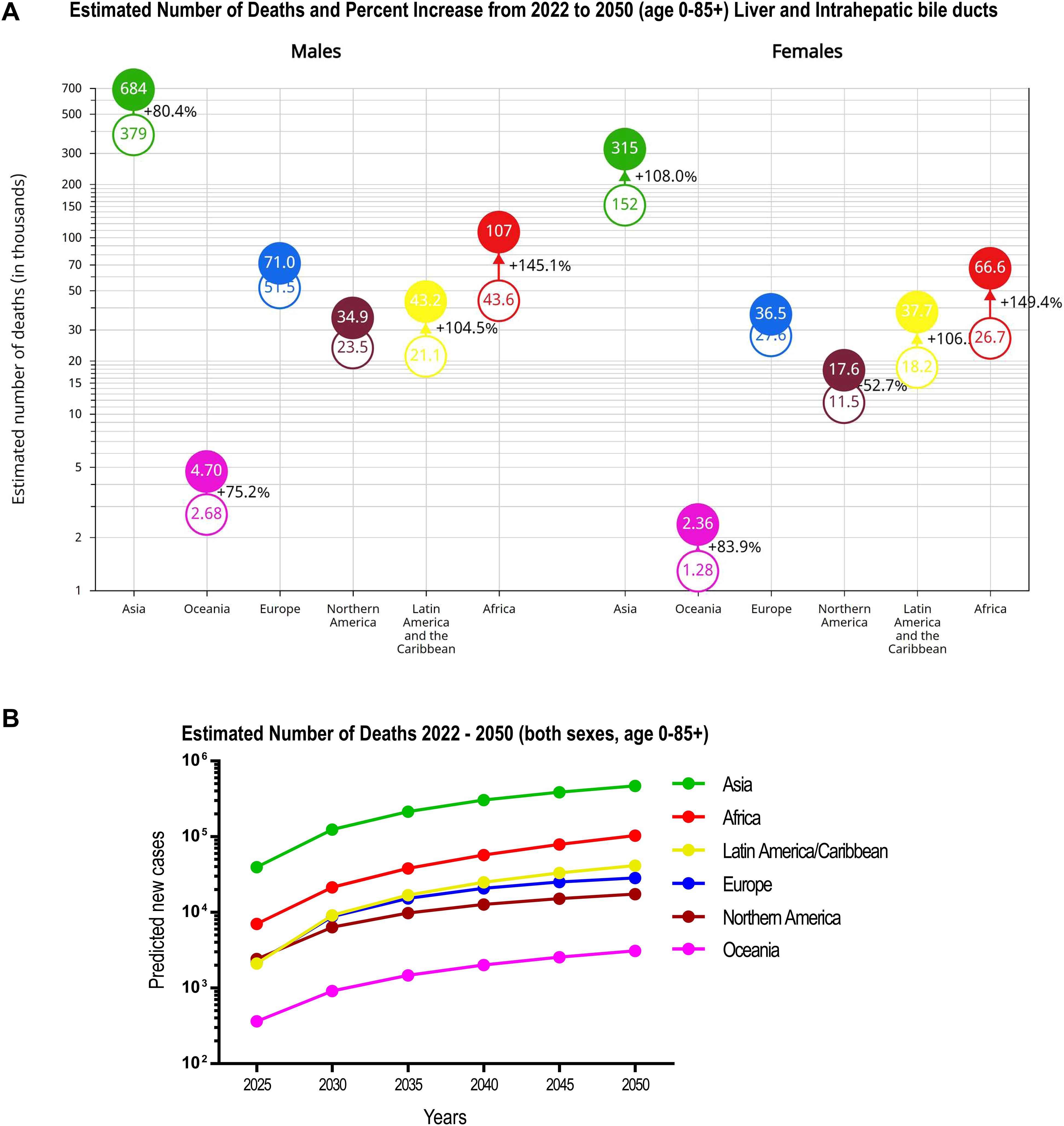
Figure 2. Estimated number of deaths and percent increase projection from 2022 to 2050 for liver and intrahepatic bile duct cancer. (A) Bubble chart showing estimated number of deaths and % increase in risk change. (B) Estimated timeline of new predicted cases of liver-cancer-related deaths (2025 through 2050) in five continents. Predicted numbers, risk and % change in five continents are shown in Supplementary Table S2. Data were obtained from the Global Cancer Observatory: Cancer Tomorrow, GLOBOCAN 2022, version 1.1 (August 02, 2024) (9). Data predict the future incidence and mortality from the current estimates in 2022 up until 2050, in 185 countries or territories grouped in five continents for both sexes. The key assumptions are that the national rates, as estimated in 2022, and the national population projections do not change throughout the 2022–2050 period. The expected number of new liver cancer-related deaths is calculated by multiplying age-specific mortality rates estimated for 2022 by the expected population for a given year extracted from the United Nations, World Population Prospects (2019 revision) (9).
Pathophysiological features of hepatocellular carcinoma and intrahepatic cholangiocarcinoma
Although over the last years considerable progress has been made in the understanding of the molecular pathways driving liver carcinogenesis; early diagnosis, treatment, surveillance and prognosis of liver cancers remain major public health challenges worldwide. Notably, clinical and pathological features of liver cancers are subject to geographic variations in exposure to risk factors, at-risk patient surveillance, management and access to potentially curative treatments (8).
HCC and intrahepatic cholangiocarcinoma (iCCA) have different pathogenesis; hence the modes of presentation, clinical, morphological and imaging features differ. A background of chronic fibroinflammatory liver diseases lead to repeated bouts of inflammation and hepatocyte death, followed by hepatocyte regeneration, fibrogenesis and ultimately to HCC (Figure 3). Over time, this process results in hepatocyte retro-differentiation and amplification of a contingent of bipotent epithelial liver progenitor cells, which promotes genomic instability and thus the emergence of preneoplastic and neoplastic foci. The lobular liver tissue architecture progressively changes into regenerative hepatocyte nodules outlined by fibroinflammatory septa, wherein biliary metaplasia of hepatocytes and ductular reaction are commonly observed. These architectural changes, together with sinusoidal capillarization, impair vascular flow and hepatocyte metabolic functions. Chronic inflammatory liver disease has been compared to a minefield; hence the term “field effect” to embody the risk of emergence of HCC in a backdrop of severe, life-threatening liver disease (18, 19).
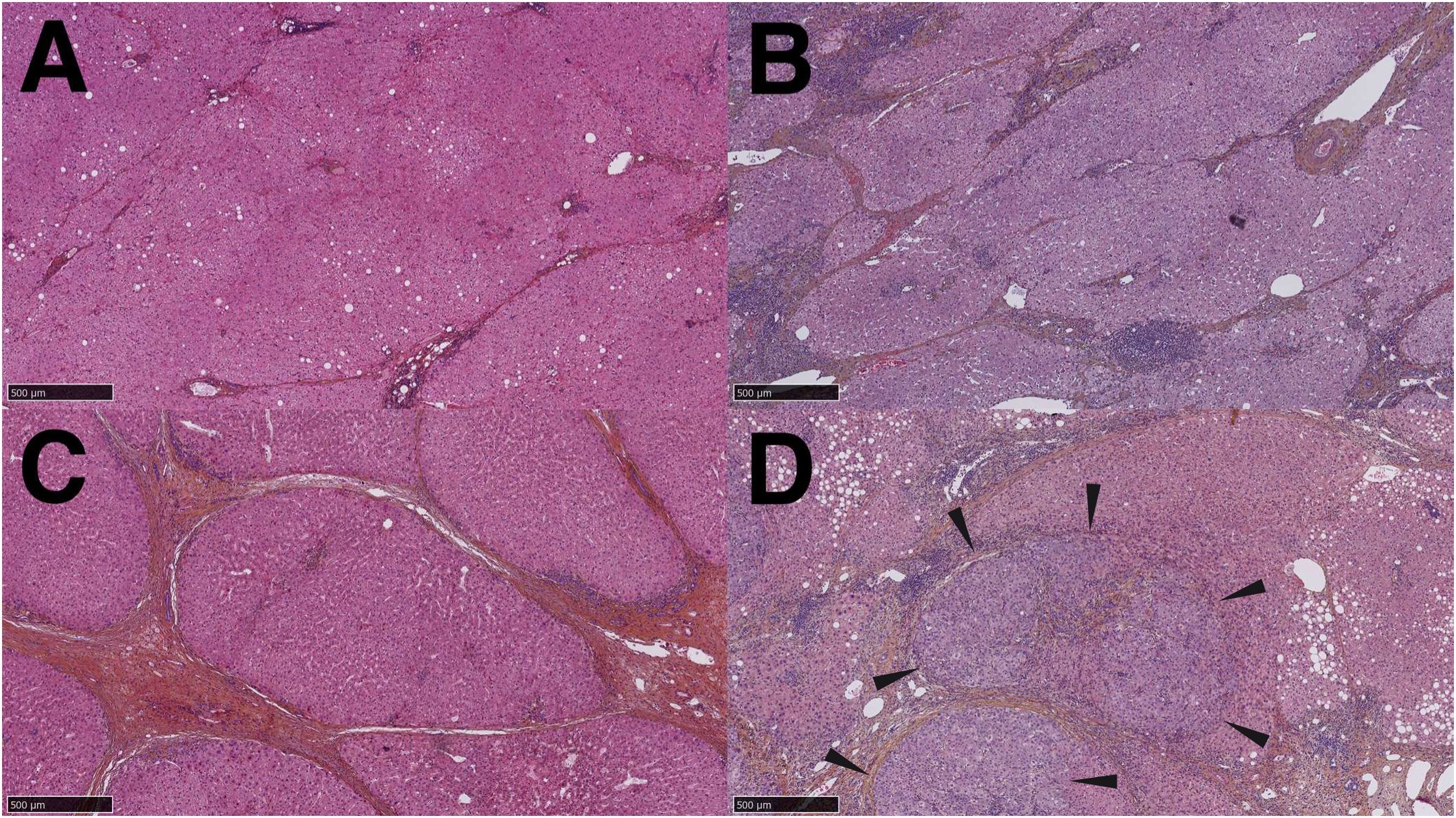
Figure 3. Stages of progression from chronic fibroinflammatory liver disease to hepatocellular carcinoma. (A). Hepatic fibrosis stage 2, small and thin fibrous bands extend from the portal tracts. (B). Stage 3 liver fibrosis, thick fibrous tracts partially outline regenerative nodules. (C). Stage 4 liver fibrosis (cirrhosis), fibrous bands completely outline regenerative nodules. (D). Hepatocellular carcinoma arising within a cirrhotic nodule: a tumor with architectural and cytological features of hepatocellular carcinoma is clearly distinguished from the surrounding parenchyma (arrowheads).
Intrahepatic cholangiocarcinoma (iCCA) is a primary liver malignancy with increasing global incidence, particularly in Western countries. Major risk factors include chronic liver diseases (HBV, HCV, MASLD/MASH), primary sclerosing cholangitis, parasitic infections affecting the liver, and exposure to carcinogens. It involves key genetic alterations such as FGFR2 fusions, IDH1/2 mutations, KRAS, TP53, and BAP1 mutations, although it shares with HCC inflammatory and fibrotic pathways promoting tumor progression. Like HCC, the diagnosis of iCCA relies on MRI and CT scans with contrast enhancement patterns; however, histopathology remains essential; in particular in cases where the differential diagnosis between HCC and iCCA is difficult on the basis of imaging features. Surgical resection is the only curative option, but it is feasible in less than 30% of cases. Like HCC, the prognosis of iCCA remains poor, with 5-year survival under 20%. Thus, early detection and molecular profiling are critical for improving outcomes (20, 21).
Geographic and socioeconomic diversity versus ethnic origin in the appraisal of liver cancer heterogeneity
Applied to Humans, the concepts of “race” and “ethnic origin” are not substantiated by scientific data and they infringe legislation in some European countries. Definition of these populational categories on the basis of skin color and/or facies poses both an ethical and a scientific problem (22). Collection of ethnic data and definition of ethnicity by a third party raises fundamental human rights issues, such as the non-respect of the right of individual self-identification (23). Therefore, we focused this review as much as possible on the reported locations of patient care, that in most cases correspond to the nearest referral hospital to the patient’s dwelling-place. This approach has the advantage of being related to the patient’s exposure to xenobiotics, infectious agents, lifestyle and access to healthcare systems.
Self-reported ancestry respects the fundamental right of self-identification (23) and has a measurable impact on liver cancer epidemiological data. For example, Latin America has a mixed population from different ancestries based on waves of European colonization and African forced emigration through transatlantic slave trade during the 18th century. Later on, in the 19th and 20th centuries, voluntary migration from Europe, Asia and the Middle East, led to high cultural and genetic diversity (24, 25). Thus, data from six countries in the ESCALON European-Latin American network prospectively following 429 hepatobiliary cancer patients (26, 27) showed important differences in liver cancer risk factors in self-reported European and non-European patients. These data will be discussed in the section addressing risk factors. Similarly, in North America, particularly in the USA, immigrants from Eastern, Southern, and Central Europe during the late 19th to early 21st centuries, and more recently from Latin America, Asia, and the Caribbean, have significantly shaped American society (28). While not legally classified as immigration, the transatlantic slave trade, which began in the early 17th century and continued until 1865, played a crucial role in forming a diverse population with varied ancestries (29). Therefore, as discussed below, integrating analyses from cancer and risk factor registries with self-reported ancestry data offers valuable insights into how risk factors differently impact various communities in the USA (30, 31).
Analyzing the impact of population groups, ancestry, and geographic origin of immigrants on the incidence of etiology-specific HCC reveals two key insights: First, immigrants bring with them not only their genetic background but also risk factors associated with their original country or region. While the influence of these risk factors may diminish over time, interactions between past and current environmental exposures can result in unique combinations of risk factors. Second, categorizing patients based on skin color, language, or continent and country of origin highlights the complexity of defining population classes, particularly in the Americas. This complexity underscores the need for a more nuanced understanding of the interplay between genetic, environmental, and social factors in health outcomes (32).
Socioeconomic diversity greatly impacts liver cancer incidence and mortality
Globally, in high/very high human development index (HDI) countries, liver cancer ASIR/ASMR per 100,000 individuals are 15.3/12.9 in males and 5.3/4.5 in females; whereas in low/medium HDI countries, liver cancer ASIR/ASMR are 6.2/5.9 in males and 3.1/3.0 in females (9). These data call for cautious interpretation, as ASIR/ASMR in low HDI regions are based on estimations from fragmented data, as pointed out above, and cannot be validated with the same robustness as data in high HDI regions. Rather, the take-home message here is that socioeconomic inequalities also impact the resources allocated to data collection leading to considerable heterogeneity (33).
In an effort to quantify the impact of social inequities, a socio-demographic index (SDI) has been used as a composite indicator of development status, which is strongly correlated with health outcomes. It is calculated as a geometric means of 0–1 indices of income per capita, average years of schooling over the age of 15 and total fertility under the age of 25. Thus, an SDI=0 indicates minimum and SDI=1 indicates maximum levels. SDI is often completed with the universal health coverage index (UHCI), which is reported on a 0–100 scale. It is based on indicators such as direct measures of therapeutic coverage, outcomes, such as mortality-to-incidence ratios and access to quality care (34).
Using the above indicators, a study based on the Global Burden of Liver Cancer (35) revealed an increasing trend in ASIR of primary liver cancer between 1990 and 2019 in nearly half (91/204) of the countries and territories included in the Global Burden of Disease (GBD) study. Surprisingly, using Pearson correlation analysis, the authors detected a low but significant positive correlation of SDI with an increase in ASIR (ρ=0.31) and in ASMR (ρ=0.26) of primary liver cancer among countries or territories with an SDI ≥ 0.7. No correlation was found in regions with SDI < 0.7. Similarly, the authors revealed a significant positive correlation of an increase in ASIR (ρ=0.49) and in ASMR (ρ=0.47) of primary liver cancer among countries or territories with an UHCI ≥ 70. No correlation was identified with UHCI <70. The authors explain this trend by the contribution of immigrant minorities in high SDI Western countries, as seen in the USA, Australia and Canada, where the highest incidence of liver cancer was identified among immigrants from high-risk countries (35). However, and as pointed out above, data issued from high versus low socioeconomic development regions of the world need cautious interpretation, as they are biased by the heterogeneity of the sources feeding estimation models (33). Conversely, registry-based studies in the USA showed that Latin American foreign-born patients with HCC (36) or liver cancer (37) had better survival than US-born patients, and that increasing generational status of Mexican Americans in the USA was associated with a higher risk of HCC (38), suggesting that USA birthplace is a risk factor for liver cancer death in Mexican Americans.
A meta-analysis of 20 studies between 1994 and 2024 including cross-sectional, cohort and case-control studies showed that patients in disadvantaged areas faced delayed treatments and worse outcomes (39). Similarly, a study involving 1,485 patients in China showed that low household income was independently associated with advanced disease and poor outcome in patients with HCC (40).
We thus studied the relationship between Gross Domestic Products (GDPs) per capita and the Mortality-to-Incidence Ratios (MIRs) for liver cancer in 181 countries in 2022. We found that the MIRs for liver cancer were inversely proportional to the GDPs per capita. That is, the higher the GDP, the lower the MIR (Figure 4A). MIRs and GDPs in current US$ for 181 countries in 2022 are listed in Supplementary Table S3. GDPs were obtained from the World Bank Group.

Figure 4. Socioeconomic diversity greatly impacts liver cancer incidence and mortality. (A) Relationship between Mortality-to-Incidence ratios (MIRs) and Gross Domestic Product (GDP) per capita for 181 countries in 2022. MIRs for each country= (ASR mortality per 105 individuals/ASR incidence per 105 individuals) x 100. GDPs and MIRs for each country, obtained from GLOBOCAN 2022, are shown in Supplementary Table S3. GDP is the sum of gross value added by all resident producers in the economy plus any product taxes and minus any subsidies not included in the value of the products. GDPs per capita for the year 2022 in current US$ is the GDP divided by midyear population. Publicly available GDPs per capita were obtained from the World Bank Group under the Creative Commons Attribution 4.0 International license (CC-BY 4.0). The source of the raw data are World Bank national accounts and OECD National Account data files. (B) Fractions of hepatocellular carcinoma etiologies by World regions (41). Regions, continents and etiologies are color-coded. “Number of HCCs” indicates the approximate number of HCC cases studied, which is proportional to circle radius. Circle radius, r= ln (HCC number) x 0.7; i.e., natural logarithm of HCC number x 0.7. Quantitative data for the 21 World regions are shown in Supplementary Table S4.
The impact of poverty is not exclusive to low-income countries. In France, higher HCC incidence is related to an unfavorable socioeconomic environment (42). This evidence highlights the interactions between social inequities and the aforementioned pathological background of comorbidities leading to HCC: alcohol and intravenous drug addictions, unchecked viral infections, as well as imbalanced nutritional and physical activity lifestyles. These risk factors are reviewed in detail in the next section.
Global landscape of risk factors associated with liver cancer
The Population Attributable Fraction (PAF) is the proportion of a disease in a population that can be attributed to a specific risk factor. It estimates the potential reduction in the outcome if the risk factor were eliminated, assuming a causal relationship. PAF is calculated using the prevalence of the risk factor and its relative risk. It helps prioritize public health interventions by identifying the impact of reducing exposure to modifiable risks on overall population health.
Figure 4B presents an overview of HCC etiologies by World regions and quantitative data for 21 World regions are shown in Supplementary Table S4 (41). Figures 5A-C shows World maps with the population attributable fractions of liver cancer by country related to alcohol, HBV and HCV (43, 44).The study of the fraction of liver cancer cases attributable to five major risk factors by geographic region and gender showed that 44% of the World total liver cancer cases were attributable to HBV, 21% to HCV; 26% to alcohol, 13% to tobacco smoking, 9% to obesity and 7% to diabetes (Supplementary Table S4) (41). By contrast, subnational studies in the USA, covering either Florida or California, and based on actual cancer and hospital discharge registries, as well as serologically proven HCV infections, report that over 40% of all HCC cases are attributed to HCV (30, 31). As shown in Figure 5, PAFs are subject to important geographical variations. Below, we present a detailed overview of the global landscape of the major risk factors leading to liver cancer.
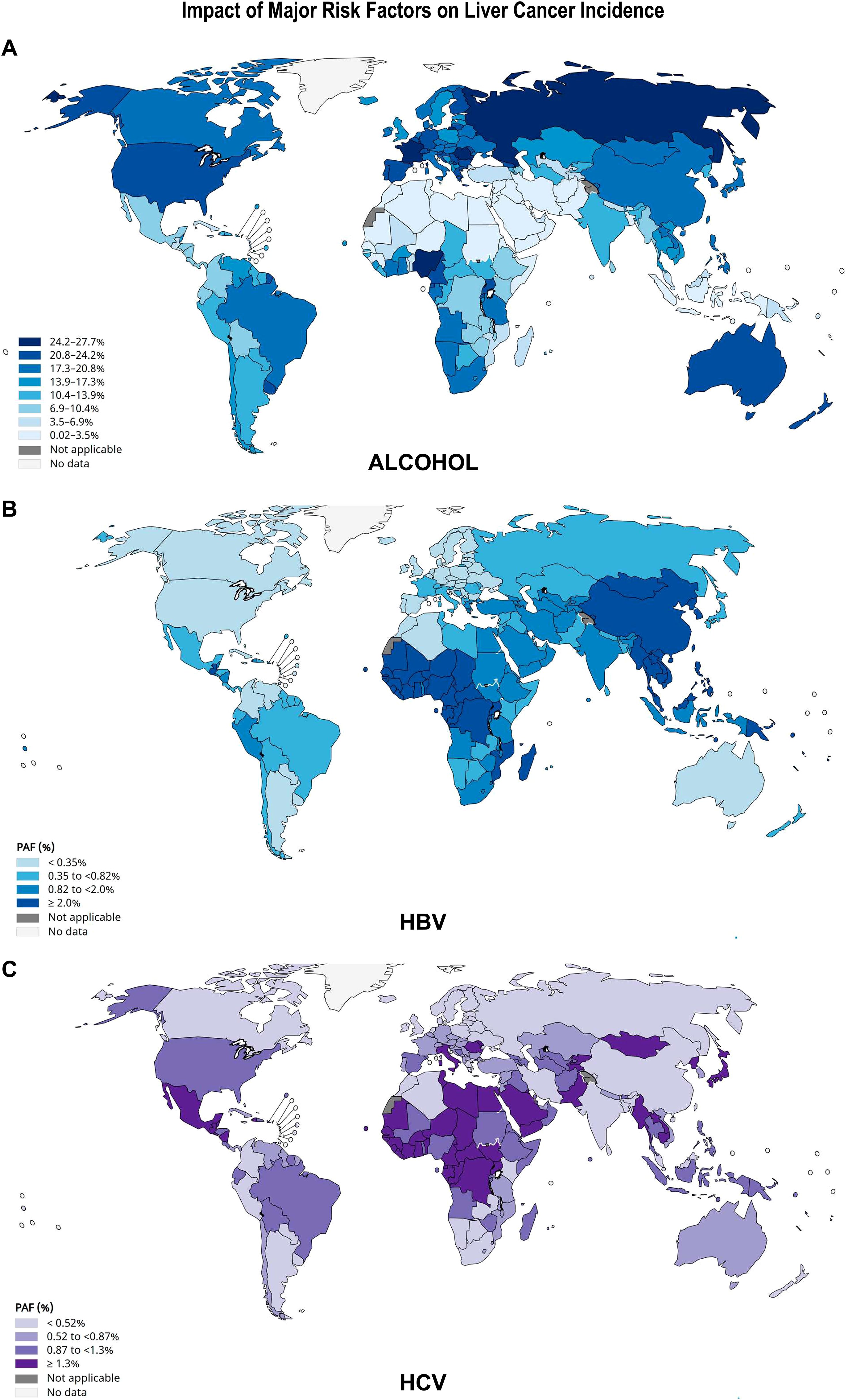
Figure 5. Impact of alcohol, HBV and HCV infections on liver cancer incidence. The population attributable fraction (PAF) is the proportion of cases of liver cancer that could theoretically be avoided if exposure to (A) alcohol; (B) HBV or (C) HCV was removed. Alcohol-related risk was modelled with an upper integration limit of 150 g per day. The authors also estimated the contribution of different levels of alcohol drinking by categorizing consumption into three levels: moderate (0.1–20 g/day; risky, 20–60; heavy, >60 g/day) (43). For HBV and HCV, the number of new cancer cases attributable to each infection was calculated by multiplying incidence estimates by the PAF, according to (44). The scales of color shades on the left are based on an absolute method, each group has the same number of observations. Source: GLOBOCAN 2022.
Alcohol
Chronic alcohol consumption can be categorized into three levels: moderate (0.1–20 g/day; risky, 20–60; heavy, >60 g/day) (43). Numerous studies indicate that, in different concentrations, alcohol has harmful effects on health. A meta-analysis published by Corrao et al. (45) showed that with daily alcohol intake > 25 g/day, there is a relative increase in the development of chronic liver disease. IARC also indicates that higher amounts have direct hepatocarcinogenic effects (46).
Global data on alcohol misuse vary according to the definition of geographic locations and the amounts of alcohol intake considered: 6% in the Middle East; up to 14% in North Africa, between 50% and 60% in Eastern Europe, whereas in Southern Europe (Spain and Italy), the prevalence is about 20% (47). According to the situational report on alcohol consumption published by the WHO in 2016, the highest rate of active drinkers was found in Europe, with 60% of the total population > 15 years old; followed by the Americas with 54.1%, the Western Pacific area with 53.8%, South East-Asia with 33.1% and finally Africa with 32.2% (48).
Alcoholic liver disease (ALD) comprises a spectrum of conditions from reversible fatty liver to acute alcoholic hepatitis, chronic inflammation, fibrosis and cirrhosis, a late stage of disease during which HCC often develops. ALD is a major cause of HCC worldwide (47). In ALD patients, the diagnosis of HCC is often delayed with symptomatic cases at presentation. Patients present with a poorer general condition, more severely impaired liver function and higher prevalence of comorbidities. However, when HCC is diagnosed during surveillance in ALD patients, the rate of allocation to first-line curative treatments is high; although it has to be considered that in these patients there are higher surveillance failure rates; in part related to decreased sensitivity of ultrasound screening. Notably, patients with moderate alcohol consumption (≤ 60 g/day) associated with one or more metabolic risk factors exhibit an aggravated HCC risk profile due to the synergistic effect of MASLD and alcohol (49). Similar results were reported by a worldwide meta-analysis including 86,345 patients, showing that 30.4% of HCCs were ALD-associated, with the highest proportion in Europe and the lowest in the Americas. ALD-related HCCs had a more advanced BCLC (Barcelona Clinic Liver Cancer) stage and higher mortality rates when compared with other causes (50).
The importance of ALD-related HCC led to the development of statistical methods to compare the impact of worldwide public health policies on ALD prevention. Thus, an alcohol preparedness index (API) was recently proposed in a multi-national study including 169 countries between 2010–2019; analyzing data from the Global Burden of Disease database. High APIs were inversely correlated with alcohol use disorder, alcohol-related liver disease mortality and alcohol-attributable HCC. The highest associations were found in the Americas, Africa and Europe. Importantly, alcohol-attributable HCC incidence decreased after 8 years from baseline assessment. Thus, liver cancer-related mortality in regions with API ≤ 10 was about 15 cases per 100,000; whereas in regions with API=100, it was about 3 cases per 100,000 (51).
ALD prevention also involves alcohol rehabilitation to reduce ALD-related HCC risk. The effect of alcohol rehabilitation and abstinence on cancer incidence was assessed in people with alcohol dependence in a nationwide retrospective cohort study. Among 24 million patients discharged from French hospitals between 2018–2021, alcohol dependence was identified in 6.3% men and 1.6% women and was strongly associated with liver, oral, pharyngeal, laryngeal, esophageal and colorectal cancers. As expected, rehabilitation treatment or abstinence was associated with lower cancer risk (52).
Although most Western European countries benefit from the highest worldwide APIs, there is room for improvement, at least in France. In 2022, an average of 165 g of pure alcohol per week per individual over 15 years of age was sold in France. This is 1.65-fold higher than the upper limit of “reasonable” alcohol consumption recommended by health authorities. The documented impact of alcohol rehabilitation on cancer risk reduction suggests that most individuals, although aware of the health risks related with alcohol misuse, are not aware of their own risk. The reason for this cognitive bias could an underestimation of the amount and frequency of alcohol intake. In France, despite a high API, the information on ALD prevention that the media deliver in compliance with Public Health regulations is stigmatizing (53) and lacks precision: “alcohol abuse is dangerous for your health” and “drink with moderation”. These slogans lead the population to a complacent self-definition of “moderation” and “frequency” of alcohol consumption and, ultimately, to a lack of self-awareness of alcohol misuse and alcohol addiction (54).
The above evidence leads to the core problem of ALD, which is alcohol use disorder (AUD), as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), that lists 11 behavioral criteria for diagnosis and assessment of the severity of AUD. Still, AUD can be difficult to diagnose because many patients do not disclose alcohol use, frequently through lack of self-awareness or denial, and remain oligosymptomatic with subclinical but progressive disease (55).
Taken together, alcohol misuse is a cofactor in the development of chronic liver disease, fibrogenesis and liver cancer. ALD increases the harmful effects of other comorbidities, such as viral hepatitis and/or MASLD. In particular, the growing incidence of MASLD and combined ALD-MASLD worldwide call for public health policies focusing on the social determinants of alcohol dependence and nutritional behavior disorders.
Other toxic substances
Other risk factors not necessarily related to a chronic inflammatory background and fibrogenesis are associated with HCC development, such as tobacco smoking, exposure to genotoxins like aflatoxins and aristolochic acid through the consumption of agricultural products (56–58). Exposure of patients to these chemical compounds can be suspected by the detection of typical mutational signatures in HCCs (59).
The association of tobacco smoking ranges from 4% to 14%, with the highest values in Southeast Asia (Supplementary Table S4) (41). Cigarette smoking is associated with a 70% increased risk of liver cancer (60); the risk progressively decreasing and effectively disappearing after 30 years of tobacco arrest (61). Several studies have identified tobacco smoking as an independent risk factor for liver fibrosis, thereby contributing to liver carcinogenesis. In the liver, smoking leads to inflammation, insulin resistance and tissue hypoxia. Smoking is also seen as an aggravating cofactor in patients with chronic hepatitis, obesity and/or alcohol misuse (62).
Aflatoxins are mycotoxins that show strong hepatocarcinogenic effects. They are produced by Aspergillus parasiticus and Aspergillus flavus fungi that grow in staple cereals and oilseeds stored under favorable moisture and temperature conditions. Aflatoxin contamination is widespread in tropical and subtropical areas around the World, such as Southeast Asia and Sub-Saharan Africa (63, 64).
Four major types of aflatoxins are known: B1, B2, G1 and G2. The B1 aflatoxin (AFB1) is the most potent liver carcinogen (65). The mechanism associated with carcinogenic effects of AFB1 is DNA adducts formation, which cause a mutation (AGG to AGT) in codon 249 in the tumor suppressor gene TP53, resulting in substitution of arginine for serine (R249S) (66).
In Europe, the European Prospective Investigation into Cancer and Nutrition study cohort consisted of 521,323 adults throughout 23 centers in 10 European countries. This study found an association between deoxynivalenol (a mycotoxin produced by Fusarium species of fungi) and HCC risk. Deoxynivalenol can be found in cereal grains and products and derived thereof, such as wheat, barley, maize, oats and rye. However, this study did not statistical associations of HCC risk with the other mycotoxins in Europe (67).
Aristolochic acid (AA) is an abundant compound found in plants of the genders Aristolichia, Bragantia and Asarum, which are commonly used in traditional Chinese medicine (68). Several studies showed a relationship between AA and the development of urothelial and renal carcinomas (69, 70). Furthermore, AA is responsible for liver cancer development as demonstrated in Taiwan and in Asia (71). A possible mechanism explaining this association is that metabolites of AA could bind purines, thus contributing to the formation of adducts that result in transversion of adenine-to-thymine mutations (72, 73). However, other chemical compounds have similar mechanisms of carcinogenesis, such as vinyl chloride, 4-aminobiphenyl 1,3-butadiene, ethylene oxide (74, 75).
Hepatitis B virus infection
HBV is a virus that contains a partial double strand of DNA belonging to the family of Hepadnaviridae (76). It was estimated that 1 in 3 people in the World had been infected with HBV (77), of which only 5% would develop chronic carrier status (78). HBV was responsible for at least 50~80% cases of HCC worldwide (79), mainly because chronic HBV infection is endemic in developing countries owing to suboptimal resources to implement prevention policies (80). HBV ranges from 6% to 14% in North and South America, with 24% in Andean Latin America; 12% in Western Europe; 39% in Eastern Europe to 50–80% in Eastern Asia, Sub-Saharan Africa and Oceania (Figures 4B; 5B; Supplementary Table S4). Custer et al. (81) classified countries according to the prevalence rates of HBV infection:
● Low, <2% positivity for surface antigen B (HBsAg) in countries such as North America, Northern Europe, Australia and New Zealand.
● Intermediate, 2-7% of HBsAg positivity in Japan, the Middle East, Eastern Europe, Southern Europe and some areas in South America.
● High, > 8% HBsAg positivity in sub-Saharan Africa, the Amazon Basin, China, Korea, and Taiwan.
There are also important differences regarding the presentation of HBV infection and the geographic location. For example, in Africa, higher prevalence rates are reported in rural compared to urban areas (82). This diversity also extends among different African countries; for example in Burkina Faso, the estimated prevalence is 17.3% (83), compared to Cameroon, where the prevalence of HBV is 10.1% (84). Altogether, over the last 30 years, a high risk of contracting HBV by children has been described in Africa, in particular in rural areas with different socioeconomic conditions (85, 86).
At the molecular level, there are 5 viral genotypes (A, B, C, D, and E) (82, 87) most frequently described. A geographic distribution associated with genotypes also emerges, highlighting genotype A in the South East of Africa and in some regions of Northern Europe. Genotype D shows a predilection for Northern Africa, as well as southern Europe, including countries such as Italy, Greece, Serbia and Montenegro. Thus, high levels of incidence and prevalence were found in Syria, Iran, Turkey, Iraq, Mongolia, Kazakhstan, Russia and India (88). Genotypes B and C were the most frequent in China and also the Asia pacific region (89), whereas genotype E was prevalent in Africa, from Senegal to Namibia (87, 90, 91).
Despite geographic heterogeneity, global HBV immunization programs show favorable results, which gives a more encouraging outlook on the foreseeable future. In the 21st century, China has seen a decrease in HBsAg carriers from 9.8% to 7.18% (92–94). However, there was still a broad breach to cover since the program had only protected 20% of rural areas. In South Korea, seroprevalence was 4%, with a reduction of chronic infection rates from 2.2 to 0.12% (95, 96). India showed seroprevalence rates of 3.1% in non-tribal populations and 11.85% in tribal populations (97, 98). Likewise, in Southwestern Asia, mainly in the territories of the Arabian Peninsula, rates ranged from 1.5% to 8% (99, 100).
An even more florid reality is found in Latin America, where it has been estimated that between 7–12 million people were chronic HBV carriers (101, 102). HBV infection rates are particularly alarming in the Amazon basin. This region extends over 8 countries: Perú, Brazil, Colombia, Bolivia, Ecuador, Venezuela, Guyana and Surinam (103), where it has been estimated that the HBsAg infection rate was greater than 8%. A study on 37 Peruvian aboriginal communities distributed in 12 areas reported infection rates of 59.7% in patients whose mean age was 22.7 years (102). Notably, combined vertical and horizontal transmission result in high rates of HBV infection in children under 10 years of age, reaching 82.5% positivity in patients over 45 years (104, 105).
The WHO’s global hepatitis strategy aims to reduce hepatitis seroprevalence by 90% and related deaths by 65% by 2030. By the end of 2022, HBV vaccine had been applied in 190 states with a global coverage of 84% for three doses. With the decline in the seroprevalence of HBV and HCV infections, as well as aflatoxin exposure, liver cancer rates have been declining in high-risk countries within the last 50 years. By contrast, incidence rates in formerly low-risk countries have increased in recent years because of the increasing prevalence of MASLD and ALD (9).
Hepatitis C virus infection
Hepatitis C virus is a single stranded RNA virus, which was identified in 1989 (106). As shown in Figure 5, despite the advent of direct antiviral agents (DAA), HCV is still considered a public health problem worldwide and a major cause of chronic liver disease and HCC in most developing countries and most regions of USA (107, 108). Thanks to the use DAAs, the global estimate of the prevalence of HCV decreased from over 100 million in 2015 to 58 million infected adults in 2019 (108–110). This evolution explains in part the decrease in the global HCV-related liver cancer burden (Figure 6). HCV PAFs range from 50–56% in Central Asia, West Sub-Saharan Africa, North Africa and Middle East, through 39% in Eastern Europe, to 15–20% in Western Europe, North and South America (Figures 4B; 5C; Supplementary Table S4). However, the estimated 15-20% PAFs for North America were recently challenged by data from actual cancer registries. These registries, linked to hospital discharge agencies and viral hepatitis departments, indicate that over 40% of all HCC cases are attributed to HCV, according to recent studies in Florida and California (30, 31).
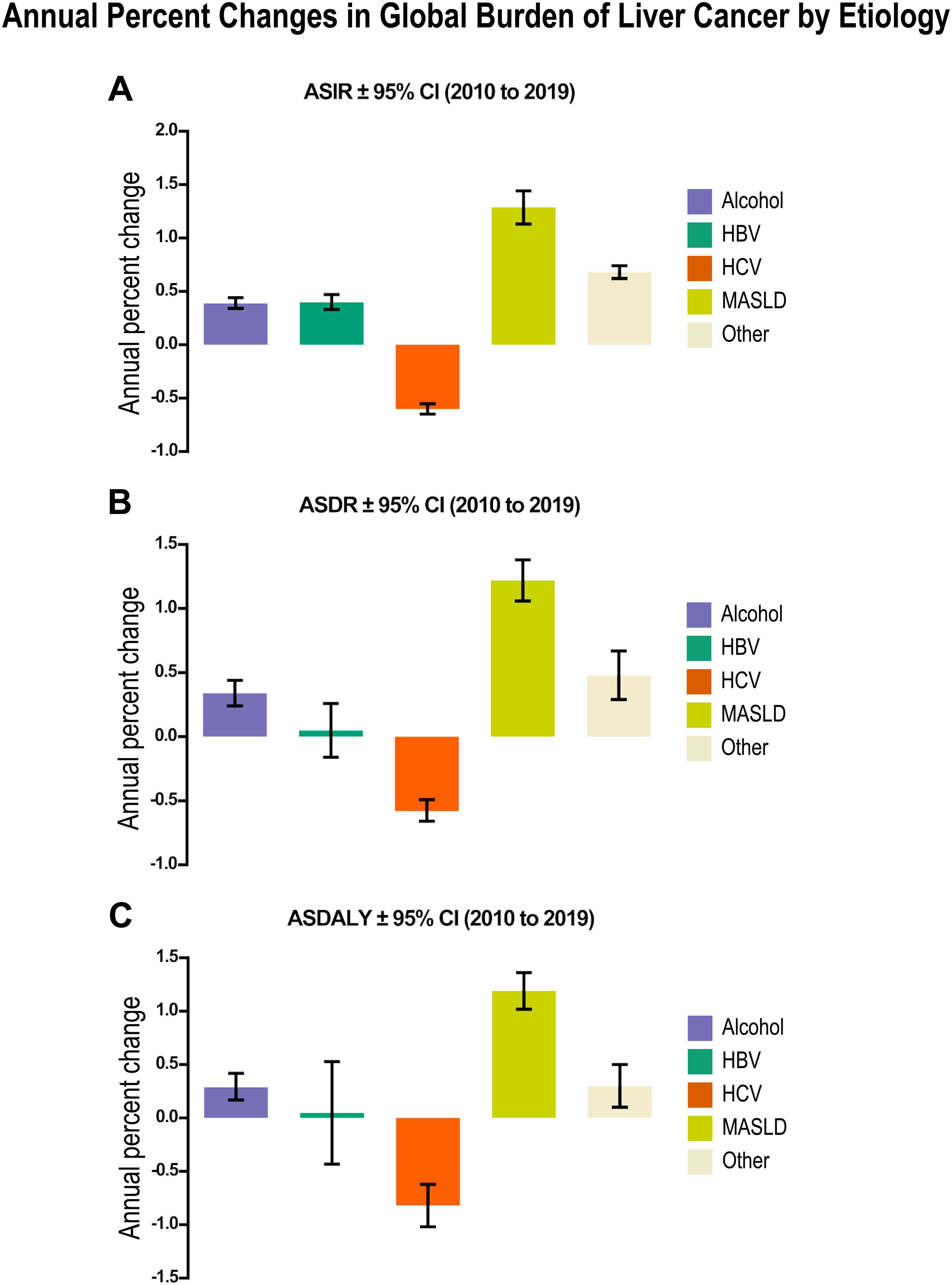
Figure 6. Annual percent changes in global burden of liver cancer by etiology from 2010 to 2019 in men reveals a major impact of MASLD in liver cancer incidence, death and patient disability. (A) Age-standardized incidence rates (ASIR) per 1x105 ± 95% CI of liver cancer. (B) Age-standardized death rates (ASDR) per 1x105 ± 95% CI of liver cancer. (C) Age-standardized disability-adjusted life-years (ASDALY) per 1x105 ± 95 CI related to each etiological factor. Relevant tabular data extracted from (111) are presented here in chart form.
In the pre-DAA era, disparities in the prevalence of HCV infection were however revealed when comparing high-income countries (HIC), where the prevalence was generally below 2% (112), with low-middle income countries (LMIC), where the prevalence was higher than 5% like, for example, in Egypt, 4.4-15% (113), Cameroon, 4.9-13.8% (114), and Mongolia, 9.6-10.8% (115).
Blood transfusions played a significant role in the spread of HCV infections, particularly when blood screening practices were not yet optimal. For example, the risk of being infected with HCV in a blood transfusion was 2.5% per 1000 units in sub-Saharan Africa (116). Also, high-risk behaviors such as sharing syringes and needles by intravenous drug users were responsible for high transmission rates, with 1.8 to 46.7 cases of HCV infection/100 people/year, mainly in people inside prisons or detention centers. Finally, other potential forms of transmission could be: intranasal use of cocaine, tattooing, body piercings, sexual intercourse with blood exchange and cupping (56).
HCV does not integrate into the host genome (117) and constantly replicates during chronic infection. Genetic studies have shown that polymorphisms or mutations in the genes LEPR, MICA/HCP5 and IFNL3 loci (118–122) are more likely to develop HCC. In particular, mutations in the IFNL3 gene present a particular geographic distribution pattern, with lower frequencies in African and higher in Asian populations (123, 124).
Another factor is the genetic makeup of HCV itself. Certain HCV genotypes have shown a strong association with the development of HCC: the genotype 3a is mostly associated with HCC in a MASH context (125), whereas the genotype 1b is associated almost exclusively with a more frequent progression towards HCC (126). Geographically, the different genotypes of HCV follow a preferential distribution, since type 1b is most prevalent in Central Asia and Asia Pacific, with Japan and Mongolia being the countries with the highest prevalence. In turn, genotype 3a has been mostly detected in India, Pakistan, Malaysia, Thailand and some regions of Western Europe (109). Genotype HCV-4 is common in the Middle East and Africa, where it is associated with over 80% of infections, but has also spread to Europe. It is also a major cause of chronic hepatitis, liver fibrogenesis and HCC (127). Genotype HCV-6 also increases the risk of HCC, particularly in Asian patients (128).
In patients with post-sustained virologic response (SVR) HCC, regional differences were observed in clinical presentation and prognosis (129). Among 8796 patients with advanced fibrosis (F3/F4) who achieved SVR from 30 sites in Europe, North America, South America, the Middle East, South Asia, East Asia and Southeast Asia, 583 (6.6%) patients developed HCC between 2015 and 2021. Patient outcome varied by region, with a hazard ratio range from 1.82 to 9.92. The best outcomes were in East Asia, North America and South America. The worst outcomes were in the Middle East and South Asia. HCC surveillance was associated with early-stage detection and lower mortality rates. The findings agree with the concept of the “field effect”, whereby despite the eradication of the HCV in the liver and despite a significant decrease in HCC incidence is SVR patients; pre-neoplastic changes persist in some patients, leading to HCC (19). Thus, the persistence of pre-neoplastic changes justifies surveillance in patients having obtained sustained viral response and in particular in those who combine multiple risk factors, some of which may remain after viral clearance, e.g., alcohol misuse, overweight and obesity.
Indeed, the importance of the interaction of multiple risk factors on the emergence of liver cancer requires careful attention. It is highlighted by the evidence that while heavy alcohol misuse alone was associated with an odds ratio of HCC of 4.96; combined exposure to alcohol plus HBV and HCV resulted in an odds ratio of 74.63 (130), this implies that after sustained viral response, the combined effect of alcohol and viral infections may have already generated pre-neoplastic lesions that require surveillance.
Metabolic disfunction-associated steatotic liver disease
Steatosis is a pathological sign that characterizes a wide spectrum of metabolic liver diseases, frequently associated with obesity or overweight, sedentary life style, alcohol misuse, nutritional imbalance and type II diabetes (131). Steatosis encompasses a spectrum of liver diseases, where more than 5% of hepatocytes show macro-vesicular steatosis, i.e., large fat droplets filling the hepatocyte cytoplasm and displacing the nucleus to the periphery. Depending on how the predisposing risk factors have been grouped to form a syndrome, the nomenclature has evolved in recent years (132).The diagnoses of Nonalcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) required exclusion of alcohol misuse. However, the frequent coexistence of steatosis with viral hepatitis, autoimmune diseases, and alcohol misuse led to the proposal of the term metabolic-associated fatty liver disease (MAFLD) in 2020 (133, 134). Three years later, a consensus among experts from 56 countries agreed on the terms Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) and Metabolic Dysfunction-Associated Steatohepatitis (MASH). These terms were considered less stigmatizing and will hopefully improve the identification of patients with steatosis. The diagnosis of MASLD is based on steatosis (≥ 5% of hepatocytes affected) plus at least one of five cardiometabolic criteria, including body mass index (BMI), insulin resistance, blood pressure, plasma triglycerides, and HDL cholesterol levels, allowing for moderate alcohol consumption (20–50 g/day for women; 30–60 g/day for men) (3, 135). The nomenclature was further redefined in 2024: in case of moderate alcohol intake, the recommended term is MetALD, while ALD is used for cases consuming > 50 g/day (women) or > 60 g/day (men) (136). An important point is, however, that the diagnosis of ALD is defined by a pattern of liver injury occurring in the setting of significant and substantial alcohol consumption, drinking history being a major discriminant factor. Other important diagnostic elements are the presence of alcoholic hepatitis and alcohol-specific markers, such as ethyl glucuronide, phosphatidyl ethanol, and carbohydrate-deficient transferrin (136).
In this review, when referring to studies applying NAFLD and/or NASH diagnostic criteria, we will keep the original terms. However, when referring generically to steatosis plus the criteria mentioned above, we will use the new MASLD/MASH terminology.
Whatever the nomenclature, a continuum of inflammatory activity and variable fibrosis may lead to HCC (137). In this regard, we recently assembled and analyzed a human liver MASLD meta-dataset (n=243) and characterized immune cell infiltrates by deconvolution of transcriptomic data. MASLD and MASH showed enhanced positive immune checkpoint levels, innate immune reactivity and extracellular matrix remodeling. In this series, the expression of fibrogenic markers was correlated with total liver fat area and the inflammation score (138).
In the recent years, MASLD has become a major risk factor, which is frequently associated with varying degrees of chronic inflammation, but not necessarily major changes in liver tissue architecture (139–141). In particular, as described above, MASH creates a chronic inflammatory microenvironment enriched in cytokines, chemokines and growth factors (142) promoting hepatocyte proliferation and retro-differentiation (143), which favors genomic instability, the emergence of preneoplastic lesions and, ultimately, HCCs.
The analysis of the annual percent changes in global burden of liver cancer by etiology from 2010 to 2019 (Figure 6) revealed a major impact of MASLD on age-standardized incidence, death and patient disability rates; whereas the impact of alcohol remained stable and that of HCV infection considerably decreased (111). These findings agree with a previous independent study on 532,000 patients showing similar trends (144).
Currently, global prevalence of MASLD is approximately 25% with wide variations around the World (145). For example, in South America and in the Middle East, the prevalence fluctuates around 30%; in contrast to 13% in Africa (57, 146), while in North America, the prevalence lies between 21-24.7% (147). MASLD and MASH are major public health problems in the USA and Western Europe, affecting 80 to 85 million people (148, 149). However, the Asian landscape is more heterogeneous. Population expansion and rural exodus in recent years have led to rates of MASLD ranging from 12.5 to 38% in China (150), 23 to 26% in Japan (151), 27% in Korea (152), 12-51% in Taiwan and 9 to 32% in India (153).
MASLD frequently emerges in the context of sedentary lifestyle, obesity and metabolic syndrome (145, 154). In turn, metabolic syndrome with associated obesity, insulin resistance and type II diabetes, is associated with the emergence of HCC (155). In this context, excessive hepatocyte uptake of triglycerides, decreased β-oxidation of fatty acids and lipid peroxidation lead to production of reactive oxygen species, which trigger hepatic inflammation, fibrogenesis and hepatocarcinogenesis (156, 157).
A subgroup of MASLD patients progress to MASH, characterized by hepatocyte ballooning, inflammation and variable degrees of fibrogenesis (158, 159). Over the recent years, compelling evidence has accumulated to unequivocally demonstrate that MASLD is an increasingly important cause of HCC (160–162). Notably, a substantial proportion of patients with MASLD progress to HCC in the absence of cirrhosis (163).
The impact of MASLD on HCC emergence can be viewed as a dynamic balance with other risk factors, particularly viral infections, throughout the 1980–2024 timeline. Countries with low rates of HBV or HCV infections show a strong association of HCC incidence with MASLD (58, 164, 165). In the United States and in Western Europe, even though HCV is still an important risk factor (166), the incidence of MASLD-related HCC is rapidly and steadily increasing and alcohol misuse remains a major cause (167).
Surprisingly, the Burden of Disease study (1990 to 2019) (137) revealed that MASLD-related liver cancer was not associated with the sociodemographic index. This evidence needs to be interpreted cautiously. It probably results from the multifactorial risk factors leading to MASLD, as suggested by its worldwide increase in incidence. We hypothesize that data acquisition may be affected by suboptimal patients’ self-awareness of at-risk nutritional and physical activity behaviors.
Another variable influencing the impact of MASLD as a risk factor for liver cancer and, in particular HCC, is ancestry. Data from six countries in the ESCALON European-Latin American network prospectively following 429 HCC patients (26) showed that in self-reported non-European patients, MASLD was the most common etiology of HCC (52%); while in self-reported European patients, MASLD accounted only for 15% of HCCs. By contrast, in patients of self-reported European ancestry, the most common etiology of HCC was HCV (38%) (27).
Taken together, the present trend toward an increase in liver cancer, and in particular, HCC incidence in high-income countries seems to result from a combination of Western lifestyle and population aging. The impact of MASLD is predicted to be amplified in the wake of the expected decrease in chronic viral hepatitis, resulting from direct antiviral therapy for HCV and progress in HBV vaccination.
Age-related risk of HCC
The age spectrum of HCC patients extends beyond that commonly described as an old adult with a longstanding history of pre-existing liver disease. The age of HCC onset is also subject to geographic variations. For example, the BRIDGE study analyzed a total of 18,031 patients with HCC from 14 different countries highlighting differences in the age of HCC onset, being above 60 years in Japan, USA and in Europe, but between 52 and 57 years in China and South Korea (168). Age at diagnosis of HCC is in turn related to the geographic distribution of risk factors (81, 103). In Sub-Saharan Africa, a multicenter study including patients from 14 centers in 7 countries, showed that the mean age at diagnosis was 45 years. Notably, a large number of these patients (49%) were infected with HBV (169). A similar picture was found in 1,541 Peruvian patients, where age at diagnosis was 44 in 50% of patients, with a 71% rate of HBV infection (170). A study of 59,907 patients showed that the country of birth was independently associated with age at the time of HCC diagnosis in the United States, with birth in Sub Saharan Africa and Oceania being strongly associated with early-onset HCC (171).
HCV-related HCC has had a major impact on the “baby boomer” generation (individuals born between 1945 and 1965), that exhibit a notably higher prevalence of HCV infection. This birth cohort effect has led to higher HCC incidence in individuals aged 50–69 years, which led to recommendations on HCV screening in the USA (172).
Increasing age is related to increasing HCC risk of cryptogenic HCCs over HBV-related HCCs. Comparing the 1980–2005 with 2006–2017 periods, the ratio of cryptogenic/HBV HCCs increased from 1:7 to 1:4. Cryptogenic HCCs were detected in older patients, with a lower proportion of male subjects and a higher incidence of smoking and unifocal HCC (173).
An association study between aging-related genes and HCC prognosis revealed a 7-gene score predicting patient outcome. Patients with high scores had HCCs with lower tumor differentiation, higher stage and worse prognosis in both the TCGA and ICGC datasets. The high-risk score was related to metabolism and tumor immunity (174).
One of the age-related molecular pathways may be telomere maintenance. Telomeres are repeated DNA sequences important for chromosomal integrity that shorten during aging as a result of the inactivation of telomerase (TERT). The activation of TERT is one of the earliest events in HCC emergence. Aging, liver fibrosis, male sex and excessive alcohol consumption are related to liver telomere shortening (175).
The worldwide burden of cancer is increasing in younger populations. A recent analysis of the Global Burden of Disease (GBD) study between 2010 and 2019 in young adults (15–49 years old) found a global estimate of 78,299 primary liver cancer cases and 60,602 deaths resulting in 2.90 million disability-adjusted years. More than half of the countries worldwide have undergone an increase in incidence rates in young adults with around 12% of deaths due to primary liver cancer occurring in this population. Despite a decline in liver cancer mortality due to most etiologies, MASLD- and alcohol-attributable liver cancer increased by 0.87% and 0.21%, respectively. The highest frequencies of liver cancer incidence, deaths and induced disability were observed in middle SDI countries, but age-standardized death rates attributable to primary liver cancer decreased in high SDI, with an annual percent change of -1.65%. Notably, the highest age-standardized incidence, mortality and disability rates attributable to primary liver cancer were attributable to alcohol misuse. According to this GBD study, alcohol-related liver cancer incidence, mortality and disability were higher by about 6.5-; 6- and 7-fold, respectively, than HBV-, HCV- and MASLD-related liver cancer (176).
As pointed out above in the Data sources and Methods section, the validity of the data from the Global Burden of Disease (GBD) study has been questioned because the distinction between primary and metastatic liver cancer is often based on verbal autopsy reports (33). Also, the estimates of incidence, mortality and disability in low SDI areas are based on limited evidence of cause-of-death certification. By contrast, in high SDI areas, a large number of countries provide valid data for primary liver cancer certification. In this context, 2019 data from the WHO mortality database showed age-standardized death rates estimates for Europe and the Americas 30% and 40% lower than those from the GBD study, highlighting the importance of external validation using the IARC cancer registry network and the GLOBOCAN or WHO databases (33).
Gender-related risk of liver cancer
Gender-related risk of liver cancer is also subject to geographic variations. Globally, liver cancer incidence is 2 to 7 times higher in men than in women (56). However, in Zimbabwe, the male/female ratios are 1.2/1, compared to France with a 5/1 ratio (177). The globally higher incidence of liver cancer in men hypothetically results from their greater risk of exposure to carcinogenic agents (alcohol, tobacco and HBV or HCV infections); whereas in women, estrogens could suppress inflammation (178). In turn, androgens could promote HCC by inducing DNA damage and oxidative stress (179). In line with these findings, recent data indicate that despite being diagnosed at an older age, women with HCC show better survival (180).
Genetic predispositions
Over most other biological markers, genetic markers provide risk information before clinically detectable disease, remain stable and may not be influenced by the course of liver or intercurrent extrahepatic disease. They also provide information on pathogenic mechanisms. A number of recent studies associate genetic variants with the risk of HCC occurrence in different etiological backgrounds. A retrospective case-control study compared genotype frequencies between HCC cases and HCC-free controls matched for age, sex, HBV and HCV infections. The authors identified polymorphisms predicting individual HCC susceptibility in high-risk HBV and HCV patients, such as ERCC1, GSTP1, CYP17A1, XRCC3 and ABCB1. These findings could contribute to HCC surveillance and early detection (181). Germline HNF1A or G6PC mutations predispose to liver adenomas with potential HCC emergence in a non-fibrotic background. On the other hand, other germline mutations predispose to chronic liver disease, leading to fibrogenesis, cirrhosis and eventually to HCC, for example, hemochromatosis due to HFE mutations, Wilson disease due to ATP7B mutations, alpha-1 antitrypsin deficiency due to SERPINA1 mutations and tyrosinemia due to FAH mutations (182).
The picture is more complex in MASLD, which arises in a backdrop of multiorgan damages linked to obesity and metabolic syndrome. Given the high prevalence of MASLD, it is important to search for biological markers sorting out patients at higher risk of developing chronic liver disease and HCC. Although the bulk of the HCC risk in fatty liver disease relies on chronic inflammation and fibrogenesis, partially determined by genetic variants, about 20% of HCCs arise in non-fibrotic or mildly fibrotic livers in patients with fatty liver disease. In the latter population, genetic polymorphisms have pleiotropic effects on obesity, metabolic syndrome and diabetes, which confound the eventual association between HCC and fatty liver disease. This reason probably explains why there is at present no evidence for genetic variants with major effect size. These findings highlight the relevance of polygenic scores to predict MASLD-related disorders. Therefore, a common strategy is to carry out Genome Wide Association Studies (GWAS) to analyze the association of millions of polymorphisms with disease phenotypes. These gene variants can group into tightly correlated linkage groups. Thus, polygenic models can be used to calculate risk scores using regression models or other refined statistical procedures that associate genetic and non-genetic covariates (183). Notably, polygenic risk scores need to be re-evaluated and, eventually, validated in different geographic settings and populations, for which allele penetrance, xenobiotic exposure and lifestyle could differ, leading to different effect size of the models. Also, given the effect size of polygenic scores and the major effect size of some clinical covariates such as age, sex, and presence of cirrhosis, robust prediction models will require very large discovery and validation cohorts, followed by external validations worldwide (184). With these caveats in mind, robust polygenic scores have been developed to gain insight into the emergence of HCC in a MASLD context and to improve HCC risk stratification. Analysis of European at-risk versus general population individuals combined PNPLA3-TM6SF2-GCKR-MBOAT7 variants in a hepatic fat polygenic risk score adjusted for HSD17B13 to predict HCC even in the absence of severe fibrosis (185). With a similar approach, a GWAS identified common variants for alcohol-related HCC. A two-stage case-control study was conducted in a cohort of 2107 European patients with alcohol-related liver disease with and without HCC. Data were adjusted for age, sex and liver fibrosis. The authors identified susceptibility alleles in WNT3A-WNT9A, which were associated with HCC regardless of liver fibrosis and confirmed previously reported genes associated with alcohol-related HCC risk, namely TM6SF2, PNPLA3 and HSK17B13 (186).
Clinical and epidemiological landscape of liver cancer in different world regions
Global liver cancer incidence and mortality have substantially declined between 2000 and 2020, which is mainly explained by a decrease in incidence and mortality in Southeast Asia, East Asia and Oceania. This steep decline is offset by an increase in Central, Eastern Europe, Central Asia, Latin America and Caribbean and a stabilized plateau in South Asia, North Africa and Middle East. In turn, Sub-Saharan Africa showed a mild decline between 2000 and 2020 (8). These data confirm previous predictions whereby an anticipated decrease driven by the control of HBV and HCV infections was expected to be offset by higher rates of metabolic syndrome (187). However, regional variations in the epidemiological dynamics warrant a closer look at the timeline of liver cancer incidence and mortality in recent years.
Asia
Taking into account the data from the national cancer sample survey during the years 1998-2007, the incidence of liver cancer was 25.84 per 100,000 individuals, with an age-standardized death rate of 18.82 per 100,000 individuals, given that 80% of the patients have progressed to advanced stages of the disease at the time of diagnosis (188).
Chinese epidemiologists cited viral infection, aflatoxin exposure, water pollution (blue-green algae toxins), excessive alcohol consumption and NAFLD as the most common possible causes (189). However, HBV infection was the most frequent cause with 10% of the general population infected (190), which contributed to the early onset of the disease, since the mean age of diagnosis was about 53 years (191).
In Hong-Kong, the median age was of 68 years for both sexes, with a male: female ratio of 2.7:1. Standardized mortality rates were 13 and 3 per 100,000, for men and women respectively, which was considerably lower than in other parts of the World (192), and the median survival in advanced cases was 11 months (193).
In Japan, 94% of all primary liver cancers were HCCs. Japan showed the highest rates of chronic HBV and HCV infections related to HCC, with most of HCC patients showing positive serology for HCV (about 67.7%), according to the Japan Liver Cancer Study Group (194). However, HCC cases in Japan were most frequently observed in older patients; 63% of HCC patients being over 65 years old (195), an age distribution quite similar to that in Hong Kong.
In other parts of the continent and, in particular, in five Indian cities (Mumbai, Bangalore, Chennai, Delhi, and Bhopal), liver cancer was the fifth most frequent cancer for both genders (196). Age-standardized incidence rates of HCC in India were 0.9–3.4 per 100,000 individuals for men and 0.2–1.8 per 100,000 individuals for women (195), with similar rates in 2022 (197). Among patients with background cirrhosis, the incidence rate increased by 1.6 per 100 person-year and chronic HBV and HCV infections were present in 71% and 16% of patients; respectively, but these data varied from rural to urban areas (198).
In a retrospective analysis of 191 HCC cases (199), the mean age at diagnosis in India was 52 years and the spectrum of clinical presentation overlapped with signs of decompensation of cirrhosis, such as ascites (57%) and gastrointestinal bleeding (22%). In addition, massive hepatomegaly was seen in more than half of the patients (56%), with a mean AFP value of 320 ng/ml. The mean ± SD size of the tumors was 6.8 ± 3.4 cm, with tumors larger than 5 cm in three-fourths of the cases.
More recent reports from India showed high intratumor heterogeneity, with coexistence of different histo-morphological patterns (200). The death rates attributable to liver cancer in deaths per million people have increased from 15.5 in the year 2000 to 23.6 in 2016, with about 1.5-fold increases in HBV- and HCV-related liver cancers and 1.7-fold increase in alcohol-related liver cancer. Notably, the death rates from cirrhosis rose by 1.08-fold within the same time lapse, with a 1.2-fold increase in HBV- and HCV-related cirrhosis and 1.05-fold in alcohol-related cirrhosis. Thus, viral hepatitis remains a major cause of cirrhosis and liver cancer in India (201).
These data imply that liver cancers and, most frequently HCCs, are detected at advanced tumor stages with clinical manifestations and indicate that there is room for implementing surveillance policies for detection and follow-up of patients at risk. In others parts of Asia, such as Philippines (202, 203) and Taiwan (1), HCC is considered among the most lethal oncological diseases and risk factors are the same as those described for the rest of Asia (203–206), except that, in Philippines, patients with cryptogenic causes represent 24.9% of all HCC cases (195).
Africa
Primary liver cancer in this continent is considered the fourth most frequent neoplasm; however, its prevalence and etiologies present great differences between Northern and Sub-Saharan Africa (SSA) (207). In Northern Africa, the incidence of liver cancer is high, due to the high prevalence of HCV infection (208), with affects over 10% of the general population in Egypt (209, 210), where the median HCC age incidence is 58 years, with a 84% HCV positivity rate. In SSA, where HBV infection remains the major risk factor, 95% of the patients present with advanced or terminal disease (211). The high prevalence of HBV infection leads to a high HCC incidence in patients under 45 years old.
Another very important cause in Africa is exposure to aflatoxin, the most important being B1, derived from Aspergillus flavus and Aspergillus parasiticus (66). Several studies have shown a potential synergistic effect between aflatoxin B1 and HBV infection (212–214), because the tropical climate favors the proliferation of Aspergillus in stored peanuts, corn and other grains for human and animal consumption (215–218).
Notably, iron overload is a risk factor very important in SSA, which is independent from the underlying liver disease. The release of the metal, after the supersaturation of its regulation and storage mechanisms, generates hepatocyte damage due to an increase in free radicals (219). Iron overload in the African diet has been previously described and called Bantu siderosis (220–222). This disease was initially identified in the Central and Southern regions of SSA Africa, mainly in rural areas, where home-made liquor is consumed from sorghum, corn among others; fermented in recycled iron barrels, which had been used mainly for the storage of chemicals. Thus, these liquors contain iron concentrations between 46–82 mg/ml (223, 224).
Lifestyle factors also affect the African population, although obesity and type 2 diabetes occur almost exclusively in urban areas (209). However, in 2019, the prevalence of metabolic syndrome and obesity were, respectively, of 78% and 27% in various SSA countries, associated also with an increase in type 2 diabetes rates (225). SSA is considered the global epicenter of HIV, with HIV and HBV coinfection being common, which leads to the rapid development of the disease and increased lethality (226).
It is important to mention that Uganda has the lowest average age at HCC diagnosis, being 32 years. In addition, 2% of the population affected by HBV-related HCC develops the disease before the age of 20 (227). The usual age of presentation is between 5 and 15 years, with a male-female ratio of 2-3: 1. The clinical manifestations do not differ significantly from those in adults (228). Surprisingly, the fibrolamellar variant, which is typical of young populations, has only been reported in 9% of all cases of HCC in children in SSA (229).
Central and South America
Approximately 4.8% of liver cancer cases worldwide occur in Latin America. The mean ASIR was 4.8 cases per 100,000 according to GLOBOCAN 2022, which is subject to regional variations (15). In Central America, Guatemala has the highest ASIRs/ASMRs per 100,000 individuals (15.5/14.9), followed by Nicaragua (9.8/9.4), Haiti (9.3/7.8) and Belize (7.0/6.7), with high mortality-to-incidence ratios. This is probably related to nutritional aflatoxin contamination in Central America (230, 231). By contrast, ASIRs/ASMRs are lower in most of South American countries, with Brazil (4.5/4.3), Argentina (3.7/3.1), Perú (4.9/4.2), Colombia (3.6/3.3), Bolivia (6.7/6.0), Venezuela (4.0/3.6) and Chile (4.7/4.5) covering 92% of the surface and 93% of the population of the South American continent. Notably, between the 2010–2019 period, analysis of the WHO database revealed alarming increases in primary liver cancer ASMR in young adults at age 15–49 in some Latin American countries: Mexico (11.5%); Colombia (16.7%); Chile (23%); Uruguay (26.9%) and Argentina (60%) (33).
In relation to risk factors, a first multicentric study, developed in 6 different countries (Brazil, Argentina, Colombia, Peru, Ecuador and Uruguay), pooling information from 14 hospitals and from a total of 1,336 patients, revealed that the main risk factor for the development of HCC was chronic infection by HCV (48%), followed by chronic alcohol misuse (22%), HBV (14%) and NAFLD (9%) (232). More recently, the South American Liver Research Network recently provided a clinical overview of HCC in South America. Although the evaluated cohort is limited to 339 HCC cases diagnosed between 2019–2021 in six countries in South America (Argentina, Perú, Ecuador, Chile, Brazil and Colombia), it is representative of major referral centers. The median patient age was 67 years and 61% were male. The most common risk factors were MASLD (37%), HCV (21%), ALD (17%), HBV (12%), and 13% of the cases were related to unidentified etiologies; 80% of HCC arose in a background of cirrhosis. Notably, HBV-related HCCs occurred in younger patients, with a median age of 46 years. About 27% of the patients received trans arterial chemoembolization or radio-embolization. Only 13% of patients benefited from resection and 6% from liver transplantation, with 9% receiving local HCC ablation, 12% systemic treatment and 17% palliative treatment (233).
A key limitation is that these data are subject to regional variations, as higher rates of HBV-related HCCs have been reported in Perú (34%) and in Brazil (38%) (232). Both countries have the greatest extension in the Amazon basin, known for being an endemic area for HBV infection. Both countries have extensive indigenous communities living in this geographic area.
The particular case of the Peruvian population
Several studies show that the first cause of HCC in Perú is chronic HBV infection, with a positivity of about 50% (170, 234, 235). HCC patients show a bimodal age distribution with respect to a 44-year-old cut-off (170). A bimodal distribution has also been described in patients from Sub-Saharan Africa (SSA), where younger patients show high prevalence of HBV positivity. However, in SSA, older patients, who represent 84% of all HCCs are predominantly HCV positive (169, 209). In Perú, patients with a negative HBV serology present positive molecular tests for HBV DNA (234). This pattern of occult infection is mainly seen in older patients compared to younger ones. In contrast, the HCV positivity rate in Peruvian patients is between 2 and 4% (170, 234).
An important clinical presentation feature in Perú is tumor size, with a mean diameter of 14 cm and high levels of AFP, with 29,000 ng/ml/tumor-cm in younger and 4,300 ng/ml/tumor-cm in older patients (170, 236). These tumors emerge on a non-cirrhotic liver, which matches the presentation pattern of HBV-related HCC in other regions of the World. An interesting feature in Perú is the presence of lesions in the non-tumor liver of these patients called “clear cell foci”, characterized by a clear and abundant Periodic-Acid-Schiff-positive cytoplasm and disruptive architecture with respect to normal hepatic parenchyma, as evidenced by a loss of the reticuline network (235). Clear cell foci may probably bear pre-neoplastic potential because they are closely related to the development of HCC in a background of chronic HBV infection.
In a recent study (237), we revealed higher concentrations of toxic metals, such as Cadmium and Arsenic, in HCCs from Peruvian than in those from French patients. Hypothetically, toxic metals could have a pathogenic role in HCC arising in non-cirrhotic livers. Although further studies are required to test this hypothesis, environmental exposure of young Peruvian individuals to toxic metals is unfortunately frequent because of the importance of the mineral industry in Peruvian economy.
Trends in two high-income countries in Europe and the Americas: the cases of United States of America and France
The USA and France are two high-development-index countries and they both share, according to GLOBOCAN 2022, similarly high age-standardized incidence and mortality rates for liver cancer. Likewise, they both show sub-national geographic and populational heterogeneities in liver cancer presentations and etiologies. In the USA, high population diversity resulting from recent and continued immigration is related to high variations in HCC incidence. In 2001, the HCC incidence in people from Asia and Pacific Islands was the highest (11.3 per 100,000 individuals), but declined by 2.2 points in the following decade. As a result, Alaskan Natives and American Indians had the highest incidence in 2016 (11.4 per 100,000 individuals), while immigrants from Spanish-speaking countries represented 9.8 per 100,100, in contrast with the category described as “non-Hispanic whites” (4.6 per 100,000 individuals) (32). More recently, significant heterogeneities by populational groups were revealed in the incidence of etiology-specific HCCs. Because the USA (in contrast to France) research practice guidelines allow for classification of populational groups by race and ethnicity, ASIRs were used to analyze the intersection between etiology and race-ethnicity in 14,420 clinically documented HCC cases from the Florida cancer registry from 2010 to 2018. Within this period, HCV remained the leading cause of HCC among men, but MASLD became the leading cause of HCC in women since 2017. HCV-related HCCs were high in USA-born minority men (with ASIRs between 7.6 and 10.9 per 100,000 individuals). However, ALD-related HCC remained high among specific “Hispanic” male groups. Since 2015, an increase in the population-based ALD-related HCC rates (+6.0%) and MALSD-related HCC rates (+4.3%) and a rapid decrease of HCV-related HCC rates (-9.6% annually) were observed. Thus, the effect of direct acting anti-HCV treatments on HCV-related ASIRs highlighted the important rise in ALD- and MASLD-related HCCs, particularly in “Hispanic” patients (30). These results were further confirmed by a retrospective cohort analysis of the 31,671 patients diagnosed with HCC in California within the same time frame (2010 to 2018). Patients of Indian, Asian, Pacific Island and Latin American origins were disproportionately affected by HCC. In this cohort, 46% of HCCs were due to HCV, 23% to MASLD, 12% to ALD and 10% to HBV, but by 2017 to 2018, MASLD accounted for 27% of HCCs, confirming the notion that HCV-related HCC decrease is offset by MASLD- and ALD-related progression (31), in line with the data illustrated in Figure 6 above.
Population-specific inequalities in the USA are also illustrated by a report published in 2021: among 1620 patients, 68% were diagnosed with HCC as outpatients and 32% in emergency departments, the latter presenting more frequently with advanced clinical stages, decompensated disease and aggressive features. In this context, unfavorable predictors applied in the emergency departments at diagnosis were male sex, black skin color, immigrant from Spanish-speaking countries, > 25% below poverty line, uninsured, lack of primary care physician/patient navigator guiding the patient through the healthcare system (238).
Moreover, in a retrospective study of 1117 HCC patients in the USA from 2008 through 2017, 36% had been self-identified as “White”; 34% as “Black” and 30% as “Hispanic”. Patients self-identified as “Hispanic” and “Black” were less likely to be diagnosed with early-stage HCC than those self-identified as “White”. Self-identified “Hispanics” were less likely to benefit from curative treatments than self-identified “Whites”. Moreover, self-identified “Black” and “Hispanic” patients had shorter survival times than self-identified “White” patients (239). Furthermore, a meta-analysis performed in August 2020 from studies on HCC outcomes according to “race and ethnicity” described 563,097 ethnically self-identified patients (53% “White”, 17% “Black”, 18% “Hispanic” and 5% “Asian”). It concluded that “racial and ethnic” disparities had an impact on HCC presentation and prognosis in the US (240).
In France, HCC patients benefiting from early detection and potentially curative treatments show a median survival of 50 months. However, median survival and access to potentially curative treatments in France are subject to regional variations. The French region Brittany has a high HCC incidence, with a median survival of less than 9 months and access to potentially curative treatments in less than 22% of patients (241–243). In a retrospective cohort study of 20,083 incident HCC patients in France between 2015 and 2017, the mean patient age was 69 years and 82% were men. The most frequent etiologies were alcohol-related liver disease in 51%, MASLD/MASH in 44% and viral hepatitis in 20%. Only 33% of patients received curative therapy, with a 1-year survival of 89.5%; 38% of the patients received only best supportive care, with a 1-year survival of 13%. Thus, at least until very recently in France, HCC was still most often diagnosed at an advanced disease stage (244).
Improving surveillance for at-risk patients
Under sociopolitical contexts that ease the access to the healthcare system, HCC detection is based on screening of patients at risk: chronic hepatitis, MASLD, MASH or other conditions leading to severe liver fibrosis/cirrhosis. In this population, patients with detection of a liver nodule > 1 cm in diameter on abdominal ultrasound imaging and/or serum alpha-fetoprotein > 20 ng/ml are proposed quarterly short-term follow-up. For patients with nodules ≥ 1 cm in diameter, quadruple-phase computed tomography or dynamic contrast enhanced magnetic resonance imaging are proposed. The outcome of a patient with HCC relies on tumor stage (tumor size, number, vascular invasion) and on the underlying liver function. Thus, careful selection of patients for HCC resection with curative intention may reach a 5-year survival ~ 70% for very early- (i.e., BCLC 0) or early- (i.e., BCLC A) stage HCCs, as reported for HCCs ≤ 2 cm (8, 245, 246). This is in contrast with the poor outcome of advanced, symptomatic HCCs, with a median survival about 6 to 18 months. At the individual level, these facts justify follow-up of at-risk patients. Notably, development and implementation of public health screening policies is offset by HCC incidence thresholds in the different categories of at-risk patients to justify cost-effectiveness (5).
As a whole, HCC treatment options are important sources of inequalities because they require infrastructure, logistics, trained personnel and, in view of their cost, the development of performant health insurance policies protecting socially vulnerable populations. For example, in West Africa, curative treatment is impossible, focusing mostly on palliative care. The vast majority of patients diagnosed with HCC enter a medical center in advanced stages of the disease, where focusing on patient comfort is frequently the only option (247). Moreover, there is a clear challenge when implementing palliative strategies; for example, Gambia reports that only 48% of HCC patients receive palliative treatments (248). Improvements in HCC surveillance in the Asia-Pacific region have spurred the adaption of management protocols to the features of the population; in particular, for intermediate stages of the disease, defined as: a single tumor with a maximum size of ≥5 cm or two or three nodules > 3 cm in diameter or > 3 nodules. This strategy prioritizes the preservation of liver function by recommending super-selective conventional TACE with curative intent as first choice of treatment (249).
In South America, despite marked heterogeneity in available resources, health care systems and reimbursement policies, progress has been made in the surveillance of at-risk patients and in the management and treatment of HCC. As described in a multicenter study conducted in 14 hospitals in Argentina (250), treatments applied for advanced HCC were trans-arterial embolization as well as tyrosine kinase inhibitors. In addition, liver transplantation is being routinely and successfully practiced in referral centers applying standardized guidelines (251). Similarly, Brazil has developed its own recommendations for the management of patients with HCC (252) based on Barcelona criteria with some modifications, according to the reality of their country. Although radiofrequency ablation is the treatment of choice for patients with initial stages of the disease and surgery is the treatment of choice for HCC developed on non-cirrhotic liver, liver transplantation procedures have entered routine practice in recent years.
Despite the fact that Argentina has been considered a high HDI country by the IARC (9), a recent study including 301 HCC patients with a mean age of 64, reports that at the time of diagnosis, only 43% of the patients were under HCC surveillance. Among the factors related with the absence (but not the failure of) surveillance on univariate analysis, health care insurance, attention in a private hospital and follow-up by an hepatologist showed significant associations. Among patients with complete surveillance, failure occurred in 25% of the patients; the only variable significantly associated with surveillance dropout was AFP ≥ 20 ng/ml (253). This seminal study suggests that self-awareness of HCC risk factors, if implemented by Public Health policies (media, education), could be determinant and cost-effective in controlling the predicted rise in HCC incidence in South America. Finally, the fact that surveillance of at-risk patients was significantly associated with health care insurance and attention in a private hospital highlights the impact of socioeconomic inequities.
Conclusion
Primary liver cancers are lethal diseases that have shown an increase in incidence in the recent years. Risk factors as well as patient management strategies vary across the World and are frequently the result of social and economic inequalities. So far, success in patient management relies on screening and early tumor detection in at-risk populations, with curative intent. However, appraisal of tumor heterogeneity and of the mechanisms of cancer progression remain two major challenges to be solved before efficient prediction of liver cancer clinical behavior can routinely be applied across the World. Overcoming these difficulties may help the development of adaptive therapeutic approaches. These challenges could be addressed by the development of cost-effective non-invasive HCC surveillance methods that, in a near future, would allow to appraise tumor heterogeneity and potential for aggressiveness.
The rise in HCC incidence worldwide calls for evolution of HCC risk models to inform surveillance decisions (26, 254). At present, surveillance decisions are currently based on the stage of liver fibrosis and consist on biannual ultrasound plus monitoring serum AFP levels. However, liver fibrosis is not the only determinant of HCC emergence; particularly in the large and ever-growing MASLD population. Thus, alternative HCC screening decisions will need to be based on individual HCC risk, that may be figured out integrating susceptibility factors. The ideal mathematical formula integrating susceptibility factors may include not only etiological, genetic and biological markers assessing liver damage, but also consider environmental and socioeconomic risk factors. The weight of these risk factors drives cancer inequities in Europe (255) and convergent evidence presented here suggests that this is the case worldwide.
Author contributions
LC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. FF: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. OM: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Inserm; Univ Rennes, Défis Scientifiques 2021, Défis Scientifiques 2024; Ministère de l’Enseignement Supérieur; Institut National du Cancer, Grant # INCa_12688; Ligue Nationale Contre le Cancer 2018, Comités d’Ille-et-Vilaine, Vendée et Côtes d’Armor; Ligue Nationale Contre le Cancer 2024, Comités d’Ille-et-Vilaine, Vendée et Côtes d’Armor.
Acknowledgments
We thank Michèle Le Guennec, Patricia Jouas, Adina Pascu and Thomas Poussou for logistics and administrative support. We thank Inès Musso, MSc in International and European Human Rights Law, for insightful discussion inspiring this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The racial and ethnic categories mentioned in this article are cited from external research not conducted in France and are presented here within quotation marks to indicate their origin from the referenced sources. The authors do not endorse the methodology of collecting or processing personal data that reveals the racial or ethnic origin of individuals. This approach aligns with Article 6 of French Law No. 78-17 of January 6, 1978, known as “Informatique et Libertés”, which prohibits such practices. Furthermore, we acknowledge the limitations and potential biases inherent in such categorizations and emphasize the importance of interpreting the data with cultural sensitivity and awareness of geopolitical contexts.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1565692/full#supplementary-material
Supplementary Table 1 | Estimated age-standardized incidence, mortality rates and Mortality-to-Incidence ratios (MIRs) of liver and intrahepatic bile duct cancers worldwide per 105 individuals, for both sexes in 2022. Data source: GLOBOCAN 2022, Cancer Today, International Agency for Research on Cancer, World Health Organization (https://gco.iarc.fr/en). Alpha-3 population codes (ISO/UN) and MIR equation are provided.
Supplementary Table 2 | Estimated number of deaths and percent increase projections from 2022 to 2050 for liver and intrahepatic bile duct cancer. Estimated timeline of predicted new cases of liver-cancer-related deaths (2025 through 2050) in five continents. Continents, years, predicted numbers, risk change and % are shown. Data sources and processing are described in the legend to Figure 3.
Supplementary Table 3 | Gross Domestic Product (GDP) per capita in current US$ for 181 countries in 2022 and Mortality-to-Incidence ratios (MIRs) of liver and intrahepatic bile duct cancers worldwide, for both sexes in 2022. Data source: GLOBOCAN 2022, Cancer Today, International Agency for Research on Cancer, World Health Organization (https://gco.iarc.fr/en). Alpha-3 codes, population codes (ISO/UN) and MIR equation are provided. For further details on GDP refer to the legend of Figure 4.
Supplementary Table 4 | Fractions of hepatocellular carcinomas related to different etiologies from 21 World regions (41).
References
1. Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, et al. Management of hepatocellular carcinoma: A review. JAMA Surg. (2023) 158:410–20. doi: 10.1001/jamasurg.2022.7989
2. Shah PA, Patil R, and Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. (2023) 77:323–38. doi: 10.1002/hep.32542
3. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
4. Kanzaki H, Katz C, and Hoshida Y. Matrisomic characterization of HCC to inform individualized patient management. Hepatology. (2023) 78:691–3. doi: 10.1097/HEP.0000000000000443
5. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primer. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
6. Désert R, Rohart F, Canal F, Sicard M, Desille M, Renaud S, et al. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology. (2017) 66:1502–18. doi: 10.1002/hep.29254
7. Desert R, Chen W, Ge X, Viel R, Han H, Athavale D, et al. Hepatocellular carcinomas, exhibiting intratumor fibrosis, express cancer-specific extracellular matrix remodeling and WNT/TGFB signatures, associated with poor outcome. Hepatology. (2023) 78:741–57. doi: 10.1097/HEP.0000000000000362
8. Singal AG, Kanwal F, and Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
9. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
10. Filho AM, Laversanne M, Ferlay J, Colombet M, Piñeros M, Znaor A, et al. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. (2025) 156:1336–46. doi: 10.1002/ijc.v156.7
11. Flaxman AD, Joseph JC, Murray CJL, Riley ID, and Lopez AD. Performance of InSilicoVA for assigning causes of death to verbal autopsies: multisite validation study using clinical diagnostic gold standards. BMC Med. (2018) 16:56. doi: 10.1186/s12916-018-1039-1
12. Li ZR, McCormick TH, and Clark SJ. Non-confirming replication of “Performance of InSilicoVA for assigning causes of death to verbal autopsies: multisite validation study using clinical diagnostic gold standards,” by Flaxman et al. BMC Med. (2020) 18:69. doi: 10.1186/s12916-020-01518-9
13. Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, et al. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. (2019) 70:674–83. doi: 10.1016/j.jhep.2018.12.001
14. Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PKH, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. (2023) 164:766–82. doi: 10.1053/j.gastro.2023.01.033
15. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4:1553–68. doi: 10.1200/JCO.2018.36.15_suppl.1568
16. Mathers CD and Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PloS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
17. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. (2022) 77:1598–606. doi: 10.1016/j.jhep.2022.08.021
18. Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primer. (2016) 2:16018. doi: 10.1038/nrdp.2016.18
19. Hernandez-Gea V, Toffanin S, Friedman SL, and Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. (2013) 144:512–27. doi: 10.1053/j.gastro.2013.01.002
20. Sirica AE, Strazzabosco M, and Cadamuro M. Intrahepatic cholangiocarcinoma: Morpho-molecular pathology, tumor reactive microenvironment, and Malignant progression. Adv Cancer Res. (2021) 149:321–87. doi: 10.1016/bs.acr.2020.10.005
21. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primer. (2021) 7:65. doi: 10.1038/s41572-021-00300-2
22. European Commission, Directorate General for Justice and Consumers, and Farkas L. Analysis and comparative review of equality data collection practices in the European Union: data collection in the field of ethnicity. Luxembourg: Publications Office (2017). Available at: https://data.europa.eu/doi/10.2838/447194 (Accessed April, 04 2025).
23. Sakolciová S and Máčaj A. Combating discrimination through Big Data – future of equality? Vilnius Univ Open Ser. (2020) 6:120–31. doi: 10.15388/OS.LAW.2020.11
24. Conomos MP, Laurie CA, Stilp AM, Gogarten SM, McHugh CP, Nelson SC, et al. Genetic diversity and association studies in US Hispanic/Latino populations: applications in the hispanic community health study/study of Latinos. Am J Hum Genet. (2016) 98:165–84. doi: 10.1016/j.ajhg.2015.12.001
25. Moya JC. Immigrants and associations: A global and historical perspective. J Ethics Migration Stud. (2005) 31:833–64. doi: 10.1080/13691830500178147
26. Debes JD, Boonstra A, Balderramo D, Mattos AZ, Arrese M, Roa JC, et al. Hepatobiliary cancers in South America: disparity strikes. Lancet Gastroenterol Hepatol. (2019) 4:581. doi: 10.1016/S2468-1253(19)30188-8
27. Loon E, Narvaez-Barbecho C, Ortiz JP, Balderramo D, Carrera E, Diaz-Ferrer J, et al. Impact of self-reported ancestry on the epidemiology of hepatocellular carcinoma. J Gastroenterol Hepatol. (2024) 39:1201–2. doi: 10.1111/jgh.16578
28. Foner N. Immigration past & Present. In: Daedalus, Immigration & the Future of America. Cambridge, Massachusetts, USA: American Academy of Arts & Sciences, vol. 142. Summer (2013). p. 16–25. doi: 10.1162/DAED_a_00216
29. Magee R. Slavery as immigration. Univ San Franc Law Rev. (2010) 44:273–306. Available online at : https://repository.usfca.edu/usflawreview/vol44/iss2/4/ (Accessed September 4, 2025)
30. Pinheiro PS, Jones PD, Medina H, Cranford HM, Koru-Sengul T, Bungum T, et al. Incidence of etiology-specific hepatocellular carcinoma: diverging trends and significant heterogeneity by race and ethnicity. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. (2024) 22:562–571.e8. doi: 10.1016/j.cgh.2023.08.016
31. Pinheiro PS, Zhang J, Setiawan VW, Cranford HM, Wong RJ, and Liu L. Liver cancer etiology in Asian subgroups and American Indian, black, Latino, and white populations. JAMA Netw Open. (2025) 8:e252208. doi: 10.1001/jamanetworkopen.2025.2208
32. McGlynn KA, Petrick JL, and El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. (2021) 73:4–13. doi: 10.1002/hep.31288
33. La Vecchia C and Santucci C. Liver cancer in young adults: Validity of global data sets. Hepatology. (2024) 80:766. doi: 10.1097/HEP.0000000000000909
34. Lozano R, Fullman N, Mumford JE, Knight M, Barthelemy CM, Abbafati C, et al. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1250–84. doi: 10.1016/S0140-6736(20)30750-9
35. Cao G, Liu J, and Liu M. Global, regional, and national trends in incidence and mortality of primary liver cancer and its underlying etiologies from 1990 to 2019: results from the global burden of disease study 2019. J Epidemiol Glob Health. (2023) 13:344–60. doi: 10.1007/s44197-023-00109-0
36. Zhou K, Song Z, Rostomian N, Dodge JL, Stern MC, Setiawan VW, et al. Association of nativity with survival among adults with hepatocellular carcinoma. JNCI J Natl Cancer Inst. (2023) 115:861–9. doi: 10.1093/jnci/djad067
37. Chen H, Wu AH, Wang S, Bookstein A, Le Marchand L, Wilkens LR, et al. Cancer mortality patterns by birthplace and generation status of Mexican Latinos: the multiethnic cohort. J Natl Cancer Inst. (2022) 114:959–68. doi: 10.1093/jnci/djac078
38. Acuna N, Zhou K, Pinheiro PS, Cheng I, Shariff-Marco S, Lim T, et al. Increasing risk of hepatocellular carcinoma with successive generations in the United States among Mexican American adults: The Multiethnic Cohort. Cancer. (2024) 130:267–75. doi: 10.1002/cncr.v130.2
39. Escobar Gil T and Aguilar GJ. Exploring social determinants and hepatocellular carcinoma: a scoping review of evidence and implications. Bayl Univ Med Cent Proc. (2025) 38:53–60. doi: 10.1080/08998280.2024.2406030
40. Shen Y, Guo H, Wu T, Lu Q, Nan KJ, Lv Y, et al. Lower education and household income contribute to advanced disease, less treatment received and poorer prognosis in patients with hepatocellular carcinoma. J Cancer. (2017) 8:3070–7. doi: 10.7150/jca.19922
41. Baecker A, Liu X, La Vecchia C, and Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. (2018) 27:205–12. doi: 10.1097/CEJ.0000000000000428
42. Comoz B, Ollivier-Hourmand I, Bouvier AM, Nousbaum JB, Nguyen TTN, Launoy G, et al. Impact of socio-economic environment on incidence of primary liver cancer in France between 2006 and 2016. Liver Int Off J Int Assoc Study Liver. (2024) 44:446–53. doi: 10.1111/liv.15784
43. Rumgay H, Shield K, Charvat H, Ferrari P, Sornpaisarn B, Obot I, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. (2021) 22:1071–80. doi: 10.1016/S1470-2045(21)00279-5
44. de Martel C, Georges D, Bray F, Ferlay J, and Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. (2020) 8:e180–90. doi: 10.1016/S2214-109X(19)30488-7
45. Corrao G, Bagnardi V, Zambon A, and Torchio P. Meta-analysis of alcohol intake in relation to risk of liver cirrhosis. Alcohol Alcohol Oxf Oxfs. (1998) 33:381–92. doi: 10.1093/oxfordjournals.alcalc.a008408
46. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. (2010) 96:3–1383. Available online at: https://publications.iarc.who.int/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Alcohol-Consumption-And-Ethyl-Carbamate-2010 (Accessed September 4, 2025).
47. Ganne-Carrié N and Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. (2019) 70:284–93. doi: 10.1016/j.jhep.2018.10.008
48. World Health Organization. Global Status Report on Alcohol and Health 2018 . Available online at: https://www.who.int/publications/i/item/9789241565639 (Accessed April 10, 2025).
49. Ganne-Carrié N and Nahon P. Differences between hepatocellular carcinoma caused by alcohol and other aetiologies. J Hepatol. (2024) 82:909–17. doi: 10.1016/j.jhep.2024.12.030
50. Zeng RW, Ong CEY, Ong EYH, Chung CH, Lim WH, Xiao J, et al. Global prevalence, clinical characteristics, surveillance, treatment allocation, and outcomes of alcohol-associated hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2024) 22:2394–402.e15. doi: 10.1016/j.cgh.2024.06.026
51. Díaz LA, Fuentes-López E, Idalsoaga F, Ayares G, Corsi O, Arnold J, et al. Association between public health policies on alcohol and worldwide cancer, liver disease and cardiovascular disease outcomes. J Hepatol. (2024) 80:409–18. doi: 10.1016/j.jhep.2023.11.006
52. Schwarzinger M, Ferreira-Borges C, Neufeld M, Alla F, and Rehm J. Alcohol rehabilitation and cancer risk: a nationwide hospital cohort study in France. Lancet Public Health. (2024) 9:e461–9. doi: 10.1016/S2468-2667(24)00107-5
53. Kelly JF and Westerhoff CM. Does it matter how we refer to individuals with substance-related conditions? A randomized study of two commonly used terms. Int J Drug Policy. (2010) 21:202–7. doi: 10.1016/j.drugpo.2009.10.010
54. Hill C. Alcohol in France: room for improvement. Lancet Public Health. (2024) 9:e416–7. doi: 10.1016/S2468-2667(24)00124-5
55. Patel PV and Flamm SL. Screening, diagnosis, and treatment of alcohol-related liver disease and alcohol-associated hepatitis. Gastroenterol Hepatol. (2022) 18:409–17.
56. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, and Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
57. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, and Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
58. Seyda Seydel G, Kucukoglu O, Altinbasv A, Demir OO, Yilmaz S, Akkiz H, et al. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. (2016) 15:662–72.
59. Letouzé E, Shinde J, Renault V, Couchy G, Blanc JF, Tubacher E, et al. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis. Nat Commun. (2017) 8:1315. doi: 10.1038/s41467-017-01358-x
60. Alberg AJ, Shopland DR, and Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. (2014) 179:403–12. doi: 10.1093/aje/kwt335
61. Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. (2018) 118:1005–12. doi: 10.1038/s41416-018-0007-z
62. Premkumar M and Anand AC. Tobacco, cigarettes, and the liver: the smoking gun. J Clin Exp Hepatol. (2021) 11:700–12. doi: 10.1016/j.jceh.2021.07.016
63. Liu Y and Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. (2010) 118:818–24. doi: 10.1289/ehp.0901388
64. Benkerroum N. Aflatoxins: producing-molds, structure, health issues and incidence in Southeast Asian and Sub-Saharan African countries. Int J Environ Res Public Health. (2020) 17:1215. doi: 10.3390/ijerph17041215
65. López C, Ramos L, Bulacio L, Ramadán S, and Rodríguez F. Aflatoxin B1 content in patients with hepatic diseases. Medicina (Mex). (2002) 62:313–6.
66. Hamid AS, Tesfamariam IG, Zhang Y, and Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol Lett. (2013) 5:1087. doi: 10.3892/ol.2013.1169
67. Huybrechts I, Jacobs I, Biessy C, Aglago EK, Jenab M, Claeys L, et al. Associations between dietary mycotoxins exposures and risk of hepatocellular carcinoma in a European cohort. PloS One. (2024) 19:e0315561. doi: 10.1371/journal.pone.0315561
68. Zhang HM, Zhao XH, Sun ZH, Li GC, Liu GC, Sun LR, et al. Recognition of the toxicity of aristolochic acid. J Clin Pharm Ther. (2019) 44:157–62. doi: 10.1111/jcpt.2019.44.issue-2
69. Chen CH, Dickman KG, Moriya M, Zavadil J, Sidorenko VS, Edwards KL, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci U S A. (2012) 109:8241–6. doi: 10.1073/pnas.1119920109
70. Hoang ML, Chen CH, Chen PC, Roberts NJ, Dickman KG, Yun BH, et al. Aristolochic acid in the etiology of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. (2016) 25:1600–8. doi: 10.1158/1055-9965.EPI-16-0219
71. Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med. (2017) 9:eaan6446. doi: 10.1126/scitranslmed.aan6446
72. Lu ZN, Luo Q, Zhao LN, Shi Y, Wang N, Wang L, et al. The mutational features of aristolochic acid-induced mouse and human liver cancers. Hepatology. (2020) 71:929–42. doi: 10.1002/hep.30863
73. Debelle FD, Vanherweghem JL, and Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. (2008) 74:158–69. doi: 10.1038/ki.2008.129
74. Barbin A, Froment O, Boivin S, Marion MJ, Belpoggi F, Maltoni C, et al. p53 gene mutation pattern in rat liver tumors induced by vinyl chloride. Cancer Res. (1997) 57:1695–8.
75. Zhou G and Zhao X. Carcinogens that induce the A:T > T:A nucleotide substitutions in the genome. Front Med. (2018) 12:236–8. doi: 10.1007/s11684-017-0611-y
76. Kay A and Zoulim F. Hepatitis B virus genetic variability and evolution. Virus Res. (2007) 127:164–76. doi: 10.1016/j.virusres.2007.02.021
77. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
78. Sharma SK, Saini N, and Chwla Y. Hepatitis B virus: inactive carriers. Virol J. (2005) 2:82. doi: 10.1186/1743-422X-2-82
79. Chen Y and Tian Z. HBV-induced immune imbalance in the development of HCC. Front Immunol eCollection. (2019) 10:2048. doi: 10.3389/fimmu.2019.02048
80. Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E, et al. Hepatitis B virus burden in developing countries. World J Gastroenterol. (2015) 21:11941–53. doi: 10.3748/wjg.v21.i42.11941
81. Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, and Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. (2004) 38:S158–68. doi: 10.1097/00004836-200411003-00008
82. McNaughton AL, Lourenço J, Bester PA, Mokaya J, Lumley SF, Obolski U, et al. Hepatitis B virus seroepidemiology data for Africa: Modelling intervention strategies based on a systematic review and meta-analysis. PloS Med. (2020) 17:e1003068. doi: 10.1371/journal.pmed.1003068
83. Lingani M, Akita T, Ouoba S, Nagashima S, Boua PR, Takahashi K, et al. The changing epidemiology of hepatitis B and C infections in Nanoro, rural Burkina Faso: a random sampling survey. BMC Infect Dis. (2020) 20:46. doi: 10.1186/s12879-019-4731-7
84. Bigna JJ, Amougou MA, Asangbeh SL, Kenne AM, Noumegni SRN, Ngo-Malabo ET, et al. Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open. (2017) 7:e015298. doi: 10.1136/bmjopen-2016-015298
85. Ugwuja E and Ugwu N. Seroprevalence of hepatitis B surface antigen and liver function tests among adolescents in Abakaliki, South Eastern Nigeria. Internet J Trop Med. (2010) 6:1726–32. Available online at: https://ispub.com/IJTM/6/2/8952 (Accessed September 4, 2025)
86. Bile K, Mohamud O, Aden C, Isse A, Norder H, Nilsson L, et al. The risk for hepatitis A, B, and C at two institutions for children in Somalia with different socioeconomic conditions. Am J Trop Med Hyg. (1992) 47:357–64. doi: 10.4269/ajtmh.1992.47.357
87. Kramvis A and Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res Off J Jpn Soc Hepatol. (2007) 37:S9–19. doi: 10.1111/j.1872-034X.2007.00098.x
88. Ozaras R, Inanc Balkan I, Yemisen M, and Tabak F. Epidemiology of HBV subgenotypes D. Clin Res Hepatol Gastroenterol. (2015) 39:28–37. doi: 10.1016/j.clinre.2014.06.005
89. Xiang Y, Huang S, Xia J, Ye D, Chen P, and Zhang L. Characterization of hepatitis B virus molecular genotypes in Chongqing and quantitative serological markers in patients during natural phases of chronic hepatitis B infection. Intervirology. (2012) 55:68–72. doi: 10.1159/000323524
90. Olinger CM, Venard V, Njayou M, Oyefolu AOB, Maïga I, Kemp AJ, et al. Phylogenetic analysis of the precore/core gene of hepatitis B virus genotypes E and A in West Africa: new subtypes, mixed infections and recombinations. J Gen Virol. (2006) 87:1163–73. doi: 10.1099/vir.0.81614-0
91. Forbi JC, Ben-Ayed Y, liang XG, Vaughan G, Drobeniuc J, Switzer WM, et al. Disparate distribution of hepatitis B virus genotypes in four sub-Saharan African countries. J Clin Virol Off Publ Pan Am Soc Clin Virol. (2013) 58:59–66. doi: 10.1016/j.jcv.2013.06.028
92. Luo Z, Li L, and Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. (2012) 16:e82–8. doi: 10.1016/j.ijid.2011.10.009
93. Yan YP, Su HX, Ji ZH, Shao ZJ, and Pu ZS. Epidemiology of hepatitis B virus infection in China: current status and challenges. J Clin Transl Hepatol. (2014) 2:15–22. doi: 10.14218/JCTH.2013.00030
94. Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. (2019) 19:811. doi: 10.1186/s12879-019-4428-y
95. Yim SY and Kim JH. The epidemiology of hepatitis B virus infection in Korea. Korean J Intern Med. (2019) 34:945–53. doi: 10.3904/kjim.2019.007
96. Pak S C, Alastal Y, Khan Z, and Darr U. Viral hepatitis in South Korea. Euroasian J Hepato-Gastroenterol. (2017) 7:163–5. doi: 10.5005/jp-journals-10018-1240
97. Batham A, Gupta MA, Rastogi P, Garg S, Sreenivas V, and Puliyel JM. Calculating prevalence of hepatitis B in India: using population weights to look for publication bias in conventional meta-analysis. Indian J Pediatr. (2009) 76:1247–57. doi: 10.1007/s12098-009-0246-3
98. Ray G. Current scenario of hepatitis B and its treatment in India. J Clin Transl Hepatol. (2017) 5:277–96. doi: 10.14218/JCTH.2017.00024
99. Ott JJ, Stevens GA, Groeger J, and Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. (2012) 30:2212–9. doi: 10.1016/j.vaccine.2011.12.116
100. Gasim GI. Hepatitis B virus in the Arab world: where do we stand? Arab J Gastroenterol Off Publ Pan-Arab Assoc Gastroenterol. (2013) 14:35–43. doi: 10.1016/j.ajg.2013.04.002
101. Alvarado-Mora MV and Pinho JRR. Epidemiological update of hepatitis B, C and delta in Latin America. Antivir Ther. (2013) 18:429–33. doi: 10.3851/IMP2595
102. Roman S. Hepatitis B virus infection in Latin America: A genomic medicine approach. World J Gastroenterol. (2014) 20:7181. doi: 10.3748/wjg.v20.i23.7181
103. Oh JK and Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. (2014) 80:384–92. doi: 10.1016/j.aogh.2014.09.013
104. Davis LG, Weber DJ, and Lemon SM. Horizontal transmission of hepatitis B virus. Lancet Lond Engl. (1989) 1:889–93. doi: 10.1016/S0140-6736(89)92876-6
105. Cabezas SC, Suárez JM, Romero CG, Carrillo PC, García MP, Reátegui SJ, et al. Hiperendemicidad de Hepatitis Viral B y Delta en pueblos indigenas de la Amazonía Peruana. Rev Peru Med Exp Salud Pública. (2006) 23:114–22. Available online at: http://www.scielo.org.pe/scielo.php?script=sci_abstract&pid=S1726-46342006000200007&lng=es&nrm=iso&tlng=es (Accessed September 4, 2025)
106. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, and Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. (1989) 244:359–62. doi: 10.1126/science.2523562
107. Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. (2003) 98:2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x
108. Stroffolini T and Stroffolini G. Prevalence and modes of transmission of hepatitis C virus infection: A historical worldwide review. Viruses. (2024) 16:1115. doi: 10.3390/v16071115
109. Gower E, Estes C, Blach S, Razavi-Shearer K, and Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. (2014) 61:S45–57. doi: 10.1016/j.jhep.2014.07.027
110. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, and Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. (2016) 22:7824–40. doi: 10.3748/wjg.v22.i34.7824
111. Tan DJH, Setiawan VW, Ng CH, Lim WH, Muthiah MD, Tan EX, et al. Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH. Hepatology. (2023) 77:1150–63. doi: 10.1002/hep.32758
112. Hanafiah KM, Groeger J, Flaxman AD, and Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. (2013) 57:1333–42. doi: 10.1002/hep.26141
113. Abd Elrazek AE, Bilasy SE, Elbanna AEM, and Elsherif AEA. Prior to the oral therapy, what do we know about HCV-4 in Egypt: A randomized survey of prevalence and risks using data mining computed analysis. Med (Baltimore). (2014) 93:e204. doi: 10.1097/MD.0000000000000204
114. Riou J, Ahmed MA, Blake A, Vozlinsky S, Brichler S, Eholié S, et al. Hepatitis C virus seroprevalence in adults in Africa: a systematic review and meta-analysis. J Viral Hepat. (2016) 23:244–55. doi: 10.1111/jvh.2016.23.issue-4
115. Tserenpuntsag B, Nelson K, Lamjav O, Triner W, Smith P, Kacica M, et al. Prevalence of and risk factors for hepatitis B and C infection among Mongolian blood donors. Transfusion (Paris). (2010) 50:92–9. doi: 10.1111/j.1537-2995.2009.02387.x
116. Abdella Y, Riedner G, Hajjeh R, and Sibinga CTS. Blood transfusion and hepatitis: what does it take to prevent new infections? East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. (2018) 24:595–7. doi: 10.26719/2018.24.6.595
117. Bandiera S, Bian CB, Hoshida Y, Baumert TF, and Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. (2016) 20:99–105. doi: 10.1016/j.coviro.2016.09.010
118. Goto K and Kato N. MICA SNPs and the NKG2D system in virus-induced HCC. J Gastroenterol. (2015) 50:261–72. doi: 10.1007/s00535-014-1000-9
119. Lange CM, Bibert S, Dufour JF, Cellerai C, Cerny A, Heim MH, et al. Comparative genetic analyses point to HCP5 as susceptibility locus for HCV-associated hepatocellular carcinoma. J Hepatol. (2013) 59:504–9. doi: 10.1016/j.jhep.2013.04.032
120. Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. (2011) 43:455–8. doi: 10.1038/ng.809
121. Ikeda A, Shimizu T, Matsumoto Y, Fujii Y, Eso Y, Inuzuka T, et al. Leptin receptor somatic mutations are frequent in HCV-infected cirrhotic liver and associated with hepatocellular carcinoma. Gastroenterology. (2014) 146:222–232.e35. doi: 10.1053/j.gastro.2013.09.025
122. Chang KC, Tseng PL, Wu YY, Hung HC, Huang CM, Lu SN, et al. A polymorphism in interferon L3 is an independent risk factor for development of hepatocellular carcinoma after treatment of hepatitis C virus infection. Clin Gastroenterol Hepatol. (2015) 13:1017–24. doi: 10.1016/j.cgh.2014.10.035
123. Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. (2009) 461:798–801. doi: 10.1038/nature08463
124. Indolfi G, Azzari C, and Resti M. Polymorphisms in the IFNL3/IL28B gene and hepatitis C: From adults to children. World J Gastroenterol WJG. (2014) 20:9245–52. doi: 10.3748/wjg.v20.i28.9245
125. Goossens N and Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. (2015) 21:105–14. doi: 10.3350/cmh.2015.21.2.105
126. Raimondi S, Bruno S, Mondelli MU, and Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: A meta-analysis. J Hepatol. (2009) 50:1142–54. doi: 10.1016/j.jhep.2009.01.019
127. Khattab MA, Ferenci P, Hadziyannis SJ, Colombo M, Manns MP, Almasio PL, et al. Management of hepatitis C virus genotype 4: recommendations of an international expert panel. J Hepatol. (2011) 54:1250–62. doi: 10.1016/j.jhep.2010.11.016
128. Lee MH, Hsiao TI, Subramaniam SR, Le AK, Vu VD, Trinh HN, et al. HCV genotype 6 increased the risk for hepatocellular carcinoma among Asian patients with liver cirrhosis. Am J Gastroenterol. (2017) 112:1111–9. doi: 10.1038/ajg.2017.123
129. Toyoda H, Kanneganti M, Melendez-Torres J, Parikh ND, Jalal PK, Piñero F, et al. Regional differences in clinical presentation and prognosis of patients with post–sustained virologic response hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2024) 22:72–80.e4. doi: 10.1016/j.cgh.2023.06.026
130. Franceschi S, Montella M, Polesel J, La Vecchia C, Crispo A, Dal Maso L, et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. (2006) 15:683–9. doi: 10.1158/1055-9965.EPI-05-0702
131. Kumar V, Abbas AK, and Aster JC eds. Robbins & Cotran pathologic basis of disease. Tenth edition. Philadelphia, PA: Elsevier (2021). p. 1379.
132. Targher G, Byrne CD, and Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and Malignant complications. Gut. (2024) 73:691–702. doi: 10.1136/gutjnl-2023-330595
133. Eslam M, Sanyal AJ, George J, and International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
134. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
135. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. (2023) 78:1966–86. doi: 10.1097/HEP.0000000000000520
136. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. (2024) 81:492–542. doi: 10.1016/j.jhep.2024.04.031
137. Huang M, Chen H, Wang H, Wang X, Wang D, Li Y, et al. Worldwide burden of liver cancer due to metabolic dysfunction-associated steatohepatitis from 1990 to 2019: insights from the Global Burden of Disease study. Front Oncol. (2024) 14:1424155. doi: 10.3389/fonc.2024.1424155
138. Cano L, Desquilles L, Ghukasyan G, Angenard G, Landreau C, Corlu A, et al. SARS-CoV-2 receptor ACE2 is upregulated by fatty acids in human MASH. JHEP Rep. (2024) 6:100936. doi: 10.1016/j.jhepr.2023.100936
139. Huang DQ, El-Serag HB, and Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
140. Baffy G, Brunt EM, and Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J Hepatol. (2012) 56:1384–91. doi: 10.1016/j.jhep.2011.10.027
141. Agosti P, Sabbà C, and Mazzocca A. Emerging metabolic risk factors in hepatocellular carcinoma and their influence on the liver microenvironment. Biochim Biophys Acta BBA - Mol Basis Dis. (2018) 1864:607–17. doi: 10.1016/j.bbadis.2017.11.026
142. Dubois-Pot-Schneider H, Fekir K, Coulouarn C, Glaise D, Aninat C, Jarnouen K, et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology. (2014) 60:2077–90. doi: 10.1002/hep.27353
143. Cabillic F and Corlu A. Regulation of transdifferentiation and retrodifferentiation by inflammatory cytokines in hepatocellular carcinoma. Gastroenterology. (2016) 151:607–15. doi: 10.1053/j.gastro.2016.06.052
144. Liu Y, Zheng J, Hao J, Wang RR, Liu X, Gu P, et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019. Cancer Med. (2022) 11:1310–23. doi: 10.1002/cam4.v11.5
145. Mitra S, De A, and Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. (2020) 5:16. doi: 10.21037/tgh.2019.09.08
146. Vernon G, Baranova A, and Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. (2011) 34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x
147. Cotter TG and Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. (2020) 158:1851–64. doi: 10.1053/j.gastro.2020.01.052
148. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, and Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. (2017) 23:8263–76. doi: 10.3748/wjg.v23.i47.8263
149. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. (2018) 69:896–904. doi: 10.1016/j.jhep.2018.05.036
150. Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. (2013) 28 Suppl 1:11–7. doi: 10.1111/jgh.12036
151. Kojima SI, Watanabe N, Numata M, Ogawa T, and Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. (2003) 38:954–61. doi: 10.1007/s00535-003-1178-8
152. Jeong EH, Jun DW, Cho YK, Choe YG, Ryu S, Lee SM, et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol. (2013) 19:266–72. doi: 10.3350/cmh.2013.19.3.266
153. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. (2019) 16:57–73. doi: 10.1038/s41575-018-0055-0
154. Diehl AM and Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. (2017) 377:2063–72. doi: 10.1056/NEJMra1503519
155. Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. (2004) 10:S69–73. doi: 10.1002/lt.20033
156. Balkwill F and Mantovani A. Inflammation and cancer: back to Virchow? Lancet Lond Engl. (2001) 357:539–45. doi: 10.1016/s0140-6736(00)04046-0
157. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. (2002) 420:333–6. doi: 10.1038/nature01137
158. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, and Ikramuddin S. Nonalcoholic steatohepatitis: A review. JAMA. (2020) 323:1175–83. doi: 10.1001/jama.2020.2298
159. Rinella ME. Nonalcoholic fatty liver disease: A systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
161. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. (2015) 62:1723–30. doi: 10.1002/hep.28123
162. Dhamija E, Paul SB, and Kedia S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J Med Res. (2019) 149:9–17. doi: 10.4103/ijmr.IJMR_1456_17
163. Yang JD, Ahmed Mohammed HF, Harmsen WS, Enders F, Gores GJ, and Roberts LR. Recent trends in the epidemiology of hepatocellular carcinoma in olmsted county, minnesota: A US population-based study. J Clin Gastroenterol. (2017) 51:742–8. doi: 10.1097/MCG.0000000000000810
164. Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: A systematic review and meta-analysis. Am J Clin Oncol. (2018) 41:874–81. doi: 10.1097/COC.0000000000000388
165. Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. (2016) 122:1757–65. doi: 10.1002/cncr.v122.11
166. Hajarizadeh B, Grebely J, and Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. (2013) 10:553–62. doi: 10.1038/nrgastro.2013.107
167. El-Serag HB and Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. (2014) 60:1767–75. doi: 10.1002/hep.27222
168. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. (2015) 35:2155–66. doi: 10.1111/liv.2015.35.issue-9
169. Yang JD, Gyedu A, Afihene MY, Duduyemi BM, Micah E, Kingham TP, et al. Hepatocellular carcinoma occurs at an earlier age in africans, particularly in association with chronic hepatitis B. Am J Gastroenterol. (2015) 110:1629–31. doi: 10.1038/ajg.2015.289
170. Bertani S, Pineau P, Loli S, Moura J, Zimic M, Deharo E, et al. An atypical age-specific pattern of hepatocellular carcinoma in Peru: A threat for Andean populations. PloS One. (2013) 8:e67756. doi: 10.1371/journal.pone.0067756
171. Yang JD, Altekruse SF, Nguyen MH, Gores GJ, and Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. (2017) 123:81–9. doi: 10.1002/cncr.v123.1
172. Kasting ML, Giuliano AR, Reich RR, Roetzheim RG, Duong LM, Thomas E, et al. Hepatitis C virus screening trends: A 2016 update of the National Health Interview Survey. Cancer Epidemiol. (2019) 60:112–20. doi: 10.1016/j.canep.2019.03.007
173. Liew ZH, Goh GBB, Hao Y, Chang PE, and Tan CK. Comparison of hepatocellular carcinoma in patients with cryptogenic versus hepatitis B etiology: A study of 1079 cases over 3 decades. Dig Dis Sci. (2019) 64:585–90. doi: 10.1007/s10620-018-5331-x
174. Chen X, Wang L, Hong L, Su Z, Zhong X, Zhou H, et al. Identification of aging-related genes associated with clinical and prognostic features of hepatocellular carcinoma. Front Genet. (2021) 12:661988. doi: 10.3389/fgene.2021.661988
175. Ningarhari M, Caruso S, Hirsch TZ, Bayard Q, Franconi A, Védie AL, et al. Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic target. J Hepatol. (2021) 74:1155–66. doi: 10.1016/j.jhep.2020.11.052
176. Danpanichkul P, Aboona MB, Sukphutanan B, Kongarin S, Duangsonk K, Ng CH, et al. Incidence of liver cancer in young adults according to the Global Burden of Disease database 2019. Hepatology. (2024) 80:828. doi: 10.1097/HEP.0000000000000872
177. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
178. Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in myD88-dependent IL-6 production. Science. (2007) 317:121–4. doi: 10.1126/science.1140485
179. Keng VW, Largaespada DA, and Villanueva A. Why men are at higher risk for hepatocellular carcinoma? J Hepatol. (2012) 57:453–4. doi: 10.1016/j.jhep.2012.03.004
180. Busso C, Nault JC, Layese R, Demory A, Blaise L, Nkontchou G, et al. Prolonged survival in women with hepatocellular carcinoma: A French observational study. Clin Res Hepatol Gastroenterol. (2024) 48:102498. doi: 10.1016/j.clinre.2024.102498
181. De Mattia E, Cecchin E, Polesel J, Bignucolo A, Roncato R, Lupo F, et al. Genetic biomarkers for hepatocellular cancer risk in a caucasian population. World J Gastroenterol. (2017) 23:6674–84. doi: 10.3748/wjg.v23.i36.6674
182. Müller M, Bird TG, and Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. (2020) 72:990–1002. doi: 10.1016/j.jhep.2020.01.019
183. Lewis CM and Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. (2020) 12:44. doi: 10.1186/s13073-020-00742-5
184. Dessein A. Clinical utility of polygenic risk scores for predicting NAFLD disorders. J Hepatol. (2021) 74:769–70. doi: 10.1016/j.jhep.2021.02.005
185. Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. (2021) 74:775–82. doi: 10.1016/j.jhep.2020.11.024
186. Trépo E, Caruso S, Yang J, Imbeaud S, Couchy G, Bayard Q, et al. Common genetic variation in alcohol-related hepatocellular carcinoma: a case-control genome-wide association study. Lancet Oncol. (2022) 23:161–71. doi: 10.1016/S1470-2045(21)00603-3
187. Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, and Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. (2018) 67:600–11. doi: 10.1002/hep.29498
188. Wu Q and Qin SK. Features and treatment options of Chinese hepatocellular carcinoma. Chin Clin Oncol. (2013) 2:8. doi: 10.3978/j.issn.2304-3865.2013.09.07
189. Sanyal AJ, Yoon SK, and Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. (2010) 15:14–22. doi: 10.1634/theoncologist.2010-S4-14
190. Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. (2011) 21:401–16. doi: 10.2188/jea.JE20100190
191. Yang M, Parikh ND, Liu H, Wu E, Rao H, Feng B, et al. Incidence and risk factors of hepatocellular carcinoma in patients with hepatitis C in China and the United States. Sci Rep. (2020) 10:20922. doi: 10.1038/s41598-020-77515-y
192. Hong Kong Cancer Registry. Report of Stage-specific Survival of Liver Cancer in Hong Kong Hong Kong Hospital Authority. Available online at: https://www3.ha.org.hk/cancereg (Accessed January 16, 2025).
193. Cheung TK, Lai CL, Wong BCY, Fung J, and Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. (2006) 24:573–83. doi: 10.1111/j.1365-2036.2006.03029.x
194. Ikai I, Kudo M, Arii S, Omata M, Kojiro M, Sakamoto M, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. (2010) 40:1043–59. doi: 10.1111/j.1872-034X.2010.00731.x
195. Yuen MF, Hou JL, and Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. (2009) 24:346–53. doi: 10.1111/j.1440-1746.2009.05784.x
196. Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev APJCP. (2008) 9:97–100.
197. Singh SP, Madke T, and Chand P. Global epidemiology of hepatocellular carcinoma. J Clin Exp Hepatol. (2025) 15:102446. doi: 10.1016/j.jceh.2024.102446
198. Paul SB, Sreenivas V, Gulati MS, Madan K, Gupta AK, Mukhopadhyay S, et al. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from a tertiary care center in northern India. Indian J Gastroenterol Off J Indian Soc Gastroenterol. (2007) 26:274–8.
199. Kumar R, Saraswat MK, Sharma BC, Sakhuja P, and Sarin SK. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM Int J Med. (2008) 101:479–85. doi: 10.1093/qjmed/hcn033
200. Rastogi A, Maiwall R, Ramakrishna G, Modi S, Taneja K, Bihari C, et al. Hepatocellular carcinoma: Clinicopathologic associations amidst marked phenotypic heterogeneity. Pathol - Res Pract. (2021) 217:153290. doi: 10.1016/j.prp.2020.153290
201. Mondal D, Das K, and Chowdhury A. Epidemiology of liver diseases in India. Clin Liver Dis. (2022) 19:114–7. doi: 10.1002/cld.1177
202. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Today (version 1.1). Lyon, France: International Agency for Research on Cancer. (2024). Available online at: https://gco.iarc.who.int/today (Accessed October 1, 2025).
203. Wong LL and Tsai N. Hepatocellular carcinoma in Filipinos. Asia Pac J Clin Oncol. (2007) 3:84–8. doi: 10.1111/j.1743-7563.2007.00091.x
204. Carpio GCA, Lomboy ARB, Libuit JM, Vicente IMG, Firmalino GC, and Ong JP. Demographic profile and treatment outcomes of Filipino patients with hepatocellular carcinoma in a liver tumor registry. Clin Gastroenterol Hepatol. (2015) 13:e110. doi: 10.1016/j.cgh.2015.04.130
205. Lucas ZDF, Pangan CP, Patal PC, and Ong J. The clinical profile of hepatocellular carcinoma patients at the Philippine general hospital. Philipp J Intern Med. (2009) 47:1–9. doi: 10.3860/pjim.v47i1.877
206. Wanich N, Vilaichone RK, Chotivitayatarakorn P, and Siramolpiwat S. High prevalence of hepatocellular carcinoma in patients with chronic hepatitis B infection in Thailand. Asian Pac J Cancer Prev APJCP. (2016) 17:2857–60.
207. Parkin DM, Bray F, Ferlay J, and Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2014) 23:953–66. doi: 10.1158/1055-9965.EPI-14-0281
208. Chaabna K, Cheema S, Abraham A, Alrouh H, Lowenfels AB, Maisonneuve P, et al. Systematic overview of hepatitis C infection in the Middle East and North Africa. World J Gastroenterol. (2018) 24:3038–54. doi: 10.3748/wjg.v24.i27.3038
209. Okeke E, Davwar PM, Roberts L, Sartorius K, Spearman W, Malu A, et al. Epidemiology of liver cancer in Africa: current and future trends. Semin Liver Dis. (2020) 40:111–23. doi: 10.1055/s-0039-3399566
210. Bahri O, Ezzikouri S, Alaya-Bouafif NB, Iguer F, Feydi AEE, Mestiri H, et al. First multicenter study for risk factors for hepatocellular carcinoma development in North Africa. World J Hepatol. (2011) 3:24–30. doi: 10.4254/wjh.v3.i1.24
211. Jonas E, Bernon M, Robertson B, Kassianides C, Keli E, Asare KO, et al. Treatment of hepatocellular carcinoma in sub-Saharan Africa: challenges and solutions. Lancet Gastroenterol Hepatol. (2022) 7:1049–60. doi: 10.1016/S2468-1253(22)00042-5
212. Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int Off J Int Assoc Study Liver. (2003) 23:405–9. doi: 10.1111/j.1478-3231.2003.00869.x
213. Madden CR, Finegold MJ, and Slagle BL. Altered DNA mutation spectrum in aflatoxin B1-treated transgenic mice that express the hepatitis B virus X protein. J Virol. (2002) 76:11770–4. doi: 10.1128/JVI.76.22.11770-11774.2002
214. Qi LN, Bai T, Chen ZS, Wu FX, Chen YY, De Xiang B, et al. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China : role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int Off J Int Assoc Study Liver. (2015) 35:999–1009. doi: 10.1111/liv.2015.35.issue-3
215. Selim MI, Popendorf W, Ibrahim MS, el Sharkawy S, and el Kashory ES. Aflatoxin B1 in common Egyptian foods. J AOAC Int. (1996) 79:1124–9. doi: 10.1093/jaoac/79.5.1124
216. Darwish WS, Ikenaka Y, Nakayama SMM, and Ishizuka M. An overview on mycotoxin contamination of foods in Africa. J Vet Med Sci. (2014) 76:789–97. doi: 10.1292/jvms.13-0563
217. Wagacha JM and Muthomi JW. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol. (2008) 124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008
218. Atawodi SE, Atiku AA, and Lamorde AG. Aflatoxin contamination of Nigerian foods and feedingstuffs. Food Chem Toxicol. (1994) 32:61–3. doi: 10.1016/0278-6915(84)90038-3
219. Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. (1996) 20:553–66. doi: 10.1016/0891-5849(95)02111-6
220. Gangaidzo IT and Gordeuk VR. Hepatocellular carcinoma and African iron overload. Gut. (1995) 37:727–30. doi: 10.1136/gut.37.5.727
221. Kew MC and Asare GA. Dietary iron overload in the African and hepatocellular carcinoma. Liver Int. (2007) 27:735–41. doi: 10.1111/j.1478-3231.2007.01515.x
222. Mandishona E, MacPhail AP, Gordeuk VR, Kedda MA, Paterson AC, Rouault TA, et al. Dietary iron overload as a risk factor for hepatocellular carcinoma in black africans. Hepatology. (1998) 27:1563–6. doi: 10.1002/hep.510270614
223. Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett. (2009) 286:38–43. doi: 10.1016/j.canlet.2008.11.001
224. Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. (2014) 3:31–40. doi: 10.1159/000343856
225. Sinclair A. Sub-Sahara Africa—The impact and challenge of type 2 diabetes mellitus requiring urgent and sustainable public health measures. EClinicalMedicine. (2019) 16:6–7. doi: 10.1016/j.eclinm.2019.10.005
226. Singh KP, Crane M, Audsley J, and Lewin SR. HIV-Hepatitis B virus co-infection: epidemiology, pathogenesis and treatment. AIDS Lond Engl. (2017) 31:2035–52. doi: 10.1097/QAD.0000000000001574
227. Yang JD, Mohamed EA, Aziz AOA, Shousha HI, Hashem MB, Nabeel MM, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol. (2017) 2:103–11. doi: 10.1016/S2468-1253(16)30161-3
228. Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol. (2015) 12:173–82. doi: 10.1016/S1665-2681(19)31354-7
229. Moore SW, Davidson A, Hadley GP, Kruger M, Poole J, Stones D, et al. Malignant liver tumors in South African children: a national audit. World J Surg. (2008) 32:1389–95. doi: 10.1007/s00268-008-9526-8
230. Alvarez CS, Ortiz J, Bendfeldt-Avila G, Xie Y, Wang M, Wu D, et al. Analysis of TP53 aflatoxin signature mutation in hepatocellular carcinomas from Guatemala: A cross-sectional study (2016-2017). Health Sci Rep. (2020) 3:e155. doi: 10.1002/hsr2.v3.2
231. Alvarez CS, Rivera-Andrade A, Kroker-Lobos MF, Florio AA, Smith JW, Egner PA, et al. Associations between aflatoxin B1-albumin adduct levels with metabolic conditions in Guatemala: A cross-sectional study. Health Sci Rep. (2022) 5:e495. doi: 10.1002/hsr2.v5.1
232. Chan AJ, Balderramo D, Kikuchi L, Ballerga EG, Prieto JE, Tapias M, et al. Early age hepatocellular carcinoma associated with hepatitis B infection in South America. Clin Gastroenterol Hepatol. (2017) 15:1631–2. doi: 10.1016/j.cgh.2017.05.015
233. Farah M, Anugwom C, Ferrer JD, Baca EL, Mattos AZ, Possebon JPP, et al. Changing epidemiology of hepatocellular carcinoma in South America: A report from the South American liver research network. Ann Hepatol. (2023) 28:100876. doi: 10.1016/j.aohep.2022.100876
234. Marchio A, Cerapio JP, Ruiz E, Cano L, Casavilca S, Terris B, et al. Early-onset liver cancer in South America associates with low hepatitis B virus DNA burden. Sci Rep. (2018) 8:12031. doi: 10.1038/s41598-018-30229-8
235. Cano L, Cerapio JP, Ruiz E, Marchio A, Turlin B, Casavilca S, et al. Liver clear cell foci and viral infection are associated with non-cirrhotic, non-fibrolamellar hepatocellular carcinoma in young patients from South America. Sci Rep. (2018) 8:9945. doi: 10.1038/s41598-018-28286-0
236. Pineau P, Ruiz E, Deharo E, and Bertani S. On hepatocellular carcinoma in South America and early-age onset of the disease. Clin Res Hepatol Gastroenterol. (2019) 43:522–6. doi: 10.1016/j.clinre.2018.10.019
237. Cano L, Bertani S, Island ML, Cerapio JP, Ruiz E, Pineau P, et al. Metallomic profile in non-cirrhotic hepatocellular carcinoma supports a phenomenon of metal metabolism adaptation in tumor cells. Sci Rep. (2021) 11:14195. doi: 10.1038/s41598-021-93369-4
238. Kronenfeld JP, Ryon EL, Goldberg D, Lee RM, Yopp A, Wang A, et al. Disparities in presentation at time of hepatocellular carcinoma diagnosis: A United States safety-net collaborative study. Ann Surg Oncol. (2021) 28:1929–36. doi: 10.1245/s10434-020-09156-4
239. Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2019) 17:551–559.e1. doi: 10.1016/j.cgh.2018.05.039
240. Rich NE, Carr C, Yopp AC, Marrero JA, and Singal AG. Racial and ethnic disparities in survival among patients with hepatocellular carcinoma in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. (2022) 20:e267–88. doi: 10.1016/j.cgh.2020.12.029
241. Costentin C, Ganne-Carrié N, Rousseau B, Gérolami R, and Barbare JC. Parcours de soins du patient atteint de carcinome hépatocellulaire en France: état des lieux en 2017. Bull Cancer (Paris). (2017) 104:752–61. doi: 10.1016/j.bulcan.2017.06.008
242. Goutté N, Sogni P, Bendersky N, Barbare JC, Falissard B, and Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. (2017) 66:537–44. doi: 10.1016/j.jhep.2016.10.015
243. Nay O, Béjean S, Benamouzig D, Bergeron H, Castel P, and Ventelou B. Achieving universal health coverage in France: policy reforms and the challenge of inequalities. Lancet. (2016) 387:2236–49. doi: 10.1016/S0140-6736(16)00580-8
244. Mathurin P, de Zélicourt M, Laurendeau C, Dhaoui M, Kelkouli N, and Blanc JF. Treatment patterns, risk factors and outcomes for patients with newly diagnosed hepatocellular carcinoma in France: A retrospective database analysis. Clin Res Hepatol Gastroenterol. (2023) 47:102124. doi: 10.1016/j.clinre.2023.102124
245. Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. (2013) 57:1426–35. doi: 10.1002/hep.25832
246. Reveron-Thornton RF, Teng MLP, Lee EY, Tran A, Vajanaphanich S, Tan EX, et al. Global and regional long-term survival following resection for HCC in the recent decade: A meta-analysis of 110 studies. Hepatol Commun. (2022) 6:1813–26. doi: 10.1002/hep4.1923
247. O’Brien M, Mwangi-Powell F, Adewole IF, Soyannwo O, Amandua J, Ogaja E, et al. Improving access to analgesic drugs for patients with cancer in sub-Saharan Africa. Lancet Oncol. (2013) 14:e176–182. doi: 10.1016/S1470-2045(12)70343-1
248. Ladep NG, Lesi OA, Mark P, Lemoine M, Onyekwere C, Afihene M, et al. Problem of hepatocellular carcinoma in West Africa. World J Hepatol. (2014) 6:783–92. doi: 10.4254/wjh.v6.i11.783
249. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-pacific primary liver cancer expert consensus statements. Liver Cancer. (2020) 9:245–60. doi: 10.1159/000507370
250. Piñero F, Marciano S, Fernández N, Silva J, Anders M, Zerega A, et al. Intermediate-advanced hepatocellular carcinoma in Argentina: Treatment and survival analysis. World J Gastroenterol. (2019) 25:3607–18. doi: 10.3748/wjg.v25.i27.3607
251. Piñero F, Tanno M, Aballay Soteras G, Tisi Baña M, Dirchwolf M, Fassio E, et al. Argentinian clinical practice guideline for surveillance, diagnosis, staging and treatment of hepatocellular carcinoma. Ann Hepatol. (2020) 19:546–69. doi: 10.1016/j.aohep.2020.06.003
252. Chagas AL, de Mattos AA, Carrilho FJ, and Bittencourt PL. Brazilian society of hepatology updated recommendations for diagnosis and treatment of hepatocellular carcinoma. Arq Gastroenterol. (2020) 57:1–20. doi: 10.1590/s0004-2803.202000000-20
253. Dirchwolf M, Marciano S, Ruf AE, Singal AG, D’Ercole V, Coisson P, et al. Failure in all steps of hepatocellular carcinoma surveillance process is frequent in daily practice. Ann Hepatol. (2021) 25:100344. doi: 10.1016/j.aohep.2021.100344
254. Innes H and Nahon P. Statistical perspectives on using hepatocellular carcinoma risk models to inform surveillance decisions. J Hepatol. (2023) 79:1332–7. doi: 10.1016/j.jhep.2023.05.005
Keywords: liver fibrogenesis, tumor progression, phenotypic diversity, hepatitis, alcohol misuse, alcohol use disorder, alcohol-related liver disease, MAFLD
Citation: Cano L, Foucher F and Musso O (2025) Geographic diversity of human liver cancers mirrors global social inequalities. Front. Oncol. 15:1565692. doi: 10.3389/fonc.2025.1565692
Received: 27 January 2025; Accepted: 22 April 2025;
Published: 16 May 2025.
Edited by:
Paulo S. Pinheiro, University of Miami, United StatesReviewed by:
Qinran Liu, American Cancer Society, United StatesNicholas Acuna, Cedars Sinai Medical Center, United States
Copyright © 2025 Cano, Foucher and Musso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orlando Musso, b3JsYW5kby5tdXNzb0BpbnNlcm0uZnI=
†These authors have contributed equally to this work
 Luis Cano
Luis Cano Fabien Foucher
Fabien Foucher Orlando Musso
Orlando Musso