- 1Specialized Medical Department, Jiangxi Cancer Hospital & Institute (The Second Affiliated Hospital of Nanchang Medical College), Nanchang, China
- 2Department of Breast Oncology Surgery, Jiangxi Cancer Hospital & Institute (The Second Affiliated Hospital of Nanchang Medical College), Nanchang, China
- 3Department of Head and Neck Oncology Radiation Therapy, Jiangxi Cancer Hospital & Institute (The Second Affiliated Hospital of Nanchang Medical College), Nanchang, China
Background: Central venous access for cancer chemotherapy is crucial for patients undergoing long-term treatment. The internal jugular vein (IJV) and subclavian vein (SCV) are commonly used for implantable port insertion, though the optimal choice remains debated. This meta-analysis aims to compare the safety and effectiveness of IJV and SCV for central venous implantable port insertion in cancer chemotherapy patients based on randomized controlled trials (RCTs).
Methods: We systematically reviewed RCTs comparing IJV and SCV for implantable port insertion. The primary endpoint was complication, while secondary endpoints included procedure failure rate, procedure duration, patient satisfaction, and pain perception.
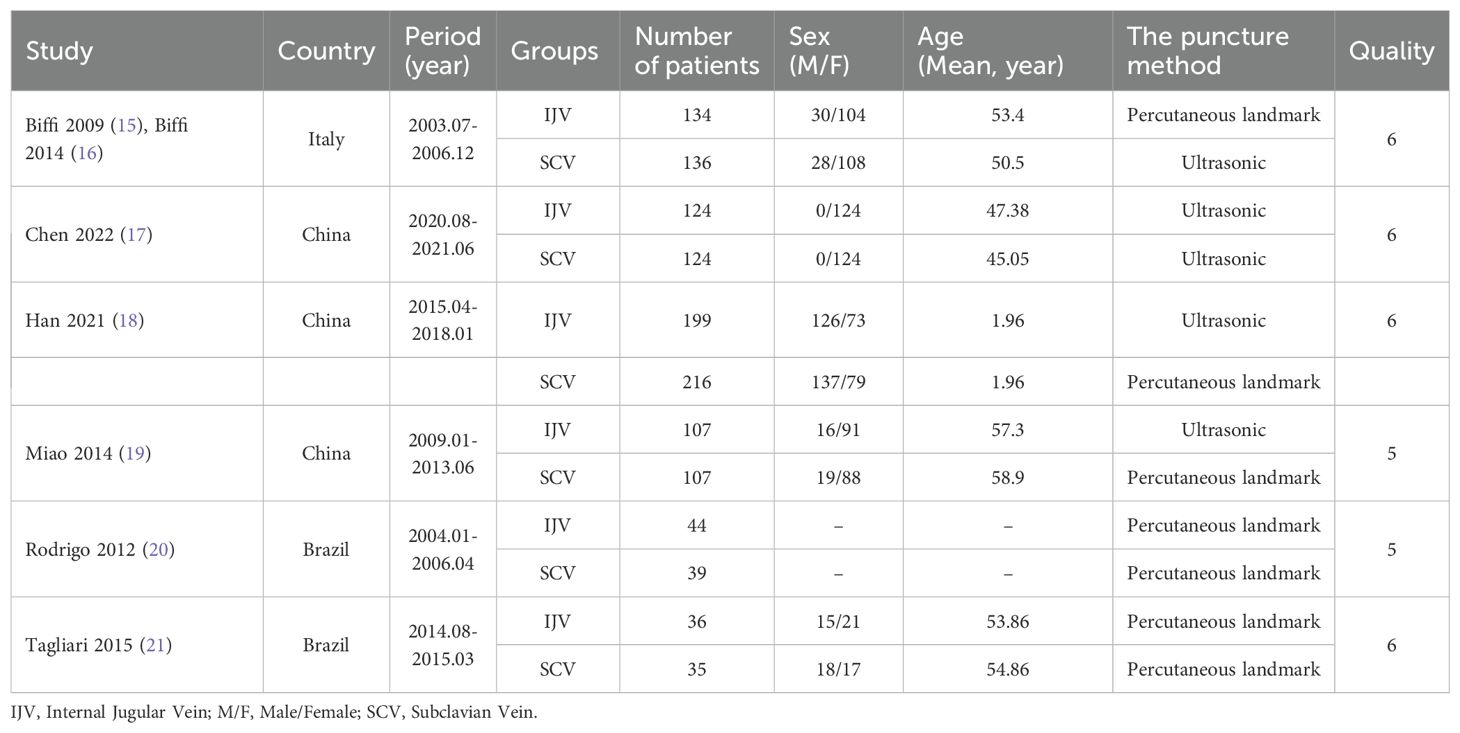
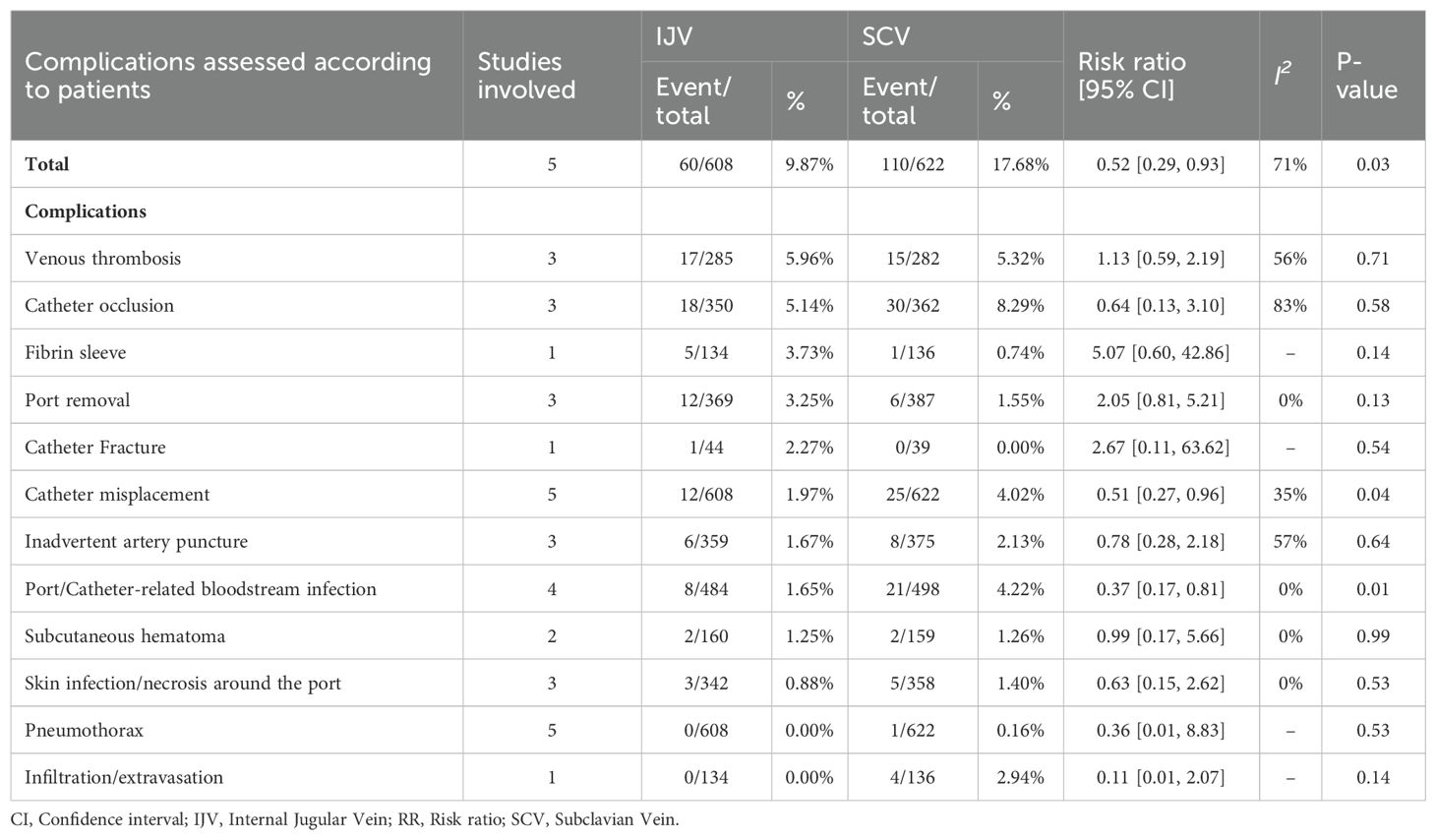
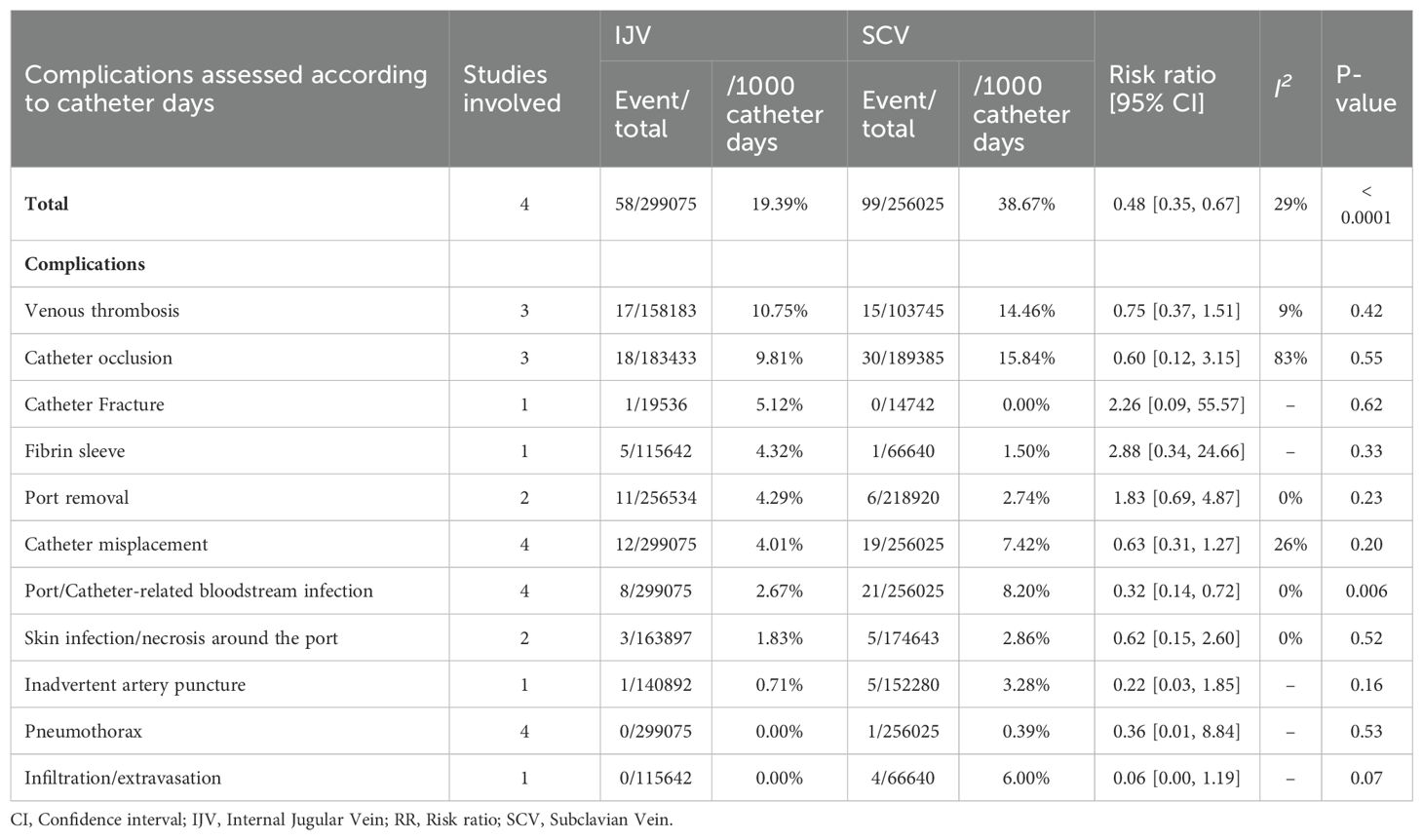
Results: A total of 7 studies based on 6 RCTs met the inclusion criteria. The baseline characteristics (age, sex, port side, and duration of implant) of patients in both groups were comparable. According to patient-reported outcomes, the SCV group experienced higher rates of total complications (risk ratio [RR]: 0.52 [0.29, 0.93], P = 0.03, I2 = 71%), catheter misplacement (RR: 0.51 [0.27, 0.96], P = 0.04, I2 = 35%), and port/catheter-related bloodstream infections (RR: 0.37 [0.17, 0.81], P = 0.01, I2 = 0%). Similarly, according to catheter days, the SCV group achieved higher rates of total complications (RR: 0.48 [0.35, 0.67], P < 0.0001, I2 = 29%) and port/catheter-related bloodstream infections (PRBIs) (RR: 0.32 [0.14, 0.72], P = 0.006, I2 = 0%). Pain perception (mean difference [MD]: -1.60 [-1.93, -1.27], P < 0.00001) was also worse in the SCV group. However, the duration of the procedure (MD: 11.55 [0.57, 22.54] minutes, P = 0.04, I2 = 97%) was longer in the IJV group. The procedural failure rate was comparable between the two groups.
Conclusions: For cancer chemotherapy patients, the IJV appears to be a safer and less painful alternative to the SCV for central venous port insertion.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42025641904.
Introduction
For cancer patients receiving chemotherapy, central venous access is critical, providing a reliable route for drug administration and blood sampling (1). Among the various methods, implantable ports (IPs) are preferred due to their lower complication rates and longer patency compared to other central venous devices (2). The internal jugular vein (IJV) and subclavian vein (SCV) are frequently used for IP insertion, but the optimal choice remains a subject of debate, as each approach presents distinct advantages and challenges (3). The IJV is often favored due to its more straightforward anatomical path and direct connection to the superior vena cava, which reduces the risk of catheter malposition. However, it is linked to an increased risk of complications, such as pneumothorax, particularly in patients with anatomical variations (4). The SCV, while deeper and more challenging to access, is less prone to displacement. However, it has been associated with a higher incidence of thrombosis and long-term catheter dysfunction (5).
Despite the widespread use of both venous sites, there remains a lack of high-quality, evidence-based studies comparing the clinical outcomes of IJV versus SCV for IP insertion. Several studies have explored various complications associated with these two approaches, including infection rates, thrombosis, and catheter migration, but the findings remain inconsistent. Mansfield et al. reported a lower incidence of pneumothorax with IJV access, while Rixecker et al. found a reduced infection rate with SCV access, likely due to its deeper anatomical location (6, 7). Additionally, Kaul et al. observed a higher incidence of thrombosis with SCV access, which complicates chemotherapy administration (8). Long-term complications, such as port migration and dysfunction, also differ between the two approaches. Becker et al. noted a higher risk of port migration with IJV insertion, which may require reintervention (9), whereas Adrian et al. found that SCV access is more prone to catheter occlusion over time, thereby reducing the port’s functionality for chemotherapy (10).
This meta-analysis aims to address the gap in current literature by comparing the safety and efficacy of IJV versus SCV for IP insertion in cancer patients. We focus on complications such as thrombosis and infection, along with clinical outcomes like pain perception. By synthesizing data from high-quality randomized controlled trials (RCTs), this study will provide a comprehensive evaluation of these two central venous access methods, offering evidence-based guidance for clinical practice.
Materials and methods
Search strategy
A comprehensive search was conducted across the Web of Science, EMBASE, Cochrane Library, PubMed, ScienceDirect, and Scopus databases to identify RCTs comparing IJV and SCV for central venous implantable port insertion in cancer chemotherapy, up to January 2, 2025. The MeSH terms used in the search included “Internal Jugular Vein”, “Subclavian Vein”, and “Randomized”. Furthermore, eligible studies were identified by screening the reference lists of the included articles. The complete search strategies for each database, including exact search strings, date ranges, and language filters, are presented in Supplementary Table S1.
Selection criteria
The inclusion criteria were set as follows: 1. RCTs; 2. Studies involving cancer patients receiving implantable ports and chemotherapy; 3. Direct comparison between IJC and SCV; 4. Includes the following outcomes: primary outcomes (complications), secondary outcomes (characteristics, data of procedures, etc).
Articles were excluded if they lacked primary data, or were meta-analyses, conference abstracts, case reports, or reviews.
Data extraction
Data were extracted independently by two investigators, including study characteristics (country, duration, etc.), patient characteristics (e.g. sex, age), safety (e.g. total and individual complications), and procedural data (e.g. failure rates, procedure duration). Any discrepancies were resolved through data re-evaluation.
Outcome assessments
Complications were evaluated based on patient count or catheter days. Pain perception was measured using the Faces Pain Scale-Revised (FPS-R), which ranges from 0 to 10, with higher scores indicating greater levels of perceived pain (the minimal clinically important difference [MCID] was 1.0) (11).
Quality assessment for included studies
The RCTs were assessed for quality using the Cochrane Risk of Bias Tool and the Jadad scale, with a maximum score of 7 points. A score of 4 or above indicates high quality (12, 13). The overall quality of evidence was evaluated using the GRADE approach (14).
Statistical analysis
Statistical analyses were performed using Stata 14.0 and RevMan 5.3 software. I² > 50% or P < 0.1 indicating substantial heterogeneity, in line with current meta-analytic guidelines (15). A random-effects model was applied in cases of high heterogeneity, while a fixed-effects model was used when heterogeneity was low. For dichotomous data, pooled risk ratios (RR) were calculated, whereas for continuous data, mean differences (MD) were used. In addition, publication bias was evaluated using funnel plot asymmetry test. A p-value of less than 0.05 was considered statistically significant for all tests.
Results
Search results
After an initial screening of 687 studies, 7 papers derived from 6 RCTs were included in the analysis (IJV group: 644 patients; SCV group: 657 patients) (Figure 1) (16–22). The baseline characteristics are summarized in Table 1. Of the studies, three were conducted in Asia, two in South America, and one in Europe. When comparing baseline characteristics, age, sex, port side, and implant duration were comparable across both groups (Figure 2). Each of the studies was considered to be of high quality (Supplementary Figure S1, Supplementary Table S2). Additionally, based on the GRADE system, all results were rated as moderate to high quality (Supplementary Table S3).
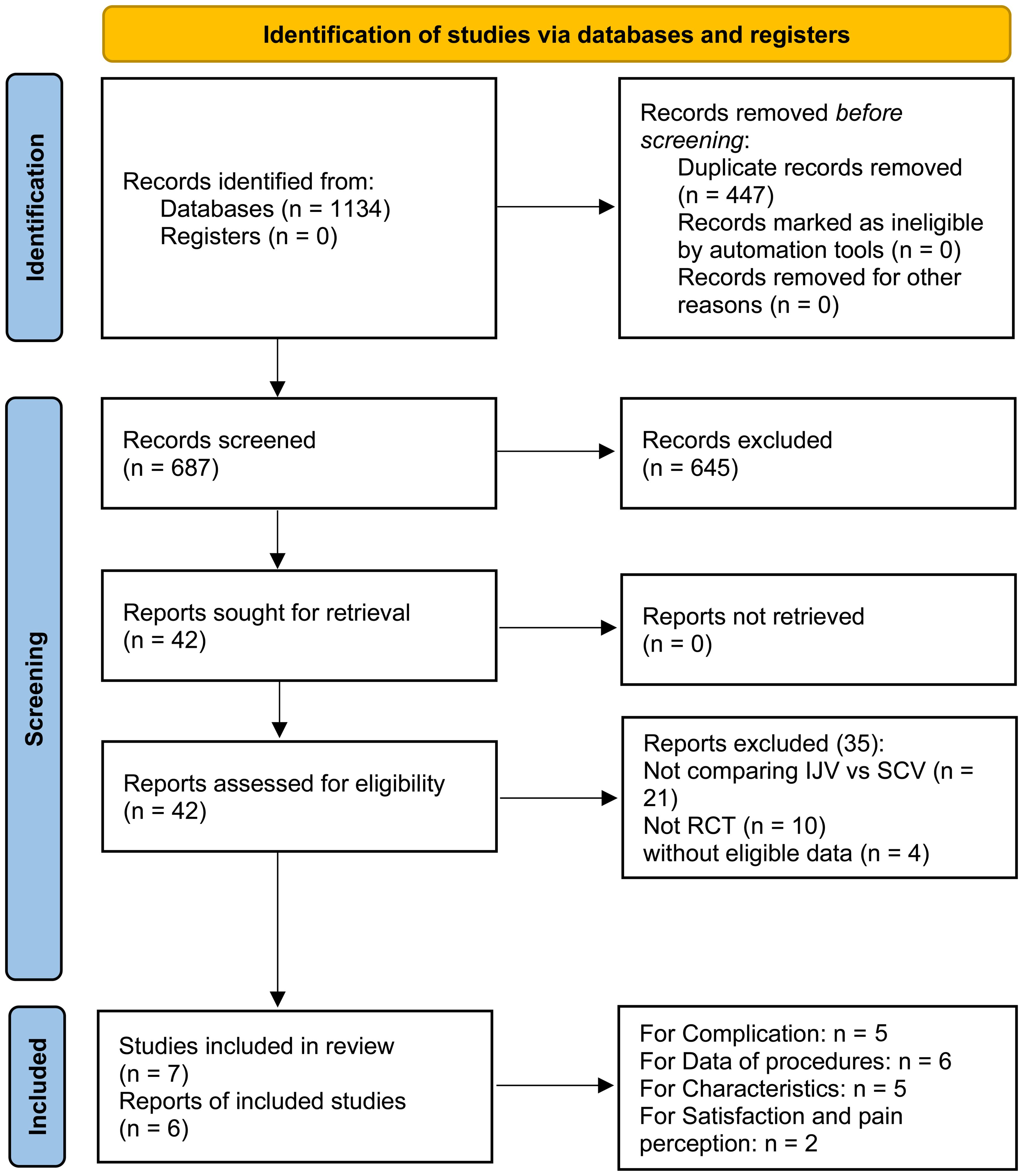
Figure 1. Flow chart. After an initial screening of 687 studies, 7 papers derived from 6 RCTs were included in the analysis.
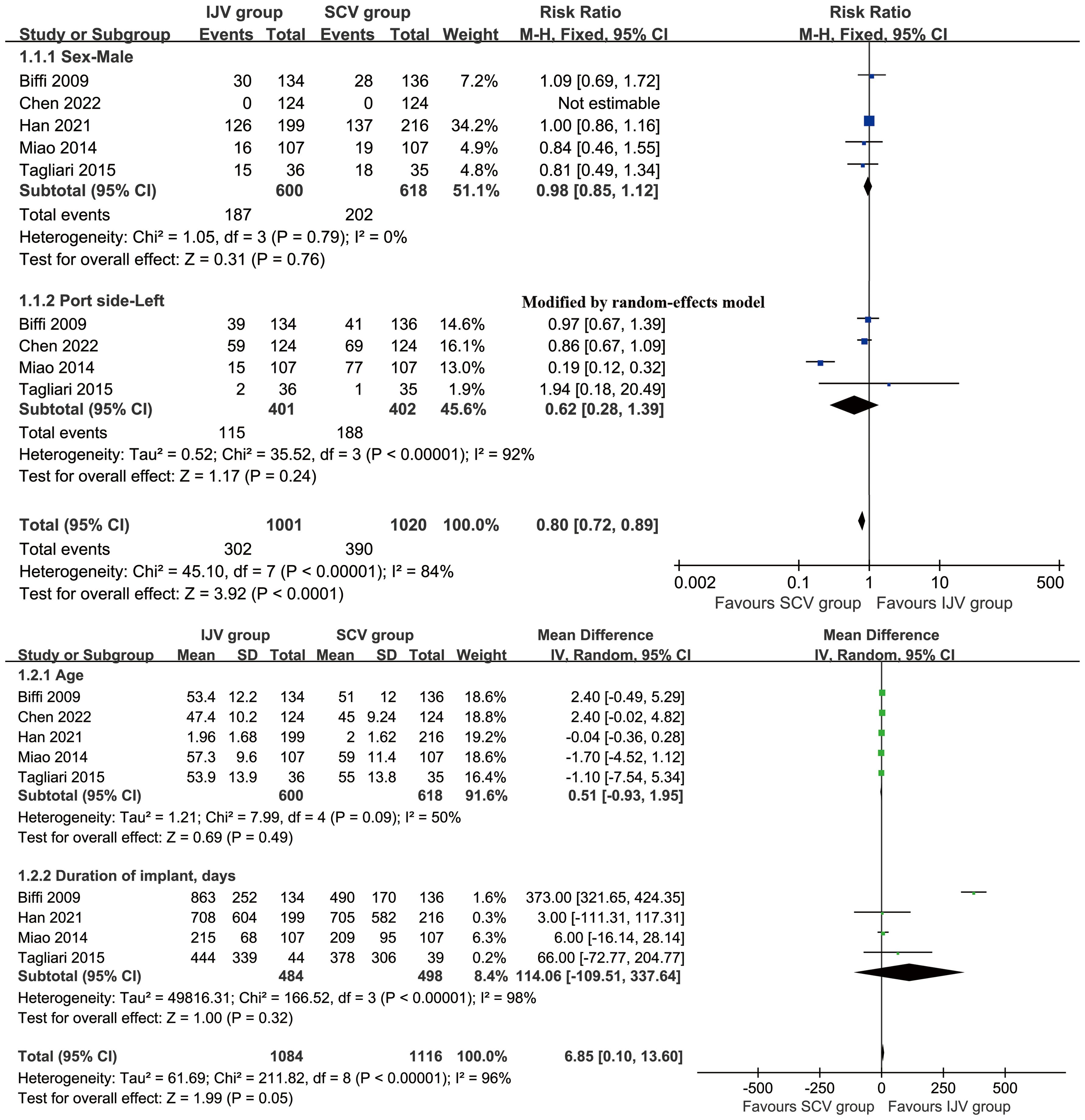
Figure 2. Forest plots of baseline characteristics associated with IJV versus SCV. Baseline age, sex, port side, and implant duration were comparable across both group.
Complications assessed according to patients
According to patient data, the SCV group exhibited higher rates of total complications (RR: 0.52 [0.29, 0.93], P = 0.03, I2 = 71%), catheter misplacements (RR: 0.51 [0.27, 0.96], P = 0.04, I2 = 35%), and port/catheter-related bloodstream infections (PRBIs) (RR: 0.37 [0.17, 0.81], P = 0.01, I2 = 0%) (Figure 3). The incidence of venous thrombosis, catheter occlusion, fibrin sleeve, port removal, catheter fracture, inadvertent artery puncture, subcutaneous hematoma, skin infection/necrosis around the port, pneumothorax, and infiltration/extravasation were comparable between the two groups (Table 2, Supplementary Figure S2).
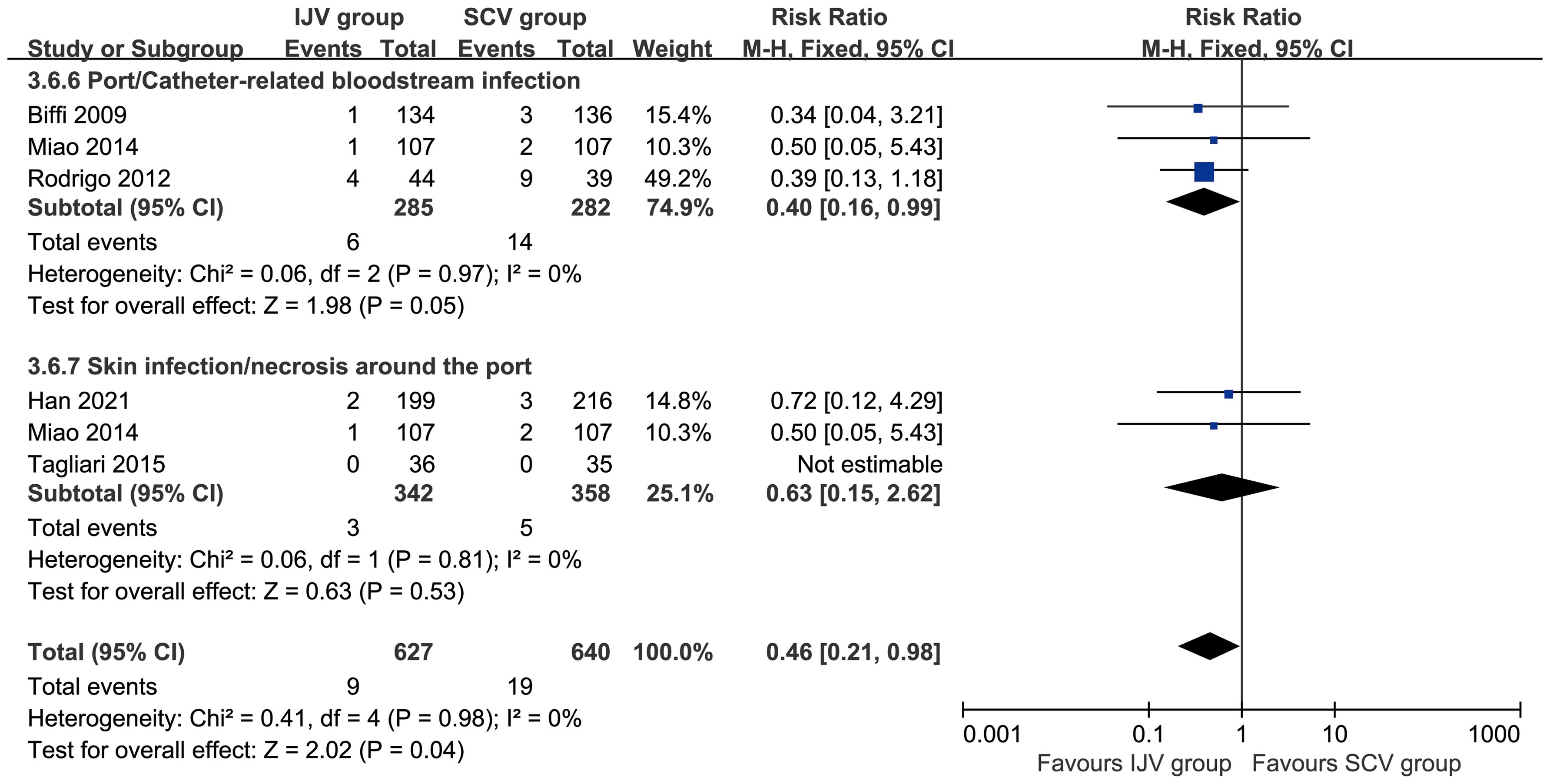
Figure 3. Forest plots of infections associated with IJV versus SCV. According to patient data, the SCV group exhibited higher rates of total complications, catheter misplacements, and port/catheter-related bloodstream infections.
Complications assessed according to catheter days
According to catheter days, the SCV group showed higher rates of total complications (RR: 0.48 [0.35, 0.67], P < 0.0001, I2 = 29%) and PRBIs (RR: 0.32 [0.14, 0.72], P = 0.006, I2 = 0%). The rates of venous thrombosis, catheter occlusion, catheter fracture, fibrin sleeve, port removal, catheter misplacement, skin infection/necrosis around the port, inadvertent artery puncture, pneumothorax, and infiltration/extravasation were comparable between the two groups (Table 3, Supplementary Figure S3).
Data of procedures
The SCV group reported higher pain perception (MD: -1.60 [-1.93, -1.27], P < 0.00001), which indicating a perceptible and clinically meaningful increase in procedural pain. However, the duration of the procedure (MD: 11.55 [0.57, 22.54] minutes, P = 0.04, I2 = 97%) was longer in the IJV group. The procedural failure rate was comparable between the two groups (Figure 4).
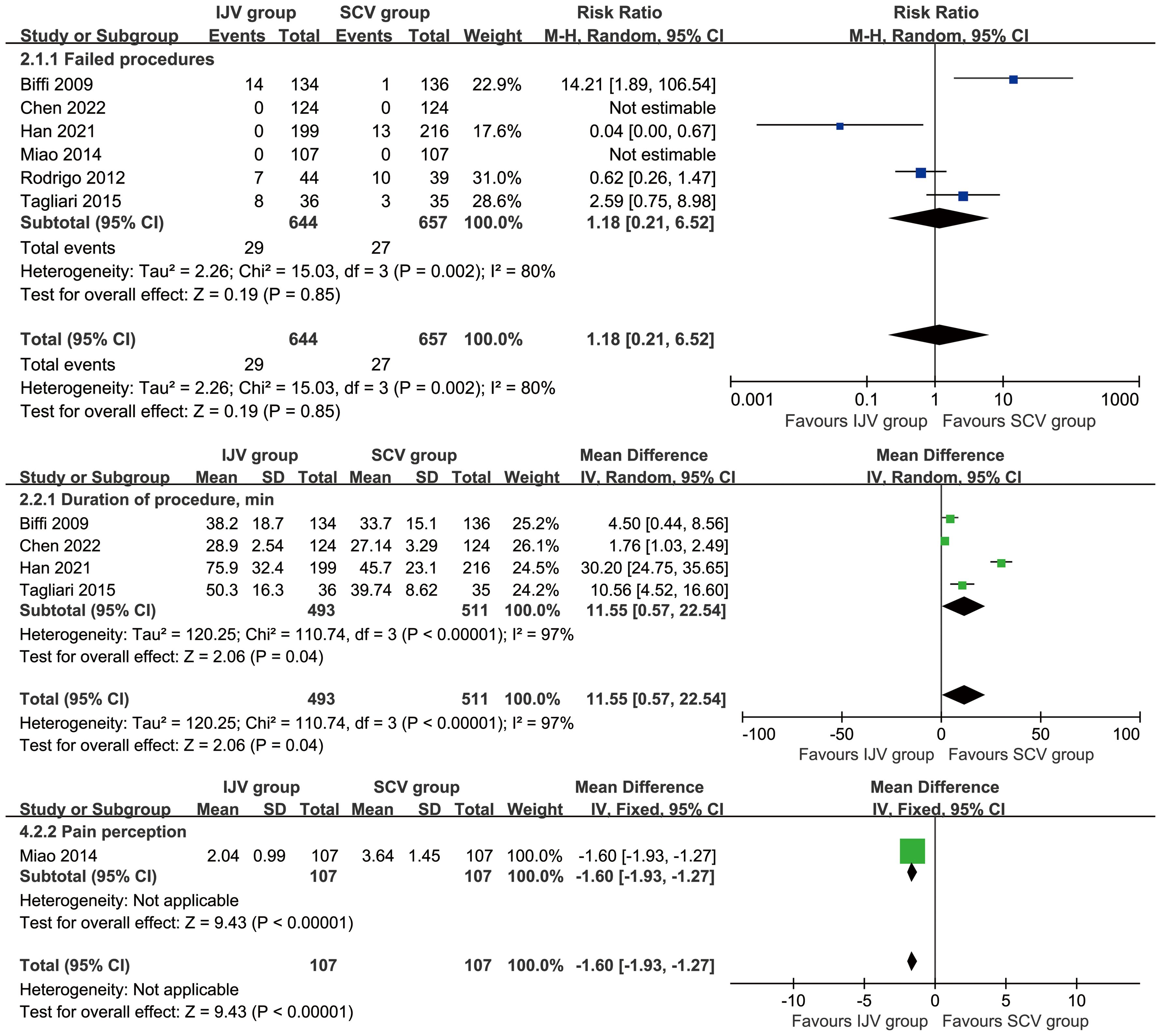
Figure 4. Forest plots of data of procedures associated with IJV versus SCV. The SCV group reported higher pain perception, shorter duration of the procedure, and similar procedural failures as compared with the IJV group.
Sensitivity analysis
The results for total complications assessed according to patients, duration of the procedure, and failed procedures, remained consistent after excluding individual studies in the sensitivity analysis (Supplementary Figure S4).
Publication bias
The funnel plot for complications assessed according to patients/catheter days, showed no significant publication bias (Figure 5).
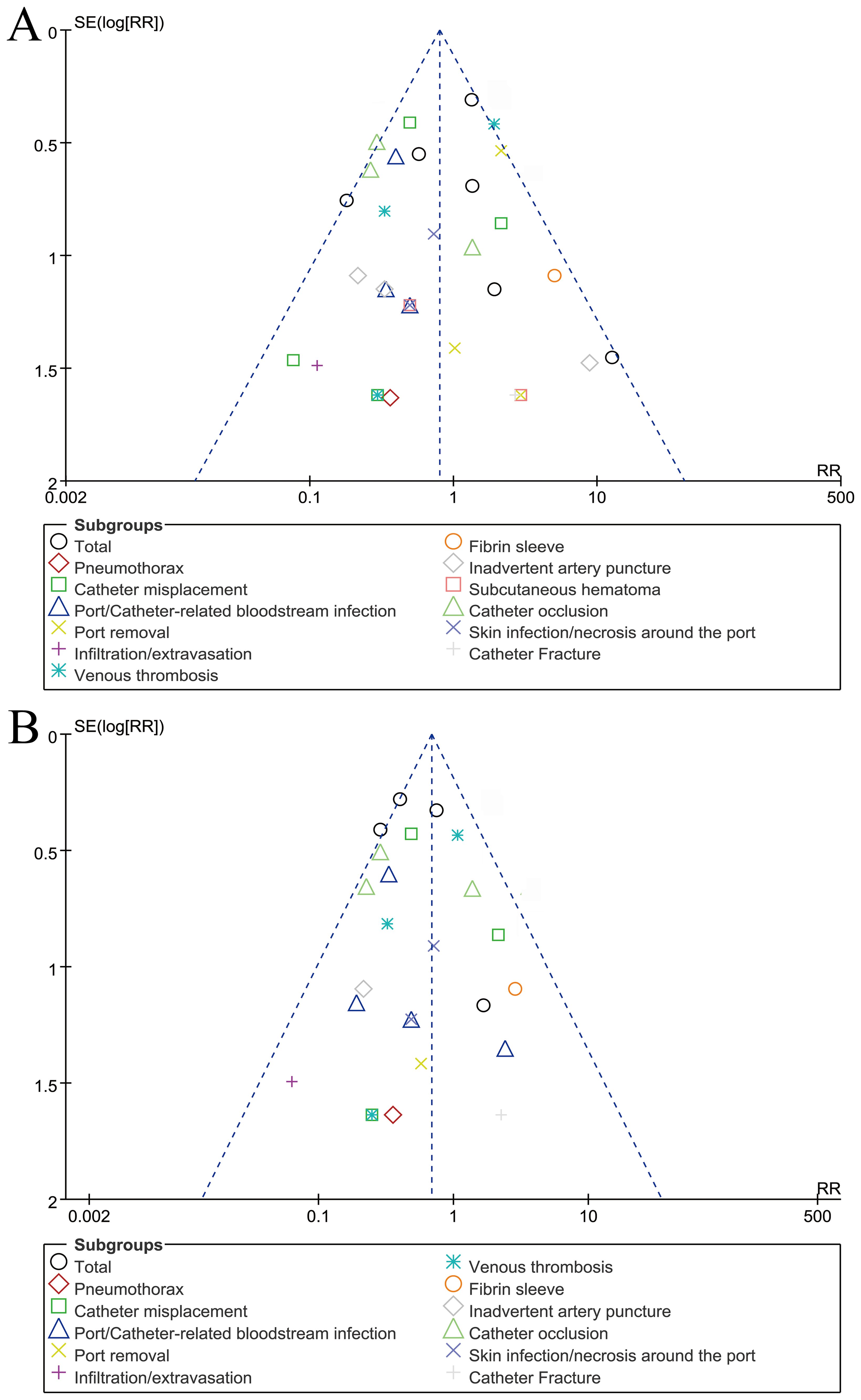
Figure 5. Funnel plots of complications assessed according to patients (A) or catheter days (B). Visual inspection suggests symmetry, indicating a low risk of publication bias.
Discussion
Implantable ports for cancer chemotherapy play a critical role in patients undergoing long-term therapy (2). The choice of venous access site, whether the IJV or SCV, remains a topic of ongoing clinical debate, with no clear consensus on the optimal approach (23). Although previous studies have suggested differences in complications associated with these two approaches, no meta-analysis based on RCTs has comprehensively compared them to provide clear evidence for clinicians (16–22). Our meta-analysis suggests that IJV insertion is associated with fewer complications, including catheter misplacement and PRBIs, compared to SCV. However, IJV insertion is associated with a longer procedure duration, while pain perception is higher in the SCV group. These findings provide clinically relevant insights and contribute to the ongoing debate regarding the optimal central venous access site for cancer patients undergoing chemotherapy.
Complications related to central venous access play a crucial role in the decision-making process between the IJV and SCV for implantable port insertion. Our meta-analysis highlights significant differences in complications between the two venous access sites, with SCV being associated with a higher incidence of overall complications, catheter misplacement, and port/catheter-related bloodstream infections. Specifically, the total complication rate was lower in the IJV group, indicating that IJV insertion is relatively safer in terms of adverse events. Previous studies have reported similar findings, with a systematic review by Zhou et al. showing a lower risk of pneumothorax and catheter misplacement with IJV access (24). Moreover, several studies have pointed out that the IJV's more direct alignment with the superior vena cava reduces the chances of malposition and improves the overall success rate of the procedure (19). In contrast, while SCV insertion is associated with fewer complications related to catheter misplacement, it has been demonstrated to increase the likelihood of thrombosis and long-term dysfunction, as our results corroborate. Several studies have demonstrated that SCV access tends to lead to a higher risk of catheter occlusion, complicating chemotherapy administration (20, 21). Accurate tip positioning under ultrasound guidance has been shown to reduce both mechanical and infectious complications. Authors proposed an empirical ultrasonographic index for optimal tip placement in a surgical oncology setting, which could further enhance safety outcomes when used alongside site selection strategies (25). This can necessitate additional interventions, thus affecting long-term patient management. Therefore, clinicians must carefully weigh these potential risks when considering venous access routes.
Thrombosis and infection remain the most concerning complications of central venous access, as both can significantly impact the course of chemotherapy and the overall prognosis of cancer patients. Our study showed no substantial difference in thrombosis rates between the two groups. Velioğlu et al. reported a significant association between SCV access and thrombosis (26). The deeper anatomical location of the SCV and its proximity to the clavicle may contribute to the increased risk of catheter-induced thrombosis due to mechanical irritation and endothelial injury, a finding also corroborated by Ma et al. (27). Infection, particularly PRBIs, is a serious concern for patients with implantable ports. In our analysis, the SCV group showed a significantly higher incidence of PRBIs, supporting the findings of previous studies by Yanık et al., who noted that the deeper placement of catheters in the SCV increases the risk of bacterial colonization and infection due to longer catheter dwell times and reduced ability to perform routine maintenance (28). The higher infection rates in the SCV group underscore the importance of strict aseptic techniques during the insertion and management of central venous devices. Clinicians should carefully consider these risks when selecting the insertion site, particularly for patients with compromised immune systems undergoing cancer treatment.
Our study also assessed other procedural factors, including the duration of the procedure, the rate of failed procedures, and pain perception. The IJV group had a longer procedure duration, which aligned with previous research by Han et al., who reported that IJV insertion often requires more time due to its relatively more challenging anatomical positioning compared to the SCV (19). While the increased procedure time may be a disadvantage, it should be considered in the context of the overall complication profile, as the lower complication rate in the IJV group may justify the additional time required for insertion. Furthermore, the clinical impact of this prolonged duration deserves attention. Although the average extension of approximately 11.5 minutes may appear modest, it could contribute to increased patient discomfort during the procedure, especially in cases requiring multiple attempts or prolonged positioning. From a resource perspective, extended procedure times may affect operating room turnover, scheduling, and personnel workload in high-volume centers. Operator experience is also an important factor influencing procedure duration. As highlighted by Mey et al., ultrasound-guided IJV catheterization involves a learning curve, and increased familiarity with the technique can significantly reduce the time needed for successful cannulation (29). Therefore, the longer duration should be interpreted in light of institutional experience, patient tolerance, and overall clinical efficiency. Furthermore, pain perception associated with the procedure was notably higher in the SCV group. This could be attributed to the deeper anatomical location of the SCV, which may cause more discomfort during catheter placement (30). Pain management strategies should be carefully considered in clinical practice, particularly for patients undergoing repeated procedures. The study by Miao et al. further supports the finding that SCV access is associated with increased pain perception, likely due to the need for greater manipulation of the catheter during insertion (20).
Emerging technologies such as ECG-guided catheter placement, magnetic navigation tools, and ultrasound-based real-time tip confirmation are transforming the landscape of central venous access (31). These techniques have demonstrated high accuracy in tip positioning, thereby reducing complications such as catheter malposition, vascular trauma, and bloodstream infections (32). Their use also contributes to shorter procedure times and potentially fewer post-insertion adjustments (33). Incorporating these technologies into future clinical trials and meta-analyses will be crucial for refining evidence-based recommendations and achieving greater standardization across institutions. As these methods gain broader adoption, their comparative effectiveness and cost-efficiency should be critically evaluated to inform future updates of clinical practice guidelines.
There are several limitations that must be acknowledged. First, despite our systematic approach, heterogeneity in patient populations, surgical techniques, and postoperative care, as well as study design variability (e.g., lack of blinding, operator dependence, and follow-up duration differences), may affect the generalizability and reliability of our findings. These limitations highlight the need for higher-quality trials with standardized methodologies, including blinding, uniform operator training, and longer follow-up periods, to reduce bias and enhance evidence reliability. Second, although we focused on RCTs, which are considered the gold standard in clinical research, the sample sizes in some studies were relatively small, limiting the statistical power of our analyses. Future large-scale, multicenter trials are needed to confirm these findings and provide more reliable data on the safety and efficacy of IJV compared to SCV access. Third, due to the lack of stratified data and limited number of RCTs reporting complications, subgroup analyses by age, technique, or region could not be performed. Furthermore, long-term follow-up data were unavailable for most of the studies, limiting our ability to assess the long-term effects of these procedures.
Conclusions
In summary, IJV access is associated with fewer complications, including catheter misplacement and PRBIs, compared to SCV. Although IJV insertion results in a longer procedure duration, it appears to be a less painful option for central venous access in cancer chemotherapy patients. Clinicians should carefully weigh the benefits and risks of each approach, considering patient-specific factors such as anatomy, the anticipated duration of chemotherapy, and the risk of complications. Further research with larger cohorts and longer follow-up is required to confirm these results and provide more robust clinical recommendations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
QL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XP: Conceptualization, Data curation, Formal Analysis, Writing – original draft. XL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. SX: Conceptualization, Data curation, Formal Analysis, Writing – original draft. MM: Conceptualization, Data curation, Formal Analysis, Writing – original draft. SW: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (NSFC, Grant number: 81560345). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors appreciate all the public health workers who participated in the databases (PubMed, ScienceDirect, the Cochrane Library, Scopus, EMBASE, and Web of Science).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1566757/full#supplementary-material
Supplementary Figure S1 | Cochrane risk assessment. Each of the studies was considered to be of high quality.
Supplementary Figure S2 | Forest plots of complications assessed according to patients associated with IJV versus SCV.
Supplementary Figure S3 | Forest plots of complications assessed according to catheter days associated with IJV versus SCV.
Supplementary Figure S4 | Sensitivity analysis. Total complications assessed according to patients (A), duration of the procedure (B), and failed procedures (C). The results remain consistent after excluding individual studies in the sensitivity analysis.
Supplementary Table S1 | Search strategy.
Supplementary Table S2 | Methodological quality assessments (Jadad scale) of the included studies.
Supplementary Table S3 | GRADE quality assessment by therapeutic strategy and study design for the outcomes.
Abbreviations
CI, Confidence interval; FPS-R, Faces Pain Scale-Revised; GRADE, Grading of Recommendations Assessment, Development and Evaluation; IJV, Internal Jugular Vein; MD, Mean difference; MCID, Minimal clinically important difference; M/F, Male/Female; NOS, Newcastle-Ottawa Scale; PRBIs, Port/catheter-related bloodstream infections; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized controlled trial; RR, Risk ratio; SCV, Subclavian Vein.
References
1. Freire MP, Assis DB, Carlesse F, Belizario JC, Germano PCP, Virolli JM, et al. A surveillance program for long-term central venous access-associated infections in outpatient chemotherapy services. Infect Control Hosp Epidemiol. (2023) 44:1555–61. doi: 10.1017/ice.2023.13
2. Abou-Mrad A, Marano L, and Oviedo RJ. A monocentric analysis of implantable ports in cancer treatment: five-year efficacy and safety evaluation. Cancers (Basel). (2024) 16:2802. doi: 10.3390/cancers16162802
3. Matsushima H, Adachi T, Iwata T, Hamada T, Moriuchi H, Yamashita M, et al. Analysis of the outcomes in central venous access port implantation performed by residents via the internal jugular vein and subclavian vein. J Surg Educ. (2017) 74:443–9. doi: 10.1016/j.jsurg.2016.11.005
4. Wu S, Huang J, Jiang Z, Huang Z, Ouyang H, Deng L, et al. Internal jugular vein versus subclavian vein as the percutaneous insertion site for totally implantable venous access devices: a meta-analysis of comparative studies. BMC Cancer. (2016) 16:747. doi: 10.1186/s12885-016-2791-2
5. Nagasawa Y, Shimizu T, Sonoda H, Mekata E, Wakabayashi M, Ohta H, et al. A comparison of outcomes and complications of totally implantable access port through the internal jugular vein versus the subclavian vein. Int Surg. (2014) 99:182–8. doi: 10.9738/INTSURG-D-13-00185.1
6. Mansfield SA, Staszak J, Murphy AJ, Talbot L, Abdelhafeez A, Prajapati H, et al. Impact of insertion site on complications in central venous access devices. Pediatr Surg Int. (2023) 39:118. doi: 10.1007/s00383-023-05399-w
7. Rixecker T, Lesan V, Ahlgrimm M, Thurner L, Bewarder M, Murawski N, et al. Insertion site of central venous catheter correlates with catheter-related infectious events in patients undergoing intensive chemotherapy. Bone Marrow Transplant. (2021) 56:195–201. doi: 10.1038/s41409-020-01003-0
8. Kaul P, Tiwari AR, Kaul P, Kumar R, and Garg PK. Revisiting the anatomical landmark-guided central venous access device insertion: A retrospective cohort study. World J Surg. (2023) 47:2562–7. doi: 10.1007/s00268-023-07088-0
9. Becker F, Wurche LA, Darscht M, Pascher A, and Struecker B. Totally implantable venous access port insertion via open Seldinger approach of the internal jugular vein-a retrospective risk stratification of 500 consecutive patients. Langenbecks Arch Surg. (2021) 406:903–10. doi: 10.1007/s00423-021-02097-w
10. Adrian M, Borgquist O, Kröger T, Linné E, Bentzer P, Spångfors M, et al. Mechanical complications after central venous catheterisation in the ultrasound-guided era: a prospective multicentre cohort study. Br J Anaesth. (2022) 129:843–50. doi: 10.1016/j.bja.2022.08.036
11. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, and Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. (2001) 93:173–83. doi: 10.1016/S0304-3959(01)00314-1
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
14. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, and Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
15. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, and Welch VA eds. Cochrane handbook for systematic reviews of interventions version 6.5 (updated august 2024). Cochrane (2024). Available at: www.training.cochrane.org/handbook (Accessed January 10, 2025).
16. Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. (2009) 20:935–40. doi: 10.1093/annonc/mdn701
17. Biffi R, Pozzi S, Bonomo G, Della Vigna P, Monfardini L, Radice D, et al. Cost effectiveness of different central venous approaches for port placement and use in adult oncology patients: evidence from a randomized three-arm trial. Ann Surg Oncol. (2014) 21(12):3725–31. doi: 10.1245/s10434-014-3784-5
18. Chen YB, Bao HS, Hu TT, He Z, Wen B, Liu FT, et al. Comparison of comfort and complications of Implantable Venous Access Port (IVAP) with ultrasound guided Internal Jugular Vein (IJV) and Axillary Vein/Subclavian Vein (AxV/SCV) puncture in breast cancer patients: a randomized controlled study. BMC Cancer. (2022) 22:248. doi: 10.1186/s12885-022-09228-6
19. Han L, Zhang J, Deng X, Kong X, Yang C, Peng L, et al. Totally implantable venous access ports: A prospective randomized study comparing subclavian and internal jugular vein punctures in children. J Pediatr Surg. (2021) 56:317–23. doi: 10.1016/j.jpedsurg.2020.04.021
20. Miao J, Ji L, Lu J, and Chen J. Randomized clinical trial comparing ultrasound-guided procedure with the Seldinger's technique for placement of implantable venous ports. Cell Biochem Biophys. (2014) 70:559–63. doi: 10.1007/s12013-014-9956-x
21. Ribeiro RC, Abib SC, Aguiar AS, and Schettini ST. Long-term complications in totally implantable venous access devices: randomized study comparing subclavian and internal jugular vein puncture. Pediatr Blood Cancer. (2012) 58:274–7. doi: 10.1002/pbc.23220
22. Tagliari AP, Staub FL, Guimarães JR, Migliavacca A, and Mossmann Dda F. Evaluation of three different techniques for insertion of totally implantable venous access device: A randomized clinical trial. J Surg Oncol. (2015) 112:56–9. doi: 10.1002/jso.23962
23. Liu W, Han Q, Li L, Chi J, Liu X, and Gu Y. Catheter malposition analysis of totally implantable venous access port in breast cancer patients. Front Surg. (2023) 9:1061826. doi: 10.3389/fsurg.2022.1061826
24. Zhou Y, Lan Y, Zhang Q, Song J, He J, Peng N, et al. Totally implantable venous access ports: A systematic review and meta-analysis comparing subclavian and internal jugular vein punctures. Phlebology. (2022) 37:279–88. doi: 10.1177/02683555211069772
25. Marano L, Izzo G, Esposito G, Petrillo M, Cosenza A, Marano M, et al. Peripherally inserted central catheter tip position: a novel empirical-ultrasonographical index in a modern surgical oncology department. Ann Surg Oncol. (2014) 21:656–61. doi: 10.1245/s10434-013-3391-x
26. Velioğlu Y, Yüksel A, and Sınmaz E. Complications and management strategies of totally implantable venous access port insertion through percutaneous subclavian vein. Turk Gogus Kalp Damar Cerrahisi Derg. (2019) 27:499–507. doi: 10.5606/tgkdc.dergisi.2019.17972
27. Ma LI, Liu Y, Wang J, Chang Y, Yu L, and Geng C. Totally implantable venous access port systems and associated complications: A single-institution retrospective analysis of 2,996 breast cancer patients. Mol Clin Oncol. (2016) 4:456–60. doi: 10.3892/mco.2016.726
28. Yanık F, Karamustafaoğlu YA, Karataş A, and Yörük Y. Experience in totally implantable venous port catheter: Analysis of 3,000 patients in 12 years. Turk Gogus Kalp Damar Cerrahisi Derg. (2018) 26:422–8. doi: 10.5606/tgkdc.dergisi.2018.15299
29. Mey U, Glasmacher A, Hahn C, Gorschlüter M, Ziske C, Mergelsberg M, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer. (2003) 11:148–55. doi: 10.1007/s00520-002-0399-3
30. Plumhans C, Mahnken AH, Ocklenburg C, Keil S, Behrendt FF, Günther RW, et al. Jugular versus subclavian totally implantable access ports: catheter position, complications and intrainterventional pain perception. Eur J Radiol. (2011) 79:338–42. doi: 10.1016/j.ejrad.2009.12.010
31. Gullo G and Qanadli SD. ECG-based techniques to optimize peripherally inserted central catheters: rationale for tip positioning and practical use. Front Cardiovasc Med. (2022) 9:765935. doi: 10.3389/fcvm.2022.765935
32. Thomsen KK, Krause L, Breitfeld P, Kouz K, Herrmann J, Chindris V, et al. Additionally using transthoracic echocardiography during ultrasound-guided jugular central venous catheter placement and the odds of catheter misplacement in children: A before-and-after study. Paediatr Anaesth. (2023) 33:89–90. doi: 10.1111/pan.14592
33. Sun W, Li J, Liu B, Liu Y, Ge R, Wang K, et al. Effects of indwelling centrally inserted central catheter on tip location of peripherally inserted central catheter with intracavitary electrocardiogram guidance: A retrospective case-control study. J Vasc Access. (2023) 24:379–84. doi: 10.1177/11297298211015088
Keywords: internal jugular vein, subclavian vein, implantable port, complication, meta-analysis, randomized controlled trials
Citation: Li Q, Peng X, Leng X, Xiao S, Min M, Wan S and Wen J (2025) Comparing internal jugular vein and subclavian vein for central venous insertion of implantable ports in cancer chemotherapy: a meta-analysis of RCTs. Front. Oncol. 15:1566757. doi: 10.3389/fonc.2025.1566757
Received: 27 January 2025; Accepted: 05 May 2025;
Published: 26 May 2025.
Edited by:
George Pappas-Gogos, Democritus University of Thrace, GreeceReviewed by:
Xingwei Sun, Second Affiliated Hospital of Soochow University, ChinaLuigi Marano, Academy of Applied Medical and Social Sciences, Poland
Huseyin Kemal Rasa, Anadolu Medical Center Hospital, Türkiye
Copyright © 2025 Li, Peng, Leng, Xiao, Min, Wan and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyun Wen, d2Vuamlhbnl1bjE5ODVAMTYzLmNvbQ==
 Qianqian Li1
Qianqian Li1 Jianyun Wen
Jianyun Wen