- 1Hospital Pharmacy, Vittorio Veneto Hospital, AULSS2 Marca Trevigiana, Vittorio Veneto, Italy
- 2Italian Society of Clinical Pharmacy and Therapeutics (SIFaCT), Turin, Italy
- 3Oncological Pharmacy Unit, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 4Department of Pharmacy, School of Specialization in Hospital Pharmacy, University of Pisa, Pisa, Italy
- 5Scientific Committee, Italian Society of Clinical Pharmacy and Therapeutics, Turin, Italy
- 6Department of Pharmaceutical and Pharmacological Sciences, University of Padua, Padua, Italy
- 7Medical Oncology, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Sacro Cuore Don Calabria, Negrar di Valpolicella, Italy
- 8Health Technology Assessment (HTA) Unit, Regional Health Service, Florence, Italy
Introduction: Anaplastic lymphoma kinase (ALK) inhibitors (ALKi) are the standard treatment for metastatic, ALK-positive non-small cell lung cancer (NSCLC). Second- and third-generation ALKi, including alectinib, brigatinib, ensartinib, envonalkib, and lorlatinib, have shown better efficacy than crizotinib. However, due to the lack of direct head-to-head comparisons among these agents, the optimal treatment for metastatic ALK-positive NSCLC remains unclear.
Methods: This study used the IPDfromKM (Individual Patient Data from Kaplan-Meier) method to reconstruct patient-level data from Kaplan-Meier curves of seven randomized phase III trials, involving a total of 3,850 patients. Crizotinib arms were pooled as the common comparator. Progression-free survival (PFS) was the primary endpoint, assessed using Cox proportional hazards models and restricted mean survival time (RMST). Subgroup analyses focused on patients with baseline central nervous system (CNS) metastases.
Results: All ALKi significantly improved PFS compared to crizotinib. Lorlatinib showed the most meaningful improvement, with the greatest benefit in both overall PFS (HR=0.28; 95% CI 0.21-0.38) and CNS PFS (HR=0.09; 95% CI 0.04-0.2). In direct comparisons, lorlatinib outperformed brigatinib (HR=0.59; 95% CI 0.39-0.87) and envonalkib (HR=0.52; 95% CI 0.35-0.77) in terms of PFS. While lorlatinib also showed improved PFS compared to alectinib (HR=0.72; 95% CI 0.50–1.04) and ensartinib (HR=0.73; 95% CI 0.48–1.10), these differences were not statistically significant. Lorlatinib demonstrated the greatest benefit in PFS among patients with baseline CNS metastases.
Conclusion: In this indirect comparison using reconstructed patient data, lorlatinib emerged as the most effective ALKi, showed the most favorable HR for PFS compared to the other ALKi, although it did not reach statistical significance versus alectinib and ensartinib. Additionally, lorlatinib showed the highest efficacy in the control of CNS progression.
1 Introduction
Non-Small Cell Lung Cancer (NSCLC) is a leading cause of cancer-related mortality worldwide (1).
Approximately 3–5% of NSCLC tumors harbor rearrangements of the anaplastic lymphoma kinase (ALK) gene, which defines a distinct molecular subtype primarily associated with adenocarcinoma histology. This subtype is more commonly observed in younger patients with no or limited smoking history and is characterized by a high tropism for the central nervous system (CNS) (2, 3).
Crizotinib, a first-generation ALK inhibitor (ALKi), has been the standard of care for metastatic ALK-positive NSCLC for several years. Its efficacy was demonstrated in the PROFILE 1014 phase III randomized trial, which showed a significant progression-free survival (PFS) benefit with crizotinib compared to platinum-based chemotherapy (10.9 vs. 7.0 months; HR 0.45, 95% CI 0.35–0.60) in patients with metastatic ALK-rearranged NSCLC (4, 5).
Since then, second-generation ALKi (alectinib, brigatinib, envonalkib, ensartinib) and the third-generation ALKi lorlatinib have been developed, demonstrating superior efficacy over crizotinib in terms of intracranial activity and PFS in randomized phase 3 clinical trials (6–14). However, in the absence of direct head-to-head trials comparing these agents, the relative efficacy of each drug remains unclear. The aim of this study is to perform a head-to-head treatment comparison analysis of different ALKi based on PFS results from randomized phase III trials the IPDfromKM method (15, 16). This approach enables the reconstruction of individual data points based on the Kaplan-Meier survival curves, facilitating cross-trial comparisons using reconstructed patient-level data. One of the key benefits of using IPDfromKM is its ability to indirectly assess time-to-event outcomes over extended follow-up periods, considering the exact time each event occurs. Furthermore, this method permits to pool data deriving from patients treated with the same regimen but enrolled in different RCTs. This pooling increases the sample size, accounts for variations in follow-up duration, and facilitates a more thorough evaluation of time-to-event outcomes. The survival curves for each regimen analyzed are then displayed on a multi-treatment Kaplan-Meier plot, offering a clear summary of the findings.
In addition, to investigate the ability to penetrate the central nervous system (CNS), a comparative sub-analysis of CNS progression outcomes is performed. Here we report the results of a comparative overview of different ALKi in patients with ALK-positive NSCLC who have received no prior treatment.
2 Materials and methods
2.1 Literature search
We searched the PubMed database to identify clinical trials for our analysis (last search on 01 October 2024). The search term was ((“Lung Neoplasms”[Mesh] OR “non-small cell lung cancer”[tiab] OR NSCLC[tiab] OR “lung cancer”[tiab]) AND (“Anaplastic Lymphoma Kinase”[Mesh] OR ALK[tiab] OR “ALK mutation”[tiab] OR “ALK-positive”[tiab]) AND (“first-line”[tiab] OR “first line”[tiab] OR “initial treatment”[tiab] OR “primary treatment”[tiab]) AND (“Antineoplastic Agents”[Mesh] OR “Protein Kinase Inhibitors”[Mesh] OR “targeted therapy”[tiab] OR “TKI”[tiab] OR crizotinib[tiab] OR alectinib[tiab] OR brigatinib[tiab] OR lorlatinib[tiab])). Our search identified 708 records. Clinical trials were selected using the automated flag filter options, resulting in 47 clinical trials. The main inclusion criteria were: (a) phase III trial; (b) first-line treatment of locally advanced or metastatic ALK-positive NSCLC; (c) TKI comparator arm (not chemotherapy); (d) PFS endpoint; (e) presented as a KM curve. For each included trial, we recorded the number of patients enrolled and the number of events (being either disease progression or death). To avoid duplicate inclusion of patients from the same trial, we considered the most recent publication.
2.2 Reconstructing patient-level data
The individual patient data (IPD) reconstruction method from Kaplan-Meier (KM) curves, known as IPDfromKM, was employed to derive individual patient data from the KM survival curves representing treatment and control arms across selected randomized clinical trials (RCTs) (15, 16).
Initially, the KM curves were digitized using WebPlotDigitizer (version 4.7, accessed online at https://apps.automeris.io/wpd/ on November 10, 2024). The digitized X and Y coordinates, along with the total number of patients and events, were inputted into the IPDfromKM software (version 1.2.3.0, last updated on March 22, 2022). The software then generated individual patient survival times (calculated as the time from enrolment to the last follow-up) and classified patient outcomes as alive, dead, or censored. This process yielded reconstructed patient-level data for each treatment arm of the RCT. In each trial, crizotinib was considered the treatment against which all other therapies were compared. Patients receiving crizotinib in the respective trials were pooled and constitute the common comparator of this analysis (control group).
2.3 Study design
The aim of this analysis was to determine which second or third-generation ALKi provides the best PFS when compared to crizotinib, which is the first-generation ALKi considered a standard of care during the design of RCT for novel ALKi. Kaplan-Meier PFS curves for each ALKi were compared both to each other and to a pooled control curve representing patients treated with crizotinib. Furthermore, we also analyze PFS in patients with brain metastasis at baseline. Restricted mean survival time (RMST) was calculated at two time points: 35 months on the overall population and 23 months when analyzing the cohort with brain metastasis at baseline.
2.4 Statistical analysis
To evaluate the efficacy of the various treatments, we used the Cox proportional hazards model to analyze PFS data, comparing each treatment to the pooled crizotinib control group. The outcomes were expressed as hazard ratios (HR) with 95% confidence intervals (95% CI). The homogeneity of the control groups was assessed through Likelihood ratio tests and concordance statistics. For indirect comparisons between the active treatments (covering all head-to-head comparisons), a Cox regression model was employed, along with the calculation of RMST. The statistical analyses were carried out using the survival package in R (version 4.3.2).
3 Results
Literature search identified 708 records which were then selected according to our inclusion and exclusion criteria to identify the most recent randomized clinical trials (RCTs) investigating the efficacy of ALKi in patients with oncogene-driven NSCLC. The study selection process is shown in Figure 1 according to PRISMA guidelines. Seven RCTs were identified for our analysis of indirect comparisons based on PFS endpoint.
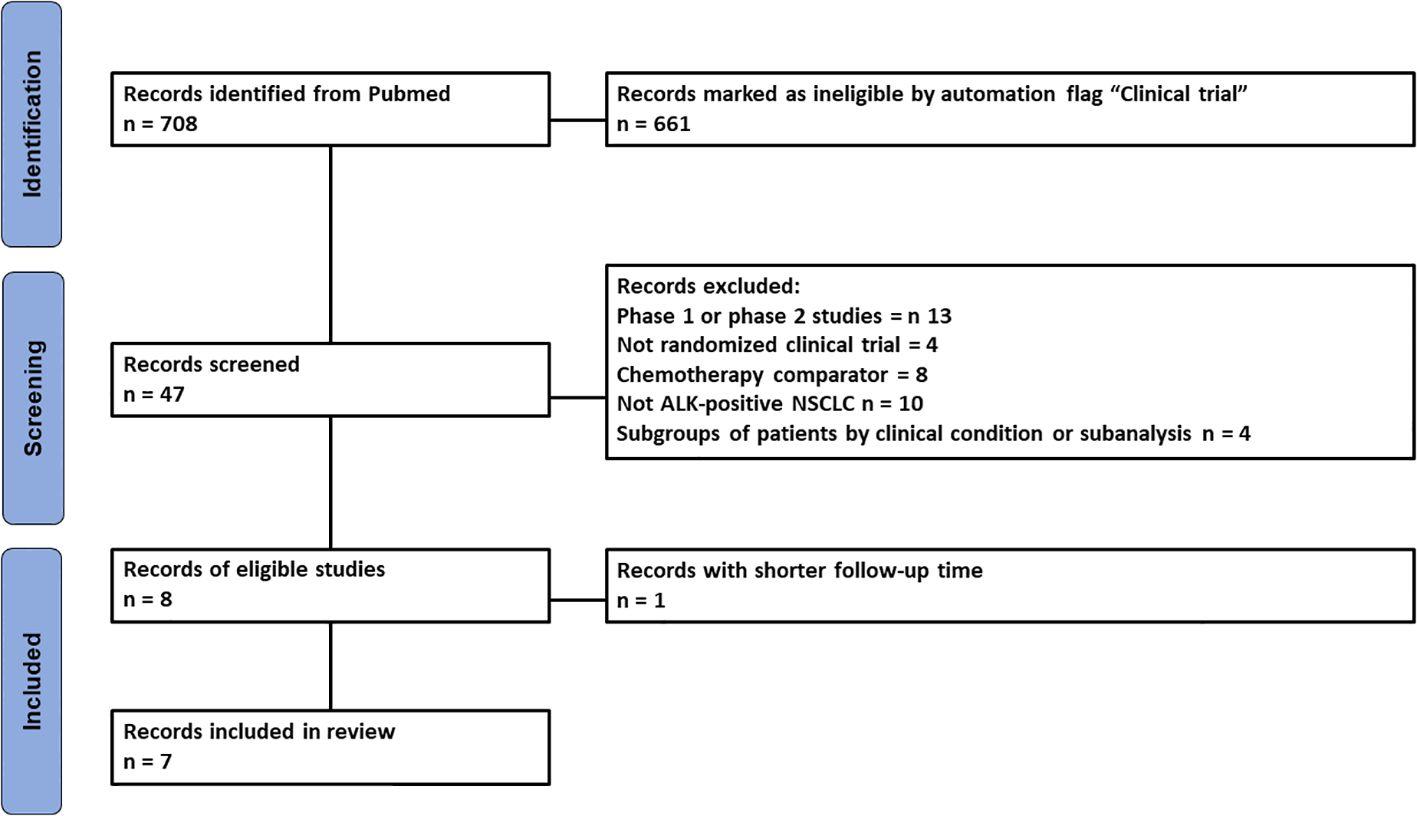
Figure 1. PRISMA flowchart of the process of trial selection. ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer.
Most patients had metastatic NSCLC and were treated with a second- or third-generation ALKi. Three trials (ALEX, J-ALEX and ALESIA) include patients treated with alectinib (6, 8, 9), while the remaining trials include the following inhibitors: ensartinib (eXalt-3) (10), lorlatinib (CROWN) (12), brigatinib (ALTA-1L) (13) and envonalkib (TQ-B3139-III-01) (14). All studies evaluated the efficacy of ALKi compared to crizotinib, which was used as the common comparator in our analysis. The results of literature search and the selection of are reported. The main clinical and demographic characteristics of the patients included in the trials are summarized in Table 1.
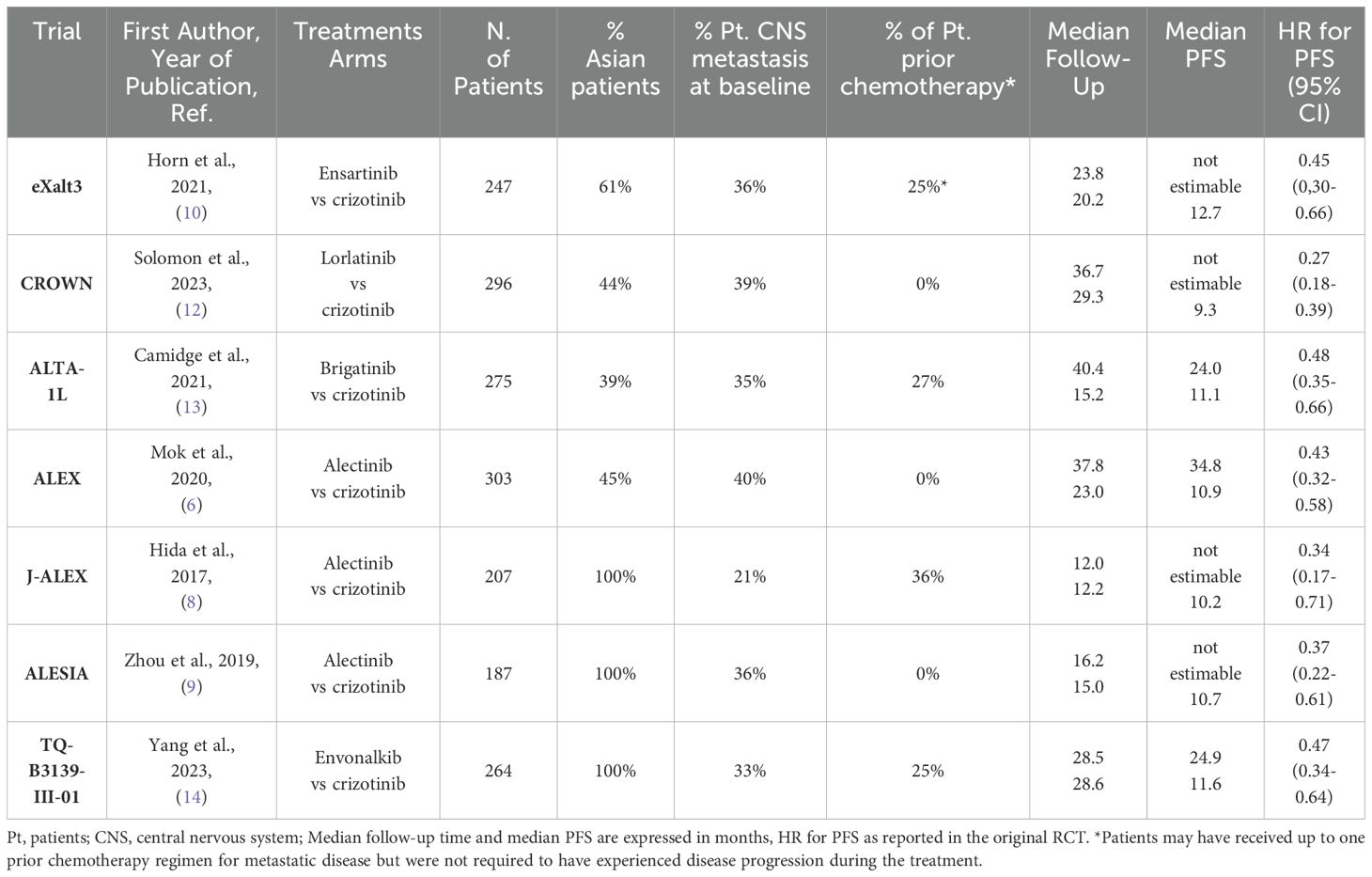
Table 1. The main clinical characteristics of patients treated with ALKi and included in the analysis are reported.
The populations included in the studies are not homogeneous in terms of ethnicity, prior chemotherapy treatments, and the percentage of patients with CNS metastases. In fact, Asian patients enrolled across studies varied from 39% in ALTA-1 to 100% in J-ALEX, ALESIA and TQ-B3139-III-01 trials. Most patients received the treatment in the first-line setting for metastatic disease, but in 4 trials (J-ALEX, ALTA-1L, eXalt-3 and TQ-B3139-III-01) a proportion of patients (25 to 36%) had also received prior chemotherapy; finally, the incidence of baseline CNS metastases varies across studies, ranging from 21% to 40%. In view of these potential sources of heterogeneity in patient selection, we first performed a heterogeneity analysis to test whether patients enrolled in the different trials behaved similarly when treated with the same drug. To do this, we compared the PFS of the crizotinib arms of the different RCTs included in the analysis. We then performed a heterogeneity test. Despite the different clinical characteristics of the patients enrolled in the various studies, there were no significant differences between the HRs for PFS of the control arms and the heterogeneity of the results was low (likelihood ratio test=4.32 with 6 degrees of freedom, p=0.6). The Kaplan-Meier (KM) curves for PFS in the crizotinib arms overlap markedly, as shown in Figure 2.
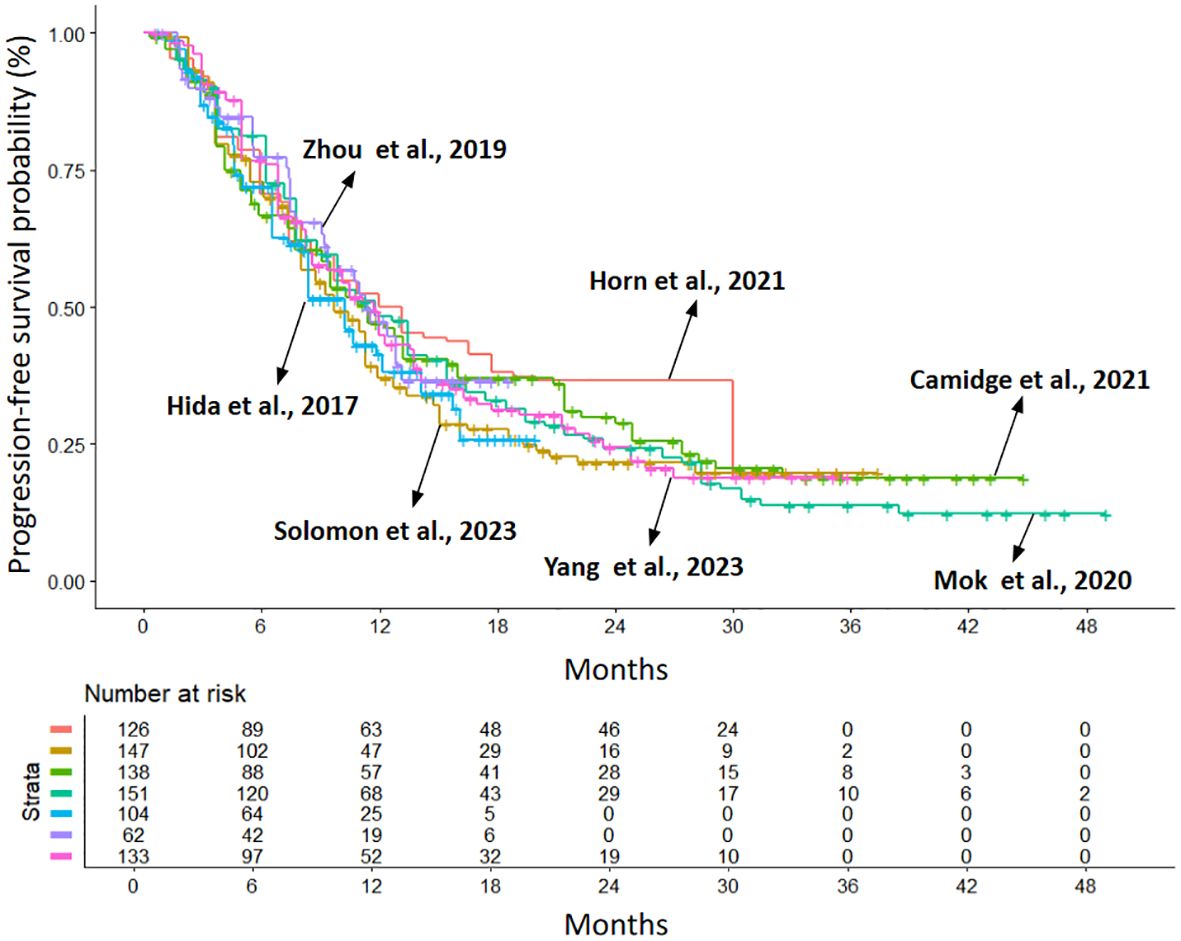
Figure 2. Kaplan–Meier curves generated after reconstructing patient-level data from the control arms of the included trials (crizotinib treated): eXalt-3 (n = 126; in red (10)); CROWN (n = 147; in gold (12)); ALTA-1L (n = 138; in light green (13)); ALEX (n = 151; in dark green (6)); J-ALEX (n = 104; in light blue (8)); ALESIA (n = 62; in purple (9)); TQ-B3139-III-01 (n = 133; in violet (14)). Endpoint: progression-free survival (PFS), time in months. n, number of patients.
After ensuring the comparability of the RCTs, we compared the efficacy of second- and third-generation ALKi with crizotinib and with each other. The primary endpoint was PFS. All inhibitors included in the analysis showed a significant PFS advantage over crizotinib. PFS KM curves for each inhibitor are shown in Figure 3, with the KM curve from pooling crizotinib-treated patients shown in red.
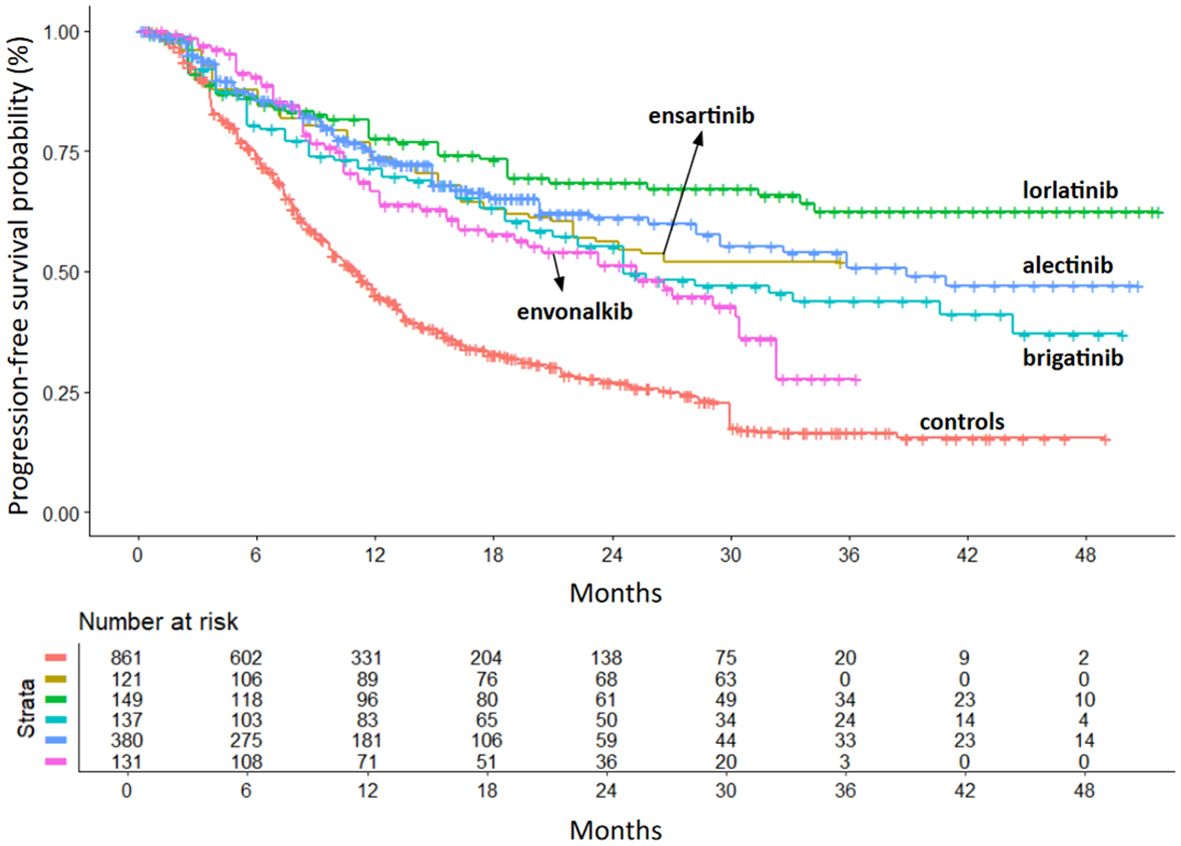
Figure 3. PFS of second- and third-generation ALKi compared to crizotinib controls. After reconstruction of individual patient data from seven trials, the following PFS KM curves were generated: crizotinib (n = 861; 7 cohorts (6, 8–10, 12–14); in red); ensartinib (n = 121 from eXalt-3 study (10); in gold); lorlatinib (n = 149 from CROWN study (12); in green); brigatinib (n = 137 from ALTA-1L study (13); in turquoise); alectinib (n = 380 from ALEX study (6), J-ALEX study (8) and ALESIA study (9); in blue); envonalkib (n = 131 from TQ-B3139-III-01 study (14); in pink). n, number of patients.
Lorlatinib showed the greatest benefit (HR=0.28; 95%IC 0.21-0.38 vs. crizotinib). Ensartinib and alectinib showed similar efficacy, followed by brigatinib and envonalkib (Table 2, Figure 4).
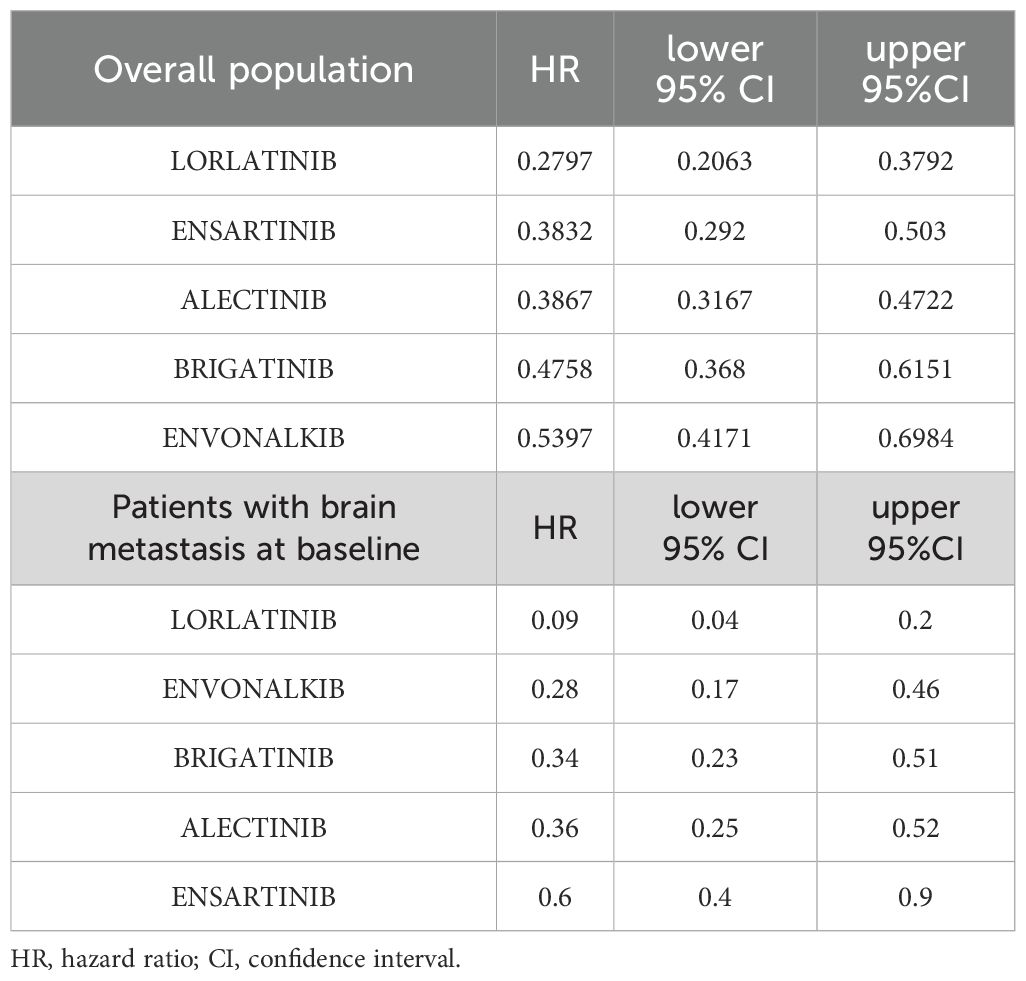
Table 2. PFS estimates of second- and third-generation ALKi compared to crizotinib are reported as HR with 95% CI in the overall population and in patients with brain metastasis at baseline.
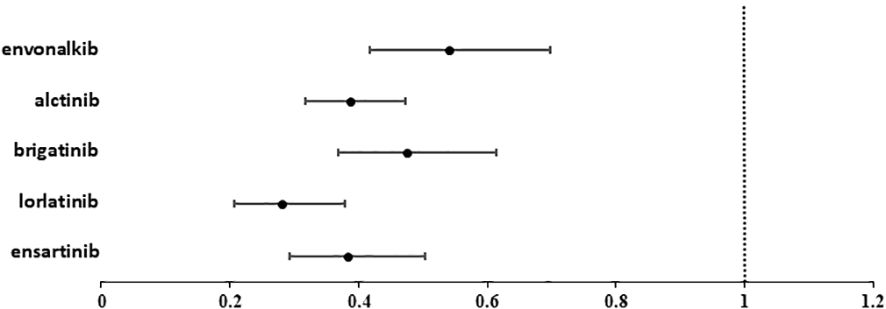
Figure 4. Forest plot showing the hazard ratio (HR) for PFS with 95% confidence interval (95%CI) of the different ALKi versus crizotinib.
In the cross-treatment comparisons of HR for PFS, lorlatinib was significantly superior to brigatinib (HR= 0.59, 95%CI= 0.40-0.86) and envonalkib (HR= 0.52, 95%CI= 0.35-0.77) but not to alectinib (HR=0.72; 95%CI=0.50-1.04) and ensartinib (HR= 0.73, 95%CI= 0.49- 1.1). All other inter-treatment comparisons did not show significant differences, except for alectinib versus envonalkib, which showed superiority of alectinib (HR= 0.72, 95%CI= 0.52-0.99). Results are reported in Table 3.
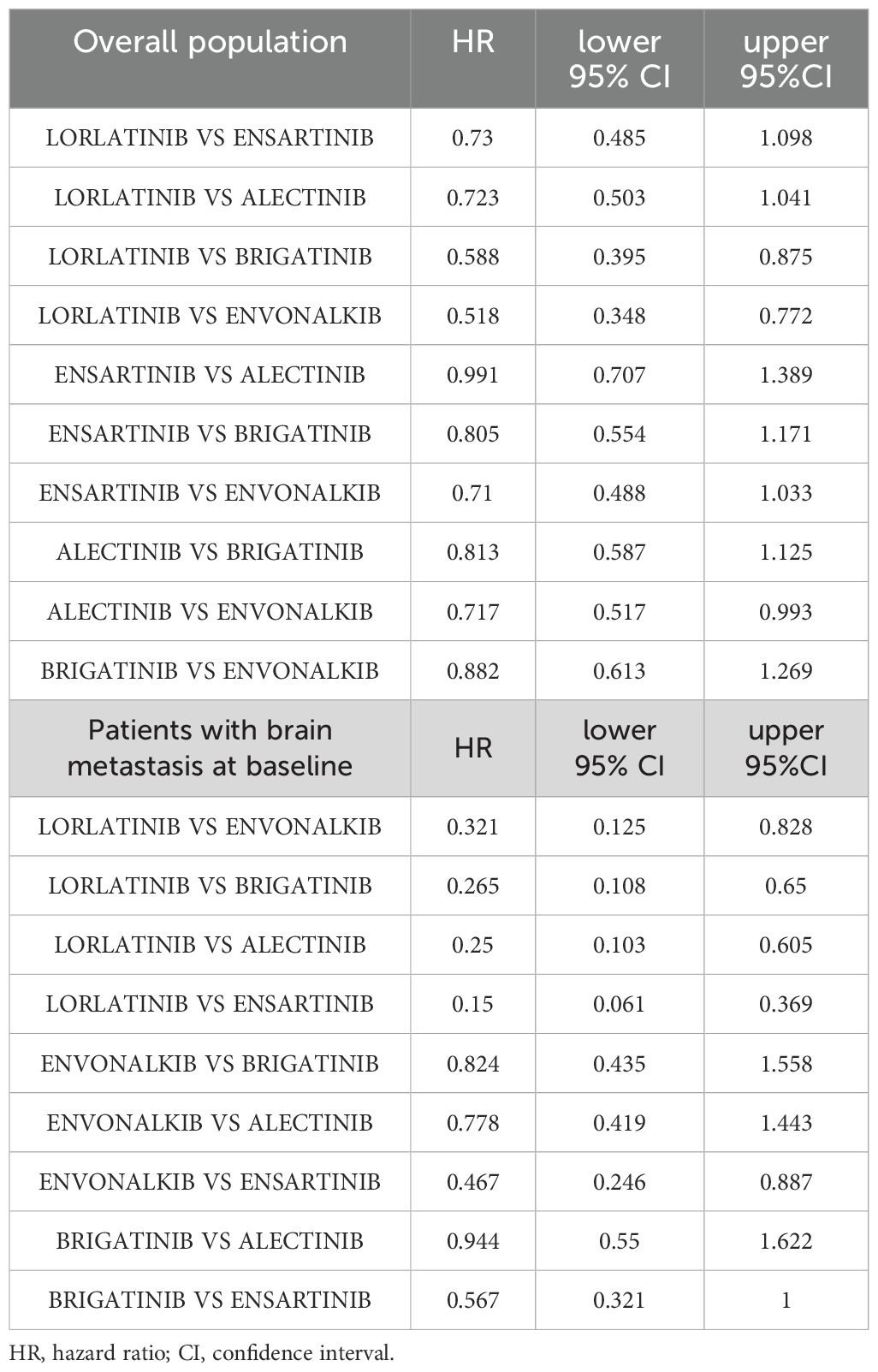
Table 3. Inter-treatment comparisons of PFS for second- and third-generation ALKi are reported as HR with 95% CI in the overall population and in patients with brain metastasis at baseline.
Given that median PFS was not achieved for some of the inhibitors and that a significant proportion of patients-maintained disease control over time, we also performed a PFS analysis using restricted mean survival at 35 months (RMST). The results are shown in Table 4.
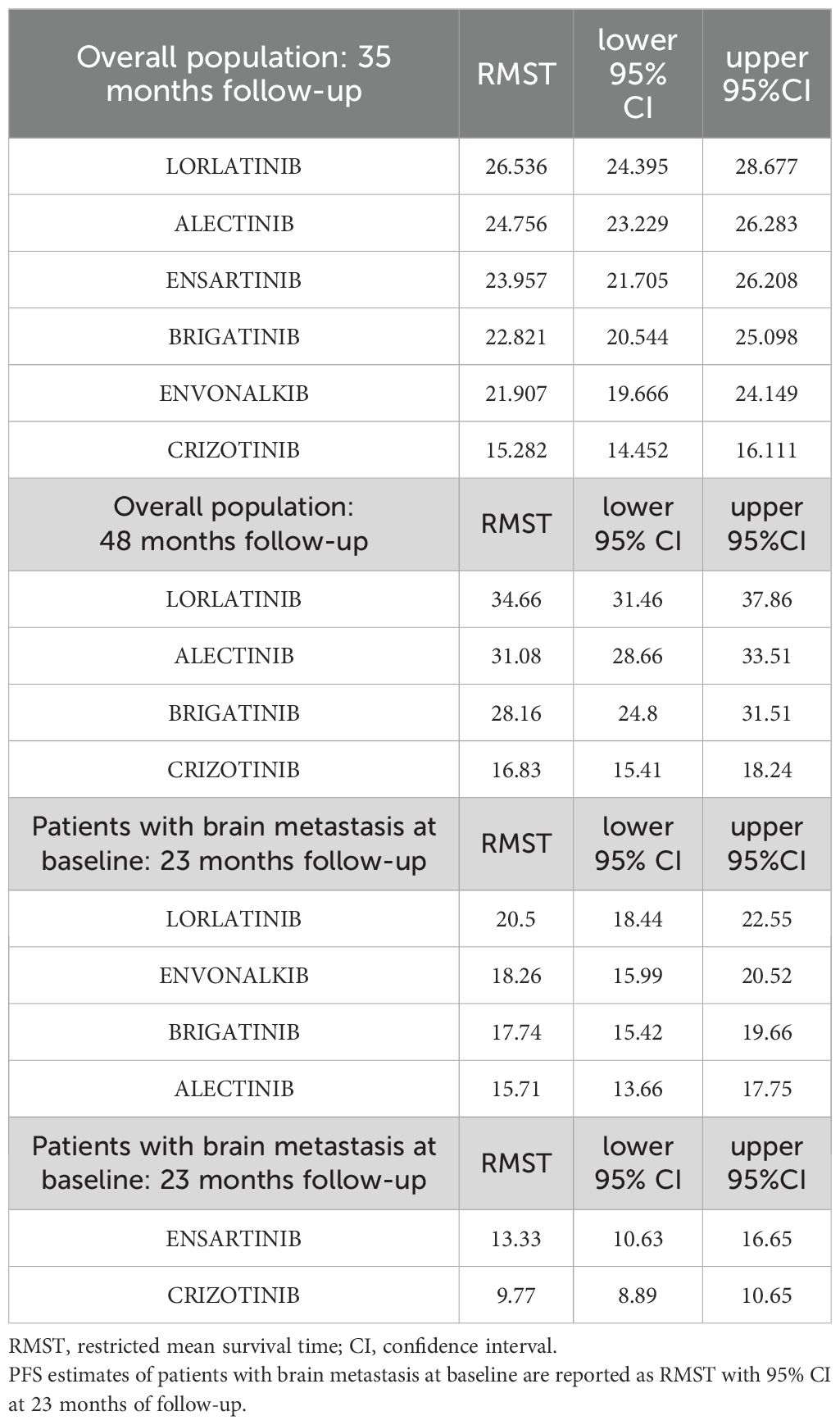
Table 4. PFS estimates of second- and third-generation ALKi compared to crizotinib are reported as RMST (expressed in months) with 95% CI in the overall population at 35 months of follow-up and at 48 months for RCT with an appropriate follow-up time.
Once again, lorlatinib showed a significant advantage over crizotinib with an RMST of 26.5 months (95%CI 24.4-28.7) compared to 15.3 months of crizotinib (95%CI 14.5-16.1). Lorlatinib also showed a modest RMST advantage over alectinib (+1.78 months) and good advantages over ensartinib (+2.6 months), brigatinib (+3.7 months) and envonalkib (+4.6 months). The results are shown in Table 4. As long-term PFS results are available for brigatinib, alectinib and lorlatinib, we repeated the RMST analysis at 48 months. At this longer follow-up time, lorlatinib showed an RMST of 34.66 months (95%CI=31.46-37.86), twice as long as crizotinib, 3.6 months longer than alectinib and 6.5 months longer than brigatinib.
We then assessed whether second- and third-generation ALKi and crizotinib differed in controlling brain metastases. For patients with brain metastases at baseline, KM curves were available for all trials. However, there were subtle differences in outcome assessment. TheTQ-B3139-III-01 and CROWN trials consider only intracranial progression, the ALTA-1L trial combines intracranial progression with death from all causes, while the eXalt-3 and alectinib trials combine progression at all sites with death from all causes.
As these differences in outcome assessment may raise concerns about the comparability of the results, we first assessed whether patients with brain metastases enrolled in the different trials and treated with the same treatment (crizotinib) resulted in similar CNS PFS profiles. The median CNS PFS in the control arms ranged from a maximum of 10.6 months (95% CI 8.76-26.90) for patients in the J-ALEX trial to a minimum of 5.5 months (95% CI 4.16-7.68) for patients in the ALTA-1L trial. However, the inter-treatment comparison showed no significant differences in PFS between the two studies and the analysis of heterogeneity showed that although the definition of CNS PFS was slightly different across the studies, the results for patients treated with crizotinib were homogeneous (likelihood ratio test=8.96 with 5 degrees of freedom, p=0.1).
When we looked at the efficacy of the different active treatments in the selected RCTs, lorlatinib was significantly superior to crizotinib (HR=0.09, 95%CI 0.05-0.2) and all other ALKi in the control of brain metastases (Figure 5). The HR for PFS in patients with brain metastases at baseline compared to crizotinib is shown in Table 2, while the results of the comparison between treatments are shown in Table 3.
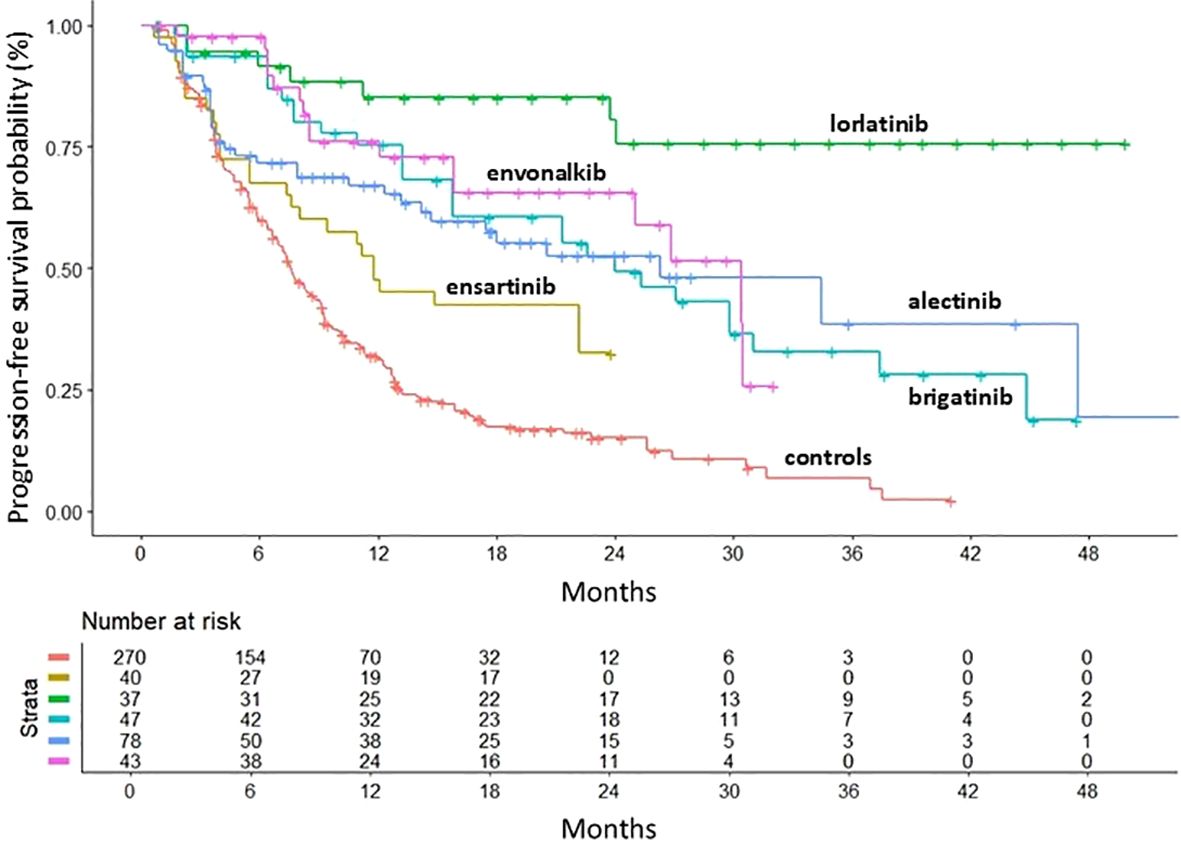
Figure 5. PFS of second- and third-generation ALKi compared to crizotinib controls in patients with brain metastasis at baseline. PFS KM curves are reported: crizotinib (n = 270; 7 cohorts (6, 8–10, 12–14); in red); ensartinib (n = 40 from eXalt-3 study (10); in gold); lorlatinib (n = 37 from CROWN study (12); in green); brigatinib (n = 47 from ALTA-1L study (13); in turquoise); alectinib (n = 78 from J-ALEX study (8); in blue); envonalkib (n = 43 from TQ-B3139-III-01 study (14); in pink). n, number of patients.
Envonalkib ranked second (HR vs. crizotinib = 0.28, 95%CI 0.11-0.46). However, its efficacy in patients with brain metastases was significantly higher only when compared to ensartinib (HR= 0.47, 95%CI= 0.25-0.89). Brigatinib and alectinib showed a similar benefit compared to crizotinib, with a reduction of 65% of the risk of progression, but no significant differences between them. This trend was also confirmed when CNS PFS was assessed by RMST at 23 months. Lorlatinib was the most effective treatment for controlling brain metastases in ALK-mutated NSCLC patients with an RMST of 20.5 months (95%CI=18.44-22.55 months), followed by envonalkib and brigatinib which showed similar RMST with 2 months less than lorlatinib. Alectinib and ensartinib showed shorter RMST, with a benefit of 4.8 and 7.7 months, respectively, compared to crizotinib.
4 Discussion
The standard first-line treatment for patients with metastatic ALK-positive NSCLC consists of an ALKi. Several second- and third-generation ALKi, including alectinib, brigatinib, ensartinib, envonalkib, and lorlatinib, have demonstrated superiority over the first-generation inhibitor crizotinib. However, due to the lack of head-to-head comparative studies among these agents, definitive conclusions cannot be drawn regarding the optimal first-line treatment.
In the present analysis, based on reconstructed patient data from randomized phase III trials, lorlatinib emerged as the ALKi with the most favorable HR for PFS compared to all other ALKi, with a RMST of 34.66 months at a follow-up of 48 months, although the difference in PFS between lorlatinib and alectinib or ensartinib was not statistically significant. A critical aspect of ALK-positive NSCLC is its tropism for the CNS. Unfortunately, the development of brain metastases is associated with a poor prognosis and, if symptomatic, can significantly impair quality of life. For this reason, the prevention and management of brain metastases are crucial in the treatment of ALK-positive NSCLC. In this context, lorlatinib demonstrated the highest efficacy in controlling CNS disease in the present analysis.
However, differences in efficacy must always be evaluated in the context of the specific toxicity profiles of the various treatments. In this regard, a key limitation of our analysis is its exclusive focus on efficacy endpoints, without consideration of toxicity. However, some speculations can be made based on the safety data reported in RCT. In fact, regarding the safety profiles of the three ALKi with the most favorable PFS HRs compared to crizotinib, RCT reported the following incidences of treatment-related grade ≥3 adverse events: 67% among patients treated with lorlatinib, 52% among those treated with alectinib (in the international ALEX study), and 5.04% among those treated with ensartinib. Treatment discontinuation rates due to adverse events were 7% for lorlatinib, 11% for alectinib and 9.1% for ensartinib (6, 8–10). The toxicity profiles differ significantly among these agents: lorlatinib was associated with hypercholesterolemia (any grade, 70%; G3-G4, 16%), hypertriglyceridemia (any grade, 64%; G3-G4,20%), edema (any grade, 55%; G3-G4, 4%), increased weight (any grade, 38%; G3-G4, 17%), cognitive effects (any grade, 21%; G3-G4, 2%) and hypertension (any grade, 18%; G3-G4, 10%) (11); alectinib was associated with anemia (any grade, 20%; G3-G5, 7%), edema (any grade, 17%; G3-G5 0%), ALT increased (any grade, 15%; G3-G4 5%), AST increased (any grade, 14%; G3-G5, 5%), and bilirubin increased (any grade, 15%; G3-G5, 2%), as reported in the international ALEX trial (6); ensartinib was associated with rash (any grade, 67.8%; G3-G4, 11.2%), ALT increased (any grade, 48.3%; G3-G4, 4.2%), AST increased (any grade, 37.8%; G3-G4, 0.7%), pruritus (any grade, 26.6%; G3-G4, 2.1%), constipation (any grade, 20.3%; grade 3-4, 0%), mostly G1-G2 (10).
Another critical aspect not addressed in our analysis is the impact of subsequent lines of therapy on overall survival. For instance, patients who progress on second-generation ALKi such as alectinib may still derive significant benefit from lorlatinib. A phase II trial reported a response rate of 29%, an intracranial response rate of 53%, and a median PFS of 6.9 months with lorlatinib in patients previously treated with two or three ALKi (17). Conversely, for patients progressing on lorlatinib, current treatment options are limited to platinum-based doublet chemotherapy (18), although novel ALKi are under development to address resistance mutations associated with lorlatinib (19). To assess the impact of subsequent lines of treatment on patient outcomes, OS would have been the preferred endpoint. However, OS data from RCTs for some ALK, including lorlatinib, envonalkib and ensartinib, are still immature, and Kaplan-Meier OS curves are not yet available for an IPDfromKM-based analysis (10, 12, 14). Therefore, a meaningful OS analysis will require more mature data. In the absence of reliable OS comparisons, it is unclear whether the optimal strategy for advanced ALK-positive NSCLC is upfront use of a third-generation ALK TKI or a sequential approach with a second-generation inhibitor followed by lorlatinib at progression. However, data from first-line RCTs show that at least 40% of patients do not receive further treatment after progression, limiting the feasibility of a sequencing approach to about 60% of cases. This highlights the importance of selecting the first-line treatment with the best PFS (20).
Further efforts in research should be made to personalize first-line treatment based on potential predictive biomarkers of efficacy and primary resistance to ALK inhibitors. Several studies suggest that the ALK fusion variant and co-mutations may influence clinical outcomes. In particular, TP53 mutations and the EML4-ALK v3 variant have been associated with a poorer prognosis (21). Among second- and third-generation inhibitors, in the absence of direct comparisons and recognizing the limitations of indirect comparisons across studies, the longest reported PFS for patients with the EML4-ALK v3 variant is 60 months with lorlatinib, compared to 16–18 months with alectinib and brigatinib (13, 22, 23). In patients with TP53 mutations, PFS was 51.6 months with lorlatinib, whereas it was only 18 months with brigatinib (13, 23). These findings suggest that in patients with molecular features associated with greater aggressiveness, such as the EML4-ALK v3 variant or TP53 mutations, first-line treatment with lorlatinib may be preferable to second-generation inhibitors.
The IPDfromKM method used in this analysis offers significant advantages by reconstructing individual patient data from Kaplan-Meier (KM) curves, and is increasingly being used in the field of survival indirect comparisons in oncology (24–27). First, this method allows crizotinib-treated patients to be pooled, thus increasing the sample size of the common comparator, while maintaining randomization as each patient in the active arms found their randomized counterpart in the control arm. Secondly, a key advantage is that it preserves the exact timing of events, which is often lost in binary meta-analyses that rely on simplified measures such as odds ratios. In addition, the multi-KM plot resulting from the comparison between treatments provides a clear and effective way to communicate the results, making them easy to interpret for both researchers and clinicians. One of the main limitations of survival analysis based on the IPDfromKM method is its dependence on the availability of subgroup-specific KM curves. Without these subgroup curves in the original studies, it becomes difficult to perform survival analyses for specific patient subgroups. However, in our context, it was also possible to analyze the efficacy of ALKi in a cohort of patients with CNS metastases at baseline, as KM curves were available. Some approximations were made as disease progression was expressed in slightly different ways in the selected RCTs, but the positive results of the heterogeneity analysis allowed for overall comparability of the results.
In conclusion, in our indirect comparison of second- and third-generation ALKi, lorlatinib achieved the best HR for overall PFS compared to the other ALKi, although it did not reach statistical significance versus alectinib and ensartinib, and the best HR for CNS PFS. However, in daily clinical practice, clinicians should carefully evaluate the balance between efficacy and side-effect profiles and determine the most appropriate treatment sequence, taking into account the characteristics and preferences of the individual patient. Despite some limitations, the IPDfromKM method is a powerful and easy-to-use tool for performing indirect treatment comparisons, which improves the understandability of indirect comparative survival analyses when individual patient data are not available.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
VD: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Project administration, Software, Supervision, Visualization. LG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. LD: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. AO: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, Methodology. AI: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Italian Society for Clinical Pharmacy and Therapeutics (SIFaCT), Turin, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. (2007) 448:561–6. doi: 10.1038/nature05945
3. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. (2009) 27:4247–53. doi: 10.1200/JCO.2009.22.6993
4. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
5. Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. (2018) 36:2251–8. doi: 10.1200/JCO.2017.77.4794
6. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. (2020) 31:1056–64. doi: 10.1016/j.annonc.2020.04.478
7. Gadgeel S, Peters S, Mok T, Shaw AT, Kim DW, Ou SI, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. (2018) 29:2214–22. doi: 10.1093/annonc/mdy405
8. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. (2017) 390:29–39. doi: 10.1016/S0140-6736(17)30565-2
9. Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. (2019) 7:437–46. doi: 10.1016/S2213-2600(19)30053-0
10. Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. (2021) 7:1617–25. doi: 10.1001/jamaoncol.2021.3523
11. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. (2020) 383:2018–29. doi: 10.1056/NEJMoa2027187
12. Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. (2023) 11:354–66. doi: 10.1016/S2213-2600(22)00437-4
13. Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in ALKi-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. (2021) 16:2091–108. doi: 10.1016/j.jtho.2021.07.035
14. Yang Y, Min J, Yang N, Yu Q, Cheng Y, Zhao Y, et al. Envonalkib versus crizotinib for treatment-naive ALK-positive non-small cell lung cancer: a randomized, multicenter, open-label, phase III trial. Signal Transduct Target Ther. (2023) 8:301. doi: 10.1038/s41392-023-01538-w
15. Messori A, Damuzzo V, Rivano M, Cancanelli L, Di Spazio L, Ossato A, et al. Application of the IPDfromKM-Shiny method to compare the efficacy of novel treatments aimed at the same disease condition: a report of 14 analyses. Cancers (Basel). (2023) 15:1633. doi: 10.3390/cancers15061633
16. Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
17. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. (2018) 19:1654–67. doi: 10.1016/S1470-2045(18)30649-1
18. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
19. Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALKis: mechanisms of resistance and management. Nat Rev Clin Oncol. (2022) 19:499–514. doi: 10.1038/s41571-022-00639-9
20. Bearz A, Bertoli E, Stanzione B, De Carlo E, Del Conte A, Bortolot M, et al. EML4-ALK: update on ALK inhibitors. Int J Mol Sci. (2025) 26:308. doi: 10.3390/ijms26010308
21. Bearz A, Martini JF, Jassem J, Kim SW, Chang GC, Shaw AT, et al. Efficacy of lorlatinib in treatment-naive patients with ALK-positive advanced NSCLC in relation to EML4::ALK variant type and ALK with or without TP53 mutations. J Thorac Oncol. (2023) 18:1581–93. doi: 10.1016/j.jtho.2023.07.023
22. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. (2019) 14:1233–43. doi: 10.1016/j.jtho.2019.03.007
23. Solomon BJ, Liu G, Felip E, Mok TSK, Soo RA, Mazieres J, et al. Lorlatinib versus crizotinib in patients with advanced ALK-positive non-small cell lung cancer: 5-year outcomes from the phase III CROWN study. J Clin Oncol. (2024) 42:3400–9. doi: 10.1200/JCO.24.00581
24. Nichetti F, Rota S, Ambrosini P, Pircher C, Gusmaroli E, Droz Dit Busset M, et al. NALIRIFOX, FOLFIRINOX, and gemcitabine with nab-paclitaxel as first-line chemotherapy for metastatic pancreatic cancer: a systematic review and meta-analysis. JAMA Netw Open. (2024) 7:e2350756. doi: 10.1001/jamanetworkopen.2023.50756
25. Vychopen M, Güresir A, Basaran AE, Güresir E, Wach J. Impact of levetiracetam use in glioblastoma: an individual patient-level meta-analysis assessing overall survival. Neurosurg Rev. (2024) 47:897. doi: 10.1007/s10143-024-03137-x
26. Zhang S, Li S, Cheng Y. The efficacy and safety of immunotherapy as first-line treatment for extensive-stage small cell lung cancer: evaluating based on reconstructed individual patient data. Front Oncol. (2024) 14:1371313. doi: 10.3389/fonc.2024.1371313
27. Deng L, Jiang C, Attwood K, Zhao JJ, Perimbeti S, Hu C, et al. Risk of further progression or death among durable progression-free survivors with melanoma or non-small-cell lung cancer in PD-1 blockade trials: implications for imaging surveillance. JCO Oncol Pract. (2023) 19:871–81. doi: 10.1200/OP.23.00353
Keywords: NSCLC, ALK-inhibitors, IPDfromKM, indirect comparison, Shiny method
Citation: Damuzzo V, Gasperoni L, Del Bono L, Ossato A, Inno A and Messori A (2025) Treatment of metastatic ALK-positive non-small cell lung cancer: indirect comparison of different ALK inhibitors using reconstructed patient data. Front. Oncol. 15:1566816. doi: 10.3389/fonc.2025.1566816
Received: 25 January 2025; Accepted: 14 April 2025;
Published: 09 May 2025.
Edited by:
Gianluca Russo, University of Naples Federico II, ItalyReviewed by:
Roberto Borea, Ospedale Policlinico San Martino, ItalyMarco Siringo, Sapienza University of Rome, Italy
Copyright © 2025 Damuzzo, Gasperoni, Del Bono, Ossato, Inno and Messori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Ossato, YW5kcmVhLm9zc2F0b0BzdHVkZW50aS51bmlwZC5pdA==
 Vera Damuzzo
Vera Damuzzo Lorenzo Gasperoni
Lorenzo Gasperoni Luna Del Bono4
Luna Del Bono4 Andrea Ossato
Andrea Ossato Alessandro Inno
Alessandro Inno Andrea Messori
Andrea Messori