- 1Department of Lymphoma and Myeloma Research Center, Beijing GoBroad Hospital, Beijing, China
- 2Cytology Laboratory, Beijing GoBroad Boren Hospital, Beijing, China
- 3Department of Medical Laboratory, Beijing GoBroad Boren Hospital, Beijing, China
Background: Relapsed or refractory (R/R) Burkitt lymphoma (BL) in adults is aggressive and lacks standardized salvage options. Data on the efficacy and safety of chimeric antigen receptor T (CAR-T) cell therapy in this population remains limited.
Methods: We retrospectively analyzed 25 adult patients with relapsed or refractory Burkitt lymphoma who received CAR T-cell therapy. Clinical data, treatment responses, and survival outcomes were collected from medical records. Bridging therapy and lymphodepleting regimens varied based on disease status. Treatment-related toxicities and CAR-T expansion were monitored. Primary endpoints included efficacy, safety, and survival. Risk factors associated with treatment outcomes were explored using univariate analyses.
Results: One month objective response rate (ORR) was 52%(13/25)(95%CI: 31.3–72.2), with a complete response rate (CRR) of 28% (7/25). Sixteen patients (64%) received sequential consolidation therapy including 9 who received a second CAR-T infusion, and 7 who proceeded to autologous or allogeneic hematopoietic stem cell transplantation. The median follow-up time was 26.10 months (range 14.50-57.17). The median OS was 5.49 months(95%CI 1.74-9.25), and the median PFS was 2.96(95%CI 1.62-4.3)months. At last follow-up(2024-08-22), 28% achieved disease-free survival, with one patient disease-free for 5 years.
Conclusions: CAR-T therapy shows promising activity in relapsed/refractory Burkitt lymphoma, but its effectiveness is limited by short response duration. High-risk features may predict poor outcomes, and a higher number of long-term survivors were observed in patients who received transplant sequential consolidation. However, due to the small sample size, larger studies are needed to validate these findings.
Introduction
Burkitt lymphoma (BL) is a highly aggressive subtype of B-cell non-Hodgkin lymphoma characterized by rapid proliferation and a high propensity for extranodal involvement, including the bone marrow and central nervous system (CNS) (1). It is strongly associated with MYC gene rearrangements, which drive uncontrolled cellular proliferation (2, 3). First-line treatment typically involves dose-intensive, short-cycle chemotherapy regimens, such as CODOX-M/IVAC, Hyper-CVAD, or DA-EPOCH-R, specifically tailored to target BL’s rapid proliferation and achieve durable remission. These regimens have demonstrated excellent outcomes in newly diagnosed BL, with five-year overall survival (OS) rates of 70–91% (4–12). In contrast, standard regimens like R-CHOP, which are effective in diffuse large B-cell lymphoma (DLBCL), are not recommended for BL due to their lower efficacy in this highly aggressive disease (6).Despite these encouraging results in newly diagnosed patients, the prognosis for relapsed or refractory (R/R) BL remains extremely poor. Patients with refractory disease or early relapse (within six months) often show resistance to conventional salvage chemotherapy, leaving limited effective options (13). Salvage regimens such as R-ICE, GDP, or even EPOCH, while occasionally providing transient disease control, rarely achieve long-term remission. For eligible patients, high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) may offer some benefit, but outcomes are generally suboptimal (14, 15). Furthermore, the role of targeted agents, such as BTK inhibitors and PI3K inhibitors, remains largely investigational and has not yet been established as standard therapy in R/R BL. In recent years, chimeric antigen receptor T-cell (CAR-T) therapy has revolutionized the treatment landscape for relapsed/refractory large B-cell lymphoma (LBCL), demonstrating durable responses and five-year survival rates of up to 40% in some studies (16, 17). However, evidence supporting the efficacy of CAR-T therapy in BL remains sparse. BL’s unique biological characteristics, including its rapid doubling time, high tumor burden, and distinct microenvironment, may pose additional challenges to CAR-T efficacy. Most available data on CAR-T in BL come from isolated case reports or small series, highlighting a critical unmet need for systematic evaluation (18–20). In this study, we conducted a retrospective analysis of patients with R/R BL treated with CAR-T therapy, including the sequential or combined use of additional targeted CAR-T products and/or hematopoietic stem cell transplantation (HSCT). Our primary objectives were to assess the efficacy, safety, and long-term survival outcomes of CAR-T therapy in this patient population. Additionally, we aimed to identify factors influencing treatment responses and explore opportunities to optimize therapeutic strategies for this challenging disease.
Method
Patients and study design
We conducted a retrospective analysis of 25 patients with relapsed or refractory (R/R) Burkitt lymphoma who received CAR-T cell therapy at Beijing GoBroad Hospital between December 13, 2018, and May 31, 2023.The inclusion criteria were as follows: (1) age between 18 and 80 years; (2) diagnosed with R/R Burkitt lymphoma, where refractory disease was defined as failure to achieve at least partial remission after first-line therapy or disease progression during treatment, and relapse was defined as the reappearance or progression of the disease after an initial response or remission; (3) MYC rearrangement or translocation detected by Fluorescence in situ hybridization (FISH); The trial protocol was approved by the Ethics Committee of Beijing GoBroad Hospital in accordance with the Declaration of Helsinki. The study participants were derived from two clinical trials (ChiCTR2200058972 and ChiCTR2100055062) with ethics approval number WZ2024-001-001. All participants or their legal guardians provided written informed consent.
CAR-T cell manufacturing
All patients underwent lymphocyte apheresis and CAR-T cell preparation, which were conducted in the laboratory of Beijing GoBroad Hospital. Peripheral blood mononuclear cells (PBMNCs) were isolated from eligible patients, and CD3+ T lymphocytes were separated using antigen-coated immunomagnetic beads. The detailed preparation process has been described in previous studies (21–24).
Bridging and lymphodepletion therapy
Prior to CAR-T cell infusion, bridging therapy was permitted. Bridging therapy was defined as lymphoma-directed treatment administered between leukapheresis and lymphodepletion (LD). The choice of bridging therapy depended on tumor burden and included drugs not previously used or those without resistance to guide an individualized regimen combining chemotherapy and targeted molecular agents.
CAR-T cell infusion and monitoring
Patients were hospitalized for at least 14 days after CAR-T infusion to monitor cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), hematologic toxicity, and infections. Hematologic toxicity was evaluated based on levels of neutrophils, platelets, and hemoglobin within the first month post-infusion and graded according to CTCAE 5.0. CRS and ICANS were assessed and graded following ASTCT consensus criteria. Corticosteroids and/or tocilizumab were administered based on the severity of CRS/ICANS and patient tolerance.
CAR-T expansion and response evaluation
CAR-T cell expansion was monitored using flow cytometry and PCR on days 3, 7, 11, 14, 21, 30, 60, and 90 after infusion. Treatment efficacy was assessed one month after CAR-T infusion based on imaging evaluations following the Lugano criteria, and flow cytometry was employed for bone marrow and cerebrospinal fluid evaluation.
Consolidation and follow-up
Consolidative treatments, if needed, included additional CAR-T therapies targeting other antigens, autologous or allogeneic hematopoietic stem cell transplantation, targeted drugs, multi-agent chemotherapy, or radiotherapy. The remission rates and long-term survival outcomes of patients receiving CAR-T therapy and subsequent integrated treatments were analyzed. Progression-free survival (PFS) was defined as the time from the first CAR-T infusion to disease progression, death, or last follow-up, while overall survival (OS) was defined as the time from the first CAR-T infusion to death or last follow-up.
Data collection
Baseline characteristics, treatment-related toxicities, clinical responses, and survival outcomes were extracted from the REDCap (Research Electronic Data Capture) database based on detailed data extracted from patients’ medical records.
Statistical analysis
All statistical analyses were performed using SPSS 26.0,SAS 9.4 and GraphPad Prism 9.0. Continuous variables were reported as medians and ranges, while categorical variables were presented as frequencies and percentages. Median progression-free survival (PFS) and overall survival (OS), as well as 1-year PFS and OS rates and their 95%CI were estimated using the Kaplan-Meier method. Cox regression were conducted to identify prognostic factors for PFS and OS.
Results
Baseline demographic characteristics
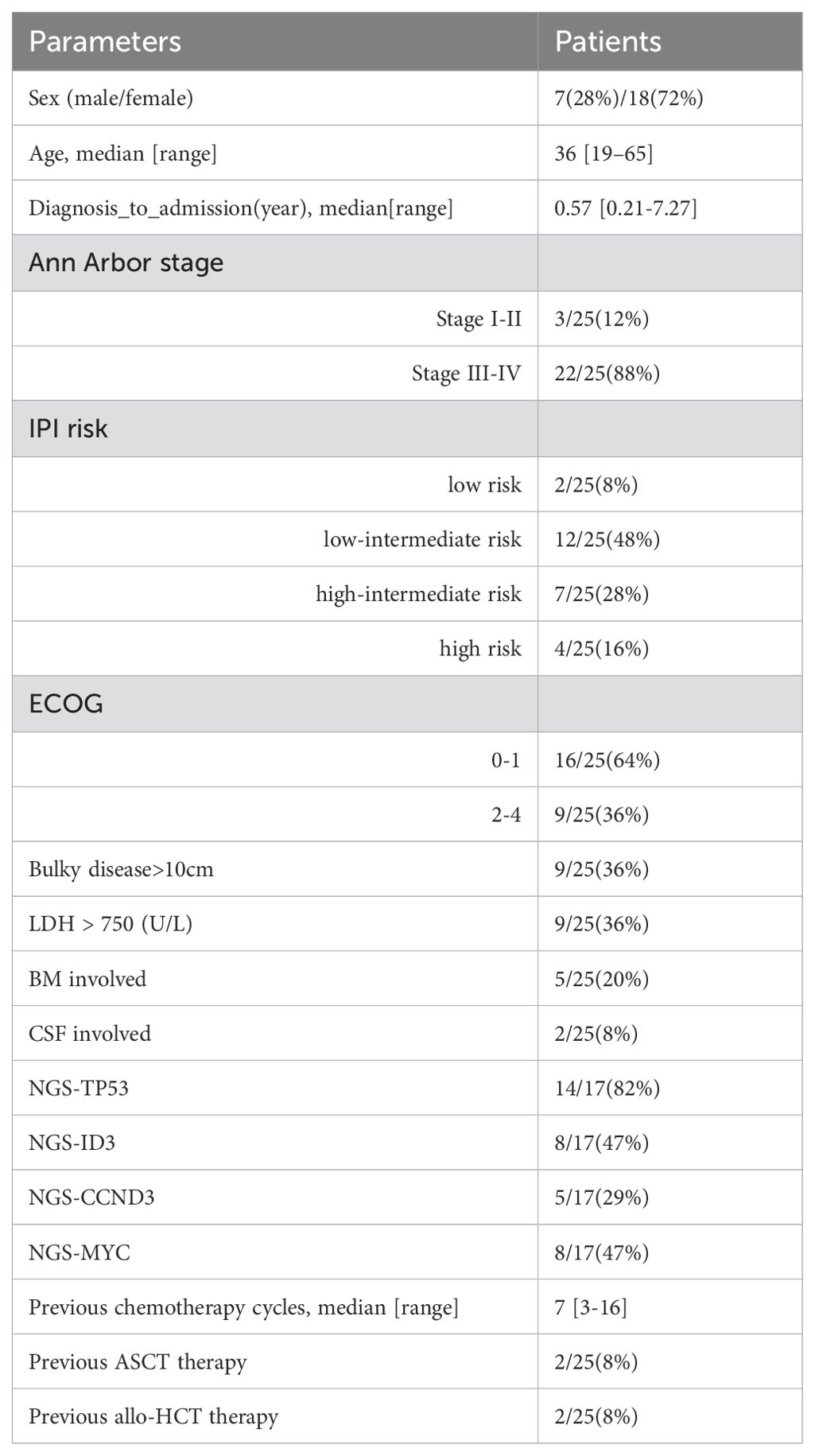
Of the 25 patients, 72% (18/25) were male, with a median age of 36 years (range 19–65). One patient had HIV, two had EBV, five had bone marrow involvement, and two had central nervous system involvement. Advanced-stage disease (stage III–IV) was observed in 88% (22/25), and 96% (24/25) had received ≥3 lines of prior therapy. The cohort included 64% (16/25) refractory cases and 36% (9/25) relapsed cases, with 44% (4/9) relapsing within six months. The median time from diagnosis to admission was 0.57 months (range 0.21–7.27), and nine patients had tumor sizes >10 cm. Induction therapies included R ± CHOP (15/25), Hyper-CVAD-AB (4/25), EPOCH (5/25), and ABVD (1/25). Two patients had a history of autologous transplantation, and two had undergone allogeneic transplantation. Nine out of 25 patients had lactate dehydrogenase levels(LDH) exceeding three times the normal value at the time of lymphocyte collection. Next-generation sequencing (17/25) revealed frequent mutations in TP53 (14/17), ID3 (8/17), MYC (8/17), and CCND3 (5/17). Bridging therapy was administered to 19 patients. 12 patients received platinum-based chemotherapy regimens, and 7 patients received anthracycline-based regimens. Notably, 10 patients received a regimen incorporating polatuzumab vedotin (POLA). The median number of bridging therapy cycles was one (range: 1–3).Five achieved partial response (PR), three had stable disease (SD), and 11 showed disease progression (PD). Lymphodepletion regimens varied among patients: 18 received fludarabine and cyclophosphamide (FC) and 7 patients did not receive additional lymphodepletion because their absolute lymphocyte count had already dropped below 0.1 × 10⁹/L following bridging chemotherapy. The baseline clinical characteristics are summarized in (Table 1).
Response
One month after CAR-T therapy, the objective response rate (ORR) was 52% (95%CI: 31.3–72.2)(13/25), with a complete response rate (CRR) of 28% (7/25). The median follow-up time was 26.10 months (range 14.50-57.17 months). The median overall survival (OS) for the 25 patients was 5.49 months (95% CI 1.734 – 9.25), and the median progression-free survival (PFS) was 2.96 months (95% CI 1.62 – 4.30). The estimated 1-year progression-free survival and overall survival rates were 16%(95%CI 5.02-32.54) and 28%(95%CI 12.42-45.98), respectively. As of the last follow-up, 28% (7/25) were in disease-free survival (Figure 1).
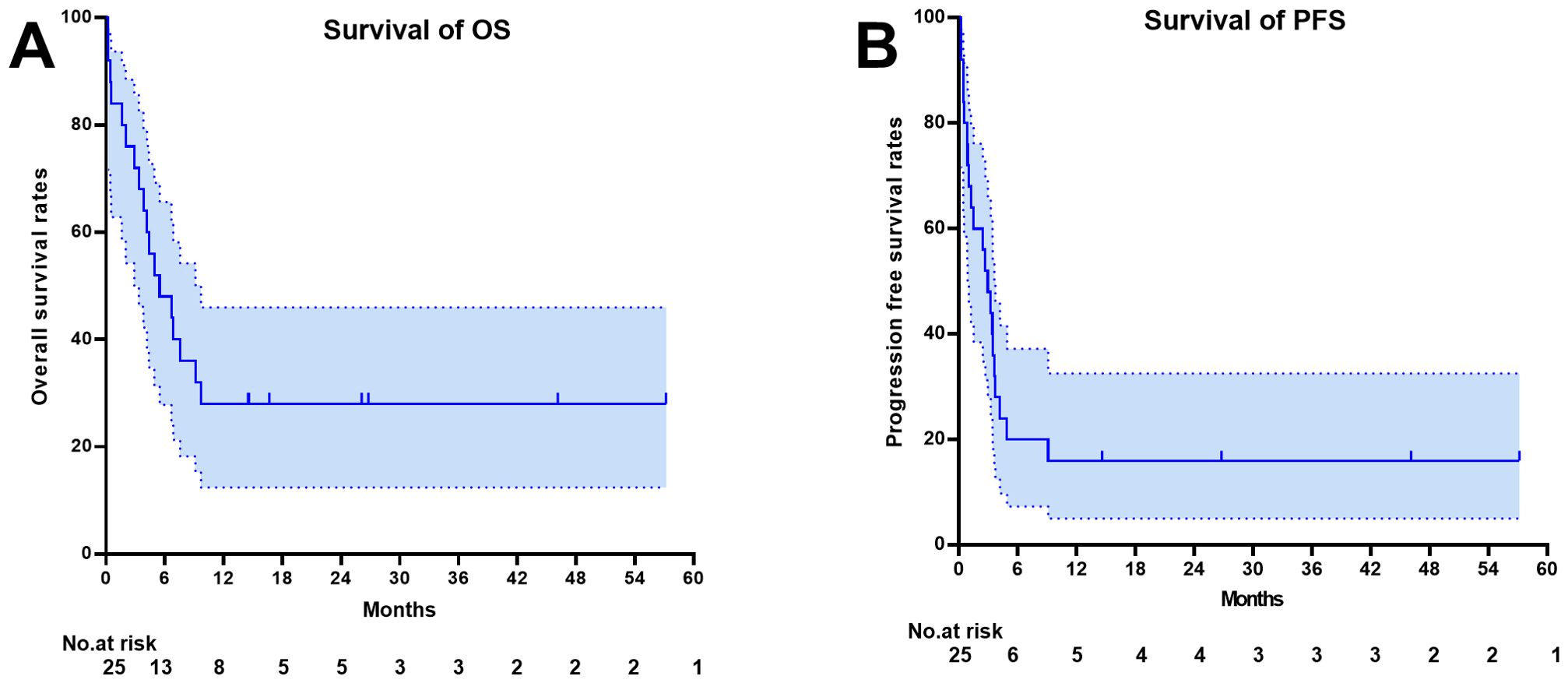
Figure 1. Survival outcome depicted by the Kaplan-Meier curve. (A) Overall Survival (B) Progression Free Survival.
After 1 month CAR-T evaluation, 68% (17/25) received sequential consolidation therapy. The median duration of sequential treatment was 54.5 days (range: 26-127). Figure 2 shows the swim plot of all patients, detailing the timeline and response following the first CAR-T treatment.
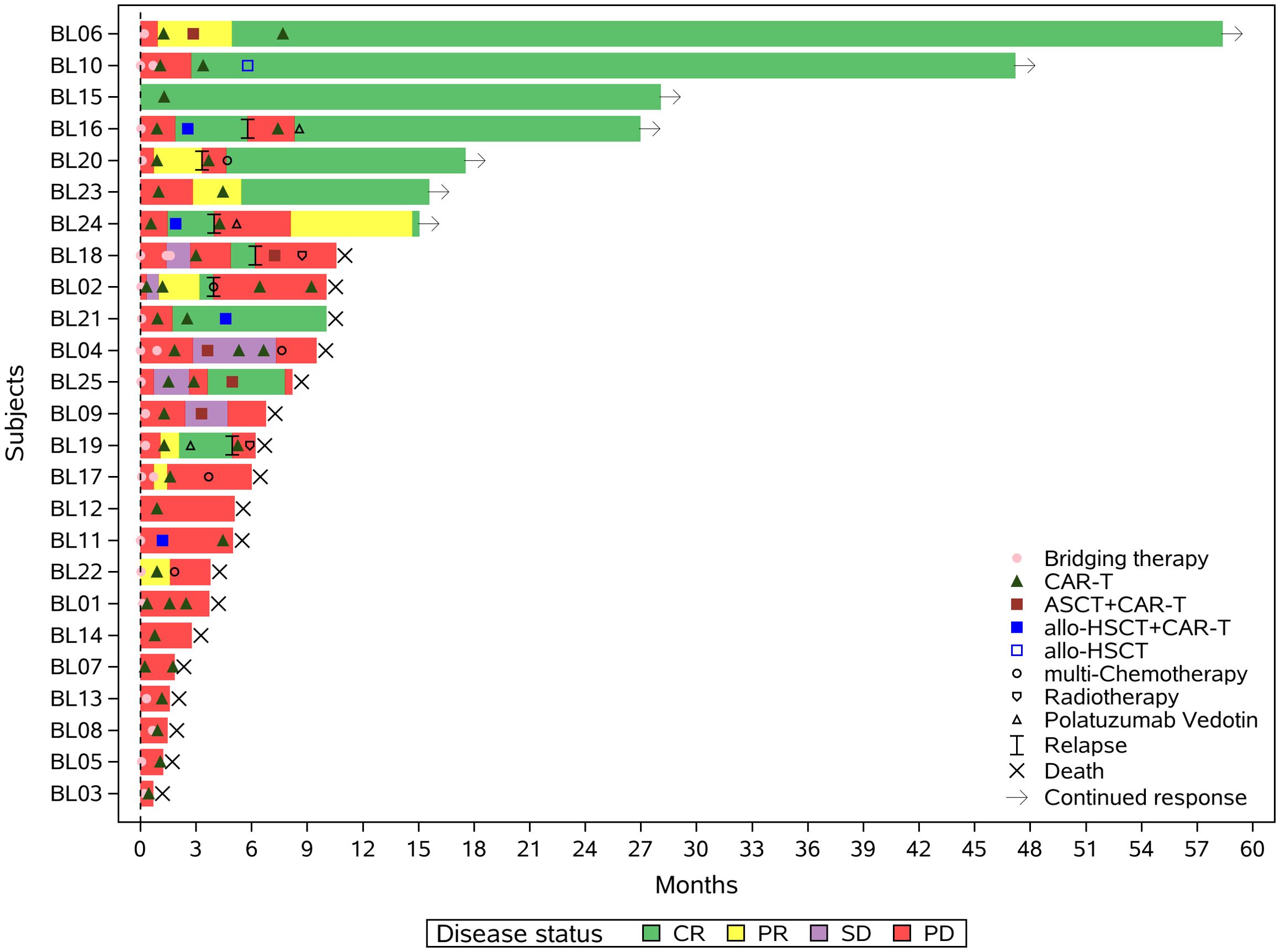
Figure 2. Swimmer plot illustrating the clinical response and follow-up of patients treated with the proposed regimen. Each bar represents an individual patient, with colors indicating different therapeutic interventions following CD19-directed CAR-T cell therapy, including alternative-target CAR-T products (e.g., CD20, CD22), autologous stem cell transplantation (ASCT), allogeneic hematopoietic stem cell transplantation (allo-HSCT), targeted agents, chemotherapy, and radiotherapy. The timing and sequence of interventions are shown along the timeline.allo-HSCT+CAR-T:Allogeneic Hematopoietic Stem Cell Transplantation combined with Chimeric Antigen Receptor T-Cell Therapy;ASCT+CAR-T:Autologous Stem Cell Transplantation combined with Chimeric Antigen Receptor T-Cell Therapy.
AE and toxicities
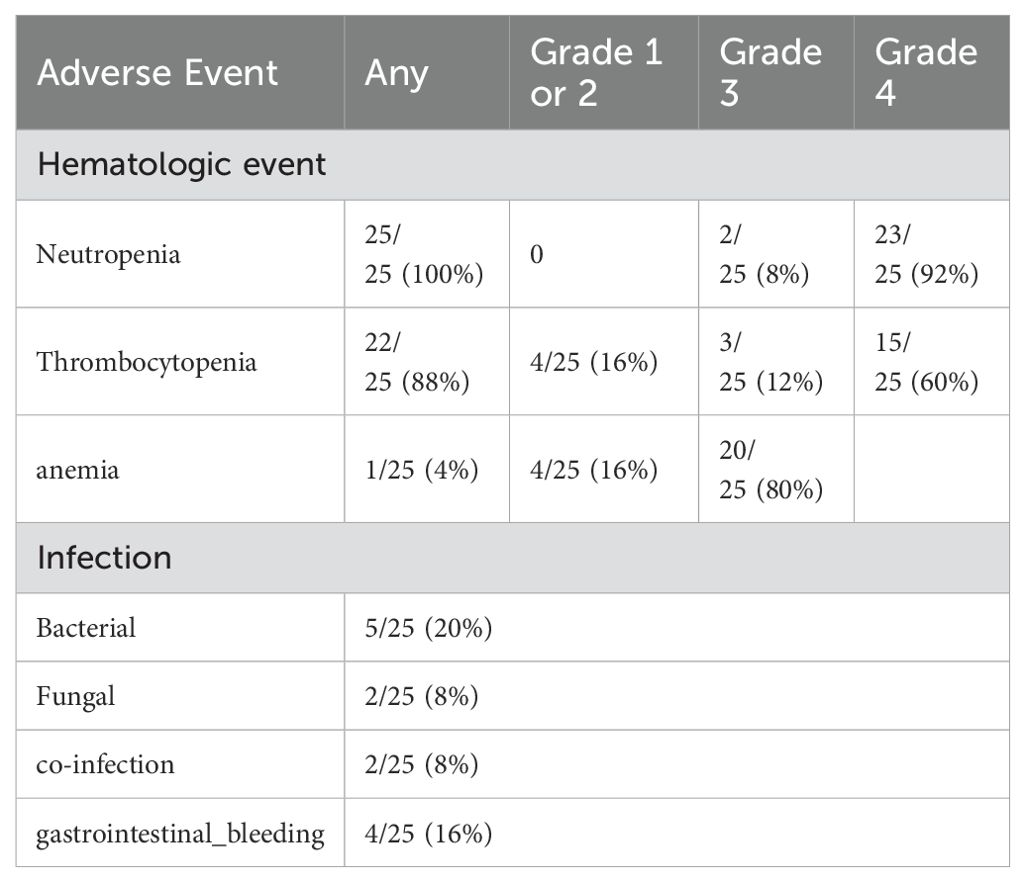
All patients received CD19-targeted CAR-T, with 21 using murine-derived and 4 using human-derived constructs. The median infusion dose was 1.46 × 10^6/kg (range: 0.0126–7.22 × 10^6/kg).Among patients receiving their first CAR-T cell therapy, 80% (20/25) experienced cytokine release syndrome (CRS), and 15% (3/20) had grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (ICANS), with 5/25 experiencing ICANS and 3/5 having grade ≥ 3 ICANS. The duration of CRS was 8 days (range 1-30). Pre-infusion IL-6 levels were measured in 20 patients. Although patients who developed grade ≥3 CRS (n=3) had higher IL-6 levels than those with grade ≤2 CRS (n=17)(29.52 vs. 6.61 pg/mL), the small sample size of the high-grade CRS group limits firm conclusions. Fifteen patients received corticosteroid treatment for CRS, with a median total steroid usage of 73.5 mg (range 5-243).Hematologic toxicity included 100% (25/25) incidence of grade 3–4 neutropenia, 18/25 for grade 3–4 thrombocytopenia, and 20/25 for grade 3–4 anemia. Grade ≥3 cytopenias were observed in 100% (25/25) of patients. The median time to recovery of grade ≥3 cytopenias (defined as return to grade ≤2 or baseline) was as follows:Neutropenia (NEU): median 21 days (range 7–72);Thrombocytopenia (PLT): median 18 days (range 7–62);Anemia (HGB): median 13 days (range 5–86).Granulocyte colony-stimulating factor (G-CSF) was administered in 7 patients (28%), and 17 patients (68%) received platelet or red blood cell transfusions as supportive care. These findings highlight the expected, yet significant, burden of hematologic toxicity in this population. Although supportive care was employed, the retrospective nature of the study limited the ability to fully characterize toxicity management protocols and their correlation with pre-treatment factors or clinical outcomes. While hematologic toxicity was systematically recorded, the current analysis did not assess its correlation with pre-infusion clinical predictors such as tumor burden or inflammatory cytokine levels, which warrants further investigation. Infections occurred in 36% (9/25) of patients, with 5 cases of bacterial infections, 2 fungal infections, 1 case of bacterial and COVID-19, and 1 case of bacterial and fungal infections. Among these, 4 patients experienced gastrointestinal bleeding during treatment. Four patients had seizures. There was 1 treatment-related death due to lower gastrointestinal bleeding. (Table 2).
Risk factors associated with the outcome
Univariate logistic regression analysis showed that several clinical factors were associated with the overall response rate (ORR) (Figure 3). For example, patients with ECOG score ≥2 had a significantly lower ORR compared to those with ECOG <2 (22.2% vs 68.8%), with an OR of 0.130 (95% CI: 0.020–0.863). The use of pola-based bridging therapy was associated with a higher response rate (80.0% vs 33.3%), with an OR of 8.000 (95% CI: 1.215–52.691). In addition, patients with baseline IL-6 ≥20 pg/mL had a lower ORR (28.6% vs 69.2%, OR: 0.178, 95% CI: 0.024–1.339); those with bulky disease >10 cm had a modestly lower ORR (44.4% vs 56.3%, OR: 0.622, 95% CI: 0.120–3.222); and those who had received more than three prior lines of therapy before CAR-T infusion showed a numerically reduced ORR (46.2% vs 58.3%, OR: 0.612, 95% CI: 0.126–2.982).
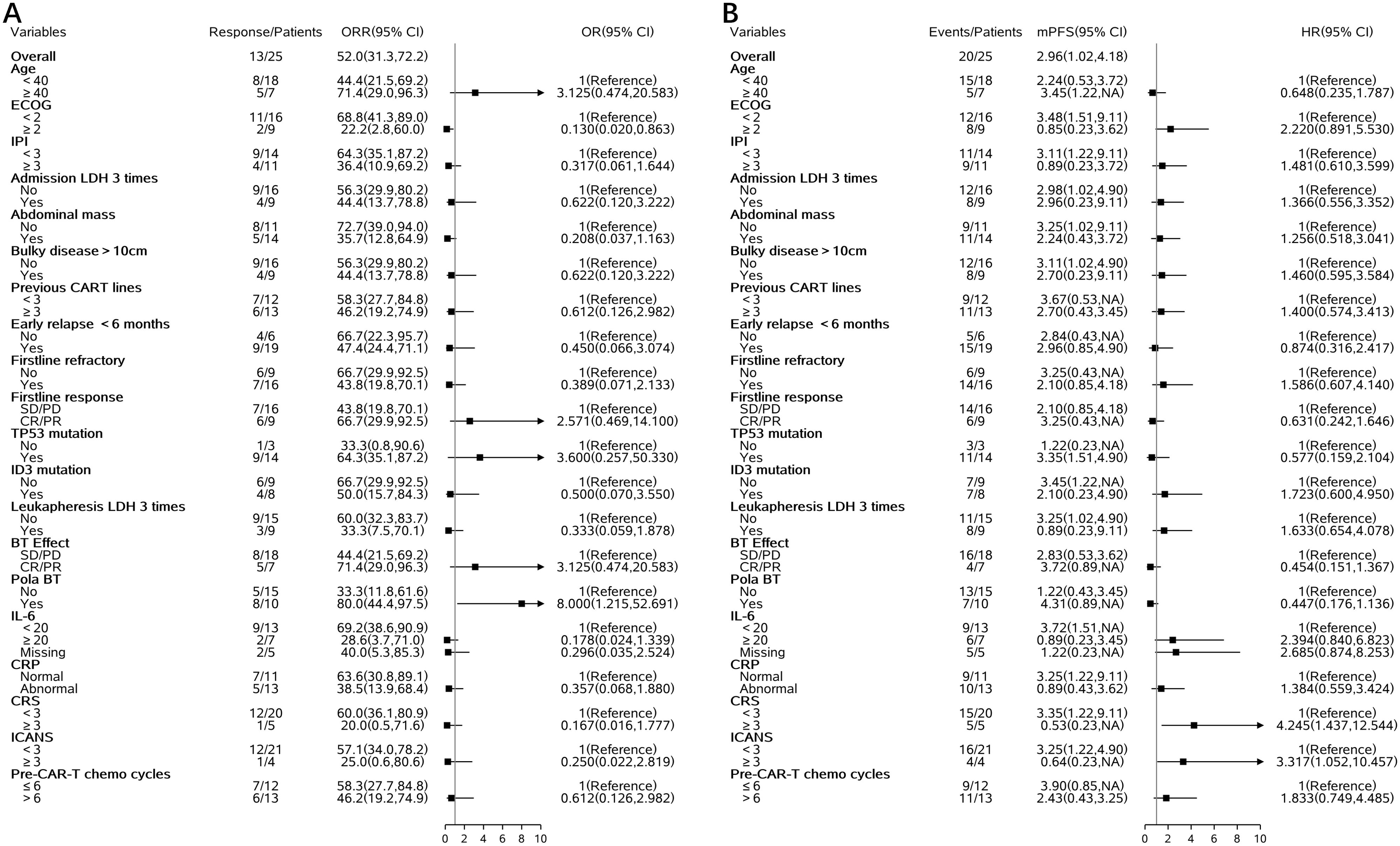
Figure 3. Forest plots of clinical and molecular factors associated with ORR and PFS. (A) ORR (B) PFS.
Univariate Cox regression analysis demonstrated that several baseline factors were also associated with progression-free survival (PFS) (Figure 3). Patients with ECOG score ≥2 had shorter PFS compared to those with better performance status (median PFS: 2.2 vs 3.5 months, HR: 2.220, 95% CI: 0.891–5.530). Baseline IL-6 ≥20 pg/mL was associated with inferior PFS (HR: 2.394, 95% CI: 0.840–6.823). Patients who achieved CR or PR after bridging therapy tended to have improved PFS (HR: 0.454, 95% CI: 0.151–1.367), as did those who received pola-based bridging therapy (HR: 0.447, 95% CI: 0.176–1.136). Furthermore, ID3 mutation was associated with a trend toward shorter PFS (HR: 1.723, 95% CI: 0.600–4.950).
Biomarker analysis
PCR methods detected 20/25 patients, all of whom had successful amplification (20/20), with a median peak value of 4966 copies and a median time to peak of day 14.5 (range 7-34). Flow cytometry detected 23/25 patients, all of whom had successful amplification (23/23), with a median peak value of 68.1 × 10^6 and a median time to peak of day 9 (range 7-28). Additionally, 6/25 patients received pomalidomide, low-dose radiotherapy, or PD-1 stimulation for CAR-T expansion on days 7 or 14 after CAR-T infusion. Six patients received stimulatory interventions, among whom four achieved successful CAR-T cell expansion (Supplementary Figure 1). Figure 4 show the amplification of CAR-T cells detected by flow cytometry method (Figure 4A) and PCR method (Figure 4B), respectively.
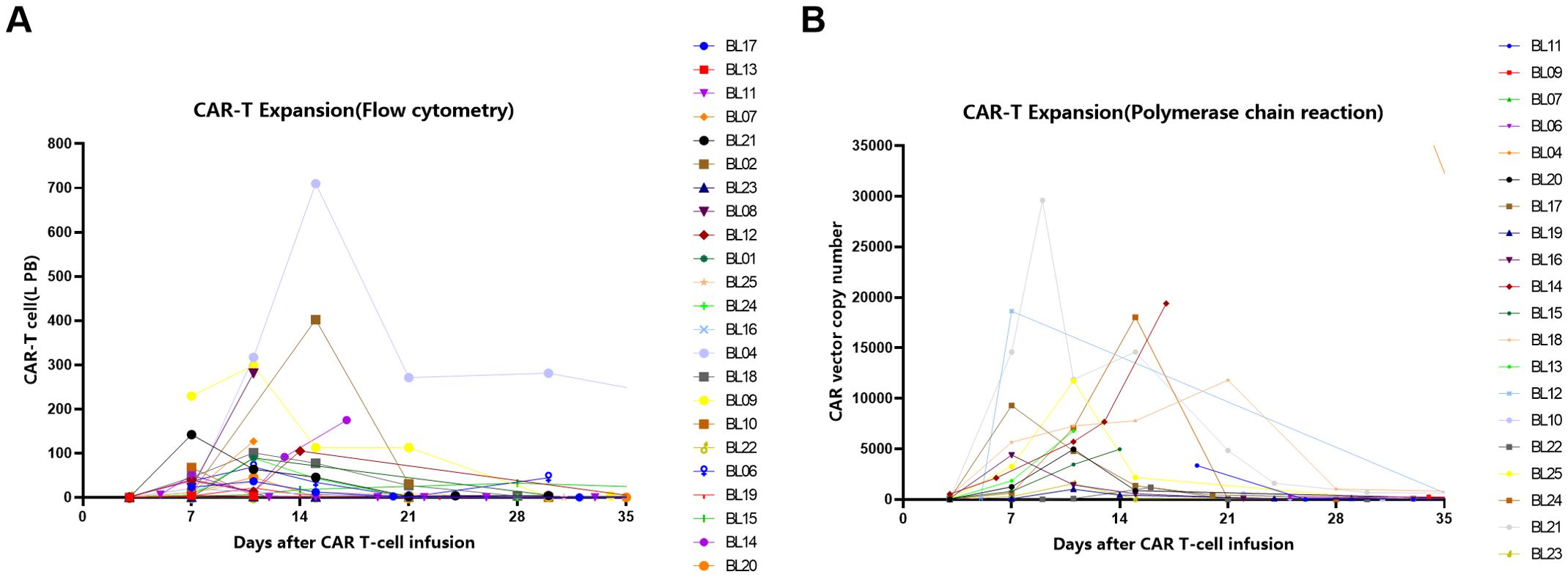
Figure 4. The amplification of CAR-T cells detected by Flow Cytometry method (A) and Polymerase chain reaction(PCR) method (B).
Gene characteristics and survival
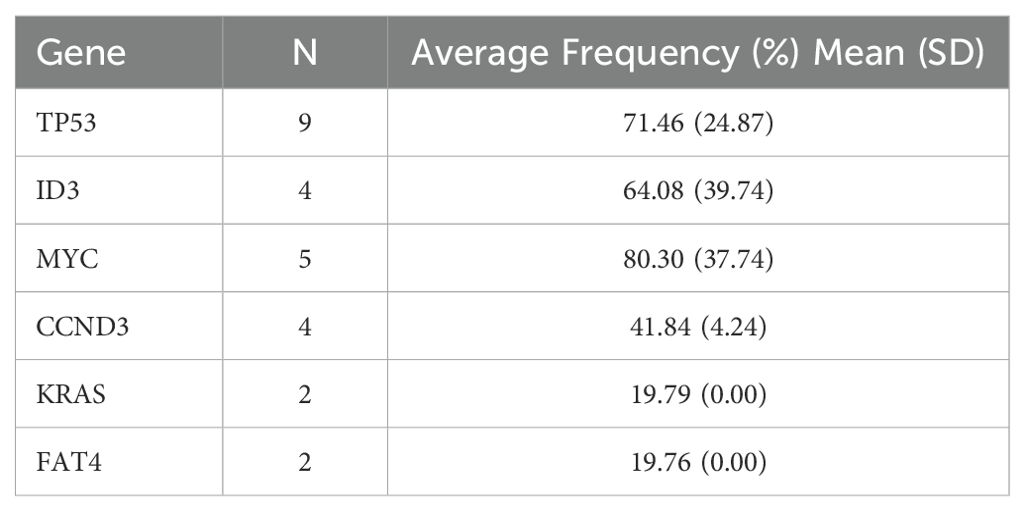
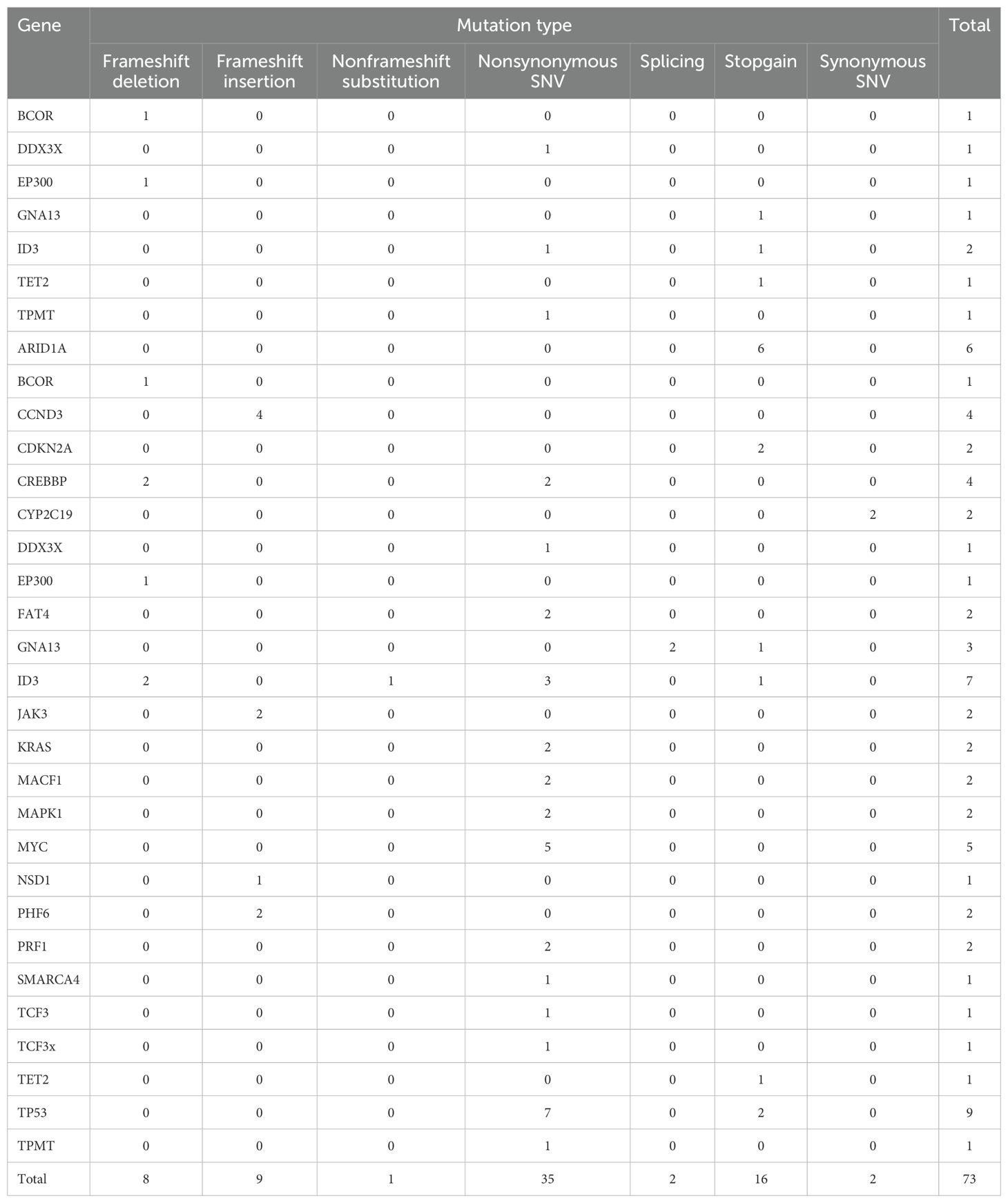
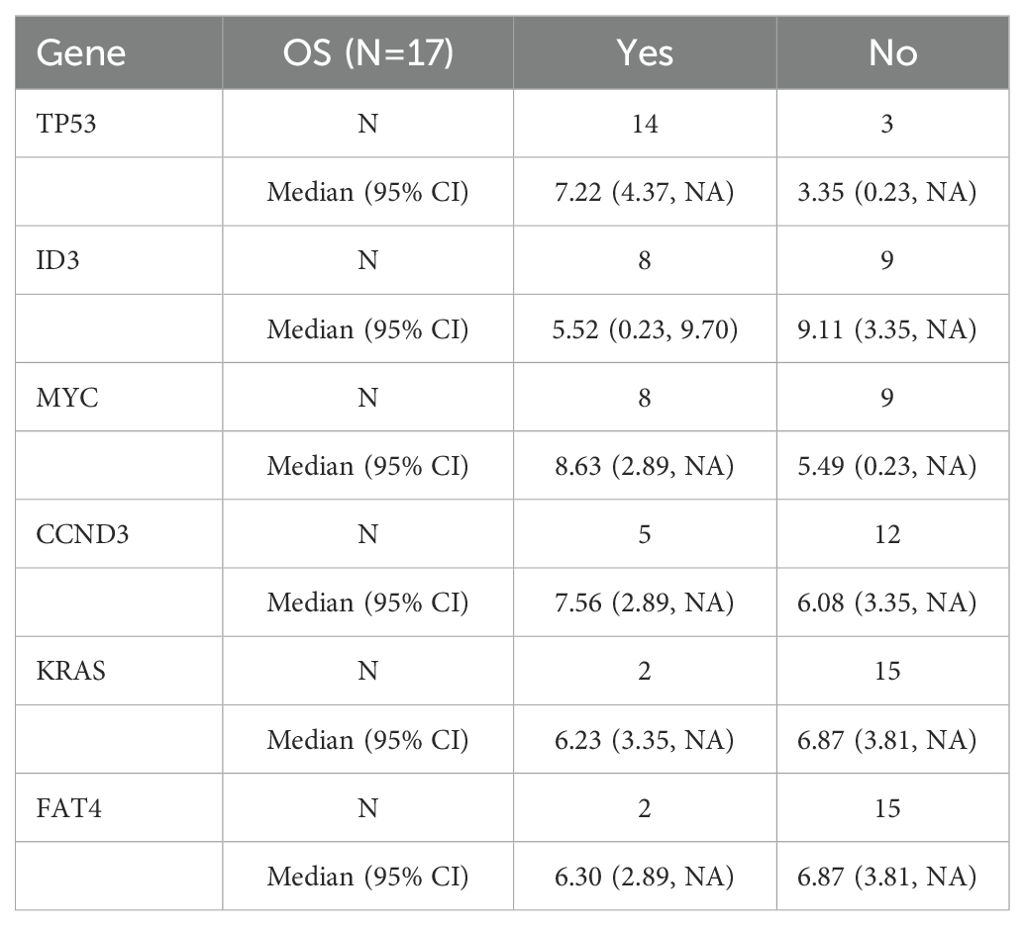
17 out of 25 patients underwent second-generation gene sequencing, with high-frequency mutations identified in genes including TP53, ID3, MYC, CCND3, KRAS, and FAT4. The mutation frequency, mutation types, and their association with survival were analyzed (Tables 3–5).
Sequential consolidation therapy
Among the 25 patients, 16 received sequential consolidation therapy, while 9 did not. The median overall survival (OS) was significantly longer in the sequential consolidation group compared to the non-consolidation group (8.34 vs. 2.01 months; p = 0.0033). Similarly, the median progression-free survival (PFS) was 3.54 months in the consolidation group and 0.53 months in the non-consolidation group (p = 0.0378).With a median follow-up of 26.10 months (range, 14.50–57.17 months), 6 out of 16 patients who received sequential consolidation therapy remained alive, compared to only 1 out of 9 in the non-consolidation group. Among patients receiving sequential consolidation, 9 underwent hematopoietic stem cell transplantation (HSCT), and 7 received additional CAR-T cell therapy. In the HSCT group, 4 of 9 patients were alive at the last follow-up. Specifically, 5 patients underwent autologous HSCT (1/5 survived), and 4 received allogeneic HSCT (3/4 survived). In the CAR-T consolidation group, 2 of 7 patients remained alive. The median OS for CAR-T consolidation and transplantation consolidation were 4.93 months and 9.11 months (Figure 5), respectively (p = 0.3026). Despite this, a higher number of patients who underwent bone marrow transplantation consolidation achieved long-term survival, suggesting that it may contribute to improved long-term outcomes.
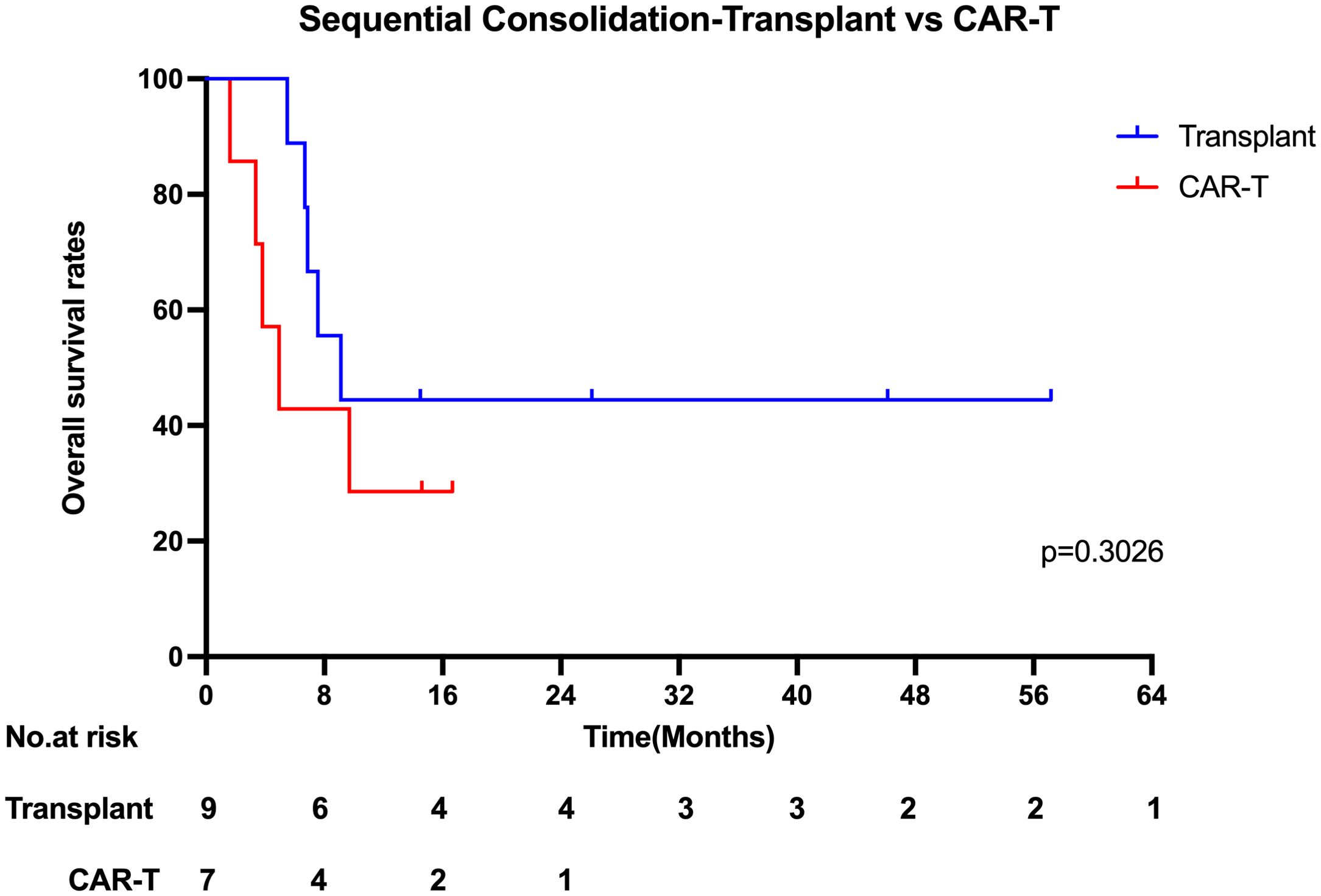
Figure 5. Kaplan-Meier estimates of the overall survival who underwent sequential Consolidation therapy.
Individualized sequential consolidation and long-term survivors
Of the 9 without sequential therapy, only one (BL15) achieved CR during bridging and remained disease-free for 2+ years post-CD19-CAR-T without consolidation. The other 8 patients experienced PD, including 6 who were unable to receive further treatment and 2 who underwent 2 cycles of POLA-based chemotherapy but showed continued progression. The median survival for these 8 patients was 1.27 months (range 0.23–4.37). Figure 2 shows individualized therapies for patients with sequential treatments, including alternative-target CAR-T, ASCT, allo-HSCT, targeted agents, multi-agent chemotherapy, and radiotherapy. Seven patients remain alive:BL06 achieved PR post-CD19-CAR-T, followed by ASCT and CD22-CAR-T, and later received CD20-CAR-T at 6 months. The patient has been in remission for 5 years.BL10 achieved CR post-CD19-CAR-T, followed by CD22-CAR-T and allo-HSCT at 5 months, and remains in remission.BL15 achieved CR post-CD19-CAR-T without further treatment and has been disease-free for 26 months.BL16 achieved CR post-CD19-CAR-T and underwent allo-HSCT with CD19&CD22-CAR-T. Despite initial remission at 1–2 months, relapse occurred at 3 months. The patient received CD20-CAR-T followed by 11 cycles of POLA maintenance therapy and has been disease-free for 22 months.BL20 achieved PR post-CD19-CAR-T, relapsed, and achieved CR post-CD20-CAR-T. The patient then received 2 cycles of R-ICE and has been disease-free for 16 months.BL23 achieved PR post-CD19-CAR-T, followed by CD20-CAR-T, and has been disease-free for 14 months.BL24 achieved remission post-CD19-CAR-T, underwent allo-HSCT with CD20-CAR-T, but progressed at 104 days. The patient subsequently received CD19&CD22-CAR-T and achieved remission for 14 months.
Discussion
Refractory relapsed Burkitt lymphoma (R/R BL) remains a clinical challenge,with limited efficacy from conventional chemotherapy and targeted agents. In our cohort of 25 patients, bridging chemotherapy showed poor effectiveness, with only 21% achieving a partial response. This highlights the chemo-resistance of this population. After the first CAR-T infusion, the overall response rate (ORR) was 52%, and the complete response (CR) rate was 28%. Although CAR-T therapy provided a certain degree of tumor control, durable remission was limited.
Our findings align with recent data from Samples et al. (2025), who reported on adult patients with R/R BL treated with CAR-T therapy. Their study demonstrated an ORR of 58% and a CR rate of 41.9%, with a median progression-free survival (PFS) of 2.3 months and a median overall survival (OS) of 6.0 months. These results, comparable to ours, underscore the limited durability of response and high rate of early relapse following CAR-T therapy in this setting (25). Additionally, case reports and small cohorts have shown that early responses to CAR-T in both adult and pediatric R/R BL can be encouraging, but long-term remission is rare without consolidation (18–20, 26, 27).
Consistent with this, Liu Y, et al. analyzed outcomes of 21 adult R/R BL patients receiving CAR-T therapy with or without autologous hematopoietic stem cell transplantation (HCT). The group receiving CAR-T therapy combined with autologous HCT (n=8) had an ORR of 87.5% and significantly improved 1-year OS and PFS compared to the CAR-T alone group (n=13) (p = 0.014 and p = 0.045, respectively), suggesting that combination strategies may enhance long-term disease control (34).Specific follow-up treatment strategies should be tailored according to changes in the patient’s condition. These strategies may include allogeneic hematopoietic stem cell transplantation, alternative targeted CAR-T therapies, or autologous hematopoietic stem cell transplantation, aiming to reduce the risk of relapse. Early initiation of consolidation therapy could play a crucial role in maintaining long-term remission (33). However, our statistical analysis did not show a significant difference between CAR-T consolidation and transplantation consolidation. The median OS for CAR-T consolidation and transplantation consolidation were 4.93 months and 9.11 months, respectively (p = 0.3026). Despite this, a higher number of patients who underwent bone marrow transplantation consolidation achieved long-term survival, suggesting that it may contribute to improved long-term outcomes. Given the limited sample size, further studies with larger cohorts are required to confirm the potential benefits of transplantation.
We conducted genetic mutation analysis on 17 patients who underwent next-generation sequencing and found common mutations, including TP53, ID3, MYC, and CCND3. These mutations may have some impacts on treatment response and prognosis. In this cohort of relapsed/refractory Burkitt lymphoma patients, the prognostic impact of mutations in TP53, MYC, ID3, CCND3, KRAS, and FAT4 remains unclear. Due to the small sample size in this study, further prospective research is needed to validate the relationship between these genetic mutations and prognosis (28, 29). The safety of CAR-T cell therapy is a key consideration in clinical application. In our study, 84% of patients experienced cytokine release syndrome (CRS), with 20% having grade ≥ 3. Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 24% of patients, with 16% being grade ≥ 3. Regarding hematologic toxicity, 100% of patients experienced neutropenia, 18 patients had grade 3–4 thrombocytopenia, and 20 patients had grade 3–4 anemia. Severe CRS and ICANS appeared to be negatively associated with survival. Grade ≥3 CRS was associated with shorter PFS (HR: 4.245; 95% CI: 1.437–12.544) and a trend toward lower ORR (OR: 0.167; 95% CI: 0.016–1.777). Similarly, grade ≥3 ICANS was associated with inferior PFS (HR: 3.317; 95% CI: 1.052–10.457) and a numerically lower ORR (OR: 0.250; 95%).CI: 0.022–2.819 ≤ These toxic reactions emphasize the need for strict monitoring and timely intervention for patients. We recommend a multidisciplinary team approach to manage CAR-T-treated patients to address potential severe adverse reactions (30).In addition to the common side effects mentioned, the risk of infection following CAR-T treatment is also a major safety concern. Because CAR-T cell therapy significantly suppresses the immune system, patients may face an increased risk of bacterial, fungal, and viral infections. Therefore, prophylactic anti-infective treatment and close infection monitoring are essential (31, 32). Several baseline factors—including ECOG performance status ≥2, elevated baseline IL-6 levels, and failure to achieve remission following bridging therapy—were associated with inferior outcomes after CAR-T cell treatment. In contrast, bridging regimens incorporating polatuzumab vedotin (POLA) demonstrated a trend toward improved response rates and progression-free survival (PFS). Incorporating POLA into bridging strategies may enhance pre-CAR-T disease control, thereby increasing the likelihood of achieving remission following CAR-T infusion.
In addition to these baseline indicators, prior treatment intensity may also impact CAR-T efficacy. The median number of prior chemotherapy cycles was 7 (range, 3–16). We hypothesized that intensive early-line treatment might compromise subsequent CAR-T efficacy. Patients were stratified by whether they had received >6 cycles of chemotherapy before CAR-T infusion. Among them, 13 of 25 (52%) received >6 cycles. This group showed a trend toward shorter progression-free survival (HR 1.833, 95% CI: 0.749–10.457) and a lower overall response rate (OR 0.612, 95% CI: 0.126–2.982).
To further improve outcomes, future strategies may involve the use of multi-targeted CAR-T products (e.g., CD19/CD22/CD20), innovative CAR designs, expedited manufacturing platforms, and integration with consolidative or maintenance therapies. The identification of predictive biomarkers—such as baseline IL-6, ECOG status, and molecular mutations—may facilitate more accurate risk stratification and individualized treatment planning. In addition, optimizing pre-CAR-T treatment regimens—such as minimizing overly intensive frontline chemotherapy—may help preserve T-cell fitness and enhance CAR-T efficacy. Given the aggressive biology and propensity for early relapse in BL, intensified post-CAR-T management should be a focus of future clinical investigations.
Conclusion
CAR-T therapy shows promising activity in relapsed/refractory Burkitt lymphoma, but its effectiveness is limited by short response duration. High-risk features may predict poor outcomes, and a higher number of long-term survivors were observed in patients who received transplant sequential consolidation. However, due to the small sample size, larger studies are needed to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Boren Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Ethical statement
All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This study was approved by the ethics committee of the Beijing GoBroad Boren Hospital (numbered: WZ2024-001-001). Patient informed consent was exempted. All methods were carried out in accordance with relevant guidelines and regulations.
Author contributions
RL: Data curation, Writing – original draft, Writing – review & editing. FY: Writing – review & editing. LM: Writing – review & editing. YG: Writing – review & editing. MC: Writing – review & editing. ZF: Data curation, Writing – review & editing. BD: Writing – review & editing. QZ: Writing – review & editing. CC: Writing – review & editing, Software. DL: Software, Writing – review & editing. XK: Writing – review & editing. KH: Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis, Methodology, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1566938/full#supplementary-material
References
1. Crombie J and LaCasce A. The treatment of Burkitt lymphoma in adults. Blood. (2021) 137:743–50. doi: 10.1182/blood.2019004099
2. Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, and Barth TFE. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. (2006) 354:2419–30. doi: 10.1056/NEJMoa055351
3. Nguyen L, Papenhausen P, and Shao H. The role of c-MYC in B-cell lymphomas: diagnostic and molecular aspects. Genes (Basel). (2017) 8(4):116. doi: 10.3390/genes8040116
4. Blum KA, Lozanski G, and Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. (2004) 104:3009–20. doi: 10.1182/blood-2004-02-0405
5. Mead G M, Sydes MR, Walewski J, Grigg A, Hatton CS, and Pescosta N. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. (2002) 13:1264–74. doi: 10.1093/annonc/mdf253
6. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, and Menon G. Burkitt’s lymphoma. Lancet. (2012) 379:1234–44. doi: 10.1016/S0140-6736(11)61177-X
7. Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, and Esposito G. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. (2012) 156:234–44. doi: 10.1111/j.1365-2141.2011.08947.x
8. Magrath I, Adde M, Shad A, Venzon D, Seibel N, and Gootenberg J. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. (1996) 14:925–34. doi: 10.1200/JCO.1996.14.3.925
9. Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, and Sposto R. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. (2007) 109:2773–80. doi: 10.1182/blood-2006-07-036673
10. Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, and Harrison L. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. (2012) 30:387–93. doi: 10.1200/JCO.2010.33.3369
11. Woessmann W, Zimmermann M, Meinhardt A, Müller S, Hauch H, and Knörr F. Progressive or relapsed Burkitt lymphoma or leukemia in children and adolescents after BFM-type first-line therapy. Blood. (2020) 135:1124–32. doi: 10.1182/blood.2019003591
12. Moleti ML, Testi AM, and Foa R. Treatment of relapsed/refractory pediatric aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. (2020) 189:826–43. doi: 10.1111/bjh.v189.5
13. Malfona F, Testi AM, Chiaretti S, and Moleti ML. Refractory burkitt lymphoma: diagnosis and interventional strategies. Blood Lymphat Cancer. (2024) 14:1–15. doi: 10.2147/BLCTT.S407804
14. Griffin TC, Weitzman S, Weinstein H, Chang M, Cairo M, and Hutchison R. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. (2009) 52:177–81. doi: 10.1002/pbc.21753
15. Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, and Grant C. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. (2013) 369:1915–25. doi: 10.1056/NEJMoa1308392
16. Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. (2023) 141:2307–15. doi: 10.1182/blood.2022018893
17. Chavez JC, Bachmeier C, and Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. (2019) 10:2040620719841581. doi: 10.1177/2040620719841581
18. Ye M, Gao L, Wang T, Yu J, Gui J, and Yang J. CD19 chimeric antigen receptor T-cell therapy following autologous stem cell transplantation against relapsed or refractory Burkitt lymphoma/leukemia: A case report and literature review. Front Oncol. (2022) 12:932254. doi: 10.3389/fonc.2022.932254
19. Geerts PAF, Hart N, Visser O, Ortiz-Maldonado V, and Chamuleau MED. Chemotherapy resistant Burkitt lymphoma: Possible novel therapies including CAR-T cell infusion. Clin Case Rep. (2023) 11:e7361. doi: 10.1002/ccr3.v11.6
20. Cheng L, Huang M, Cao Y, Wang J, Xu H, and Jiang L. Allogeneic CAR-T bridging to allo-HSCT as a treatment strategy for refractory adult Burkitt’s lymphoma: a case report. Transl Cancer Res. (2022) 11:2996–3002. doi: 10.21037/tcr-22-174
21. Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. (2017) 31:2587–93. doi: 10.1038/leu.2017.145
22. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, and Bunin NJ. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
23. Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, and Feldman SA. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. (2010) 116:4099–102. doi: 10.1182/blood-2010-04-281931
24. Xue F, Zheng P, Liu R, Feng S, Guo Y, Shi H, et al. The autologous hematopoietic stem cells transplantation combination-based chimeric antigen receptor T-cell therapy improves outcomes of relapsed/refractory central nervous system B-cell lymphoma. J Oncol 2022. (2022) p:2900310. doi: 10.1155/2022/2900310
25. Samples L, Sadrzadeh H, Frigault MJ, Jacobson CA, Hamadani M, Gurumurthi A, et al. Outcomes among adult recipients of CAR T-cell therapy for burkitt lymphoma. Blood. (2025). doi: 10.1182/blood.2024026831
26. Liu Y, Deng B, Hu B, Zhang W, Zhu Q, and Liu Y. Sequential different B-cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. (2022) 6:717–30. doi: 10.1182/bloodadvances.2021004557
27. Zhou X, Ge T, Li T, Huang L, Cao Y, Xiao Y, et al. CAR19/22 T cell therapy in adult refractory Burkitt’s lymphoma. Cancer Immunol Immunother. (2021) 70:2379–84. doi: 10.1007/s00262-021-02850-6
28. Yang P, Dong F, Zhao W, Tian L, Wan W, and Li Q-H. Clinical characteristics and prognosis affecting-factors of the patients with burkitt lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2021) 29:1498–503. doi: 10.19746/j.cnki.issn.1009-2137.2020.02.024
29. Panea RI, Love CL, Shingleton JR, Reddy A, Bailey JA, and Moormann AM. The whole-genome landscape of Burkitt lymphoma subtypes. Blood. (2019) 134:1598–607. doi: 10.1182/blood.2019001880
30. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. (2019) 37 Suppl 1:48–52. doi: 10.1002/hon.2595
31. Hill JA, Li D, Hay KA, Green ML, Cherian S, and Chen X. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. (2018) 131:121–30. doi: 10.1182/blood-2017-07-793760
32. Arya S and Shahid Z. Overview of infectious complications among CAR T- cell therapy recipients. Front Oncol. (2024) 14:1398078. doi: 10.3389/fonc.2024.1398078
33. Negishi S, Girsch JH, Siegler EL, Bezerra ED, Miyao K, and Sakemura RL. Treatment strategies for relapse after CAR T-cell therapy in B cell lymphoma. Front Pediatr. (2023) 11:1305657. doi: 10.3389/fped.2023.1305657
Keywords: Burkitt lymphoma (BL), car-t, Allo-HSCT, allogeneic hematopoietic stem cell transplantation, auto-HSCT, mutation - genetics, IL-6
Citation: Liu R, Yang F, Ma L, Guo Y, Cao M, Fu Z, Deng B, Zheng Q, Chen C, Li D, Ke X and Hu K (2025) CAR T- cell therapy provides an opportunity for further consolidation treatment for relapsed or refractory adult Burkitt lymphoma patients. Front. Oncol. 15:1566938. doi: 10.3389/fonc.2025.1566938
Received: 26 January 2025; Accepted: 30 April 2025;
Published: 23 May 2025.
Edited by:
Jeffrey J. Pu, Upstate Medical University, United StatesReviewed by:
Justin Boucher, Moffitt Cancer Center, Tampa, United StatesJacopo Mariotti, Humanitas Research Hospital, Italy
Copyright © 2025 Liu, Yang, Ma, Guo, Cao, Fu, Deng, Zheng, Chen, Li, Ke and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Ke, a2V4eUBnb2Jyb2FkaGVhbHRoY2FyZS5jb20=; Kai Hu, aHVrQGdvYnJvYWRoZWFsdGhjYXJlLmNvbQ==
 Rui Liu1
Rui Liu1 Qinlong Zheng
Qinlong Zheng Kai Hu
Kai Hu