- 1Department of Ultrasound, Qilu Hospital of Shandong University Dezhou Hospital, Dezhou, China
- 2Clinical Training Center, Qilu Hospital of Shandong University Dezhou Hospital, Dezhou, China
Gynecological diseases (GyD) are related to reproductive tissue disorders such as the cervix, vagina, fallopian tubes, and ovaries, which can affect fertility. Among these diseases, we can mention endometriosis, ovarian laziness, primary ovarian insufficiency, cancers related to these tissues, and even Asherman’s disease. Considering the impact of these diseases on the population’s youth, it is imperative to develop effective methods for diagnosing, treating, monitoring, and preventing their progression. In the past, ultrasound-based methods have been used for early diagnosis of GyD, including ovarian cancer. However, in today’s era, it is essential to enhance the features of this method to ensure that patients are screened more effectively and their treatment responses can be tracked. In recent years, the spread of artificial intelligence has led to its application in various branches of medicine. Many studies have increased their efficiency by combining ultrasound and artificial intelligence methods. Additionally, the simultaneous use of ultrasound and surgery can help improve patient recovery and the success of the procedure. Additionally, various studies have utilized the combination of ultrasound-based methods and different drugs to treat GyD. In this manuscript, we will discuss the pathology of gynecological diseases, the use of ultrasound-based methods, and their combination with other methods.
1 Introduction
Gynecological diseases (GyD) are of great importance due to their impact on women’s health and fertility and, ultimately, the youth of the population (1). GyD is generally categorized into three distinct groups, each with its own unique pathological pathways. These include diseases related to tumors (cervical tumors, ovarian tumors) (2)Infectious diseases (fungal infections such as vaginitis) (3), and endocrine gland-related diseases (4, 5). Also, the reason for some GyD is unknown; it can be Vulvodynia with symptoms such as pain in the vulva and itching (6). Some other diseases, such as endometriosis (7), can also occur due to a combination of different mechanisms. In endometriosis, due to retrograde menstruation, cellular remains are poured into the peritoneal cavity, and endometrial-like lesions are formed there due to the dysfunction of the immune system, which can lead to abnormal bleeding and pelvic pain (8).
To prevent the progression of these diseases, early diagnosis is critical (9). However, many diagnostic methods, such as laparoscopy, are invasive and require patient-friendly treatment. In the case of GyD, methods based on ultrasound (US) can be used as non-invasive methods (10). This method is preferentially used to diagnose benign or malignant abdominal and pelvic masses caused by the ovary, adnexa, and uterus (11). There are different types of US, which can be done in two- or three-dimensional ways. However, the results of the studies have shown that the use of three-dimensional US has a higher sensitivity than the two-dimensional type in diagnosing fibroids, cysts, adnexal torsion, endometrial thickness, and uterine congenital abnormalities (nearly 100 percent) (12).
As mentioned, ultrasound can diagnose the tumor and check its exact staging. However, the use of complementary methods such as computerized tomography, artificial intelligence, and magnetic resonance imaging (MRI) can be used for correct and differential diagnosis of endometrial cancer and other GyD (13, 14). The combination of these methods is crucial for doctors to make decisions. Considering that the US can play an important role in classifying GyD stages, using it with artificial intelligence can help choose between surgery and drug treatment (15). This review will first discuss the basics of using the US to diagnose GyD types and then how to combine it with artificial intelligence for medical decision-making.
2 Clinical applications of ultrasound in gynecological disease diagnosis
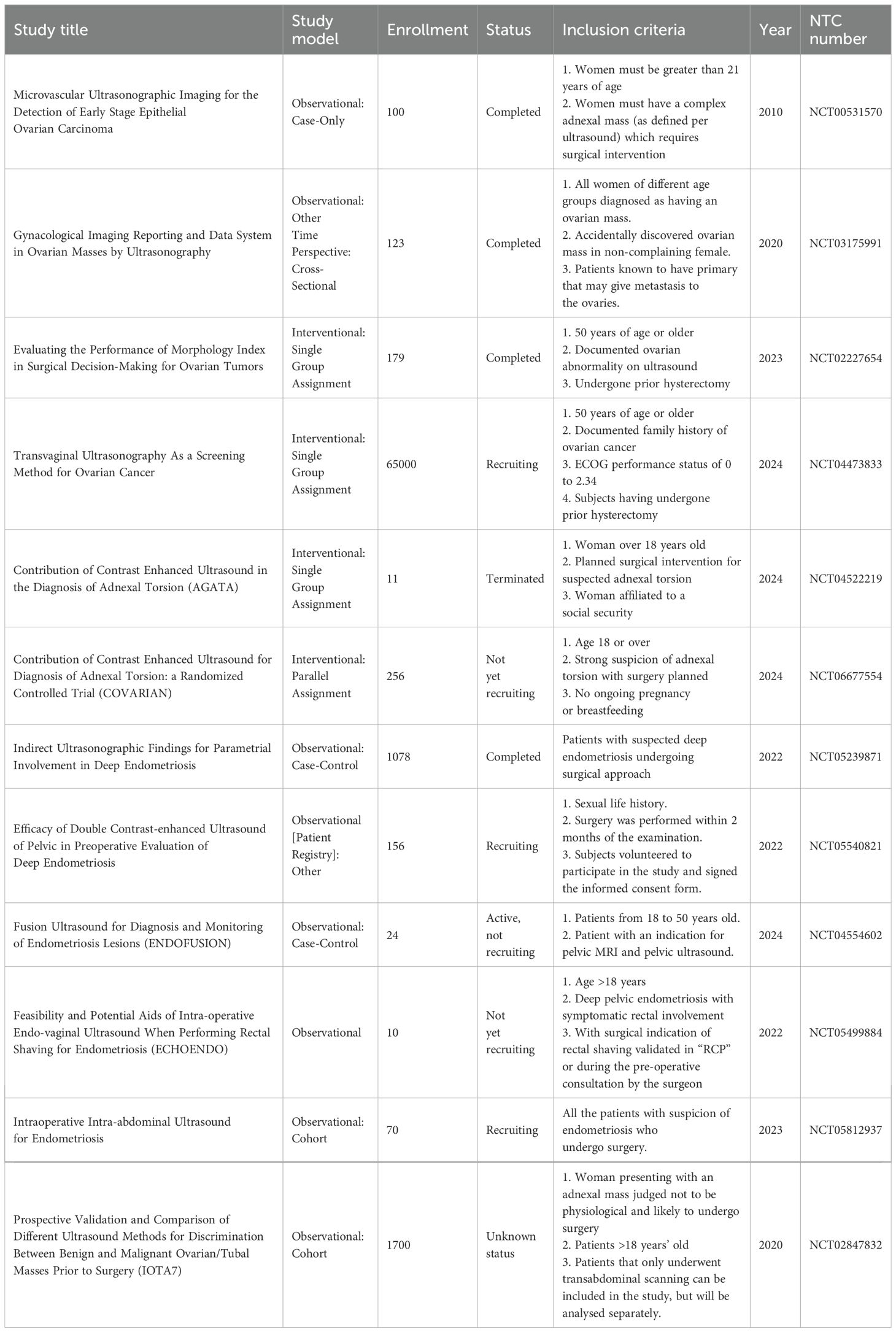
The US is a less invasive method with minimal side effects used to diagnose various diseases (16). If an experienced operator performs in the US, their results are precious in diagnosing diseases such as tumors, their size, metastasis, and follow-up treatment (17). In addition to the operator, the type of US device can also influence the diagnostic results. The conducted reviews should be recorded and used for further offline or artificial intelligence reviews. Another variable that can affect the rejection of US results is the patient (18). In some cases, obesity can create shadows that prevent correct diagnosis, and complementary methods must be used to confirm US results (Table 1) (19). In general, it is possible to use US in GyD such as fibroma and fibrosarcoma, adnexal torsion, acute abdomen, struma ovarii, ovarian dysgerminoma, decidualized endometriomas, extragastrointestinal stromal tumors, serous cystadenofibromas in adnexa, ovarian mature cystic teratomas, recurrent ovarian stromal cell tumors, ovarian yolk sac tumors, benign retroperitoneal pelvic peripheral-nerve-sheath tumors, etc (11, 20). The following section will explain this method for some of the mentioned diseases. Figure 1 summarizes the characteristics of ultrasound in the diagnosis of gynecological diseases. (Figure 1).
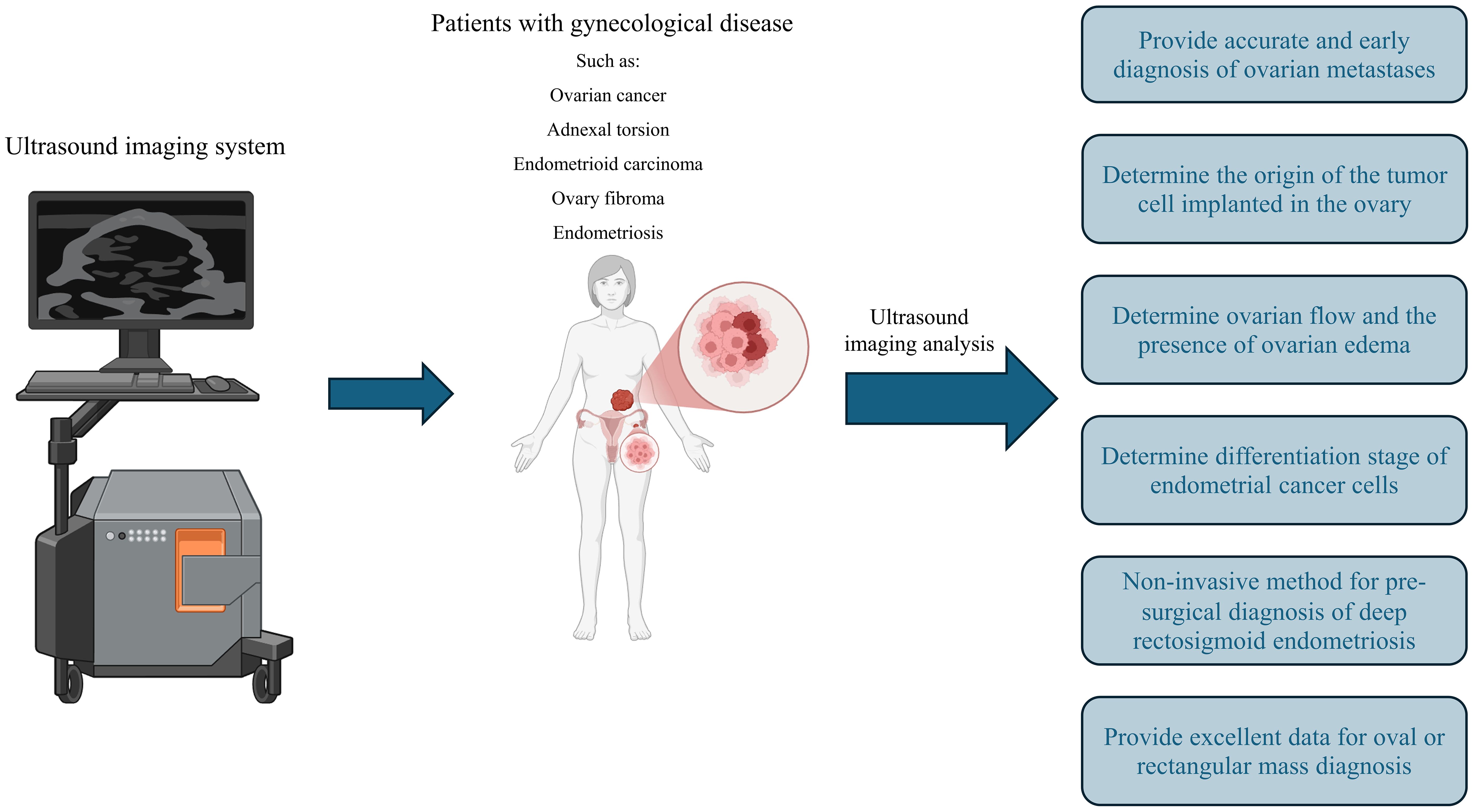
Figure 1. The application of ultrasound in gynecological disease. Ultrasound imaging can play a significant role in diagnosing numerous gynecological diseases, such as various cancers, endometriosis, and more. It can also assist in determining cancer stage and assessing ovarian reserve.
2.1 Ovarian cancer and metastases
The results of transvaginal gray and color Doppler ultrasound examinations in patients with metastatic ovarian tumors originating from other tumors indicate that these tumors can be solid, multilocular, or solid-multilocular (21). The type of tumors that metastasize to the ovary can vary based on the origin of the tumor cells. Thus, tumors originating from the breast, uterus, stomach, or lymphoma are of solid type. This is while ovarian tumors originating from bile ducts, colon, appendix, or rectum are solid-multilocular or multilocular. Therefore, using ultrasound to check ovarian metastases can determine the origin of the tumor cells implanted in the ovary (22). Also, in another study, color Doppler was used to investigate the spread of a peripheral vessel called a lead vessel to the center of the mass formed in the ovary. The results of this study show that the lead vessel is observed in 35% of patients with solid mass and 52% of patients with solid-multilocular or multilocular tumors (23). Also, ultrasound has been used to compare primary ovarian tumors and tumors caused by metastasis from endometrial cancer. The results of this study show that the sonomorphological characteristics of these two types of tumors are different (24). Such diagnoses can help choose the type of treatment.
However, the use of molecular methods in combination with ultrasound can help in more accurate and early diagnosis of ovarian metastases. In a study conducted by F. Moro et al., CA125/CEA was combined with ultrasound to detect metastases of multilocular ovarian masses. After confirming ovarian tumors in patients, the levels of CA125, CEA, and the CA125/CEA ratio were evaluated. The results showed that, based on ultrasound data, CEA alone can be used for the differential diagnosis of ovarian metastases and ovarian neoplasms (25).
2.2 Adnexal torsion
This disease can occur due to the twisting of the adnexal vessels, which leads to irreparable damage to the ovarian tissue by cutting off the blood flow to the ovary (26, 27). Therefore, early diagnosis is essential in this disease. One of the primary methods for diagnosing this disease is through clinical and surgical examinations, which can be supplemented with ultrasound using Color Doppler (26, 28). The results of the retrospective studies conducted by F. Moro and colleagues investigated the torsion of the adnexal vessels in patients, which was confirmed using surgery. The results of this study indicate that the common symptoms can be diagnosed by ultrasound. This disease is characterized by the presence of free fluid in the pelvis, the whirlpool sign, enlarged adnexa, and ovarian stromal edema (OSE) (29). In another study, ultrasound was used to diagnose adnexal torsion with acute abdominal pain (30). The results of this study have shown that the sensitivity of ultrasound for diagnosing adnexal torsion is 84.4%, indicating a high diagnostic value (30, 31). Additionally, a 2023 meta-analysis study, which examined articles from 1990 to 2021, demonstrated that ultrasound examinations can be used to identify symptoms such as the reduction or absence of ovarian Doppler flow and the presence of ovarian edema. It was used to diagnose adnexal torsion with high sensitivity (32).
2.3 Endometrioid carcinoma
This cancer is the most common type of endometrial cancer in women and has recognizable molecular, macroscopic, and microscopic features (33). The presence of squamous differentiation, ciliated cells, and secretory cells usually recognizes this type of cancer (34). In addition, molecular features such as microsatellite instability (MSI), mutations in proto-oncogene genes like PTEN and k-RAS, and cell growth induction mutations such as FGFR2 can be used to identify endometrial carcinoma (35). The main treatment criterion for this disease is stage and histopathology. Surgery followed by radiotherapy can prevent the spread of tumor cells (36). It is imperative to diagnose this disease and prevent its progression. One of the primary methods for diagnosing this disease is biopsy, along with methods based on immunohistochemistry (33, 37). Thus, the observation of epithelial and mesenchymal malignant differentiation, as well as the immunohistochemical examination based on DNA mismatch repair (MMR) proteins, p53, and p16, can aid in diagnosing this disease (38, 39). However, performing a biopsy is invasive, and it seems that it can lead to the stimulation of tumor tissue and sometimes increase the metastasis of tumor cells. Therefore, the need for less invasive methods such as ultrasound is quite evident. In a retrospective study by F. Moro et al., ultrasound was used to diagnose endometrial carcinoma (20). This study evaluated the information of 239 patients examined by ultrasound. The results of this study show that endometrial carcinoma typically presents as large, multilocular-solid tumor masses (20).
Transvaginal ultrasound (TVS) or transrectal ultrasound (TRS) to check the differentiation stage of endometrial cancer cells also shows the good power of ultrasound in diagnosing two types of differentiation, well and moderate, and its results are consistent with the results of the biopsy of patients (40). In the case of endometrial carcinoma, it seems that one of the characteristics associated with high risk is myometrial infiltration. In the study conducted by L. Pineda, ultrasound was used to investigate this issue in hospitals (41). Also, the results have been compared with those obtained from the macroscopic mean. The extracted tissue was evaluated by pathologists who were unaware of the ultrasound results. The results showed that the use of TVS could be used as a method for assessing myometrial infiltration and as an alternative to intraoperative macroscopic examination, primarily when performed by an experienced examiner (41).
Additionally, a study by M. Cubo-Abert utilized TVS to investigate and compare its effectiveness with other methods, including magnetic resonance imaging (MRI), in the diagnosis of endometrial carcinoma. The results demonstrate that TVS is a reliable and suitable diagnostic method for low-grade endometrial carcinoma, and it can be used as the initial line of diagnosis (42). Therefore, it appears that using ultrasound to diagnose endometrial carcinoma can assist surgeons in making informed medical decisions and treating these patients.
2.4 Ovary fibroma and fibrothecoma
Fibroids and fibrothecoma are benign tumors of the ovary, which have a prevalence of about 4% among tumors related to the ovary (43). These diseases are typically associated with acid reflux and are referred to as Meigs syndrome. Considering the benign nature of these tumors, their early detection can help in the management of the disease (44). Fibroma and fibrothecoma can also be associated with Guerlain-Goltz syndrome, which affects the nature of the cells in the tumor mass and is usually related to the appearance of keratinocytes and basal cells (45). These diseases typically do not cause symptoms in patients (46). A study conducted by D. Paladini to diagnose fibroma and fibrothecoma using ultrasound reveals that approximately 50% of patients have ascites. The presence of fluid in the pouch of Douglas, the increase of CA125, and high color content in the diagnosis of this disease by ultrasound can lead to misdiagnosis of fibroid and fibrothecoma as malignancy (47). For this reason, it is said that the diagnostic features of these diseases using ultrasound are non-specific and need to be combined with other methods.
2.5 Endometriosis and adenomyosis
In recent years, TVS has been proposed as a primary test for pelvic ectopic endometriosis and endometriosis. In endometriosis, ectopic lesions form in different parts of the peritoneal cavity, ovaries, and other areas. Additionally, the presence of endometrial lesions in the myometrium is referred to as adenomyosis (48). Due to the effect of these diseases on the disruption of hormone levels and induction of infertility in patients, the use of alternative laparoscopic (ultrasound) methods, which are invasive, is essential. Also, in new studies, ultrasound has been used for high-precision diagnosis of deep ectopic lesions of the pelvis (49). Additionally, reviews and analyses conducted in a meta-analysis study have demonstrated that TVS, with or without the use of previous bowel preparation, is an accurate test for the non-invasive and pre-surgical diagnosis of deep rectosigmoid endometriosis (50, 51).
2.6 Tubal cancer
In a study, patients who were confirmed for tubal cancer by macroscopic and histopathological examinations were evaluated by color Doppler ultrasound and grayscale before surgery (52). The results of this study show that the images captured by ultrasound have three types of differential appearance in tubal cancer: a sausage-shaped cystic structure with solid tissue protruding into it like a papillary protrusion, a sausage-shaped cystic structure with a large solid component filling part of the cyst cavity and an excellent oval or rectangular mass. Therefore, tubal cancer can be diagnosed in patients by analyzing ultrasound images (53).
3 Artificial intelligence-assisted diagnosis
With the expansion and advancement of methods based on artificial intelligence (AI), these methods have found their way into medical fields (54, 55). In the past, algorithms and programming based on medical boards were used in diagnosis and treatment. However, conventional general programming algorithms generate outputs using given input data and rules, while artificial intelligence can create regulations and patterns using input and output data (56). Hence, AI can reliably predict outcomes from new input. It has been stated in many studies that with the specific development of artificial intelligence in a particular disease, it can be used in diagnosis, deciding on the type of treatment, and examining the treatment process (57). For example, in some studies, AI has been used to diagnose skin cancer and retinopathy in diabetic patients with high accuracy (58, 59). The use of AI in gynecology and obstetrics has had challenges (Table 2). However, recent advances have turned this method into a powerful tool, and using its potential can help improve the health of women and babies.
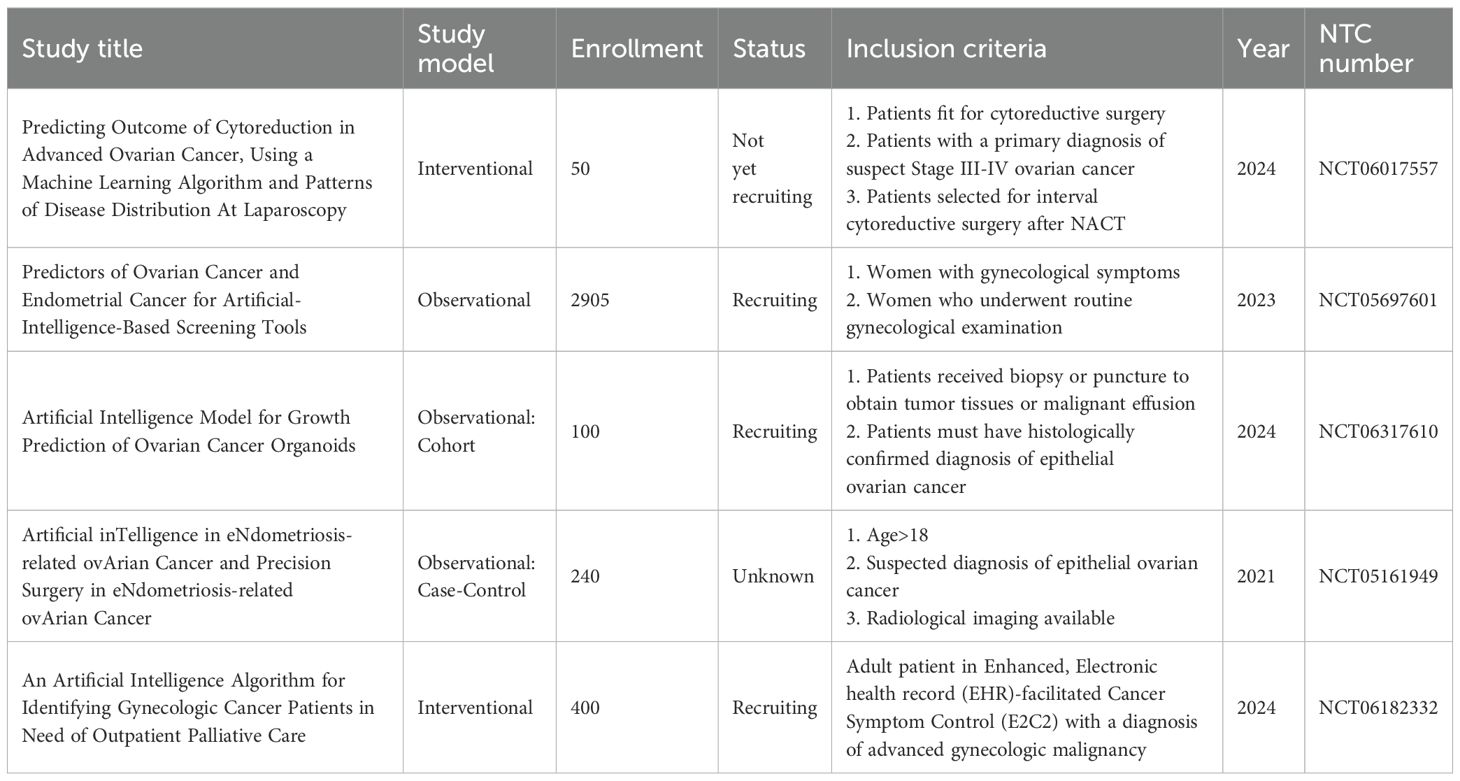
Table 2. The application of artificial intelligence in diagnosis and treatment of gynecological disease.
Artificial intelligence encompasses a set of digital computer systems equipped with features such as the ability to process information, exhibit intelligent behavior, demonstrate comparative performance, and engage in critical thinking, which enables the user to obtain a series of output data from the input data (60). In the case of medical issues, the input data are the results of patient examinations, and the output data can include test results, treatment decisions, tumor location, angiogenesis, fetal characteristics (complete or premature), and other cases. When discussing artificial intelligence, it is essential to understand that concepts such as machine learning (ML), deep learning (DL), and convolutional neural networks (CNN) overlap to a significant extent (61). However, there are also differences between them (62, 63). Considering that these concepts are used in the following text, their explanation can help to understand the content as well as possible. In applications based on machine learning, computer systems and software use input data to create a series of patterns.
Machine learning algorithms in AI can be divided into three categories: Unsupervised learning, supervised learning, and reinforcement learning (64, 65). The functions of these functions can be used in combination to diagnose and treat. During training, supervised learning algorithms learn from input data and labeled output targets to create a model that can classify or predict new data based on learned relationships. Usually, in this type of learning, some data whose results are known to the researchers are given to the used algorithm, and the information processed by it is compared with the real results that were already available to confirm the efficiency of the constructed algorithm (66). Unsupervised machine learning models differ from supervised models in that they learn and interpret relationships between key pieces of information in a dataset without predefined output data. They can reveal associations or clusters in data and complement supervised learning by identifying patterns that may not have been detected. This information can improve supervised algorithms and create new models (67).
Demographic and genetic factors significantly influence breast cancer risk. Studies emphasize age as a key criterion; middle-aged and older women exhibit higher incidence rates (68). Susceptibility varies by race, ethnicity, and geographic location, affected by genetic, environmental, and socioeconomic factors. Key risk factors necessitating tailored prevention plans include a family history of cancer and genetic mutations, such as those associated with BRCA1 and BRCA2 (69). Noteworthy economic disparities also impact access to care. Early menarche, late menopause, and hormonal treatments influence risk through hormonal exposure. The development of breast cancer also relies on metabolic factors such as insulin resistance and diabetes. Risk analysis is partly based on a patient’s medical history, including breast density and a history of previous malignancies.
Artificial intelligence enhances detection, diagnosis, treatment, risk assessment, and prevention, particularly through improved mammography (70). While there are concerns about false positives, it aids radiologists in identifying lesions. Despite challenges such as data privacy and ethical concerns, artificial intelligence also aids in radiotherapy planning and genetic risk analysis (71).
Although there are few investigations, AI in mammography screening looks to have some potential (72). In women 50–69 at 12 locations in Germany, the PRAIM study matched AI-supported double reading against conventional double reading (73). Between July 2021 and February 2023, 463,094 women were screened, and 260,739 of them received AI assistance. At 6.7 per 1,000, the artificial intelligence team had a breast cancer detection rate that was 17.6% higher than the control group (73). The recall rate of the AI group was lower than that of the control, and the positive predictive values for biopsies and recalls were also higher. Artificial intelligence could raise mammography screening indicators (74).
4 Ultrasound and artificial intelligence in gynecological disease
One of the primary methods used in gynecology and obstetrics is the combination of ultrasound and AI (75). The retrospective analysis of ultrasound images from patients, combined with the creation of a database that can be integrated with AI, can facilitate a more accurate diagnosis of women’s diseases by comparing new ultrasound images. This type of method also leads to a reduction in the time from imaging to diagnosis (76). Additionally, the use of this technology can lead to a reduction in human error in the diagnosis of GyD. Therefore, by utilizing various types of artificial intelligence tools, ultrasound images can serve as input data to create an algorithm (77). The combined use of different AI tools will significantly help obstetrics and gynecology in the future. In the following, we will discuss some artificial hash tools that GyD can use (Table 3).
Table 3. Application of ultrasound and artificial intelligence in combination for diagnosis and treatment of gynecological disease.
Probe guidance, which is also called AI-GUIDE, is one of the most widely used AI tools, which is used in echocardiography and in working with probes that are necessary for taking pictures (78). Using a Probe guidance application, which guides the operator in orientation and how to manipulate the ultrasound probes to obtain a suitable biometric screen for photographing the fetus, is beneficial. Therefore, this application can help teach ultrasound and entrust the initial scans to general doctors who do not perform this test (Figure 2). Thus, the need for the permanent presence of experts is reduced, and errors caused by individual mistakes are also minimized (79). In the study conducted by Richard Droste et al. on Probe guidance and using probe movement data obtained from 464 tests performed by expert operators, it was shown that this application, by combining the motion signal of the Inertial Measurement Unit (IMU) and creating the US-GuideNet network, helps guide the operator (80).
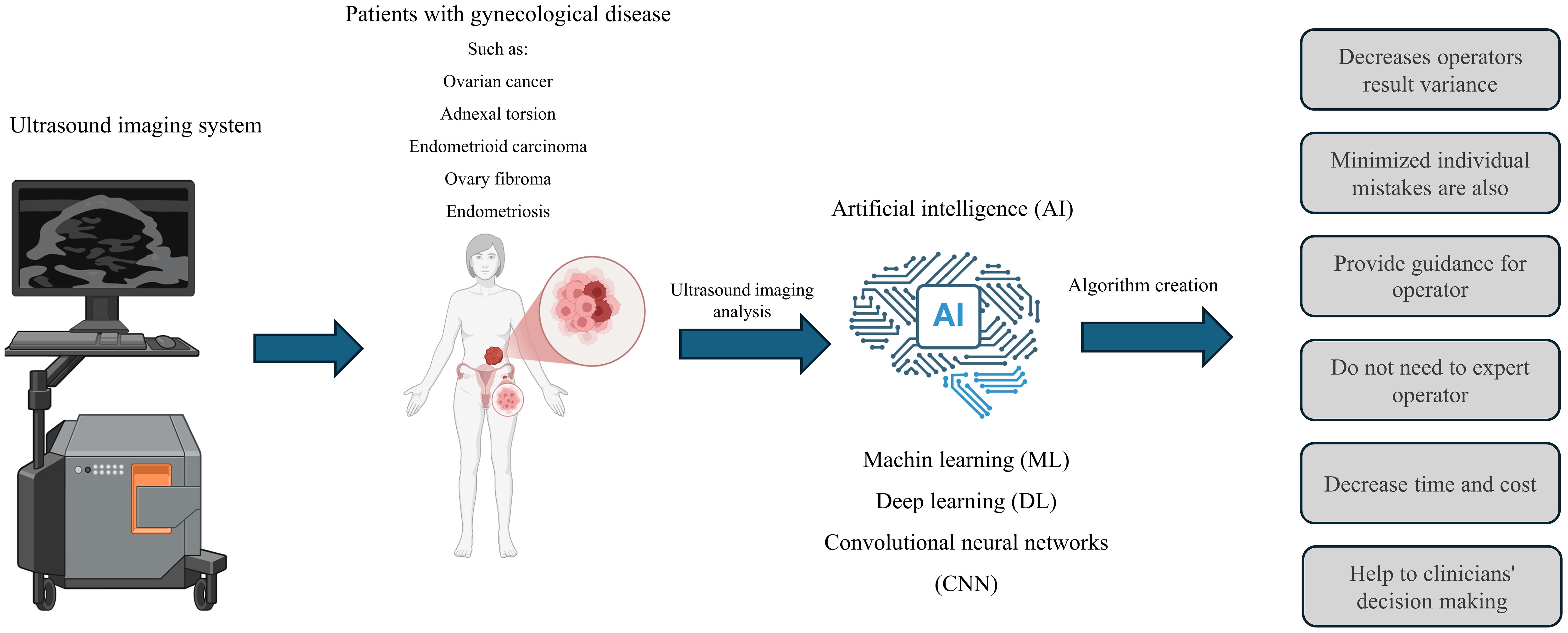
Figure 2. The application of ultrasound and its integration with artificial intelligence in gynecological disease. The use of artificial intelligence can lead to the development of algorithms that assist in diagnosing women’s diseases. This combination can enhance diagnostic accuracy, decrease the reliance on professionals, speed up the diagnostic process, and lower costs. However, artificial intelligence cannot replace experts.
In another study, two common standard methods were employed to examine the participants and their fetal anomalies: one used by a professional operator and the other with the assistance of artificial intelligence. The results show that using Intelligent Fetal Imaging and Diagnosis (iFIND) to separate freehand scanning from image capture and measurement leads to faster scanning and improved workflow. It appears that AI can automate repetitive tasks, potentially leading to increased attention being paid to identifying fetal abnormalities (81). The study conducted by L. Drukker and colleagues also used deep learning algorithms to track the movement of the operator’s eyes and create an algorithm for performing ultrasound examinations. They also used the resulting data to develop an algorithm. The results of this study indicate that the operator’s influence can impact the outcome, and artificial intelligence can help mitigate this variation (82). Another application of artificial intelligence is anomaly highlighting, which can identify and report unusual findings about the fetus during ultrasound scans in standard planes and aid in sonographic referrals (83).
Another artificial intelligence tool that has many users is Cyst classification. The cysts in the ovaries in polycystic ovary syndrome (PCOS) and cancers can affect ovarian functions in fertility as well as women’s health (84). Various factors such as genetics, nutrition, activity level, and smoking can have a significant effect on the onset and spread of this disease (85). Classification of cysts is essential because they require different treatments. One of the primary methods of classifying cysts in PCOS and various types of ovarian cancers is the method described by the International Ovarian Tumor Analysis (IOTA) (86). The diagnosis is based on IOTA ultrasound criteria. According to IOTA, ovarian cysts are divided into benign and malignant categories, depending on their characteristics (87). The ovarian cysts are divided into two categories: complex and simple (88). However, intermediate states have also been defined. In addition, the AROMA study classified ovarian masses into three groups: solid, cystic, and motley, and the diagnostic accuracies were separately compared to other studies that classified ovarian masses into benign and malignant (89).
In a retrospective study using archived data of IOTA-related criteria for the diagnosis of fetal ovarian cysts in 51 patients (90). With the advancement of AI-based methods, giving IOTA-related criteria to artificial intelligence tools can be used for cyst classification. The use of these applications can lead to increasing the decision-making speed of doctors, reducing human errors in diagnosis, and helping to choose the proper treatment (90).
One of the primary diseases that can be evaluated, diagnosed, treated, and followed up by the combination of ultrasound and AI is ovarian and uterine cancers (91). A meta-analysis study published by Sian Mitchell et al. reports that, as of October 2022, 14 articles have utilized AI to analyze data from ultrasound scans, investigating anomalies and ovarian cancer in patients. The statistical analyses of these studies show that AI with high overall sensitivity (81%) and high specificity (92%) can diagnose these cases in patients (89). In a systematic review study published by F. Moro et al. in 2024, they reviewed studies that utilized ultrasound and artificial intelligence in gynecologic oncology. After selecting articles based on existing criteria and using RAYYAN QCRI and QUADAS-AI software, 50 articles were extracted, and their results were reported. A notable point is that most studies were conducted in the field of ovarian cancer (37 out of 50). In most of these studies, machine learning-based methods have been used to detect ovarian masses. Therefore, this study has introduced artificial intelligence in combination with ultrasound as a powerful and efficient tool for diagnosing ovarian tomographic masses (92). Additionally, Francesca Arezzo and colleagues stated that, in addition to the role that AI combined with ultrasound can play in diagnosing tumor masses, it can also predict progression-free survival (PFS) in ovarian cancer patients. For this purpose, researchers retrospectively combined data from ultrasound (2019 and 2018) with machine learning-based modeling to predict 12-month progression-free survival (PFS). The data analysis results show that using the Random Forest algorithm (RFF) with 90% accuracy and 90% recall can accurately determine the 12-month progression-free survival (PFS) of patients with epithelial ovarian cancer (93). Between 2009 and 2023, the research group of Amor and colleagues conducted approximately 11 studies evaluating the performance of artificial intelligence in classifying and analyzing data from ultrasound (Gynecologic Imaging Reporting and Data System (GI-RADS)) in adnexal masses (94).
5 Medical decision-making using ultrasound and artificial intelligence
Artificial intelligence has been a new and transformative tool since its inception. From its primary conditions to today’s advanced states, it has been in medicine and related services (95, 96). One field in which artificial intelligence for making treatment decisions has been tested is diseases related to gynecology and obstetrics (97). As mentioned in the previous section, the use of artificial intelligence tools enhances the speed and accuracy of disease diagnosis. Also, this tool can help in treatment after diagnosis (98). In such a way, by entering information related to medical history, lifestyle, and genetics, it is possible to personalize disease-based decisions in people (99). However, issues such as privacy may be questioned in these approaches, and informed consent must be obtained from patients to use the information and enter it into a database (100). Algorithms related to decision-making in artificial intelligence should also be well-evaluated so as not to make mistakes (77). One of the areas where artificial intelligence can be used in the treatment of GyD is its help in surgeries.
Artificial intelligence can be used in various stages of surgery, including 1) preoperative planning and 2) real-time guidance during procedures (54, 101). One of the primary pieces of information used in artificial intelligence for surgical applications is images obtained through ultrasound. By analyzing these images with artificial intelligence tools, the location of masses and vessels is determined to perform more accurate surgeries and prevent operator errors (102).
In addition, artificial intelligence can be integrated into robotic surgery through advanced algorithmic programming, allowing surgical interventions and treatments to be performed entirely by the robot and transferring all the information from the surgery to the specialist in real-time (103, 104). Therefore, in general, artificial intelligence and machine learning have the potential to significantly improve the field of GyD-related surgeries by reducing risk, increasing accuracy, reducing complications, and improving patient outcomes (77). With the continued development of this technology, in the not-too-distant future, we will witness an increasing number of surgical systems and applications that utilize artificial intelligence in clinical practice, including tumor removal surgeries, the removal of lesions related to endometriosis, and fully automated laparoscopy by a robotic surgeon (105).
6 Limitations and future directions
Artificial Intelligence cannot replace doctors; it can merely assist in clinical practice by supporting doctors’ decisions and preventing other errors. The performance of AI algorithms depends on the availability of high-quality and representative datasets. In oncogynecology, AI holds promise for early diagnosis and improved patient outcomes. Such problems include but are not limited to the following: much data is needed, biased data results in biased AI models, and limited interpretability exists in the AI model, besides the fact that it is hard to handle uncertainty (106). AI raises ethical challenges in the diagnosis of GyD due to concerns over data privacy, potential algorithmic bias, transparency, and liability. Confidentiality and protection of patient data are paramount, as AI models trained on sensitive data may prove vulnerable to data breaches. Algorithmic bias arising from biased training data will lead to biased predictions and exacerbate health inequities (107).
Furthermore, there is an ethical concern about discrimination and the replacement of human doctors by AI systems (108). In other specialized areas, such as gynecology and obstetrics, where data is relatively scarce, the development of highly accurate AI models becomes limited. Any biased data used for training AI models may result in less reliable predictions for specific patient groups (109). AI models are large “black boxes,” and it is challenging even for doctors to comprehend their predictive results, let alone be confident in them (97, 110).
The clinical use of AI presents several advantages, but also a long list of challenges and unknowns. One significant issue will likely be the impact of AI on jobs, rather than the creation of unemployment. Automation, including AI, will enhance efficiency and job satisfaction for professionals, such as sonographers, by automating routine scanning tasks, thereby allowing them to devote more time to patient care (110, 111). Machines offer a consistency that human clinicians may lack for various reasons. Early applications of AI are likely to involve repetitive tasks in imaging, addressing the shortage of imaging experts, and meeting the surging demand for diagnostic imaging. Concerns are also raised regarding the generalization of AI into clinical diagnosis and management, as AI models often depend entirely on imaging data without considering vital clinical context, such as age or familial risk (110).
Other issues are related to the safety of adaptive AI systems, as existing regulations prescribe static models that do not change over time. AI’s “black box” nature is unnerving to clinicians who want to understand possible biases in AI models, as human input can also bias it (112). Moreover, resource-intensive processes for annotating data result in prejudice and need strategies such as using pre-trained models or smaller model sizes for deployability (113, 114). While high expectations for AI exist, convincing clinical studies have yet to be widely reported. The last one deals with professional liability in the light of AI-assisted incorrect diagnoses, which has raised heated and ongoing debates among regulators, lawyers, and clinicians (115, 116).
7 Conclusion and future perspective
In recent years, AI technologies in gynecologic surgery have revolutionized the way surgeries are performed. Artificial intelligence in this field should no longer be viewed as a dream of the future but as a reality shaping the course of planning, conducting, and evaluating gynecologic surgery (114). Robotics, such as the da Vinci Surgical System (117), has been used in increasingly complex procedures, like hysterectomies and myomectomies, with even greater precision and control (118, 119). It also involves preoperative planning and decision-making, utilizing machine learning and predictive analytics to analyze imaging data and develop personalized surgical plans that take into account each patient’s unique anatomy and medical conditions. Imaging and diagnostics utilize AI to enhance the diagnostic accuracy of gynecologic conditions, including ovarian cysts, fibroids, and cancers. With the power of AI, minute information from imaging data that might have escaped the human naked eye can be analyzed, resulting in the early diagnosis of diseases.
AI and human experts each offer unique skills in the field of gynecological illnesses. In particular, AI excels at analyzing large datasets to identify trends and aid in the early detection of disorders like cervical and ovarian cancer. Conversely, humans provide essential elements such as clinical judgment, nuanced interpretation, and patient-centered care. AI-assisted tools can enhance human capabilities, potentially resulting in faster and more accurate diagnoses, improved treatment strategies, and ultimately better patient outcomes.
AI also enhances postoperative care by monitoring recovery, predicting complications, and providing personalized recommendations. Challenges, however, include ethical issues, data privacy concerns, misinterpretation of clinical scenarios, high costs, and the need for specialized training among healthcare professionals. Notwithstanding, AI technologies are shaping gynecological surgery with precision, efficiency, and personalization; hence, they promise to improve surgical outcomes and patient care in gynecology. These ever-evolving AI technologies will further revolutionize this most essential field of medicine. The integration of AI in gynecological surgery shows great potential for innovation and improvement in patient care.
AI technologies, such as machine learning and robotics, can enhance personalized medicine by analyzing patient data in real-time and offering customized surgical plans. AI-driven virtual reality simulations can provide surgeons with realistic training experiences, while AI-enhanced surgical tools promise enhanced precision and flexibility during procedures. However, challenges such as regulating AI integration for patient safety and data privacy must be addressed through collaboration among the medical community, regulators, and ethicists. The future of AI in gynecological surgeries appears promising. Still, it is crucial to strike a balance between technological advancements and ethical considerations, as well as patient-centric care, through ongoing collaboration among gynecologists, AI researchers, and policymakers.
Author contributions
SS: Writing – original draft. CD: Writing – original draft. LD: Writing – original draft. XL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Silverberg SG, Major FJ, Blessing JA, Fetter B, Askin FB, Liao SY, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: a Gynecologic Oncology Group pathologic study of 203 cases. Int J gynecological Pathol. (1990) 9:1–19. doi: 10.1097/00004347-199001000-00001
3. Sweet RL. Gynecologic conditions and bacterial vaginosis: implications for the non-pregnant patient. Infect Dis obstetrics gynecology. (2000) 8:184–90. doi: 10.1002/1098-0997(2000)8:3/4<184::AID-IDOG16>3.0.CO;2-P
4. Zhao Y, Zhao F, and Yan L. Clinical analysis and nursing points of gynecological endocrine disorders. Adv Obstetrics Gynecology Res. (2023) 1:8–13. doi: 10.26689/aogr.v1i3.5745
5. Murphy AR, Campo H, and Kim JJ. Strategies for modelling endometrial diseases. Nat Rev Endocrinol. (2022) 18:727–43. doi: 10.1038/s41574-022-00725-z
6. Bergeron S, Reed BD, Wesselmann U, and Bohm-Starke N. Vulvodynia. Nat Rev Dis Primers. (2020) 6:36. doi: 10.1038/s41572-020-0164-2
7. Vercellini P, Vigano P, Somigliana E, and Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. (2014) 10:261–75. doi: 10.1038/nrendo.2013.255
8. Taylor HS, Kotlyar AM, and Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397:839–52. doi: 10.1016/S0140-6736(21)00389-5
9. Holcakova J, Bartosik M, Anton M, Minar L, Hausnerova J, Bednarikova M, et al. New trends in the detection of gynecological precancerous lesions and early-stage cancers. Cancers. (2021) 13:6339. doi: 10.3390/cancers13246339
10. Derchi LE, Serafini G, Gandolfo N, Gandolfo NG, and Martinoli C. Ultrasound in gynecology. Eur Radiol. (2001) 11:2137–55. doi: 10.1007/s003300101080
11. Mitrofanova PV, Ramazanova KS, Beshkok MB, Goroeva AZ, Sidorenko PO, Khodova ME, et al. Modern methods of diagnosis of gynecological diseases. Cardiometry. (2024) 31:138–44. doi: 10.18137/CM
12. Turkgeldi E, Urman B, and Ata B. Role of three-dimensional ultrasound in gynecology. J Obstetrics Gynecology India. (2015) 65:146–54. doi: 10.1007/s13224-014-0635-z
13. Singla M and Sit N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrasonics Sonochemistry. (2021) 73:105506. doi: 10.1016/j.ultsonch.2021.105506
14. Recker F, Gembruch U, and Strizek B. Clinical ultrasound applications in obstetrics and gynecology in the year 2024. Journal of Clinical Medicine, MDPI (2024). p. 1244.
15. Fischerova D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: a review. Ultrasound obstetrics gynecology. (2011) 38:246–66. doi: 10.1002/uog.10054
16. Izadifar Z, Babyn P, and Chapman D. Mechanical and biological effects of ultrasound: a review of present knowledge. Ultrasound Med Biol. (2017) 43:1085–104. doi: 10.1016/j.ultrasmedbio.2017.01.023
17. Hansmann M, Hackelöer B-J, and Staudach A. Ultrasound diagnosis in obstetrics and gynecology. books.google.com, Springer Science & Business Media (2012).
18. Papp Z and Fekete T. The evolving role of ultrasound in obstetrics/gynecology practice. Int J Gynecology Obstetrics. (2003) 82:339–46. doi: 10.1016/S0020-7292(03)00224-8
19. Hassani SN. Ultrasound in gynecology and obstetrics. books.google.com, Springer Science & Business Media (2012).
20. Moro F, Magoga G, Pasciuto T, Mascilini F, Moruzzi M, Fischerova D, et al. Imaging in gynecological disease (13): clinical and ultrasound characteristics of endometrioid ovarian cancer. Ultrasound Obstetrics Gynecology. (2018) 52:535–43. doi: 10.1002/uog.19026
21. Alcázar JL, Galán MJ, Ceamanos C, and García-Manero M. Transvaginal gray scale and color Doppler sonography in primary ovarian cancer and metastatic tumors to the ovary. J ultrasound Med. (2003) 22:243–7. doi: 10.7863/jum.2003.22.3.243
22. Testa AC, Ferrandina G, Timmerman D, Savelli L, Ludovisi M, Van Holsbeke C, et al. Imaging in gynecological disease (1): ultrasound features of metastases in the ovaries differ depending on the origin of the primary tumor. Ultrasound Obstetrics Gynecology: Off J Int Soc Ultrasound Obstetrics Gynecology. (2007) 29:505–11. doi: 10.1002/uog.4020
23. Testa AC, Mancari R, Di Legge A, Mascilini F, Salutari V, Scambia G, et al. The ‘lead vessel’: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound obstetrics gynecology. (2008) 31:218–21. doi: 10.1002/uog.5251
24. Moro F, Leombroni M, Pasciuto T, Trivellizzi I, Mascilini F, Ciccarone F, et al. Synchronous primary cancers of endometrium and ovary vs endometrial cancer with ovarian metastasis: an observational study. Ultrasound Obstetrics Gynecology. (2019) 53:827–35. doi: 10.1002/uog.20213
25. Moro F, Pasciuto T, Djokovic D, Di Legge A, Granato V, Moruzzi M, et al. Role of CA125/CEA ratio and ultrasound parameters in identifying metastases to the ovaries in patients with multilocular and multilocular-solid ovarian masses. Ultrasound Obstetrics Gynecology. (2019) 53:116–23. doi: 10.1002/uog.19174
26. Huchon C and Fauconnier A. Adnexal torsion: a literature review. Eur J Obstetrics Gynecology Reprod Biol. (2010) 150:8–12. doi: 10.1016/j.ejogrb.2010.02.006
27. Sasaki KJ and Miller CE. Adnexal torsion: review of the literature. J minimally invasive gynecology. (2014) 21:196–202. doi: 10.1016/j.jmig.2013.09.010
28. Hibbard LT. Adnexal torsion. Am J obstetrics gynecology. (1985) 152:456–61. doi: 10.1016/S0002-9378(85)80157-5
29. Moro F, Bolomini G, Sibal M, Vijayaraghavan S, Venkatesh P, Nardelli F, et al. Imaging in gynecological disease (20): clinical and ultrasound characteristics of adnexal torsion. Ultrasound Obstetrics Gynecology. (2020) 56:934–43. doi: 10.1002/uog.21981
30. Bardin R, Perl N, Mashiach R, Ram E, Orbach-Zinger S, Shmueli A, et al. Prediction of adnexal torsion by ultrasound in women with acute abdominal pain. Ultraschall der Medizin-European J Ultrasound. (2020) 41:688–94. doi: 10.1055/a-1014-2593
31. Kupesic S and Plavsic BM. Adnexal torsion: color Doppler and three-dimensional ultrasound. Abdominal Imaging. (2010) 35:602–6. doi: 10.1007/s00261-009-9573-0
32. Garde I, Paredes C, Ventura L, Pascual MA, Ajossa S, Guerriero S, et al. Diagnostic accuracy of ultrasound signs for detecting adnexal torsion: systematic review and meta-analysis. Ultrasound Obstetrics Gynecology. (2023) 61:310–24. doi: 10.1002/uog.24976
33. Murali R, Davidson B, Fadare O, Carlson JA, Crum CP, Gilks CB, et al. High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int J Gynecological Pathol. (2019) 38:S40–63. doi: 10.1097/PGP.0000000000000491
34. Andrade DAP, Silva VD, Matsushita GM, Lima MA, Vieira MA, Andrade CEMC, et al. Squamous differentiation portends poor prognosis in low and intermediate-risk endometrioid endometrial cancer. PloS One. (2019) 14:e0220086. doi: 10.1371/journal.pone.0220086
35. Azueta A, Gatius S, and Matias-Guiu X. Endometrioid carcinoma of the endometrium: pathologic and molecular features. in Seminars in diagnostic pathology. 2010. (2010) Elsevier: Seminars in diagnostic pathology.
36. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. (2000) 355:1404–11. doi: 10.1016/S0140-6736(00)02139-5
37. Lim D, Ip PP, Cheung AN, Kiyokawa T, and Oliva E. Immunohistochemical comparison of ovarian and uterine endometrioid carcinoma, endometrioid carcinoma with clear cell change, and clear cell carcinoma. Am J Surg Pathol. (2015) 39:1061–9. doi: 10.1097/PAS.0000000000000436
38. Kounelis S, Kapranos N, Kouri E, Coppola D, Papadaki H, Jones MW, et al. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Modern Pathol. (2000) 13:379–88. doi: 10.1038/modpathol.3880062
39. Huvila J, Pors J, Thompson EF, and Gilks CB. Endometrial carcinoma: molecular subtypes, precursors and the role of pathology in early diagnosis. J Pathol. (2021) 253:355–65. doi: 10.1002/path.5608
40. Alcázar J, Pineda L, Caparrós M, Utrilla-Layna J, Juez L, Mínguez J, et al. Transvaginal/transrectal ultrasound for preoperative identification of high-risk cases in well-or moderately differentiated endometrioid carcinoma. Ultrasound Obstetrics Gynecology. (2016) 47:374–9. doi: 10.1002/uog.14912
41. Pineda L, Alcázar J, Caparrós M, Mínguez J, Idoate M, Quiceno H, et al. Agreement between preoperative transvaginal ultrasound and intraoperative macroscopic examination for assessing myometrial infiltration in low-risk endometrioid carcinoma. Ultrasound Obstetrics Gynecology. (2016) 47:369–73. doi: 10.1002/uog.14909
42. Cubo-Abert M, Díaz-Feijoo B, Bradbury M, Rodríguez-Mías NL, Vera M, Pérez-Hoyos S, et al. Diagnostic performance of transvaginal ultrasound and magnetic resonance imaging for preoperative evaluation of low-grade endometrioid endometrial carcinoma: prospective comparative study. Ultrasound Obstetrics Gynecology. (2021) 58:469–75. doi: 10.1002/uog.23607
43. Troiano RN, Lazzarini KM, Scoutt LM, Lange RC, Flynn SD, McCarthy S, et al. Fibroma and fibrothecoma of the ovary: MR imaging findings. Radiology. (1997) 204:795–8. doi: 10.1148/radiology.204.3.9280262
44. Cho YJ, Lee HS, Kim JM, Joo KY, and Kim ML. Clinical characteristics and surgical management options for ovarian fibroma/fibrothecoma: a study of 97 cases. Gynecologic Obstetric Invest. (2013) 76:182–7. doi: 10.1159/000354555
45. Bazot M, Ghossain MA, Buy JN, Deligne L, Hugol D, Truc JB, et al. Fibrothecomas of the ovary: CT and US findings. J Comput assisted tomography. (1993) 17:754–9. doi: 10.1097/00004728-199309000-00017
46. Sivanesaratnam V, Dutta R, and Jayalakshmi P. Ovarian fibroma—clinical and histopathological characteristics. Int J Gynecology Obstetrics. (1990) 33:243–7. doi: 10.1016/0020-7292(90)90009-A
47. Paladini D, Testa A, Van Holsbeke C, Mancari R, Timmerman D, Valentin L, et al. Imaging in gynecological disease (5): clinical and ultrasound characteristics in fibroma and fibrothecoma of the ovary. Ultrasound Obstetrics Gynecology: Off J Int Soc Ultrasound Obstetrics Gynecology. (2009) 34:188–95. doi: 10.1002/uog.6394
48. Chokoshvili D, Vears D, and Borry P. Growing complexity of (expanded) carrier screening: Direct-to-consumer, physician-mediated, and clinic-based offers. Best Pract Res Clin Obstetrics Gynaecology. (2017) 44:57–67. doi: 10.1016/j.bpobgyn.2017.02.006
49. Reid S and Condous G. Update on the ultrasound diagnosis of deep pelvic endometriosis. Eur J Obstetrics Gynecology Reprod Biol. (2017) 209:50–4. doi: 10.1016/j.ejogrb.2016.02.040
50. Noventa M, Saccardi C, Litta P, Vitagliano A, D'Antona D, Abdulrahim B, et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta-analysis. Fertility sterility. (2015) 104:366–383. e2. doi: 10.1016/j.fertnstert.2015.05.002
51. Hudelist G, English J, Thomas A, Tinelli A, Singer C, Keckstein J, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound obstetrics gynecology. (2011) 37:257–63. doi: 10.1002/uog.8858
52. Lee Y, Medeiros F, Kindelberger D, Callahan MJ, Muto MG, Crum CP, et al. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv anatomic Pathol. (2006) 13:1–7. doi: 10.1097/01.pap.0000201826.46978.e5
53. Ludovisi M, De Blasis I, Virgilio B, Fischerova D, Franchi D, Pascual M, et al. Imaging in gynecological disease (9): clinical and ultrasound characteristics of tubal cancer. Ultrasound Obstetrics Gynecology. (2014) 43:328–35. doi: 10.1002/uog.12570
54. Hamet P and Tremblay J. Artificial intelligence in medicine. metabolism. (2017) 69:S36–40. doi: 10.1016/j.metabol.2017.01.011
55. Szolovits P. Artificial intelligence and medicine. In: Artificial intelligence in medicine. Routledge (2019). p. 1–19.
56. Gore JC. Artificial intelligence in medical imaging. Magnetic resonance imaging, Elsevier (2020) p. A1–4.
57. Holzinger A, Langs G, Denk H, Zatloukal K, and Müller H. Causability and explainability of artificial intelligence in medicine. Wiley Interdiscip Reviews: Data Min Knowledge Discov. (2019) 9:e1312. doi: 10.1002/widm.1312
58. Rossi JG, Rojas-Perilla N, Krois J, and Schwendicke F. Cost-effectiveness of artificial intelligence as a decision-support system applied to the detection and grading of melanoma, dental caries, and diabetic retinopathy. JAMA Network Open. (2022) 5:e220269–e220269. doi: 10.1001/jamanetworkopen.2022.0269
59. Semerci ZM, Toru HS, Çobankent Aytekin E, Tercanlı H, Chiorean DM, Albayrak Y, et al. The role of artificial intelligence in early diagnosis and molecular classification of head and neck skin cancers: A multidisciplinary approach. Diagnostics. (2024) 14:1477. doi: 10.3390/diagnostics14141477
60. Helm JM, Swiergosz AM, Haeberle HS, Karnuta JM, Schaffer JL, Krebs VE, et al. Machine learning and artificial intelligence: definitions, applications, and future directions. Curr Rev musculoskeletal Med. (2020) 13:69–76. doi: 10.1007/s12178-020-09600-8
61. Jakhar D and Kaur I. Artificial intelligence, machine learning and deep learning: definitions and differences. Clin Exp Dermatol. (2020) 45:131–2. doi: 10.1111/ced.14029
62. Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smaïl-Tabbone M, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. (2020) 158:76–94. e2. doi: 10.1053/j.gastro.2019.08.058
63. Yang YJ and Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. (2019) 25:1666. doi: 10.3748/wjg.v25.i14.1666
64. Mascarenhas M, Afonso J, Andrade P, Cardoso H, and Macedo G. Artificial intelligence and capsule endoscopy: unravelling the future. Ann Gastroenterol. (2021) 34:300. doi: 10.20524/aog.2021.0606
65. Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H, et al. eD octor: machine learning and the future of medicine. J Internal Med. (2018) 284:603–19. doi: 10.1111/joim.12822
66. Rashidi HH, Tran N, Albahra S, and Dang LT. Machine learning in health care and laboratory medicine: General overview of supervised learning and Auto-ML. Int J Lab Hematol. (2021) 43:15–22. doi: 10.1111/ijlh.13537
67. Sidey-Gibbons JA and Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction. BMC Med Res Method. (2019) 19:1–18. doi: 10.1186/s12874-019-0681-4
68. Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, et al. Breast cancer: pathogenesis and treatments. Signal transduction targeted Ther. (2025) 10:49. doi: 10.1038/s41392-024-02108-4
69. Panet F, Papakonstantinou A, Borrell M, Vivancos J, and Vivancos MA. Use of ctDNA in early breast cancer: analytical validity and clinical potential. NPJ Breast Cancer. (2024) 10:50. doi: 10.1038/s41523-024-00653-3
70. AlSamhori JF, AlSamhori ARF, Duncan LA, Qalajo A, Alshahwan HF, Al-abbadi M, et al. Artificial intelligence for breast cancer: Implications for diagnosis and management. J Medicine Surgery Public Health. (2024) 3:100120. doi: 10.1016/j.glmedi.2024.100120
71. Uchikov P, Khalid U, Dedaj-Salad GH, Ghale D, Rajadurai H, Kraeva M, et al. Artificial intelligence in breast cancer diagnosis and treatment: advances in imaging, pathology, and personalized care. Life. (2024) 14:1451. doi: 10.3390/life14111451
72. Dileep G and Gyani SGG. Artificial intelligence in breast cancer screening and diagnosis. Cureus. (2022) 14:1–6. doi: 10.7759/cureus.30318
73. Eisemann N, Bunk S, Mukama T, Baltus H, Elsner SA, Gomille T, et al. Nationwide real-world implementation of AI for cancer detection in population-based mammography screening. Nat Med. (2025) 31:1–8. doi: 10.1038/s41591-024-03408-6
74. Nicolis O, De Los Angeles D, and Taramasco C. A contemporary review of breast cancer risk factors and the role of artificial intelligence. Front Oncol. (2024) 14:1356014. doi: 10.3389/fonc.2024.1356014
75. Dhombres F, Bonnard J, Bailly K, Maurice P, Papageorghiou AT, Jouannic JM, et al. Contributions of artificial intelligence reported in obstetrics and gynecology journals: systematic review. J Med Internet Res. (2022) 24:e35465. doi: 10.2196/35465
76. Gumilar KE and Tan M. The promise and challenges of Artificial Intelligence-Large Language Models (AI-LLMs) in obstetrics and gynecology. Majalah Obstetri Ginekologi (Journal Obstetrics Gynecology Science). (2024) 32:128–35. doi: 10.20473/mog.V32I22024.128-135
77. Vemulavada S, Karthikvatsan S, Babu A, Kadalmani B, Devi TR, Sasipraba T, et al. Role of artificial intelligence in the diagnosis and therapy of gynecological disorders: opportunities and challenges. Trans Res Biomed Sciences: Recent Prog Future Prospects. (2024) p:121–44.
78. Ferraz S, Coimbra M, and Pedrosa J. Assisted probe guidance in cardiac ultrasound: A review. Front Cardiovasc Med. (2023) 10:1056055. doi: 10.3389/fcvm.2023.1056055
79. Krull A, Hirsch P, Rother C, Schiffrin A, and Krull C. Artificial-intelligence-driven scanning probe microscopy. Commun Phys. (2020) 3:54. doi: 10.1038/s42005-020-0317-3
80. Droste R, Drukker L, Papageorghiou AT, and Noble JA. (2020). Automatic probe movement guidance for freehand obstetric ultrasound, in: Medical Image Computing and Computer Assisted Intervention–MICCAI 2020: 23rd International Conference, Lima, Peru, October 4–8, 2020, Proceedings, Part III 23, . Springer.
81. Matthew J, Skelton E, Day TG, Zimmer VA, Gomez A, Wheeler G, et al. Exploring a new paradigm for the fetal anomaly ultrasound scan: Artificial intelligence in real time. Prenatal diagnosis. (2022) 42:49–59. doi: 10.1002/pd.6059
82. Drukker L, Droste R, Noble A, and Papageorghiou A. VP40. 20: Standard biometric planes: what are the salient anatomical landmarks? Ultrasound Obstetrics Gynecology. (2020) 56:235–5. doi: 10.1002/uog.22958
83. Ryou H, Yaqub M, Cavallaro A, Roseman F, and Papageorghiou AJA. (2016). Automated 3D ultrasound biometry planes extraction for first trimester fetal assessment, in: Machine Learning in Medical Imaging: 7th International Workshop, MLMI 2016, Held in Conjunction with MICCAI 2016, . Athens, Greece: Springer.
84. Escobar Morreale HF. Polycystic ovary syndrome: treatment strategies and management. Expert Opin Pharmacotherapy. (2008) 9:2995–3008. doi: 10.1517/14656560802559932
85. Artini PG, Di Berardino O, Simi G, Papini F, Ruggiero M, Monteleone P, et al. Best methods for identification and treatment of PCOS. Minerva ginecologica. (2010) 62:33.
86. Kaijser J, Bourne T, Valentin L, Sayasneh A, Van Holsbeke C, Vergote I, et al. Improving strategies for diagnosing ovarian cancer: a summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound obstetrics gynecology. (2013) 41:9–20. doi: 10.1002/uog.12323
87. Garg S, Kaur A, Mohi JK, Sibia PK, and Kaur N. Evaluation of IOTA simple ultrasound rules to distinguish benign and Malignant ovarian tumours. J Clin Diagn Research: JCDR. (2017) 11:TC06. doi: 10.7860/JCDR/2017/26790.10353
88. Patel-Lippmann KK, Sadowski EA, Robbins JB, Paroder V, Barroilhet L, Maddox E, et al. Comparison of international ovarian tumor analysis simple rules to society of radiologists in ultrasound guidelines for detection of Malignancy in adnexal cysts. Am J Roentgenology. (2020) 214:694–700. doi: 10.2214/AJR.18.20630
89. Mitchell S, Nikolopoulos M, El-Zarka A, Al-Karawi D, Al-Zaidi S, Ghai A, et al. Artificial intelligence in ultrasound diagnoses of ovarian cancer: A systematic review and meta-analysis. Cancers. (2024) 16:422. doi: 10.3390/cancers16020422
90. Romiti A, Moro F, Ricci L, Codeca C, Pozzati F, Viggiano M, et al. Using IOTA terminology to evaluate fetal ovarian cysts: analysis of 51 cysts over 10-year period. Ultrasound Obstetrics Gynecology. (2023) 61:408–14. doi: 10.1002/uog.26061
91. Shrestha P, Poudyal B, Yadollahi S, Wright DE, Gregory AV, Warner JD, et al. A systematic review on the use of artificial intelligence in gynecologic imaging–background, state of the art, and future directions. Gynecologic Oncol. (2022) 166:596–605. doi: 10.1016/j.ygyno.2022.07.024
92. Moro F, Ciancia M, Zace D, Vagni M, Tran HE, Giudice MT, et al. Role of artificial intelligence applied to ultrasound in gynecology oncology: A systematic review. Int J Cancer. (2024) 155:1832–45. doi: 10.1002/ijc.35092
93. Arezzo F, Cormio G, La Forgia D, Santarsiero CM, Mongelli M, Lombardi C, et al. A machine learning approach applied to gynecological ultrasound to predict progression-free survival in ovarian cancer patients. Arch Gynecology Obstetrics. (2022) 306:2143–54. doi: 10.1007/s00404-022-06578-1
94. Amor F, Vaccaro H, Alcázar JL, León M, Craig JM, Martinez J, et al. Gynecologic imaging reporting and data system: a new proposal for classifying adnexal masses on the basis of sonographic findings. J Ultrasound Med. (2009) 28:285–91. doi: 10.7863/jum.2009.28.3.285
95. Giordano C, Brennan M, Mohamed B, Rashidi P, Modave F, Tighe P, et al. Accessing artificial intelligence for clinical decision-making. Front digital Health. (2021) 3:645232. doi: 10.3389/fdgth.2021.645232
96. Garcia-Vidal C, Sanjuan G, Puerta-Alcalde P, Moreno-García E, and Soriano A. Artificial intelligence to support clinical decision-making processes. EBioMedicine. (2019) 46:27–9. doi: 10.1016/j.ebiom.2019.07.019
97. Delanerolle G, Yang X, Shetty S, Raymont V, Shetty A, Phiri P, et al. Artificial intelligence: a rapid case for advancement in the personalization of gynaecology/obstetric and mental health care. Women’s Health. (2021) 17:17455065211018111. doi: 10.1177/17455065211018111
98. Emin EI, Emin E, Papalois A, Willmott F, Clarke S, Sideris M, et al. Artificial intelligence in obstetrics and gynaecology: is this the way forward? Vivo. (2019) 33:1547–51. doi: 10.21873/invivo.11635
99. Desai GS. Artificial intelligence: the future of obstetrics and gynecology. J Obstetrics Gynecology India. (2018) 68:326–7. doi: 10.1007/s13224-018-1118-4
100. Loftus TJ, Tighe PJ, Filiberto AC, Efron PA, Brakenridge SC, Mohr AM, et al. Artificial intelligence and surgical decision-making. JAMA Surg. (2020) 155:148–58. doi: 10.1001/jamasurg.2019.4917
101. Patel VL, Shortliffe EH, Stefanelli M, Szolovits P, Berthold MR, Bellazzi R, et al. The coming of age of artificial intelligence in medicine. Artif Intell Med. (2009) 46:5–17. doi: 10.1016/j.artmed.2008.07.017
102. Sadeghi-Goughari M, Rajabzadeh H, Han JW, and Kwon HJ. Artificial intelligence-assisted ultrasound-guided focused ultrasound therapy: a feasibility study. Int J Hyperthermia. (2023) 40:2260127. doi: 10.1080/02656736.2023.2260127
103. Iftikhar M, Saqib M, Zareen M, and Mumtaz H. Artificial intelligence: revolutionizing robotic surgery. Ann Med Surg. (2024) 86:5401–9. doi: 10.1097/MS9.0000000000002426
104. Pavone M, Seeliger B, Teodorico E, Goglia M, Taliento C, Bizzarri N, et al. Ultrasound-guided robotic surgical procedures: a systematic review. Surg Endoscopy. (2024) 38:2359–70. doi: 10.1007/s00464-024-10772-4
105. Yazdani A, Costa S, and Kroon B. Artificial intelligence: Friend or foe. Australian and New Zealand: Wiley Online Library (2023) p. 127–30.
106. Shailieva SL, Mamchueva DK, Vishnevskaya A, Dzhalaeva KS, Ramazanova E, Kokaeva Y, et al. An opportunity for using artificial intelligence in modern gynecology. Obstetrics Gynecology Reprod. (2024). doi: 10.17749/2313-7347/ob.gyn.rep.2024.511
107. Ratwani RM, Sutton K, and Galarraga JE. Addressing AI algorithmic bias in health care. JAMA. (2024) 332:1051–2. doi: 10.1001/jama.2024.13486
108. Prabha C. Role of artificial intelligence in gynecology and obstetrics. In: Artificial intelligence and machine learning for women’s health issues. Elsevier (2024). p. 1–15.
109. Parikh RB, Teeple S, and Navathe AS. Addressing bias in artificial intelligence in health care. Jama. (2019) 322:2377–8. doi: 10.1001/jama.2019.18058
110. Drukker L, Noble J, and Papageorghiou A. Introduction to artificial intelligence in ultrasound imaging in obstetrics and gynecology. Ultrasound Obstetrics Gynecology. (2020) 56:498–505. doi: 10.1002/uog.22122
111. Pfob A, Griewing S, Seitz K, Hillen C, Becker S, Bayer C, et al. Current landscape of hospital information systems in gynecology and obstetrics in Germany: a survey of the commission Digital Medicine of the German Society for Gynecology and Obstetrics. Arch Gynecology Obstetrics. (2023) 308:1823–30. doi: 10.1007/s00404-023-07223-1
112. Fischer A, Rietveld A, Teunissen P, Hoogendoorn M, and Bakker P. What is the future of artificial intelligence in obstetrics? A qualitative study among healthcare professionals. BMJ Open. (2023) 13:e076017. doi: 10.1136/bmjopen-2023-076017
113. Eoh KJ, Kwon GY, Lee EJ, Lee J, Lee I, Kim YT, et al. Efficacy of large language models and their potential in Obstetrics and Gynecology education. Korean J Obstetrics Gynecology. (2024). doi: 10.5468/ogs.24211
114. Sinha R, Raina R, Bag M, and Rupa B. Empowering gynaecologists with Artificial Intelligence: Tailoring surgical solutions for fibroids. Eur J Obstetrics Gynecology Reprod Biol. (2024) 299:72–7. doi: 10.1016/j.ejogrb.2024.06.001
115. Idowu IO, Fergus P, Hussain A, Dobbins C, Khalaf M, Eslava RVC, et al. (2015). Artificial intelligence for detecting preterm uterine activity in gynecology and obstetric care, in: 2015 IEEE international conference on computer and information technology; ubiquitous computing and communications; dependable, autonomic and secure computing; pervasive intelligence and computing, . IEEE.
116. Lee Y and Kim SY. Potential applications of ChatGPT in obstetrics and gynecology in Korea: a review article. Obstetrics Gynecology Sci. (2024) 67:153–9. doi: 10.5468/ogs.23231
117. Surgical I and da Vinci. Surgical system (2013). Available online at: http://www.intusurg.com/html/davinci.html (Accessed May 25, 2013).
118. Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg. (2010) 252:254–62. doi: 10.1097/SLA.0b013e3181e6239e
Keywords: gynecological disease, ultrasound, artificial intelligence, diagnosis, treatment
Citation: Shi S, Dai C, Liu D and Liu X (2025) Application of ultrasound in combination with other methods in gynecological disease: artificial intelligence, surgery, and drugs. Front. Oncol. 15:1567024. doi: 10.3389/fonc.2025.1567024
Received: 26 January 2025; Accepted: 04 August 2025;
Published: 01 September 2025.
Edited by:
Andrea Tinelli, Veris delli Ponti Hospital, ItalyReviewed by:
Somya Srivastava, ABES Engineering College, IndiaMarcel Vasconcellos, Federal University of Rio de Janeiro, Brazil
Copyright © 2025 Shi, Dai, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingjie Liu, eGluZ2ppZWxpdTg5MkBnbWFpbC5jb20=
 Shaohua Shi1
Shaohua Shi1 Xingjie Liu
Xingjie Liu