- 1Department of Pharmacy, National Cancer Center Hospital East, Kashiwa, Japan
- 2Department of Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 3Department of Experimental Therapeutics, National Cancer Center Hospital East, Kashiwa, Japan
- 4Department of General Internal Medicine, National Cancer Center Hospital East, Kashiwa, Japan
- 5Department of Thoracic Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 6Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 7Department of Diagnostic Radiology, National Cancer Center Hospital East, Kashiwa, Japan
Background: Drug-induced interstitial lung disease (DIILD) is a serious complication of cancer treatment that is primarily treated with corticosteroids. However, effective standardized regimens for corticosteroid-refractory DIILD have not been established. Cyclophosphamide (CPA) is an immunosuppressant that is potentially effective against DIILD, but supporting evidence is limited, particularly for diseases induced by novel chemotherapeutic drugs. In this study, we examined the efficacy and safety of CPA in corticosteroid-refractory DIILD caused by various anticancer drugs.
Methods: We retrospectively reviewed the medical records of patients who underwent CPA therapy for corticosteroid-refractory DIILD at the National Cancer Center Hospital East between January 2013 and October 2023. Corticosteroid-refractory DIILD was defined as cases of DIILD classified as grade ≥3 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, in which no improvement was observed within 48 hours after initiating corticosteroid therapy. The primary endpoint was 30-day survival post-CPA. The secondary endpoints included radiological improvements and changes in oxygen supplementation.
Results: Fifteen patients (median age 73 years; 80% male) were included in the analysis. Patients were classified into molecular-targeted drugs (MT; 20%, 3/15), MT + cytotoxic drugs (33%, 5/15), immune checkpoint inhibitors (ICI) ± cytotoxic drugs (27%, 4/15), and cytotoxic drugs alone (20%, 3/15) groups. The overall 30-day survival rate was 47% (7/15). Improvement of oxygen demand allowed 20% (3/15) of patients to discontinue oxygen supplementation. CPA demonstrated drug class-dependent efficacy: highest in the MT group (67% survival, 2/3), less benefit in the cytotoxic drugs alone group (0% survival, 0/3). Adverse events included grade 3 anemia (n=2), grade 4 neutropenia (n=1), and grade 2 cytomegalovirus infection (n=1), with no treatment-related deaths.
Conclusion: CPA exhibited potential efficacy for corticosteroid-refractory DIILD, particularly in patients with MT-induced DIILD, with manageable toxicity. The differential responses based on drug category suggest tailored approaches to DIILD management may be warranted. These findings may contribute to optimizing the management of severe DIILD during cancer treatment.
1 Introduction
Interstitial lung diseases (ILDs) are a heterogeneous group of disorders that affect pulmonary parenchyma and exhibit various clinical, radiological, and pathological features. Drug-induced interstitial lung disease (DIILD) is an ILD subtype that occurs as a result of exposure to certain drugs. Anticancer drugs are a major cause of DIILDs (23–51% of cases), followed by antirheumatic agents and antibiotics (1). DIILD is one of the most serious complications of anticancer treatment (2) and is associated with treatment-related deaths (3). The pathogenic mechanisms underlying DIILD remain incompletely defined; however, its development is primarily attributed to two principal mechanisms: direct cytotoxic injury to lung tissue and dysregulated immune-mediated damage (4). These processes can result in inflammation, fibrosis, and pulmonary scarring, particularly in severe cases.
Historically, a variety of cytotoxic agents, including bleomycin, gemcitabine, and irinotecan, have been reported to be the major causes of DIILD, with high incident rates ranging from 10% to 20% (5). However, with the development of molecular-targeted therapies (MT) and immunotherapies, the number of causative anticancer agents has increased. Notably, the HER2-targeted antibody-drug conjugate, trastuzumab deruxtecan (T-DXd) as well as immune checkpoint inhibitors (ICI) are effective against a variety of cancer types and are commonly used in clinical practice (6, 7); however, a high incidence of ILD and related deaths has been observed (8, 9). Other drugs, such as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors for lung cancer and cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors for breast cancer demonstrate high efficacy (10, 11), but they are also associated with DIILD (12, 13). This highlights the ongoing issue of high DIILD incidence and mortality rates associated with novel agents, which underscores the need for more effective management strategies for DIILD.
The management of DIILD involves the discontinuation of causative drug therapy and the initiation of immunosuppressive agents, depending on the severity of the symptoms (14). Although evidence supporting the benefit of corticosteroids is largely observational (15), their use for DIILD is widely accepted. However, some patients exhibit resistance to corticosteroid therapy. When no clinical improvement is observed after administering high-dose corticosteroids (prednisone 1–2 mg/kg/day) within 48 hours, the condition is defined as corticosteroid-refractory DIILD (14, 16). For patients with corticosteroid-refractory DIILD, alternative immunosuppressive agents, such as azathioprine, cyclophosphamide (CPA), mycophenolate mofetil, and infliximab, should be considered. All of these agents have been evaluated in studies in the context of corticosteroid-refractory, nonidiopathic pulmonary fibrosis (17). In particular, CPA, which acts as an immunosuppressant by alkylating DNA and inhibiting the growth of fast-dividing immune cells, such as T- and B-lymphocytes (18), improves pulmonary function in patients with scleroderma-related ILD or interstitial pneumonia with autoimmune features (IPAF) (17, 19). Given that the pathogenesis of DIILD likely involves immune-mediated mechanisms, the anti-inflammatory and antifibrotic properties of CPA may be beneficial in controlling disease progression. However, clinical evidence regarding the use of CPA specifically in corticosteroid-refractory DIILD remains limited (20).
Corticosteroid-refractory DIILD is associated with a particularly poor prognosis (21), evidence-based treatment strategies for this disease are urgently needed. Therefore, we conducted a retrospective analysis to explore the efficacy and safety of CPA for corticosteroid-refractory DIILD in a clinical setting.
2 Materials and methods
2.1 Patients and data collection
This retrospective cohort study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (22, Supplementary Table 1). The medical records of patients treated with CPA for corticosteroid-refractory DIILD at the National Cancer Center Hospital East (NCCHE). Patients were eligible for inclusion if: 1) treated at the NCCHE between January 2013 and October 2023; 2) DIILD diagnosis via multidisciplinary assessment (clinical/radiologica30l); 3) had corticosteroid-refractory DIILD, defined as cases classified as grade ≥3 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 that showed no clinical or radiological improvement within 48 hours after initiating high-dose corticosteroid therapy (prednisone ≥1 mg/kg/day) (14, 16); 4) received CPA for corticosteroid-refractory DIILD; and 5) sufficient data available. Patients were excluded if: 1) concurrently enrolled in an interventional clinical trial at the time of CPA administration, or 2) a confirmed active infection as the primary cause of the respiratory condition. Patient demographics and disease characteristics were collected at the time of CPA administration. These included age, gender, primary tumor, performance status (Eastern Cooperative Oncology Group, ECOG), smoking index, radiation history, and the number of prior treatments. The causative drugs, imaging patterns of DIILD, and oxygen supplementation amount were also collected. The approval for the study and waiver for patient informed consent were received from the Institutional Review Board (IRB) of the National Cancer Center (IRB number 2023-308), in accordance with the principles stated in Japan’s Ethics Guidelines for Epidemiological Research.
2.2 Assessment
2.2.1 Severity of the DIILD
DIILD was diagnosed based on laboratory and imaging findings through a multidisciplinary discussion involving the attending physician, radiologist, and pulmonologist. To classify grade ≥3 DIILD according to CTCAE more objectively, severity in this study was categorized based on the levels of respiratory support required, reflecting the degree of hypoxemia and respiratory compromise. Five severity groups were established: I) no oxygen supplementation; II) nasal cannula oxygenation; III) oxymask (OM) or Oxymizer®; IV) reservoir mask (RM); or V) high flow nasal cannula (HFNC) or noninvasive positive pressure ventilation (NIPPV; including two-stage or continuous positive pressure). This classification system provides an objective, clinically relevant assessment of DIILD severity that correlates with increasing impairment of gas exchange and allows for standardized comparison across patients.
2.2.2 Imaging pattern of the DIILD
The imaging patterns of DIILD were categorized as follows: i) acute respiratory distress syndrome (ARDS)/diffuse alveolar damage (DAD); ii) nonspecific interstitial pneumonia (NSIP); iii) organizing pneumonia (OP); iv) acute eosinophilic pneumonia; and v) hypersensitivity pneumonitis (HP) revised by radiologist (T.S) according to previous reports (23–25).
2.2.3 Efficacy of cyclophosphamide on DIILD
The primary endpoint was survival rate after 30 days of CPA administration. The secondary endpoint was improvement detected by clinical and imaging response. Clinical response was defined by alterations in oxygen supplementation and was evaluated pre- and 30 days post-CPA administration. Overall survival following CPA administration was estimated using the Kaplan-Meier method. The efficacy of CPA based on imaging response was evaluated by radiologist (T.S.) who was blinded to the patients’ clinical outcomes. The assessment was performed by comparing baseline and follow-up imaging patterns to determine radiological improvement or progression. Baseline imaging was defined as the imaging patterns obtained at the time of cancer diagnosis. Based on the causative drugs, the patients were categorized into MT, MT + cytotoxic, ICI ± cytotoxic, and cytotoxic drugs alone groups. Statistical analyses were not performed because of the small sample size and retrospective nature of the study. Instead, descriptive analyses were conducted to provide an overview of the data.
2.2.4 Adverse event assessment
Adverse events related to CPA administration were evaluated and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
3 Results
3.1 Patients
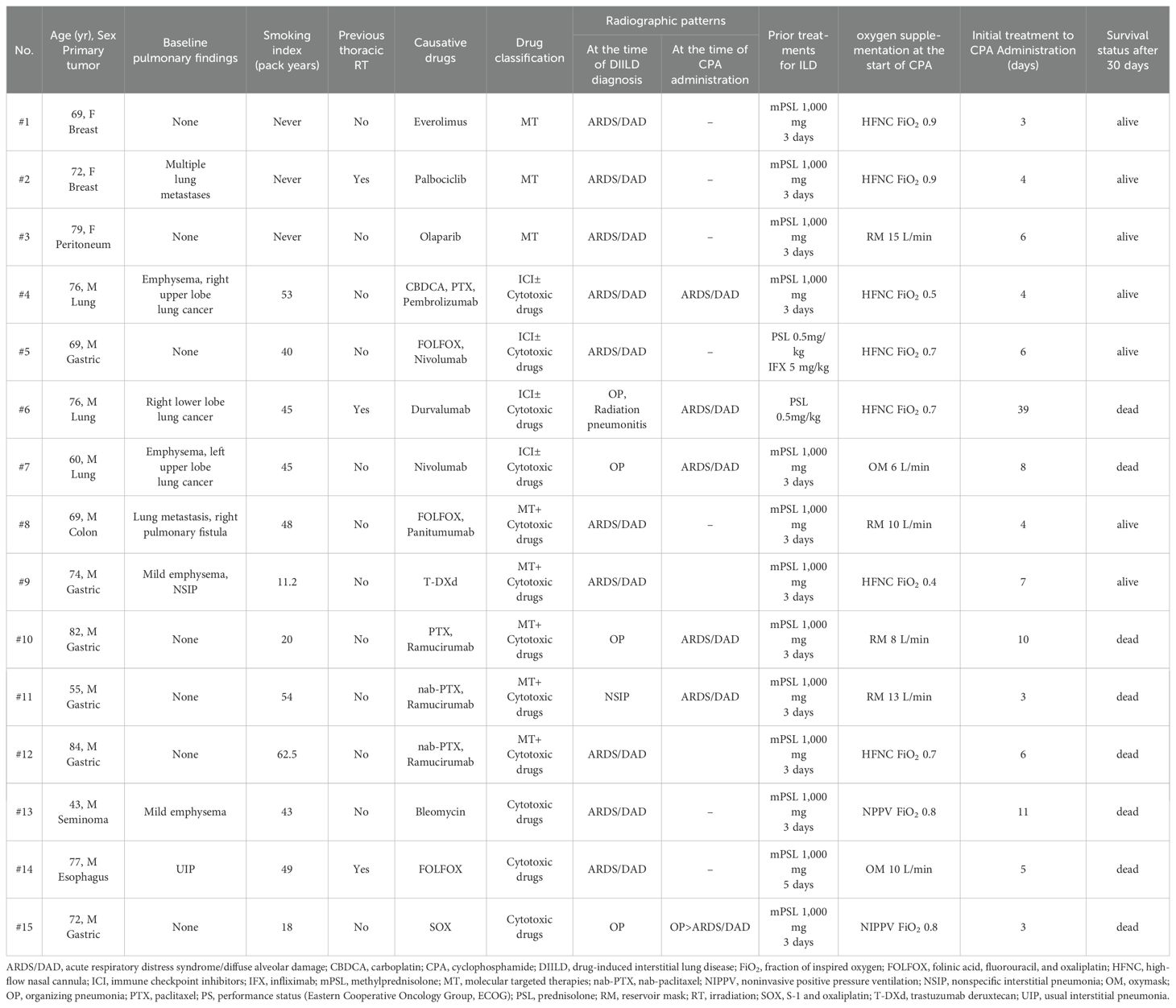
We identified 15 patients with corticosteroid-refractory DIILD who underwent treatment with CPA (Table 1, Supplementary Figure 1). The median age at diagnosis was 73 years (range: 43–84 years), with males comprising 80% (12 patients) of the cohort. Among the patients, 80% (12 patients) were current or former smokers, with a mean smoking index (packs/year) of 40.7 (range: 11.25–62.5). The primary cancer types were gastric (40%, 6 patients), lung (20%, 3 patients), breast (13%, 2 patients), and others (27%, 4 patients). In addition, 20% (3 patients) of the patients had a history of chest radiotherapy.
At the initial diagnosis of DIILD, radiographic findings predominantly showed ARDS/DAD patterns (67%, 10 patients), followed by OP (27%, 4 patients) and NSIP patterns (7%, 1 patient).
Based on the DIILD-causative drugs, the patients were classified into the following four groups: MT (20%, 3 patients), MT + cytotoxic drugs (33%, 5 patients), ICI ± cytotoxic drugs (27%, 4 patients), and cytotoxic drugs alone (20%, 3 patients). DIILD occurred between 38 and 548 days (median: 83 days) after initial drug administration. All patients received high-dose corticosteroids (methylprednisolone 1,000 mg for 3–5 days) before CPA, and one patient also received infliximab (5 mg/kg). The patients did not show improvement within 48 hours after initiating corticosteroid therapy. The interval between the initial DIILD treatment and CPA administration ranged from 3 to 39 days (median: 6 days). CPA was administered as a single dose in all patients, with the majority receiving 500 mg/body and two receiving 500 mg/m², determined based on institutional practice and clinical judgment for these severe, refractory cases, considering patient condition and potential hematologic toxicity.
3.2 Clinical outcomes of CPA therapy for DIILD
Figure 1 shows the dynamics of oxygen supplementation, treatment intervention, and the final survival outcomes following CPA administration to each patient.
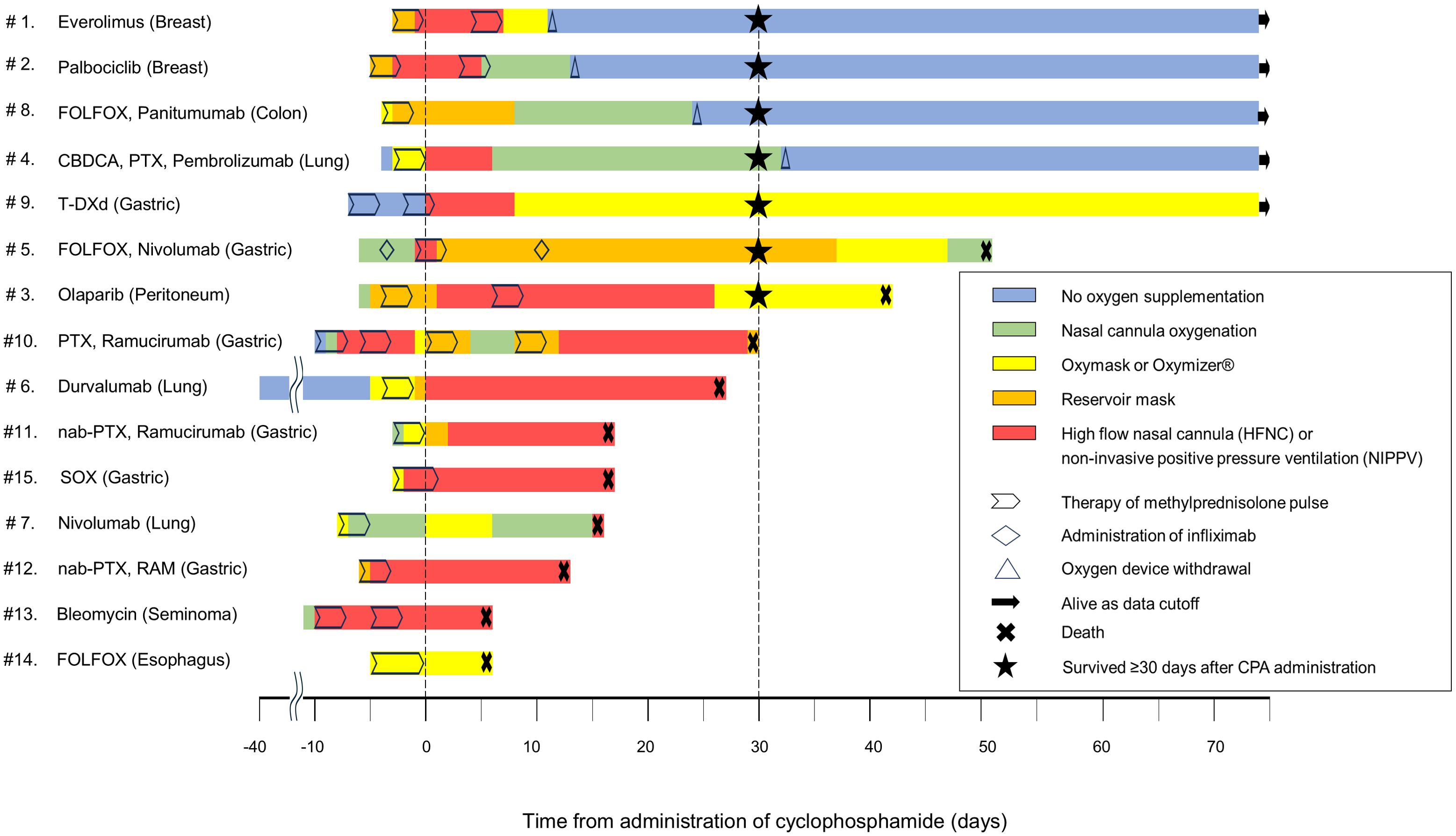
Figure 1. Swimmer plot showing changes in clinical outcomes and oxygen requirements in patients following CPA administration for DIILD. The swimmer plot demonstrates the clinical course of each patient following CPA administration, including the type and duration of oxygen supplementation, survival outcome, and key therapeutic interventions. The day of CPA administration was defined as day 0. Negative time points denote events before CPA administration, whereas the events following the administration are marked as positive. CBDCA, carboplatin; CPA, cyclophosphamide; DIILD, drug-induced interstitial lung disease; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; HFNC, high-flow nasal cannula; nab-PTX, nab-paclitaxel; NIPPV, noninvasive positive pressure ventilation; PTX, paclitaxel; SOX, S-1 and oxaliplatin; T-DXd, trastuzumab deruxtecan.
3.2.1 Survival status after 30 days
The 30-day survival rate post-CPA administration was 47% (7 patients). DILD was the cause of all the patient deaths within 30 days post-CPA administration. Among the seven surviving patients, five were discharged from the hospital, whereas two remained hospitalized and subsequently died on days 41 and 50 after CPA administration. Supplementary Figure 2 shows the overall survival curve for the cohort.
3.2.2 Oxygen supplementation
At the time of CPA administration, all patients required oxygen support to varying degrees. The highest oxygen supplementation during the period from the initiation of corticosteroid therapy to the start of CPA administration was as follows: OM or Oxymizer® 13% (2 patients), RM 20% (3 patients), and HFNC or NIPPV 67% (10 patients). Subsequently, 20% (3 patients) of the patients no longer required any oxygen supplementation, whereas 27% (4 patients) continued to require some form of oxygen therapy, showing improvement. However, the remaining 53% (8 patients) did not experience any benefit (Figure 2A).
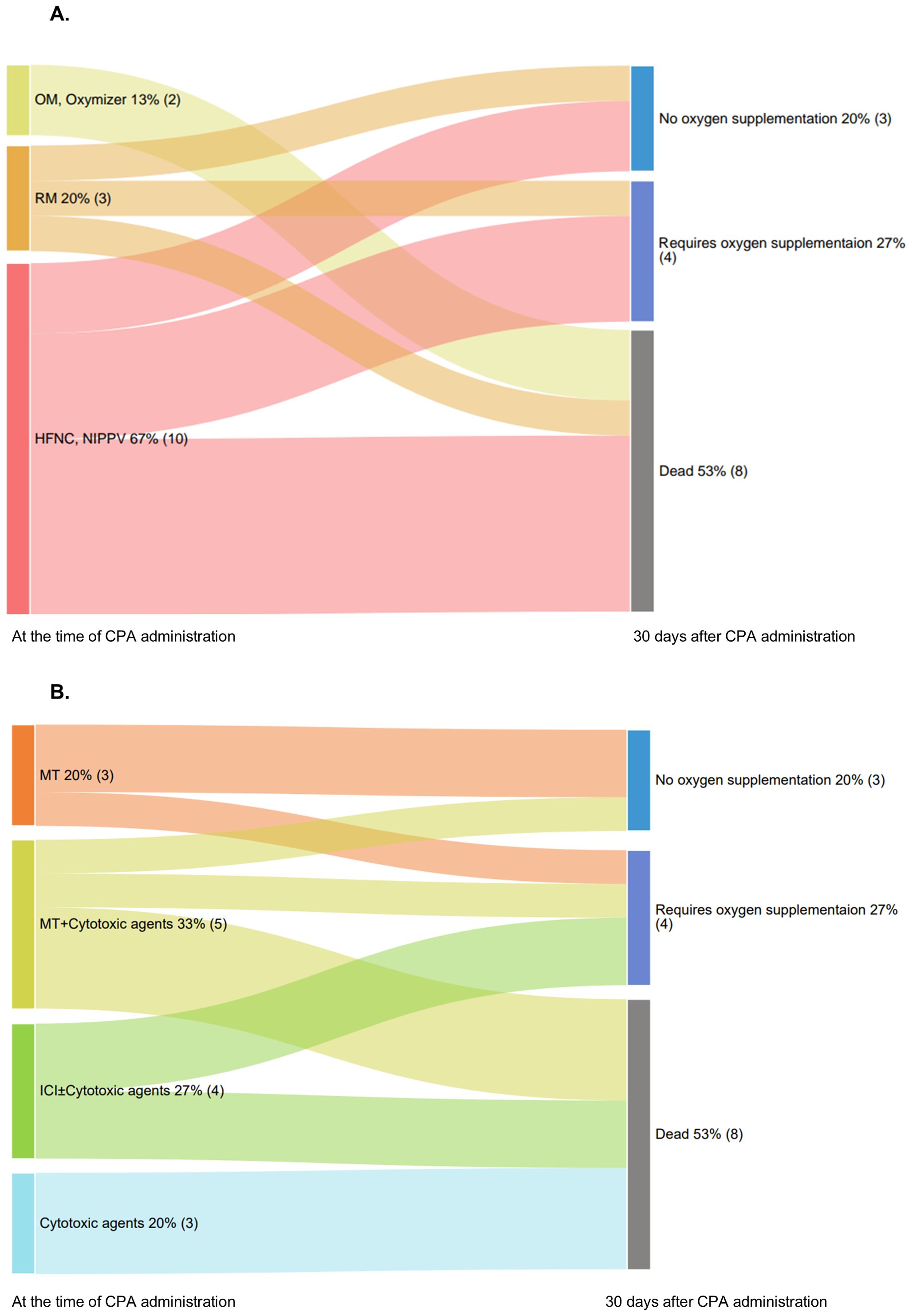
Figure 2. Oxygen supplementation status after CPA administration. (A) Sankey diagram illustrating the transition in oxygen supplementation status of patients at the time of CPA administration and 30 days later. (B) Sankey diagram illustrating patient outcomes 30 days after CPA administration and categorized by causative drugs. CPA, cyclophosphamide; HFNC, high-flow nasal cannula; ICI, immune checkpoint inhibitors; NIPPV, noninvasive positive pressure ventilation; OM, oxymask; RM, reservoir mask.
The outcomes at 30 days following CPA administration, categorized by the causative drugs, are shown in Figure 2B. In the MT group, 67% (2/3) no longer required oxygen supplementation, whereas 33% (1/3) still required it. In the MT + cytotoxic drugs group, 20% (1/5) experienced improvement without oxygen supplementation, whereas 80% (4/5) had different outcomes: one continued oxygen supplementation, and four died. In the ICI ± cytotoxic drugs group, 50% (2/4) of the patients continued requiring oxygen supplementation, whereas the other 50% (2/4) died. In the cytotoxic drugs alone group, all three patients died (Figure 2B).
3.2.3 Imaging pattern
Figure 3 shows a detailed comparison of sequential CT scans of patients, who developed DIILD, at four distinct time points: baseline, initial diagnosis of DIILD, post-corticosteroid treatment, and post-CPA administration. The CT images show the radiologic patterns, treatment response, and 30-day survival status of four patients with various cancer types treated with different causative agents.
● Case No. 2: A 72-year-old female patient with breast cancer treated with palbociclib in combination with endocrine therapy. At the time of DIILD diagnosis, the imaging findings revealed diffuse bilateral ground-glass opacities (GGO) consistent with ARDS/DAD pattern. Despite corticosteroid therapy, her oxygen demand increased from 10 L/min via RM to HFNC with an FiO2 of 90%. After CPA treatment, the GGO markedly decreased, and oxygen supplementation was discontinued within 13 days. The patient survived >30 days post-CPA administration and remained alive at the cutoff date.
● Case No. 5: A 69-year-old male patient with gastric cancer treated with FOLFOX and nivolumab. At DIILD diagnosis, imaging findings were consistent with ARDS/DAD pattern, showing diffuse bilateral GGO and patchy consolidation predominantly in lower lobes. Despite treatment with infliximab and corticosteroids, oxygen demand rose from 2 L/min via nasal cannula to HFNC with an FiO2 of 60%. After CPA administration, despite improvements in GGO but persistent consolidation and fibrotic changes, oxygen supplementation was maintained at 7 L/min via RM for 30 days following CPA administration. The patient survived 30 days, but ultimately died from DIILD after 50 days of CPA administration.
● Case No. 9: A 74-year-old male patient with gastric cancer treated with T-DXd with NSIP pattern at baseline. At the time of DIILD diagnosis, imaging showed ARDS/DAD pattern with diffuse bilateral GGO. Despite corticosteroid therapy, his oxygen needs increased to HFNC with an FiO2 of 40%. After CPA administration, oxygen supplementation was maintained at 5 L/min via Oxymizer®, with partial radiographic improvement but complications from cytomegalovirus infection and pneumothorax. The patient survived post-CPA administration, but died from pneumothorax 279 days later.
● Case No. 12: A 55-year-old male patient with gastric cancer received nab-paclitaxel and ramucirumab. At the time of DIILD diagnosis, imaging showed ARDS/DAD pattern with diffuse bilateral GGO. Despite corticosteroid therapy, the oxygen needs became as high as 10 L/min via RM to HFNC with a FiO2 of 70%. CPA administration resulted in minimal radiographic improvements and oxygen requirement was not improved. The patient unfortunately died 12 days after the CPA administration because of interstitial pneumonia.
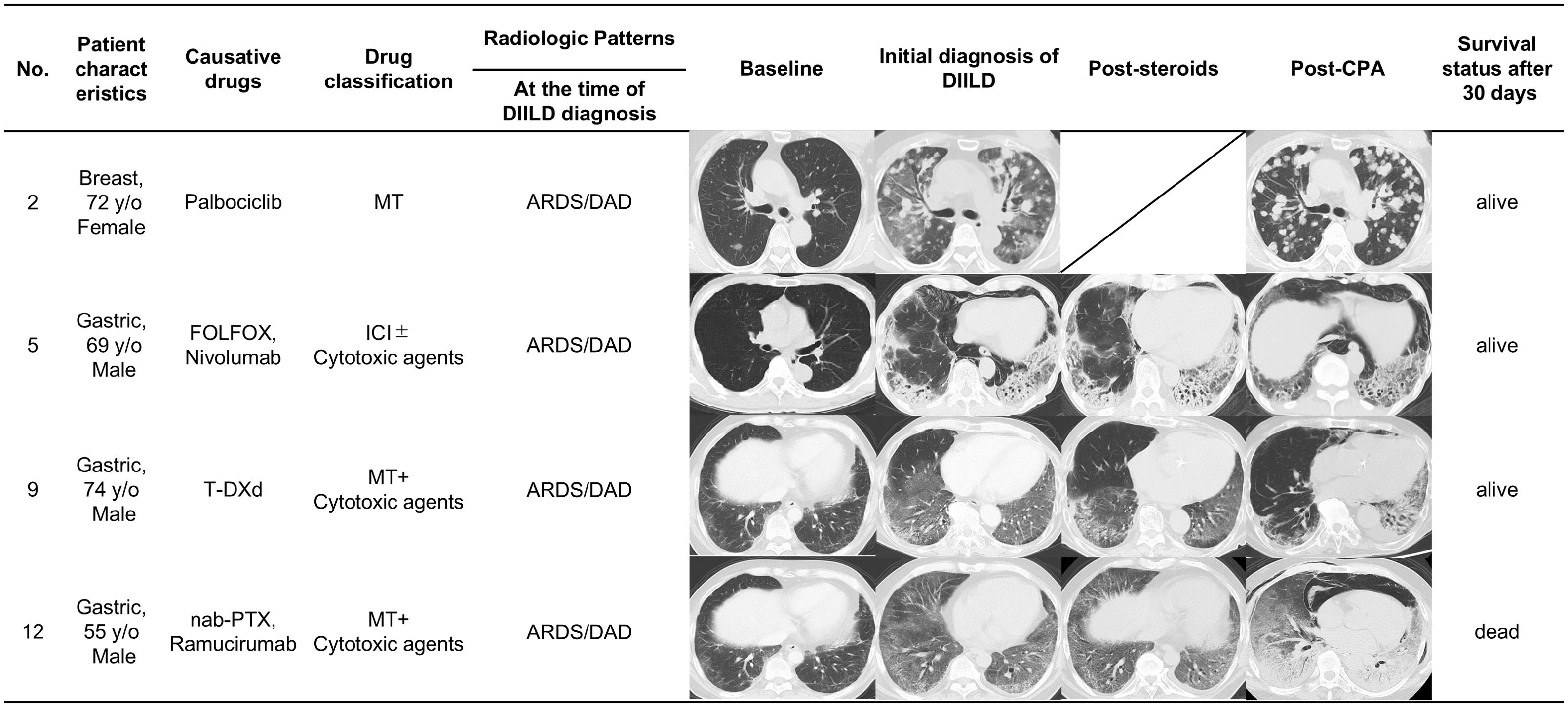
Figure 3. Serial radiologic imaging of patients with corticosteroid-refractory DIILD. Serial CT images showing radiologic patterns in four representative patients with corticosteroid-refractory DIILD who were treated with CPA. ARDS/DAD, acute respiratory distress syndrome/diffuse alveolar damage; CPA, cyclophosphamide; DIILD, drug-induced interstitial lung disease; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; ICI, immune checkpoint inhibitors; MT, molecular-targeted therapies; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; PTX, paclitaxel; T-DXd, trastuzumab deruxtecan.
3.3 Adverse events
Adverse events associated with CPA included grade 3 anemia in two patients, grade 4 neutropenia in 7% (1 patient), and grade 2 cytomegalovirus infection in 7% (1 patient). No deaths were attributed to adverse events.
4 Discussion
This study examined the effectiveness of CPA in 15 patients with DIILD caused by anticancer agents. To our knowledge, it is the first to report on the efficacy of CPA in DIILD induced by modern therapies, including MT and ICI. Although the efficacy of CPA in DIILD remains unclear, these real-world clinical data suggest that CPA may be an option for the treatment of corticosteroid-resistant DIILD provoked by modern anticancer drugs.
The development of DIILD is related to cytotoxicity and the immune responses which act independently or synergistically (4). The cytotoxic mechanisms include reactive oxygen species induced by drug exposure, such as bleomycin (26), impaired alveolar repair (27), reduced detoxification of pulmonary metabolites (28), and cytokine release (29). These mechanisms are frequently associated with cytotoxic anticancer drugs. In addition, T-DXd, an antibody-drug conjugate widely used to treat various cancers, is thought to be non-specifically taken up by alveolar macrophages via Fcγ receptors, releasing DXd within lung tissue, where it accumulates and causes alveolar damage (30). In contrast, immune-mediated mechanisms, including T-cell activation, dysregulation of regulatory T-cells (Tregs), and the inflammatory cytokine storm (31), often result in lung injury, particularly with ICIs (32), CDK4/6 inhibitors (33), mTOR inhibitors (34), and other MTs (35).
Our findings demonstrate drug category-dependent efficacy of CPA in DIILD treatment. This observed variable efficacy across drug categories can be explained by distinct pathophysiological mechanisms underlying DIILD development. For MT-induced DIILD (exemplified by CDK4/6 inhibitors, mTOR inhibitors, and PARP inhibitors), the pathogenesis primarily involves dysregulation of specific immune pathways, including T-cell activation and cytokine production. CPA’s potent suppression of T and B lymphocyte proliferation and function directly targets these mechanisms, potentially explaining the higher efficacy observed in this subgroup (18, 36). The established use of CPA in treating autoimmune disease and graft-versus-host diseases further supports its potential effectiveness against DIILD mediated by immune-related mechanisms (37).
In contrast, cytotoxic drug-induced DIILD (particularly with agents like bleomycin) involves direct cellular toxicity (the cytotoxic mechanisms), such as oxidative stress, alveolar epithelial cell death, and impaired tissue repair (21, 26), rather than immune-mediated inflammation, mechanisms that are largely independent of immune cell proliferation. This likely explains why all patients in the cytotoxic drugs alone group failed to respond to CPA therapy, suggesting that alternative approaches targeting antioxidant pathways or tissue repair mechanisms may be more appropriate for this subgroup.
In the ICI ± cytotoxic drugs group, the observed mixed response patterns (50% survival with continued oxygen requirements) likely reflect the complex immunopathology involving dual mechanisms: DIILD caused by immune-related mechanisms from ICIs and cytotoxic mechanisms from conventional agents. This mixed etiology may explain why CPA, with its primarily immunosuppressive mechanism of action, yielded only partial benefits in this subgroup. Furthermore, the poor outcomes in the two patients who developed DIILD after ICI monotherapy underscore the inherent challenges and severity of corticosteroid-refractory immune-related pneumonitis; however, conclusions based specifically on these two cases are limited. This observation aligns with previous studies investigating other immunosuppressants (e.g., infliximab, mycophenolate mofetil) for refractory ICI pneumonitis (20, 38, 39), which also reported variable effectiveness and often modest resolution rates. Our findings, along with case reports (20, 40), suggest that CPA could be considered an additional therapeutic option in this difficult refractory setting, although appropriate patient selection is likely critical.
This study has several limitations. First, it was from a single center and had a retrospective design, with a small sample size. Second, significant heterogeneity existed within the cohort regarding patient background factors, the specific anticancer drugs suspected of causing DIILD, and the timing of CPA administration. This heterogeneity complicates interpretation, particularly in attributing DIILD causality definitively to a single drug category when combination regimens involving multiple drug classes (e.g., MT + cytotoxic ICI + cytotoxic) were used. The possibility that the observed effects may result from a delayed response to corticosteroids cannot be ruled out. Third, the lack of a control group to compare CPA therapy with that of other immunosuppressive agents limited the assessment of the efficacy of CPA. Fourth, the predominantly male cohort (80%) limited our ability to explore potential sex-based differences. Fifth, the small number of patients receiving the 500 mg/m² CPA dose (n=2) compared to the 500 mg fixed dose (n=13) precluded any meaningful comparison of efficacy between these dosing strategies. A standardized, evidence-based dosing protocol for CPA does not currently exist for this specific clinical indication. Finally, bronchoalveolar lavage was not performed in these patients, making it difficult to definitively evaluate immune mechanisms and the role of CPA in immune modulation. Despite these limitations, this study provides valuable real-world insights into the use of CPA and highlights its potential role in managing immune-mediated DIILD. It also indicates that CPA may not be effective in treating corticosteroid-resistant DIILD caused by cytotoxic anticancer drugs. To further validate these findings, prospective studies with larger patient cohorts are needed.
In summary, this study suggests that, even in the current era of rapidly emerging anticancer therapies, CPA remains a promising option for managing corticosteroid-resistant DIILD caused by anticancer drugs. These real-world findings particularly highlight its potential efficacy in cases associated with MT. However, because of the variability in patient responses, further studies are needed to tailor treatment strategies according to the patient’s condition and the pathophysiology of DIILD.
Data availability statement
The datasets generated for this study are not publicly available but can be obtained from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Name of Ethics Committee/Institutional Review Board: Ethics Review Board of the National Cancer Center Affiliation: National Cancer Center Hospital East, Kashiwa, Chiba, Japan. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The requirement for written informed consent was waived because this study was a retrospective analysis of medical records. The study design posed no risk to participants, and obtaining individual consent was impractical due to the retrospective nature of the research. This waiver was approved by the Ethics Review Board of the National Cancer Center (approval number: 2023-308), in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
Author contributions
HK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. KB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing, Data curation, Formal Analysis, Visualization. HN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. CF: Conceptualization, Writing – review & editing. CK: Conceptualization, Writing – review & editing. YN: Conceptualization, Writing – review & editing. HU: Data curation, Writing – review & editing. KS: Data curation, Writing – review & editing. TS: Data curation, Formal Analysis, Visualization, Writing – review & editing. TK: Conceptualization, Writing – review & editing. TM: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Enago (www.enago.jp) for English language editing. This research also acknowledges the use of OpenAI’s ChatGPT (version 4.0) as a generative AI tool to support the writing process. The AI model was utilized for initial language drafting and refinement. The prompts provided to the AI and its outputs have been documented and submitted in the Supplementary Files accompanying this manuscript.
Conflict of interest
HN has received a consulting fee from Terumo Corporation and honoraria from Chugai, Takeda, Pfizer, Eli Lilly, AstraZeneca, Ono Pharmaceutical, Sanofi/Regeneron, and MSD. HN also reports that an immediate family member works for Otsuka Pharmaceutical Co., Ltd. CK has received honoraria from Merck & Co., Daiichi Sankyo, Ono Pharmaceutical, Takeda, Astra Zeneca, Sanofi, Astellas, Bristol-Myers Squibb, Chugai, Janssen, Merck KGaA, and Bayer. YN has received research funding (all to institution) from ABBVIE, Ono, Daiichi Sankyo,Taiho, Pfizer, Boehringer Ingelheim, Eli Lilly, Eisai, AstraZeneca, Chugai, and Bayers; and receiving honoraria from AstraZeneca, Eisai, Ono, Guardant, Takeda, Eli Lilly, Novartis, Pfizer, Chugai, PDR pharma, Nihon Kayaku, Taiho, Bristol, Bayer,Daiichi Sankyo, and MSD. HU has received research funding (all to institution) from Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Amgen K.K., Taiho Pharmaceutical Co., Ltd., MSD K.K., and AbbVie GK. and honoraria from DAIICHI SANKYO COMPANY, LIMITED, CHUGAI PHARMACEUTICAL CO., LTD., Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd., MSD K.K., and AstraZeneca K.K. KS has received personal fees for consulting and advisory roles from Bristol Myers Squibb, Takeda, Ono Pharmaceutical, Novartis, Daiichi Sankyo, Amgen, Boehringer Ingelheim, Merck Pharmaceutical, Astellas, Guardant Health Japan, Janssen, Astra Zeneca, Zymeworks Biopharmaceuticals, ALX Oncology Inc.,Bayer, GlaxoSmithKline K.K., HEALIOS K.K., Moderna.Inc, Arcus Biosciences Inc.; receiving honoraria from Bristol-Myers Squibb, Janssen, AstraZeneca, Eli Lilly, Ono Pharmaceutical, and Astellas; and receiving research funding (all to institution) from Merck Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, Ono Pharmaceutical, Astellas, Eisai, Amgen, PRA Health Sciences, AstraZeneca, PPD-SNBL K.K. and TORAY, outside the submitted work. TS has received honoraria from Canon Medical Systems; and has received research funding from Grant-in-Aid for Scientific Research C (Grant No. 22K07708). TM reports receiving honoraria from Eisai, Pfizer, Novartis, Chugai, Eli Lilly, AstraZeneca, Kyowa-Kirin, Taiho, Daiichi-Sankyo; and receiving research funding from Sysmex, Sanofi, MSD, Pfizer, Novartis, Chugai, Astra Zeneca, Ono Pharmaceutical, Daiichi Sankyo, Gilead Sciences. The other authors indicated no financial relationships.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript.
This research also acknowledges the use of OpenAI’s ChatGPT (version 4.0) as a generative AI tool to support the writing process. The AI model was utilized for initial language drafting and refinement.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1567317/full#supplementary-material
References
1. Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: A systematic review. J Clin Med. (2018) 7:356. doi: 10.3390/jcm7100356
2. Erasmus JJ, McAdams HP, and Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am. (2002) 40:61–72. doi: 10.1016/s0033-8389(03)00109-x
3. Fujimoto D, Kato R, Morimoto T, Shimizu R, Sato Y, Kogo M, et al. Characteristics and prognostic impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PloS One. (2016) 11:e0168465. doi: 10.1371/journal.pone.0168465
4. Lee W-I and Hissaria P. Drug-induced interstitial lung disease. Indian J Rheumatol. (2019) 14:19. doi: 10.4103/0973-3698.272161
5. Dimopoulou I, Bamias A, Lyberopoulos P, and Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol. (2006) 17:372–9. doi: 10.1093/annonc/mdj057
6. Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
7. Yi M, Zheng X, Niu M, Zhu S, Ge H, and Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. (2022) 21:28. doi: 10.1186/s12943-021-01489-2
8. Abuhelwa Z, Alloghbi A, Alqahtani A, and Nagasaka M. Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-positive advanced solid Malignancies: A systematic review. Drugs. (2022) 82:979–87. doi: 10.1007/s40265-022-01736-w
9. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, and Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
10. Xia B, Zhang S, and Ma S. Management of non-small cell lung cancer with EGFR mutation: the role of radiotherapy in the era of tyrosine kinase inhibitor therapy-opportunities and challenges. J Thorac Dis. (2017) 9:3385–93. doi: 10.21037/jtd.2017.09.67
11. Kappel C, Elliott MJ, Kumar V, Nadler MB, Desnoyers A, and Amir E. Comparative overall survival of CDK4/6 inhibitors in combination with endocrine therapy in advanced breast cancer. Sci Rep. (2024) 14:3129. doi: 10.1038/s41598-024-53151-8
12. Suh CH, Park HS, Kim KW, Pyo J, Hatabu H, and Nishino M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer. (2018) 123:60–9. doi: 10.1016/j.lungcan.2018.06.032
13. Raschi E, Fusaroli M, Ardizzoni A, Poluzzi E, and De Ponti F. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. (2021) 186:219–27. doi: 10.1007/s10549-020-06001-w
14. Conte P, Ascierto PA, Patelli G, Danesi R, Vanzulli A, Sandomenico F, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. (2022) 7:100404. doi: 10.1016/j.esmoop.2022.100404
15. Camus P, Bonniaud P, Fanton A, Camus C, Baudaun N, and Foucher P. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med. (2004) 25:479–519, vi. doi: 10.1016/j.ccm.2004.05.006
16. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
17. van den Bosch L, Luppi F, Ferrara G, and Mura M. Immunomodulatory treatment of interstitial lung disease. Ther Adv Respir Dis. (2022) 16:17534666221117002. doi: 10.1177/17534666221117002
18. Hurd ER and Giuliano VJ. The effect of cyclophosphamide on B and T lymphocytes in patients with connective tissue diseases. Arthritis Rheum. (1975) 18:67–75. doi: 10.1002/art.1780180113
19. Wiertz IA, van Moorsel CHM, Vorselaars ADM, Quanjel MJR, and Grutters JC. Cyclophosphamide in steroid refractory unclassifiable idiopathic interstitial pneumonia and interstitial pneumonia with autoimmune features (IPAF). Eur Respir J. (2018) 51:1702519. doi: 10.1183/13993003.02519-2017
20. Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. (2021) 9:e001731. doi: 10.1136/jitc-2020-001731
21. Kubo K, Azuma A, Kanazawa M, Kameda H, Kusumoto M, Genma A, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Invest. (2013) 51:260–77. doi: 10.1016/j.resinv.2013.09.001
22. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
23. Kashiwada T, Saito Y, Terasaki Y, Hisakane K, Takeuchi S, Sugano T, et al. Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol. (2019) 49:165–73. doi: 10.1093/jjco/hyy180
24. Sakai F, Johkoh T, Kusumoto M, Arakawa H, and Takahashi M. Drug-induced interstitial lung disease in molecular targeted therapies: high-resolution CT findings. Int J Clin Oncol. (2012) 17:542–50. doi: 10.1007/s10147-012-0489-2
25. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. (2016) 22:6051–60. doi: 10.1158/1078-0432.CCR-16-1320
26. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. (2012) 13:39. doi: 10.1186/1465-9921-13-39
27. Miettinen PJ, Warburton D, Bu D, Zhao J-S, Berger JE, Minoo P, et al. Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev Biol. (1997) 186:224–36. doi: 10.1006/dbio.1997.8593
28. Cooper JAD, Zitnik RJ, and Matthay RA. Mechanisms of drug-induced pulmonary disease. Annu Rev Med. (1988) 39:395–404. doi: 10.1146/annurev.me.39.020188.002143
29. Kim Y-J, Song M, and Ryu J-C. Mechanisms underlying methotrexate-induced pulmonary toxicity. Expert Opin Drug Saf. (2009) 8:451–8. doi: 10.1517/14740330903066734
30. Kumagai K, Aida T, Tsuchiya Y, Kishino Y, Kai K, and Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. (2020) 111:4636–45. doi: 10.1111/cas.14686
31. Kim S and Lim JU. Immune checkpoint inhibitor-related interstitial lung disease in patients with advanced non-small cell lung cancer: systematic review of characteristics, incidence, risk factors, and management. J Thorac Dis. (2022) 14:1684–95. doi: 10.21037/jtd-22-93
32. Rapoport BL, Shannon VR, Cooksley T, Johnson DB, Anderson L, Blidner AG, et al. Pulmonary toxicities associated with the use of immune checkpoint inhibitors: an update from the immuno-oncology subgroup of the neutropenia, infection & Myelosuppression study group of the multinational association for supportive care in cancer. Front Pharmacol. (2021) 12:743582. doi: 10.3389/fphar.2021.743582
33. Luo X, Nie J, Wang S, Chen Z, Chen W, Li D, et al. Poly(ADP-ribosyl)ation of FOXP3 protein mediated by PARP-1 protein regulates the function of regulatory T cells. J Biol Chem. (2015) 290:28675–82. doi: 10.1074/jbc.M115.661611
34. Molas-Ferrer G, Soy-Muner D, Anglada-Martínez H, Riu-Viladoms G, Estefanell-Tejero A, and Ribas-Sala J. Interstitial pneumonitis as an adverse reaction to mTOR inhibitors. Nefrologia. (2013) 33:297–300. doi: 10.3265/Nefrologia.pre2013.Jan.11439
35. Ishiguro Y, Ishiguro H, and Miyamoto H. Epidermal growth factor receptor tyrosine kinase inhibition up-regulates interleukin-6 in cancer cells and induces subsequent development of interstitial pneumonia. Oncotarget. (2013) 4:550–9. doi: 10.18632/oncotarget.939
36. Brinkman CJ, Nillesen WM, and Hommes OR. The effect of cyclophosphamide on T lymphocytes and T lymphocyte subsets in patients with chronic progressive multiple sclerosis. Acta Neurol Scand. (1984) 69:90–6. doi: 10.1111/j.1600-0404.1984.tb07784.x
37. Madondo MT, Quinn M, and Plebanski M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat Rev. (2016) 42:3–9. doi: 10.1016/j.ctrv.2015.11.005
38. Ogusu S, Harutani Y, Tozuka T, Saito R, Koyama J, Sakamoto H, et al. Second-line immunosuppressant administration for steroid-refractory immune-related adverse events in patients with lung cancer. Cancer Immunol Immunother. (2023) 72:3765–72. doi: 10.1007/s00262-023-03528-x
39. Luo J, Beattie JA, Fuentes P, Rizvi H, Egger JV, Kern JA, et al. Beyond steroids: immunosuppressants in steroid-refractory or resistant immune-related adverse events. J Thorac Oncol. (2021) 16:1759–64. doi: 10.1016/j.jtho.2021.06.024
Keywords: drug-induced interstitial lung disease, cyclophosphamide, corticosteroid-refractory, immune-related pneumonitis, immunosuppressant
Citation: Katahara H, Baba K, Nakajima H, Funasaka C, Kondoh C, Naito Y, Udagawa H, Shitara K, Sasaki T, Kawasaki T and Mukohara T (2025) Cyclophosphamide for anticancer therapy-induced interstitial lung disease in the modern era: a retrospective cohort study. Front. Oncol. 15:1567317. doi: 10.3389/fonc.2025.1567317
Received: 27 January 2025; Accepted: 28 April 2025;
Published: 16 May 2025.
Edited by:
Kaige Chen, Wake Forest University, United StatesReviewed by:
Panneerselvam Jayabal, The University of Texas Health Science Center at San Antonio, United StatesYen Ari Indrawijaya, Universitas Islam Negeri Maulana Malik Ibrahim, Indonesia
Copyright © 2025 Katahara, Baba, Nakajima, Funasaka, Kondoh, Naito, Udagawa, Shitara, Sasaki, Kawasaki and Mukohara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiromichi Nakajima, aGlyb21pbmFAZWFzdC5uY2MuZ28uanA=
 Honami Katahara
Honami Katahara Kaede Baba1
Kaede Baba1 Hiromichi Nakajima
Hiromichi Nakajima Chikako Funasaka
Chikako Funasaka Yoichi Naito
Yoichi Naito Kohei Shitara
Kohei Shitara Tomoaki Sasaki
Tomoaki Sasaki Toru Mukohara
Toru Mukohara