- Department of General Surgery, ChengFei Hospital, Chengdu, Sichuan, China
Objective: Minimally invasive pancreaticoduodenectomy is becoming more and more popular among surgeons, but whether robotic pancreatoduodenectomy (RPD) is superior to laparoscopic surgery remains controversial. The study aims to assess the available literature and compare the perioperative outcomes of RPD and laparoscopic pancreatoduodenectomy (LPD).
Methods: A systematic literature search was performed in the PubMed, Cochrane Library, Embase, Web of Science databases (October 2024). Risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs) were calculated.
Results: The 29 studies that met inclusion criteria included 15137 PDs, out of which 8935 were LPD and 6202 were RPD. Compared with LPD, RPD has lower overall complications (RR, 0.87), conversion rates (RR, 0.47) and blood transfusion rates (RR, 0.56), shorter length of stay (MD, -0.80 days), and higher number of harvested lymph nodes (MD, 1.77). There were no significant differences observed in 90-day mortality (RR, 0.92), major complications (RR, 1.00), operative time (MD, 3.93 mins), blood loss (MD, -22.50 mL), reoperation (RR, 0.96), bile leak (RR, 0.87), postoperative pancreatic fistula (RR, 1.00), delayed gastric emptying (RR, 1.19), and R0 resection (RR, 0.99) between the groups.
Conclusions: Robotic-assisted surgery for PD is safe and feasible. Compared to LPD, it offers better short-term outcomes.
Introduction
Pancreatoduodenectomy (PD) is a challenging surgical procedure associated with high postoperative complications and mortality (1). With the advancement of surgical techniques and perioperative management, although the postoperative mortality rate of PD has been reduced to 5%, the postoperative complications is still as high as 40% (2). Postoperative complications will not only prolong hospital stay and increase hospital cost, but also affect the long-term prognosis of patients (3). Therefore, how to reduce postoperative complications is the key concern of pancreatic surgeons.
Compared with traditional open surgery, minimally invasive surgery (including laparoscopic surgery and robotic surgery) may have potential advantages in reducing postoperative complications and blood loss, and shortening hospital stay (4–6). Since Gagner et al. reported the first case of laparoscopic pancreatoduodenectomy (LPD) in 1994, LPD has been widely used in the world (7). However, laparoscopic surgery has disadvantages such as unstable camera platform, limited range of motion and two-dimensional imaging (3). The robotic surgical platform has a three-dimensional visual field of view and more flexible and precise manipulation of instruments, so it retains the advantages of minimally invasive surgery while overcoming the disadvantages of laparoscopic surgery (1, 8). Several studies have compared the effectiveness and safety of robotic and laparoscopic surgery in PD. However, whether robotic pancreatoduodenectomy (RPD) is superior to LPD remains controversial. Farah et al. ‘s (4) cohort study found that RPD significantly reduced the incidence of postoperative complications compared with LPD (51% vs. 38.9%, respectively). An international multicenter retrospective study by Emmen et al. (9), including 2,082 patients from 50 centers in 12 European countries, showed that the incidence of postoperative pancreatic leakage and delayed gastric emptying was higher in the RPD group than in the LPD group.
Therefore, in order to clarify the effectiveness and safety of robotic surgery in PD and to provide evidence-based medical evidence for surgeons when selecting surgical approaches. We comprehensively collected published evidence and conducted a meta-analysis to evaluate the potential benefits of RPD versus LPD in short-term outcomes.
Methods
Search strategy
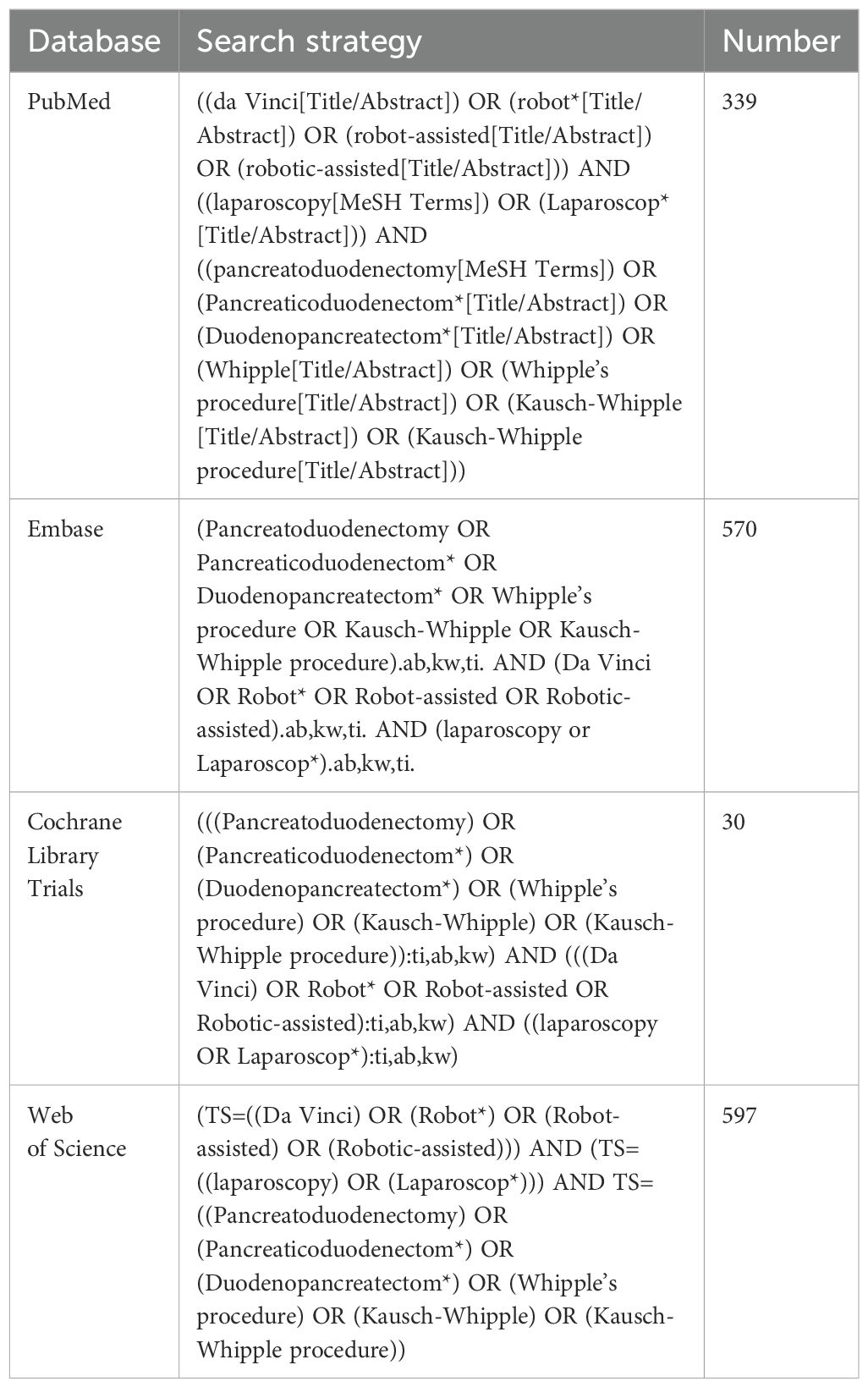
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (10). Two authors (Faying Liu and Yang Zou) independently conducted a comprehensive literature search using the EMBASE, Web of Science, PubMed, and Cochrane Library databases to identify studies published before October 24, 2024. The search strategy is presented in Table 1. In addition, we checked the reference lists of the identified articles and related reviews to further screen for eligible studies. No language restrictions were applied during the search process.
Study selection
Studies included in this meta-analysis were chosen according to the PICOS criteria:
a. Patient: patients undergoing pancreatoduodenectomy;
b. Intervention: robotic pancreatoduodenectomy;
c. Comparison: laparoscopic pancreatoduodenectomy;
d. Outcomes: assessing any of the short-term outcomes of interest. Studies focusing solely on long-term survival or those without direct comparison between RPD and LPD were excluded. Primary outcomes included 90-day mortality, overall complications, and major complications (Clavien-Dindo III-V) (9). Secondary outcomes included blood loss, length of stay, operative duration, conversion, reoperation, bile leak, postoperative pancreatic fistula (POPF), delayed gastric emptying, blood transfusion, number of harvested lymph nodes, and R0 resection. 90-day mortality was defined as any death within 90 days from surgery.The overall complications were defined as any complications and classified according to the Clavien-Dindo classification (including both surgical complications and medical complications).
e. Study type: RCTs, cohort studies, and case-control studies.
The exclusion criteria were as follows: reviews, case reports, editorials, conference abstracts, letters, single-arm studies, animal studies, and repeated publications. Studies with fewer than 10 patients in each group were excluded.
Data extraction
Data from all eligible studies were independently extracted by two investigators (Faying Liu and Yang Zou), and any disagreements were resolved by discussion with a third-party independent reviewer (Qi Ruan). The extracted data included author name, year of publication, country, study design, study population (sample size, age, body mass index, and sex), and short-term outcomes. When data of interest were unavailable, the corresponding author was contacted to obtain the necessary data.
Quality assessment
The risk of bias in RCTs was assessed independently by two authors (Faying Liu and Yang Zou) using the Cochrane risk-of-bias tool 2 (11): (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) selection of reported results, and (6) overall risk of bias. For non-RCTs, the quality assessment was conducted independently by two authors using the Newcastle-Ottawa Scale (NOS), which assigns a score on a 9-point scale. A score of ≥7 indicates high quality, and scores of 5–6 indicate moderate quality. Any discrepancies were resolved through discussion, with intervention by a third author (Qi Ruan) whenever necessary.
Statistical analysis
The meta-analysis was performed using the Review Manager software (version 5.3). Risk ratios (RR) with corresponding 95% confidence intervals (CI) were calculated for qualitative variables and mean difference (MD) for quantitative data. The I² statistic was used to assess the degree of heterogeneity. A random-effects model was used if I² > 50%; otherwise, a fixed-effects model was employed (12). To explore the robustness of the results, we adopted the 1-study exclusion method to evaluate the impact of each study on the pooled effect size. When zero events were observed in one or both treatment groups in a trial, we excluded these studies to verify the robustness of our results. Publication bias was assessed using funnel plot for primary outcomes. Statistical significance was set at p < 0.05.
Results
Literature retrieval
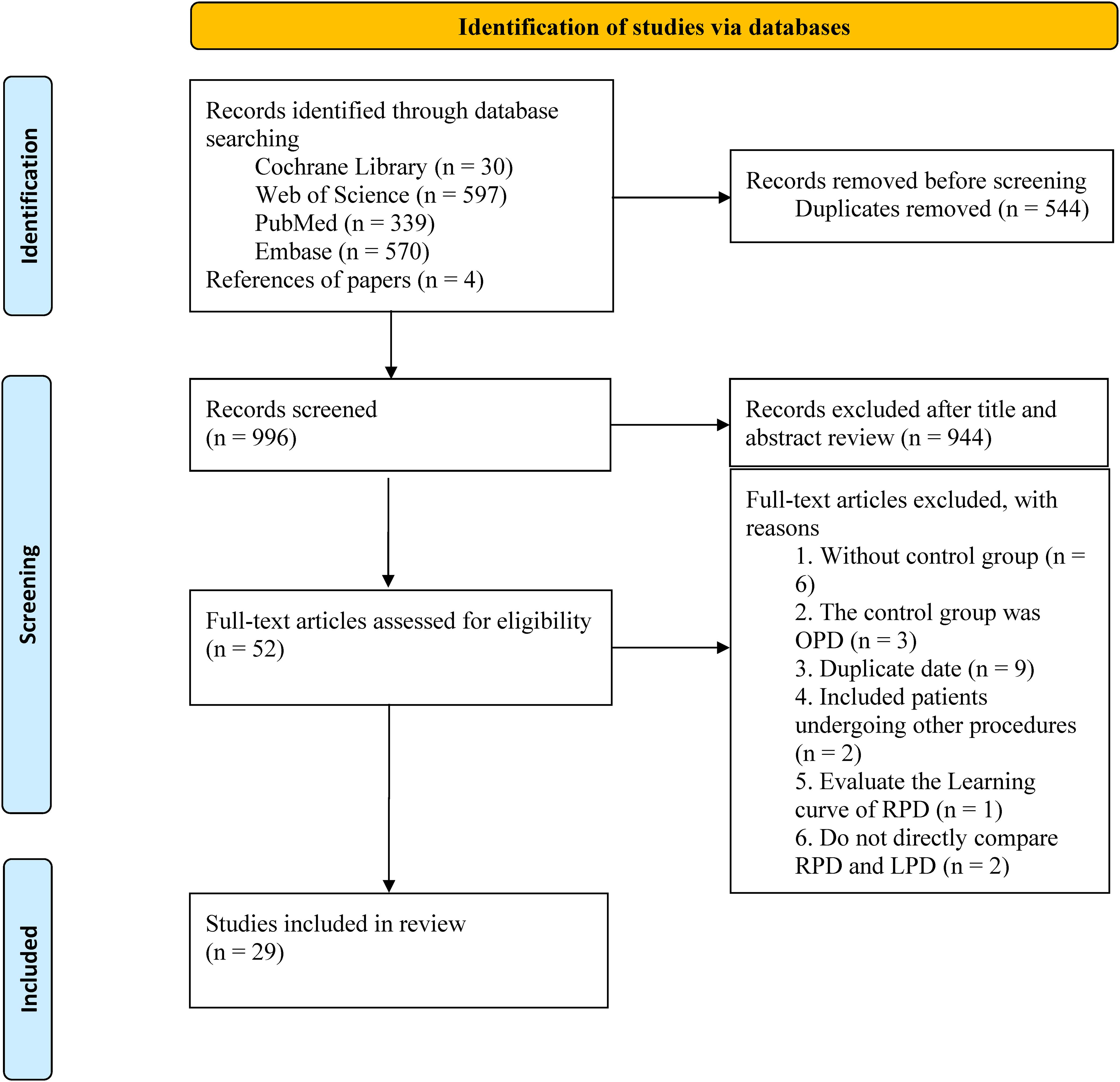
The search strategy retrieved 1540 studies, of which 544 duplicates were excluded. After reviewing titles and abstracts, 944 studies were excluded, and the full texts of the remaining 52 studies were evaluated. Finally, 29 studies (1, 4, 9, 13–38) were included in the final analysis (Figure 1).
Study characteristics and quality assessment
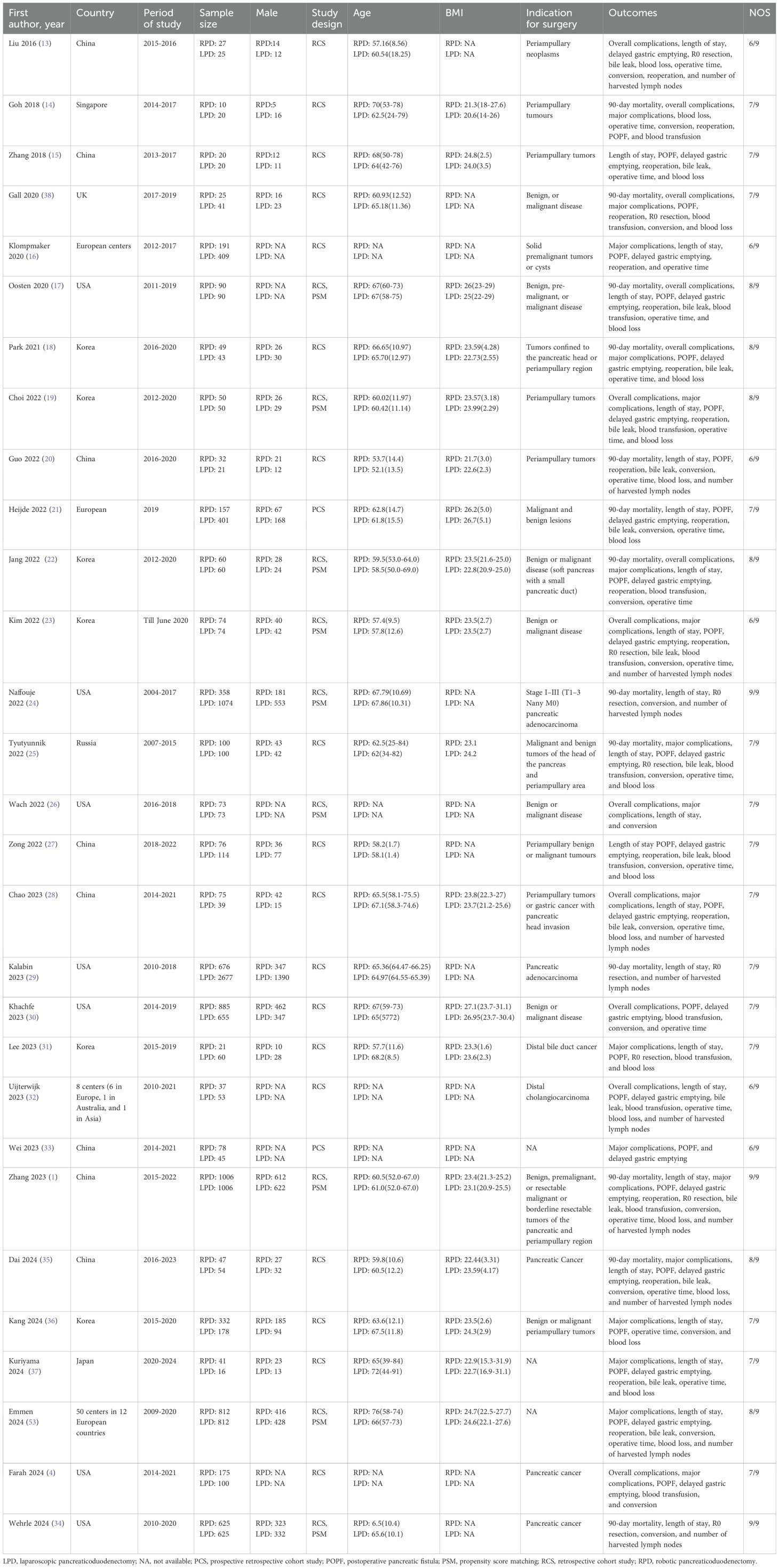
The main characteristics of the 29 included studies are summarized in Table 2. The studies were published between 2016 and 2024 and included 15137 patients (RPD group: 6202 patients; LPD group: 8935 patients). Among the included studies, 27 were retrospective cohort studies and 2 were prospective cohort studies. Nine studies adopted the PSM design. The included patients were mainly from the United States, China, Korea, The Netherlands, UK, Russia, Japan, and Singapore. All studies were considered of moderate to high quality, achieving a score of ≥6 based on the NOS.
Meta-analysis
90-day mortality
Thirteen studies reported data on 90-day mortality. The combined results of the 13 studies showed that there was no significant difference between the RPD group and the LPD group regarding this outcome with low heterogeneity (RR 0.92, 95% CI 0.74, 1.15; Heterogeneity: I2 = 0%, P = 0.46) (Figure 2A).
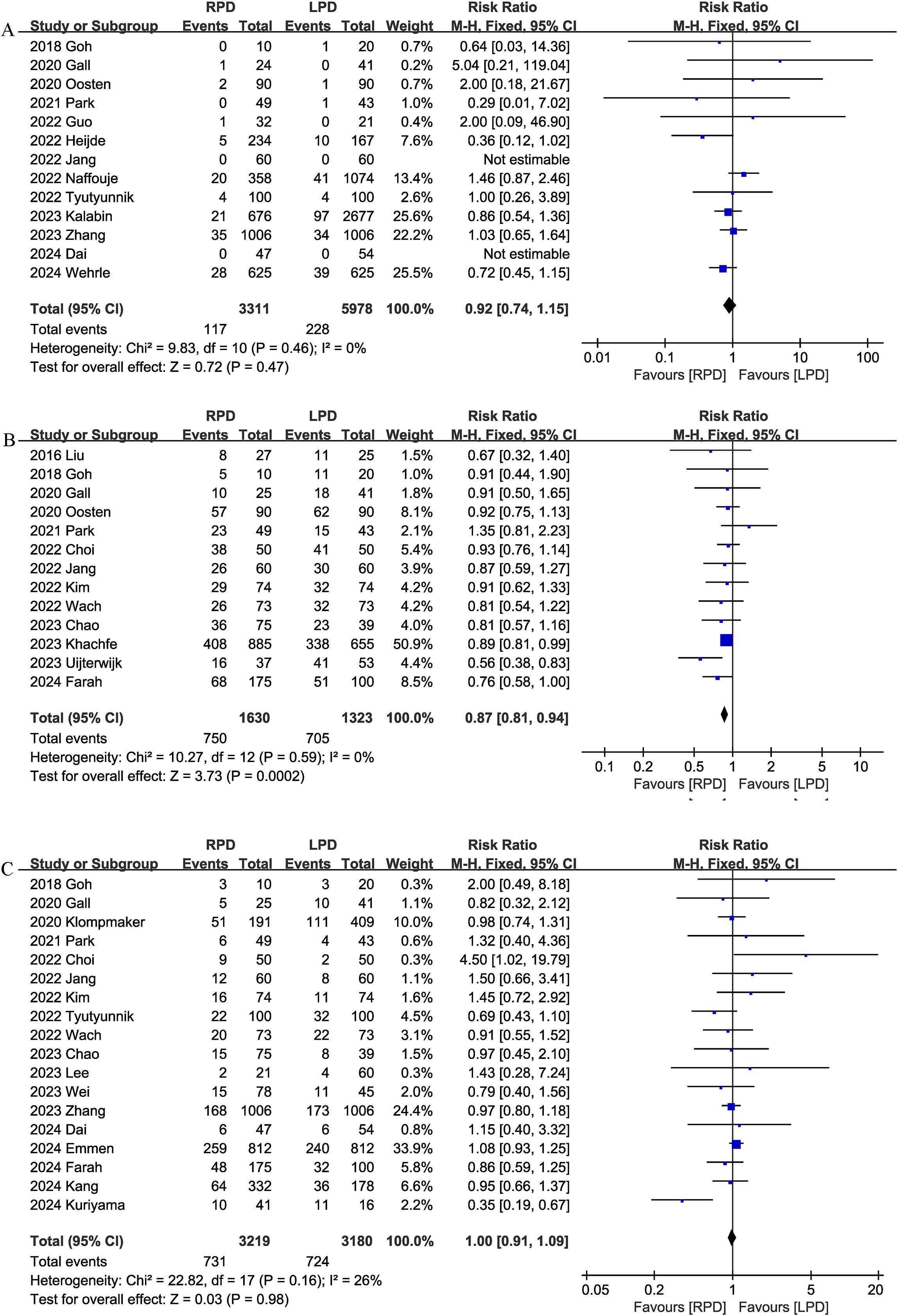
Figure 2. Comparison of primary outcomes between the two groups. (A) 90-day mortality, (B) overall complications, and (C) major complications.
Overall complications
Thirteen studies assessed overall complications. The pooled results suggested that RPD significantly reduced the overall complication rates (RR 0.87, 95% CI 0.81, 0.94, P = 0.0002), with low heterogeneity (I2 = 0%, P = 0.59) (Figure 2B).
Major complications
Combined data from 18 studies showed that the rates of major complications (Clavien–Dindo ≥ 3) were comparable between the RPD and LPD groups (RR 1.00, 95% CI 0.91, 1.09; Heterogeneity: I2 = 26%, P = 0.16) (Figure 2C).
Length of stay
The length of the hospital stay was reported in 23 studies. According to the results of this meta-analysis, RPD significantly reduced the length of the hospital stay as compared with the LPD group (MD, -0.80 days; 95% CI, -1.30, -0.29, P = 0.002) (Figure 3A).
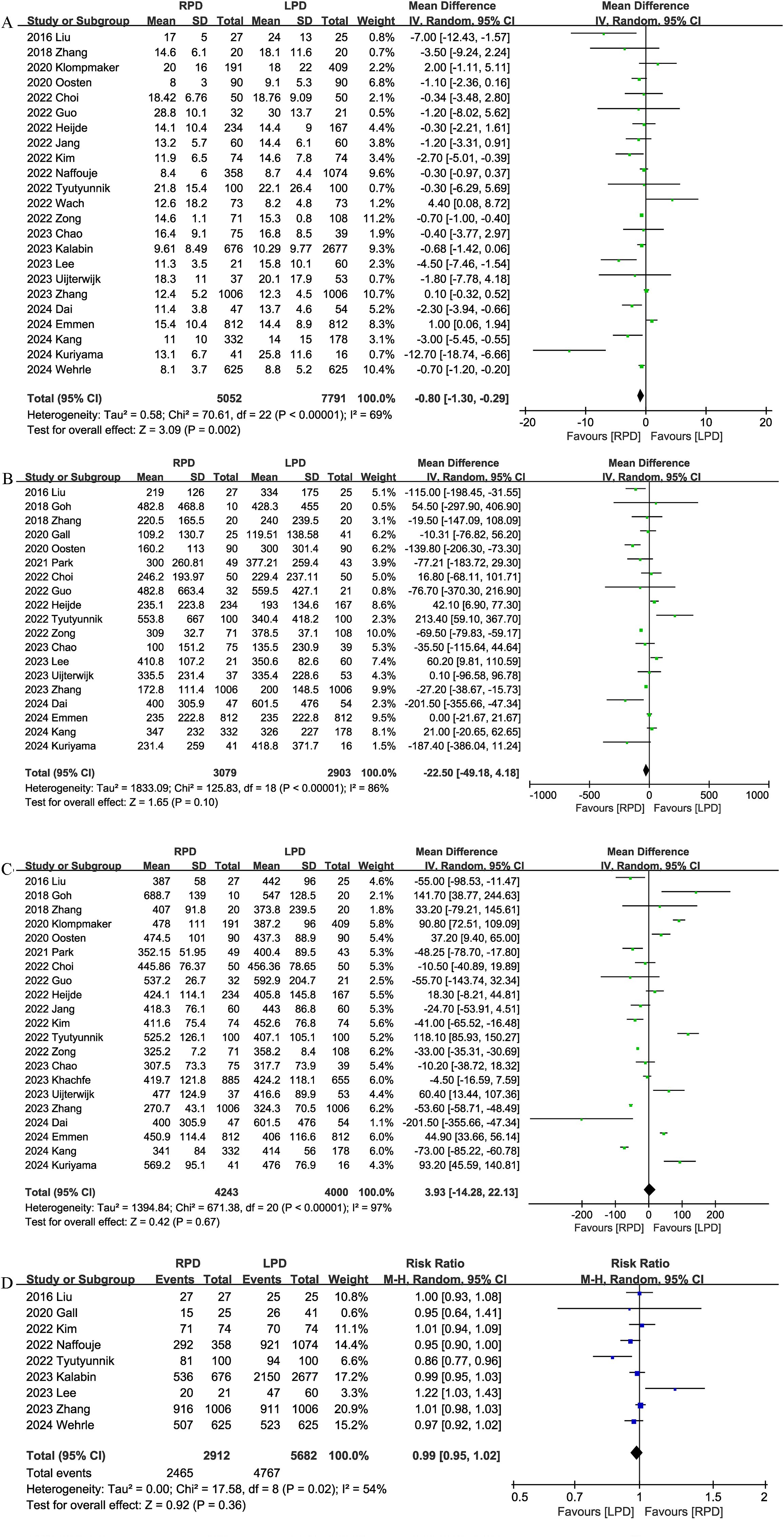
Figure 3. Comparison of secondary outcomes between the two groups. (A) length of stay, (B) intraoperative blood loss, (C) operative time, and (D) R0 resection.
Blood loss
Nineteen studies provided information on intraoperative blood loss. The combined results showed that the RPD group has similar intraoperative blood loss as compared with the LPD group (MD, -22.50 mL; 95% CI, -49.18, 4.18, P = 0.10; I2 = 86%) (Figure 3B).
Operation time
The operation time was reported in 21 trials. The combined results showed that the RPD group has similar operation time as compared with the LPD group (MD, 3.93 mins; 95% CI, -14.28, 22.13, P = 0.67) (Figure 3C).
R0 resection
R0 resection was reported in 9 studies, and the combined effect size suggested that the R0 resection rates were comparable between the two groups (RR 0.99, 95% CI 0.95, 1.02, P = 0.36; I2 = 54%) (Figure 3D).
Number of lymph nodes harvested
Eleven trials reported the number of lymph nodes harvested. Compared with LPD, RPD significantly increased the number of lymph nodes harvested (MD, 1.77; 95% CI, 0.66, 2.88, P = 0.002; I2 = 85%) (Figure 4A).
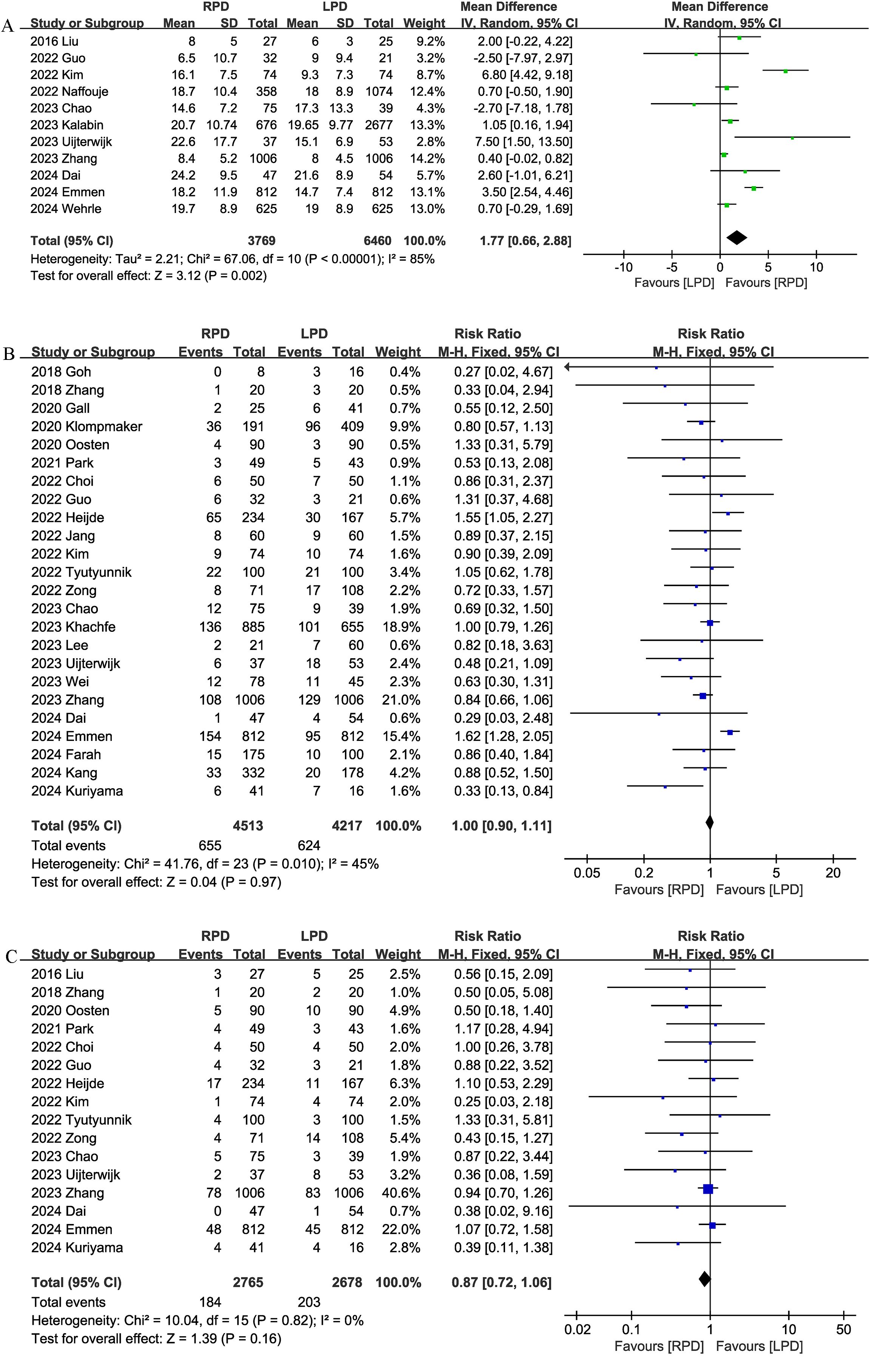
Figure 4. Comparison of secondary outcomes between the two groups. (A) number of lymph nodes harvested, (B) postoperative pancreatic fistula, and (C) bile leak.
Postoperative pancreatic fistula
Twenty-four studies evaluated the POPF. There was no significant difference in the incidence of POPF (RR 1.00, 95% CI 0.90, 1.11, P = 0.97) (Figure 4B) between the RPD and LPD groups.
Bile leak
Sixteen studies reported bile leaks. No significant differences were observed between the two groups (RR 0.87, 95% CI 0.72, 1.06, P = 0.16), and heterogeneity was low (I2 = 0%, P = 0.82) (Figure 4C).
Conversion rate
Conversion rate was evaluated in 19 studies, and the pooled results showed that RPD had lower conversion rate than LPD (RR 0.47, 95% CI 0.38, 0.59; heterogeneity: I2 = 58%, P = 0.0010) (Figure 5A).
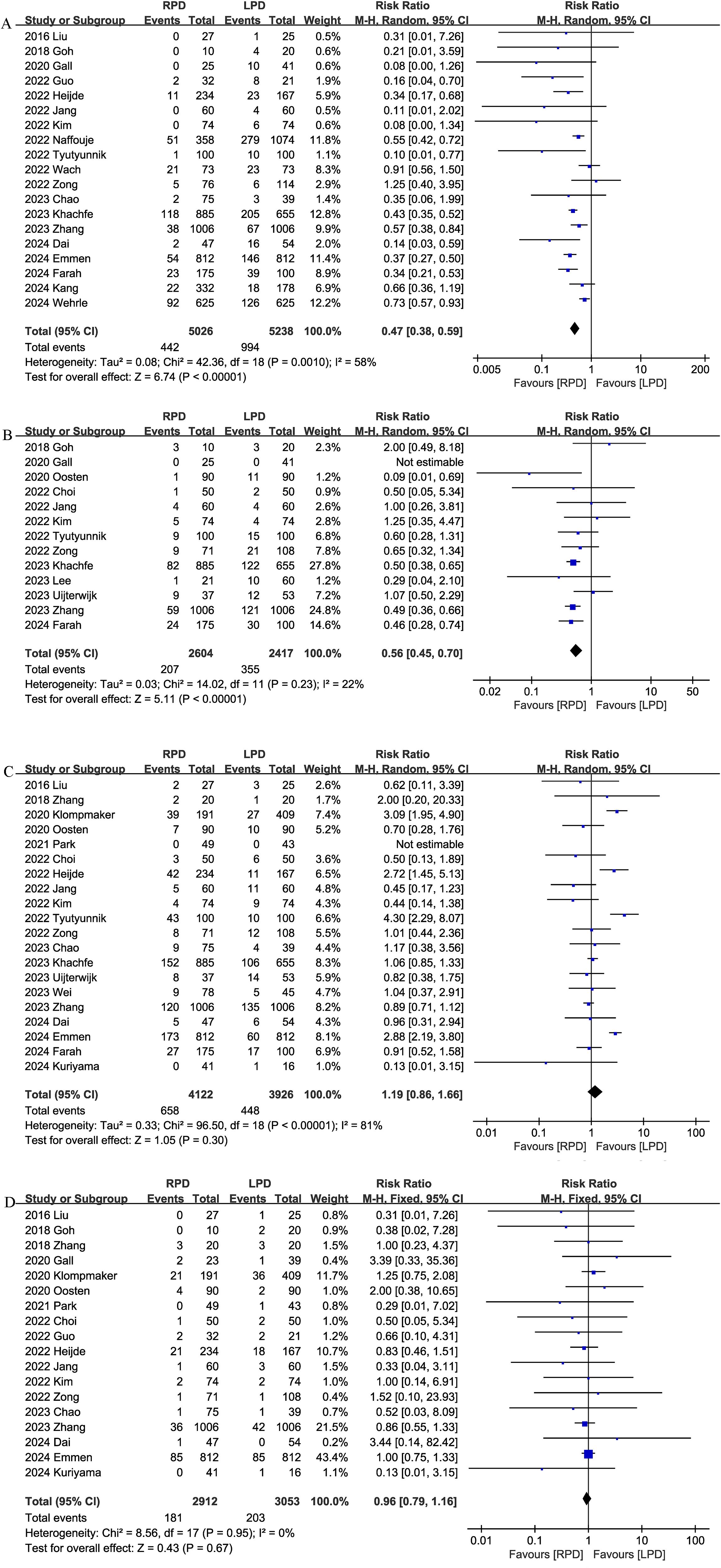
Figure 5. Comparison of secondary outcomes between the two groups. (A) Conversion rate, (B) blood transfusion, (C) delayed gastric emptying, and (D) reoperation.
Blood transfusion
Thirteen studies compared blood transfusion rates between the RPD and LPD groups. The combined results showed that RPD was effective in reducing the blood transfusion rate (RR 0.56, 95% CI 0.45, 0.70, P<0.00001) (Figure 5B).
Delayed gastric emptying
Delayed gastric emptying was reported in 20 studies, and there was no significant difference in the incidence of delayed gastric emptying (RR 1.19, 95% CI 0.86, 1.66, P = 0.30) (Figure 5C) between the two groups.
Reoperation
Eighteen trials reported the reoperation rates. There were no significant differences between the two groups, and heterogeneity was low (RR 0.96, 95% CI 0.79, 1.16; Heterogeneity: I2 = 0%, P = 0.95; Figure 5D).
Sensitivity analysis
According to the funnel plots (Figure 6) and Egger tests, and no significant publication bias was observed for 90-day mortality, overall complications, and major complications. Sensitivity analysis showed that no single study affected the overall effect size of the length of stay, blood transfusion, conversion rate, 90-day mortality, overall complications, major complications, reoperation, bile leak, operation time, delayed gastric emptying, POPF, number of lymph nodes harvested, blood loss, or R0 resection. Excluding these studies with no events in one or both groups did not change the total effect size of blood transfusion, conversion rate, 90-day mortality, reoperation, bile leak, delayed gastric emptying, and POPF.
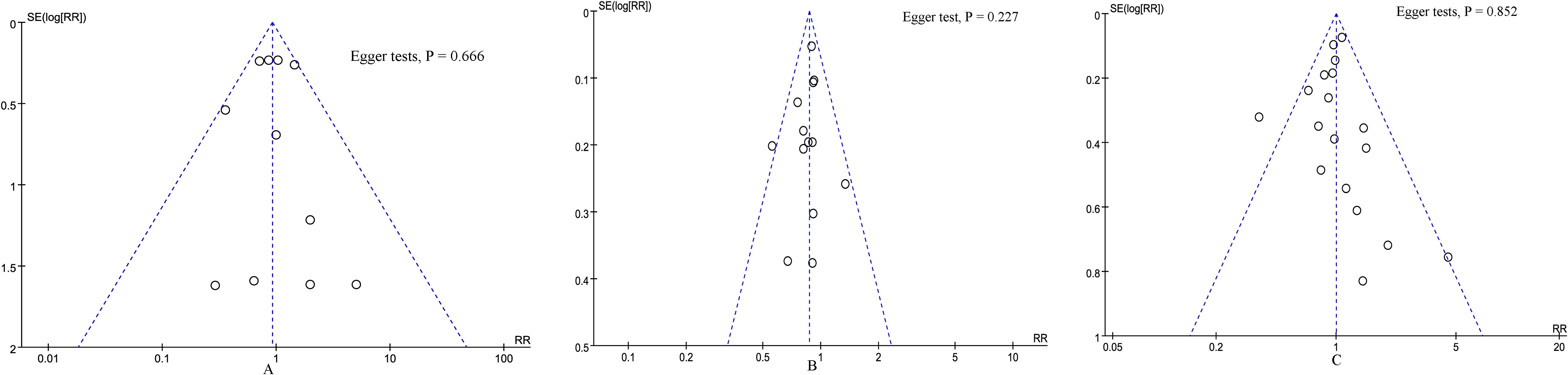
Figure 6. Funnel plot of primary outcomes. (A) 90-day mortality, (B) overall complications, and (C) major complications.
Discussion
In recent years, minimally invasive surgery has been widely used in pancreatic surgery. However, whether RPD is superior to LPD remains controversial. Although two previous meta-analyses (39, 40) were conducted, they included only six and nine studies, respectively, limiting the reliability of their conclusions. In comparison, our study included 29 studies, including data from 15137 patients. Our meta-analysis showed that compared with traditional LPD, RPD effectively reduced postoperative complications, blood transfusion, and conversion rates, shortened hospital stay, and increased the number of lymph nodes harvested. In addition, there were no significant differences in postoperative mortality, reoperation rates, operation time, intraoperative blood loss, and R0 resection rates between the two groups. Our results have important clinical value as we provide evidence that RPD is not inferior to LPD in the short term and can provide potential benefits. These results may help pancreatic surgeons in their choice of surgical approaches.
Postoperative complications are associated with a poorer long-term prognosis (3). Cho et al. (41) analyzed 200 patients with periampullary cancer who underwent pancreatoduodenectomy and showed that 3-year overall survival and disease-free survival were significantly lower in patients with postoperative complications (31.0% and 22.3%, respectively) than in patients without postoperative complications (49.0% and 40.0%, respectively). The high complication rate after PD is troubling pancreatic surgeons, and minimally invasive surgery may be a potential strategy to improve the postoperative morbidity of PD. Surgeons’ enthusiasm for LPD waned due to the high mortality rates reported in the LEOPARD-2 trial (42). In addition, subsequent meta-analyses (43) based on RCTs have also failed to demonstrate the benefit of LPD in terms of postoperative complications, leading to increasing hopes for RPD. Our results showed that RPD significantly reduced the incidence of postoperative complications compared with LPD. Given the impact of postoperative complications on long-term survival, lower postoperative complications may have potential benefits for patients’ long-term outcomes. In 2020, Kamarajah et al. (39) conducted a meta-analysis of six non-RCTs, involving 3,462 patients. Their results indicated that there was no significant difference in the incidence of postoperative complications and POPF compared with LPD and RPD. In 2022, Ouyang et al. (40) conducted an updated meta-analysis, and their study included nine retrospective studies. The results of the meta-analysis indicated that there were no significant differences between RPD and LPD in terms of total postoperative complications, major complications, POPF, delayed gastric emptying, and reoperation. Furthermore, the meta-analysis by Armengor-Garcia et al. (44) included 17 studies involving a total of 5,483 patients. The results indicated that compared with LPD, RPD did not significantly reduce postoperative hemorrhage, delayed gastric emptying, mortality, or readmission rates. However, Armengol-Garcia et al. did not evaluate the data of total postoperative complications and major complications. A meta-analysis by Tang et al. (45), which included 17 studies and 9,417 subjects, indicated that RPD could significantly reduce postoperative complications. Compared with previous studies, our meta-analysis has the following innovations. On the one hand, the number of studies and sample sizes included in the previously published meta-analyses were limited, which affected the statistical power and failed to draw convincing conclusions. In contrast, we included a larger number of studies (29 studies) and a larger sample size (15137 subjects), making our results more reliable. On the other hand, the population we included was broader, including patients with non-ampullary tumors, which made our conclusion more universal. In addition, Conversion to open is associated with an increased risk of postoperative complications (39). Our summarized results suggest that the conversion rates in the RPD group is significantly lower than that in the LPD group. Similarly, several previously published studies have observed the benefit of robotic surgery in reducing conversion rates in a variety of procedures (46–48). POPF is the most common and destructive complication after PD surgery, with an incidence of up to 20% (49). POPF is classified by the International Pancreatic Surgery Research Group (ISGPS) into clinically relevant POPF (Grade B and C) and biochemical POPF (Grade A) (50). Our study showed no significant difference between RPD and LPD in the incidence of clinically relevant POPF. This is consistent with the results of two previous meta-analyses (39, 40).
Increased intraoperative blood loss is significantly associated with poor prognosis in PD, and reducing intraoperative blood loss is helpful to improve perioperative outcomes (51). One of the advantages of minimally invasive surgery is that it is less invasive and less bleeding during the operation (52). Compared to LPD, RPD has a wider field of view, fewer tremors, and can perform detailed anatomy with less surgical trauma (40, 52). These advantages may lead to benefits in reducing intraoperative blood loss. Our findings showed that RPD significantly reduced the blood transfusion rate.
Some researchers are concerned that robotic surgery may prolong the operation time because of the additional time required to assemble the equipment (52, 53). However, a recent study (3) found that when the surgical team goes beyond the learning curve and gains enough experience, the surgical time for RPD is significantly reduced. A previous meta-analysis by Kamarajah et al. (39) found that RPD did not extend surgery time compared to LPD. The results of this study also indicated that the operation time was comparable between the RPD group and the LPD group. In addition, previous evidence has shown that robot-assisted gastrointestinal surgery can improve gastrointestinal function recovery and shorten hospital stays compared to laparoscopic surgery (54). In PD surgery, we also demonstrated the benefit of RPD in reducing the length of hospital stay.
Complete tumor resection and appropriate lymph node dissection are the keys of PD. R0 resection is an important predictor of long-term survival (49). A previous meta-analysis (49) showed no significant difference in R0 resection rates between different surgical approaches (open PD, LPD, and RPD). This is similar to the results of this study. Obtaining a sufficient number of lymph nodes is critical for accurate assessment of lymph node status, and the number of lymph nodes obtained is significantly associated with accurate staging and long-term patient survival (55). Our study showed that RPD significantly increased the number of lymph nodes acquired compared with LPD. This may be due to the robotic platform’s ability to provide enlarged 3D images that eliminate arm tremors and aid in precise lymph node dissection (40).
The high cost may be a factor limiting the further adoption of RPD. Due to the lack of data related to hospitalization costs in the included studies, we did not assess the difference in total costs between RPD and LPD. In fact, the increase in the cost of robotic surgery is mainly due to the installation and maintenance of the equipment (30). For example, in other areas such as hepatectomy and distal pancreatectomy, some studies have found that the surgical cost of robotic surgery is higher than laparoscopic surgery, while the hospital cost of robotic surgery is lower than laparoscopic surgery (56, 57). With the development of technology and the popularity of robotic surgery, the equipment cost of RPD is expected to decrease. In addition, the benefits of robotic surgery (lower postoperative complications and shorter hospital stays) may further reduce hospital costs. Therefore, the economic benefits of RPD deserve further evaluation in future studies.
This study has the following strengths. On the one hand, we conducted an extensive literature search, incorporating all the evidence currently available. On the other hand, we confirmed the robustness of the main results through sensitivity analysis.
There are some limitations to this study. First, most of the studies included in this meta-analysis are retrospective studies and lack RCTs. Second, high heterogeneity was found in some outcome measures (length of hospital stay, number of lymph nodes harvested, and operation time), which hindered accurate estimation of outcomes. The included studies originate from different countries, which may introduce variability in surgical standards, healthcare infrastructure, and patient management protocols. These differences may be the sources of heterogeneity. However, the sensitivity analysis still confirmed the stability of our main results. Furthermore, most of the included studies originated from high-volume centers. The availability of robotic surgery is limited in some developing countries. Considering the differences among regions, the conclusions of our research may not be directly generalized to some low-volume units. These low-volume centers need to undergo further training with RPD and go through the learning curve in order to bring out the true benefits of RPD. Among the 29 studies we included, 9 studies adopted the PSM design, while the remaining studies did not. The failure to adopt the PSM design may lead to differences in some preoperative basic characteristics (such as age, gender and weight), and these factors may have an impact on the results of the study. In the future, well-designed RCTs are needed to further balance the differences between the experimental group and the control group to verify the benefits of RPD. Finally, although our meta-analysis suggests that RPD is no less safe and effective than LPD in the perioperative period, few studies have evaluated the difference in long-term oncology outcomes between RPD and LPD. Given the potential benefits of RPD, future well-designed studies investigating the long-term oncology prognosis of RPD are warranted.
In conclusion, this meta-analysis suggests that compared with LPD, RPD can significantly reduce postoperative complications, blood transfusion, conversion, and hospital stay, and increase the number of lymph nodes harvested. In addition, there were no significant differences in mortality, reoperation rates and R0 resection rates between the two procedures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
FL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. QC: Data curation, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. TC: Conceptualization, Formal Analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. HX: Conceptualization, Data curation, Investigation, Software, Validation, Writing – original draft, Writing – review & editing. TX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. QR: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WL: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang XP, Xu S, Zhao ZM, Yu GS, Han B, Chen X, et al. Outcomes of robotic versus laparoscopic pancreatoduodenectomy following learning curves of surgeons: A multicenter study on 2255 patients. Ann Surg. (2023) 111:1214–30. doi: 10.1097/sla.0000000000006167
2. Peng L, Lin S, Li Y, and Xiao W. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg Endosc. (2017) 31:3085–97. doi: 10.1007/s00464-016-5371-2
3. Tang G, Zhang L, Xia L, Zhang J, Chen R, and Zhou R. Comparison of short-term outcomes of robotic versus open Pancreaticoduodenectomy: A Meta-Analysis of randomized controlled trials and Propensity-Score-Matched studies. Int J Surg. (2024) 111:1214–123. doi: 10.1097/js9.0000000000001871
4. Farah E, Al Abbas A, Abreu AA, Cheng M, Yopp A, Wang S, et al. Minimally invasive pancreaticoduodenectomy: A favorable approach for frail patients with pancreatic cancer. Surgery. (2024) 175:1168–75. doi: 10.1016/j.surg.2023.12.022
5. Uijterwijk BA, Wei K, Kasai M, Ielpo B, Hilst JV, Chinnusamy P, et al. Minimally invasive versus open pancreatoduodenectomy for pancreatic ductal adenocarcinoma: Individual patient data meta-analysis of randomized trials. Eur J Surg Oncol. (2023) 49:1351–61. doi: 10.1016/j.ejso.2023.03.227
6. Yan JF, Pan Y, Chen K, Zhu HP, and Chen QL. Minimally invasive pancreatoduodenectomy is associated with lower morbidity compared to open pancreatoduodenectomy: An updated meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Med (Baltimore). (2019) 98:e16730. doi: 10.1097/md.0000000000016730
7. Gagner M and Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. (1994) 8:408–10. doi: 10.1007/bf00642443
8. Wang K, Dong SS, Zhang W, Ni YY, Xie F, Wang JC, et al. Surgical methods influence on the risk of anastomotic fistula after pancreaticoduodenectomy: a systematic review and network meta-analysis. Surg Endosc. (2023) 37:3380–97. doi: 10.1007/s00464-022-09832-4
9. Emmen A, Zwart MJW, Khatkov IE, Boggi U, Groot Koerkamp B, Busch OR, et al. Robot-assisted versus laparoscopic pancreatoduodenectomy: a pan-European multicenter propensity-matched study. Surgery. (2024) 175:1587–94. doi: 10.1016/j.surg.2024.02.015
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
11. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
12. Higgins JP and Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
13. Liu R, Zhang T, Zhao ZM, Tan XL, Zhao GD, Zhang X, et al. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc. (2017) 31:2380–6. doi: 10.1007/s00464-016-5238-6
14. Goh BKP, Low TY, Kam JH, Lee SY, and Chan CY. Initial experience with laparoscopic and robotic surgery for the treatment of periampullary tumours: single institution experience with the first 30 consecutive cases. ANZ J Surg. (2019) 89:E137–e41. doi: 10.1111/ans.15033
15. Zhang Y, Hong D, Zhang C, and Hu Z. Total laparoscopic versus robot-assisted laparoscopic pancreaticoduodenectomy. Biosci Trends. (2018) 12:484–90. doi: 10.5582/bst.2018.01236
16. Klompmaker S, van Hilst J, Wellner UF, Busch OR, Coratti A, D’Hondt M, et al. Outcomes after minimally-invasive versus open pancreatoduodenectomy: A pan-European propensity score matched study. Ann Surg. (2020) 271:356–63. doi: 10.1097/sla.0000000000002850
17. van Oosten AF, Ding D, Habib JR, Irfan A, Schmocker RK, Sereni E, et al. Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. (2021) 25:1795–804. doi: 10.1007/s11605-020-04869-z
18. Park SE, Choi HJ, You YK, and Hong TH. Effectiveness and stability of robot-assisted anastomosis in minimally invasive pancreaticoduodenectomy. Ann Surg Treat Res. (2021) 100:329–37. doi: 10.4174/astr.2021.100.6.329
19. Choi M, Rho SY, Kim SH, Hwang HK, Lee WJ, and Kang CM. Total laparoscopic versus robotic-assisted laparoscopic pancreaticoduodenectomy: which one is better? Surg Endosc. (2022) 36:8959–66. doi: 10.1007/s00464-022-09347-y
20. Guo W, Ye X, Li J, Lu S, Wang M, Wang Z, et al. Comparison of surgical outcomes among open, laparoscopic, and robotic pancreatoduodenectomy: a single-center retrospective study. BMC Surg. (2022) 22:348. doi: 10.1186/s12893-022-01797-4
21. van der Heijde N, Vissers FL, Manzoni A, Zimmitti G, Balsells J, Berrevoet F, et al. Use and outcome of minimally invasive pancreatic surgery in the European E-MIPS registry. HPB (Oxford). (2023) 25:400–8. doi: 10.1016/j.hpb.2022.07.015
22. Jang JY, Kang CM, Kim H, Choi M, Lee JH, and Choi SH. Which one is better? Laparoscopic versus robotic reconstruction in the remnant soft pancreas with a small pancreatic duct following pancreaticoduodenectomy: a multicenter study with propensity score matching analysis. Surg Endosc. (2023) 37:4028–39. doi: 10.1007/s00464-022-09602-2
23. Kim H, Choi SH, Jang JY, Choi M, Lee JH, and Kang CM. Multicenter comparison of totally laparoscopic and totally robotic pancreaticoduodenectomy: Propensity score and learning curve-matching analyses. J Hepatobiliary Pancreat Sci. (2022) 29:311–21. doi: 10.1002/jhbp.1078
24. Naffouje SA, Kamarajah SK, Denbo JW, Salti GI, and Dahdaleh FS. Surgical Approach does not Affect Return to Intended Oncologic Therapy Following Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: A Propensity-Matched Study. Ann Surg Oncol. (2022) 29:7793–803. doi: 10.1245/s10434-022-12347-w
25. Tyutyunnik P, Klompmaker S, Lombardo C, Lapshyn H, Menonna F, Napoli N, et al. Learning curve of three European centers in laparoscopic, hybrid laparoscopic, and robotic pancreatoduodenectomy. Surg Endosc. (2022) 36:1515–26. doi: 10.1007/s00464-021-08439-5
26. Wach MM, Myneni AA, Miller L, Boccardo J, Ibrahim-Zada I, Schwaitzberg SS, et al. An assessment of perioperative outcomes for open, laparoscopic, and robot-assisted pancreaticoduodenectomy in New York State. J Surg Oncol. (2022) 126:1434–41. doi: 10.1002/jso.27075
27. Zong K, Luo K, Chen K, Ye J, Liu W, and Zhai W. A comparative study of robotics and laparoscopic in minimally invasive pancreatoduodenectomy: A single-center experience. Front Oncol. (2022) 12:960241. doi: 10.3389/fonc.2022.960241
28. Chao YJ, Lu WH, Liao TK, Su PJ, Wang CJ, Lai CH, et al. Feasibility of simultaneous development of laparoscopic and robotic pancreaticoduodenectomy. Sci Rep. (2023) 13:6190. doi: 10.1038/s41598-023-33269-x
29. Kalabin A, Mani VR, Kruse RL, Schlesselman C, Li KY, Staveley-O’Carroll KF, et al. New perspectives on robotic pancreaticoduodenectomy: An analysis of the National Cancer Database. World J Gastrointest Surg. (2023) 15:60–71. doi: 10.4240/wjgs.v15.i1.60
30. Khachfe HH, Nassour I, Hammad AY, Hodges JC, AlMasri S, Liu H, et al. Robotic pancreaticoduodenectomy: increased adoption and improved outcomes: is laparoscopy still justified? Ann Surg. (2023) 278:e563–e9. doi: 10.1097/sla.0000000000005687
31. Lee W, Song KB, Hong S, Park Y, Kwak BJ, Jun E, et al. Minimally invasive versus open pancreaticoduodenectomy for distal bile duct cancer: an inverse probability of treatment weighting analysis of outcomes. Surg Endosc. (2023) 37:881–90. doi: 10.1007/s00464-022-09533-y
32. Uijterwijk BA, Lemmers DHL, Bolm L, Luyer M, Koh YX, Mazzola M, et al. Long-term outcomes after laparoscopic, robotic, and open pancreatoduodenectomy for distal cholangiocarcinoma: an international propensity score-matched cohort study. Ann Surg. (2023) 278:e570–e9. doi: 10.1097/sla.0000000000005743
33. Wei TH, Su PJ, Lu WH, Liao TK, Wang CJ, Lai CH, et al. Minimally invasive approaches increase postoperative complications in obese patients undergoing pancreaticoduodenectomy during the initial development period: a propensity score matching study. Surg Endosc. (2023) 37:2770–80. doi: 10.1007/s00464-022-09773-y
34. Wehrle CJ, Chang JH, Gross AR, Woo K, Naples R, Stackhouse KA, et al. Comparing oncologic and surgical outcomes of robotic and laparoscopic pancreatoduodenectomy in patients with pancreatic cancer: a propensity-matched analysis. Surg Endosc. (2024) 38:2602–10. doi: 10.1007/s00464-024-10783-1
35. Dai M, Chen L, Xu Q, Cui M, Li P, Liu W, et al. Robotic versus laparoscopic pancreaticoduodenectomy for pancreatic cancer: evaluation and analysis of surgical efficacy. Ann Surg Oncol. (2024) 31:7043–51. doi: 10.1245/s10434-024-15764-1
36. Kang JS, Lee M, Lee JS, Han Y, Sohn HJ, Lee B, et al. Perioperative outcomes of robot-assisted pancreatoduodenectomy (PD) and totally laparoscopic PD after overcoming learning curves with comparison of oncologic outcomes between open PD and minimally invasive PD. Ann Hepatobiliary Pancreat Surg. (2024) 28:508–15. doi: 10.14701/ahbps.24-121
37. Kuriyama N, Fujii T, Kaluba B, Sakamoto T, Komatsubara H, Noguchi D, et al. Short-term surgical outcomes of open, laparoscopic, and robot-assisted pancreatoduodenectomy: A comparative, single-center, retrospective study. Asian J Endosc Surg. (2025) 18:e13397. doi: 10.1111/ases.13397
38. Gall TM, Pencavel TD, Cunningham D, Nicol D, and Jiao LR. Transition from open and laparoscopic to robotic pancreaticoduodenectomy in a UK tertiary referral hepatobiliary and pancreatic centre - Early experience of robotic pancreaticoduodenectomy. HPB (Oxford). (2020) 22:1637–44. doi: 10.1016/j.hpb.2020.03.008
39. Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, et al. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. (2020) 46:6–14. doi: 10.1016/j.ejso.2019.08.007
40. Ouyang L, Zhang J, Feng Q, Zhang Z, Ma H, and Zhang G. Robotic versus laparoscopic pancreaticoduodenectomy: an up-to-date system review and meta-analysis. Front Oncol. (2022) 12:834382. doi: 10.3389/fonc.2022.834382
41. Cho JY, Han HS, Yoon YS, Hwang DW, Jung K, and Kim YK. Postoperative complications influence prognosis and recurrence patterns in periampullary cancer. World J Surg. (2013) 37:2234–41. doi: 10.1007/s00268-013-2106-6
42. van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. (2019) 4:199–207. doi: 10.1016/s2468-1253(19)30004-4
43. Sattari SA, Sattari AR, Makary MA, Hu C, and He J. Laparoscopic versus open pancreatoduodenectomy in patients with periampullary tumors: A systematic review and meta-analysis. Ann Surg. (2023) 277:742–55. doi: 10.1097/sla.0000000000005785
44. Armengol-García C, Blandin-Alvarez V, Sharma E, Salinas-Ruiz LE, González-Méndez ML, Monteiro Dos Santos M, et al. Perioperative outcomes of robotic vs laparoscopic pancreatoduodenectomy: a meta-analysis and trial sequential analysis. Surg Endosc. (2025) 39:1462–72. doi: 10.1007/s00464-024-11515-1
45. Tang G, Chen F, Chen R, Zhou R, and Zhang J. Robotic versus laparoscopic pancreaticoduodenectomy for pancreatic and periampullary tumors: a meta-analysis. Front Oncol. (2024) 14:1486504. doi: 10.3389/fonc.2024.1486504
46. Liang B, Peng Y, Yang W, Yang Y, Li B, Wei Y, et al. Robotic versus laparoscopic liver resection for posterosuperior segments: a systematic review and meta-analysis. HPB (Oxford). (2024) 26:1089–102. doi: 10.1016/j.hpb.2024.06.003
47. Zhu XM, Bai X, Wang HQ, and Dai DQ. Comparison of efficacy and safety between robotic-assisted versus laparoscopic surgery for locally advanced mid-low rectal cancer following neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Int J Surg. (2024) 111:54–1166. doi: 10.1097/js9.0000000000001854
48. Li Z, Zhou W, Yang W, Miao Y, Zhang Y, Duan L, et al. Efficacy and safety of robotic vs. laparoscopic gastrectomy for patients with gastric cancer: systematic review and meta-analysis. Int J Surg. (2024) 110:8054–6. doi: 10.1097/js9.0000000000001826
49. Kamarajah SK, Bundred JR, Marc OS, Jiao LR, Hilal MA, Manas DM, et al. A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB (Oxford). (2020) 22:329–39. doi: 10.1016/j.hpb.2019.09.016
50. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
51. Seykora TF, Ecker BL, McMillan MT, Maggino L, Beane JD, Fong ZV, et al. The beneficial effects of minimizing blood loss in pancreatoduodenectomy. Ann Surg. (2019) 270:147–57. doi: 10.1097/sla.0000000000002714
52. Kabir T, Tan HL, Syn NL, Wu EJ, Kam JH, and Goh BKP. Outcomes of laparoscopic, robotic, and open pancreatoduodenectomy: A network meta-analysis of randomized controlled trials and propensity-score matched studies. Surgery. (2022) 171:476–89. doi: 10.1016/j.surg.2021.07.020
53. Emmen A, de Graaf N, Khatkov IE, Busch OR, Dokmak S, Boggi U, et al. Implementation and outcome of minimally invasive pancreatoduodenectomy in Europe: a registry-based retrospective study - a critical appraisal of the first 3 years of the E-MIPS registry. Int J Surg. (2024) 110:2226–33. doi: 10.1097/js9.0000000000001121
54. Loureiro P, Barbosa JP, Vale JF, and Barbosa J. Laparoscopic versus robotic gastric cancer surgery: short-term outcomes-systematic review and meta-analysis of 25,521 patients. J Laparoendosc Adv Surg Tech A. (2023) 33:782–800. doi: 10.1089/lap.2023.0136
55. Zhang X, Sun C, Zhao L, Niu P, Li Z, Fei H, et al. At least 16 lymph nodes are recommended to examine during pancreaticoduodenectomy in ampullary adenocarcinoma. Am J Cancer Res. (2023) 13:340–51.
56. Koh YX, Zhao Y, Tan IE, Tan HL, Chua DW, Loh WL, et al. Evaluating the economic efficiency of open, laparoscopic, and robotic distal pancreatectomy: an updated systematic review and network meta-analysis. Surg Endosc. (2024) 38:3035–51. doi: 10.1007/s00464-024-10889-6
Keywords: robotic pancreatoduodenectomy, laparoscopic pancreatoduodenectomy, mortality, postoperative complications, meta-analysis
Citation: Liu F, Zou Y, Chen Q, Chen T, Xiao H, Xie T, Zheng L, Ruan Q and Liu W (2025) Robotic pancreatoduodenectomy provides better short-term outcomes as compared to its laparoscopic counterpart: a meta-analysis. Front. Oncol. 15:1568957. doi: 10.3389/fonc.2025.1568957
Received: 31 January 2025; Accepted: 02 June 2025;
Published: 18 June 2025.
Edited by:
Veria Khosrawipour, Cornell University, United StatesReviewed by:
Patrycja Salata, Wroclaw University of Environmental and Life Sciences, PolandCarolina Khosrawipour, Wroclaw Medical University, Poland
Copyright © 2025 Liu, Zou, Chen, Chen, Xiao, Xie, Zheng, Ruan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Ruan, cWlydWFuMjAyNEAxNjMuY29t; Wang Liu, bGl1d2FuZzE5ODExQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Faying Liu†
Faying Liu† Qi Ruan
Qi Ruan