- 1Department of Hematology, the First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Department of Hematology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 3Institute of Clinical Pharmacology, Anhui Medical University, Key Laboratory of Anti-Inflammatory and Immune Medicine, Ministry of Education, Anhui Collaborative Innovation Centre of Anti-Inflammatory and Immune Medicine, Hefei, China
Background: The combination of anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) has been administered for graft-versus-host disease (GVHD) prophylaxis of haploidentical hematopoietic stem cell transplantation (haplo-HSCT) in recent years. Varied doses of ATG and PTCy were applied in multiple studies with promising outcomes.
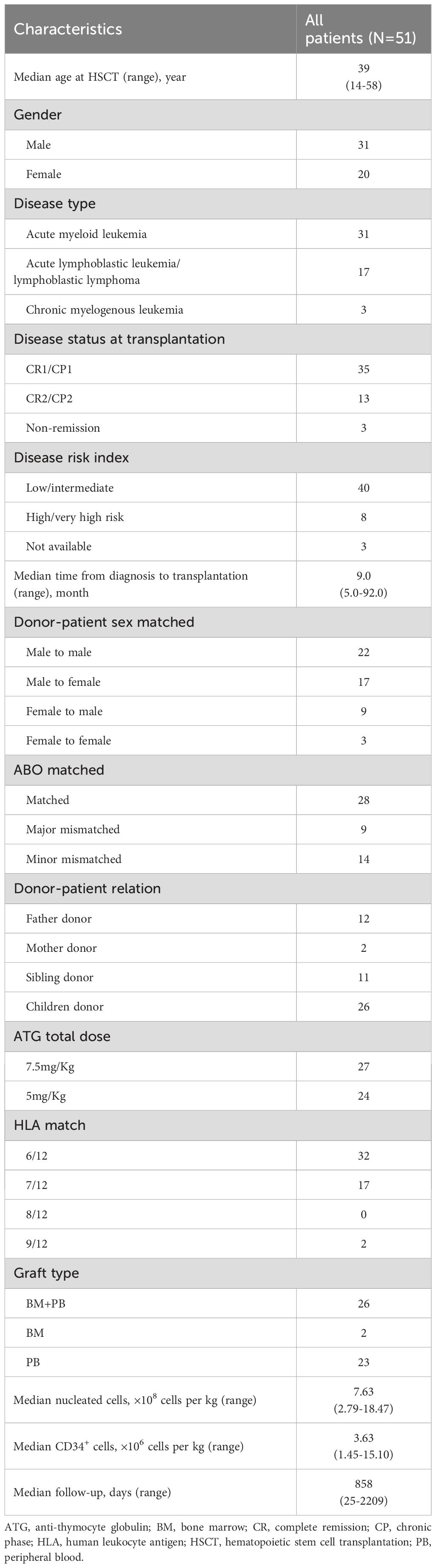
Methods: We retrospectively analyzed 51 consecutive leukemia patients who underwent haplo-HSCT with the joint use of low-dose ATG (27 patients with 7.5 mg/Kg and 24 patients with 5 mg/Kg) and PTCy (29 mg/Kg) for GVHD prophylaxis in our center. The impact of different ATG doses and absolute lymphocyte count (ALC) before ATG infusion was also evaluated.
Results: The 100-day cumulative incidences (CIs) of grade I-IV, II-IV and III-IV acute GVHD of the whole cohort were 42.9%, 34.7% and 12.2%, respectively. The 2-year CIs of overall and moderate-to-severe chronic GVHD were 44.7% and 27.7%, respectively. The 2-year overall survival, disease-free survival, non-relapse mortality and CI of relapse were 66.7%, 54.8%, 25.5% and 19.7%, respectively. Between 7.5 and 5 mg/Kg ATG groups, no significant difference on CIs of acute GVHD was observed. Interestingly, pre-ATG ALC impacted the occurrence of acute GVHD. With a cutoff point of 0.585×109/L, low ALC group showed reduced CIs of grade I-IV (16.7% versus 58.0%, p=0.01), II-IV (16.7% versus 45.1%, p=0.06) and III-IV (0 versus 19.4%, p=0.05) acute GVHD as compared to high ALC group.
Conclusions: The results suggested that this low-dose ATG/PTCy regimen was feasible and pre-ATG ALC levels could influence the occurrence of acute GVHD in this regimen.
1 Introduction
During the past 20 years, the outcome of haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has been dramatically improved, thanks to effective graft-versus-houst disease (GVHD) prophylaxis strategies with anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy). In China, ATG-based regimen is much more widely used. Compared to identical sibling HSCT, haplo-HSCT with ATG may achieve similar overall survival (OS) and disease-free survival (DFS) in acute myeloid leukemia (AML) patients, but incidences of acute and chronic GVHD were still significantly higher (1). In addition, the application of ATG is usually associated with higher risk of infections, especially virus reactivation (2–5).
In recent years, the combination of ATG and PTCy was reported to effectively reduce GVHD in HSCT patients. Low-dose PTCy (14.5 mg/Kg for 2 days) was demonstrated to augment the protective effect of ATG on GVHD and boost the reconstitution of regulatory T cells in a HSCT mouse model (6). In subsequent prospective trials for haplo-HSCT with maternal or collateral relative donors, the group with low-dose PTCy plus standard-dose ATG (2.5 mg/Kg for 4 days) showed significantly lower incidences of GVHD and comparable relapse and OS, as compared to ATG alone (6, 7). Besides, the joint use of ATG and PTCy at other doses and timings was also implemented in multiple studies with haplo- or unrelated donor-HSCT, resulting in promising outcomes (8–14).
Here, we report the low-dose ATG/PTCy regimen for haplo-HSCT in our center, which includes 2.5 mg/Kg ATG for 2 or 3 days (from day -4 or -3 to day -2) and 14.5 mg/Kg PTCy for 2 days (day +3 and +4). In addition, the impact of different total ATG doses (7.5 mg/Kg versus 5 mg/kg) and absolute lymphocyte count (ALC) before ATG infusion was analyzed in attempt to further optimize this ATG/PTCy regimen.
2 Methods
2.1 Patient selection
We included 51 consecutive leukemia patients who underwent haplo-HSCT with the low-dose ATG/PTCy regimen in the First Affiliated Hospital of Anhui Medical University, Hefei, China, from February 2018 to March 2022. All patients had a Karnofsky Performance Score (KPS) >60%. Clinical features, post-transplant outcomes and adverse events were collected retrospectively. This study was conducted in accordance with the Declaration of Helsinki and approved by the Hospital Ethics Committee. Informed consent was obtained from patients or their legal guardians before HSCT.
2.2 HLA typing and donors
High-resolution molecular typing for human leukocyte antigen (HLA)-A, -B, -C, -DRB1, -DQB1 and –DPB1 was done for both recipients and donors. Young and male donor is the first choice for the transplant. Donors were mobilized with granulocyte colony-stimulating factor (7.5-10 μg/kg per day), and collection of bone marrow/peripheral blood stem cells or both, which usually took one or two days, started from the 5th day of mobilization. A minimum dose of 2×106 CD34+ cells/Kg recipient body weight was required for the transplant.
2.3 Transplant procedures
The conditioning regimen consisted of the following: busulfan (BU), 3.2 mg/kg intravenously for 4 days from day -7 to -4; cyclophosphamide (Cy), 50 mg/Kg intravenously for 2 days from day -3 to -2; rabbit ATG (thymoglobulin; Genzyme-Sanofi, Lyon, France), 2.5 mg/Kg for 2 or 3 days from day -4 or -3 to day -2; simustine, 250 mg/m2 orally on day -8 for all acute lymphoid leukemia (ALL) patients and a proportion of AML patients. Two doses of 14.5 mg/Kg Cy were given on days +3 and +4. In addition, patients received cyclosporine, mycophenolate mofetil and short-term methotrexate for GVHD prophylaxis. Cyclosporine was administered at a dose of 2.5 mg/Kg intravenously from day -2 and adjusted to achieve a therapeutic level of 200 to 300 μg/L. Cyclosporine taping started around day +60 to +75 for all patients without GVHD. Mycophenolate mofetil was given orally at a dose of 0.5g q12h from day -2 and discontinued on around day +30 if no acute GVHD was present.
Granulocyte colony-stimulating factor (5 μg/kg) and recombinant human thrombopoietin (300 U/Kg) were administered subcutaneously from day +5. Prophylactic anti-bacterial (levofloxacin), anti-fungal (micafungin or voriconazole), anti-viral (ganciclovir from day -7 to -2, and acyclovir after stem cell infusion) and anti-pneumocystis Jiroveci pneumonia (oral septra) therapies were administered to all patients. Pre-emptive therapy with ganciclovir was given once cytomegalovirus (CMV) deoxyribonucleic acid (DNA) was > 1000 IU/ml by quantitative polymerase chain reaction (PCR). Rituximab was pre-emptively given once Epstein–Barr virus (EBV) DNA was > 105 IU/ml.
2.4 Engraftment and chimerism analysis
Neutrophil engraftment was defined as the first of 3 consecutive days when absolute neutrophil count exceeds 0.5×109/L, and platelet engraftment was defined as the first day of 7 consecutive days with a platelet count more than 20×109/L without platelet transfusion. The whole blood and leukocyte chimerism after the transplant was analyzed using PCR of short tandem repeats.
2.5 Statistical analysis
The last follow-up was updated in March 2024. Patient characteristics were reported as descriptive statistics. Chi-square and Fisher’s exact tests were used to compare categorical variables between two ATG groups, while the Mann-Whitney test was used for continuous variables.
Competing risk analysis was applied for the calculation of cumulative incidences (CIs) of GVHD, relapse (CIR), non-relapse mortality (NRM) and CMV/EBV viremia. Death from any cause was treated as the competing risk for acute GVHD, chronic GVHD and CMV/EBV viremia, while relapse and NRM were as the competing risk for each other. Excluding two patients with graft failure, 49 patients were eligible for acute GVHD evaluation. Forty-seven patients survived for more than 100 days after transplant and were eligible for chronic GVHD evaluation. Kaplan-Meier method was applied to estimate DFS and OS. Receiver operating characteristic (ROC) analysis was performed to determine an optimal cutoff value of ALC for acute GVHD of any grade.
All p-values are two-tailed, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 20.0 (SPSS, Inc. Chicago, IL, NY) and R software version 3.5.3 (http://www.r-project.org).
3 Results
3.1 Patient characteristics
Characteristics of total 51 patients are summarized in Table 1. The median age of all patients at transplantation was 39 years (range, 14–58 years). Thirty-one patients were diagnosed with acute myeloid leukemia, 17 with acute lymphoblastic leukemia/lymphoblastic lymphoma and 2 with chronic myelogenous leukemia. The median follow-up was 858 days (range, 25–2209 days). Before December of 2020, we applied 7.5 mg/Kg of ATG in our transplant protocol, and thereafter changed the dose of ATG to 5 mg/Kg. In total, 27 patients received 7.5 mg/Kg ATG and 24 patients received 5.0 mg/Kg ATG for GVHD prophylaxis. The median follow-up was 1359 days (range, 25–2209 days) and 792 days (range, 55–1157 days) for 7.5 mg/Kg and 5 mg/Kg ATG groups, respectively.
3.2 Hematopoietic recovery, regimen-related toxicity and virus reactivation
Median time to neutrophil and platelet engraftment was 11 days (range, 9–26 days) and 12 days (range, 9–135 days), respectively. In total, 1 patient had primary graft failure, 1 had secondary graft failure and 2 patients had primary delayed platelet engraftment. The patient with primary graft failure received 7.5 mg/Kg ATG and had positive class I donor-specific anti-HLA antibody (mean fluorescence intensity 8447) and desensitization treatments were performed before the transplant. The patient with secondary graft failure, who received 7.5 mg/Kg ATG, was possibly precipitated by virus infections (CMV and human herpesvirus 6).
Hemorrhagic cystitis occurred in 20 patients with 3 of grade III-IV. Transplant-associated thrombotic microangiopathy and capillary leak syndrome were documented in 1 and 2 patients, respectively. By day 180 after transplant, 4 patients had fungal infection. The 180-day CIs of CMV and EBV reactivation were 42.0% (95%CI, 28.1%-55.3%) and 62.7% (95%CI, 47.7%-74.6%), respectively. CMV disease was observed in 2 patients, presenting as CMV pneumonia in one case and CMV enteritis in the other. No post-transplant lymphoproliferative disorder was developed.
3.3 GVHD and transplant outcomes
The 100-day CIs of grade I-IV, II-IV and III-IV acute GVHD were 42.9% (95%CI, 28.8%-56.9%), 34.7% (95%CI, 21.2%-48.2%) and 12.2% (95%CI, 3.0%-21.5%), respectively (Figures 1A–C). The median time to onset of grade I-IV acute GVHD was 22 days (range, 8–97 days). Forty-seven patients survived for more than 100 days after transplant and were eligible for chronic GVHD evaluation. The 2-year CIs of overall and moderate-to-severe chronic GVHD were 44.7% (95%CI, 30.2%-59.1%) and 27.7% (95%CI, 14.7%-40.6%), respectively (Figures 1D, E). The median time to onset of chronic GVHD was 154 days (range, 100–341 days).
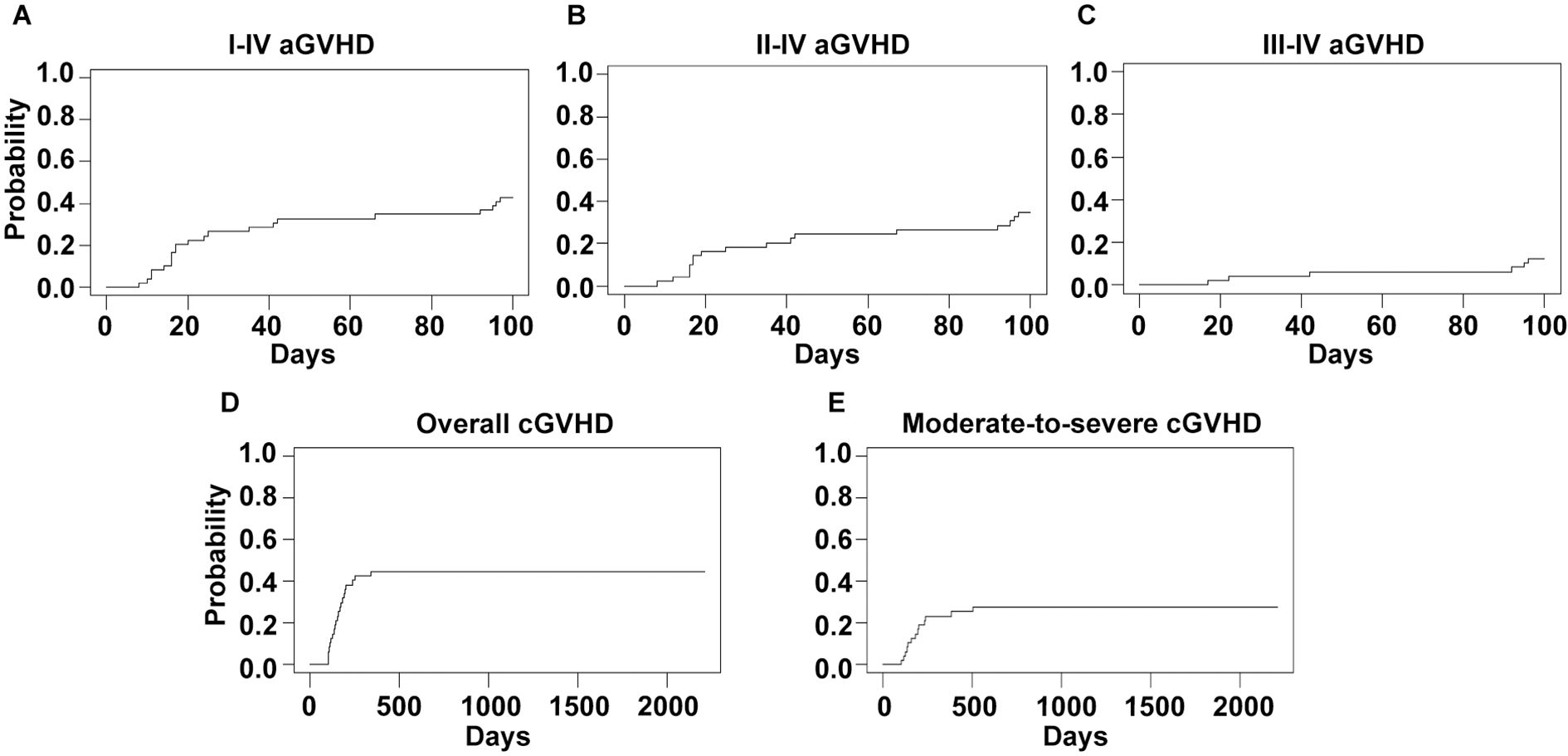
Figure 1. GVHD incidences of the whole cohort. Cumulative incidences of I-IV (A), II-IV (B) and III-IV (C) acute GVHD and overall (D) and moderate-to-severe (E) chronic GVHD are shown.
The 2-year OS, DFS, NRM and CIR were 66.7% (95%CI, 54.9- 80.9%), 54.8% (95%CI, 42.7-70.4%), 25.5% (95%CI, 13.4%-37.6%) and 19.7% (95%CI, 8.6%-30.8%), respectively (Figure 2). Up to the final follow-up, 10 patients relapsed. The median time to relapse was 359 days (range, 82–726 days). Fourteen patients experienced NRM. Causes of death were relapse in 8 (36%) patients, GVHD in 7 (32%) patients, infection in 5 (23%) patients and graft failure in 2 (9%) patients.
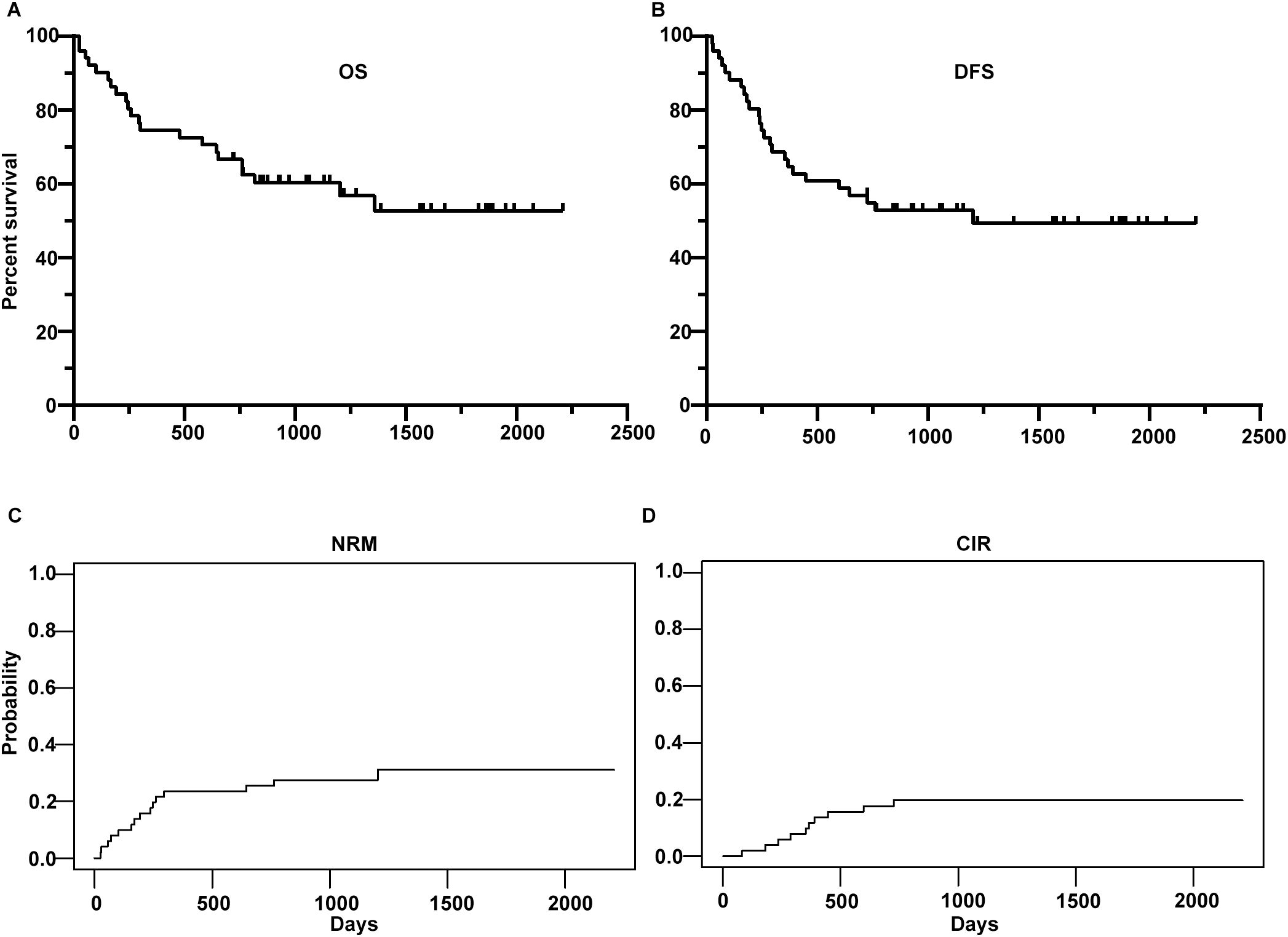
Figure 2. Overall survival (OS) (A), disease-free survival (DFS) (B), non-relapse mortality (NRM) (C) and cumulative incidence of relapse (CIR) (D) of the whole cohort.
3.4 Effect of ATG doses and pre-ATG absolute lymphocyte count on GVHD
In this study, two total ATG doses (7.5 and 5 mg/Kg) were applied in the conditioning regimen. However, no significant difference was observed regarding the CIs of grade I-IV (44.0% versus 41.7%, p=1.00), II-IV (32.0% versus 37.5%, p=0.69) or III-IV (12.0% versus 12.5%, p=0.97) acute GVHD (Figures 3A–C). The CIs of overall (37.5% versus 52.2%, p=0.31) and moderate-to-severe (20.8% versus 34.8%, p=0.28) chronic GVHD were similar as well (Figures 3D, E).
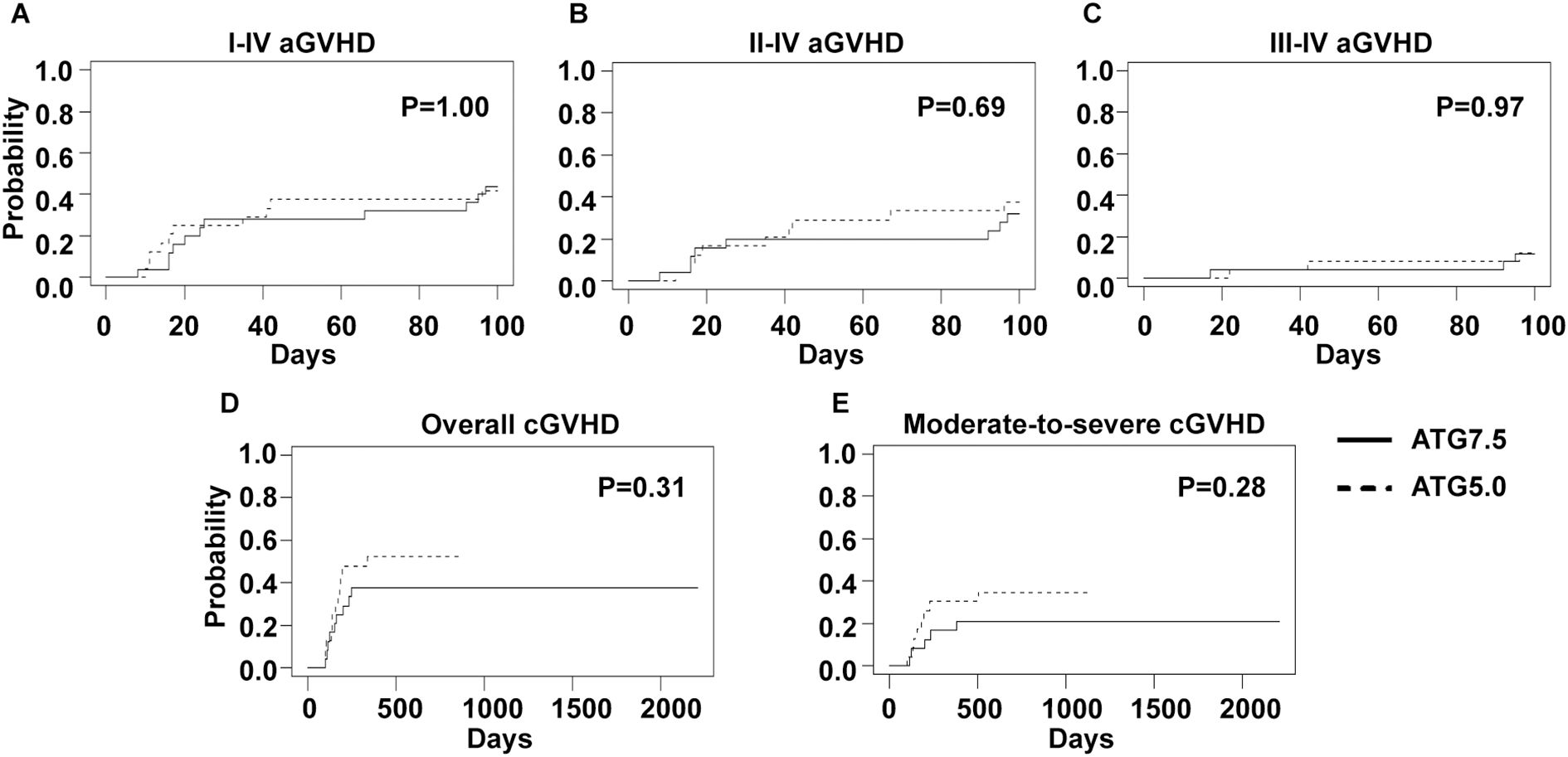
Figure 3. GVHD incidences of 7.5 mg/Kg and 5 mg/Kg ATG groups. Cumulative incidences of I-IV (A), II-IV (B) and III-IV (C) acute GVHD and overall (D) and moderate-to-severe (E) chronic GVHD are shown.
As absolute lymphocyte count on the first day of ATG administration (pre-ATG ALC) was reported as a risk factor for GVHD in the literature, we also explored its effect on GVHD incidences. The median value of pre-ATG ALC was 0.83×109/L (range, 0.01-2.48×109/L) and showed no significant difference between 7.5 mg/Kg and 5.0 mg/Kg ATG groups (median, 0.90×109/L versus 0.76×109/L, p=0.73). Then, an optimal pre-ATG ALC cutoff value of 0.585×109/L for grade I-IV and II-IV acute GVHD was calculated using ROC analysis. With this cutoff value, the whole cohort was divided into low (18 patients) and high (31 patients) pre-ATG ALC groups. Low ALC group showed lower CIs of grade I-IV (16.7% versus 58.0%, p=0.01), II-IV (16.7% versus 45.1%, p=0.06) and III-IV (0 versus 19.4%, p=0.05) acute GVHD (Figures 4A–C). However, CIs of chronic GVHD showed no significant difference between two ALC groups (Figures 4D, E).
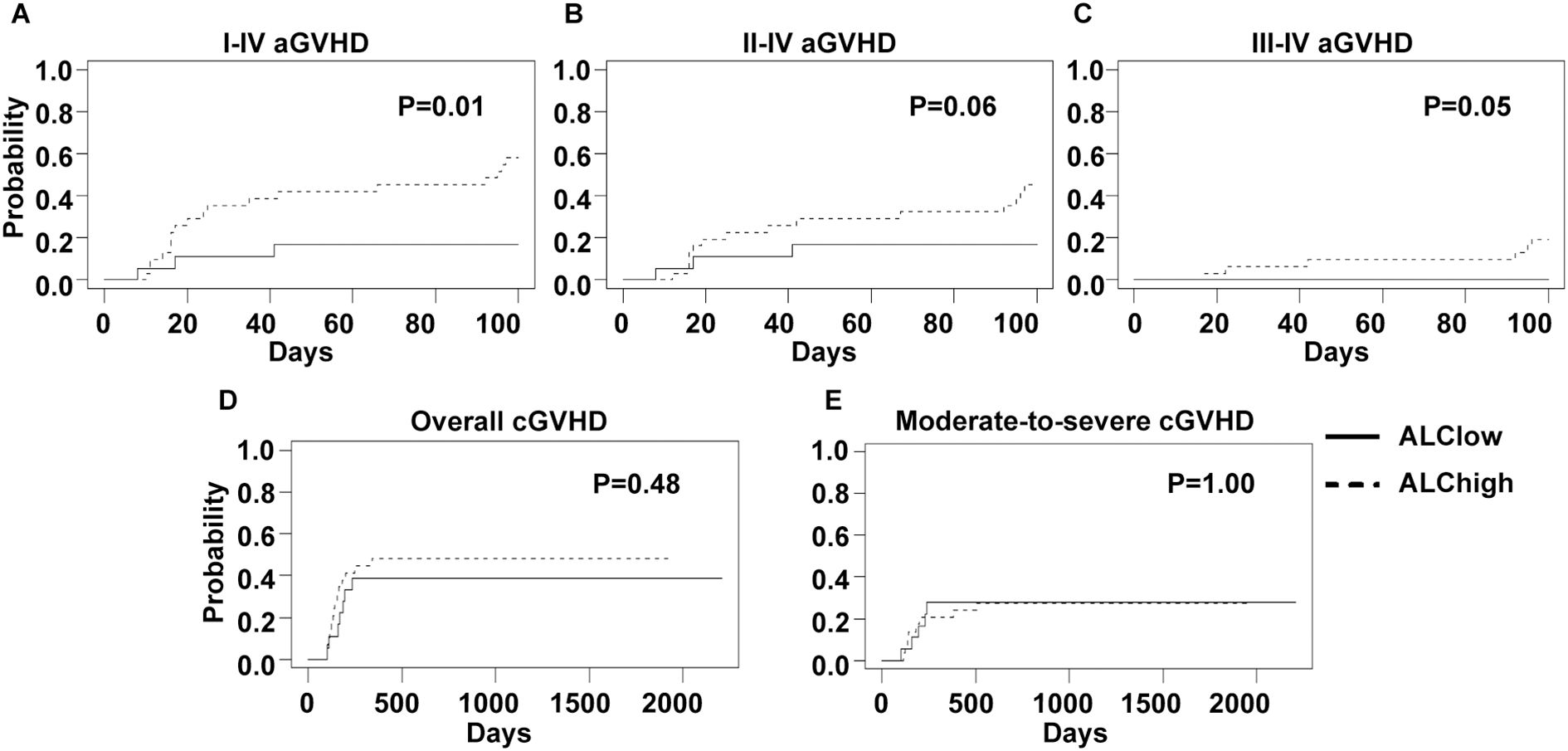
Figure 4. GVHD incidences of low and high ALC groups. An optimal ALC cutoff point of 0.585×109/L, calculated by ROC analysis, was used to divide all patients into low and high ALC groups. Cumulative incidences of I-IV (A), II-IV (B) and III-IV (C) acute GVHD and overall (D) and moderate-to-severe (E) chronic GVHD of two ALC groups are shown.
Furthermore, we analyzed the impact of different ATG doses and pre-ATG ALC levels on OS, DFS, NRM, CIR and CMV/EBV reactivation (Supplementary Figures 1, 2), and no significant differences were observed. The impact of disease status at transplantation (CR1 and non-CR1) and stem cell source (bone marrow included or not) on GVHD and survival was also analyzed. The patients in CR1 showed significant superior 2-year OS (77.1% versus 43.8%, p=0.03) and DFS (65.6% versus 31.3%, p=0.004) with similar GVHD incidences compared to those in non-CR1 (Supplementary Figure 3). The inclusion of bone marrow as stem cell source did not result in significant difference in GVHD incidence and survival (Supplementary Figure 4).
4 Discussion
ATG and PTCy are two main GVHD-prophylaxis strategies for unmanipulated haplo-HSCT. controversies still exist whether ATG or PTCy brings better survival after transplant (15–17). Alternatively, many transplant centers attempted to combine ATG and PTCy to further optimize their GVHD-prophylaxis regimens, in which various doses and timings of ATG and PTCy were applied (6, 13, 18). In the present study, we report the low-dose ATG/PTCy regimen for haplo-HSCT in our center and the data are comparable to those in the literature, in which ATG and/or PTCy were used for GVHD prophylaxis, regarding GVHD and survival (4, 17–20).
In late 2020, we modified the ATG dose of our protocol from 7.5 mg/Kg to 5.0 mg/Kg, based on the results of a multicenter randomized study reported by Lin et al. In this study, two doses (7.5 mg/kg and 10 mg/kg) of ATG without PTCy were compared in haplo-HSCT and 7.5 mg/kg ATG resulted in reduced EBV/CMV infections without increased incidence of GVHD (20). In our study, the influence of two ATG doses were also analyzed, and no differences were observed regarding incidences of GVHD, CMV/EBV reactivation and survival.
Interestingly, when the whole cohort was divided into two groups by an optimal pre-ATG ALC cutoff point of 0.585×109/L, low ALC group showed lower incidences of acute GVHD. In several studies on population pharmacokinetics of ATG, pre-ATG ALC was determined as an important factor impacting ATG levels (21, 22). As lymphocytes are the main “targets” of ATG, it is reasonable that ALC may influence the ATG level through target-mediated drug disposition (23). In addition, the correlation of pre-ATG ALC with transplant outcomes was also reported by several studies (24–26).
However, conflicting data also exist. Heelan et al. reported a retrospective study which included 111 patients receiving matched unrelated donor HSCT with ATG, and pre-ATG ALC did not correlate with GVHD, relapse or mortality (27). It is noteworthy that, in this study, the range of ALC was 0 - 0.19×109/L, much lower than that in other studies mentioned above. Takahashi et al. reported a novel population pharmacokinetics model for ATG in the HSCT setting with minimal pre-ATG ALC (range 0–0.058×109/L) (23). In this model, influential covariates include ideal body weight, baseline serum albumin level, baseline serum IgG level and CD4+ T cell graft dose, rather than ALC. Taken together, it seems that severe lymphopenia may diminish the impact of ALC on ATG level and HSCT outcomes.
In our study, the data favor low pre-ATG ALC level, which correlated with lower acute GVHD and similar survival outcomes, in line with the study of Jamani et al. (22) However, Woo et al. reported that, in the setting of matched related donor HSCT with 4.5 mg/kg total ATG dose, low ALC group (cutoff point 0.50×109/L) had significantly higher NRM and inferior OS, although GVHD incidences were lower (26). We consider that total ATG dose in our regimen is relatively lower than the “optimal” ATG dose, therefore patients may benefit from lower ALC. Therefore, the impact of ALC is supposed to be carefully evaluated for each transplant population and transplant regimen.
In addition, based on our data, it seems that 2.5 mg/kg more ATG dose (7.5 mg/Kg as compared to 5 mg/Kg) is not enough to overcome the increased risk of acute GVHD which high ALC brings. Other strategies should be tried to further optimize GVHD prophylaxis, for example, addition of other cytotoxic drugs, e.g. fludarabine, before ATG infusion to adjust pre-ATG ALC levels, or dose adjustment of PTCy based on pre-ATG ALC levels.
Besides the ATG dose and pre-ATG ALC, we also analyzed the impact of disease status at transplantation and stem cell source on GVHD and survival. Patients in CR1 showed superior survival outcomes compared to those in non-CR1, in line with the literature (19, 28). Bone marrow as stem cell source is generally thought to result in reduced GVHD incidence, though conflicting data still exist (28–30). In our study, inclusion of bone marrow as stem cell source did not show any advantage in GVDH incidence and survival. This might be because bone marrow was applied together with peripheral blood stem cell in most cases, and this is kind of a standard procedure in haplo-HSCT “Beijing protocol”.
Our study has limitations. First, it is a retrospective analysis with a limited number of HSCT patients. Multivariate analysis was not performed and further large prospective clinical trials are needed to confirm the impact of ATG doses and ALC in our low-dose ATG/PTCy regimen. Second, we did not monitor post-transplant immune reconstitution, e.g. CD4+/CD8+ T cell and NK cell recovery. These objective indicators might better show the impact of ATG doses and ALC. Third, it would be better to monitor ATG levels in this study. Data on ATG pharmacokinetics might provide more evidence to support our findings.
In summary, our study shows that our low-dose ATG/PTCy regimen for GVHD prophylaxis of haplo-HSCT is feasible and pre-ATG ALC has a great impact on acute GVHD incidence in our transplant system. Further attempts to improve GVHD prophylaxis based on ALC levels could be made in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Data curation, Formal analysis, Funding acquisition, Writing – original draft. XL: Data curation, Investigation, Writing – review & editing. JN: Data curation, Investigation, Writing – review & editing. MR: Data curation, Investigation, Writing – review & editing. ZL: Data curation, Investigation, Writing – review & editing. JD: Data curation, Investigation, Writing – review & editing. LL: Data curation, Investigation, Writing – review & editing. MyY: Data curation, Investigation, Writing – review & editing. ZZ: Data curation, Writing – review & editing. SZ: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. MzY: Conceptualization, Supervision, Writing – review & editing. QL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Anhui Province (NO. 2208085QH243), the Basic and Clinical Collaborative Research Improvement Project of Anhui Medical University (No. 2022xkjT021) and 2021 Clinical Research Startup Program of the First Affiliated Hospital of Anhui Medical University (LCYJ2021YB007).
Acknowledgments
We thank all patients and their families, and our transplant team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1569149/full#supplementary-material
References
1. Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: A multicenter, prospective study. Blood. (2015) 125:3956–62. doi: 10.1182/blood-2015-02-627786
2. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: A randomized trial. Bone Marrow Transplant. (2014) 49:426–33. doi: 10.1038/bmt.2013.191
3. Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological Malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. (2016) 17:164–73. doi: 10.1016/S1470-2045(15)00462-3
4. Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: long-term outcomes of a prospective randomized trial. Cancer. (2017) 123:2881–92. doi: 10.1002/cncr.30540
5. Bosch M, Dhadda M, Hoegh-Petersen M, Liu YP, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. (2012) 14:1258–75. doi: 10.3109/14653249.2012.715243
6. Wang Y, Chang YJ, Chen L, Xu LP, Bian ZL, Zhang XH, et al. Low-dose post-transplant cyclophosphamide can mitigate GVHD and enhance the G-CSF/ATG induced GVHD protective activity and improve haploidentical transplant outcomes. Oncoimmunology. (2017) 6:e1356152. doi: 10.1080/2162402X.2017.1356152
7. Wang Y, Wu D-P, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. (2019) 12:88. doi: 10.1186/s13045-019-0781-y
8. Salas MQ, Prem S, Atenafu EG, Law AD, Lam W, Al-Shaibani Z, et al. Dual T-cell depletion with ATG and PTCy for peripheral blood reduced intensity conditioning allo-hsct results in very low rates of GVHD. Bone Marrow Transplant. (2020). 55(9):1773–83. doi: 10.1038/s41409-020-0813-9
9. Law AD, Salas MQ, Lam W, Michelis FV, Thyagu S, Kim D, et al. Reduced-intensity conditioning and dual T lymphocyte suppression with antithymocyte globulin and post-transplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical hematopoietic stem cell transplants for hematological Malignancies. Biol Blood Marrow Transplant. (2018) 24:2259–64. doi: 10.1016/j.bbmt.2018.07.008
10. Deotare U, Atenafu EG, Loach D, Michelis FV, Kim D, Thyagu S, et al. Reduction of severe acute graft-versus-host disease using a combination of pre transplant anti-thymocyte globulin and post-transplant cyclophosphamide in matched unrelated donor transplantation. Bone Marrow Transplant. (2018) 53:361–5. doi: 10.1038/s41409-017-0053-9
11. Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral blood stem cell transplants. Eur J Haematol. (2019) 102:486–93. doi: 10.1111/ejh.13230
12. Dulery R, Menard AL, Chantepie S, El-Cheikh J, Francois S, Delage J, et al. Sequential conditioning with thiotepa in T cell-replete hematopoietic stem cell transplantation for the treatment of refractory hematologic Malignancies: comparison with matched related, haplo-mismatched, and unrelated donors. Biol Blood Marrow Transplant. (2018) 24:1013–21. doi: 10.1016/j.bbmt.2018.01.005
13. Zu Y, Gui R, Li Z, Wang J, Zhang Y, Yu F, et al. Low-dose PTCy plus low-dose ATG as GVHD prophylaxis after UD-PBSCT for hematologic Malignancies: A prospective, multicenter, randomized controlled trial. Blood Cancer J. (2023) 13:10. doi: 10.1038/s41408-022-00771-w
14. Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic Malignancies: A prospective, phase II study. Bone Marrow Transplant. (2019) 54:1049–57. doi: 10.1038/s41409-018-0382-3
15. Tang F, Xu Y, Chen H, Xu L, Zhang X, Wang Y, et al. Comparison of the clinical outcomes of hematologic Malignancies after myeloablative haploidentical transplantation with G-CSF/ATG and posttransplant cyclophosphamide: results from the Chinese bone marrow transplantation registry group (CBMTRG). Sci China Life Sci. (2019) 63:571–81. doi: 10.1007/s11427-019-9594-7
16. Arnon N, Abraham SK, Myriam L, Fabio C, Emanuele A, Yener K, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin for graft-versus-host disease prevention in haploidentical transplantation for adult acute lymphoblastic leukemia. Haematologica. (2021) 106:1591–8. doi: 10.3324/haematol.2020.247296
17. Ruggeri A, Sun YQ, Labopin M, Bacigalupo A, Lorentino F, Arcese W, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin as graft-versus-host disease prophylaxis in haploidentical transplant. Haematologica. (2017) 102:401–10. doi: 10.3324/haematol.2016.151779
18. Li X, Yang J, Cai Y, Huang C, Xu X, Qiu H, et al. Low-dose anti-thymocyte globulin plus low-dose post-transplant cyclophosphamide-based regimen for prevention of graft-versus-host disease after haploidentical peripheral blood stem cell transplants: A large sample, long-term follow-up retrospective study. Front Immunol. (2023) 14:1252879. doi: 10.3389/fimmu.2023.1252879
19. Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-Term Follow-up of Haploidentical Hematopoietic Stem Cell Transplantation without in Vitro T Cell Depletion for the Treatment of Leukemia Nine Years of Experience at a Single Center. Cancer. (2013) 119:978–85. doi: 10.1002/cncr.27761
20. Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two dose levels of rabbit antithymocyte globulin as graft-versus-host disease prophylaxis in haploidentical stem cell transplantation: A multicenter randomized study. BMC Med. (2019) 17:156. doi: 10.1186/s12916-019-1393-7
21. Admiraal R, van Kesteren C, Jol-van der Zijde CM, van Tol MJD, Bartelink IH, Bredius RGM, et al. Population pharmacokinetic modeling of thymoglobulin(®) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. (2015) 54:435–46. doi: 10.1007/s40262-014-0214-6
22. Jamani K, Dabas R, Kangarloo SB, Prokopishyn NL, Luider J, Dharmani-Khan P, et al. Rabbit antithymocyte globulin serum levels: factors impacting the levels and clinical outcomes impacted by the levels. Biol Blood Marrow Transplant. (2019) 25:639–47. doi: 10.1016/j.bbmt.2018.12.065
23. Takahashi T, Teramoto M, Matsumoto K, Jaber MM, Tamaki H, Ikegame K, et al. Population pharmacokinetics of total rabbit anti-thymocyte globulin in non-obese adult patients undergoing hematopoietic cell transplantation for hematologic Malignancy. Clin Pharmacokinet. (2023) 62:1081–91. doi: 10.1007/s40262-023-01252-4
24. Kennedy VE, Chen H, Savani BN, Greer J, Kassim AA, Engelhardt BG, et al. Optimizing antithymocyte globulin dosing for unrelated donor allogeneic hematopoietic cell transplantation based on recipient absolute lymphocyte count. Biol Blood Marrow Transplant. (2018) 24:150–5. doi: 10.1016/j.bbmt.2017.08.029
25. Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. (2017) 4:e183–e91. doi: 10.1016/s2352-3026(17)30029-7
26. Woo GU, Hong J, Kim H, Byun JM, Koh Y, Shin DY, et al. Preconditioning absolute lymphocyte count and transplantation outcomes in matched related donor allogeneic hematopoietic stem cell transplantation recipients with reduced-intensity conditioning and antithymocyte globulin treatment. Biol Blood Marrow Transplant. (2020) 26:1855–60. doi: 10.1016/j.bbmt.2020.06.005
27. Heelan F, Mallick R, Bryant A, Radhwi O, Atkins H, Huebsch L, et al. Does lymphocyte count impact dosing of anti-thymocyte globulin in unrelated donor stem cell transplantation? Biol Blood Marrow Transplant. (2020) 26:1298–302. doi: 10.1016/j.bbmt.2020.02.026
28. Sanz J, Labopin M, Blaise D, Raiola AM, Busca A, Vydra J, et al. Haploidentical stem cell donor choice for patients with acute myeloid leukemia: A study from the ALWP of the EBMT. Blood Adv. (2024) 8:2332–41. doi: 10.1182/bloodadvances.2023012133
29. Amin MK, Shahzad M, Al-Ramahi JS, DeJarnette MS, Lutfi F, Ahmed N, et al. Outcomes after bone marrow versus peripheral blood haploidentical hematopoietic stem cell transplantation using posttransplant cyclophosphamide-based GVHD prophylaxis. Transplant Cell Ther. (2024) 30:S241–S2. doi: 10.1016/j.jtct.2023.12.316
Keywords: anti-thymocyte globulin, post-transplant cyclophosphamide, graft-versus-host disease, haploidentical hematopoietic stem cell transplantation, absolute lymphocyte count
Citation: Hong J, Liang X, Ni J, Ruan M, Long Z, Dai J, Liang L, Yang M, Zhang Z, Zhang S, Ge J, Yang M and Li Q (2025) Low-dose ATG/PTCy for graft-versus-host disease prevention in haploidentical transplantation: a single-center experience. Front. Oncol. 15:1569149. doi: 10.3389/fonc.2025.1569149
Received: 31 January 2025; Accepted: 21 May 2025;
Published: 09 June 2025.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Alejandro Majlis, Santa Maria Clinic, ChileJian Zhou, Henan Provincial Cancer Hospital, China
Copyright © 2025 Hong, Liang, Ni, Ruan, Long, Dai, Liang, Yang, Zhang, Zhang, Ge, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsheng Li, bGlxaW5nc2hlbmdAYWhtdS5lZHUuY24=
 Jian Hong
Jian Hong Xinglin Liang1
Xinglin Liang1 Zhangbiao Long
Zhangbiao Long Mingya Yang
Mingya Yang Jian Ge
Jian Ge Mingzhen Yang
Mingzhen Yang