- 1Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 2Department of Clinic Specialistic and Stomatological Sciences, Polytechnic University of Marche, Ancona, Italy
- 3Department of Biomedicine, Neurosciences and Advanced Diagnostics (Bi.N.D.), University of Palermo, Palermo, Italy
Background: Optical Coherence Tomography (OCT) is an advanced imaging technique that is widely used in ophthalmology and is increasingly being applied in other fields of medicine. In oral oncology, OCT offers high-resolution, non-invasive (uses non-ionizing light), label-free, real-time imaging, providing detailed insights into tissue microanatomy and cellular structures, thus having the potential to improve early detection, monitoring and cost-effective screening of high-risk populations. However, significant challenges remain in applying OCT to OSCC and OPMDs, particularly in clinical practice.
Methods: A comprehensive search of PUBMED, SCOPUS, and Web of Science databases was performed up to October 2024. Additional manual searches were conducted by screening article bibliographies. Inclusion criteria encompassed studies published in English involving human subjects and evaluating the role of OCT in OSCC and OPMD assessment, OCT utilization for margin resection, and artificial intelligence (AI)-assisted interpretation of OCT images. After removal of duplicates and screening of titles and abstracts, full-text analysis was conducted on eligible studies.
Results: The technique has been investigated for its accuracy in identifying malignant changes in tissues before surgery and/or evaluating resection margins during surgery. Although early studies, primarily in animal models, have been extended to humans and have demonstrated the potential of OCT to accurately assess resection margins and identify precancerous lesions, significant limitations persist. The high cost of OCT equipment reduces its accessibility, availability and widespread use as a common investigation methodology in non-experimental settings. In addition, there is significant heterogeneity in the methodologies used to interpret OCT data, which is strictly operator dependent and may affect standardization and reproducibility of results. This is further complicated by the introduction and increased trend to adopt artificial intelligence (AI) algorithms in imaging evaluation. Machine learning and deep learning algorithms have shown superior diagnostic sensitivity and accuracy compared to clinician judgment. However, especially when used to assess resection margins, these algorithms may be significantly affected by sample extension and preparation, which remains a barrier to the routine clinical application of OCT systems.
Conclusion: Addressing the advantages and challenges of this emerging technique may help focus future research on standardizing application protocols and enhancing AI-assisted analysis to improve diagnostic performance and facilitate clinical translation.
1 Introduction
Oral squamous cell carcinoma (OSCC) is a significant global health problem affecting approximately 390,000 people worldwide (1). It is the most common type of head and neck cancer and its incidence has been increasing recently, accounting for almost 90% of all oral cancer cases (2). Although the oral cavity is anatomically accessible, making mucosal assessment relatively straightforward, OSCC diagnosis is often delayed, leading to persistently high rates of prevalence, incidence and mortality (3). Some patients may develop Oral Potentially Malignant Diseases (OPMDs) prior to tumor onset. These conditions, which are associated with an increased risk of cancer, present with a range of clinical manifestations, including red, white or ulcerated areas (4), with either exophytic or endophytic growth patterns (5). Larger lesions may have heterogeneous patterns with deeper areas, which can be difficult to assess during initial biopsy procedures, potentially requiring multiple biopsies and causing patient discomfort.
Currently, histological examination of biopsy samples is used for assessment and surgery remains the primary treatment for most cases (6). To minimize the need for multiple surgeries, the integration of non-invasive preoperative techniques into clinical practice could improve lesion characterization and facilitate better decision making in biopsy site selection and initial curative surgery (7). Improved preoperative knowledge of the microscopic features of the lesion could help surgeons to refine surgical protocols, determining both the extent of surgery required and a more accurate understanding of histopathological details.
Imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) are used for this purpose. MRI, in particular, provides excellent soft tissue contrast without exposure to ionizing radiation. However, MRI has significant drawbacks, including long waiting times - typically 65 to 105 days (8) - and high costs, requiring specialized facilities. In addition, around 10% of patients cannot undergo MRI due to factors such as metal implants or claustrophobia (9). These limitations have restricted the widespread use of MRI in the preoperative evaluation of patients with suspected OSCC and OPMDs.
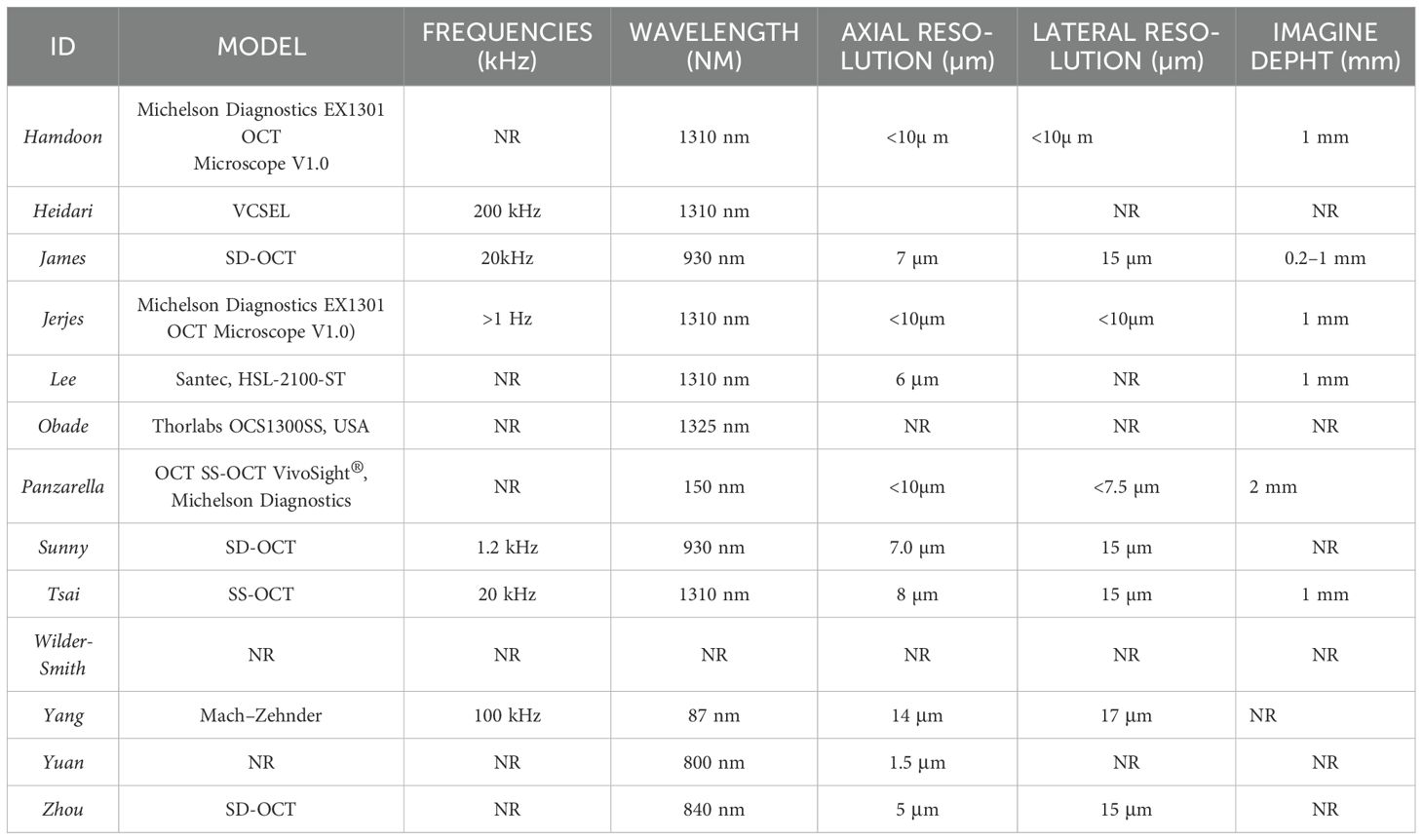
Widely recognized in ocular diagnostics, Optical Coherence Tomography (OCT) is increasingly applied in fields like dermatology, cardiology, odontology, gastroenterology, and oncology (10–12); Now, OCT’s use has been extended to the detection of early epithelial changes in head and neck, providing high-resolution images of tissue microanatomy and cellular structures of the mucous membranes (13, 14). This technique offers label-free, non-contact in vivo microscopy by utilizing non-ionizing visible light to analyze tissue optical properties (15–17) Employing a low-coherence broadband near-infrared light source, OCT achieves high spatial resolution (approximately 20 μm) and provides real-time imaging (18). The method is quick, repeatable, and well-tolerated by patients (19).overcoming the limitations associated with other imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). As a non-invasive, real-time imaging method, OCT provides valuable information suitable for assessing Oral squamous cell carcinoma (OSCC) and Oral potentially malignant diseases (OPMDs) and assists surgeons in evaluating resection margins (16).
Despite the numerous advantages mentioned above, a review of recent literature highlights several critical challenges associated with its clinical application. Therefore, the primary aim of this review is to highlight the challenges associated with the potential application of this technique in clinical practice.
2 Materials and method
A comprehensive search of PUBMED, SCOPUS, and Web of Science was performed up to October 2024, supplemented by manual searches of article bibliographies. Studies in English involving human subjects and evaluating the role of OCT in assessing OSCC and OPMD, the use of OCT in margin resection, and the application of AI for diagnosis using OCT were included.
3 Results
3.1 Study selection
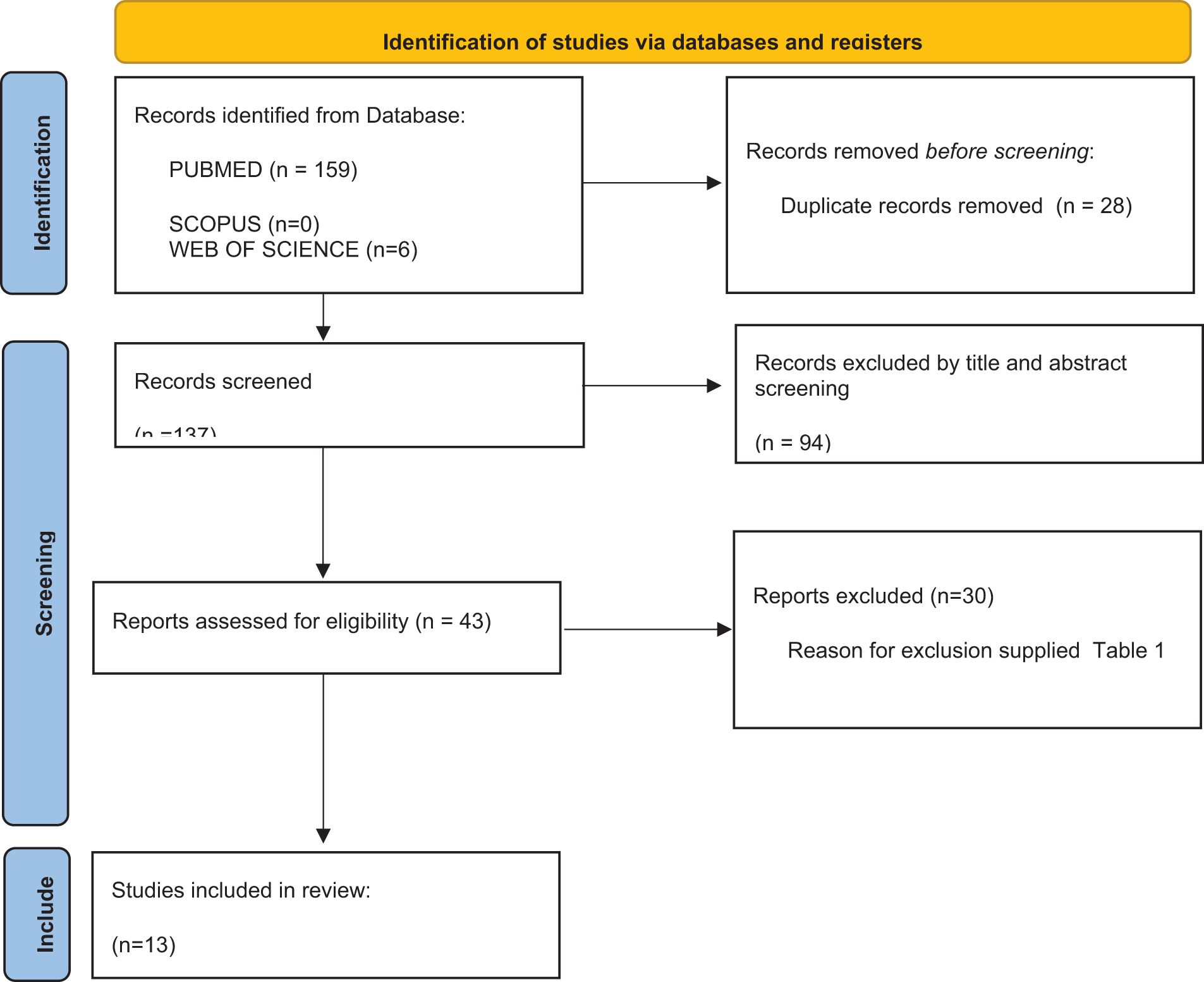
The electronic search retrieved a total of 165 articles from the electronic database search up to October 2024. After the removal of duplicates (28), title and abstract analysis was performed on 137 articles, of which 94 were excluded after the screening. Full text analysis was performed on the remaining 43 articles, and 30 articles were further excluded. The final review includes 13 articles (12, 16, 17, 20–29) (Figure 1).
3.2 Summary of included studies
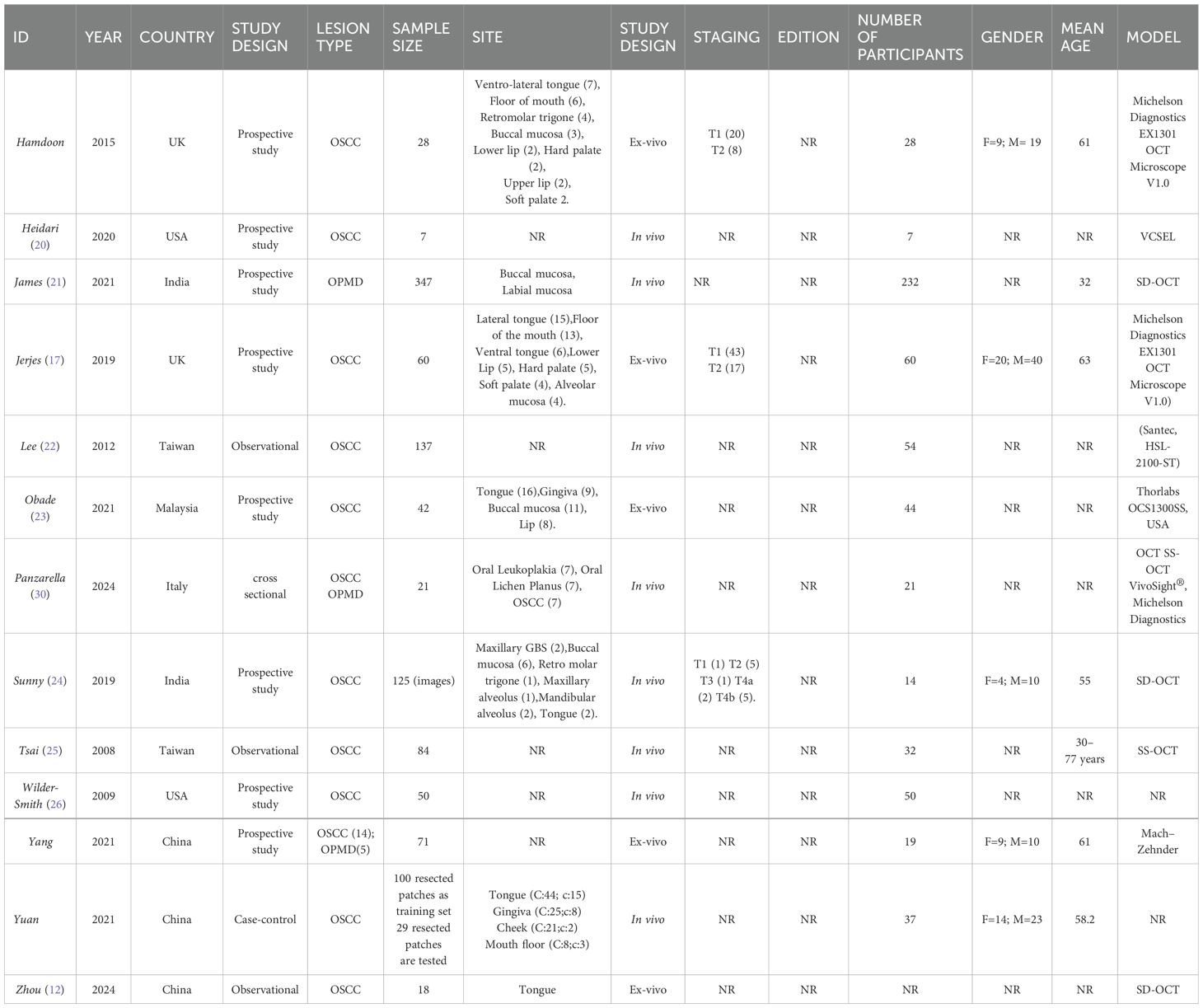
The eligible studies (Table 1) were conducted from 2008 to 2024, in various countries around the world, including: China (12, 27, 28), India (21–24), Italy (29), Malaysia (23), Taiwan (22–25), UK (16, 17) and USA (20–26).
Ten studies evaluated OSCC lesions (12, 16, 17, 20, 22–26, 28), one evaluated OPMD (21), and two evaluated both (27, 29).
In five studies (12, 16, 17, 23, 27) OCT was used ex-vivo, in the remaining eight (20–22, 24, 26, 30–32), it was used in-vivo.
The OCT data were interpreted in two different ways, by the use of AI (20, 21, 24, 27, 32) or by clinicians (12, 16, 17, 22, 23, 26, 30, 31) (Table 2).
4 Discussion
4.1 Limitations in oral clinical practice
The use of Optical Coherence Tomography (OCT) in oral pathology can be traced back to 2004, with studies conducted by Wilder-Smith, Chen, and Kim (10, 33–35); these early investigations focused exclusively on animal models.
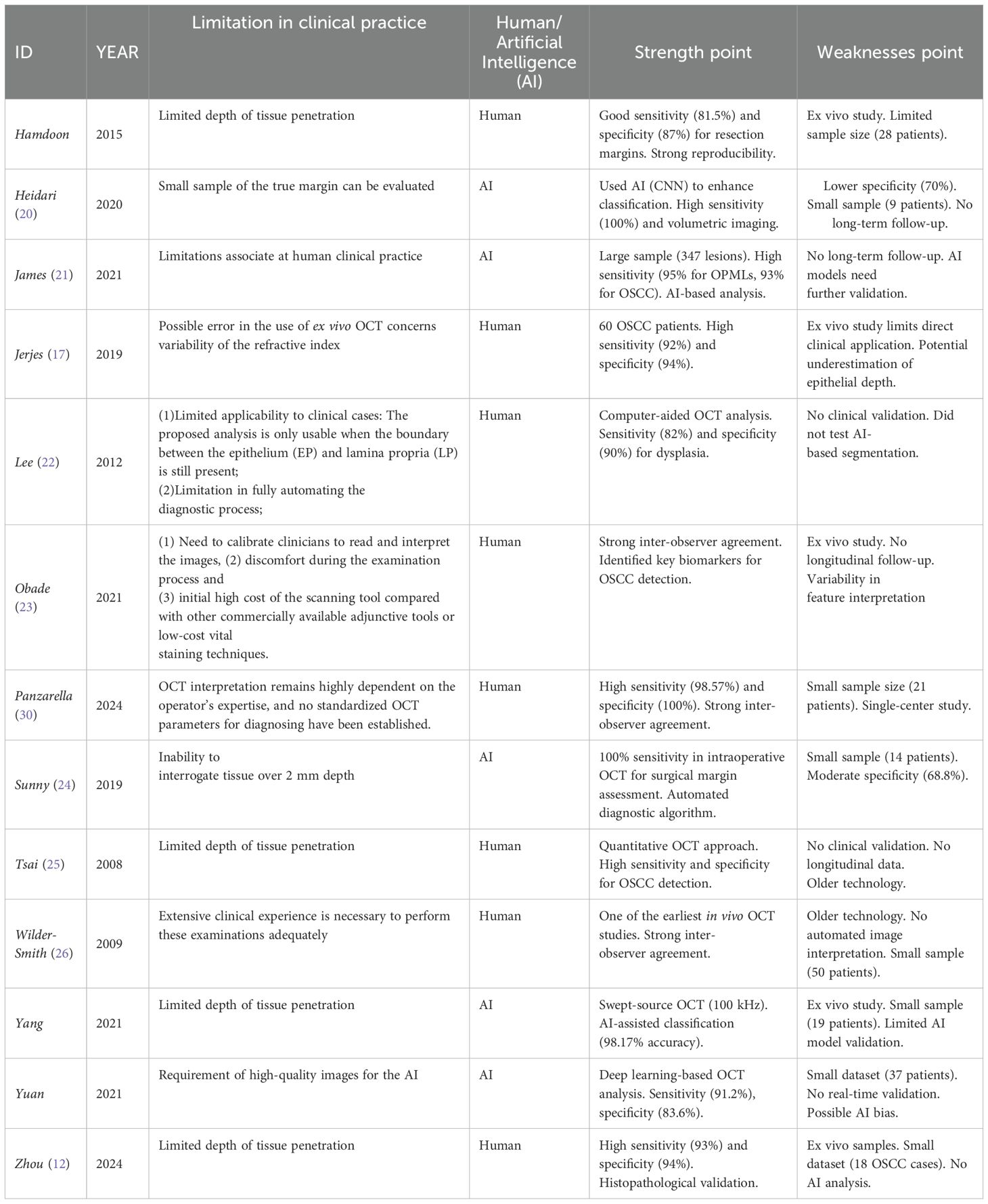
Based on the analysis of the studies available in the literature, screened according to the following inclusion criteria: studies published in English; cohort, case-control, retrospective observational, or longitudinal study designs; studies investigating the role of OCT in the assessment of OSCC and OPMD; and studies conducted on human subjects (Table 1). The first human studies using OCT were not conducted until 2006, possibly due to the high cost of the technology. A commercial OCT system can cost between $40,000 and $150,000, making it generally available only in hospitals (36); It is therefore essential to investigate the development of low-cost OCT systems to enable the application of this technology in large-scale population screening. The availability of affordable, portable, and user-friendly OCT systems will be crucial to facilitating its widespread clinical adoption (36).
The in vivo study by Ridgway et al. (37) showed that while OCT can visualize the most superficial epithelial layers, its ability to image the basement membrane and subsurface structures is significantly limited. Moreover, image quality heavily depends on the operator’s technique (38),making histological examination necessary for a more complete analysis.
The analysis of oral mucosa images obtained by OCT allowed the distinction between normal and moderately dysplastic or mildly dysplastic mucosa was analyzed in the study conducted by Lee et al. (22), with a sensitivity of 82% and a specificity of 90%, allowing near real-time diagnosis of precancerous conditions by OCT imaging. The same level of accuracy (89.6%) was also found in the study ex-vivo by Obade (23), who noted that the ability of OCT to observe the integrity of the basement membrane is a key parameter in detecting OSCC and differentiating OSCC from oral dysplasia or benign conditions. Both Wilder-Smith (26) and Tsai (25) reported an accuracy exceeding 90% (93.10% and 100%, respectively) when comparing OCT data with histological findings, highlighting OCT is potential as a valuable tool for the early detection and diagnosis of oral lesions. Similar results were observed in a more recent study (12), which found that the mean grey value (MGV) of OSCC in OCT images was significantly higher than that of the surrounding healthy tissue. This suggests that MGV could be a useful parameter for distinguishing tumors from normal tissue.
Moreover, in the studies conducted by Hamdoon Tsai, Yang, and Zhou, the most frequently criticized characteristic is the limited penetration depth (12, 16, 27, 31); which makes it impossible to analyze deeper tissue layers, a prerequisite for an accurate diagnosis. This would lead to an underestimation of the tumor staging (39).
A second aspect that has been analyzed in clinical practice is the possible use of OCT in surgical resection; the Hamdoon (16) study reported a sensitivity and specificity of 81.50% and 87% respectively for the ability of OCT to differentiate between tumor-free and tumor-involved surgical margins. The same level of accuracy was reported by Jerjes (17), who showed that the highest correlation was at 24 hours after resection (r = 0.964). In the buccal mucosal resection margins, OCT and histopathological measurements showed a much better correlation (r = 0.971) compared to other anatomical sites (floor of the mouth, soft palate, lateral tongue and ventral tongue), while the lowest correlation was found for the lower lip (r = 0.578).From Jerjes’ study, it also emerges that there is an underestimation of epithelial thickness: OCT tends to slightly underestimate epithelial thickness, with an average of 20µm in tumor-free margins and 10µm in tumor-involved margins (17). In the Sunny study, another limitation is that OCT has a limited penetration depth (approximately 2 mm), which presents a challenge in assessing deep margins (24). It is also worth noting that numerous studies (12, 16, 17, 23, 27) have used an OCT system to scan ex vivo oral tissues from tumor sections or biopsies, and compared the diagnoses made by OCT with those made by histopathological analysis. However, ex vivo studies do not consider potential motion artefacts, or the anatomical constraints associated with the clinical use of such devices.
4.2 Heterogeneity among the methodologies: human versus artificial intelligence
One of the major issues in the review of the literature regarding the use of this device in clinical practice is the great heterogeneity between studies (12, 16, 17, 20, 22–27, 30, 32) in the methods of interpretation of OCT-based imaging.
In the studies conducted by Zhou, Wilder-Smith, Tsai,Panzarella,Jerjes, Obade and Lee (12, 23, 25, 26, 30) the images obtained with OCT were compared with histological examination by two different clinicians, measuring the results obtained according to 5 parameters: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy.
Whereas, in the studies conducted by Heidari, Yuan, Yang, Sunny, and James (21, 24, 27, 32) the obtained images were interpreted using two models: the Artificial Neural Network (ANN) and Multi-Level Deep Residual Learning (MDRL). According to the study conducted by Heidari (20) Convolutional Neural Networks (CNNs) are powerful because they can identify distinct features within an image that yield the highest accuracy in classification. Also the paper of James et all (21) showed that OCT images analyzed by automated image processing algorithm (ANN-based analysis) could distinguish dysplastic-OPML and malignant lesions with a sensitivity of 95% and 93%, respectively. Sunny et al. (24) reported a potential clinical application using an artificial intelligence-based algorithm to identify tumor margin areas with 100% accuracy, achieving diagnostic results equivalent to histology (kappa, κ = 0.922); However, its inability to interrogate tissue over 2 mm depth has also been highlighted.
More recent studies (27, 28) have reached the same conclusions, stating that neural networks, both ANN and MDRL, can be used in clinical settings with an excellent diagnostic performance 99.04%,/91.2% sensitivity, 98.81%/83.6% specificity, 98.63/87.5% accuracy respectively (Table 3).
5 Limitations
This study has many limitations. One of the main limitations is the heterogeneity of the study designs, as the included studies differ significantly in their methodological approaches. Some are prospective while others are retrospective, leading to potential biases in data collection and interpretation. In addition, the comparison between in vivo and ex vivo studies introduces further variability, as differences in tissue handling and imaging conditions may affect the reliability and consistency of reported findings. Another critical limitation is the variability in the anatomical regions analyzed between studies. Different sites within the oral cavity (e.g. tongue, buccal mucosa, floor of the mouth) have different histological and structural characteristics that may influence the optical coherence tomography (OCT) signal and its interpretation. In addition, the lack of studies with complete data sets is a significant barrier to drawing definitive conclusions. Many studies do not report key patient demographics such as age and gender. The inconsistencies in data availability and reporting further complicate efforts to establish standardized diagnostic criteria and guidelines for the clinical use of OCT in oral oncology. Addressing these limitations in future research through standardized study protocols, comprehensive data collection and consistent reporting criteria will be essential to improve the reliability and applicability of OCT in clinical practice.
6 Conclusions
OCT holds significant potential for clinical applications, such as guiding biopsy site selection, monitoring lesions, and serving as a rapid, cost-effective screening tool for high-risk populations.
However, the currently available evidence in the literature remains highly heterogeneous, ranging from differences in study methodologies (in vivo vs. ex vivo) to variability in the anatomical sections analyzed. Further studies and standardization are certainly necessary for an accurate diagnosis.
OCT has demonstrated high diagnostic accuracy in differentiating between normal, dysplastic and malignant tissue, with sensitivity and specificity often exceeding 80-90%. In addition, OCT’s ability to assess basement membrane integrity has been recognized as a key factor in distinguishing OSCC from other oral conditions. However, several limitations remain, in particular the limited penetration depth of the technology, which limits its ability to analyze deeper tissue layers. This limitation could lead to underestimation of tumor staging and challenges in surgical margin assessment, as highlighted by several studies. In addition, operator-dependent image quality and underestimation of epithelial thickness further complicate its clinical application. Advanced algorithms, including machine learning, deep learning, and artificial intelligence, have demonstrated significantly higher diagnostic sensitivity and, in many cases, greater diagnostic accuracy compared to clinician expertise, but still not reliable enough Moreover, the increasing reliance on such adjuncts could potentially reduce clinicians’ expertise in interpreting OCT images.
Author contributions
FE: Writing – original draft, Writing – review & editing. DC: Data curation, Formal analysis, Writing – review & editing. ML: Data curation, Formal analysis, Writing – review & editing. AS: Validation, Writing – review & editing. LM: Validation, Writing – review & editing. GC: Supervision, Validation, Writing – review & editing. LR: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Health Extended Alliance for Innovative Therapies, Advanced Lab-research, and Integrated Approaches of Precision Medicine—acronimo HEAL ITALIA (grant number PE_00000019—CUP D73C22001230006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Almangush A, Mäkitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A, et al. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol. (2020) 107:104799. doi: 10.1016/j.oraloncology.2020.104799
3. Coppola N, Mignogna MD, Rivieccio I, Blasi A, Bizzoca ME, Sorrentino R, et al. Current knowledge, attitudes, and practice among health care providers in OSCC awareness: systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18:4506. doi: 10.3390/ijerph18094506
4. Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. (2010) 46:414–7. doi: 10.1016/j.oraloncology.2010.03.009
5. Santosh AB, Boyd D, Laxminarayana KK. Proposed clinico-pathological classification for oral exophytic lesions. J Clin Diagn Res. (2015) 9:ZE01–8. doi: 10.7860/JCDR/2015/12662.6468
6. Rocchetti F, Tenore G, Montori A, Cassoni A, Cantisani V, Di Segni M, et al. Preoperative evaluation of tumor depth of invasion in oral squamous cell carcinoma with intraoral ultrasonography: a retrospective study. Surg Med Pathol Radiol. (2021) 131:130–8. doi: 10.1016/j.oooo.2020.07.003
7. Contaldo M, Poh CF, Guillaud M, Lucchese A, Rullo R, Lam S, et al. Oral mucosa optical biopsy by a novel handheld fluorescent confocal microscope specifically developed: technologic improvements and future prospects. Surg Med Pathol Radiol. (2013) 116:752–8. doi: 10.1016/j.oooo.2013.09.006
8. Sun YC, Wu HM, Guo WY, Ou YY, Yao MJ, Lee LH. Simulation and evaluation of increased imaging service capacity at the MRI department using reduced coil-setting times. PLoS One. (2023) 18:e0288546. doi: 10.1371/journal.pone.0288546
9. Arnold TC, Freeman CW, Litt B, Stein JM. Low-field MRI: Clinical promise and challenges. J Magn Reson Imaging. (2023) 57:25–44. doi: 10.1002/jmri.28408
10. Chen SF, Lu CW, Tsai MT, Wang YM, Yang C, Chiang CP. Oral cancer diagnosis with optical coherence tomography. Conf Proc IEEE Eng Med Biol Soc. (2005) 2005:7227–9. doi: 10.1109/IEMBS.2005.1616178
11. Zhang T, Shepherd S, Huang Z, Macluskey M, Li C. Development of an intraoral handheld optical coherence tomography-based angiography probe for multi-site oral imaging. Opt Lett. (2023) 48:4857–60. doi: 10.1364/OL.497080
12. Zhou K, Zheng K, Huang L, Zheng X, Jiang C, Huang J, et al. Discrimination of healthy oral tissue from oral cancer based on the mean grey value determined by optical coherence tomography. BMC Health. (2024) 24:1004. doi: 10.1186/s12903-024-04741-5
13. Kim DH, Kim SW, Hwang SH. Efficacy of optical coherence tomography in the diagnosing of oral cancerous lesion: systematic review and meta-analysis. Head Neck. (2023) 45:473–81. doi: 10.1002/hed.27232
14. Kim JS, Kim BG, Hwang SH. Efficacy of artificial intelligence-assisted discrimination of oral cancerous lesions from normal mucosa based on the oral mucosal image: A systematic review and meta-analysis. Cancers (Basel). (2022) 14(14):3499. doi: 10.3390/cancers14143499
15. Gambino A, Martina E, Panzarella V, Ruggiero T, Haddad GE, Broccoletti R, et al. Potential use of optical coherence tomography in oral potentially Malignant disorders: in-vivo case series study. BMC Health. (2023) 23:540. doi: 10.1186/s12903-023-03263-w
16. Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn Ther. (2016) 13:211–7. doi: 10.1016/j.pdpdt.2015.07.170
17. Jerjes W, Hamdoon Z, Yousif AA, Al-Rawi NH, Hopper C. Epithelial tissue thickness improves optical coherence tomography’s ability in detecting oral cancer. Photodiagnosis Photodyn Ther. (2019) 28:69–74. doi: 10.1016/j.pdpdt.2019.08.029
18. Hsieh YS, Ho YC, Lee SY, Chuang CC, Tsai JC, Lin KF, et al. Dental optical coherence tomography. Sensors (Basel). (2013) 13:8928–49. doi: 10.3390/s130708928
19. Ramezani K, Tofangchiha M. Oral cancer screening by artificial intelligence-oriented interpretation of optical coherence tomography images. Radiol Res Pract. (2022) 2022:1614838. doi: 10.1155/2022/1614838
20. Heidari AE, Pham TT, Ifegwu I, Burwell R, Armstrong WB, Tjoson T, et al. The use of optical coherence tomography and convolutional neural networks to distinguish normal and abnormal oral mucosa. J Biophotonics. (2020) 13:e201900221. doi: 10.1002/jbio.201900221
21. James BL, Sunny SP, Heidari AE, Ramanjinappa RD, Lam T, Tran AV, et al. Validation of a point-of-care optical coherence tomography device with machine learning algorithm for detection of oral potentially Malignant and Malignant lesions. Cancers (Basel). (2021) 13(14):3583. doi: 10.3390/cancers13143583
22. Lee CK, Chi TT, Wu CT, Tsai MT, Chiang CP, Yang CC. Diagnosis of oral precancer with optical coherence tomography. BioMed Opt Express. (2012) 3:1632–46. doi: 10.1364/BOE.3.001632
23. Obade AY, Pandarathodiyil AK, Oo AL, Warnakulasuriya S, Ramanathan A. Application of optical coherence tomography to study the structural features of oral mucosa in biopsy tissues of oral dysplasia and carcinomas. Clin Investig. (2021) 25:5411–9. doi: 10.1007/s00784-021-03849-0
24. Sunny SP, Agarwal S, James BL, Heidari E, Muralidharan A, Yadav V, et al. Intra-operative point-of-procedure delineation of oral cancer margins using optical coherence tomography. Oncol. (2019) 92:12–9. doi: 10.1016/j.oraloncology.2019.03.006
25. Tsai MT, Lee HC, Lee CK, Yu CH, Chen HM, Chiang CP, et al. Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt Express. (2008) 16:15847–62. doi: 10.1364/oe.16.015847
26. Wilder-Smith P, Lee K, Guo S, Zhang J, Osann K, Chen Z, et al. In vivo diagnosis of oral dysplasia and Malignancy using optical coherence tomography: preliminary studies in 50 patients. Lasers Surg Med. (2009) 41:353–7. doi: 10.1002/lsm.20773
27. Yang Z, Shang J, Liu C, Zhang J, Liang Y. Identification of oral precancerous and cancerous tissue by swept source optical coherence tomography. Lasers Surg Med. (2022) 54:320–8. doi: 10.1002/lsm.23461
28. Yuan W, Yang J, Yin B, Fan X, Yang J, Sun H, et al. Noninvasive diagnosis of oral squamous cell carcinoma by multi-level deep residual learning on optical coherence tomography images. Oral Dis. (2023) 29:3223–31. doi: 10.1111/odi.14318
29. Panzarella V, Buttacavoli F, Gambino A, Capocasale G, Di Fede O, Mauceri R, et al. Site-coded oral squamous cell carcinoma evaluation by optical coherence tomography (OCT): A descriptive pilot study. Cancers (Basel). (2022) 14(23):5916. doi: 10.3390/cancers14235916
30. Panzarella V, Buttacavoli F, Rodolico V, Maniscalco L, Firenze A, De Caro V, et al. Application of targeted optical coherence tomography in oral cancer: A cross-sectional preliminary study. Diagnostics. (2024) 14:2247. doi: 10.3390/diagnostics14192247
31. Tsai MT, Lee CK, Lee HC, Chen HM, Chiang CP, Wang YM, et al. Differentiating oral lesions in different carcinogenesis stages with optical coherence tomography. J BioMed Opt. (2009) 14:044028. doi: 10.1117/1.320093610.1117/1.3200936
32. Yuan W, Cheng L, Yang J, Yin B, Fan X, Yang J, et al. Noninvasive oral cancer screening based on local residual adaptation network using optical coherence tomography. Med Biol Eng Comput. (2022) 60:1363–75. doi: 10.1007/s11517-022-02535-x
33. Kim CS, Wilder-Smith P, Ahn YC, Liaw LH, Chen Z, Kwon YJ. Enhanced detection of early-stage oral cancer in vivo by optical coherence tomography using multimodal delivery of gold nanoparticles. J BioMed Opt. (2009) 14:034008. doi: 10.1117/1.3130323
34. Wilder-Smith P, Jung WG, Brenner M, Osann K, Beydoun H, Messadi D, et al. In vivo optical coherence tomography for the diagnosis of oral Malignancy. Lasers Surg Med. (2004) 35:269–75. doi: 10.1002/lsm.20098
35. Wilder-Smith P, Krasieva T, Jung WG, Zhang J, Chen Z, Osann K, et al. Noninvasive imaging of oral premalignancy and Malignancy. J BioMed Opt. (2005) 10:051601. doi: 10.1117/1.2098930
36. Song G, Jelly ET, Chu KK, Kendall WY, Wax A. A review of low-cost and portable optical coherence tomography. Prog BioMed Eng (Bristol). (2021) 3:032002. doi: 10.1088/2516-1091/abfeb7
37. Ridgway JM, Armstrong WB, Guo S, Mahmood U, Su J, Jackson RP, et al. In vivo optical coherence tomography of the human oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. (2006) 132:1074–81. doi: 10.1001/archotol.132.10.1074
38. Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. (2004) 137:156–69. doi: 10.1016/s0002-9394(03)00792-x
Keywords: OCT, OSCC, OPMD, limitation, AI
Citation: Esperouz F, Ciavarella D, Lorusso M, Santarelli A, Lo Muzio L, Campisi G and Lo Russo L (2025) Critical review of OCT in clinical practice for the assessment of oral lesions. Front. Oncol. 15:1569197. doi: 10.3389/fonc.2025.1569197
Received: 31 January 2025; Accepted: 16 April 2025;
Published: 08 May 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Mohammad Hasan Yousefi, Qom University of Medical Sciences, IranJan Dirk Raguse, Fachklinik Hornheide, Germany
Copyright © 2025 Esperouz, Ciavarella, Lorusso, Santarelli, Lo Muzio, Campisi and Lo Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fariba Esperouz, ZmFyaWJhLmVzcGVyb3V6QHVuaWZnLml0
 Fariba Esperouz
Fariba Esperouz Domenico Ciavarella
Domenico Ciavarella Mauro Lorusso
Mauro Lorusso Andrea Santarelli
Andrea Santarelli Lorenzo Lo Muzio
Lorenzo Lo Muzio Giuseppina Campisi
Giuseppina Campisi Lucio Lo Russo
Lucio Lo Russo