- 1Department of Medical Oncology, The First People’s Hospital of Linping, Hangzhou, Zhejiang, China
- 2Department of Pathology, The First People’s Hospital of Linping, Hangzhou, Zhejiang, China
Lung cancer (LC) is a significant global health concern, underscoring the need for ongoing research into novel therapeutic modalities. Trophoblast cell surface antigen-2 (TROP2) is overexpressed in tumor tissues and minimally expressed in normal tissues, making it a promising target for cancer treatment. A TROP2-targeted antibody-drug conjugate (ADC) has been approved by the Food and Drug Administration (FDA). This review aims to provide a comprehensive overview of the characteristics of TROP2 and its role in cancer development. It is imperative to acknowledge the significant advancements made in the realm of LC therapy through the development of ADCs that specifically target the TROP2 antigen. The potential of the TROP2-ADC in the treatment of LC is a subject of considerable promise, suggesting a promising future in the therapeutic management of this condition.
1 Introduction
Lung cancer (LC) is a neoplasm with high global morbidity and mortality, accounting for 12.4% of new cases and 18.7% of deaths worldwide (1). The substantial basic, economic, and social burden necessitates the urgent development of effective treatment programs. The advent of targeted therapy and immunotherapy has marked a paradigm shift in the therapeutic landscape of LC (2). Nevertheless, the treatment of advanced LC patients with actionable genomic alterations and genetic mutations remains a formidable challenge. Intractable problems following include the lack of effective treatments after first-line treatment resistance, including the limited efficacy of back-line chemotherapy, chemotherapy combined with immunotherapy, and toxic side effects (3). To address these challenges, there is an urgent need to promote the development of novel drugs and treatment options.
Trophoblast cell surface antigen-2 (TROP2) is expressed at high levels in a variety of solid cancer cells and has been shown to affect signaling pathways involved in cancer proliferation, migration, invasion, and metastasis (4). TROP2 exhibits frequent overexpression across major histological subtypes of lung cancer, with particularly high prevalence observed in squamous cell carcinoma (approximately 60%), and adenocarcinoma (42%-64%) (5, 6). However, the responsiveness to TROP2 targeted therapeutics is depend on lung cancer subtype, rather than the expression of TROP2 (5). Squamous lung cancer appears to respond better to targeted TROP2 therapy than adenocarcinoma lung cancer. Therefore, TROP2 can be regarded as a viable target for the treatment of lung cancer, particularly in the context of NSCLC (7). This review will focus on the relationship between TROP2 and cancers, emphasizing the development of TROP2-ADC.
2 Structure and function of TROP2
The TACSTD family comprises TACSTD1 and TACSTD2, two genes that are highly conserved and closely related, and respectively encode TROP2 and epithelial cell-adhesion molecule (EpCAM) (8). TROP2 functions as a single transmembrane protein comprising an extracellular domain (ECD), a single transmembrane domain, and a short cytoplasmic tail (7). The ECD contains a Cysteine-Rich Domain (CRD), a Tyrosine Cluster Domain (TY), and a Cysteine-deficient Domain (CPD), which collectively contribute to the formation of a stable dimer (Figure 1) (9, 10). TROP2 exhibits low expression levels in normal epithelial cells and high expression levels in many epithelial tumors (e.g., colon, pancreas, and breast) (Figure 1) (9). The ECD of TROP2 is anchored to the cell membrane by a unidirectional transmembrane helix (TM) attached to the intracellular structural domain (ICD) (Figure 1). The ECD of TROP2 affects signaling transduction by a conserved phosphatidylinositol-4,5-bisphosphate (PIP2) binding sequence (11). The intracellular structural domains contain sites that interact with a variety of signaling proteins, such as the phosphorylation site of protein kinase C (PKC) (12).
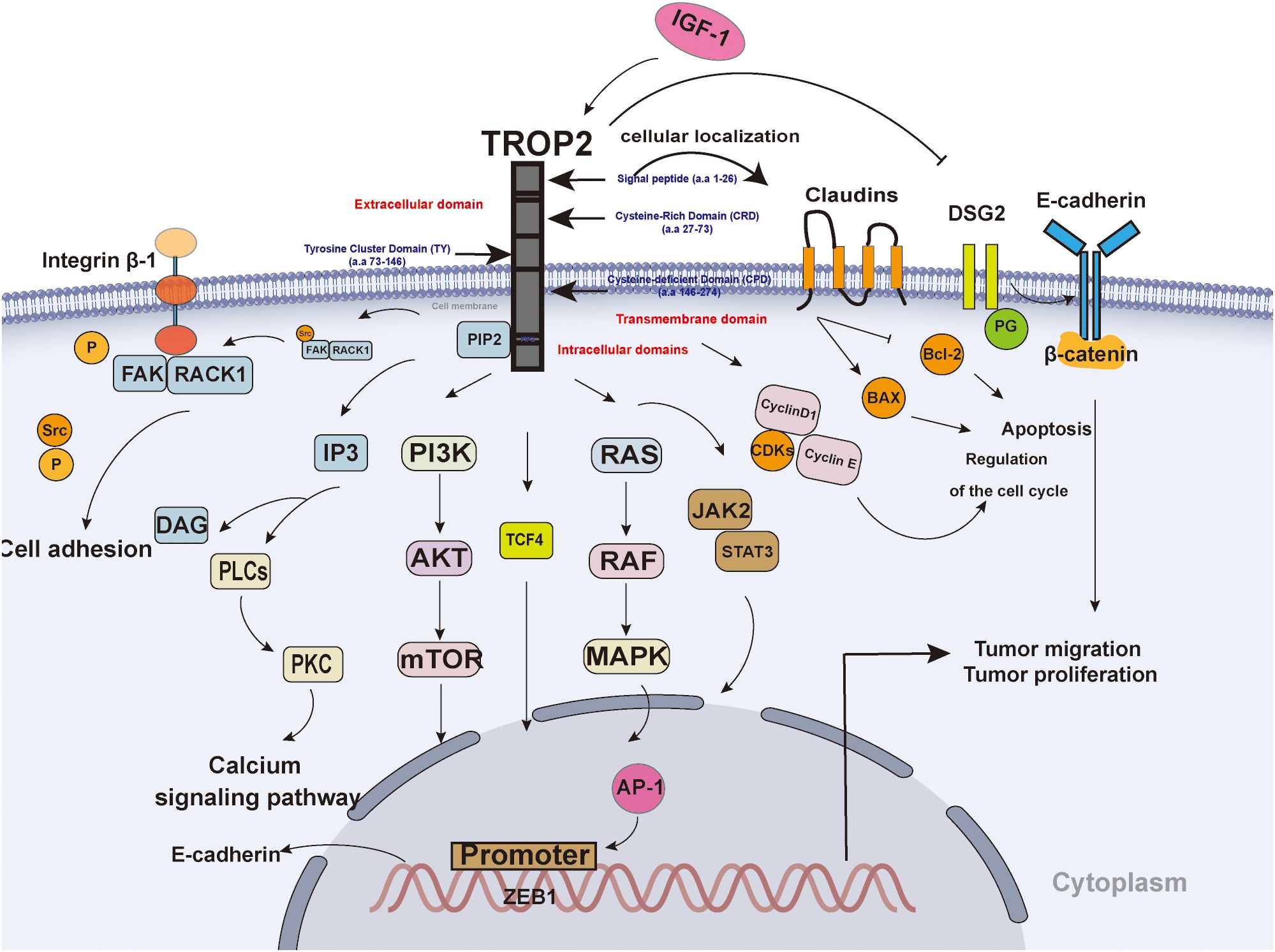
Figure 1. The Structure and signaling pathway of TROP2. TROP2 consists of an extracellular domain, a transmembrane domain, and an intracellular domain. TROP2 affects tumor proliferation and migration through multiple pathways: 1) TROP2 interacts with IGF-1)and affects downstream signaling, such as the PI3K-AKT and MAPK pathways; 2) TROP2 regulates the expression of cyclin D1, cyclin E, and CDK to promote the cell cycle; 3) TROP2 promotes the transition from PIP2 to IP3 and DAG;4) TROP2 promotes the recruitment of RACK1 and contributes to spatial coupling between FAK and β1-integrin; 5) TROP2 transcriptionally regulates ZEB1 expression, contributes to the expression of E-cadherin; 6) TROP2 decreases the expression level of DSG2 to promote tumor cell invasion and migration by EGFR-AKT and DSG2-PG-β-catenin pathways; 7) TROP2 downregulated Bcl-2 and upregulated Bax; 8) TROP2 promotes cell motility and claudin-7 localization to cellular borders; 0) TROP2 promote JAK2-STAT3 signaling pathway.
TROP2 has been shown to promote tumor cell proliferation by regulating the calcium signaling pathway, cell cycle protein expression and reducing fibronectin adhesion. TROP2 interact with insulin-like growth factor 1 (IGF-1) and its ligands to affect the upstream (Figure 1) (13). The S303 site of the intracellular domain of TROP2 undergoes phosphorylation, which in turn promotes a series of critical intracellular signals, including the transition from PIP2 to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (14). This, in turn, has been shown to stimulate the mitogen-activated protein kinase (MAPK) signaling pathway and promote the activation of activator protein 1(AP-1) (Figure 1) (14). Furthermore, the activated MAPK signaling pathway has been associated with epithelial-mesenchymal transition (EMT), which in turn affects tumor invasion (15). The transcription factor AP-1 has been identified as a contributing factor to angiogenesis (16). TROP2 promote growth and proliferation through the activation of the janus kinase 2 (JAK2)- signal transducer and activator of transcription 3(STAT3) pathway (17). In addition, TROP2 upregulated B-cell lymphoma 2 (Bcl-2) and downregulated Bcl-2-associated X protein (Bax), promoting tumorigenesis and tumor progression (18). Further, TROP2 has been shown to regulate the expression of cell cycle proteins, including CyclinD1, CyclinE, and CDK proteins such as cyclin-dependent kinase 2 (CDK2) and CDK4, thereby promoting cell cycle progression (Figure 1) (19). Ser-322 by PKCα/phosphorylates TROP2 to promote cell motility and claudin-7 localization to cellular borders (20). Further, β-conjugate protein/transcription factor 4 (TCF4) has the capacity to bind to the C-terminal fragment of TROP2 to the promoter of zinc finger E-Box binding homeobox 1 (ZEB1), and upregulate of ZEB1 expression (21). As a result, The ZEB1 contributes to the expression of E-cadherin, and influence tumor aggressiveness and metastatic capacity (21). Besides, overexpression of TROP2 decreases the expression level of desmoglein 2 (DSG2), activates EGFR-AKT and DSG2-plakoglobin(PG)-β-catenin pathways to promote tumor cell invasion and migration (22). TROP2 upregulation promotes the membrane translocation of receptor for activated C kinase 1 (RACK1), thereby establishing spatial coupling between focal adhesion kinase (FAK) and β1-integrin (Figure 1) (23). This interaction triggers FAK activation through autophosphorylation at Tyr397, which releases Src from the complex and activates it (23). Ultimately, this leads to a reduction in tumor cell adhesion (Figure 1) (23).
3 TROP2-targeted therapeutics
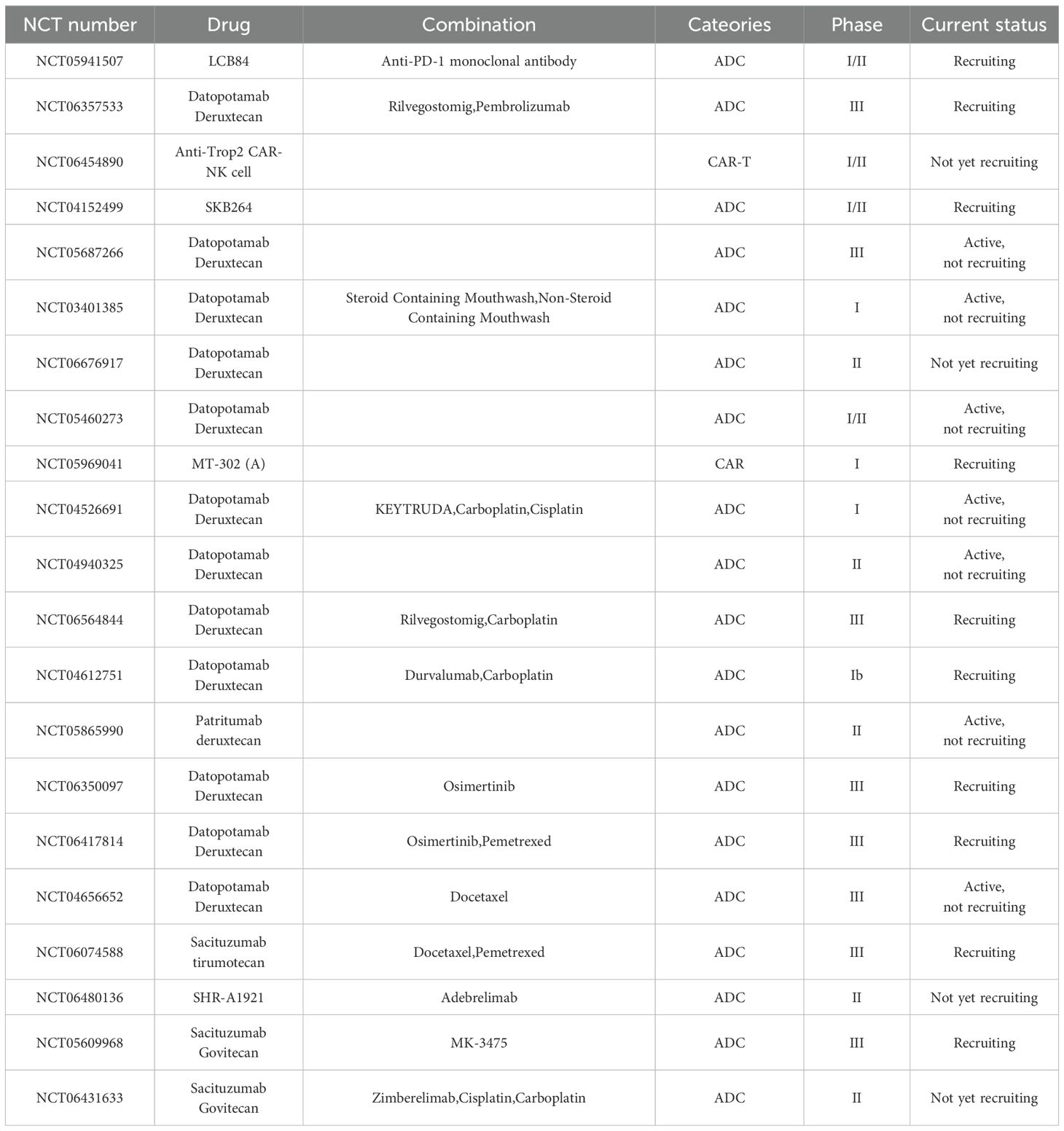
TROP2-targeted therapeutics are predominantly formulated as ADC (24) (Table 1). ADCs represent a novel class of antitumor drugs that exhibit the high specificity of monoclonal antibodies and the high activity of small-molecule cytotoxic drugs (25). These drugs consist of antibodies, linkers, and payloads. Monoclonal antibodies are capable of recognizing targeted antigens on the surface of tumor cells. The ADC complex enters the cell interior via receptor-mediated endocytosis, releasing the cytotoxic drug (26). Furthermore, the diffusion of cytotoxic drugs released by ADC through the cell membrane can also lead to the destruction of neighboring cancer cells (27). The FDA has approved several ADCs targeting TROP2 for marketing (Figure 2).
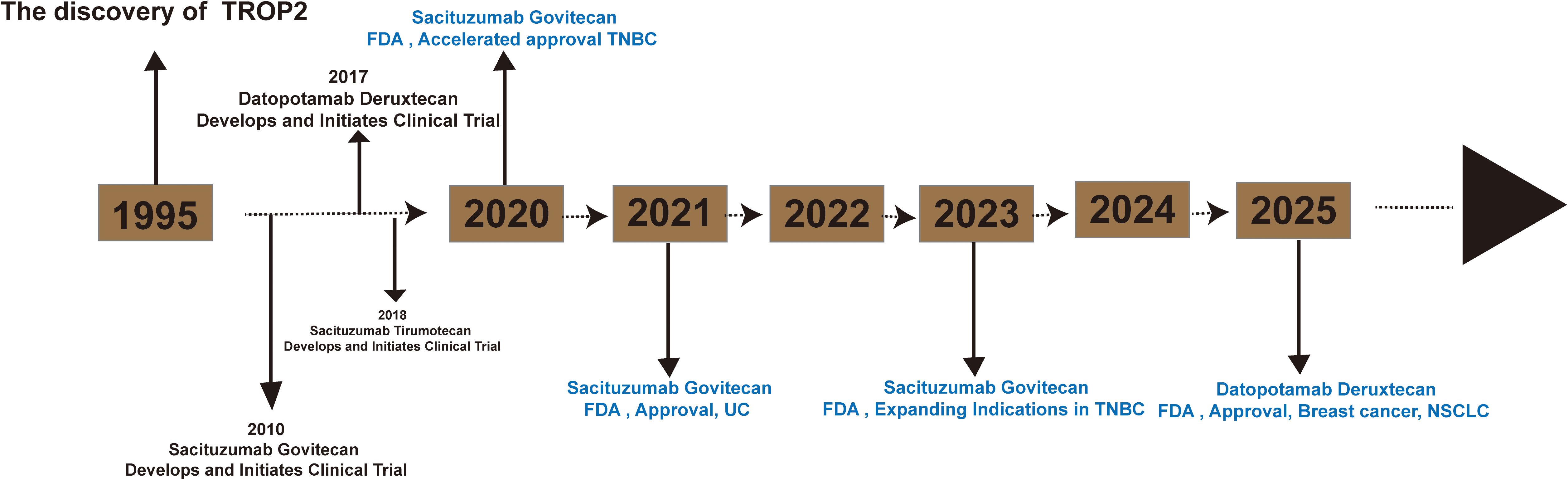
Figure 2. Timeline for the launch of TROP2-ADC (sacituzumab govitecan, datopotamab deruxtecan and sacituzumab tirumotecan).
3.1 Sacituzumab govitecan
Sacituzumab govitecan is an antibody-drug conjugate developed to treat solid tumors, including breast, lung, and urothelial cancers. It contains the irinotecan active metabolite SN-38 (govitecan) site-specifically conjugated to a humanized monoclonal antibody (hRS7) directed against the CPD domain of TROP2 (28). In vitro, sacituzumab govitecan exhibits a mechanism similar to that of free SN-38, and hRS7 shows potent broad anticancer activity in human cancer xenografts and in patients (28).
The combination of targeted drugs and chemotherapeutic drugs in sacituzumab govitecan (SG) represents a novel approach to cancer treatment. This combination has the potential to enhance the efficacy of targeted drugs in targeting tumor tissues while concurrently reducing the impact on normal tissues (29). Notably, SG is primarily target-oriented for patients diagnosed with triple-negative breast cancer (TNBC) (29, 30). The TROPiCS-02 study demonstrated that the combination of SG improved median overall survival (OS) (14.4 months vs. 11.2 months) and reduced overall risk of death by 11% (31). SG has been shown to demonstrate superior efficacy and safety in the second-line treatment of small cell lung cancer and non-small cell lung cancer, particularly in cases where patients have become resistant to conventional treatment regimens. In a subsequent TROPiCS-03 trial targeting extensive stage small cell lung cancer, the objective remission rate (ORR) was achieved at 41.9%, accompanied by a median progression-free survival (PFS) of 4.4 months and a median OS of 13.6 months (32). The clinic trial placed particular emphasis on the controlled security of SG in lung cancer (32). The efficacy of SG in the treatment of NSCLC is well-documented. A subsequent EVOKE-01 trial, which was designed to compare SG and chemotherapy, revealed that SG enhances OS, particularly among patients who are refractory to programmed cell death-ligand 1 (PD-L1) therapy, while concurrently ensuring enhanced manageable safety (33).
3.2 Datopotamab deruxtecan
Datopotamab Deruxtecan (Dato-DXd) is composed of the anti-TROP-2 IgG1 monoclonal antibody Datopotamab linked with the topoisomerase I inhibitor exatecan derivative (DXd) via a cleavable tetrapeptide linker, ensuring precise eradication of tumor cells (34). Dato-DXd suppresses the proliferation of TROP2-expressing tumors by recognizing the TROP2 ectodomain (ECD) in tumor cells. Furthermore, a study verifies that DXd exhibits more potent Topo inhibitory activity than SN-38 (35). The Dato-DXd combination has demonstrated notable antitumor activity across various tumor types, particularly in NSCLC and breast cancer (34, 36). The TROPION-Breast01 trial, which involved the utilization of Dato-DXd, demonstrated a substantial reduction in disease progression risk, with a median PFS of 6.9 months in comparison to the chemotherapy group (37).
Dato-DXd is currently engaged in testing and exploration in the domain of lung cancer. In the randomized, phase III, open-label, global study TROPION-Lung01, which enrolled 604 patients with lung cancer, the Dato-DXd treatment group demonstrated a significant increase in PFS (4.4 months vs. 3.7 months, HR=0.75, 95%Cl 0.62-0.91; P=0.004) when compared to the docetaxel group. In addition, the Dato-DXd group exhibited superior objective remission rate (ORR), median duration of remission (DOR), and disease control rate (DCR) (ORR: 26.4% vs. 12.8%, DOR: 7.1 months vs. 5.6 months, DCR: 77.3% vs. 64.9%) (38). It is anticipated that Dato-DXd will emerge as a potential treatment option for patients with NSCLC who have developed resistance to EGFR- tyrosine kinase inhibitors (TKIs). The TROPION-Lung05 study demonstrated that patients receiving the treatment of Dato-DXd achieved confirmed ORR of 35.8% and median DOR of 7.0 months (39). These findings offer a range of treatment options for patients with NSCLC who are eligible for Dato-DXd therapy. A multitude of clinical trials are currently being conducted on the subject of Dato-DXd, aimed to explore the possibility of combination (Table 1). Consequently, the FDA successively approved Dato-DXd in 2025 for the treatment of hormone receptor (HR)-positive/human epidermal growth factor receptor2 (HER2)-negative breast cancer and locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated NSCLC.
3.3 Sacituzumab tirumotecan (SKB264)
The shedding of the SN-38 payload from SG leads to off-target toxicity, including neutropenia, diarrhea, vomiting, and nausea (40). Sacituzumab Tirumotecan is a humanized IgG1 mAb hRS7 conjugated with a property cytotoxic developed using a novel DNA topoisomerase I inhibitor (KL610023) and optimized ligation methods to address off-target toxicity (41, 42). In TROP2-expressing xenograft models, sacituzumab tirumotecan demonstrates strong efficacy, a favorable safety profile, and an excellent therapeutic window (40).
Sacituzumab tirumotecan is a class of TROP2-ADC that is currently undergoing clinical trials for various cancers (Figure 1, Table 1). In a phase I/II clinical trial recruiting EGFR wild and EGFR mutation patients, the ORR was 40%, with a median PFS of 6.2 months and the efficacy benefit was even more prominent in EGFR mutant patients compared to EGFR wild-type patients (43). The efficacy of the drug has been demonstrated in patients with EGFR mutation lung cancer. In a phase II clinical trial designed to assess the efficacy of Sacituzumab tirumotecan in patients resistant to EGFR-TKI who received platinum-containing chemotherapy, the study cohort treated with sacituzumab tirumotecan achieved an ORR of 34%, a median PFS of 9.3 months (43). In a clinical trial designated as OptiTROP-Lung01, patients were administered Sacituzumab tirumotecan in combination with KL-A167, a PD-L1 inhibitor. The study observed an ORR of 59.3%, accompanied by manageable safety profiles (44).
3.4 Next-generation of TROP2-targeted therapeutics
Current developments focus on novel TROP2-targeted therapeutics, including dual antibody ADCs, bispecific T cell engager (BiTE) antibodies, and a TROP2-targeted nano-in-gel vaccine (NIGel-Vax). However, dual antibody ADCs present significant challenges. A minimal dose must be verified for each payload to obtain a response, and stable molecular structures are essential (45). BiTEs are a new type of immunotherapy that recognizes both tumor surface antigens and CD3. F7AK3, a bispecific antibody targeting TROP2 and CD3, has demonstrated remarkable antitumor efficacy in vitro and in vivo (46). Additionally, a novel bispecific antibody was designed by reducing the binding affinity of CD3 in two steps to reduce its ability to stimulate cells (47). Nectin cell adhesion protein 4 (Nectin-4), a member of the Nectin family, is specifically expressed in tumor tissues (48). Clinical trials for a Nectin-4/TROP2 dual antibody ADC are currently in development. A phase 1 clinical study is evaluating the safety, tolerability, pharmacokinetics (PK), immunogenicity, and preliminary antitumor efficacy of AK146D1 in patients with advanced solid tumors (NCT06929663). NIGel-Vax, a novel cancer immunotherapy, exerts anti-tumor effects by stimulating T cells and increasing memory T cells, among other immune amplifications (49). In TNBC models, NIGel-Vax targeting TROP2 achieved a 96% tumor suppression rate and a 50% cure rate (49).
4 The resistance mechanism of TROP2-ADC
Although TROP2-targeting ADCs have demonstrated remarkable clinical efficacy, drug resistance continues to pose a significant therapeutic challenge. However, research on the mechanisms underlyingTROP2-ADC resistance remains limited. Reduced target (TROP2) expression represents a common mechanism of resistance to TROP2-targeting ADCs. Exposure to the treatment of ADCs, tumor cells experiencing a marked decrease in antigen levels shortly (50). Therefore, the also points to the downregulation of TROP2 in treatment of TROP2-ADC. For instance, a triple-negative breast cancer patient lacking TROP2 expression exhibited de novo resistance to SG (51). Additionally, acquired resistance to SG (IMMU-132) in another patient was associated with a mutated TROP2 protein, leading to reduced ADC binding due to altered subcellular localization (52).
Efflux of the ADC payload constitutes another resistance mechanism. The payload SN-38, released from SG, is a representative topoisomerase I inhibitor. However, its unfavorable physicochemical properties—such as poor solubility and stability—hinder effective delivery to tumor sites (53). Furthermore, the upregulation of multi-drug resistance (MDR) pathways and tumor heterogeneity contribute to both inherent and acquired resistance to the SN-38 payload (54). To address this resistance, researchers have developed a novel class of ADCs utilizing frontal T moiety-exatecan conjugates, demonstrating efficacy without significant toxic side effects (54).
Furthermore, the lysosomal degradation of ADCs depends on an acidic lysosomal environment and the activity of lysosomal enzymes (55). Lysosomal dysfunction can therefore impair ADC efficacy. For example, in the enmetuximab (T‐DM1)-resistant breast cancer cell line BT474, researchers observed lysosomal alkalization and impaired activity of lysosomal proteolytic enzymes (56). Additionally, beyond lysosomal dysfunction, impaired expression of Endophilin A2 (Endo II) in HER2-positive (HER2+) breast cancer models reduced HER2 internalization and diminished the response to T-DM1 (57). As a scaffolding protein, Endo II plays a role in clathrin-independent endocytosis. In conclusion, internalization and lysosomal dysfunction also leads to drug resistance problems.
5 Conclusion and prospective
TROP2, a glycoprotein, has been shown to regulate a variety of pathological activities, including tumor growth and migration. Its distinct expression in various tumors renders it an optimal target for multiple therapeutic interventions. The elevated expression levels of TROP2 in tumors suggest that targeted therapeutic agents are more likely to bind to tumor cells, thereby enhancing the therapeutic effect and reducing adverse effects. The field of oncology has witnessed significant advancements in the development of TROP2 population particularly in the context of lung cancer and breast cancer. Emerging evidence suggests that TROP2-targeted therapeutic strategies may demonstrate clinical benefits in cancer patients regardless of detectable TROP2 expression status. However, the therapeutic targeting of TROP2 is confronted with significant challenges, including off-target toxicity and acquired resistance. To address these limitations, promising strategies involve optimizing ADC design, developing next-generation ADCs with enhanced tumor selectivity, and exploring synergistic combination therapies with immune checkpoint inhibitors or targeted agents. Concurrently, an increasing number of clinical trials are being developed to expand the utilization of these targeted drugs to various tumor populations. As a class of drugs specifically targeted to tumors, TROP2-targeted therapeutics are widely regarded as poised to transform the future of solid tumor therapy, such as gastric cancer, pancreatic cancer, breast cancer, prostate cancer (58–61).
Author contributions
X-XL: Funding acquisition, Writing – original draft, Writing – review & editing. J-LC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Zhejiang Provincial Traditional Chinese Medicine Science and Technology Plan Project (2023ZR128).
Acknowledgments
We are thankful to the patient and all the physicians and technicians who participated in this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-small cell lung cancer, version 4.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2024) 22:249–74. doi: 10.6004/jnccn.2204.0023
3. Yang JC, Lee DH, Lee JS, Fan Y, de Marinis F, Iwama E, et al. Phase III KEYNOTE-789 study of pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor–Resistant, EGFR-mutant, metastatic nonsquamous non-small cell lung cancer. J Clin Oncol. (2024) 42:4029–39. doi: 10.1200/JCO.23.02747
4. Battagin AS, Bertuzzo CS, Carvalho PO, Ortega MM, and Marson FAL. Single nucleotide variants c.-13G –> C (rs17429833) and c.108C –> T (rs72466472) in the CLDN1 gene and increased risk for familial colorectal cancer. Gene. (2021) 768:145304. doi: 10.1016/j.gene.2020.145304
5. Inamura K, Yokouchi Y, Kobayashi M, Ninomiya H, Sakakibara R, Subat S, et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget. (2017) 8:28725–35. doi: 10.18632/oncotarget.15647
6. Omori S, Muramatsu K, Kawata T, Miyawaki E, Miyawaki T, Mamesaya N, et al. Trophoblast cell-surface antigen 2 expression in lung cancer patients and the effects of anti-cancer treatments. J Cancer Res Clin Oncol. (2022) 148:2455–63. doi: 10.1007/s00432-021-03784-3
7. Liu X, Ma L, Li J, Sun L, Yang Y, Liu T, et al. Trop2-targeted therapies in solid tumors: advances and future directions. Theranostics. (2024) 14:3674–92. doi: 10.7150/thno.98178
8. Nelson BE and Meric-Bernstam F. Leveraging TROP2 antibody-drug conjugates in solid tumors. Annu Rev Med. (2024) 75:31–48. doi: 10.1146/annurev-med-071322-065903
9. Liao S, Wang B, Zeng R, Bao H, Chen X, Dixit R, et al. Recent advances in trophoblast cell-surface antigen 2 targeted therapy for solid tumors. Drug Dev Res. (2021) 82:1096–110. doi: 10.1002/ddr.21870
10. Vidmar T, Pavsic M, and Lenarcic B. Biochemical and preliminary X-ray characterization of the tumor-associated calcium signal transducer 2 (Trop2) ectodomain. Protein Expr Purif. (2013) 91:69–76. doi: 10.1016/j.pep.2013.07.006
11. Cubas R, Li M, Chen C, and Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. (2009) 1796:309–14. doi: 10.1016/j.bbcan.2009.08.001
12. Guerra E, Trerotola M, Tripaldi R, Aloisi AL, Simeone P, Sacchetti A, et al. Trop-2 induces tumor growth through AKT and determines sensitivity to AKT inhibitors. Clin Cancer Res. (2016) 22:4197–205. doi: 10.1158/1078-0432.CCR-15-1701
13. Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, et al. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med. (2012) 4:472–85. doi: 10.1002/emmm.201200222
14. Hu Y, Zhu Y, Qi D, Tang C, and Zhang W. Trop2-targeted therapy in breast cancer. biomark Res. (2024) 12:82. doi: 10.1186/s40364-024-00633-6
15. Mi K, Zeng L, Chen Y, Ning J, Zhang S, Zhao P, et al. DHX38 enhances proliferation, metastasis, and EMT progression in NSCLC through the G3BP1-mediated MAPK pathway. Cell Signal. (2024) 113:110962. doi: 10.1016/j.cellsig.2023.110962
16. Yoshitomi Y, Ikeda T, Saito-Takatsuji H, and Yonekura H. Emerging role of AP-1 transcription factor junB in angiogenesis and vascular development. Int J Mol Sci. (2021) 22(6):2804. doi: 10.3390/ijms22062804
17. Hou J, Lv A, Deng Q, Zhang G, Hu X, and Cui H. TROP2 promotes the proliferation and metastasis of glioblastoma cells by activating the JAK2/STAT3 signaling pathway. Oncol Rep. (2019) 41:753–64. doi: 10.3892/or.2018.6859
18. Wu B, Yu C, Zhou B, Huang T, Gao L, Liu T, et al. Overexpression of TROP2 promotes proliferation and invasion of ovarian cancer cells. Exp Ther Med. (2017) 14:1947–52. doi: 10.3892/etm.2017.4788
19. Yao L, Chen J, and Ma W. Decoding TROP2 in breast cancer: significance, clinical implications, and therapeutic advancements. Front Oncol. (2023) 13:1292211. doi: 10.3389/fonc.2023.1292211
20. Mori Y, Akita K, Ojima K, Iwamoto S, Yamashita T, Morii E, et al. Trophoblast cell surface antigen 2 (Trop-2) phosphorylation by protein kinase C alpha/delta (PKCalpha/delta) enhances cell motility. J Biol Chem. (2019) 294:11513–24. doi: 10.1074/jbc.RA119.008084
21. Iwamoto S, Mori Y, Yamashita T, Ojima K, Akita K, Togano S, et al. Trophoblast cell surface antigen-2 phosphorylation triggered by binding of galectin-3 drives metastasis through down-regulation of E-cadherin. J Biol Chem. (2023) 299:104971. doi: 10.1016/j.jbc.2023.104971
22. Yang T, Jia L, Bian S, Chang X, Zhang Q, Tang Q, et al. TROP2 down-regulated DSG2 to promote gastric cancer cell invasion and migration by EGFR/AKT and DSG2/PG/beta-catenin pathways. Curr Cancer Drug Targets. (2022) 22:691–702. doi: 10.2174/1568009622666220407111013
23. Trerotola M, Li J, Alberti S, and Languino LR. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the beta1 integrin-RACK1 axis. J Cell Physiol. (2012) 227:3670–7. doi: 10.1002/jcp.v227.11
24. Herbet A, Hautiere M, Jean-Alphonse F, Vivier D, Leboeuf C, Costa N, et al. Targeting the activated allosteric conformation of the endothelin receptor B in melanoma with an antibody-drug conjugate: mechanisms and therapeutic efficacy. BJC Rep. (2025) 3:3. doi: 10.1038/s44276-024-00109-y
25. Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today. (2014) 19:869–81. doi: 10.1016/j.drudis.2013.11.004
26. Brito RS, Olivier T, and Wolfer A. Oncology. Antibody-drug conjugates: a challenging innovation. Rev Med Suisse. (2025) 21:142–6. doi: 10.53738/REVMED.2025.21.902.142
27. Zhou H, Zeng Y, Hida T, Hsu R, Huang Y, and Dong X. The current landscape and prospects of antibody-drug conjugates for lung cancer brain metastases: a narrative review. Transl Lung Cancer Res. (2024) 13:3778–94. doi: 10.21037/tlcr-24-964
28. Goldenberg DM, Stein R, and Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. (2018) 9:28989–9006. doi: 10.18632/oncotarget.25615
29. Li X, Zhang L, Hu S, Liu D, Hu B, Ran J, et al. Postmarketing safety of sacituzumab govitecan: A pharmacovigilance study based on the FDA adverse event reporting system. Clin Pharmacol Ther. (2024) 115:256–68. doi: 10.1002/cpt.v115.2
30. Kang C. Sacituzumab govitecan: A review in unresectable or metastatic HR+/HER2- breast cancer. Target Oncol. (2024) 19:289–96. doi: 10.1007/s11523-024-01036-1
31. Rugo HS, Bardia A, Marme F, Cortes J, Schmid P, Loirat D, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2023) 402:1423–33. doi: 10.1016/S0140-6736(23)01245-X
32. Dowlati A, Chiang AC, Cervantes A, Babu S, Hamilton E, Wong SF, et al. Phase 2 open-label study of sacituzumab govitecan as second-line therapy in patients with extensive-stage small cell lung cancer: results from TROPiCS-03. J Thorac Oncol. (2025) 20(6):799–808. doi: 10.1016/j.jtho.2024.12.028
33. Paz-Ares LG, Juan-Vidal O, Mountzios GS, Felip E, Reinmuth N, de Marinis F, et al. Sacituzumab govitecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: the randomized, open-label phase III EVOKE-01 study. J Clin Oncol. (2024) 42:2860–72. doi: 10.1200/JCO.24.00733
34. Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, et al. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. (2021) 20:2329–40. doi: 10.1158/1535-7163.MCT-21-0206
35. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. (2016) 22:5097–108. doi: 10.1158/1078-0432.CCR-15-2822
36. Gogtay M, Aimalla N, Thirugnanasambandam RP, and Ganti AK. Therapeutic potential of datopotamab deruxtecan in the treatment of advanced non-small cell lung cancer: evidence to date. Onco Targets Ther. (2025) 18:575–84. doi: 10.2147/OTT.S466220
37. Bardia A, Jhaveri K, Im SA, Pernas S, De Laurentiis M, Wang S, et al. Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: primary results from TROPION-breast01. J Clin Oncol. (2025) 43:285–96. doi: 10.1200/JCO.24.00920
38. Ahn MJ, Tanaka K, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E, et al. Datopotamab deruxtecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: the randomized, open-label phase III TROPION-lung01 study. J Clin Oncol. (2025) 43:260–72. doi: 10.1200/JCO-24-01544
39. Sands J, Ahn M-J, Lisberg A, Cho BC, Blumenschein G, Shum E, et al. Datopotamab deruxtecan in advanced or metastatic non–small cell lung cancer with actionable genomic alterations: results from the phase II TROPION-lung05 study. J Clin Oncol. (2025) 43:1254–65. doi: 10.1200/JCO-24-01349
40. Cheng Y, Yuan X, Tian Q, Huang X, Chen Y, Pu Y, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol. (2022) 12:951589. doi: 10.3389/fonc.2022.951589
41. Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer. (2017) 123:3843–54. doi: 10.1002/cncr.v123.19
42. Pandey R, Gruslova A, Chiou J, Brenner AJ, and Tiziani S. Stable isotope dilution LC-HRMS assay to determine free SN-38, total SN-38, and SN-38G in a tumor xenograft model after intravenous administration of antibody-drug conjugate (Sacituzumab govitecan). Anal Chem. (2020) 92:1260–7. doi: 10.1021/acs.analchem.9b04419
43. Zhao S, Cheng Y, Wang Q, Li X, Liao J, Rodon J, et al. Sacituzumab tirumotecan in advanced non-small-cell lung cancer with or without EGFR mutations: phase 1/2 and phase 2 trials. Nat Med. (2025) 31:1976–86. doi: 10.1038/s41591-025-03638-2
44. Fang W, Wang Q, Cheng Y, Wang Y, Wu L, Zhu H, et al. Sacituzumab tirumotecan (sac-TMT) in combination with tagitanlimab (anti-PD-L1) in first-line (1L) advanced non-small-cell lung cancer (NSCLC): Non-squamous cohort from the phase II OptiTROP-Lung01 study. J Clin Oncol. (2025) 43:8529. doi: 10.1200/JCO.2025.43.16_suppl.8529
45. Schlam I, Moges R, Morganti S, Tolaney SM, and Tarantino P. Next-generation antibody-drug conjugates for breast cancer: Moving beyond HER2 and TROP2. Crit Rev Oncol Hematol. (2023) 190:104090. doi: 10.1016/j.critrevonc.2023.104090
46. Liu H, Bai L, Huang L, Ning N, Li L, Li Y, et al. Bispecific antibody targeting TROP2xCD3 suppresses tumor growth of triple negative breast cancer. J Immunother Cancer. (2021) 9(10):e003468. doi: 10.1136/jitc-2021-003468
47. Wang D, Zhang L, Wang B, Zhao L, Deng L, Xu W, et al. Construction of a novel TROP2/CD3 bispecific antibody with potent antitumor activity and reduced induction of Th1 cytokines. Protein Expr Purif. (2023) 205:106242. doi: 10.1016/j.pep.2023.106242
48. Chatterjee S, Sinha S, and Kundu CN. Nectin cell adhesion molecule-4 (NECTIN-4): A potential target for cancer therapy. Eur J Pharmacol. (2021) 911:174516. doi: 10.1016/j.ejphar.2021.174516
49. Liu T, Si X, Liu L, Ma S, Huang Z, Zhang Y, et al. Injectable nano-in-gel vaccine for spatial and temporal control of vaccine kinetics and breast cancer postsurgical therapy. ACS Nano. (2024) 18:3087–100. doi: 10.1021/acsnano.3c08376
50. Loganzo F, Tan X, Sung M, Jin G, Myers JS, Melamud E, et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther. (2015) 14:952–63. doi: 10.1158/1535-7163.MCT-14-0862
51. Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. (2021) 32:1148–56. doi: 10.1016/j.annonc.2021.06.002
52. Coates JT, Sun S, Leshchiner I, Thimmiah N, Martin EE, McLoughlin D, et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov. (2021) 11:2436–45. doi: 10.1158/2159-8290.CD-21-0702
53. Yang J, Jia L, He Z, and Wang Y. Recent advances in SN-38 drug delivery system. Int J Pharm. (2023) 637:122886. doi: 10.1016/j.ijpharm.2023.122886
54. Weng W, Meng T, Zhao Q, Shen Y, Fu G, Shi J, et al. Antibody-exatecan conjugates with a novel self-immolative moiety overcome resistance in colon and lung cancer. Cancer Discov. (2023) 13:950–73. doi: 10.1158/2159-8290.CD-22-1368
55. Chen YF, Xu YY, Shao ZM, and Yu KD. Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer Commun (Lond). (2023) 43:297–337. doi: 10.1002/cac2.12387
56. Rios-Luci C, Garcia-Alonso S, Diaz-Rodriguez E, Nadal-Serrano M, Arribas J, Ocana A, et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. (2017) 77:4639–51. doi: 10.1158/0008-5472.CAN-16-3127
57. Baldassarre T, Truesdell P, and Craig AW. Endophilin A2 promotes HER2 internalization and sensitivity to trastuzumab-based therapy in HER2-positive breast cancers. Breast Cancer Res. (2017) 19:110. doi: 10.1186/s13058-017-0900-z
58. Kushiyama S, Yashiro M, Yamamoto Y, Sera T, Sugimoto A, Nishimura S, et al. Clinicopathologic significance of TROP2 and phospho-TROP2 in gastric cancer. Mol Clin Oncol. (2021) 14:105. doi: 10.3892/mco.2021.2267
59. Xu C, Zhu M, Wang Q, Cui J, Huang Y, Huang X, et al. TROP2-directed nanobody-drug conjugate elicited potent antitumor effect in pancreatic cancer. J Nanobiotechnology. (2023) 21:410. doi: 10.1186/s12951-023-02183-9
60. Shen M, Liu S, and Stoyanova T. The role of Trop2 in prostate cancer: an oncogene, biomarker, and therapeutic target. Am J Clin Exp Urol. (2021) 9:73–87.
Keywords: TACSTD2, TROP2, antibody-drug conjugate, lung cancer, lung adenocarcinoma
Citation: Li X-x and Chen J-l (2025) TROP2: as a promising target in lung cancer. Front. Oncol. 15:1569897. doi: 10.3389/fonc.2025.1569897
Received: 05 March 2025; Accepted: 04 August 2025;
Published: 27 August 2025.
Edited by:
Rehan Khan, Rutgers University, United StatesReviewed by:
Petr Benes, Masaryk University, CzechiaMukulika Bose, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-xing Li, MjUxMjU4MTY1QHFxLmNvbQ==
†ORCID: Xing-xing Li, orcid.org/0009-0001-4235-9842
 Xing-xing Li
Xing-xing Li Jia-li Chen2
Jia-li Chen2