- Department of Gynecology and Obstetrics, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Zhejiang, Hangzhou, China
Recently, the global incidence of endometrial cancer is increasing. Endocrine therapy offers advantages in the management of this malignancy due to its broad applicability and favorable tolerability profile. Although conventional endocrine treatments, including progesterone, gonadotropin-releasing hormone agonists and aromatase inhibitors demonstrate efficacy in endometrial cancer, their long-term utility is limited by adverse effects such as drug resistance and disease recurrence with prolonged treatment. Novel endocrine therapeutic agents, including selective estrogen receptor modulators, selective estrogen receptor degraders, epigenetic-targeted therapies, mTOR inhibitors, cyclin-dependent kinase inhibitors, and metformin, remain in preclinical development or clinical trials. Inspiringly, the preliminary findings suggest these emerging agents may positively impact survival outcomes in endometrial cancer patients. This review examines the mechanisms, methodologies, and efficacy of both traditional and novel endocrine therapeutic approaches for endometrial cancer.
1 Introduction
Endometrial cancer (EC) comprises a group of malignancies originating from the endometrial epithelium and represents the most common gynecologic malignancy in developed countries (1). The rising incidence of EC is associated with multiple factors, including population aging, obesity, type 2 diabetes mellitus, female infertility, delayed menopause, and declining rate of hysterectomy for benign conditions (2, 3). Based on molecular analyses from the Cancer Genome Atlas (TCGA) and the Proactive Molecular Risk Classifier for EC (ProMisE), ECs are now classified into four molecular subgroups: POLE-mutated, p53 wild type (low copy number-CNL or nonspecific molecular profile-NSMP), p53 null/missense mutations (high copy number), and mismatch repair deficient (MMRd). This molecular classification has optimized clinical management of EC by defining distinct risk categories (4). Endocrine therapy, representing an early form of targeted therapy for EC, plays a crucial role in fertility-sparing approaches for early-stage disease and non-surgical management of advanced or recurrent EC (5, 6). The NSMP type predominantly manifests as low-grade, early-stage tumors with high expression of estrogen receptor (ER) and progesterone receptor (PR), demonstrating favorable response to endocrine therapy (7). However, the efficacy of monotherapy is usually limited, and resistance often develops with extended treatment cycles. Combination strategies incorporating other targeted therapies or chemotherapy may enhance quality of life and survival outcomes (8).
2 Endocrine therapeutic targets for endometrial cancer
2.1 Mechanisms of estrogen action
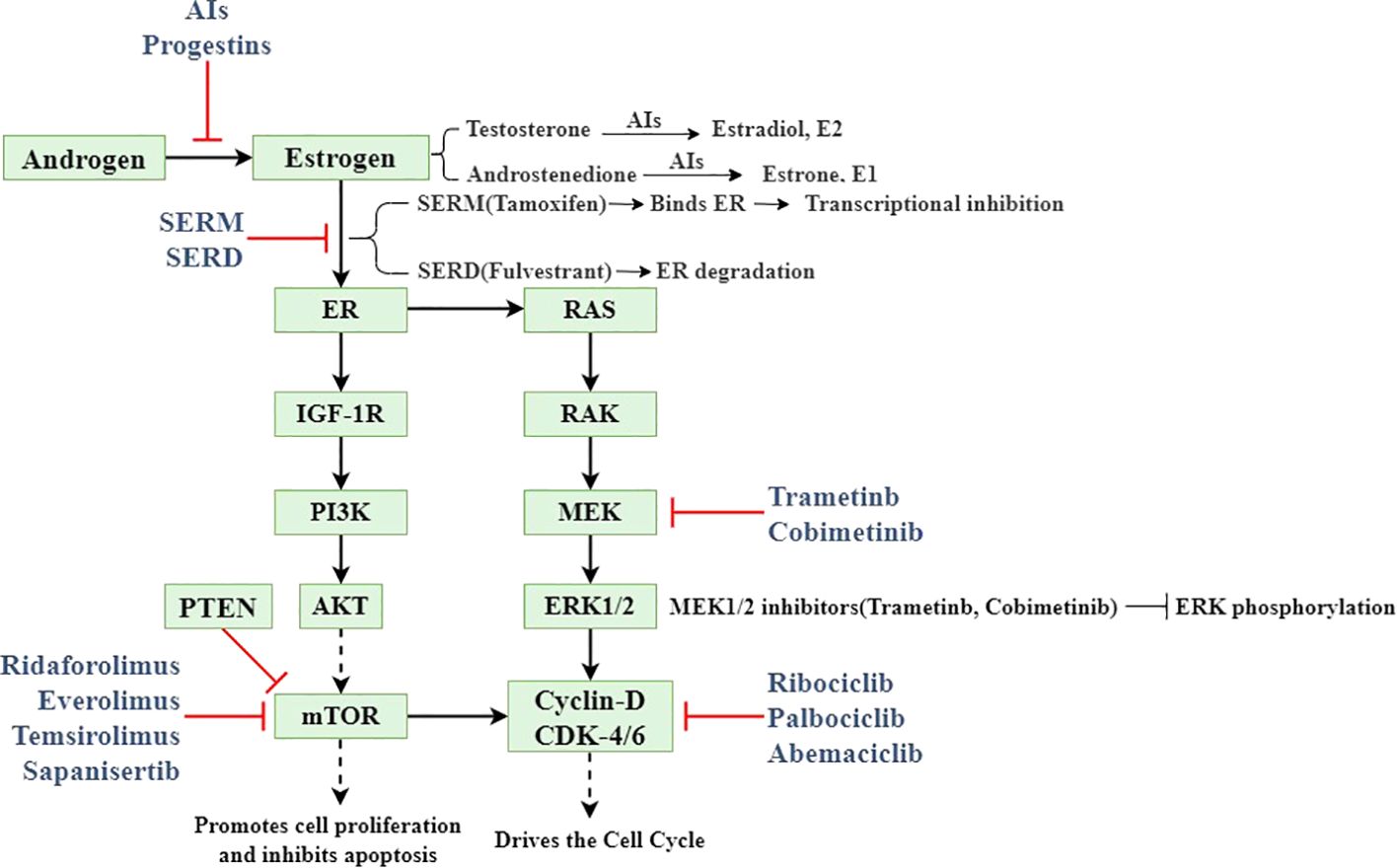
Based on clinical, endocrine and epidemiological characteristics, EC is classified into type I (estrogen-dependent) and type II (non- estrogen-dependent). Type I predominantly comprises endometrioid carcinoma, accounting for 80-90% of all EC cases (9). Intracellular estrogens include estrone (E1), estradiol (E2), and estriol (E3). In premenopausal women, E1 and E2 are primarily secreted by the ovaries, with minor contributions from adipose tissue and the adrenal glands, while E3 is predominantly produced by placental tissue during pregnancy. Ovarian estrogen secretion is regulated by the hypothalamic-pituitary-ovarian (HPO) axis. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which stimulates the synthesis of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH promotes androgen production, which is subsequently aromatized to estrogen by aromatase. Elevated estrogen levels create negative feedback on the hypothalamus and pituitary, inhibiting further estrogen production and secretion (10). Estrogen mediates its biological effects in target tissues by binding to specific receptors, including ERα, ERβ, and G protein-coupled estrogen receptor (GPER) (11). ERα has been implicated in abnormal proliferation, inflammation, and malignant transformation. Estrogen binding to ERα activates PI3K/AKT/mTOR signaling with RAS/RAK/MEK/ERK signaling pathway, directly affecting cyclin D and cyclin-dependent kinase 4/6 (CDK4/6) (12). ERβ may counteract these pro-tumorigenic effects by modulating the expression of ERα-regulated genes (13).
2.2 Mechanism of progesterone action
Progesterone, another principal reproductive hormone secreted by the ovaries, plays a critical role in in the differentiation of endometrium. When elevated estrogen levels induce excessive endometrial proliferation without progesterone-mediated differentiation, the endometrium undergoes significant thickening and may develop endometrial atypical hyperplasia (EAH), a precancerous lesion (10, 14). Research indicates that progesterone exerts anti-estrogenic effects through dual mechanisms: it reduces the quantity of estrogen receptors while decreasing the production of new receptors, and it modulates enzymatic activity by reducing the activity of sulfatase and stimulating sulfotransferase, thereby counteracting estrogen production (15).
2.3 Endocrine therapeutic targets for endometrial cancer
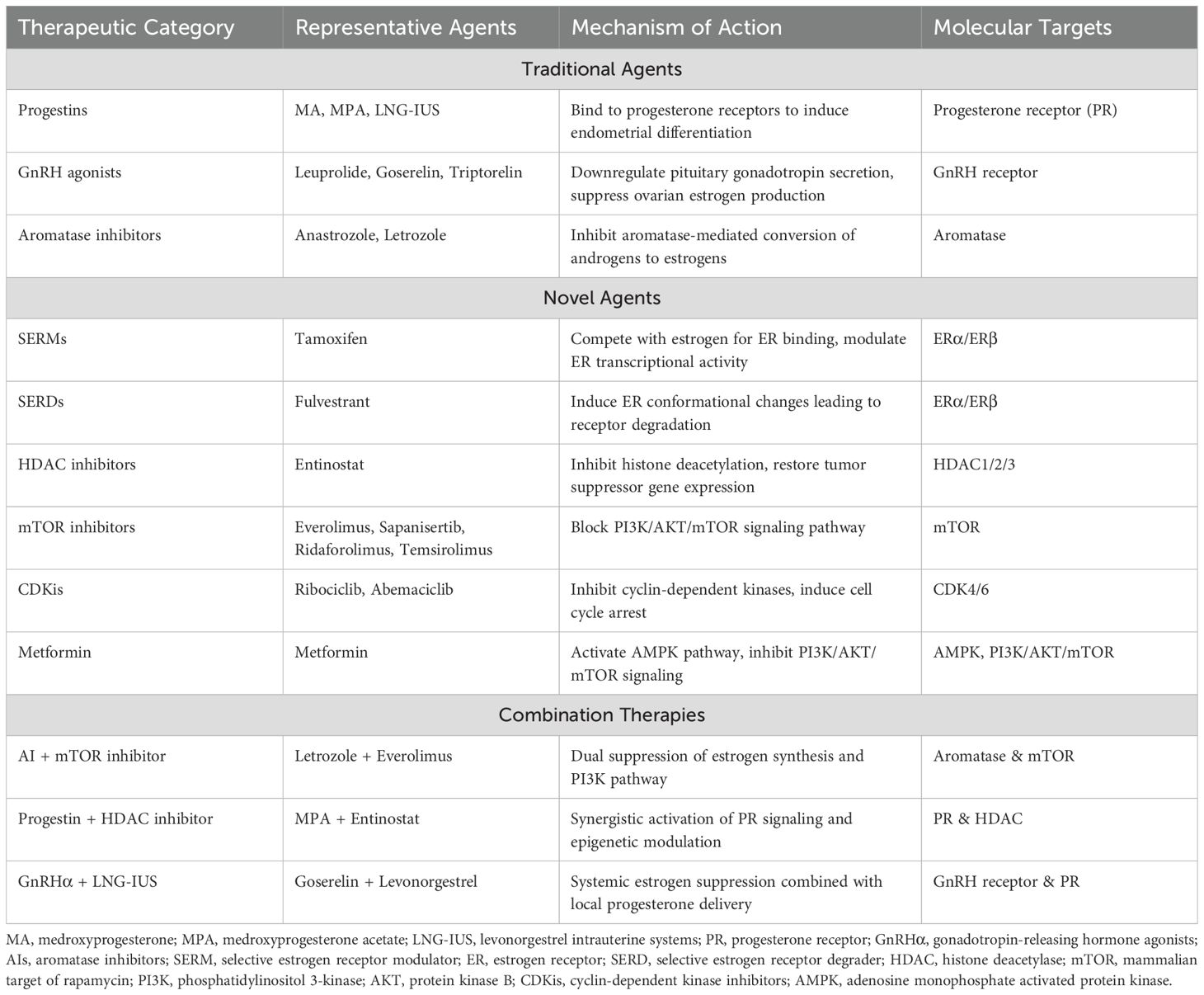
Multiple therapeutic approaches target the estrogen production pathway. Gonadotropin-releasing hormone agonists (GnRHα) and aromatase inhibitors (AIs) effectively reduce estrogen production, thereby mitigating EC progression (16). Oral progestins and/or levonorgestrel intrauterine systems (LNG-IUS) represent the primary modalities for progesterone supplementation in EC management, particularly for EAH and early-stage EC, offering effective fertility preservation (17). To prevent estrogen-receptor binding, selective estrogen receptor modulators (SERMs) regulate ER activity by competing with estrogen for ER binding and altering associated cofactors. Additionally, selective estrogen receptor degraders (SERDs) destabilize the estrogen receptor H12 domain upon binding, inducing ER degradation (18). Epigenetic approaches, including histone deacetylase (HDAC) inhibitors, DNA methylation (DNMT) inhibitors, histone methyltransferases (HMTs), and histone demethylases (HDMs), effectively alter the transcription of key genes affecting EC cell proliferation, apoptosis, and receptor expression of estrogen and progesterone (19). EC exhibits the highest frequency of mutations in the PI3K/AKT/mTOR pathway compared to other tumor types in TCGA, suggesting that inhibitors targeting this pathway may benefit EC patients (20, 21). Preclinical studies demonstrate that cyclin-dependent kinase inhibitors (CDKis) may address endocrine therapy resistance partially mediated by PI3K overactivation; however, additional clinical data regarding the efficacy and adverse effects of CDKis in EC patients are needed (22). The above mechanism of action and targets are shown in Figure 1. Additional endocrine therapeutic agents include metformin, androgen receptor (AR) antagonists, and steroid sulfatase (STS) etc. The key endocrine therapeutic agents with their respective mechanisms and molecular targets are systematically summarized in Table 1.
3 Endocrine therapy for endometrial cancer
3.1 Progesterone
Progestogens were the initial agents clinically employed in endocrine management of EC. Oral progestins, primarily medroxyprogesterone (MA) and medroxyprogesterone acetate (MPA), represent the standard approach. In early-stage EC, oral long-acting MPA reduces tumor proliferation, promotes tumor differentiation, and provides benefit during the preoperative interval (23). LNG-IUS, an intrauterine sustained-release progestin system, has gained acceptance among gynecologists due to its localized action, minimal systemic adverse effects, and high patient compliance. Gallos et al. (24) demonstrated that LNG-IUS may yield superior clinical outcomes compared to oral progestins. Janda et al. (25) reported a 61% pathological complete response rate following 6 months of LNG-IUS treatment in early-stage EC. Similarly, Westin et al. (26) observed a 12-month clinical remission rate of 83% with LNG-IUS in the treatment of EAH and early-stage EC, including 90.6% for EAH and 66.7% for early-stage EC. Despite the satisfactory short-term outcomes of progesterone therapy in EAH and early-stage EC, preventing drug resistance and disease recurrence remains a critical challenge. Research has documented recurrence rates as high as 30-40% following progesterone therapy for early-stage EC, potentially associated with PR-A and PR-B expression ratios in endometrial glands and stroma. Women with PR-A:PR-B ≤ 1 exhibited a higher recurrence risk than those with PR-A:PR-B > 1 (26, 27). Multiple clinical trials suggest beneficial effects of progestins in combination with GnRHα, HDAC inhibitors, and metformin for the treatment of EAH and early-stage EC and for recurrence prevention, detailed in subsequent sections. In conclusion, oral progestins and/or LNG-IUS effectively alleviate clinical symptoms and pathological features of EAH and early-stage EC. However, therapeutic effects are often short-lived, with prolonged use associated with drug resistance and disease recurrence. Future therapeutic strategies will focus on overcoming drug resistance through combination approaches to prevent EC progression and recurrence.
3.2 GnRHα
GnRH is a decapeptide secreted by the hypothalamus that regulates pituitary gonadotropin secretion. In preclinical models, GnRH-I and GnRH-II agonists, antagonists, and cytotoxic GnRH-I analogs have demonstrated antiproliferative effects and apoptosis induction in human EC cell lines (28). Several studies from the 1990s established that GnRHα exhibits significant antitumor activity against recurrent EC while effectively alleviating pain in EC patients (29, 30). However, subsequent in-depth investigations revealed that single-agent GnRHα therapy (leuprolide, goserelin, and triptorelin) demonstrated low response rate and limited efficacy in recurrent EC management (31–33). In the study by Asbury et al., a positive therapeutic response was observed in a patient who had previously received hormone therapy, despite the limited efficacy of single-agent goserelin acetate in recurrent EC (32). This finding suggests that GnRHα may enhance therapeutic efficacy in EC through combination with other endocrine therapies. GnRHα combined with LNG-IUS or AIs demonstrates efficacy in treating EAH and early-stage EC and represents a viable alternative to hysterectomy for fertility preservation (34, 35).
3.3 AIs
AIs interfere with endogenous estrogen production by inhibiting aromatase activity and may be employed in EC management. In a randomized trial evaluating anastrozole as a neoadjuvant therapeutic agent, significant reductions in ER, AR, and Ki67 expression in endometrial glands were observed following 14 days of anastrozole treatment, suggesting potential benefits of preoperative anastrozole in EC management (36). For elderly patients ineligible for standard surgical intervention, early administration of oral anastrozole may alleviate or stabilize disease and improve quality of life (37). Mileshkin et al. (38) conducted a phase II clinical trial assessing the efficacy of anastrozole in ER/PR-positive recurrent/metastatic EC. Although the objective response rate to monotherapy was relatively low, nearly half of the patients derived clinical benefit from treatment, and the degree of anastrozole benefit did not strongly correlate with ER/PR expression levels. Conversely, Lindemann et al. (39) found that ER-positive patients treated with the AIs exemestane demonstrated significantly higher rates of disease remission, median progression-free survival (PFS), and overall survival (OS) compared to ER-negative patients in a phase II clinical trial of ER-positive advanced/recurrent EC. To enhance efficacy, AIs are frequently combined with other agents in clinical practice. Beyond the previously mentioned combination with GnRHα for fertility-preserving treatment of EAH and early-stage EC, AIs combined with mTOR inhibitors have shown promise in advanced/recurrent EC management and survival prolongation. In summary, AIs monotherapy demonstrates greater efficacy in EAH and early-stage EC, with more limited efficacy in advanced or recurrent disease. The relationship between therapeutic efficacy and ER/PR status requires further investigation (40). Additionally, AIs combination with other agents (e.g., GnRHα and mTOR inhibitors) may enhance EC therapeutic efficacy and compensate limitations of monotherapy.
3.4 SERM and SERD
Tamoxifen, the most commonly utilized SERM analogue in clinical practice, exhibits dual effects in EC development and treatment. On one hand, tamoxifen use for other conditions increases EC risk by 1.5-6.9 fold; on the other hand, tamoxifen demonstrates utility in advanced/recurrent EC management (41). Tamoxifen may exert these effects by functioning as an agonist or antagonist through ERα, depending on cellular variations in coactivators or corepressors (42). Advanced/recurrent EC management frequently involves tamoxifen in combination with progesterone to counteract PR downregulation and enhance the antitumor activity of progesterone (8). Fulvestrant, a SERD analogue, possesses estrogen receptor antagonistic property, but its efficacy in EC remains uncertain. Results from two available phase II clinical trials suggest limited activity of fulvestrant in recurrent/metastatic EC management. Additionally, fulvestrant demonstrates poor bioavailability, and optimal therapeutic dosing remains undetermined (43–45). Combination approaches with fulvestrant may enhance therapeutic efficacy in EC; however, new clinical trials have not been conducted, and fulvestrant has not received approval for EC treatment.
3.5 Targeted epigenetics
Since Hrzenjak et al. (46) initially reported significantly higher HDAC2 expression in endometrial stromal sarcomas (ESS) compared to normal endometrial stroma, subsequent studies have demonstrated significantly elevated expression of HDAC1, HDAC2, and HDAC3 in EC (47). In vitro studies have shown that HDAC inhibitors effectively induce cell cycle arrest, reduce cell proliferation, and increase p21 expression in EC. Additionally, HDAC inhibitors enhance PR expression, which regulates cellular differentiation and exerts important anticancer effects (48). Duska et al. (49) conducted a clinical trial evaluating the HDAC inhibitor entinostat in combination with MPA during the surgical window. Results indicated that entinostat did not directly affect the expression of endometrial PR in the short term; however, Ki67 expression levels were lower with combination therapy compared to MPA monotherapy. In summary, HDAC inhibitors demonstrate promise in EC management. However, additional trials are necessary to determine the therapeutic role, utility, and safety profile of these agents. In addition, several studies have demonstrated correlations between DNA and histone methylation with EC development, although investigations have been limited to in vitro experiments (19).
3.6 mTOR inhibitors
mTOR inhibitors target diseases with PI3K/AKT/mTOR pathway mutations and are currently undergoing clinical trials for EC therapy. Based on several clinical investigations, mTOR inhibitors such as everolimus, sapanisertib, ridaforolimus, and temsirolimus have demonstrated preliminary antitumor activity in advanced/recurrent EC management (50–53). Beyond monotherapy, mTOR inhibitors combined with AIs show particular promise in EC treatment. Heudel et al. (54) found that adding the mTOR inhibitor vistusertib to anastrozole further prolonged 8-week PFS and median PFS and improved overall response rates in EC patients. The combination of everolimus and letrozole demonstrates high clinical benefit rates (CBR) and objective response rates (ORR) in patients with recurrent/progressive EC, with particularly notable efficacy in endometrioid EC harboring CTNNB1 mutations (55).
3.7 CDKis
Both the inherent endocrine characteristics of EC and its manifestation as a cell cycle dysregulation suggest potential utility for CDKis in these patients. Colon-Otero et al. (56) hypothesized that CDKis combined with AIs might be suitable for EC management based on the efficacy of this approach in breast cancer. Using clinical samples and patient-derived xenograft (PDX) models, they demonstrated that ribociclib (a CDKi) combined with letrozole produced positive survival effects in patients with recurrent ER-positive EC. Similarly, Konstantinopoulos et al. (57) found that letrozole combined with abemaciclib (a CDKi) demonstrated positive response effects in recurrent/metastatic EC, independent of tumor grade, prior hormone therapy, mismatch repair status, and PR status.
3.8 Metformin
Metformin, initially developed for diabetes management, has increasingly demonstrated importance in oncology. Multiple clinical trials have shown that metformin administration during the EC surgical window reduces Ki67 expression in tumor tissue while altering the phosphorylation status of PI3K/AKT/mTOR pathway proteins (58–60). However, Kitson et al. (61) demonstrated in a phase III clinical trial that short-term metformin treatment did not reduce Ki67 expression in EC tissues and failed to demonstrate positive effects on tumor proliferation inhibition. In combination approaches, metformin appears to offer superior efficacy compared to MA monotherapy in EAH management when used in combination with MA (62, 63). Additionally, in EAH and early-stage EC, metformin may inhibit disease recurrence following MPA treatment and contribute to fertility preservation (64).
4 Conclusion
Traditional endocrine therapy maintains a significant role in EC fertility preservation, preoperative neoadjuvant therapy, and palliative care for advanced disease; however, challenges such as high rates of treatment resistance and recurrence require continued attention. Novel therapeutic approaches may address limitations of conventional treatments, but research on emerging agents remains relatively limited, most still in preclinical development, resulting in insufficient clinical data regarding efficacy and adverse effects. Future research will continue to focus on novel targets and therapeutic modalities. Concurrently, combinations of traditional and novel agents may yield enhanced outcomes for EC patients.
Author contributions
XG: Writing – original draft, Writing – review & editing. RT: Validation, Writing – review & editing. JY: Funding acquisition, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the “Spearhead and Leading wild goose” Research Project(2024C03145) of Zhejiang Province, China. The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Katagiri R, Iwasaki M, Abe SK, Islam MR, Rahman MS, Saito E, et al. Reproductive factors and endometrial cancer risk among women. JAMA Netw Open. (2023) 6:e2332296. doi: 10.1001/jamanetworkopen.2023.32296
3. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. (2022) 399:1412–28. doi: 10.1016/S0140-6736(22)00323-3
4. Besharat AR, Giannini A, Caserta D. Pathogenesis and treatments of endometrial carcinoma. Clin Exp Obstet Gynecol. (2023) 50:229. doi: 10.31083/j.ceog5011229
5. Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning C, et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. (2019) 153:55–62. doi: 10.1016/j.ygyno.2019.01.014
6. Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. (2021) 31:12–39. doi: 10.1136/ijgc-2020-002230
7. D’Oria O, Giannini A, Besharat AR, Caserta D. Management of endometrial cancer: molecular identikit and tailored therapeutic approach. Clin Exp Obstet Gynecol. (2023) 50:210. doi: 10.31083/j.ceog5010210
8. Bestvina CM, Fleming GF. Chemotherapy for endometrial cancer in adjuvant and advanced disease settings. Oncol. (2016) 21:1250–9. doi: 10.1634/theoncologist.2016-0062
9. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol: Off J Am Soc Clin Oncol. (2013) 31:2607–18. doi: 10.1200/jco.2012.48.2596
10. Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. (2013) 75:225–40. doi: 10.1146/annurev-physiol-030212-183708
11. Yu K, Huang ZY, Xu XL, Li J, Fu XW, Deng SL. Estrogen receptor function: impact on the human endometrium. Front Endocrinol. (2022) 13:827724. doi: 10.3389/fendo.2022.827724
12. Kailasam A, Langstraat C. Contemporary use of hormonal therapy in endometrial cancer: a literature review. Curr Treat Options Oncol. (2022) 23:1818–28. doi: 10.1007/s11864-022-01031-6
13. Thomas C, Gustafsson J. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. (2011) 11:597–608. doi: 10.1038/nrc3093
14. Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol. (2020) 69:95–107. doi: 10.1016/j.bpobgyn.2020.05.003
15. Schindler AE. Progestogen deficiency and endometrial cancer risk. Maturitas. (2009) 62:334–7. doi: 10.1016/j.maturitas.2008.12.018
16. Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: An old dog with new tricks. Gynecol Oncol. (2019) 153:175–83. doi: 10.1016/j.ygyno.2018.12.018
17. Ring KL, Mills AM, Modesitt SC. Endometrial hyperplasia. Obstet Gynecol. (2022) 140:1061–75. doi: 10.1097/AOG.0000000000004989
18. Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. (2018) 186:1–24. doi: 10.1016/j.pharmthera.2017.12.012
19. Inoue F, Sone K, Toyohara Y, Takahashi Y, Kukita A, Hara A, et al. Targeting epigenetic regulators for endometrial cancer therapy: its molecular biology and potential clinical applications. Int J Mol Sci. (2021) 22:2305. doi: 10.3390/ijms22052305
20. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. (2013) 497:67–73. doi: 10.1038/nature12113
21. Barra F, Evangelisti G, Ferro Desideri L, Di Domenico S, Ferraioli D, Vellone VG, et al. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin Investig Drugs. (2019) 28:131–42. doi: 10.1080/13543784.2018.1558202
22. Giannone G, Tuninetti V, Ghisoni E, Genta S, Scotto G, Mittica G, et al. Role of cyclin-dependent kinase inhibitors in endometrial cancer. Int J Mol Sci. (2019) 20:2353. doi: 10.3390/ijms20092353
23. Fiascone S, Danilack VA, Kao MJ, Cohen M, Singh K, Kalife E, et al. While women await surgery for type I endometrial cancer, depot medroxyprogesterone acetate reduces tumor glandular cellularity. Am J Obstet Gynecol. (2018) 219:381.e1–.e10. doi: 10.1016/j.ajog.2018.07.024
24. Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod. (2013) 28:2966–71. doi: 10.1093/humrep/det320
25. Janda M, Robledo KP, Gebski V, Armes JE, Alizart M, Cummings M, et al. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: Results of a randomized clinical trial. Gynecol Oncol. (2021) 161:143–51. doi: 10.1016/j.ygyno.2021.01.029
26. Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, et al. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol. (2021) 224:191.e1–.e15. doi: 10.1016/j.ajog.2020.08.032
27. Sletten ET, Arnes M, Lyså LM, Larsen M, Ørbo A. Significance of progesterone receptors (PR-A and PR-B) expression as predictors for relapse after successful therapy of endometrial hyperplasia: a retrospective cohort study. Bjog. (2019) 126:936–43. doi: 10.1111/bjo.2019.126.issue-7
28. Emons G, Gründker C. The role of gonadotropin-releasing hormone (GnRH) in endometrial cancer. Cells. (2021) 10:292. doi: 10.3390/cells10020292
29. Gallagher CJ, Oliver RTD, Oram DH, Fowler CG, Blake PR, Mantell BS, et al. A new treatment for endometrial cancer with gonadotrophin releasing-hormone analogue. Br J Obstet Gynaecol. (1991) 98:1037–41. doi: 10.1111/j.1471-0528.1991.tb15343.x
30. Jeyarajah AR, Gallagher CJ, Blake PR, Oram DH, Dowsett M, Fisher C, et al. Long-term follow-up of gonadotrophin-releasing hormone analog treatment for recurrent endometrial cancer. Gynecol Oncol. (1996) 63:47–52. doi: 10.1006/gyno.1996.0276
31. Covens A, Thomas G, Shaw P, Ackerman I, Osborne R, Lukka H, et al. A phase II study of leuprolide in advanced/recurrent endometrial cancer. Gynecol Oncol. (1997) 64:126–9. doi: 10.1006/gyno.1996.4544
32. Asbury RF, Brunetto VL, Lee RB, Reid G, Rocereto TF. Goserelin acetate as treatment for recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Am J Clin Oncol. (2002) 25:557–60. doi: 10.1097/00000421-200212000-00004
33. Lhommé C, Vennin P, Callet N, Lesimple T, Achard JL, Chauvergne J, et al. A multicenter phase II study with triptorelin (sustained-release LHRH agonist) in advanced or recurrent endometrial carcinoma: a French anticancer federation study. Gynecol Oncol. (1999) 75:187–93. doi: 10.1006/gyno.1999.5538
34. Pashov AI, Tskhay VB, Ionouchene SV. The combined GnRH-agonist and intrauterine levonorgestrel-releasing system treatment of complicated atypical hyperplasia and endometrial cancer: a pilot study. Gynecol Endocrinol. (2012) 28:559–61. doi: 10.3109/09513590.2011.649813
35. Chen J, Cao D, Yang J, Yu M, Zhou H, Cheng N, et al. Oncological and reproductive outcomes for gonadotropin-releasing hormone agonist combined with aromatase inhibitors or levonorgestrel-releasing intra-uterine system in women with endometrial cancer or atypical endometrial hyperplasia. Int J Gynecol Cancer. (2022) 32:1561–7. doi: 10.1136/ijgc-2022-003882
36. Thangavelu A, Hewitt MJ, Quinton ND, Duffy SR. Neoadjuvant treatment of endometrial cancer using anastrozole: a randomised pilot study. Gynecol Oncol. (2013) 131:613–8. doi: 10.1016/j.ygyno.2013.09.023
37. Gardella B, Dominoni M, Bogliolo S, Cassani C, Carletti GV, De Silvestri A, et al. Palliative treatment of endometrial cancer: what is the role of anastrozole in elderly women? BMC Palliat Care. (2021) 20:28. doi: 10.1186/s12904-021-00719-0
38. Mileshkin L, Edmondson R, O’Connell RL, Sjoquist KM, Andrews J, Jyothirmayi R, et al. Phase 2 study of anastrozole in recurrent estrogen (ER)/progesterone (PR) positive endometrial cancer: The PARAGON trial - ANZGOG 0903. Gynecol Oncol. (2019) 154:29–37. doi: 10.1016/j.ygyno.2019.05.007
39. Lindemann K, Malander S, Christensen RD, Mirza MR, Kristensen GB, Aavall-Lundqvist E, et al. Examestane in advanced or recurrent endometrial carcinoma: a prospective phase II study by the Nordic Society of Gynecologic Oncology (NSGO). BMC Cancer. (2014) 14:68. doi: 10.1186/1471-2407-14-68
40. Gao C, Wang Y, Tian W, Zhu Y, Xue F. The therapeutic significance of aromatase inhibitors in endometrial carcinoma. Gynecol Oncol. (2014) 134:190–5. doi: 10.1016/j.ygyno.2014.04.060
41. Hu R, Hilakivi-Clarke L, Clarke R. Molecular mechanisms of tamoxifen-associated endometrial cancer (Review). Oncol Lett. (2015) 9:1495–501. doi: 10.3892/ol.2015.2962
42. Emons G, Mustea A, Tempfer C. Tamoxifen and endometrial cancer: A janus-headed drug. Cancers (Basel). (2020) 12:2535. doi: 10.3390/cancers12092535
43. Covens AL, Filiaci V, Gersell D, Lutman CV, Bonebrake A, Lee YC. Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. (2011) 120:185–8. doi: 10.1016/j.ygyno.2010.10.015
44. Emons G, Günthert A, Thiel FC, Camara O, Strauss HG, Breitbach GP, et al. Phase II study of fulvestrant 250 mg/month in patients with recurrent or metastatic endometrial cancer: a study of the Arbeitsgemeinschaft Gynäkologische Onkologie. Gynecol Oncol. (2013) 129:495–9. doi: 10.1016/j.ygyno.2013.02.039
45. Bogliolo S, Cassani C, Dominoni M, Orlandini A, Ferrero S, Iacobone AD, et al. The role of fulvestrant in endometrial cancer. Expert Opin Drug Metab Toxicol. (2017) 13:537–44. doi: 10.1080/17425255.2016.1244264
46. Hrzenjak A, Moinfar F, Kremser ML, Strohmeier B, Staber PB, Zatloukal K, et al. Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Mol Cancer Ther. (2006) 5:2203–10. doi: 10.1158/1535-7163.MCT-05-0480
47. Weichert W, Denkert C, Noske A, Darb-Esfahani S, Dietel M, Kalloger SE, et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. (2008) 10:1021–7. doi: 10.1593/neo.08474
48. Gujral P, Mahajan V, Lissaman AC, Ponnampalam AP. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod Biol Endocrinol. (2020) 18:84. doi: 10.1186/s12958-020-00637-5
49. Duska LR, Filiaci VL, Walker JL, Holman LL, Hill EK, Moore RG, et al. A surgical window trial evaluating medroxyprogesterone acetate with or without entinostat in patients with endometrial cancer and validation of biomarkers of cellular response. Clin Cancer Res. (2021) 27:2734–41. doi: 10.1158/1078-0432.CCR-20-4618
50. Voss MH, Gordon MS, Mita M, Rini B, Makker V, Macarulla T, et al. Phase 1 study of mTORC1/2 inhibitor sapanisertib (TAK-228) in advanced solid tumours, with an expansion phase in renal, endometrial or bladder cancer. Br J Cancer. (2020) 123:1590–8. doi: 10.1038/s41416-020-01041-x
51. Colombo N, McMeekin DS, Schwartz PE, Sessa C, Gehrig PA, Holloway R, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer. (2013) 108:1021–6. doi: 10.1038/bjc.2013.59
52. Ray-Coquard I, Favier L, Weber B, Roemer-Becuwe C, Bougnoux P, Fabbro M, et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br J Cancer. (2013) 108:1771–7. doi: 10.1038/bjc.2013.183
53. Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. (2011) 29:3278–85. doi: 10.1200/JCO.2010.34.1578
54. Heudel P, Frenel JS, Dalban C, Bazan F, Joly F, Arnaud A, et al. Safety and efficacy of the mTOR inhibitor, vistusertib, combined with anastrozole in patients with hormone receptor-positive recurrent or metastatic endometrial cancer: the VICTORIA multicenter, open-label, phase 1/2 randomized clinical trial. JAMA Oncol. (2022) 8:1001–9. doi: 10.1001/jamaoncol.2022.1047
55. Slomovitz BM, Jiang Y, Yates MS, Soliman PT, Johnston T, Nowakowski M, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. (2015) 33:930–6. doi: 10.1200/JCO.2014.58.3401
56. Colon-Otero G, Zanfagnin V, Hou X, Foster NR, Asmus EJ, Wahner Hendrickson A, et al. Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers. ESMO Open. (2020) 5:e000926. doi: 10.1136/esmoopen-2020-000926
57. Konstantinopoulos PA, Lee EK, Xiong N, Krasner C, Campos S, Kolin DL, et al. Two-stage study of letrozole and abemaciclib in estrogen receptor-positive recurrent endometrial cancer. J Clin Oncol. (2023) 41:599–608. doi: 10.1200/JCO.22.00628
58. Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer. (2016) 114:281–9. doi: 10.1038/bjc.2015.453
59. Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol Oncol. (2014) 134:607–14. doi: 10.1016/j.ygyno.2014.06.014
60. Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: a preoperative prospective trial. Cancer. (2014) 120:2986–95. doi: 10.1002/cncr.v120.19
61. Kitson SJ, Maskell Z, Sivalingam VN, Allen JL, Ali S, Burns S, et al. PRE-surgical metformin in uterine Malignancy (PREMIUM): a multi-center, randomized double-blind, placebo-controlled phase III trial. Clin Cancer Res. (2019) 25:2424–32. doi: 10.1158/1078-0432.CCR-18-3339
62. Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. Bjog. (2020) 127:848–57. doi: 10.1111/1471-0528.16108
63. Tehranian A, Ghahghaei-Nezamabadi A, Arab M, Khalagi K, Aghajani R, Sadeghi S. The impact of adjunctive metformin to progesterone for the treatment of non-atypical endometrial hyperplasia in a randomized fashion, a placebo-controlled, double blind clinical trial. J Gynecol Obstet Hum Reprod. (2021) 50:101863. doi: 10.1016/j.jogoh.2020.101863
Keywords: endometrial cancer, endocrine therapy, traditional targets, novel targets, mechanisms
Citation: Guan X, Tang R and Yang J (2025) Endocrine therapy for endometrial cancer: traditional approaches and novel targets. Front. Oncol. 15:1570011. doi: 10.3389/fonc.2025.1570011
Received: 02 February 2025; Accepted: 15 April 2025;
Published: 05 May 2025.
Edited by:
Panagiota S Filippou, Aristotle University of Thessaloniki, GreeceReviewed by:
Ilaria Cuccu, Sapienza University of Rome, ItalyCopyright © 2025 Guan, Tang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: JianHua Yang, eWpoMjAwNkB6anUuZWR1LmNu
 XiaoJing Guan
XiaoJing Guan RongRong Tang
RongRong Tang JianHua Yang
JianHua Yang