- 1Department of Oral and Maxillofacial Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Oral Medicine, School of Stomatology, Qingdao University, Qingdao, China
Objective: Giant cell-rich lesions in the maxillofacial region are relatively rare, and comprehensive clinical differential diagnostic protocols are currently lacking. This article aims to provide a reference for the clinical diagnosis and treatment of giant cell-rich lesions.
Methods: This study investigates the distinguishing features of four types of giant cell-rich lesions in differential diagnosis and treatment: giant cell tumor of bone (GCT), aneurysmal bone cyst (ABC), tenosynovial giant cell tumor (TGCT), and giant cell reparative granuloma (GCRG).
Results: Immunohistochemical (IHC) analysis reveals strong p63 positivity in the mononuclear stromal cells of GCT, but not in GCRG. The “fluid-fluid level” observed in magnetic resonance imaging (MRI) is a diagnostic indicator for ABC, reflecting variable signal intensities. TGCT is characterized by the presence of synovial monocytes, multinucleated giant cells, foam cells, and hemosiderin-laden cells.
Conclusion: Accurate diagnosis requires a comprehensive evaluation of clinical, imaging, and pathological data. While complete resection is crucial for GCT to prevent recurrence and malignant transformation, GCRG typically responds well to curettage due to its benign nature. Early surgical intervention is essential for TGCT to control its aggressive progression and minimize complications.
Introduction
Giant cell-rich lesions encompass a diverse group of tumors and neoplastic lesions characterized by the presence of varying numbers of reactive, multinucleated osteoclast-like giant cells (1, 2). The World Health Organization (WHO) classifies several osteoclast-rich lesions, including giant cell tumor of bone (GCT), aneurysmal bone cyst (ABC), tenosynovial giant cell tumor (TGCT), and giant cell reparative granuloma (GCRG), alongside non-ossifying fibromas and certain benign conditions (1, 3, 4).
These lesions are relatively uncommon in the maxillofacial region, where accurate diagnosis can be particularly challenging due to significant overlap in the clinical and histopathological features of these disorders (5, 6). Effective diagnosis requires a thorough evaluation of pathological, clinical, and radiological attributes, with special attention to the anatomical location, patient age, and lesion count. Additionally, the identification of elements beyond giant cells is crucial to avoid misdiagnosis (7–9). This article reviews clinical cases of four pertinent disorders, discusses key aspects of their differential diagnosis and management in routine practice, and offers guidance for the clinical diagnosis and treatment of GCT (6, 10, 11).
Case reports
Case 1
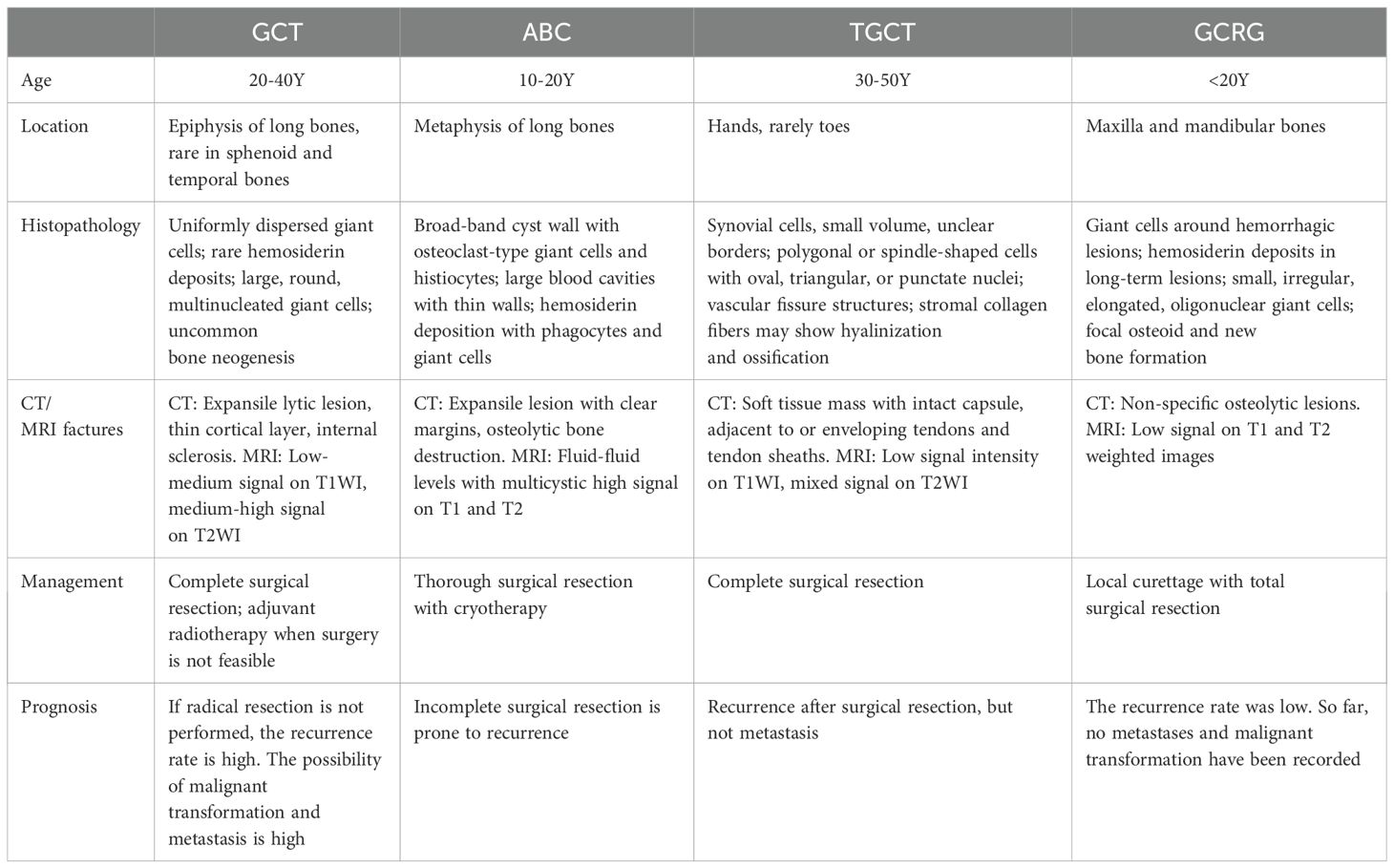
A 43-year-old woman presented with a 4-month history of a left mandibular mass. Computed tomography (CT) scans (Figures 1A–C) revealed expansive osteolytic destruction in the mandibular ramus and body, featuring soft tissue density, cortical thinning, and multifragmented bone with clear soft tissue boundaries. Surgical management involved tumor resection with free fibular flap reconstruction. Hematoxylin-eosin (H&E) staining (Figures 1D, E) demonstrated a giant cell-rich tumor composed of evenly distributed spindle or mononuclear cells and osteoclast-like giant cells, with occasional mitotic figures and sinusoidal cavities, consistent with GCT. IHC analysis (Supplementary Figure 1) yielded the following results: p63(+), S100(-), CD68(+), SMA (+), and Ki-67(+). The patient remained recurrence-free and asymptomatic during 36 months of follow-up.
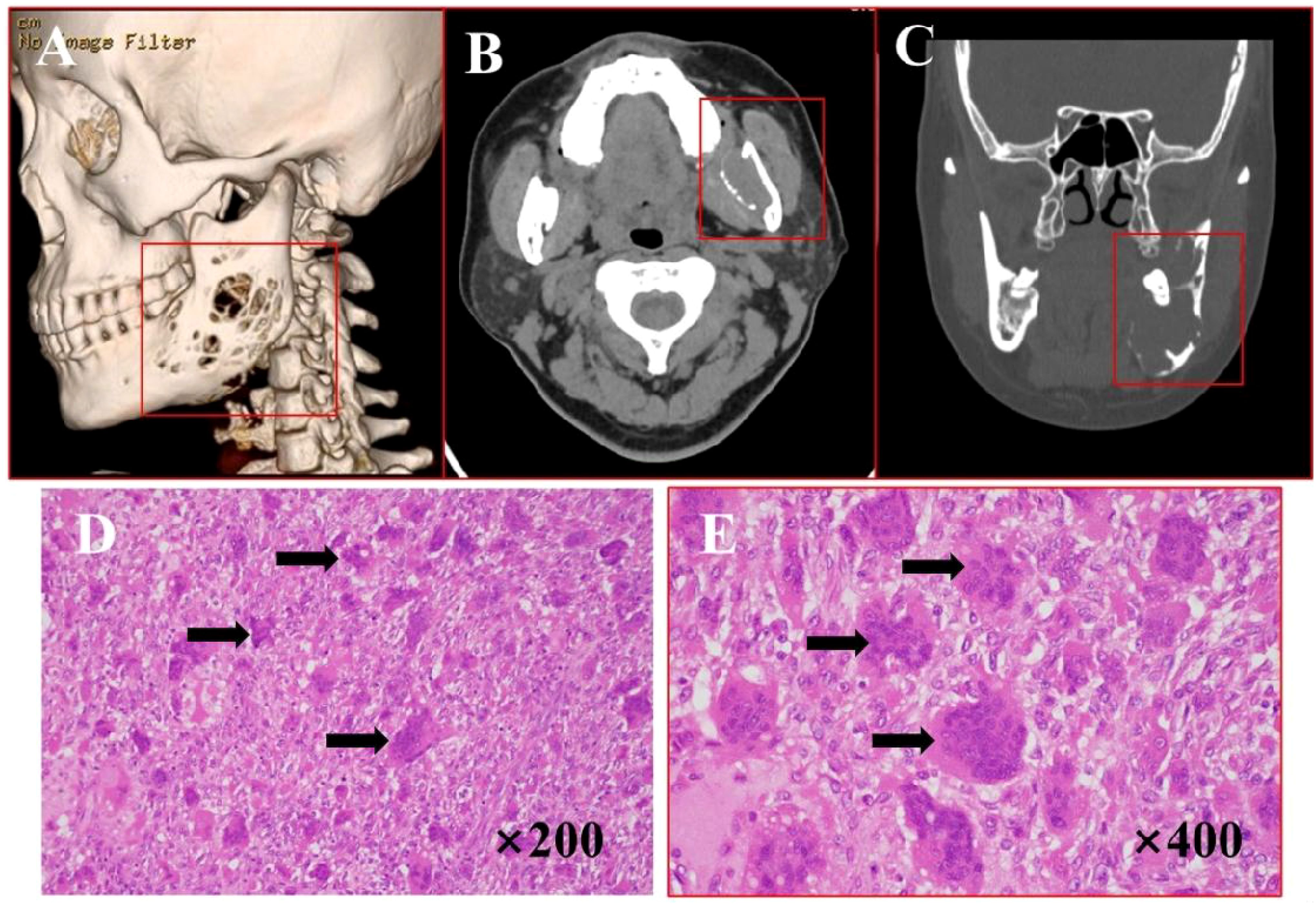
Figure 1. Imaging and histopathological features of GCT. (A) Bone CT demonstrates expansive osteolytic destruction in the left mandibular ramus and body with cortical thinning. (B, C) Axial and coronal CT reveal multiple discontinuities in the bone structure, characterized by dense soft tissue, thin cortical bone, multiple bone discontinuities, and clearly demarcated surrounding soft tissue. (D–E) H&E staining (×200, ×400): arrows points giant cells and the cells were oval.
Case 2
A 52-year-old woman presented with a 3-month history of a painless right maxillary mass. CT scans (Figures 2A–C) demonstrated extensive alveolar bone destruction with contour loss, well-defined margins, and partial bone thinning, measuring 45×52×39 mm. The lesion, containing a thin bony shell, caused right nasal cavity displacement. Surgical intervention included tumor resection under general anesthesia with titanium plate reconstruction. H&E staining (Figures 2D, E) of the tumor showed areas of active fibroproliferation with abundant reactive trabecular bone structure. Multiple cystic cavities were locally observed, indicative of ABC changes. Postoperative recovery was uneventful, and the patient remained recurrence-free and asymptomatic during 21 months of follow-up.

Figure 2. Imaging and histopathological features of ABC. (A) Bone CT demonstrates lesion involvement of the right maxilla, maxillary sinus, and palatine bone with irregular surrounding bone thickness. (B, C) Axial and coronal CT reveal osteolytic destruction with well-defined margins, partial bone thinning, and an internal thin bony shell. (D, E) H&E staining (×200, ×400): arrows points cystic cavities with reactive new bone formation.
Case 3
A 19-year-old female presented with a 2-year history of a firm, fixed left temporal mass. CT imaging (Figures 3A–C) revealed an irregular soft tissue lesion (46×30×37 mm) in the temporal squama and zygomatic region, containing speckled calcifications and associated bone destruction with areas of sclerosis. Surgical management involved complete tumor resection under general anesthesia. H&E staining (Figures 3D, E) revealed that the tumor was rich in giant cells, containing numerous phagocytic, hemosiderin-laden mononuclear cells with abundant cytoplasm and inclusion bodies. Some regions exhibited chondroid differentiation surrounded by pronounced proliferative fibrous tissue, leading to a diagnosis of diffuse TGCT with chondroid metaplasia. IHC results (Supplementary Figure 2) were as follows: SMA(+), S100 (-), p63 (-), Ki-67 (+), CD68 (-). The patient remained recurrence-free and asymptomatic during 21 months of follow-up.
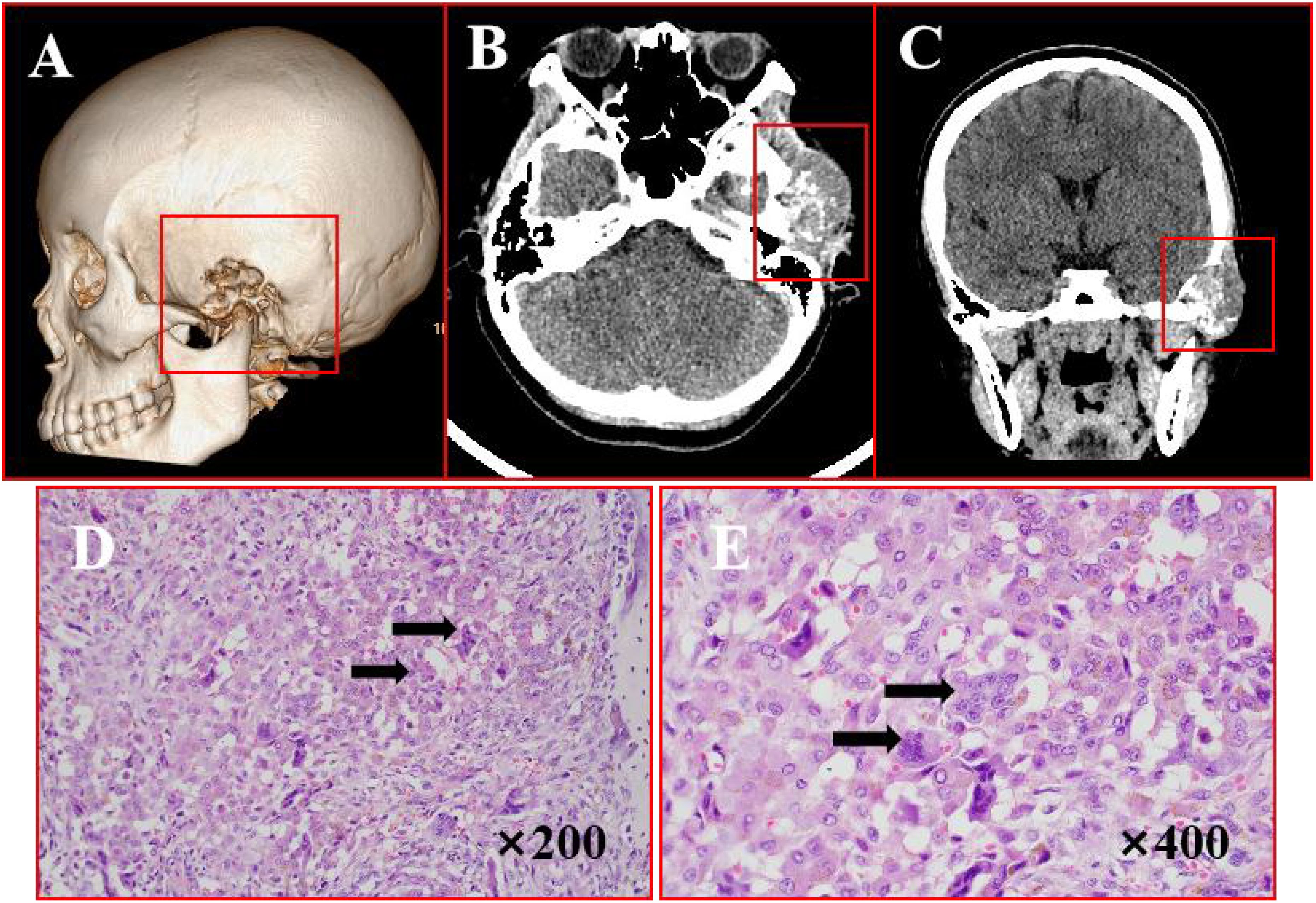
Figure 3. Imaging and histopathological features of TGCT. (A) Bone CT demonstrates osteolytic destruction involving the left mastoid air cells, zygomatic arch, and temporal bone with adjacent sclerosis. (B, C) Axial and coronal CT reveal an irregular soft tissue lesion extending into the temporal fossa, featuring poorly defined margins and internal speckled calcifications. (D, E) H&E staining (×200,×400): arrows points actively proliferating monocytes and osteoclast-like multinucleated giant cells.
Case 4
A 32-year-old male presented with a 2-year history of a painful right preauricular mass. CT imaging (Figures 4A–C) revealed a 29×45 mm cystic-solid lesion with heterogeneous enhancement, causing bone destruction in the temporal, sphenoid, and zygomatic regions, along with adjacent soft tissue changes. Surgical management included tumor resection via a hemicoronal approach, zygomatic osteotomy, and temporal fascia flap reconstruction. H&E staining (Figures 4D, E) showed extensive degeneration, fibrous proliferation, hemorrhage, and histiocytic reaction, with areas of mononuclear cell proliferation and osteoclast-like giant cells, consistent with GCRG. IHC results (Supplementary Figure 3) demonstrated: P63 (-), S100 (-), CD68 (+),Ki67 (+), EMA (-) and SMA (+) expression. The patient remained recurrence-free and asymptomatic during 21 months of follow-up.
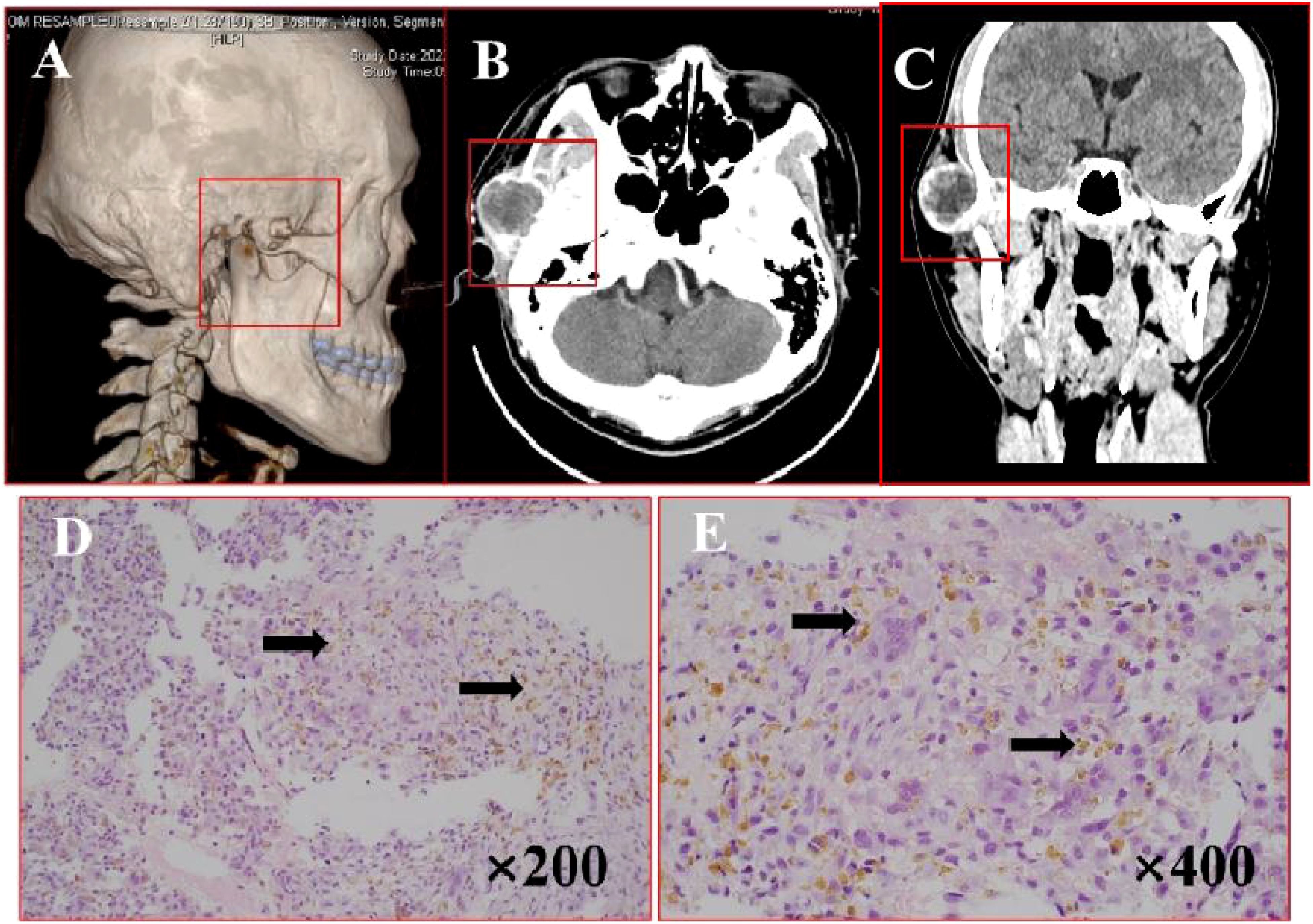
Figure 4. Imaging and histopathological features of GCRG. (A) Bone CT demonstrates right temporal expansile osteolytic destruction with cortical thinning and heterogeneous lesion density. (B, C) Axial and coronal CT reveal mastoid bone destruction with soft tissue density and a cystic-solid mass anterior to the right ear. (D, E) H&E staining (×200, ×400): arrows points fibrous tissue hyperplasia with multinucleated giant cells, and hemosiderin deposition.
Discussion
Giant cell-rich lesions, defined by the presence of multinucleated giant cells, encompass various benign, nonneoplastic, and borderline conditions. Accurate diagnosis through histopathological, imaging, and molecular analyses is critical for guiding treatment decisions and predicting recurrence risk (12, 13). This review focuses on four representative lesions: GCT, GCRG, ABC, and TGCT (Table 1).
GCT typically manifests with pain and local swelling, predominantly affecting individuals aged 20-45 years, with a strong preference for long bones and rare cranial involvement (1, 13). GCRG, a non-neoplastic condition primarily seen in children and young adults, commonly involves the jawbones, exhibiting destructive and locally invasive features that often lead to tumor misdiagnosis (4, 14). ABC presents as a blood-filled cystic lesion containing osseous tissue, mainly affecting adolescents in long bone metaphyses, characterized by distinctive subperiosteal bone deposition (15, 16). TGCT, a benign soft tissue tumor originating from synovial structures, typically occurs in 30-50-year-olds, with female predominance, and presents with joint swelling, pain, and restricted mobility (8, 9).
GCT typically demonstrates expansile bone destruction with adjacent cortical thickening or thinning, showing marked heterogeneous enhancement on MRI (4, 17). ABC is characterized by lytic destruction with multiple fluid-fluid levels on imaging - a diagnostic hallmark - often accompanied by periosteal reaction (15, 18). GCRG presents as nonspecific lytic lesions containing bony septations, typically lacking fluid-fluid levels, which helps differentiate it from GCT and ABC (4, 19). TGCT manifests as a well-defined or irregular soft tissue mass, potentially with calcifications or bone erosion, with MRI being crucial for assessing tumor extent and adjacent tissue involvement (20, 21).
Histopathologically, GCT displays uniformly distributed multinucleated giant cells, with P63 protein expression and occasional ossification/calcification serving as key diagnostic markers to differentiate it from GCRG and ABC (19, 22, 23). GCRG primarily consists of vascular-rich connective tissue containing fibroblasts and mononuclear cells, typically showing hemorrhage, hemosiderin deposition, and occasional osteoid formation. ABC, while also containing giant cells, is characterized by blood-filled cystic spaces lined with hemosiderin deposits and osteoblast-covered reactive bone, sometimes with diagnostic chondroid calcification (14, 15). TGCT exhibits synovial-like mononuclear cells mixed with multinucleated giant cells, foamy cells, and hemosiderin deposits, occasionally demonstrating cleft-like spaces and pseudoglandular structures (24, 25).
Surgical management remains the cornerstone for these lesions, with specific approaches tailored to each condition. For GCT, complete surgical excision is crucial to minimize the 25% recurrence risk and prevent potential malignant transformation or pulmonary metastasis, with adjuvant radiotherapy considered for high-risk cases (25, 26). GCRG treatment primarily involves surgical resection, achieving an 80% cure rate with local curettage, supplemented by calcitonin or bisphosphonate therapy to reduce recurrence (10, 11). ABC management centers on surgical curettage with bone grafting, though its high recurrence risk (10%-60%) may necessitate adjunctive cryotherapy or radiotherapy in complex cases (12, 13, 27). TGCT treatment relies on surgical resection of localized lesions, with postoperative radiotherapy proving particularly effective for diffuse forms, demonstrating improved prognosis despite its inherent recurrence risk. Across all conditions, comprehensive surgical strategies combined with appropriate adjuvant therapies and rigorous follow-up are essential for optimal outcomes (23, 27).
Overall, Surgery is the primary treatment for these lesions, with treatment selection guided by lesion aggressiveness, anatomical location, and patient factors. Postoperative adjuvant therapy and regular follow-up are essential to minimize recurrence and optimize outcomes.
This study highlights the importance of a multidisciplinary approach, integrating clinical, radiological, and histopathological data, to achieve accurate diagnosis and effective management. Emerging tools such as artificial intelligence (AI) in radiology and molecular markers offer significant potential to enhance diagnostic precision and treatment outcomes. Future advancements should focus on integrating multimodal data—clinical, radiological, histopathological, and molecular—to develop comprehensive diagnostic frameworks. Personalized treatment strategies, guided by molecular profiling and AI-driven predictive models, could optimize surgical and adjuvant therapies. By leveraging AI, molecular diagnostics, and multidisciplinary approaches, clinicians can achieve more precise diagnoses and tailored treatments, ultimately advancing patient care in this complex field.
Conclusion
This study explores diagnostic and management challenges of maxillofacial giant cell-rich lesions (GCT, ABC, TGCT, GCRG) through four rare cases. Radical surgical excision yielded excellent outcomes, with no recurrence over 21-36 months of follow-up. A multidisciplinary approach, combining MRI features (e.g., “fluid-fluid levels” in ABC) and immunohistochemical markers (p63, CD68, Ki-67), was crucial for accurate differentiation and pathogenic insights. Complete surgical resection is critical for GCT to avoid recurrence and potential malignant transformation, whereas GCRG, being a benign lesion, generally shows favorable outcomes with curettage. For TGCT, prompt surgical treatment is necessary to manage its aggressive behavior and reduce the risk of complications. The findings provide a comprehensive framework for accurate diagnosis and tailored treatment, enhancing patient outcomes and quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
As this study is a retrospective analysis based on previously collected data without any direct involvement of patients or interventions, formal approval from an ethics committee was not required. The data used in the study were collected from publicly available sources or previously published cases, ensuring compliance with ethical standards. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XG: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. SW: Investigation, Writing – review & editing. YL: Methodology, Project administration, Writing – review & editing. LC: Supervision, Validation, Writing – review & editing. XZ: Formal analysis, Project administration, Writing – review & editing. JS: Formal analysis, Resources, Writing – review & editing. HX: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Youth Research Fund of the Affiliated Hospital of Qingdao University (Grant No. QDFYQN2023203); Clinical Medicine +X Research Program, Affiliated Hospital of Qingdao University (Grant No. QDFY+X2024221); Shandong Province Youth Innovation Technology Support Program for Colleges and Universities (Grant No. 2023KJ357); the Peak-Climbing Project in stomatology of Qingdao; and Qingdao Science and Technology for the Benefit of the People Demonstration Special Project (Grant No. 25-1-5-smjk-19-nsh).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1572560/full#supplementary-material
References
1. Bansal K, Singh J, Gupta P, Singh S, Kumar R, Singh S. Giant cell tumor: a case series of seven patients with gct at atypical sites. J Orthopaedic Case Rep. (2023) 13:171–79. doi: 10.13107/jocr.2023.v13.i11.4032
2. Ahmed SK, Shehzad MN. Giant cell tumor of soft tissue: gct arising from periosteum of tibia. Pak J Med Sci. (2024) 40:S97–99. doi: 10.12669/pjms.40.2(ICON).8953
3. Basu Mallick A, Chawla SP. Giant cell tumor of bone: an update. Curr Oncol Rep. (2021) 23(5):51. doi: 10.1007/s11912-021-01047-5
4. Alzaidi SS, Ghafouri AM, Alayoubi SA, Rhbeini YA. Giant cell reparative granuloma of parotid region infiltrating the zygomatic bone: a case report. Ann Med Surg (Lond). (2020) 56:145–48. doi: 10.1016/j.amsu.2020.06.014
5. Chan AS, Katiyar V, Dy P, Singh V. Updates on the treatment of tenosynovial giant cell tumor. Hematology/Oncology Stem Cell Ther. (2023) 16:307–15. doi: 10.56875/2589-0646
6. Neugebauer J, Blum P, Keiler A, Süß M, Reinbacher P, Neubauer M, et al. Mismatch between preoperative mri findings and postoperative histological results in the treatment of tenosynovial giant cell tumor. Anticancer Res. (2025) 45:1087–96. doi: 10.21873/anticanres
7. Chi Y, Qin Z, Bai J, Yan J, Xu Z, Yang S, et al. Update on the nature of central giant cell granuloma of the jaw with a focus on the aggressive subtype. Pathology. (2025) S0031-3025(25)00010-8. doi: 10.1016/j.pathol.2024.10.010
8. Robert M, Farese H, Miossec P. Update on tenosynovial giant cell tumor, an inflammatory arthritis with neoplastic features. Front Immunol. (2022) 13:820046. doi: 10.3389/fimmu.2022.820046
9. Dania V, Stavropoulos NA, Gavriil P, Trikoupis I, Koulouvaris P, Savvidou OD, et al. Treatment modalities for refractory-recurrent tenosynovial giant cell tumor (tgct): an update. Medicina. (2024) 60:1675. doi: 10.3390/medicina60101675
10. Sakai K, Minabe M, Hata K, Kamemoto K, Masuda K, Hashimoto K, et al. Successful treatment of a rapidly enlarging mandibular aneurysmal bone cyst with sclerotherapy and intralesional curettage. Cureus. (2025) 17(1):e78256. doi: 10.7759/cureus.78256
11. Kumar KAJ, Humayun S, Kumar B, Rao J. Reparative giant cell granuloma of the maxilla. Ann Maxillofac Surgery. (2011) 1:181. doi: 10.4103/2231-0746.92791
12. Klienkoff P, Weingertner N, Geyer L, Gros C, Kurtz J, Bornert F. Management of a rare mandibular giant cell tumor of bone by neoadjuvant denosumab therapy and surgery: a 4-year follow-up case report. Int J Surg Case Rep. (2023) 112:108980. doi: 10.1016/j.ijscr.2023.108980
13. Ari I, Adiloglu S, Aktas A, Yasan GT, Usman E, Aksoy S. Incidence, treatment method and recurrence rate in giant cell granulomas: retrospective study. J Craniomaxillofac Surg. (2024) 52:697–703. doi: 10.1016/j.jcms.2024.03.011
14. Dareh MTB, Andisheh Tadbir A, Aghakouchakzadeh A. Evaluation of the relationship between the expression of agnor and ki67 with the recurrence rate in central granulomatous giant cell lesions: a case-control. Clin Exp Dent Res. (2024) 10(2):e870. doi: 10.1002/cre2.870
15. Woldow A, Foy VM. Aneurysmal bone cyst of the skull: a case report. Sage Open Med Case Rep. (2022) 10:2050313X221117727. doi: 10.1177/2050313X221117727
16. Yahaya JJ, Morgan ED, Abraham ZS, Othieno E. Aneurysmal bone cyst of the mandible: a rare case report and literature review. Ann Med Surgery. (2023) 85:5133–37. doi: 10.1097/MS9.0000000000001168
17. Rekhi B, Dave V. Giant cell tumor of bone: an update, including spectrum of pathological features, pathogenesis, molecular profile and the differential diagnoses. Histol Histopathol. (2023) 38:139–53. doi: 10.14670/HH-18-486
18. Nasri E, Reith JD. Aneurysmal bone cyst: a review. J Pathol Transl Med. (2023) 57:81–7. doi: 10.4132/jptm.2023.02.23
19. Liu Y, Zhou J, Shi J. Clinicopathology and recurrence analysis of 44 jaw aneurysmal bone cyst cases: a literature review. Front Surg. (2021) 8:678696. doi: 10.3389/fsurg.2021.678696
20. Mohammadi F, Bashizadehfakhar H, Aliasghari S, Gholamhoseini Z. Aggressive multiple central giant cell granulomas of the jaws. Case Rep Dent. (2023) 2023:1–07. doi: 10.1155/2023/5410229
21. Nagar SR, Bansal S, Jashnani K, Desai RS. A comparative clinicopathological study of giant cell tumour (gct), central giant cell granuloma (cgcg) and peripheral giant cell granuloma (pgcg). J Maxillofac Oral Surg. (2023) 22:485–501. doi: 10.1007/s12663-022-01724-3
22. Choi YJ, Lee C, Jeon KJ, Han S. Computed tomography and magnetic resonance imaging characteristics of giant cell tumors in the temporomandibular joint complex. Imaging Sci Dent. (2021) 51:149. doi: 10.5624/isd.20200300
23. Smit C, Robinson L, Roman TE, Roza A, Rocha AC, Santos-Silva AR, et al. Clinicoradiological spectrum of primary aneurysmal bone cysts of the maxillofacial region: a series of 31 cases. Dentomaxillofac Radiol. (2022) 51:20220071. doi: 10.1259/dmfr.20220071
24. Nagar SR, Bansal S, Jashnani K, Sinha A, Desai RS. A comparative analysis of p63 expression in giant cell tumour (gct), central giant cell granuloma (cgcg) and peripheral giant cell granuloma (pgcg). Head Neck Pathology. (2020) 14:733–41. doi: 10.1007/s12105-019-01118-x
25. Garg P, Jain J, De N, Chatterjee K. A central giant cell granuloma in posterior part of maxilla—a case report. Int J Surg Case Rep. (2017) 30:222–25. doi: 10.1016/j.ijscr.2016.11.015
26. Richardson J, Litman E, Stanbouly D, Lee KC, Philipone E. Aneurysmal bone cyst of the head & neck: a review of reported cases in the literature. J Stomatology Oral Maxillofac Surgery. (2022) 123:59–63. doi: 10.1016/j.jormas.2021.01.014
Keywords: giant cell lesions, Maxillofacial region, giant cell tumor of bone, aneurysmal bone cyst, giant cell reparative granuloma, tenosynovial giant cell tumor
Citation: Gao X, Wang S, Zhan X, Liu Y, Chen L, Sun J and Xu H (2025) Case Report: Giant cell lesions in the Maxillofacial region: diagnostic points and treatment strategies. Front. Oncol. 15:1572560. doi: 10.3389/fonc.2025.1572560
Received: 07 February 2025; Accepted: 26 March 2025;
Published: 16 April 2025.
Edited by:
Vui King Vincent-Chong, University at Buffalo, United StatesReviewed by:
Liviu Bilteanu, Carol Davila University of Medicine and Pharmacy, RomaniaSaurabh Pal, University of Maryland, United States
Copyright © 2025 Gao, Wang, Zhan, Liu, Chen, Sun and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Sun, c3VuamlhbnFkZnlAcWR1LmVkdS5jbg==; Haoyue Xu, eHVoYW95dWU5MEAxNjMuY29t
‡ORCID: Yanshan Liu, orcid.org/0009-0001-2491-3766
Jian Sun, orcid.org/0009-0001-0562-6104
 Xiaohan Gao
Xiaohan Gao Shuangyi Wang
Shuangyi Wang Xiaohong Zhan
Xiaohong Zhan Yanshan Liu1,2‡
Yanshan Liu1,2‡ Haoyue Xu
Haoyue Xu