- 1Department of Respiratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan
- 2Gunma University Heavy Ion Medical Center, Maebashi, Gunma, Japan
- 3Department of Medical Oncology, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan
- 4Gunma University Graduate School of Health Sciences, Maebashi, Gunma, Japan
Objectives: We conducted a phase I study to evaluate the recommended dose of S-1 in combination with cisplatin (SP) and concurrent carbon ion radiotherapy (CIRT) in patients with stage III locally advanced non-small cell lung cancer (LA-NSCLC).
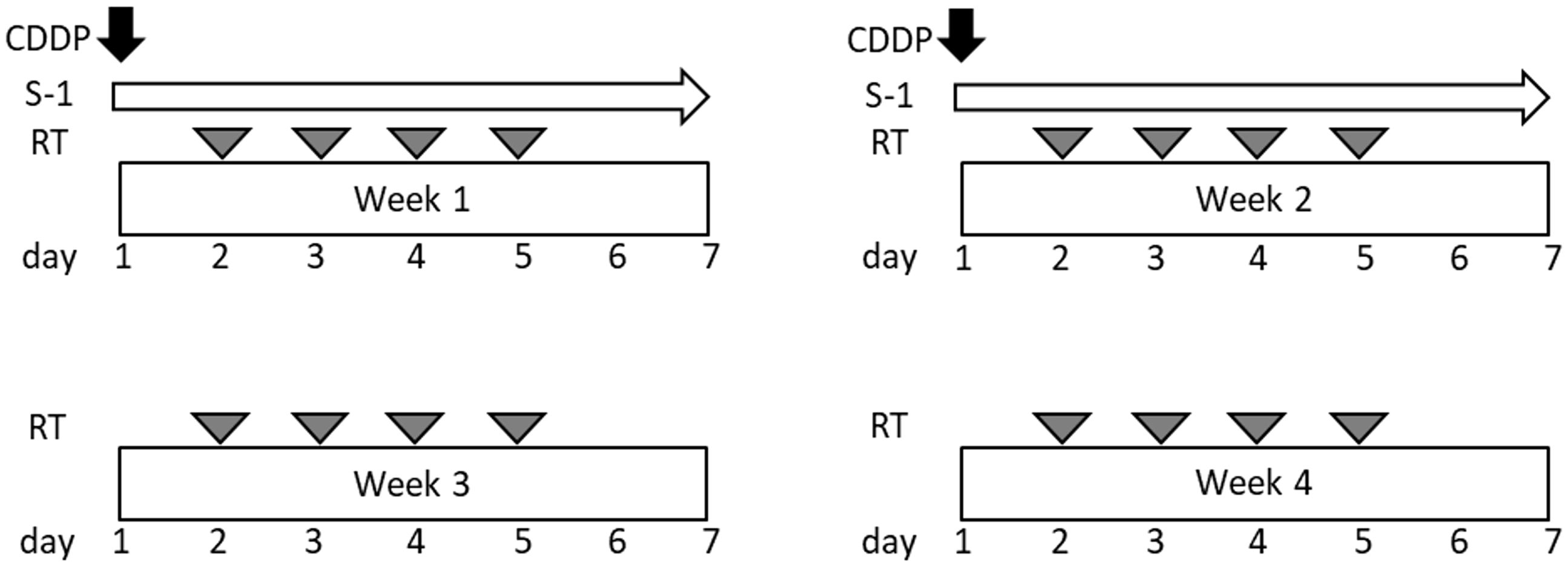
Materials and methods: S-1 was administered orally twice daily after a meal for 14 consecutive days, and cisplatin was administered on days 1 and 8. The dose of each drug in this study was planned as follows: level 0, S-1–30 mg/m2 twice daily and cisplatin 40 mg/m2; level 1, S-1–40 mg/m2 twice daily and cisplatin 40 mg/m2. CIRT was conducted at a total dose of 64 Gy (relative biological effectiveness) in 16 fractions.
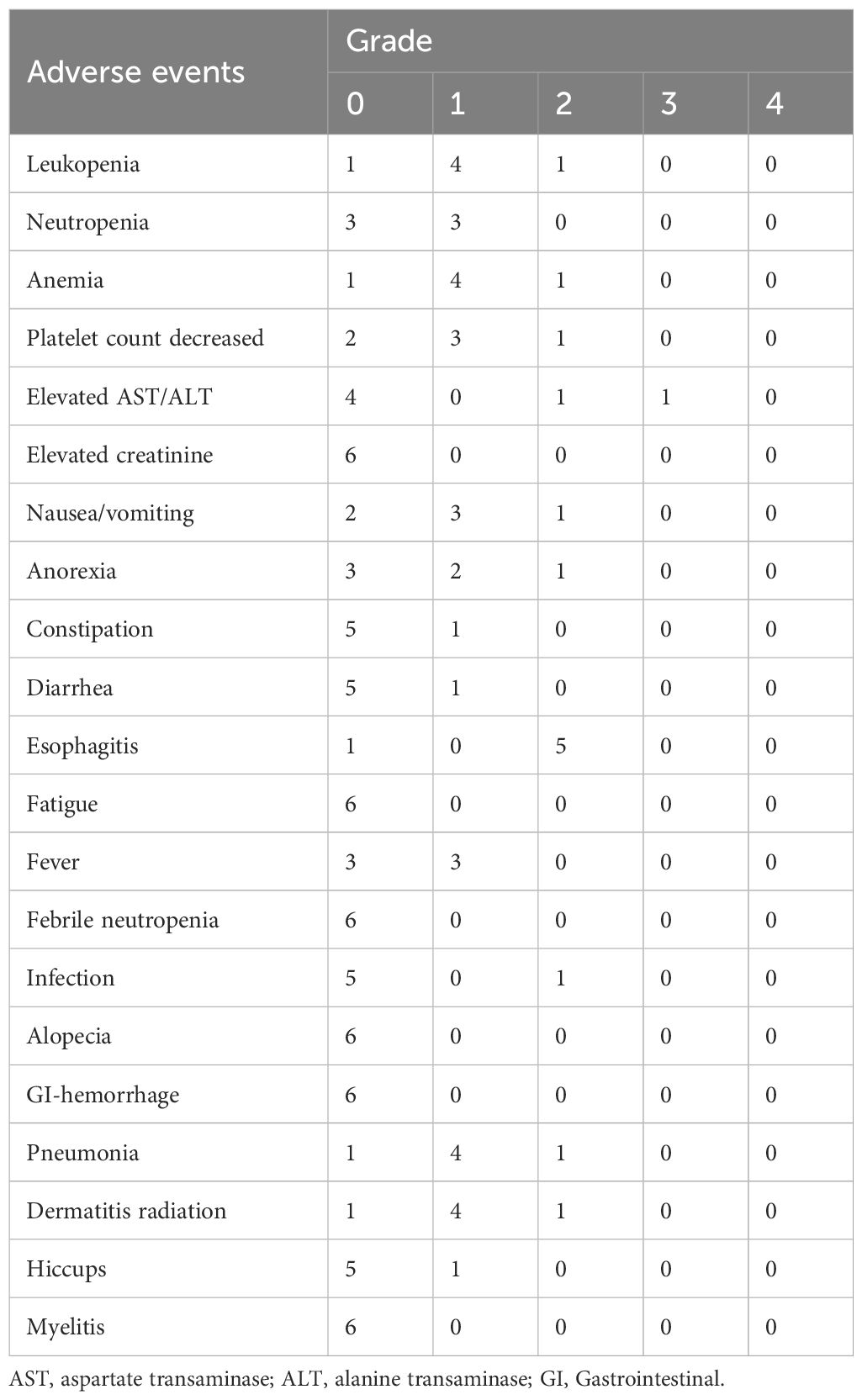
Results: Six patients were enrolled in this study. At level 1, one patient experienced grade 3 elevated alanine aminotransferase and aspartate aminotransferase levels, which is regarded as a dose-limiting toxicity. This event improved immediately. Five patients developed grade 2 esophagitis. In three of the five patients, symptoms such as pain and dysphagia due to esophagitis recurred several months after resolution of the acute esophagitis that occurred during irradiation. None of the patients experienced adverse events of ≥grade 3. Thus, level 1 was determined to be the recommended dose.
Conclusion: Chemotherapy with SP and concurrent CIRT is feasible and well-tolerated in patients with Stage III LA-NSCLC.
1 Introduction
Lung cancer is a common cause of cancer-related mortalities worldwide. The most common histological type is non-small cell lung cancer (NSCLC), which accounts for approximately 85% of all lung cancer cases. Approximately 25% of all NSCLC cases are diagnosed as unresectable locally advanced NSCLC (LA-NSCLC) (1). Currently, the standard treatment is platinum-based chemotherapy with concurrent thoracic radiotherapy followed by consolidation therapy with durvalumab. Consolidative treatment with durvalumab after concurrent chemoradiation therapy (cCRT) has significantly improved clinical outcomes compared to chemoradiation therapy (CRT) alone (2). However, 5-year progression-free survival (PFS) and overall survival (OS) rates of 33.1% and 42.9%, respectively, in patients with stage III LA-NSCLC treated with cCRT and consolidation durvalumab therapy remain unsatisfactory (3).
Compared to X-ray radiotherapy, carbon ion radiotherapy (CIRT) provides a higher conformal dose distribution to the tumor, sparing normal tissues (4). It has higher linear energy transfer, resulting in greater relative biological effectiveness (RBE) (5). CIRT without chemotherapy has been reported for patients with LA-NSCLC. Although 2-year local control rates are excellent (73.9–100%), the median survival time is 24–27.6 months due to distant metastasis (6–8). Several preclinical studies have suggested that anticancer agents can sensitize human NSCLC cells to carbon ion irradiation (9, 10). However, the effect of combined CIRT and chemotherapy in patients with LA-NSCLC remains unclear.
S-1 plus cisplatin (SP) is a great candidate for chemotherapy regimens combined with concurrent CIRT. Several phase II studies have demonstrated that the 2-year OS rate in patients undergoing cCRT with SP was 52.9–75.6% (11–14), equivalent to that of cCRT with standard chemotherapy regimens. Additionally, cCRT with SP is reportedly more feasible than cCRT with conventional chemotherapy (12).
Therefore, we conducted a phase I study to evaluate the recommended dose of SP and concurrent CIRT in patients with stage III LA-NSCLC.
2 Materials and methods
2.1 Patient eligibility
The eligibility criteria for this study were as follows: histologically or cytologically proven unresectable stage III NSCLC (Union for International Cancer Control 8th edition) (15); a performance status (PS) of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale; ages between 20 and 74 years; life expectancy of ≥3 months; adequate bone marrow activity (neutrophil count ≥1500 mm-3, hemoglobin ≥9.0 g/dL, and platelet count ≥100,000 mm-3); aspartate transaminase [AST] and alanine transaminase [ALT] ≤2.5 times the upper limit of the normal range; total serum bilirubin ≤1.5 times the upper limit of the normal range; estimated glomerular filtration rate ≥60 ml/min/m2; and oxygen saturation ≥90%. Patients were excluded if they had any of the following: malignant pericardial or pleural effusions; interstitial lung disease; active double cancer; concomitant serious illness such as uncontrolled angina pectoris; myocardial infarction in the previous 3 months; tumor invasion to the heart, large vessels, trachea, or esophagus; metastases to contralateral hilar lymph nodes; infection or other diseases contraindicating chemotherapy or radiotherapy; pregnancy; or breastfeeding. This study was approved by the local institutional ethics committee, and written informed consent was obtained from all patients. This study was registered with the Japan Registry of Clinical Trials (jRCTs031190126).
2.2 Study design
2.2.1 Chemotherapy
This was an open-label, single-center, single-arm, phase I study. The chemotherapy regimen consisted of one cycle of S-1 and cisplatin (Figure 1). S-1 was orally administered twice daily after meals for 14 consecutive days. Each capsule of S-1 contained 20 or 25 mg of tegafur. Individual doses were rounded to the nearest pill size less than the calculated dose, given the available formulation. Cisplatin was administered on days 1 and 8 via intravenous infusion for over 60 min. All the patients received prophylactic antiemetic therapy consisting of a 5-hydroxytryptamine 3 receptor antagonist, a neurokinin 1 receptor antagonist, and a steroid. The dose of each drug in this study was planned as follows: level 0, S-1–30 mg/m2 twice daily and cisplatin 40 mg/m2; and level 1, S-1–40 mg/m2 twice daily and cisplatin 40 mg/m2. Prophylactic administration of granulocyte colony-stimulating factors (G-CSF) was not permitted. G-CSF administration was permitted for patients with grade 4 and/or grade 3 febrile neutropenia.
2.2.2 CIRT
The patients were immobilized in the supine or prone position using a thermoplastic shell (Shellfitter; Sanyo Polymer Industrial, Nara, Japan) and a pillow made of water-sclerogenic polymers (Moldcare; ALCARE, Tokyo, Japan). Computed tomography (CT) images were obtained using 2-mm-thick slices. A four-dimensional CT scan was obtained to quantify respiratory motion. Gross tumor volume (GTV) included the primary gross tumor and lymph node metastasis. The clinical target volume (CTV) was defined as the GTV plus a 5 mm margin in all directions, considering the anatomical boundaries. Planning target volume 64 (PTV64), which was irradiated at 64 Gy (RBE), was defined as the primary CTV with internal and setup margins. PTV52, which was irradiated at 52 Gy (RBE), was defined as primary CTV and lymph node metastases with internal and setup margins. When the PTV was close to organs at risk (OARs), such as the esophagus or spinal cord, the dose constraint of the OAR was prioritized over the target dose. The clinical dose distribution was calculated from the physical dose and RBE based on experimental results [5]. The unit of clinical dose was described as “Gy (RBE).” The passive scattering carbon-ion dose distribution was calculated using XiO-N (ELEKTA; Mitsubishi Electric, Tokyo, Japan). A total dose of 64 Gy (RBE) in 16 fractions was administered to the isocenter of the PTV for 4 weeks (4 fractions per week). In principle, the maximum dose to the OARs is defined as follows: spinal cord, 30 Gy (RBE); the esophagus, 60 Gy (RBE); trachea and main bronchus, 60 Gy (RBE); stomach or bowel, 40 Gy (RBE); and brachial plexus, 60 Gy (RBE). Daily orthogonal two-dimensional kV image pairs and weekly CT scans were used for image-guided radiotherapy. The dose distribution for a representative case with axial images is shown in Figure 2.
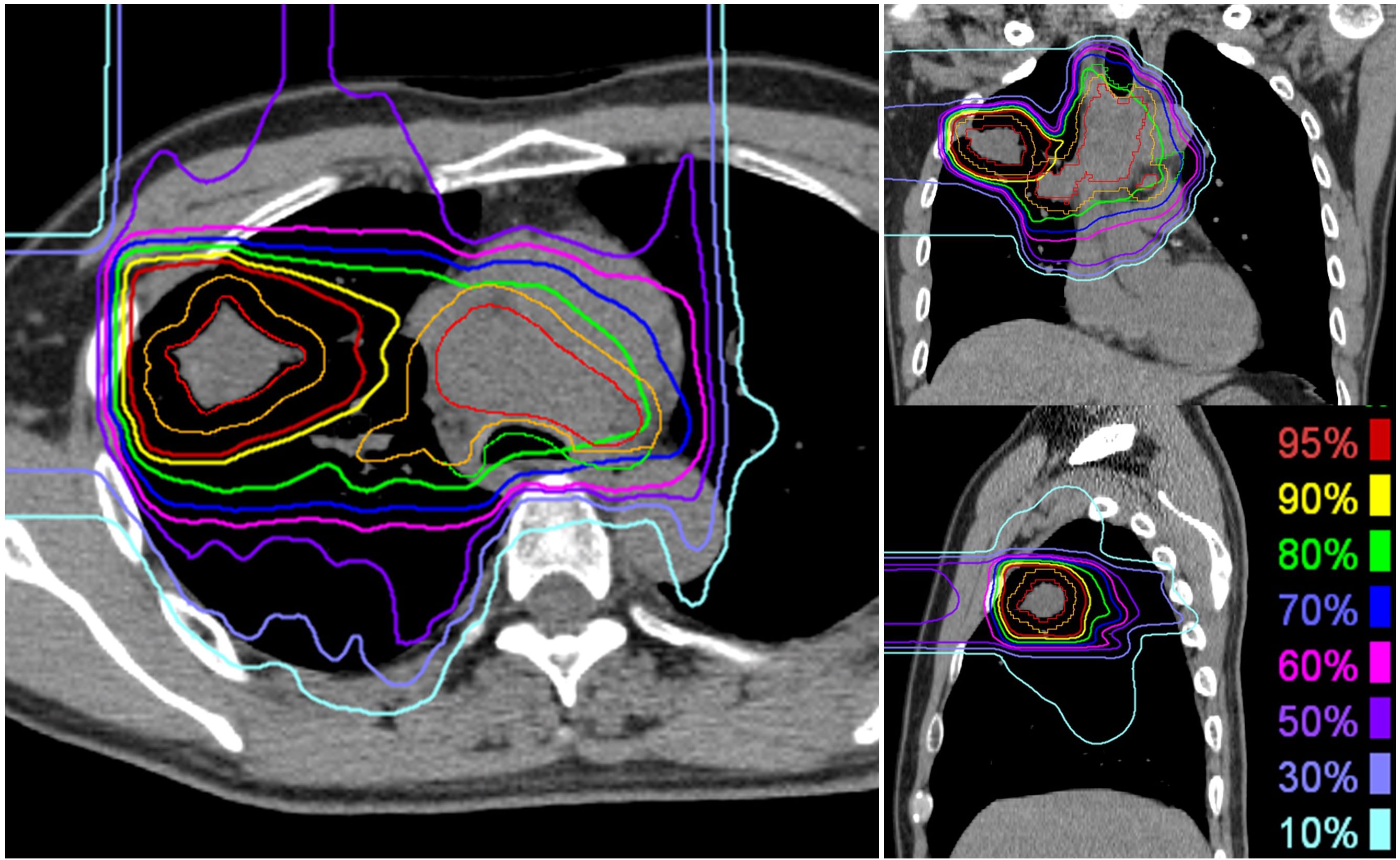
Figure 2. Representative dose distribution of carbon ion radiotherapy for locally advanced lung cancer. The primary tumor received a total dose of 64 Gy (RBE) delivered in 16 fractions, while lymph node metastases were irradiated with a total dose of 52 Gy (RBE). The gross tumor volume is delineated in red, and the clinical target volume for 52 Gy (RBE) is represented in orange.
2.2.3 Dose modification
The primary endpoint of the present study was to evaluate the recommended dose of SP and concurrent CIRT in patients with stage III LA-NSCLC. Doses were reduced from level 1 to level 0 according to the frequency of dose-limiting toxicity (DLT) evaluated between treatment initiation and 3 months after the last irradiation. Initially, six patients were treated with a dose of level 1. If fewer than two DLTs were observed in six patients, level 1 was determined to be the recommended dose (RD). If two DLTs were observed, discontinuation of patient entry at level 1 and recruitment of six patients at level 0 were planned. If fewer than two DLTs were observed in six patients, level 0 was determined as RD. If two DLTs were observed, study termination was planned. DLT was defined as: (1) ≥grade 3 nonhematologic toxicities except nausea/vomiting; (2) grade 4 thrombocytopenia; (3) grade 4 neutropenia; (4) Grade 3 or 4 febrile neutropenia; and (5) any unresolved toxicity requiring a delay in subsequent irradiation exceeding 14 days. Toxicities were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Additional treatments, including consolidative durvalumab therapy, were prohibited until recurrence was diagnosed.
2.3 Assessment
Prior to treatment, the patients were evaluated using complete blood cell count, differential count, biochemical examinations, chest radiography, chest and abdominal contrast-enhanced CT, whole-brain magnetic resonance imaging or CT, and 18F-fluorodeoxyglucose positron emission tomography. Complete blood cell counts, differential counts, biochemical and physical examinations, chest radiography, and toxicity tests were performed weekly. Toxicity was graded according to CTCAE version 5.0. The tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1 (16). Responses were defined as follows: complete response, disappearance of all target lesions; partial response, ≥30% reduction in size; stable disease, <30% decrease and <20% increase in size; and progressive disease, >20% increase in size (or the appearance of new lesions). The overall response was defined as the best response recorded from treatment initiation until disease progression or recurrence, confirmed by repeated assessments performed no less than 4 weeks after the criteria for response were first met. Survival was recorded from the first day of treatment with CIRT to the date of death or the last follow-up, and survival curves were prepared using the Kaplan–Meier method. OS was defined as the time from the date of the first administration of anticancer agents to death from any cause. PFS was defined as the time between the date of the first CIRT administration and the date of disease progression or death.
2.4 Statistical analysis
The Kaplan–Meier method was used to determine the actuarial survival rate. Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Patients’ characteristics
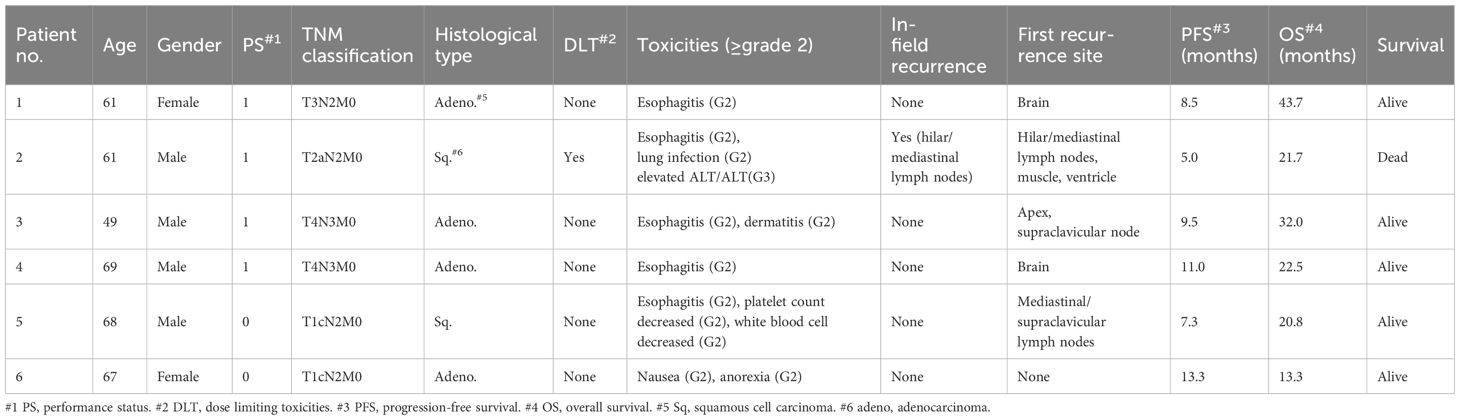
Six patients, including four men and two women with a median age of 64 years (range, 49–69 years), were enrolled in this study at a dose of level 1 between September 2019 and September 2022. Patient characteristics are summarized in Table 1. ECOG PS was 0 in 2 patients and 1 in 4 patients. All the patients had a history of smoking. The histological types were adenocarcinoma in four patients and squamous cell carcinoma in two patients. The clinical stages were IIIA in 3 patients, IIIB in 1 patient, and IIIC in 2 patients. Comorbidities included chronic obstructive pulmonary disease in two patients and old myocardial infarction and chronic heart failure in one patient. Because RD was determined as level 1 as described below, no patients were enrolled at a dose of level 0.
3.2 Treatment delivery and toxicity
We evaluated toxicity in all treated patients. All adverse events are listed in Table 2. Six patients were enrolled in Level 1. Complete doses of chemotherapy and CIRT were administered to all patients. Patient #2 experienced grade 3 elevated ALT and AST levels 21 days after completion of the protocol. At the onset of elevated ALT and AST levels, loxoprofen and vonoprazan were administered to treat pain due to esophagitis. After the withdrawal of these drugs, ALT and AST levels decreased immediately. Although DLT was observed in this case, five patients did not develop DLTs. Five patients developed grade 2 esophagitis. In three of the five patients, symptoms such as pain and dysphagia due to esophagitis recurred several months after resolution of the acute esophagitis that occurred during irradiation. Patient #1 developed an esophageal ulcer 105 days after the completion of irradiation. Since the ulcer site was within the irradiation field, this event was considered treatment-related toxicity. The swallowing pain and other symptoms improved after a few months. None of the patients experienced ≥grade 3 pneumonitis or hematological toxicities. No treatment-related death occurred. Thus, level 1 was determined as the RD for the phase II study.
3.3 Response and survival
The clinical courses of the six patients are shown in Table 1. All patients exhibited at least a partial response. One patient developed in-field recurrence, and two patients developed metastases to the lymph nodes around the PTV. Two patients (Patients #3 and #5) underwent CIRT for recurrence. Patient #3 was free of recurrence 20 months after the second CIRT for the apex and supraclavicular nodes. Patient #5 experienced multiple lung metastases 4 months after the completion of the second CIRT for the mediastinal/supraclavicular lymph nodes. Distant metastases were observed in three patients. The median PFS and OS were 8.5 months and not reached, respectively, and the median follow-up period was 22.1 months. The data cutoff date for this study was December 2023.
3.4 Dosimetric analysis
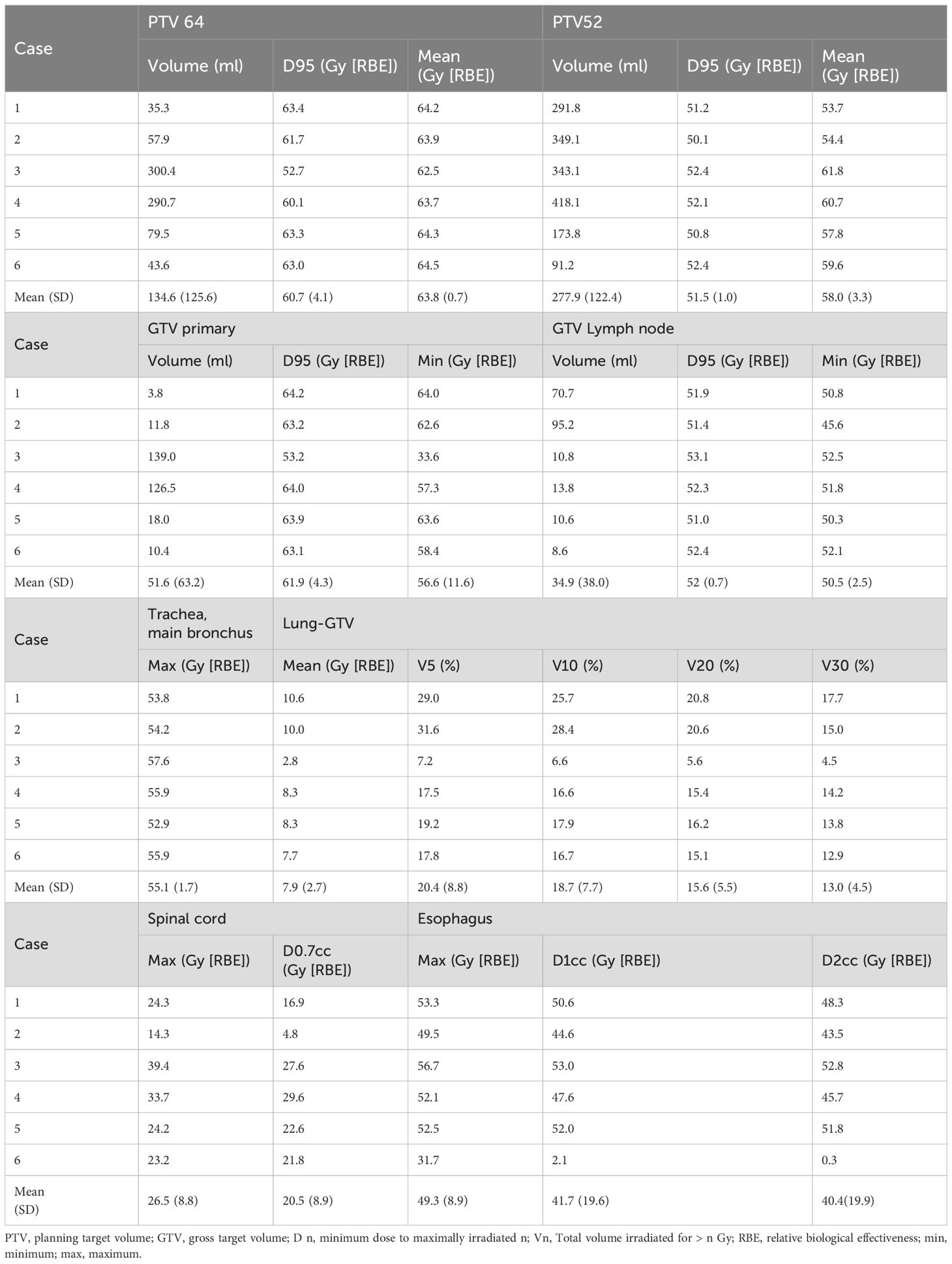
Table 3 lists the dosimetric parameters for all patients. A mean PTV dose of 64 Gy was almost achieved at the prescribed dose of 64 Gy (RBE). Patient #3 had a tumor that invaded the spinal canal and was adjacent to the spinal cord; hence, the doses of the PTV64 and GTV primary tumors were low compared to those of the others. However, the patient did not experience local recurrence near the spinal cord. The mean V5 and V20 for lung-GTV were 20.4% and 15.6%, respectively. All patients achieved D0.7cc for the spinal cord under 30 Gy (RBE). Patients #1, #3, and #5, who had recurrent esophagitis, had an esophageal D1cc greater than 50 Gy (RBE).
4 Discussion
This is the first phase I study to evaluate the tolerability of concurrent chemotherapy with SP and CIRT in patients with inoperable stage III NSCLC. This study demonstrated that one patient experienced DLT, and RD was defined as level 1. We observed that the treatment was tolerable. There were no ≥grade 3 toxicities other than elevated AST and ALT. Other notable findings are the high frequency of grade 2 esophagitis and the delayed onset of recurrent esophagitis.
Five of the six patients experienced recurrence outside the irradiated fields, and the effect of the combination of CIRT with SP in suppressing potential distant metastasis appeared to be insufficient. A possible reason for this may be that the short duration of the CIRT treatment resulted in the administration of one course of SP when used only within the concurrent use period, in contrast to two to four courses of SP for conventional radiotherapy without consolidation therapy with durvalumab (11–14). Future studies on the use of durvalumab maintenance therapy to prevent distant metastases are warranted.
Local control remains a challenge in cCRT and radiography for stage III NSCLC. The RTOG 0617 trial, which compared standard- and high-dose chemoradiotherapy, reported that about half of the patients experienced locoregional recurrence (17). Another phase III trial of cCRT reported that one-third of patients had local recurrence (18, 19). The PACIFIC trial also reported that the lungs and lymph nodes were the most common sites of recurrence (3). For CIRT without chemotherapy for locally advanced NSCLC, a study reported a 2-year local control rate of 73.9–100% (6–8). In our study, only one of six patients experienced recurrence within the irradiation field. The effectiveness of the addition of SP to CIRT for local control was not clear because of the high local control rate of CIRT alone. In the future, it is expected that a combination of immune checkpoint inhibitors will help to take advantage of the local control of CIRT if distant metastasis is reduced.
Although the standard treatment for stage III lung cancer is 1 year of consolidation therapy with durvalumab after cCRT, not all patients can complete the full course. In the PACIFIC study, only 49% of the patients who received durvalumab completed 1 year of treatment. Of the durvalumab discontinuations, 30% were due to adverse events (20). Additionally, 23–55% of patients with stage III NSCLC treated with cCRT did not meet the criteria of the PACIFIC trial, and some of these patients could not be initiated on durvalumab (21, 22). Approximately 20% of the reasons for not initiating durvalumab were due to radiation pneumonitis (23). The risk of ≥grade 3 pulmonary toxicities in stage III lung cancer with cCRT is 7–20.6% (17, 18). Taking these considerations into account, reducing radiation-induced pulmonary toxicity is important for continuing or initiating durvalumab. The radiation dose to lung tissues is associated with the incidence of radiation pneumonitis (24, 25). Theoretically, CIRT can reduce lung doses compared to X-rays (4); this might reduce pulmonary toxicity. In fact, two prospective clinical trials have reported that the incidence of ≥grade 3 radiation pneumonitis was 0–1.6%, which is lower than that in patients treated with conventional CRT (6, 7). This could increase the accessibility of durvalumab consolidation therapy and potentially improve clinical outcomes. In our study, one case of pneumonia occurred but improved with antibiotics over approximately 2 weeks without the need for steroids, suggesting no radiation-induced pneumonitis.
Our study had some limitations. First, our study could not verify the efficacy of concurrent chemotherapy with SP and CIRT owing to the nature of a phase I study with a small sample size. Second, this study did not permit consolidative durvalumab therapy after protocol treatment. Given the tolerability of concurrent chemotherapy and CIRT in the present study, prospective evaluation of the efficacy and tolerability of this treatment, followed by durvalumab consolidation, is warranted in the future.
In conclusion, our study demonstrated that concurrent chemotherapy with SP and CIRT is tolerable. The RD for the phase II study was S-1–40 mg/m2 twice daily for 14 consecutive days and cisplatin 40 mg/m2 on days 1 and day 8. Prospective studies are warranted to examine the efficacy of this treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Gunma University Hospital Clinical Research Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YM: Writing – original draft, Writing – review & editing. NK: Writing – original draft, Writing – review & editing. NS: Writing – review & editing. NO: Writing – review & editing. HT: Writing – review & editing. HK: Writing – review & editing. RS: Writing – review & editing. TM: Writing – review & editing. TH: Writing – review & editing. TO: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Research Project of Gunma University Heavy Ion Medical Center funded chemotherapy and CIRT for protocol treatment, medical writing support, and the publication.
Acknowledgments
The authors would like to acknowledge all patients and their families. The authors would also like to thank Dr. Norimitsu Kasahara, Jobu Hospital for Respiratory Diseases, for the set-up of the study and Prof. Yoshiaki Ohyama, Innovative Medical Research Center, Gunma University Hospital, for supporting data management and statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LA-NSCLC, locally advanced non-small cell lung cancer; CRT, chemoradiation therapy; PFS, progression-free survival; OS, overall survival; CIRT, carbon ion radiotherapy; RBE, relative biological effectiveness; SP, S-1 plus cisplatin; PS, performance status; ECOG, Eastern Cooperative Oncology Group; AST, aspartate transaminase; ALT, alanine transaminase; jRCT, Japan Registry of Clinical Trials; G-CSF, granulocyte colony-stimulating factors; CT, Computed tomography; GTV, Gross tumor volume; CTV, clinical target volume; PTV, planning target volume; DLT, dose-limiting toxicity; RD, recommended dose; CTCAE, Common Terminology Criteria for Adverse Events; RECIST, Response Evaluation Criteria in Solid Tumor.
References
1. Mountain CF. Revisions in the international system for staging lung cancer. Chest. (1997) 111:1710–7. doi: 10.1378/chest.111.6.1710
2. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
3. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. (2022) 40:1301–11. doi: 10.1200/JCO.21.01308
4. Kubo N, Saitoh JI, Shimada H, Shirai K, Kawamura H, Ohno T, et al. Dosimetric comparison of carbon ion and X-ray radiotherapy for stage IIIA non-small cell lung cancer. J Radiat Res. (2016) 57:548–54. doi: 10.1093/jrr/rrw041
5. Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. (1999) 44:201–10. doi: 10.1016/s0360-3016(98)00544-6
6. Saitoh JI, Shirai K, Abe T, Kubo N, Ebara T, Ohno T, et al. A Phase I Study of hypofractionated carbon-ion radiotherapy for stage III non-small cell lung cancer. Anticancer Res. (2018) 38:885–91. doi: 10.21873/anticanres.12298
7. Takahashi W, Nakajima M, Yamamoto N, Yamashita H, Nakagawa K, Miyamoto T, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer. (2015) 121:1321–7. doi: 10.1002/cncr.29195
8. Anzai M, Yamamoto N, Hayashi K, Nakajima M, Nomoto A, Ogawa K, et al. Safety and efficacy of carbon-ion radiotherapy alone for stage III non-small cell lung cancer. Anticancer Res. (2020) 40:379–86. doi: 10.21873/anticanres.13963
9. Kubo N, Noda SE, Takahashi A, Yoshida Y, Oike T, Murata K, et al. Radiosensitizing effect of carboplatin and paclitaxel to carbon-ion beam irradiation in the non-small-cell lung cancer cell line H460. J Radiat Res. (2015) 56:229–38. doi: 10.1093/jrr/rru085
10. Schlaich F, Brons S, Haberer T, Debus J, Combs SE, and Weber KJ. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol. (2013) 8:260. doi: 10.1186/1748-717X-8-260
11. Niho S, Yoshida T, Akimoto T, Sakamaki K, Ono A, Seto T, et al. Randomized phase II study of chemoradiotherapy with cisplatin + S-1 versus cisplatin + pemetrexed for locally advanced non-squamous non-small cell lung cancer: SPECTRA study. Lung Cancer. (2020) 141:64–71. doi: 10.1016/j.lungcan.2020.01.008
12. Sasaki T, Seto T, Yamanaka T, Kunitake N, Shimizu J, Kodaira T, et al. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br J Cancer. (2018) 119:675–82. doi: 10.1038/s41416-018-0243-2
13. Nogami N, Takigawa N, Hotta K, Segawa Y, Kato Y, Kozuki T, et al. A phase II study of cisplatin plus S-1 with concurrent thoracic radiotherapy for locally advanced non-small-cell lung cancer: The Okayama Lung Cancer Study Group Trial 0501. Lung Cancer. (2015) 87:141–7. doi: 10.1016/j.lungcan.2014.11.001
14. Kaira K, Tomizawa Y, Yoshino R, Yoshii A, Matsuura M, Iwasaki Y, et al. Phase II study of oral S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Lung Cancer. (2013) 82:449–54. doi: 10.1016/j.lungcan.2013.09.004
15. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
17. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG Oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. (2020) 38:706–14. doi: 10.1200/JCO.19.01162
18. Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. (2010) 28:3299–306. doi: 10.1200/JCO.2009.24.7577
19. Renard A, Noël G, and Mazeron JJ. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesin and cisplatin in unresectable stage III non small-cell lung cancer. Cancer Radiother. (2000) 4:317–8. doi: 10.1016/s1278-3218(00)80010-4
20. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. (2018) 379:2342–50. doi: 10.1056/NEJMoa1809697
21. Hosoya K, Fujimoto D, Kawachi H, Sato Y, Kogo M, Nagata K, et al. Ineligibility for the PACIFIC trial in unresectable stage III non-small cell lung cancer patients. Cancer Chemother Pharmacol. (2019) 84:275–80. doi: 10.1007/s00280-019-03885-4
22. Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer. (2020) 146:23–9. doi: 10.1016/j.lungcan.2020.05.035
23. Kubo N, Kobayashi D, Iwanaga M, Matsuura M, Higuchi K, Eishima J, et al. Radiotherapy patterns of care for locally-advanced non-small cell lung cancer in the pre- and post-durvalumab era: A region-wide survey in a Japanese prefecture. J Radiat Res. (2022) 63:264–71. doi: 10.1093/jrr/rrab116
24. Saito G, Oya Y, Taniguchi Y, Kawachi H, Daichi F, Matsumoto H, et al. Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non-small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON). Lung Cancer. (2021) 161:86–93. doi: 10.1016/j.lungcan.2021.08.019
Keywords: carbon-ion radiotherapy, concurrent chemoradiotherapy, non-small cell lung cancer, platinum-based chemotherapy, clinical trial
Citation: Miura Y, Kubo N, Sunaga N, Okano N, Tsurumaki H, Kawamura H, Sakurai R, Maeno T, Hisada T and Ohno T (2025) A phase I study of S-1 and cisplatin with concurrent hypofractionated carbon-ion radiotherapy for patients with stage III non-small cell lung cancer. Front. Oncol. 15:1573462. doi: 10.3389/fonc.2025.1573462
Received: 09 February 2025; Accepted: 14 July 2025;
Published: 05 August 2025.
Edited by:
Mohammad Rezaee, Johns Hopkins University, United StatesReviewed by:
Kyung Hwan Kim, Yonsei University, Republic of KoreaChenbin Bian, Sichuan University, China
Copyright © 2025 Miura, Kubo, Sunaga, Okano, Tsurumaki, Kawamura, Sakurai, Maeno, Hisada and Ohno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatsuya Ohno, dG9obm9AZ3VubWEtdS5hYy5qcA==
 Yosuke Miura
Yosuke Miura Nobuteru Kubo
Nobuteru Kubo Noriaki Sunaga
Noriaki Sunaga Naoko Okano2
Naoko Okano2 Hidemasa Kawamura
Hidemasa Kawamura Takeshi Hisada
Takeshi Hisada Tatsuya Ohno
Tatsuya Ohno