- 1Department of Radiation Oncology, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 2Department of Anesthesiology, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 3Department of Medical Imaging Center, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 4Department of Information, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
- 5Department of Thoracic Surgery, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing, China
Introduction: The combination of immunotherapy with neoadjuvant chemotherapy (nICT) or chemoradiotherapy (nICRT) represents a novel treatment approach for patients with locally advanced esophageal squamous cell carcinoma (LA-ESCC). This study aimed to compare postoperative complications between patients who underwent esophagectomy directly and those who received surgery following neoadjuvant immunotherapy combining treatments (nIComT) including nICT or nICRT.
Materials and methods: A retrospective analysis was conducted on patients with LA-ESCC at our center. A 1:1 propensity score matching (PSM) was used to eliminate baseline characteristics differences. The primary endpoint was postoperative complications, which were assessed based on the Esophageal Cancer Complications Consensus Group (ECCG) criteria, and the severity was evaluated according to the Clavien-Dindo classification.
Results: After PSM, 116 matched patients were analyzed in both the surgery-alone and nIComT group. The overall complication rates between the two groups were similar (51.7% vs 56.0%, P=0.510). Incidence of cardiovascular complications, most of which were grade I and II, was increased in the nIComT group compared with the surgery-alone group(P=0.003). The higher rate of cardiovascular complications mainly due to hypotension (52.6% vs 42.2%, P=0.004) requiring intervention including the use of vasopressors, or transfusion. Additionally, more patients in the nIComT group received perioperative transfusion (34.5% vs 14.7%, P<0.001), as well as an extended operation duration (276 ± 66min vs 246 ± 63min, P<0.001), when compared to the surgery-alone group. The logistic regression analyses of potential risk factors for cardiovascular complications showed that receiving neoadjuvant treatment was independently associated with cardiovascular complications (OR=2.03, 95% CI=1.15-3.57, P=0.015).
Conclusion: Our study highlights an increased risk of cardiovascular complications among patients received nIComT, underscoring the significance of postoperative circulatory interventions. Further prospective studies are needed for validation.
Introduction
Esophageal cancer (EC) ranks as the eleventh most common malignancy and seventh in mortality worldwide (1). EC is particularly prevalent in Asia, where China accounting for over half of all cases (2). Esophageal squamous cell carcinoma (ESCC) represents the predominant subtype in China, comprising approximately 90% of EC cases (3). Neoadjuvant chemotherapy (nCT) or chemoradiotherapy (nCRT) combined with esophagectomy is the standard of care for locally advanced esophageal squamous cell carcinoma (LA-ESCC). In spite of this, the 5-year overall survival (OS) rate for LA-ESCC was 30.3% as of 2015 (4). Therefore, it is essential to explore new therapeutic strategies to enhance treatment efficacy and improve survival benefits for patients with LA-ESCC.
Immune checkpoint inhibitors (ICIs) have emerged as a promising therapeutic strategy for ESCC (5, 6). Building upon the established efficacy of ICIs as adjuvant therapy in LA-ESCC patients demonstrated in the CheckMate 577 trial (7), multiple prospective clinical trials have subsequently investigated the integration of ICIs with neoadjuvant chemotherapy (nICT) or chemoradiotherapy (nICRT) (8–11). Several studies have demonstrated pathological complete response (pCR) rates of 16.7%-39.2% in LA-ESCC patients who underwent nICT (9, 12, 13), while the NICE trial reported a 2-year OS rate of 78.1% (14). Notably, nICRT have shown a trend of enhanced efficacy compared to the CROSS and NEOCRTEC5010 trials (15, 16), with pCR rates ranging from 22.6% to 55.6% (8, 11, 17). In summary, nICT or nICRT represents a promising therapeutic for patients with LA-ESCC.
Although neoadjuvant immunotherapy has shown promise for LA-ESCC (8, 10, 11), the postoperative safety implications of combining ICIs with neoadjuvant therapy contentious. A meta-analysis revealed that, compared to nCT, nICT significantly increased surgery cancellation rates due to grade≥3 treatment-related adverse events (TRAEs) (18). Furthermore, neoadjuvant immunotherapy has been identified as an independent risk factor for increased surgical complexity (19). While limited evidence in LA-ESCC, the safety profile of combining immunotherapy with conventional neoadjuvant regimens requires urgent investigation.
This retrospective study compared postoperative safety outcomes between patients underwent esophagectomy directly and those received neoadjuvant immunotherapy combining treatments (nIComT), which include nICT or nICRT, aiming to provide clinical evidence for postoperative safety management in LA-ESCC.
Materials and methods
Study design
This is a retrospective clinical study screening consecutive patients with LA-ESCC, staged cT1-4aN+M0/cT3-4aN0M0 according to the AJCC 8th edition (20), who underwent esophagectomy at our center between January 2020 to January 2022. The primary endpoint was postoperative safety. The secondary endpoint was perioperative outcomes.
This study was conducted according to the Declaration of Helsinki and received ethical approval from the Institutional Review Board and Ethics Committee of our center. The requirement for informed consent was waived due to the anonymity of the data.
Patient selection
The inclusion criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status ≤1; (2) complete clinical data available; and (3) normal blood, liver and kidney functions. Patients who had undergone endoscopic submucosal dissection (ESD) for esophageal lesions, received other neoadjuvant therapy or relevant antitumor therapy for malignancies other than ESCC were excluded.
Neoadjuvant treatment
By January 2022, all patients in the nIComT group had received 2–4 cycles of nICT prior to esophagectomy. The neoadjuvant immunotherapy regimen included five PD-1 inhibitors: sintilimab (200 mg, i.v, q3w), toripalimab (240 mg i.v, q3w), camrelizumab (200 mg i.v, q3w), pembrolizumab (200 mg i.v, q3w), and tislelizumab (200 mg i.v, q3w). The chemotherapy regimen consisted of paclitaxel (135–175 mg/m², i.v, q3w) plus carboplatin (area under the curve [AUC]=5, i.v, q3w) or cisplatin (75 mg/m², i.v, q3w) (TP regimen).
For patients received nICRT, the total radiation dose was 30 Gy, delivered as a short-course regimen in 12 fractions (2.5 Gy per fraction, 5 fractions weekly) under conventional fractionation. All cases were derived from a prospective clinical trial conducted at our center (SCALE-1 trial, ChiCTR2100045104) (17). The radiotherapy planning employed involved-field irradiation technique with gross tumor volume (GTV) encompassing primary esophageal lesions and metastatic lymph nodes (LNs) defined by radiographic criteria (≥10mm short-axis for non-special regions, ≥5mm for LNs in esophageal/tracheoesophageal groove areas, or presence of malignant features like central necrosis/ring enhancement/eccentric calcification). No clinical target volume (CTV) was defined. Planning target volume (PTV) margins were created by expanding GTV by 0.8cm for mid-upper thoracic lesions and 1.0cm for lower thoracic lesions, with additional 2.0cm longitudinal margin along esophageal axis. Lymph node PTVs were generated by 1.0cm isotropic expansion from GTV (17).
Surgery
Surgery was performed within 4–8 weeks after the end of the last neoadjuvant treatment. All patients received the Ivor-Lewis operation (right transthoracic esophagectomy with reconstruction and laparoscopic dissection) or the McKeown operation (right thoracotomy, laparoscopy dissection, and left cervical esophagectomy with reconstruction) which are the usual procedures at our center and widely used in China. Esophagectomy and cervical or thoracic anastomosis were performed on all patients using gastric reconstruction of the esophagus. The right recurrent laryngeal nerve chain was fully dissected, but the left recurrent laryngeal nerve chain was only dissected in select patients with suspected metastatic lymph nodes.
Outcomes measurement
Postoperative complications were evaluated using the Esophageal Cancer Complications Consensus Group (ECCG) criteria (21), including postoperative hypotension defined as mean arterial pressure (MAP) below 60 mmHg (22). Severity was assessed by the Clavien-Dindo classification, specifically addressing ≥grade III complications requiring surgical intervention, life-threatening conditions necessitating ICU readmission, and mortality (23). TRAEs during neoadjuvant therapy were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) (24), with immune-related adverse events (irAEs) defined as events like hepatitis, colitis, pneumonitis, and myocarditis connected to ICIs with potential immunologic causes (25, 26). Both TRAEs and irAEs were systematically evaluated in a double-blind manner by two senior radiation oncologists and two radiologists based on clinical and imaging data. Mortality was defined as postoperative death within 30 days.
Statistical analysis
PSM was employed to eliminate baseline differences between the surgery-alone group and the nIComT group. Variables including age, body mass index (BMI), gender, ECOG performance status, comorbidities, smoking history, tumor location, cTNM stage, anastomotic site, surgical procedure, and extent of lymphadenectomy were incorporated into the PSM model construction using nearest matching with a caliper value of 0.02 and a 1:1 matching ratio (27, 28). Continuous variables were described as mean ± standard deviation (SD), with group comparisons made via Wilcoxon rank-sum test or t-test. Categorical variables were presented as frequencies or percentages and compared using Pearson chi-square test or Fisher’s exact test. Conditional logistic regression analysis was utilized to validate potential risk factors, where variables with P<0.20 in univariate analysis were included in multivariate analysis (29). All statistical tests were two-sided, with P<0.05 indicating significance. Data were analyzed using R version 4.3.1 and SPSS 27.0.
Results
Patient characteristics
The patient screening process is shown in Figure 1. Between January 2020 and January 2022, a total of 1,477 patients underwent esophagectomy were screened at our center. After applying exclusion criteria, 723 patients were excluded, resulting in 754 eligible for study inclusion. Among eligible patients, 634 underwent esophagectomy directly, while 120 received nIComT prior to surgery. To minimize selection bias, a 1:1 PSM was performed between the surgery-alone and the nIComT groups. After PSM, each group comprised 116 patients. Within the nIComT group, 96 patients received nICT and 20 received nICRT. Baseline characteristics of patients before and after PSM are presented in Table 1, with features demonstrating balance between groups (P>0.05). Detailed comparisons of baseline characteristics before and after PSM adjustment are visualized in Figure 2.
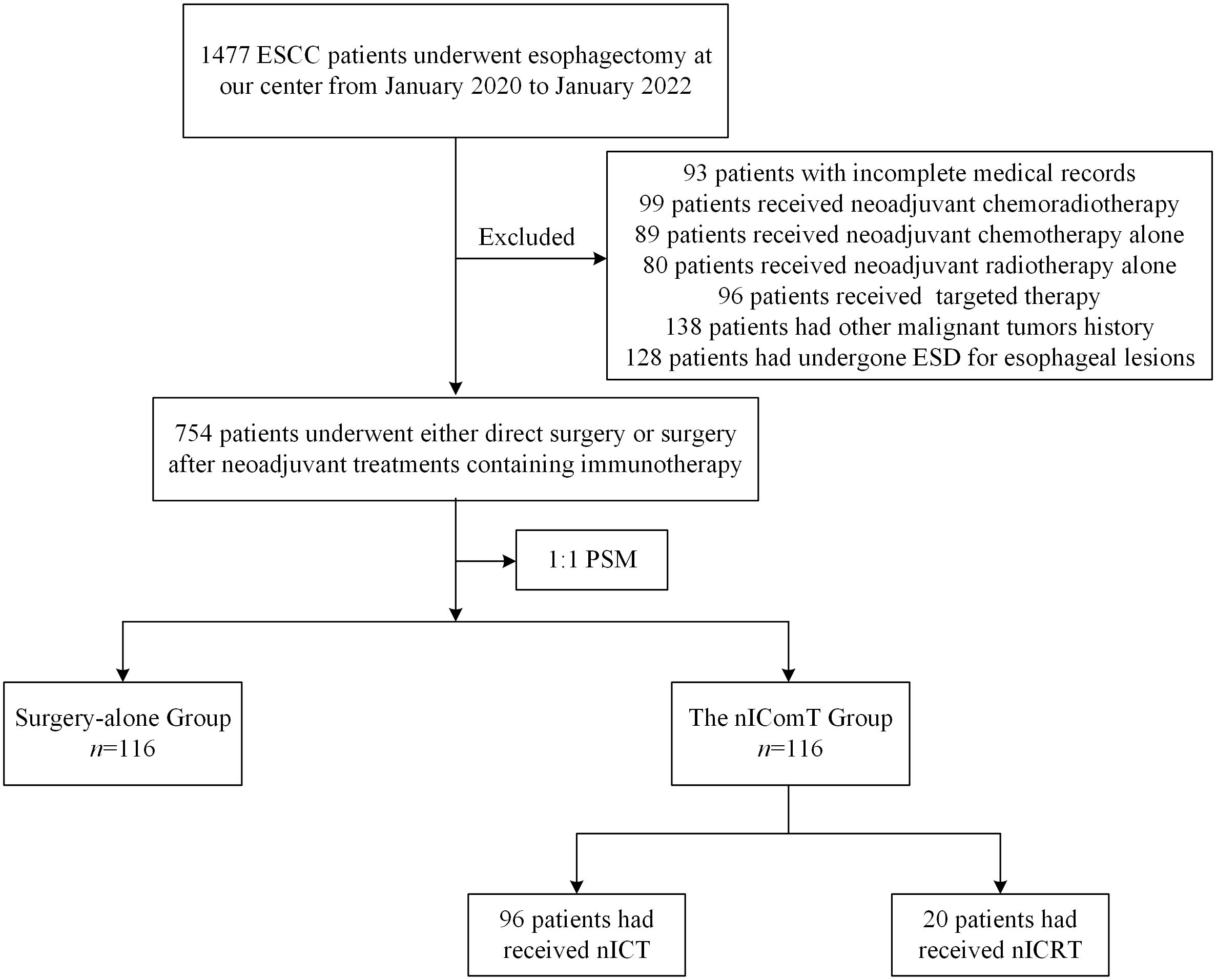
Figure 1. Patient selection flowchart. ESD, endoscopic submucosal dissection; PSM, propensity score matching; nICT, neoadjuvant immunotherapy combined with chemotherapy; nICRT, neoadjuvant immunotherapy combined with chemoradiotherapy.
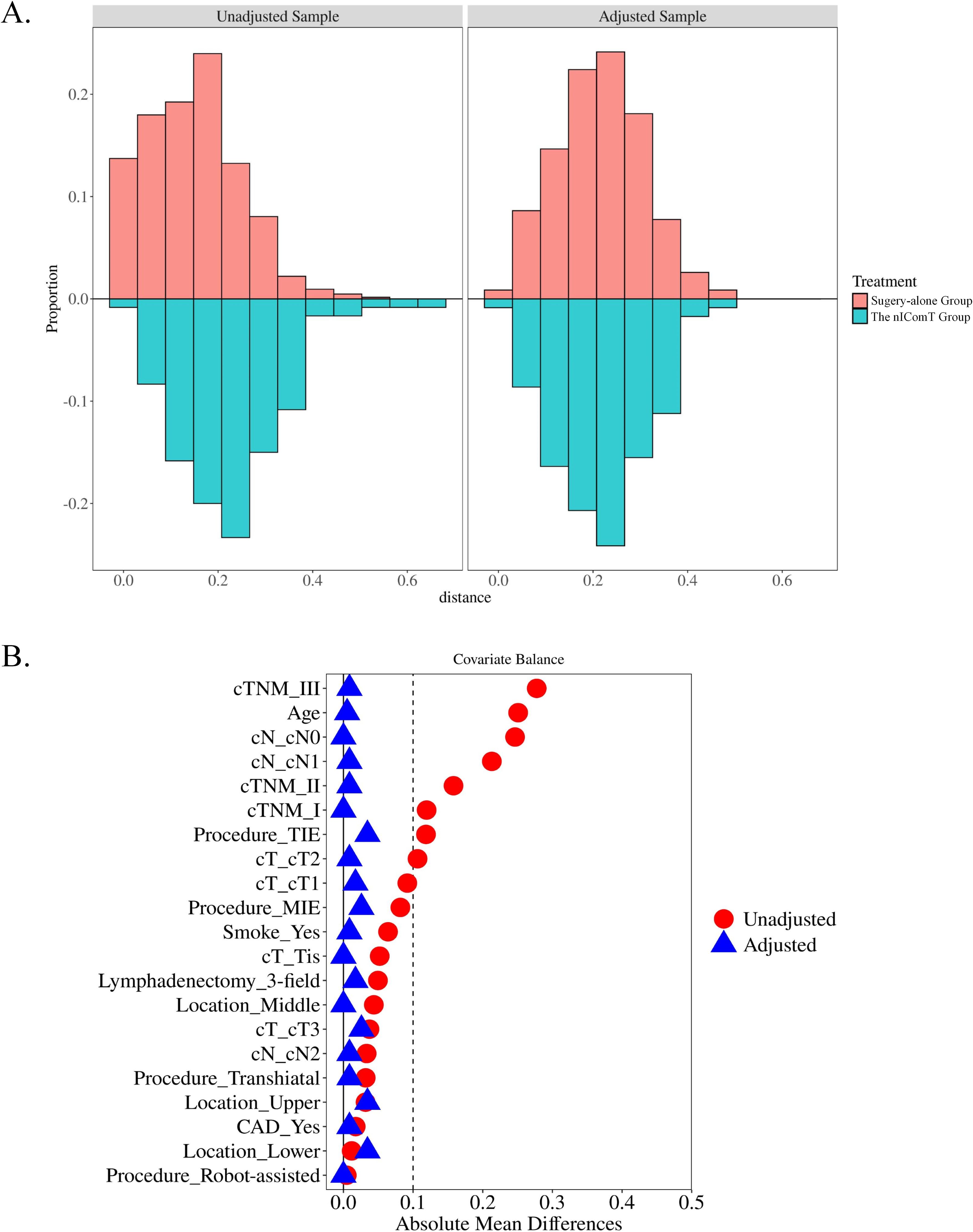
Figure 2. (A) Histogram showing the balance for the categorical variable; (B) Covariate balance measured by standardized mean difference. nIComT, neoadjuvant immunotherapy combination treatments; CAD, coronary artery disease; T, tumor; Tis, carcinoma in situ; N, node; TIE, transmediastinal esophagectomy; MIE, minimally invasive esophagectomy.
Postoperative complications
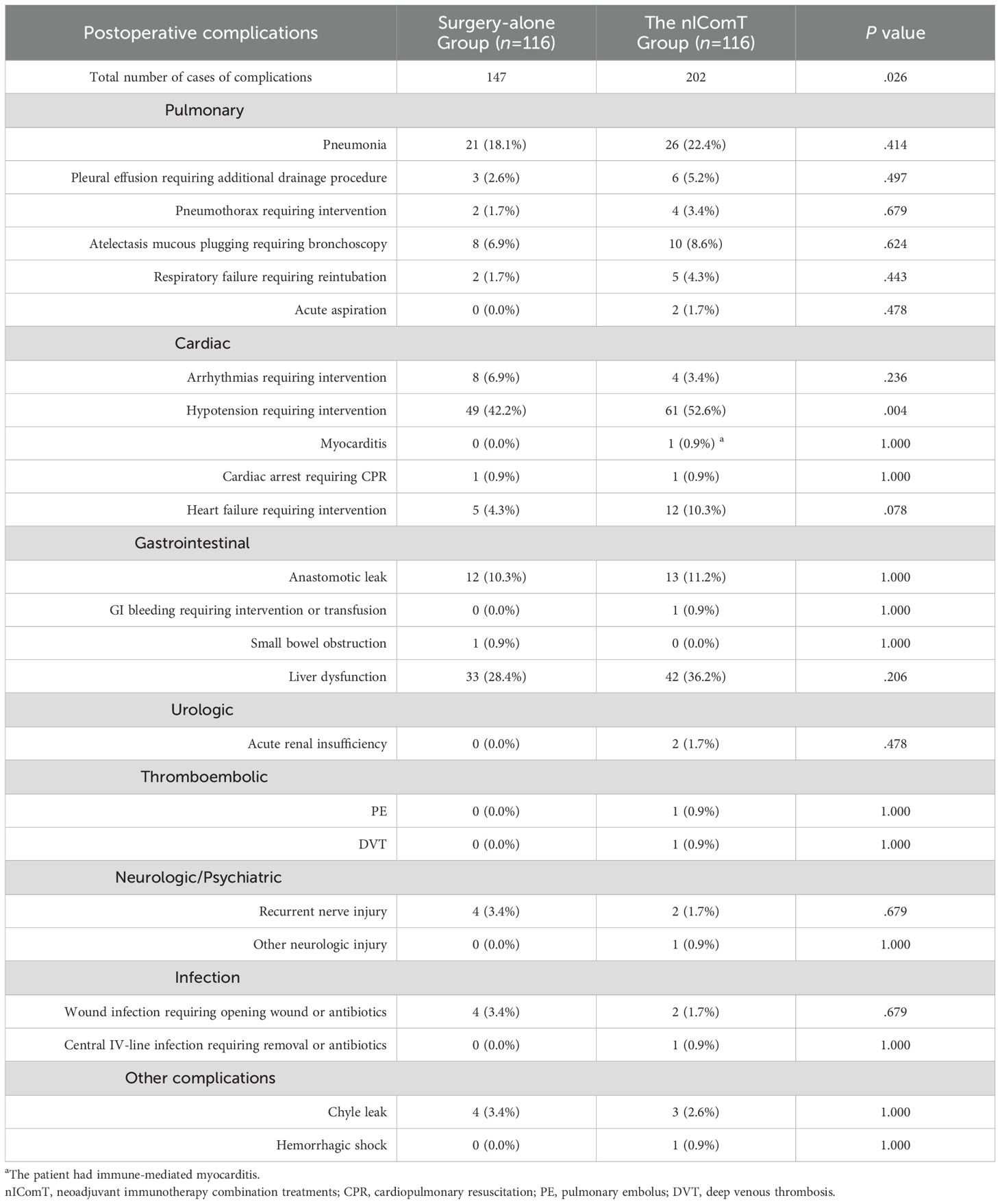
Table 2 presents the overall incidence of postoperative complications and rates of Clavien-Dindo grade ≥III complications in both groups. No significant difference was observed in total complication rates between the two groups (51.7% vs 56.0%, P=0.510). The incidence of complications in pulmonary, gastrointestinal, urologic, thromboembolic and neuropsychiatric systems, and infections was comparable between groups (P>0.05). However, the nIComT group showed a significantly higher rate of grade I-II cardiovascular complications compared to the surgery-alone group (P=0.003).
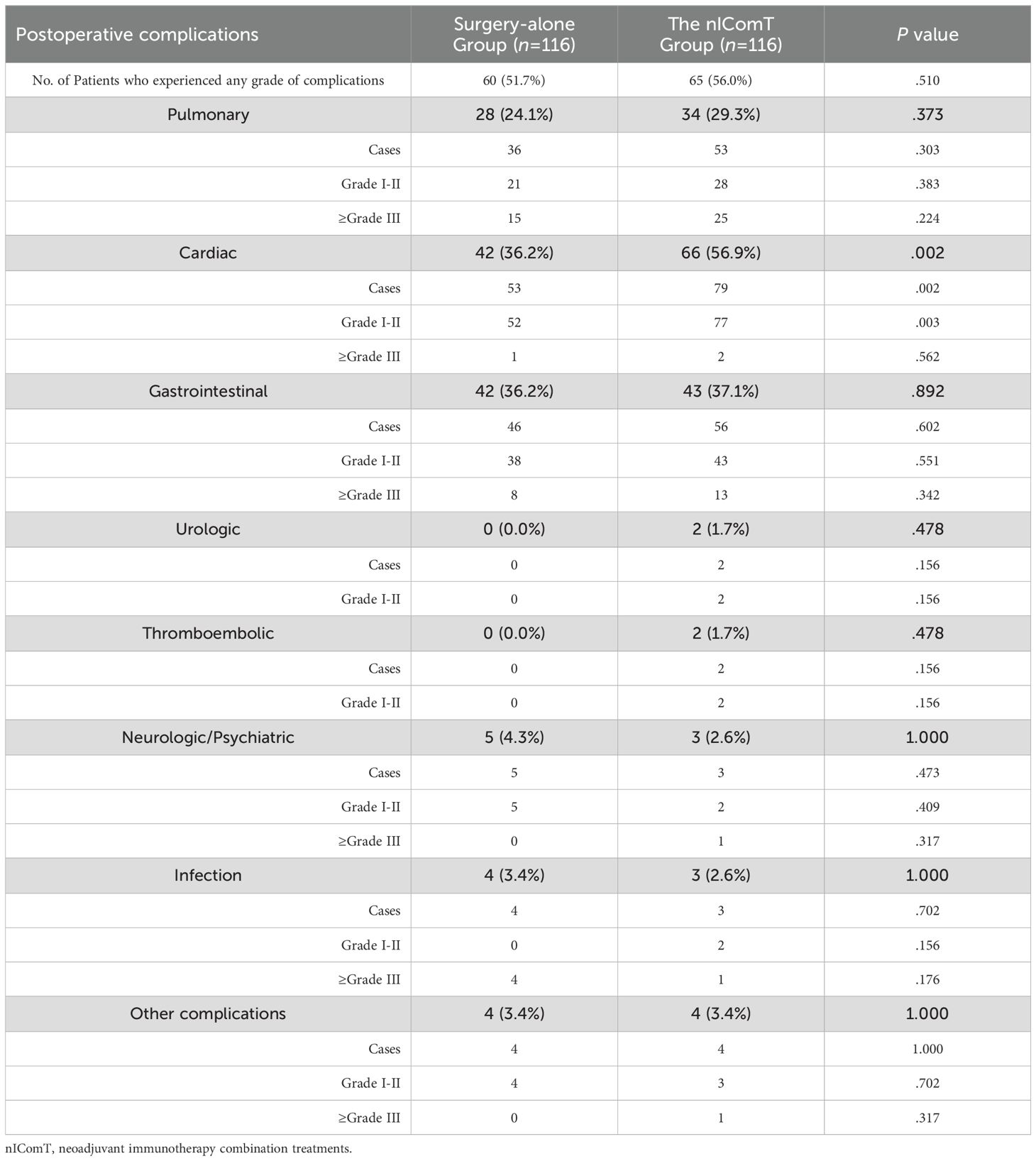
Table 2. Postoperative complications assessed according to the Clavien-Dindo classification between the surgery-alone group and the nIComT group.
During the postoperative period, 147 complications occurred in the surgery-alone group versus 202 in the nIComT group (P=0.026). Rates of common complications including respiratory, gastrointestinal, urologic, thromboembolic, neuropsychiatric, and infectious were similar (P>0.05). Hypotension was significantly more frequent in the nIComT group (52.6% vs 42.2%, P=0.004), managed via continuous vasopressor including dopamine, ephedrine, norepinephrine, or transfusion. Although not statistically significant, the nIComT group underwent a trend of higher rate of heart failure (10.3% vs 4.3%, P=0.078) compared with the surgery-alone group. One nIComT patient required ICU readmission for hemorrhagic shock within 48 hours; another developed grade 3 immune-related myocarditis one month postoperatively, presenting with elevated creatine kinase levels not observed during neoadjuvant therapy. Table 3 presents the incidences of complications of each system.
Compared to the nICT subgroup, the nICRT subgroup exhibited significantly higher overall complication rates (47.9% vs 95.0%, P<0.001), primarily driven by cardiovascular complications (54.2% vs 65.0%, P=0.068). Specifically, compared with the nICT subgroup, intervention-requiring heart failure was more frequent in the nICRT subgroup (7.3% vs 25.0%, P=0.050), with a trend toward increased pneumonia (18.8% vs 40.0%, P=0.075). In addition, the nICRT subgroup had longer operative durations, greater perioperative blood loss, increased intraoperative urine output, prolonged hospital stays, and higher costs than the nICT subgroup (P<0.05). Detailed data are provided in Supplementary Tables S1-S3.
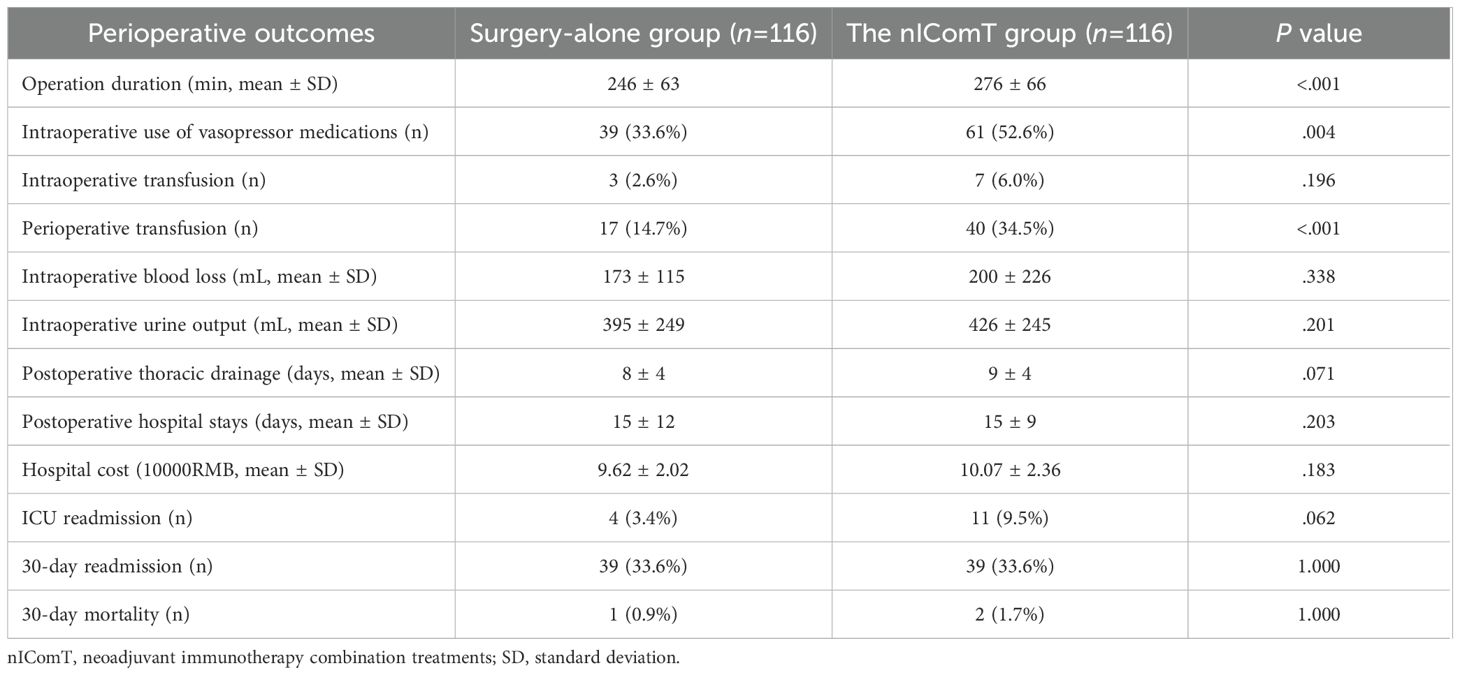
Other perioperative outcomes and mortality
Table 4 demonstrates the TRAEs that occurred in the nIComT group during neoadjuvant treatment. The most common TRAEs in the nIComT group were anemia (n=74, 63.8%), lymphocytopenia (n=72, 62.1%), and thrombocytopenia (n=42, 36.2%). Compared with the nICT subgroup, the nICRT subgroup exhibited significantly higher incidence rates of lymphocytopenia (54.2% vs 100.0%), thrombocytopenia (29.2% vs 70.0%), leukopenia (19.8% vs 75.0%), neutropenia (14.6% vs 80.0%), and elevated lactate dehydrogenase (13.5% vs 45.0%) (P<0.05). Notably, all 20 patients in the nICRT subgroup developed lymphocytopenia (100%). No irAEs were observed in the nIComT group during neoadjuvant treatment.
The other perioperative outcomes between the two groups were summarized in Table 5. The mean operative duration was significantly longer in the nIComT group, when compared with the surgery-alone group (276 ± 66min vs 246 ± 63min, P<0.001). A higher proportion of nIComT patients required intraoperative vasopressors including dopamine, ephedrine, norepinephrine (52.6% vs 33.6%, P=0.004) and perioperative transfusions (34.5% vs 14.7%, P<0.001). Postoperative thoracic drainage volume (P=0.071) and ICU readmission rates (9.5% vs 3.4%, P=0.062) showed increasing trends in the nIComT group, though non-significant. No other perioperative outcomes differed significantly between groups.
One patient in the surgery-alone group died within 48 hours after surgery due to respiratory cardiac arrest resulting from the development of atrial fibrillation complicated by respiratory failure. Two patients in the nIComT group died within 30 days of surgery, including one patient received nICT (cardiac arrest due to hypotension with sinus tachycardia within 24 hours) and one patient received nICRT (metabolic encephalopathy with secondary epilepsy). No significant difference in mortality was observed between groups (P>0.05).
Relative factors associated with cardiovascular complications
Figures 3, 4 present the univariate and multivariate logistic regression analyses of potential risk factors for cardiovascular complications. Univariate analysis identified significant associations with neoadjuvant therapy (OR=2.33, 95% CI=1.37-3.94, P=0.002), three-field lymphadenectomy (OR=4.16, 95% CI=1.11-15.53, P=0.034), operative duration (OR=1.01, 95% CI=1.01-1.01, P=0.010), and intraoperative blood loss (OR=1.01, 95% CI=1.01-1.01, P=0.013). Multivariate analysis demonstrated that neoadjuvant therapy remained independently associated with cardiovascular complications (OR=2.03, 95% CI=1.15-3.57, P=0.015).

Figure 3. Univariate logistic regression analyses of potential risk factors for cardiovascular complications. OR, odds ratio; 95% CI, 95% confidence interval; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; CAD, coronary artery disease; CI, cerebral infarction; T, tumor; Tis, carcinoma in situ; N, node; TIE, transmediastinal esophagectomy; MIE, minimally invasive esophagectomy.

Figure 4. Multivariate logistic regression analyses of potential risk factors for cardiovascular complications. OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
There is no consistent conclusion in whether nIComT increase postoperative toxicity in LA-ESCC patients. In this study, we retrospectively compared the occurrence of postoperative complications in the surgery-alone group versus nIComT group. Our findings revealed that both groups exhibited similar complication rates across systems, except for the cardiovascular system. Notably, the increasing occurrence of grade I-II cardiovascular complications was reported for the first time in the present study, underscoring the significance of postoperative circulatory interventions for patients who underwent nIComT.
As previously reported, the rates of overall systemic complications were not statistically different from the surgery-alone group as compared with neoadjuvant chemotherapy (nCT) or nCRT group (15, 30). A study comparing esophagectomy after nCT with surgery directly demonstrates that the occurrence of postoperative complications is similarly between the two groups of patients (30). Furthermore, the CROSS study also demonstrates that the incidence of postoperative complications, including those related to the cardiovascular system, is comparable between patients who received esophagectomy following nCRT and those who underwent surgery directly (15). Additionally, several studies with small sample sizes have demonstrated the acceptable safety profile of nICT or nICRT in the treatment of LA-ESCC (31–38). Consistent with previous studies, the nIComT group in our research also indicates that comparable safety profile of systemic complications and severe complications of grade III or above. However, our study is the first to discover that postoperative cardiovascular complications of grade I-II occur significantly more frequently in the nIComT group than in the control group, primarily manifesting as hypotension requiring treatment and heart failure. The NEOCRTEC5010 study also found that the nCRT group had a higher incidence of cardiovascular system complications compared to the surgery-alone group (16), but mainly arrhythmias. In conclusion, despite reports of the safety of neoadjuvant treatment, the occurrence of postoperative hypotension has received limited attention. Our study examined hypotension as part of postoperative cardiovascular complications and identified an increased risk associated with nIComT. Therefore, it is imperative to monitor for hypotension in patients receiving nIComT postoperatively.
The underlying causes of postoperative hypotension in non-cardiac surgery include decreased cardiac output due to hypovolemia, cardiac pump failure or obstruction, and decreased vascular volume resulting from inflammation, pharmacologic interventions, or sympathetic nervous system compromise (39). It was observed that esophagectomy is susceptible to hypotension and arrhythmias due to atrial pressure and cardiac rotation resulting from mediastinal maneuvers, as well as vagus nerve stimulation (40). However, it is noteworthy that there is a significant difference in the incidence of hypotension between the surgery-alone and the nIComT group as reported by the present study. Therefore, the combination of neoadjuvant treatment may be the primary factor contributing to the increased incidence of hypotension in the nIComT group.
Neoadjuvant treatment including chemotherapy, chemoradiotherapy, and immunotherapy, all can cause damage to the heart (41). Through cytotoxicity-induced myocardial damage, chemotherapy can lead to a variety of cardiovascular system complications, such as systolic and diastolic dysfunction of the heart (42). It is also undeniable that microvascular and macrovascular damage may result from ionizing radiation exposure following chest radiation therapy, which can subsequently lead to conditions such as pericarditis, myocardial injury, and myocardial ischemia (43). ICIs can cause systemic multi-system inflammation (44). Studied have demonstrated that ICIs can cause structural changes in the heart, leading to myocardial edema and apical ballooning (45). The clinical manifestations of its cardiotoxicity are highly variable, ranging from asymptomatic elevations of cardiac biomarkers to the rapid onset of cardiogenic shock, including hemodynamic failure due to myocarditis (46). A study specifically exploring the impact of nCT and nCRT on postoperative cardiac complications in LA-ESCC patients found that nCRT posed a greater risk of cardiac system complications than nCT, particularly in terms of N-terminal pro-B-type natriuretic peptide (NT-proBNP) elevation (47). Although the differences in the study did not reach statistical significance, the incidence of arrhythmia reported for nCT and nCRT in the study was 19% and 25.5%, while the incidence rate of heart failure was 6.3% and 3.5%, respectively (47). In the CROSS and NEOCRTE5010 studies, the rates among patients receiving nCRT were 21% and 14.1%, respectively (15, 16). In the nIComT group of our study, the overall incidence of cardiovascular complications reached 15.5%, with a notably higher rate of 12.4% of patients received nICT and 30.0% in patients received nICRT. The incidence of cardiovascular complications in subgroup analysis of our study was comparable or higher than that reported in previous studies. Meanwhile, studies have also reported that the combining ICIs with neoadjuvant chest radiation therapy not only improves the prognosis for patients with non-small cell lung cancer (NSCLC), but also increases the incidence of cardiovascular system complications (48), although the mechanism is not fully understood. The addition of ICIs may be a key factor contributing to the increased risk of postoperative cardiovascular complications in the nIComT group. In conclusion, the integration of neoadjuvant immunotherapy with nCT or nCRT holds the potential to synergistically exacerbate postoperative cardiovascular injury. Consequently, the toxicity associated with nIComT can be mitigated by regulating its intensity, including reducing the dosage of chemotherapy or chemoradiotherapy. Moreover, patients underwent nIComT require heightened vigilance towards the toxicity of the cardiovascular system throughout the postoperative period.
Hypotension was defined as MAP <60 mmHg, consistent with thresholds associated with adverse outcomes in non-cardiac surgery (49). Although intraoperative hemodynamic management remains controversial (50–53), our findings demonstrate significant clinical relevance: 42.2% of surgery-alone patients and 52.6% of nIComT patients required vasopressors for hypotension – a higher incidence than historical esophagectomy cohorts (54). This elevated vasopressor demand in the nIComT group likely reflects cardiovascular stress from synergistic effects of ICIs combined with chemotherapy or radiotherapy, consistent with reported T-cell-mediated myocardial injury mechanisms by PD-1 inhibitors (55). Furthermore, patients with a history of nIComT use, especially those who have received nICRT, should be monitored for vital sign changes during the perioperative period, and the occurrence of serious complications should be recognized and evaluated in a timely manner. Furthermore, postoperative hypotension can lead to oliguria, pulmonary edema, respiratory failure and other adverse consequences. Therefore, in the prospectively study currently conducted in our center, we have adopted intravenous infusion of vasoactive drugs such as norepinephrine for 3–7 days after esophagectomy to maintain blood pressure close to the preoperative normal level, and have initially achieved good results.
This study has several limitations. Firstly, as a retrospective analysis with a small sample size, it carries inherent risks of selection bias and therapeutic heterogeneity. Secondly, the underrepresentation of the nCRT cohort reflects real-world clinical patterns in China, where most LA-ESCC patients with advanced diagnoses and compromised performance status preferentially receive nCT. Lastly, the lack of systematic perioperative cardiac function parameters in some cases hindered comprehensive assessment of treatment-related cardiotoxicity. Future studies should prioritize prospective clinical trials implementing standardized protocols, incorporating comprehensive cardiovascular monitoring and mechanistic investigations to validate these findings. Future prospective studies are needed to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was conducted according to the Declaration of Helsinki and received ethical approval from the Institutional Review Board and Ethics Committee of Jiangsu Cancer Hospital. The requirement for informed consent was waived due to the anonymity of the data. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Resources, Validation, Writing – review & editing. GX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. BY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. YH: Data curation, Investigation, Resources, Software, Visualization, Writing – original draft. ZC: Data curation, Investigation, Resources, Software, Writing – review & editing. LG: Conceptualization, Formal analysis, Methodology, Resources, Writing – original draft. CK: Formal analysis, Investigation, Methodology, Resources, Writing – original draft. LZ: Conceptualization, Resources, Validation, Visualization, Writing – original draft. ZZ: Data curation, Formal analysis, Investigation, Validation, Writing – original draft. QX: Investigation, Methodology, Validation, Visualization, Writing – original draft. YC: Formal analysis, Investigation, Software, Validation, Writing – original draft. XZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MJ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NJ: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Project administration, Resources, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Research Project of Jiangsu Cancer Hospital (No. ZL202202; No. ZL202305), and Beijing Life Oasis Public Service Center (No. BH004506).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1573597/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Cli. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Qiu H, Cao S, and Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commu. (2021) 41:1037. doi: 10.1002/cac2.v41.10
3. Arnold M, Ferlay J, Van Berge Henegouwen MI, and Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. (2020) 69:1564–71. doi: 10.1136/gutjnl-2020-321600
4. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Heal. (2018) 6:e555–67. doi: 10.1016/S2214-109X(18)30127-X
5. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lance. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
6. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. (2021) 326:916. doi: 10.1001/jama.2021.12836
7. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Me. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
8. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
9. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. (2022) 10:e004291. doi: 10.1136/jitc-2021-004291
10. Van Den Ende T, De Clercq NC, Van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Re. (2021) 27:3351–9. doi: 10.1158/1078-0432.CCR-20-4443
11. Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Re. (2022) 28:3021–31. doi: 10.1158/1078-0432.CCR-22-0413
12. Gao L, Lu J, Zhang P, Hong ZN, and Kang M. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Onco. (2022) 13:478–87. doi: 10.21037/jgo-22-131
13. Chen X, Xu X, Wang D, Liu J, Sun J, Lu M, et al. Neoadjuvant sintilimab and chemotherapy in patients with potentially resectable esophageal squamous cell carcinoma (KEEP-G 03): an open-label, single-arm, phase 2 trial. J Immunother Cancer. (2023) 11:e005830. doi: 10.1136/jitc-2022-005830
14. Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, et al. Two-year outcomes of clinical N2–3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Sur. (2024) 167:838–847.e1. doi: 10.1016/j.jtcvs.2023.08.056
15. Van Hagen P, Hulshof MCCM, Van Lanschot JJB, Steyerberg EW, Henegouwen MIVB, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Me. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
16. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Onco. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
17. Jiang N, Zhang J, Guo Z, Wu Y, Zhao L, Kong C, et al. Short-course neoadjuvant radiotherapy combined with chemotherapy and toripalimab for locally advanced esophageal squamous cell carcinoma (SCALE-1): a single-arm phase Ib clinical trial. J Immunother Cancer. (2024) 12:e008229. doi: 10.1136/jitc-2023-008229
18. Aburaki R, Fujiwara Y, Chida K, Horita N, and Nagasaka M. Surgical and safety outcomes in patients with non-small cell lung cancer receiving neoadjuvant chemoimmunotherapy versus chemotherapy alone: A systematic review and meta-analysis. Cancer Treat Re. (2024) 131:102833. doi: 10.1016/j.ctrv.2024.102833
19. Bai G, Chen X, Peng Y, Ji Y, Bie F, Liu Y, et al. Surgery challenges and postoperative complications of lung cancer after neoadjuvant immunotherapy. Thorac Cancer. (2024) 15:1138–48. doi: 10.1111/1759-7714.15297
20. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, and Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Onco. (2017) 12:36–42. doi: 10.1016/j.jtho.2016.10.016
21. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, et al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Sur. (2015) 262:286–94. doi: 10.1097/SLA.0000000000001098
22. Kim JH, Lee HC, Kim Sj, Yoon SB, Kong SH, Yu HW, et al. Perioperative hemodynamic instability in pheochromocytoma and sympathetic paraganglioma patients. Sci Re. (2021) 11:18574. doi: 10.1038/s41598-021-97964-3
23. Dindo D, Demartines N, and Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Sur. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
24. National Cancer Institute (NCI). Common terminology criteria for adverse events (CTCAE.(2017). Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
25. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. (2022) 20:387–405. doi: 10.6004/jnccn.2022.0020
26. Daetwyler E, Wallrabenstein T, König D, Cappelli LC, Naidoo J, Zippelius A, et al. Corticosteroid-resistant immune-related adverse events: a systematic review. J Immunother Cancer. (2024) 12:e007409. doi: 10.1136/jitc-2023-007409
27. Sun T, Qin G, and Wu Z. Comparison of propensity score methods under different confounding structures:A simulation study. Chin J Health Sta. (2017) 3:415–20.
28. Xie L and Zhang Z. Survival benefit of combined immunotherapy and chemoradiotherapy in locally advanced unresectable esophageal cancer: an analysis based on the SEER database. Front Immuno. (2024) 15:1334992. doi: 10.3389/fimmu.2024.1334992
29. Xie L, Zhang Y, Niu X, Jiang X, Kang Y, Diao X, et al. A nomogram for predicting cancer-specific survival in patients with locally advanced unresectable esophageal cancer: development and validation study. Front Immuno. (2025) 16:1524439. doi: 10.3389/fimmu.2025.1524439
30. Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: european organisation for research and treatment of cancer randomized trial 40954. J Clin Onco. (2010) 28:5210. doi: 10.1200/JCO.2009.26.6114
31. Zhou RQ, Luo J, Li LJ, Du M, and Wu QC. Neoadjuvant camrelizumab plus chemotherapy in locally advanced oesophageal squamous cell carcinoma: a retrospective cohort study. BMC Sur. (2023) 23:114. doi: 10.1186/s12893-023-02023-5
32. Qiao Y, Zhao C, Li X, Zhao J, Huang Q, Ding Z, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immuno. (2022) 13:953229. doi: 10.3389/fimmu.2022.953229
33. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Di. (2021) 13:3518–28. doi: 10.21037/jtd-21-340
34. Wang Z, Shao C, Wang Y, Duan H, Pan M, Zhao J, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: A systematic review and meta-analysis. Int J Sur. (2022) 104:106767. doi: 10.1016/j.ijsu.2022.106767
35. Ge F, Huo Z, Cai X, Hu Q, Chen W, Lin G, et al. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: A systematic review and meta-analysis. JAMA Netw Ope. (2022) 5:e2239778. doi: 10.1001/jamanetworkopen.2022.39778
36. Ma X, Zhao W, Li B, Yu Y, Ma Y, Thomas M, et al. Neoadjuvant immune checkpoint inhibitors plus chemotherapy in locally advanced esophageal squamous cell carcinoma: perioperative and survival outcomes. Front Onco. (2022) 12:810898. doi: 10.3389/fonc.2022.810898
37. Hong ZN, Weng K, Peng K, Chen Z, Lin J, and Kang M. Neoadjuvant immunotherapy combined chemotherapy followed by surgery versus surgery alone for locally advanced esophageal squamous cell carcinoma: A propensity score-matched study. Front Onco. (2021) 11:797426. doi: 10.3389/fonc.2021.797426
38. Fan M, Dai L, Yan W, Yang Y, Lin Y, and Chen K. Efficacy of programmed cell death protein 1 inhibitor in resection transformation treatment of esophageal cancer. Thorac Cancer. (2021) 12:2182–8. doi: 10.1111/1759-7714.14054
39. Briesenick L, Flick M, and Saugel B. Postoperative blood pressure management in patients treated in the ICU after noncardiac surgery. Curr Opin Crit Car. (2021) 27:694–700. doi: 10.1097/MCC.0000000000000884
40. Nikbakhsh N, Amri P, Shakeri A, and Shakeri A. Changes in blood pressure and heart rhythm during transhiatal esophagectomy. Casp J Intern Me. (2012) 3:541.
41. Beukema JC, De Groot C, Plukker JTM, Vliegenthart R, Langendijk JA, Van Luijk P, et al. Late cardiac toxicity of neo-adjuvant chemoradiation in esophageal cancer survivors: A prospective cross-sectional pilot study. Radiother Onco. (2022) 167:72–7. doi: 10.1016/j.radonc.2021.11.029
42. Battisha A, Sawalha K, Obeidat Y, and Patel B. Role of cardiac biomarkers in monitoring cardiotoxicity in chemotherapy patients. Crit Pathw Cardiol J Evid-Based Me. (2023) 22:83–7. doi: 10.1097/HPC.0000000000000314
43. Lancellotti P, Nkomo VT, Badano LP, Bergler J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European association of cardiovascular imaging and the American society of echocardiography. J Am Soc Echocardiog. (2013) 26:1013–32. doi: 10.1016/j.echo.2013.07.005
44. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Me. (2012) 366:925. doi: 10.1056/NEJMoa1112824
45. Romitan DM, Rădulescu D, Berindan-Neagoe I, Stoicescu L, Grosu A, Rădulescu L, et al. Cardiomyopathies and arrhythmias induced by cancer therapies. Biomedicines. (2020) 8:496. doi: 10.3390/biomedicines8110496
46. Asnani A. Cardiotoxicity of immunotherapy: incidence, diagnosis, and management. Curr Oncol Re. (2018) 20:44. doi: 10.1007/s11912-018-0690-1
47. Zhang Z and Zhang H. Impact of neoadjuvant chemotherapy and chemoradiotherapy on postoperative cardiopulmonary complications in patients with esophageal cancer. Dis Esophagus. (2017) 30:1–7. doi: 10.1093/dote/dox002
48. Luo Y, Zeng Z, Liu Y, and Liu A. Reflecting on the cardiac toxicity in non-small cell lung cancer in the era of immune checkpoint inhibitors therapy combined with thoracic radiotherapy. Biochim Biophys Acta BBA - Rev Cancer. (2023) 1878:189008. doi: 10.1016/j.bbcan.2023.189008
49. Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping STJ, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. (2015) 123:307–19. doi: 10.1097/ALN.0000000000000756
50. Buitenwerf E, Boekel MF, van der Velde MI, Voogd MF, Kerstens MN, Wietasch GJKG, et al. The haemodynamic instability score: Development and internal validation of a new rating method of intra-operative haemodynamic instability. Eur J Anaesthesiol. (2019) 36:290–6. doi: 10.1097/EJA.0000000000000941
51. Godet T, Grobost R, and Futier E. Personalization of arterial pressure in the perioperative period. Curr Opin Crit Car. (2018) 24:554–9. doi: 10.1097/MCC.0000000000000548
52. Hallqvist L, Granath F, Huldt E, and Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: An observational study. Eur J Anaesthesiol. (2018) 35:273–9. doi: 10.1097/EJA.0000000000000735
53. Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. (2019) 122:563–74. doi: 10.1016/j.bja.2019.01.013
54. Yeheyis ET, Kassa S, Yeshitela H, and Bekele A. Intraoperative hypotension is not associated with adverse short-term postoperative outcomes after esophagectomy in esophageal cancer patients. BMC Sur. (2021) 21:1. doi: 10.1186/s12893-020-01015-z
Keywords: esophagectomy, esophageal squamous cell carcinoma (ESCC), neoadjuvant immunotherapy, perioperative complications, immune checkpoint inhibitors (ICIs)
Citation: Li Y, Xiao G, Yang B, Hong Y, Chen Z, Gu L, Kong C, Zhao L, Zhu Z, Xu Q, Chen Y, Jiang M, Zhu X and Jiang N (2025) Impact of neoadjuvant immunotherapy combined with chemotherapy or chemoradiotherapy on postoperative safety in locally advanced esophageal squamous cell carcinoma: a propensity score-matched retrospective cohort study. Front. Oncol. 15:1573597. doi: 10.3389/fonc.2025.1573597
Received: 09 February 2025; Accepted: 30 April 2025;
Published: 21 May 2025.
Edited by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceReviewed by:
Liangyun Xie, Henan University of Science and Technology, ChinaLorenzo Giorgi, Humanitas Research Hospital, Italy
Christina Koufopoulou, National and Kapodistrian University of Athens, Greece
Copyright © 2025 Li, Xiao, Yang, Hong, Chen, Gu, Kong, Zhao, Zhu, Xu, Chen, Jiang, Zhu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Jiang, bmppYW5nMTE3QG5qbXUuZWR1LmNu; Xiangzhi Zhu, MTMxODI5NDgwNjhAMTYzLmNvbQ==; Ming Jiang, TWluZ2ppYW5nMjAyM0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share first authorship
 Yixin Li1‡
Yixin Li1‡ Lingling Gu
Lingling Gu Cheng Kong
Cheng Kong Xiangzhi Zhu
Xiangzhi Zhu Ning Jiang
Ning Jiang