- 1Department of Pathology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Development and Related Diseases of Women and Children Key Laboratory of Sichuan Province, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Department of Pathology, West China Hospital, Sichuan University, Chengdu, China
Background: Müllerian papilloma is a rare benign genital tract tumor, and its malignant transformation is extremely rare. Due to its complex and diverse pathological morphological manifestations, it is prone to misdiagnosis.
Methods: We reported the malignant transformation of Müllerian papilloma into endometrioid carcinoma in two young girls, along with their pathological results. For the first time, we combined next-generation sequencing (NGS) technology to explore the molecular characteristics.
Results: The two cases of malignant transformation into endometrioid adenocarcinoma exhibited similar pathological morphology and immunohistochemical (IHC) markers. Morphologically, they presented complex and diverse features. The benign areas showed a mild papillary structure, while the malignant areas displayed complex papillary branches, cribriform patterns, and solid structures, accompanied by hemorrhage, necrosis, and interstitial inflammatory cell infiltration. In terms of IHC, CK7 and EMA were either focally positive or diffusely positive; Vimentin, P16, and SALL-4 were negatively expressed; P53 showed wild-type expression; the ki67 proliferation index was 35-45%. Subsequent sequencing revealed a low tumor mutation burden and stable microsatellites. However, three novel fusion genes were identified.
Conclusion: The malignant transformation of Müllerian papilloma is extremely rare, with complex and diverse morphological manifestations. High vigilance is required during diagnosis to avoid confusion with sarcomas. This tumor has a low tumor mutation burden and stable microsatellites, and the exact mechanism of malignant transformation requires further investigation.
1 Introduction
Müllerian papilloma is a rare benign genital tract tumor, commonly located in the vagina and cervix, mainly occurring in prepubertal girls. It was previously referred to as “mesonephric papilloma”, or “benign polypoid tumor”. Until 1981, based on the ultrastructure of these lesions, Ulbright et al. believed it originated from the Müllerian duct (1). Subsequent immunohistochemical characteristics also supported the Müllerian duct origin (2), and the disease name “Müllerian papilloma” was used. Clinically, patients often seek medical treatment due to vaginal bleeding, and a thorough examination must be carried out to check for the presence of malignant tumors at this time.
The malignant transformation of Müllerian papilloma is extremely rare. In 2003, Abu et al. first described a case of Müllerian papilloma malignant transformation into clear cell carcinoma, which occurred in an adult after multiple recurrences (3). Now, in our hospital, two cases of the malignant transformation of Müllerian papilloma into endometrioid adenocarcinoma have been discovered for the first time. Due to the rarity of these diseases and their diverse morphological manifestations, misdiagnosis is very likely in practical work. As far as we know, there is currently no research on the gene level of Müllerian papilloma, and the exact pathogenesis of its malignant transformation is unclear. Here, we present the clinicopathological features and molecular information of these two cases. At the same time, we reviewed the literature to improve our understanding of the disease, avoid misdiagnosis, and provide evidence for its clinical diagnosis and treatment.
2 Case presentation
2.1 Case1
2.1.1 Clinical presentation
A 12-year-old girl presented with vaginal bleeding for three months. Her past medical history was unremarkable, and there was no family history of genetic diseases. Physical examination revealed a large mass in the vagina. Pelvic magnetic resonance imaging (MRI) revealed an external cervical solid mass with unclear boundaries, protruding to the upper part of the vagina, with a size of about 7.0cm × 6.2cm × 4.0cm (Figure 1A). Routine hematological and biochemical tests showed no abnormalities.
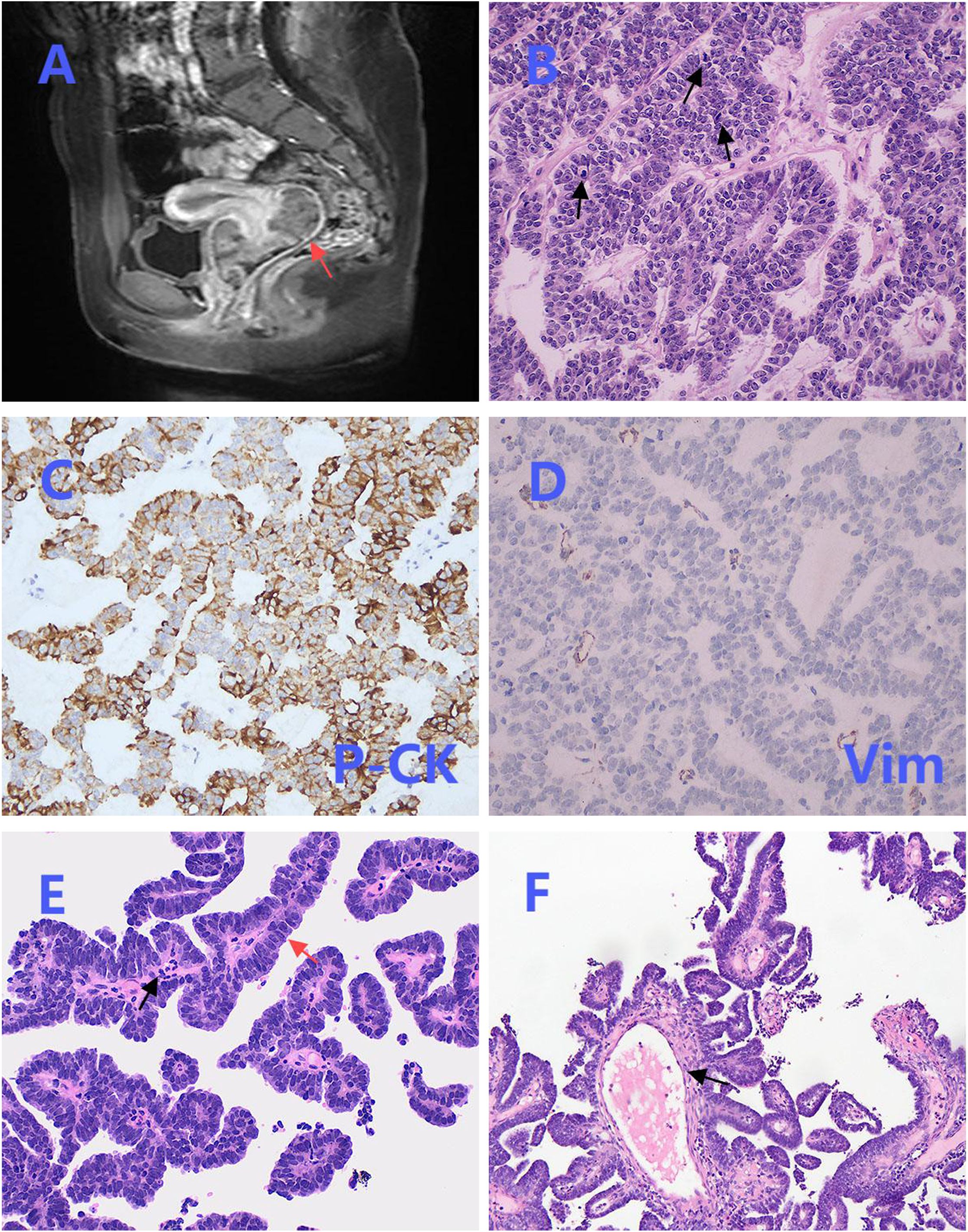
Figure 1. Magnetic resonance imaging (MRI), biopsy and partial postoperative pathological images. (A) Pelvic MRI revealed a solid mass at the external os of the cervix, with unclear boundaries (red arrow). (B) The tumor cells in the biopsy were arranged in a cord-like and glandular pattern, and mitosis was easily observed (black arrows) (×200). (C) Biopsy immunohistochemical staining of P-CK was positive (×200). (D) Biopsy immunohistochemical staining of Vimentin was negative (×200). (E) The benign area in the postoperative pathology showed a papillary structure, covered by a single layer of columnar epithelium (red arrow), with infiltration of inflammatory cells (black arrow) (×200). (F) The fibrous vascular core in the benign area was accompanied by edematous changes (black arrow) (×200).
2.1.2 Pathological diagnosis
The patient initially underwent a colposcopic biopsy in the local hospital, which suggested undifferentiated small round cell sarcoma. Subsequently, after pathological consultation in our institution, the initial diagnosis was sarcoma with epithelioid differentiation. Biopsy analysis demonstrated that the tumor cells exhibited an epithelioid morphology, characterized by cord-like and glandular arrangements, a mucinous background, and prominent mitotic figures (Figure 1B). Immunohistochemical staining revealed positive expression of P-CK (Figure 1C) and negative expression of Vimentin (Figure 1D).
Given the malignant nature of the tumor, the patient underwent trans-abdominal total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection on October 18, 2024. The final postoperative pathological diagnosis was the transformation of cervical Müllerian papilloma into endometrioid adenocarcinoma.
Hematoxylin and eosin (HE) staining demonstrated the coexistence of benign and malignant areas within the tumor tissue. The benign area displayed a normal papillary structure, lined by a single layer of columnar epithelium, with no mitotic figures, a visible fibrovascular core, edema-like changes, and chronic inflammatory cell infiltration (Figures 1E, F). In contrast, the malignant region exhibited glands arranged in a back-to-back pattern (Figure 2A) and fused into a cribriform structure (Figure 2B). In some areas, solid growth was observed, with cells showing disorganized arrangement and stratified columnar glandular epithelium characterized by enlarged nuclei and prominent nucleoli (Figure 2C). Focal areas of tissue were arranged in a cord-like pattern, with evidence of hemorrhage and necrosis (Figure 2D).
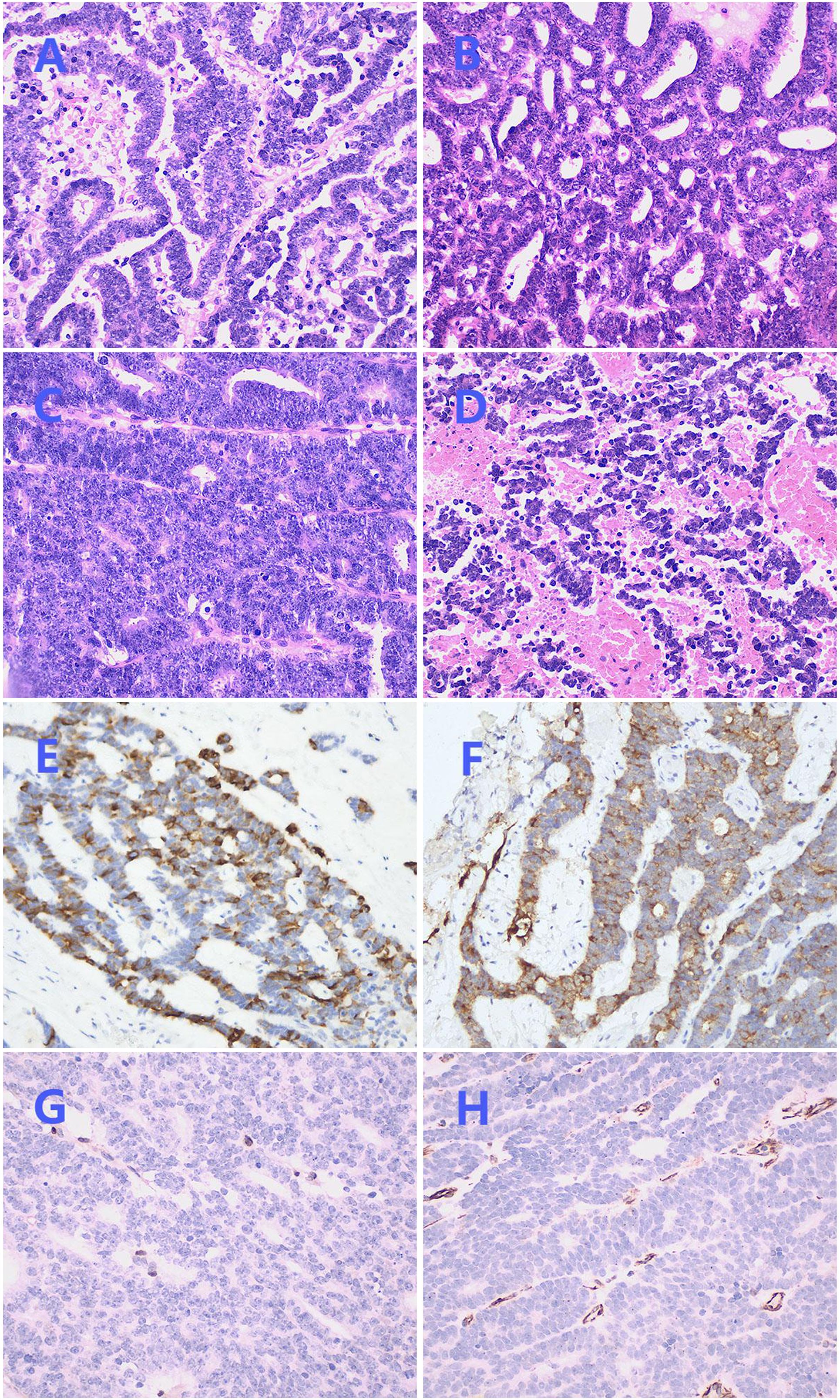
Figure 2. Postoperative pathological images. (A) Irregularly shaped glands with stratified columnar epithelium (×200). (B) Glands fused into a cribriform structure (×200). (C) Solid growth area with enlarged nucleus and obvious nucleolus (×200). (D) Focal areas were arranged in a cord-like pattern, accompanied by bleeding and necrosis (×200). (E) Immunohistochemical staining of CK7 was focally positive (×200). (F) Immunohistochemical staining of EMA was focally positive (×200). (G) Immunohistochemical staining of ER was negative (×200). (H) Immunohistochemical staining of Vimentin was negative (×200).
Immunohistochemical staining showed CK7 (Figure 2E) and EMA (Figure 2F) were focally positive, Pax-8 was strongly positive, and PR was weakly positive, while ER (Figure 2G), Vimentin (Figure 2H), P16, GATA3, CD10, TTF-1, CD56, chromogranin A, synaptophysin, WT-1, Sall-4 were negative, P53 was in wild-type, ki67 index was 45%, no expression loss of mismatch repair proteins was observed. Subsequently, NGS technology was applied to the formalin-fixed paraffin-embedded tissue of the lesion. DNA sequencing showed a low TMB and stable microsatellites. However, in RNA sequencing, we found three new gene rearrangements: MAML1 - KAT6B (EX1:EX17), KAT6B - MAML1 (EX16:EX2), and KTN1 - MAPK1IP1L (EX5:EX4).
2.1.3 Treatment and follow-up
After surgery, the patient received adjuvant radiotherapy. In the most recent follow-up, the patient had no disease recurrence but had post-radiotherapy myelosuppression.
2.2 Case2
This case was first reported in our hospital in 2019 (4). Through follow-up and genetic testing, we updated the previous case report. The patient was a 13 - year - old girl who had experienced irregular vaginal bleeding for 2 years. Both digital rectal examination and pelvic MRI revealed a mass within the vagina, and the pathological biopsy suggested rhabdomyosarcoma. After 4 cycles of neoadjuvant chemotherapy, the patient underwent transabdominal total hysterectomy and pelvic lymph node dissection in our hospital on July 28, 2016. Based on the postoperative pathology and immunohistochemistry, the final diagnosis was endometrioid adenocarcinoma resulting from the malignant transformation of Müllerian papilloma in the vagina and cervix. After the operation, the patient received regular adjuvant therapy, including 6 cycles of chemotherapy and 23 sessions of radiotherapy. In the latest follow-up, no disease recurrence or metastasis was found, and there were no obvious chemoradiotherapy reactions. It should be noted that due to the long time elapsed, which affected the quality inspection of the tumor tissue, we did not obtain the genetic test results of Case 2.
3 Discussion
Müllerian papilloma is a benign tumor originating from Müllerian epithelium. By reviewing 24 reported cases of benign Müllerian papilloma in the literature (Table 1) (1, 2, 5–22), we summarized key characteristics including patient age, symptoms, tumor size, treatment modalities, and outcomes. The majority of cases (23/24, 95.8%) occurred in prepubertal girls with a median age at presentation of 5 years (range, 9 days to 24 years). Lesions were predominantly located in the cervix (12/24, 50.0%) and vagina (11/24, 45.8%), with only one case involving both sites (1/24, 4.2%). Clinical manifestations included vaginal bleeding, discharge, and asymptomatic masses. Diagnosis primarily relied on colposcopic pathological biopsy. Morphologically, classic Müllerian papilloma exhibits a papillary structure covered by single-layer or stratified columnar epithelium or metaplastic squamous epithelium. The stroma was edematous, containing fibrovascular cores and inflammatory cells. Psammoma bodies or bone metaplasia were occasionally observed. The cytoplasm was eosinophilic, with minimal nuclear pleomorphism and mitotic figures. The prognosis was favorable after local resection of the lesion.
However, malignant transformation of Müllerian papilloma is exceedingly rare, with only three documented cases (including the current cases) reported previously (Table 2). The earliest reported case involved clear cell carcinoma in an adult with severe cerebral palsy (3). In this study, the two cases exhibit similar clinical and pathological features. Compared to benign Müllerian papilloma, the clinical symptoms are comparable, but tumors with malignant transformation tend to occur in older children and present as larger masses with poorly defined borders relative to surrounding tissues. Microscopically, both benign and malignant areas coexisted: the benign area showed mild papillary structures, while the malignant area displayed various architectures, including branched papillary, cord-like, cribriform, and solid structures. Mitotic figures and nuclear atypia were prominent, accompanied by necrosis, hemorrhage, and deep infiltration. Immunohistochemically, due to the low degree of differentiation of cancer cells, CK and EMA markers are typically only focally positive. Studies have shown that Vimentin and ER expression levels in cervical adenocarcinoma are significantly lower than those in endometrial adenocarcinoma (23, 24). Therefore, the negative expression of Vimentin and ER further supports adenocarcinoma of cervical origin.
In clinical practice, diagnosing malignant transformation of Müllerian papilloma is extremely challenging. First, cancers in young girls are rare, leading to cautious consideration of such diagnoses. Second, initial biopsies may not be fully reliable, as areas of small-round cells arranged in cords and mucus background can be mistaken for sarcomas, particularly embryonal rhabdomyosarcoma, a common cervical malignancy in children (25). Immunohistochemical analysis revealed positive expression of P-CK, whereas other markers, including P63, CK7, and Vimentin, demonstrated negative results. Consequently, during the initial biopsy evaluation, considering the patient’s age, morphological characteristics, and immunohistochemical findings, a sarcoma was highly suspected. Postoperative pathological examination of our two cases uncovered diverse morphological features. However, biopsies often represent localized lesions, which may be confounded by necrosis and hemorrhage, thereby complicating accurate diagnosis. Therefore, multi-site biopsies and meticulous microscopic observation are essential to enhance diagnostic accuracy.
Müllerian papilloma malignant transformation into endometrioid carcinoma is extremely rare, and it usually needs to be differentiated from other tumors of the female reproductive system at the time of diagnosis:
Benign Müllerian papilloma: Morphologically, the papillary structure is regular without fusion or significant atypia, and mitotic figures are uncommon. Immunohistochemically, ER and PR are typically positive, while the cell proliferation index Ki-67 remains low (4).
Mesonephric duct adenocarcinoma: A rare subtype of cervical adenocarcinoma that is HPV-independent. Tumor cells often exhibit a cuboidal or low columnar appearance, arranged in small tubular, cord-like, or solid nest patterns. Occasionally, eosinophilic secretions may be observed within the lumen, which differs from Müllerian-derived tumors regarding cellular morphology and arrangement. Immunohistochemical markers such as GATA3, PAX8, and CD10 (apical and luminal staining) can be positive, while TTF-1 and CEA are rarely positive, ER and WT1 are typically negative, and KRAS gene mutations are frequently present (26, 27).
Neuroendocrine carcinoma: Cervical neuroendocrine carcinoma mostly occurs in adult females. Morphologically, tumor cells show nested, trabecular, or rosette arrangements, with fine chromatin (“salt-and-pepper” appearance). In terms of immunophenotype, it characteristically expresses neuroendocrine markers (such as CD56, chromogranin A, synaptophysin), and due to its frequent association with HPV infection, p16 often shows diffuse positivity.
Serous carcinoma: Primary serous adenocarcinoma of the cervix is extremely rare (28). Both Müllerian papilloma malignant transformation into endometrioid carcinoma and serous carcinoma may display complex papillary structures with varying degrees of cellular atypia and mitotic figures. Serous carcinoma is more prevalent in postmenopausal women, characterized by papillae with numerous slender branches. WT-1 is often positive, and abnormal p53 protein expression and p53 gene mutations are common (29, 30).
Malignant Müllerian mixed tumor: This tumor exhibits bidirectional differentiation, containing both malignant epithelial and mesenchymal components. Epithelial components express markers such as CK and EMA, while stromal components express mesenchymal markers like Vimentin and occasionally sarcoma-specific markers (31).
For treating malignant transformation of Müllerian papilloma, no established guidelines exist, so cervical cancer protocols are referenced (32). In the earliest case of clear cell carcinoma, radical surgery and adjuvant treatments were not pursued due to the patient’s overall condition. In our two cases, both were clinically staged as II A, with favorable prognoses following radical surgery and adjuvant treatments. Case 1 did not receive neoadjuvant therapy before surgery and had bilateral adnexectomy, while Case 2, who had combined anemia, received neoadjuvant chemotherapy before surgery and preserved both adnexa. Through follow-up, no signs of recurrence or metastasis were observed in these two cases. Given the limited number of cases, further research on large cohorts is needed to determine optimal treatment strategies.
In fact, approximately 25% of prepubertal vaginal bleeding events remain undiagnosed (33). Therefore, those cases without a clear diagnosis and asymptomatic cases cannot be actively detected. The mechanism underlying the malignant transformation of Müllerian papilloma remains unclear. It is unknown whether this transformation occurs directly from benign tumors or when the critical point of transition from benign to malignant occurs. If it can be detected early and actively treated before the patient’s malignant transformation, the impact of the lesion on the patient’s reproductive system may be reduced.
In this study, we report the genetic test results of Müllerian papilloma with malignant transformation using NGS technology for the first time. DNA sequencing revealed TMB-L and MSS. RNA sequencing identified three novel fusion genes: MAML1-KAT6B (EX1:EX17), KAT6B-MAML1 (EX16:EX2), and KTN1-MAPK1IP1L (EX5:EX4). These gene fusions have been observed with other partner genes. Studies have found that MAML1/2 promotes the nuclear localization of YAP/TAZ and tumorigenesis (34). In 2023, Warmke LM et al. reported an NR1D1-MAML1-fused epithelioid and spindle cell sarcoma, which was similar to pseudomyogenic hemangioendothelioma (PHE) in core biopsies (35). Zafir et al. proposed that MAML1 is a co-regulator that changes the adhesion ability of endometrial epithelial cells (36). Based on the above studies, we believe that MAML1 may be related to the formation of endometrioid adenocarcinoma in this case. The KAT6B gene encodes histone acetyltransferase, which regulates gene expression by modifying lysine residues on histones, thereby affecting the structure of chromatin (37). Abnormal function of these genes is associated with the occurrence and development of cancer. Here, we speculate that the formation of the newly formed MAML1-KAT6B fusion gene may be related to the malignant transformation of Müllerian papilloma. Therefore, in the future, a large number of similar cases need to be collected for in-depth research, to further explore the clinical significance of these fusion genes, and to analyze in detail the mechanism of Müllerian papilloma and its malignant transformation, so as to deepen the understanding of the disease and promote its early detection and treatment.
In general, the malignant transformation of Müllerian papilloma is extremely rare, presenting complex and diverse morphologies under the microscope. High vigilance is required during diagnosis, and attention must be paid to the limitations of local biopsy diagnosis to avoid confusion with sarcomas. This tumor has a low tumor mutation burden and stable microsatellites, and the exact mechanism of malignant transformation still needs further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ST: Data curation, Writing – original draft. ZY: Conceptualization, Writing – original draft. WW: Funding acquisition, Writing – review & editing. YH: Methodology, Supervision, Writing – review & editing. LJ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of the Science and Technology Department of Sichuan Province (No. 2023NSFSC0539).
Acknowledgments
We feel grateful for the doctors and staff who have been involved in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ulbright TM, Alexander RW, and Kraus FT. Intramural papilloma of the vagina: evidence of Müllerian histogenesis. Cancer. (1981) 48:2260–6. doi: 10.1002/1097-0142(19811115)48:10<2260::AID-CNCR2820481022>3.0.CO;2-E
2. Schmedding A, Zense M, Fuchs J, and Glüer S. Benign papilloma of the cervix in childhood: immunohistochemical findings and review of the literature. Eur J Pediatr. (1997) 156:320–2. doi: 10.1007/s004310050609
3. Abu J, Nunns D, Ireland D, and Brown L. Malignant progression through borderline changes in recurrent Müllerian papilloma of the vagina. Histopathology. (2003) 42:510–1. doi: 10.1046/j.1365-2559.2003.01554.x
4. Zou J, Xie L, Xiao X, Xu L, Yang F, Qiu T, et al. Cervicovaginal Müllerian papilloma malignant transformation in a prepubertal girl. J Clin Pathol. (2019) 72:836. doi: 10.1136/jclinpath-2018-205612
5. Liu HL, Liu J, and Pan YC. Analysis of one case of Müllerian papilloma in the genital tract of a young girl. Chin J Misdiagn. (2011) 11:991.
6. Jin M, Liao SL, Zhang WP, and Luo YP. A case of Müllerian papilloma of the uterine cervix. Chin J Pathol. (2002) 1):45. doi: 10.3760/j.issn:0529-5807.2002.01.027
7. Lucchetti MC, Diomedi Camassei F, Bakhsh H, and Crucianelli S. A rare cause of vaginal bleeding in infancy: be aware of the benign Müllerian papilloma. J Pediatr Adolesc Gynecol. (2017) 30:516–7. doi: 10.1016/j.jpag.2017.01.015
8. Mierau GW, Lovell MA, Wyatt-Ashmead J, and Goin L. Benign Müllerian papilloma of childhood. Ultrastruct Pathol. (2005) 29:209–16. doi: 10.1080/01913120590951211
9. Kumar A and Kumar A. Benign Müllerian papilloma of the cervix. J Minim Invasive Gynecol. (2012) 19:541–2. doi: 10.1016/j.jmig.2011.11.004
10. Yalamanchili V, Entezami P, Langenburg S, and Stockmann P. Consider benign Müllerian papilloma: a rare cause of vaginal bleeding in children. Pediatr Surg Int. (2014) 30:1285–7. doi: 10.1007/s00383-014-3630-7
11. Hollowell ML, Goulart RA, Gang DL, Otis CN, Prior J, Sachs BF, et al. Cytologic features of müllerian papilloma of the cervix: mimic of malignancy. Diagn Cytopathol. (2007) 35:607–11. doi: 10.1002/dc.20712
12. Tumini S, Carinci S, Anzellotti MT, Chiesa PLL, Rossi C, Stuppia L, et al. Genital sanguineous discharge in prepuberty: a case of Müllerian papilloma of vagina in a nine-year-old girl. J Pediatr Endocrinol Metab. (2010) 23:831–2. doi: 10.1515/jpem.2010.133
13. McCluggage WG, Nirmala V, and Radhakumari K. Intramural müllerian papilloma of the vagina. Int J Gynecol Pathol. (1999) 18:94–5. doi: 10.1097/00004347-199901000-00016
14. McQuillan SK, Grover SR, Pyman J, and Jayasinghe YL. Literature review of benign müllerian papilloma contrasted with vaginal rhabdomyosarcoma. J Pediatr Adolesc Gynecol. (2016) 29:333–7. doi: 10.1016/j.jpag.2015.02.114
15. Smrkolj S, Sorc L, Sinkovec J, and Rakar S. Müllerian papilloma in a patient with Proteus syndrome: case report and review of the literature. Eur J Gynaecol Oncol. (2012) 33:428–32.
16. Lane BR, Ross JH, Hart WR, and Kay R. Müllerian papilloma of the cervix in a child with multiple renal cysts. Urology. (2005) 65:388. doi: 10.1016/j.urology.2004.08.023
17. Cohen M, Pedemonte L, and Drut R. Pigmented müllerian papilloma of the vagina. Histopathology. (2001) 39:541–3. doi: 10.1046/j.1365-2559.2001.1301d.x
18. Smith YR, Quint EH, and Hinton EL. Recurrent benign müllerian papilloma of the cervix. J Pediatr Adolesc Gynecol. (1998) 11:29–31. doi: 10.1016/S1083-3188(98)70104-4
19. Lüttges JE and Lübke M. Recurrent benign Müllerian papilloma of the vagina. Immunohistological findings and histogenesis. Arch Gynecol Obstet. (1994) 255:157–60. doi: 10.1007/BF02390944
20. Dudič R, Dudičová V, and Urdzík P. The rare cause of childhood bleeding - recurrent Müllerian papilloma. J Obstet Gynaecol. (2019) 39:432–3. doi: 10.1080/01443615.2018.1512084
21. Arbo E, dos Reis R, Uchoa D, Acetta SG, Rivoire WA, and Capp E. Vaginal Müllerian papilloma in a 2-year-old child. Gynecol Obstet Invest. (2004) 58:55–6. doi: 10.1159/000078491
22. Reck-Burneo CA, Villanueva J, and Velcek FT. Vaginal müllerian papilloma: an unusual cause of vaginal bleeding in a toddler. J Pediatr Adolesc Gynecol. (2009) 22:e124–126. doi: 10.1016/j.jpag.2008.10.002
23. Huang JH. Expression and clinical significance of immunohistochemical markers in the diagnosis of endometrioid adenocarcinoma and cervical adenocarcinoma. Lab Med Clinic. (2015) 12:778–779, 782. doi: 10.3969/j.issn.1672-9455.2015.06.023
24. Sheng ZN, Yuan B, and Shen QL. Clinical Significance of Immunohistochemical markers in the differential diagnosis of endometrioid adenocarcinoma and cervical adenocarcinoma. Syst Med. (2022) 7:35–39. doi: 10.19368/j.cnki.2096-1782.2022.22.035
25. Wei X and Li L. Cytological diagnosis of patients with embryonal rhabdomyosarcoma of the cervix: case report and literature review. Diagn Pathol. (2024) 19:73. doi: 10.1186/s13000-024-01497-y
26. Rabban JT, McAlhany S, Lerwill MF, Grenert JP, and Zaloudek CJ. PAX2 distinguishes benign mesonephric and Müllerian glandular lesions of the cervix from endocervical adenocarcinoma, including minimal deviation adenocarcinoma. Am J Surg Pathol. (2010) 34:137–46. doi: 10.1097/PAS.0b013e3181c89c98
27. Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, and Tavassoli FA. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. (2001) 25:379–87. doi: 10.1097/00000478-200103000-00013
28. Zhou C, Gilks CB, Hayes M, and Clement PB. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 Cases. Am J Surg Pathol. (1998) 22:113. doi: 10.1097/00000478-199801000-00015
29. Nofech-Mozes S, Rasty G, Ismiil N, Covens A, and Khalifa MA. Immunohistochemical characterization of endocervical papillary serous carcinoma. Int J Gynecol Cancer. (2006) 16:286–92. doi: 10.1136/ijgc-00009577-200602001-00046
30. Nofech-Mozes S, Khalifa MA, Ismiil N, Saad RS, Hanna WM, Covens A, et al. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. (2008) 21:1147–55. doi: 10.1038/modpathol.2008.108
31. Ribeiro-Silva A, Novello-Vilar A, Cunha-Mercante AM, and De Angelo Andrade LAL. Malignant mixed Müllerian tumor of the uterine cervix with neuroendocrine differentiation. Int J Gynecol Cancer. (2002) 12:223–7. doi: 10.1136/ijgc-00009577-200203000-00016
32. Zhou H, Liu YY, Luo M, and Lin ZQ. Update and interpretation of the 2024 NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer (Version 1). Chin J Pract Gynecol Obstet. (2023) 39:1119–21. doi: 10.19538/j.fk2023110113
33. Söderström HF, Carlsson A, Börjesson A, and Elfving M. Vaginal bleeding in prepubertal girls: etiology and clinical management. J Pediatr Adolesc Gynecol. (2016) 29:280–5. doi: 10.1016/j.jpag.2015.10.017
34. Kim J, Kwon H, Shin YK, Song G, Lee T, Kim Y, et al. MAML1/2 promote YAP/TAZ nuclear localization and tumorigenesis. Proc Natl Acad Sci U S A. (2020) 117:13529–40. doi: 10.1073/pnas.1917969117
35. Warmke LM, Collier CD, and Davis JL. NR1D1::MAML1 epithelioid and spindle cell sarcoma mimicking pseudomyogenic hemangioendothelioma in core biopsy: A case report and review of the literature. Genes Chromosomes Cancer. (2023) 62:655–62. doi: 10.1002/gcc.23186
36. Zafir S, Zhou W, Menkhorst E, Santos L, and Dimitriadis E. MAML1: a coregulator that alters endometrial epithelial cell adhesive capacity. Fertil Res Pract. (2021) 7:8. doi: 10.1186/s40738-021-00100-y
Keywords: Müllerian papilloma, malignant transformation, next-generation sequencing, case report, literature review
Citation: Tao S, Zhang Y, Wang W, He Y and Jiang L (2025) Müllerian papilloma: two case reports of malignant transformation and literature review. Front. Oncol. 15:1573747. doi: 10.3389/fonc.2025.1573747
Received: 09 February 2025; Accepted: 14 July 2025;
Published: 31 July 2025.
Edited by:
Maria Isaguliants, Riga Stradiņš University, LatviaReviewed by:
Katerina Kubelka-Sabit, CH Acibadem Sistina, North MacedoniaCinzia Giacometti, Casa di cura Pederzoli, Italy
Copyright © 2025 Tao, Zhang, Wang, He and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying He, aGV5aW5nNjI2QDE2My5jb20=; Lili Jiang, ODc5ODc2MDQ3QHFxLmNvbQ==
 Sirong Tao
Sirong Tao Yan Zhang
Yan Zhang Wei Wang1,2
Wei Wang1,2 Ying He
Ying He Lili Jiang
Lili Jiang