- 1Department of Geriatrics, Liaocheng People’s Hospital, Liaocheng, Shandong, China
- 2Department of Radiation Oncology, Liaocheng People’s Hospital, Liaocheng, Shandong, China
Mediastinal lymph node metastasis from squamous cell carcinoma (SCC) is one of the most common sites for regional metastasis in thoracic malignancies. However, SCC originating from the ileum is relatively rare. We herein report a case of mediastinal lymph node metastasis as the first manifestation of postoperative ileal SCC. No primary tumor was detected on comprehensive imaging studies and bronchoscopy in this patient, and endobronchial ultrasound (E-BUS) considered metastatic SCC. After undergoing four cycles of postoperative chemotherapy with the XELOX regimen, the patient experienced rapid disease progression; however, a subsequent change to a treatment regimen of paclitaxel and cisplatin resulted in a favorable response. This article details the patient’s clinical presentation and treatment course and includes a literature review of primary SCCs arising from the jejunum and ileum, thereby enhancing understanding of these uncommon small intestine tumors.
Introduction
During malignant tumor metastasis, lymph nodes are anatomically situated downstream of the tumor tissue and are regarded as gateways to distant metastasis. While mediastinal lymph node metastasis is more commonly associated with malignant tumors of the upper gastrointestinal tract, it is rarer in lower gastrointestinal tract cancers. Small bowel malignancies account for only 3% of gastrointestinal tumors, making them relatively uncommon (1). Most small bowel cancers develop in the duodenum, and SCC originating from the jejunum is extremely rare (2). Here, we present a case of SCC with mediastinal lymph node metastasis originating from the ileum, and summarize nine previously reported cases in the English literature.
Case presentation
A 52-year-old male patient with a 20-year history of alcohol consumption presented to our gastrointestinal surgery department in July 2023, complaining of intermittent abdominal pain lasting ten days, which had intensified over the past day. The patient has no family history of malignancy. Upon examination, the patient exhibited abdominal muscle tension, pressure pain throughout the abdomen, a drum-like sound upon percussion, and reduced bowel sounds. An abdominal CT scan indicated abdominopelvic effusion and free gas within the peritoneal cavity. With a suspicion of digestive perforation, the patient underwent an exploratory laparotomy under general anesthesia following a comprehensive preoperative evaluation. During the surgery, a mass measuring 5x5x3 cm was discovered at the terminal ileum, approximately 5 cm from the ileocecal valve, along with an enlarged lymph node measuring about 4x4x3 cm at the base of the ileocecal blood vessels. Histological examination of the surgical specimen confirmed moderately differentiated ulcerative SCC with subserosal infiltration (pT3), lymphovascular invasion, and lymph node metastases (2/26). The immunoprofile supported squamous differentiation: CK(+), CK5/6(+), P63(+), P53(-), D2-40(+), HER-2(0), SMARCA4/Brg1(+), AFP(-), CgA(-), Syn(-), CD56(-), SALL-4(-) (Figure 1), with intact mismatch repair proteins (MLH1+, PMS2+, MSH2+, MSH6+). The proliferative index (Ki-67 at 95%) indicated a high-grade biological behavior. Postoperative supplemental imaging, including a chest-enhanced CT, cranial MRI, and bone scans, revealed no metastatic lesions. Given the rarity of small intestine SCC, postoperative adjuvant chemotherapy was administered using the XELOX regimen, following the NCCN guidelines for small intestine adenocarcinoma (3). After surgery, the patient underwent four cycles of chemotherapy with the XELOX regimen starting from August 16, 2023. The patient experienced mild fatigue and nausea as adverse effects during chemotherapy, which were assessed as grade 1 according to the CTCAE 5.0 criteria.
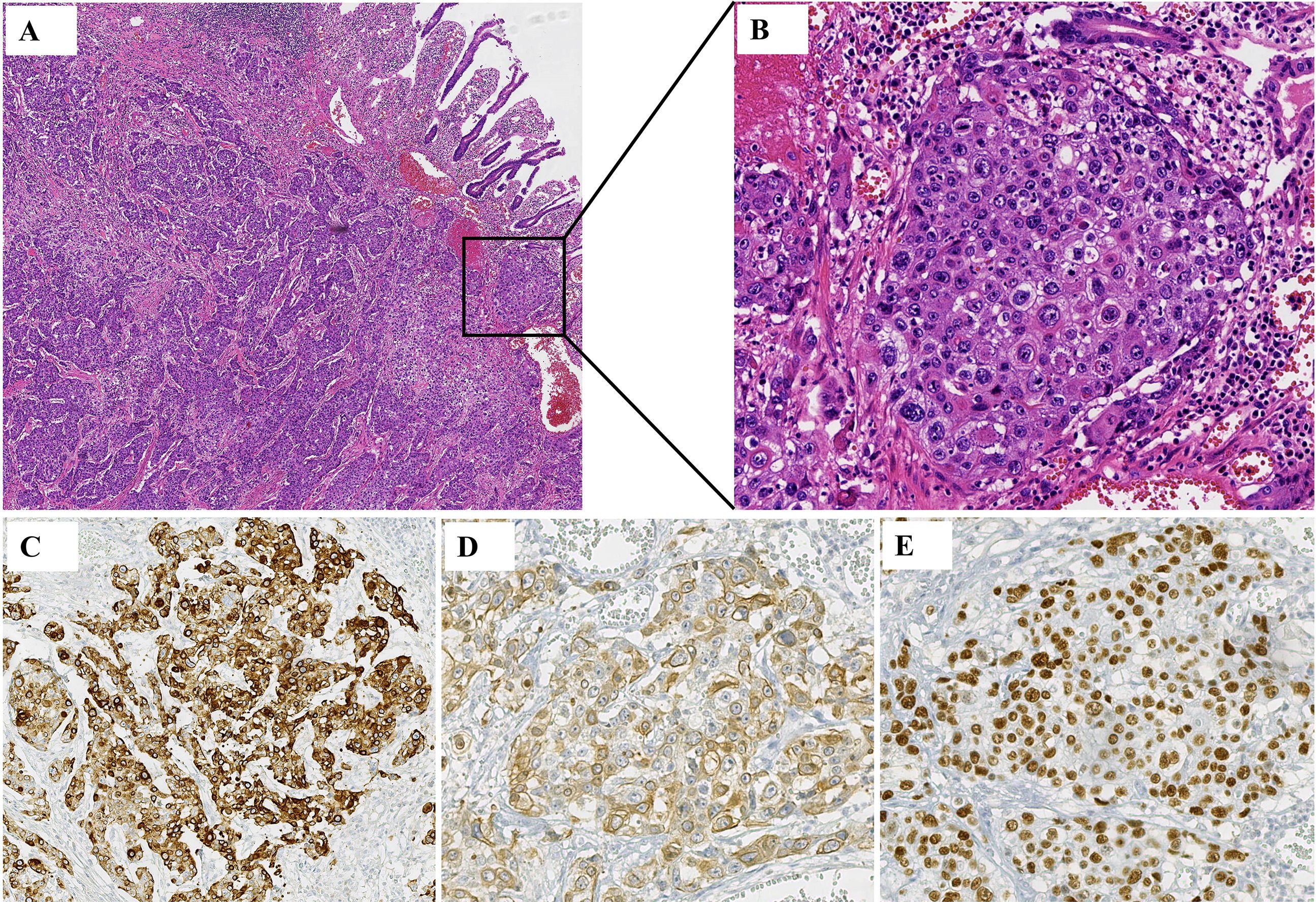
Figure 1. Histology from a postoperative ileum mass. (A, B) Squamous cell carcinoma was seen in the patient’s tissue. (A) HE, ×5; (B) HE, ×20. (C) Immunohistochemistry showed that CK was positive in the patient’s tissues. (D) Immunohistochemistry showed that CK5/6 was positive in the patient’s tissues. (E) Immunohistochemistry showed that P63 was positive in the patient’s tissues.
The patient was admitted to our hospital in January 2024 due to the detection of a 33*35 mm mediastinal mass (Figures 2A–C) on chest CT. The asymptomatic patient exhibited elevated tumor markers: CEA 5.65 ng/mL and SCC antigen 28.0 ng/mL. Diagnostic workup revealed no primary lesions, including contrast-enhanced abdominal CT and bronchoscopy (Figures 2D–F). The endobronchial ultrasound guided tranbronchial needle aspiration (EBUS-TBNA) histopathology demonstrated poorly differentiated SCC with an immunohistochemical profile: CK5/6(+), P63(+), P40(+), CK7(-), CK20(-) (Figures 2G–J).
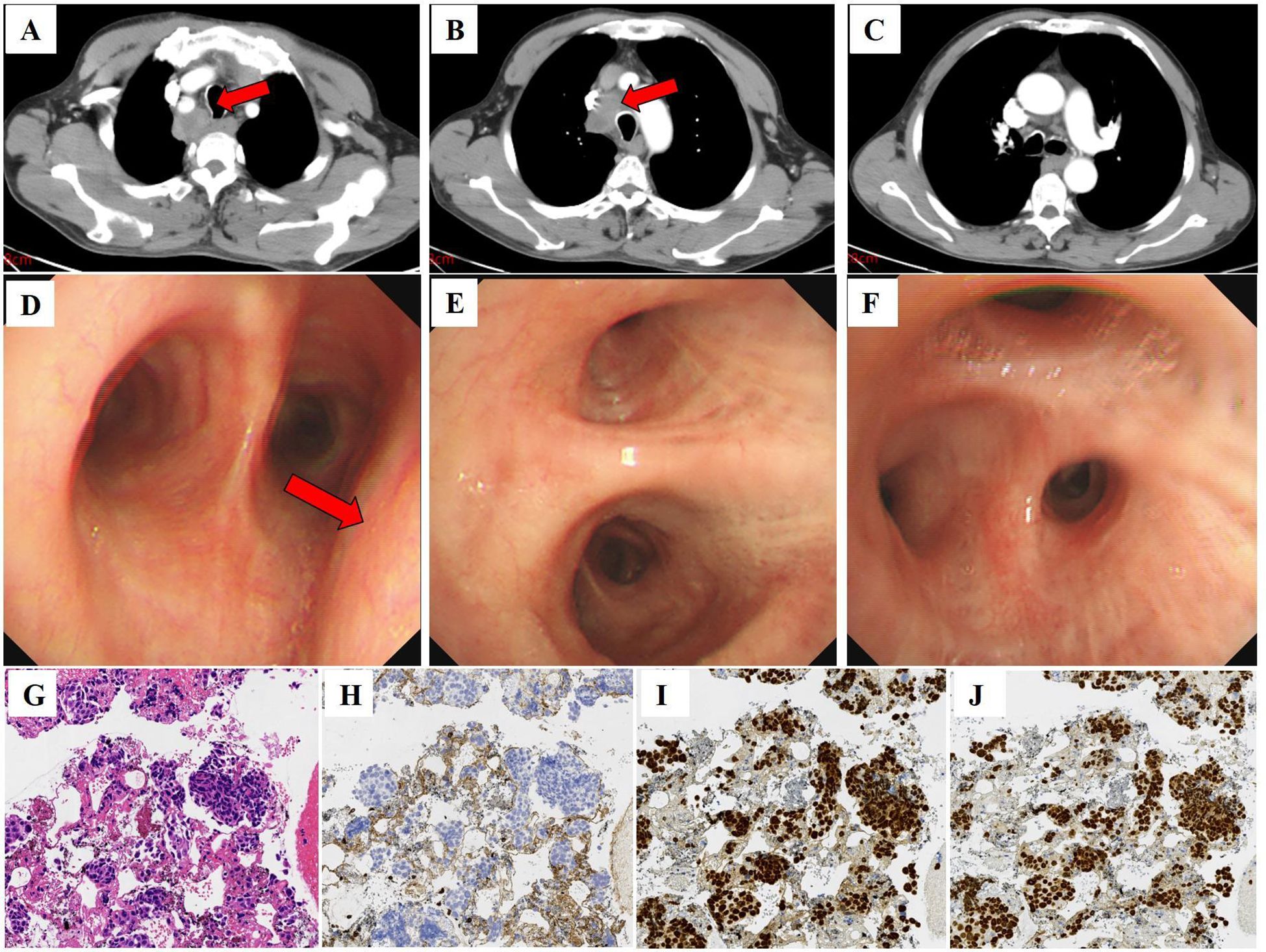
Figure 2. Computed tomography, bronchoscopy images, and histology of punctured tissue of the patient. (A–C) Computed tomography image shows enlarged lymph nodes in the mediastinum (red arrow) (January 04, 2024; slice thickness: 5 mm; window width: 360; window level: 60; arterial phase). (D–F) Bronchoscopy shows extrinsic compressive stenosis of the tracheal lumen (red arrow), with primary and segmental bronchi patency and no hemorrhage or neoplasm. (G) Squamous cell carcinoma was seen in the patient’s tissue. HE, ×20. (H) Immunohistochemistry showed that CK7 was negative in the patient’s tissues. (I) Immunohistochemistry showed that P40 was positive in the patient’s tissues. (J) Immunohistochemistry showed that P63 was positive in the patient’s tissues.
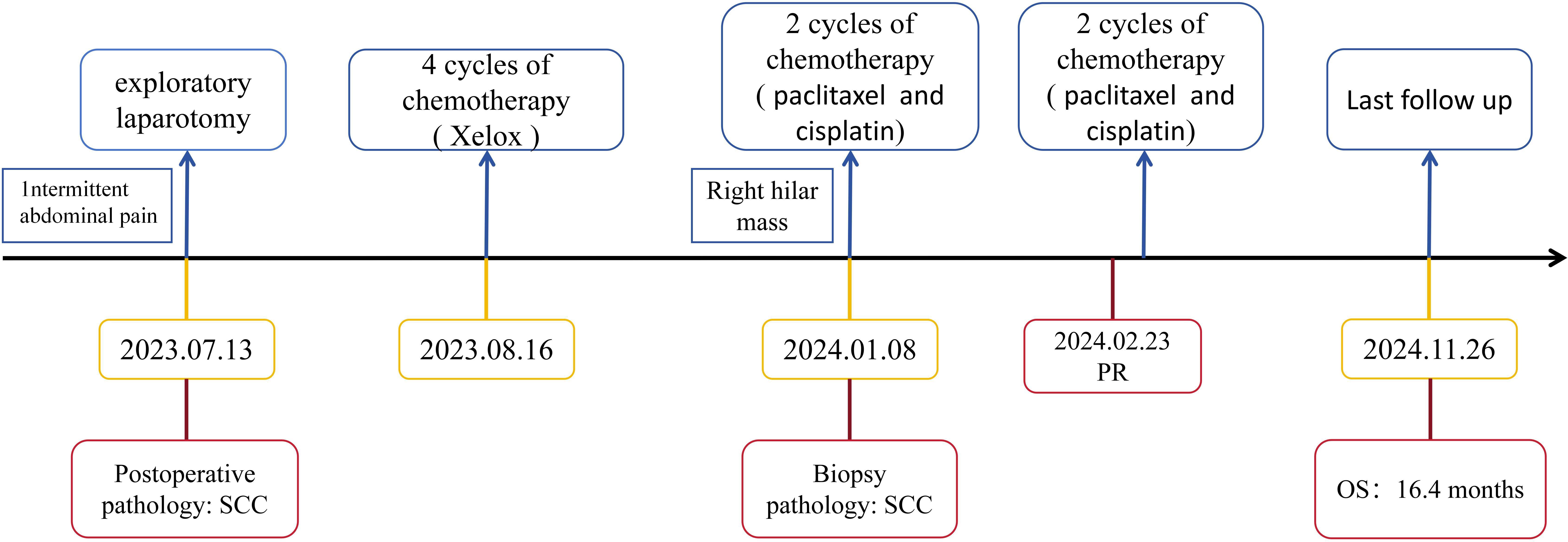
After considering the patient’s medical history, we attributed the lung hilar mass to metastatic squamous cell carcinoma (SCC) of ileal origin. Following four cycles of postoperative chemotherapy with the XELOX regimen, the patient’s condition progressed. Given the patient’s pathology, we sought a multidisciplinary team (MDT) discussion involving medical oncology, radiotherapy, thoracic surgery, pathology, imaging, and clinical nutrition. We adjusted the chemotherapy regimen to include paclitaxel (albumin-bound) (260 mg/m² on day 1 every 3 weeks) and cisplatin (80 mg/m² on day 1 every 3 weeks), starting on January 08, 2024. Throughout the chemotherapy, the patient experienced grade 1 fatigue and grade 2 bone marrow suppression, as defined by CTCAE 5.0, which both improved with symptomatic support. After two follow-up cycles, mediastinal lymph node shrinkage was observed. A Chest CT on February 23, 2024, revealed mediastinal lymph nodes measuring 18*21 mm (Figure 3). The treatment efficacy was assessed as partial remission according to Response Evaluation Criteria in Solid Tumors Version 1.1. After completing 4 cycles of chemotherapy, the patient underwent regular reviews. The last follow-up, on November 26, 2024, showed no significant progression or new metastatic lesions on chest CT. The patient’s overall survival stands at 16.4 months. Figure 4 illustrates the patient’s entire treatment course.
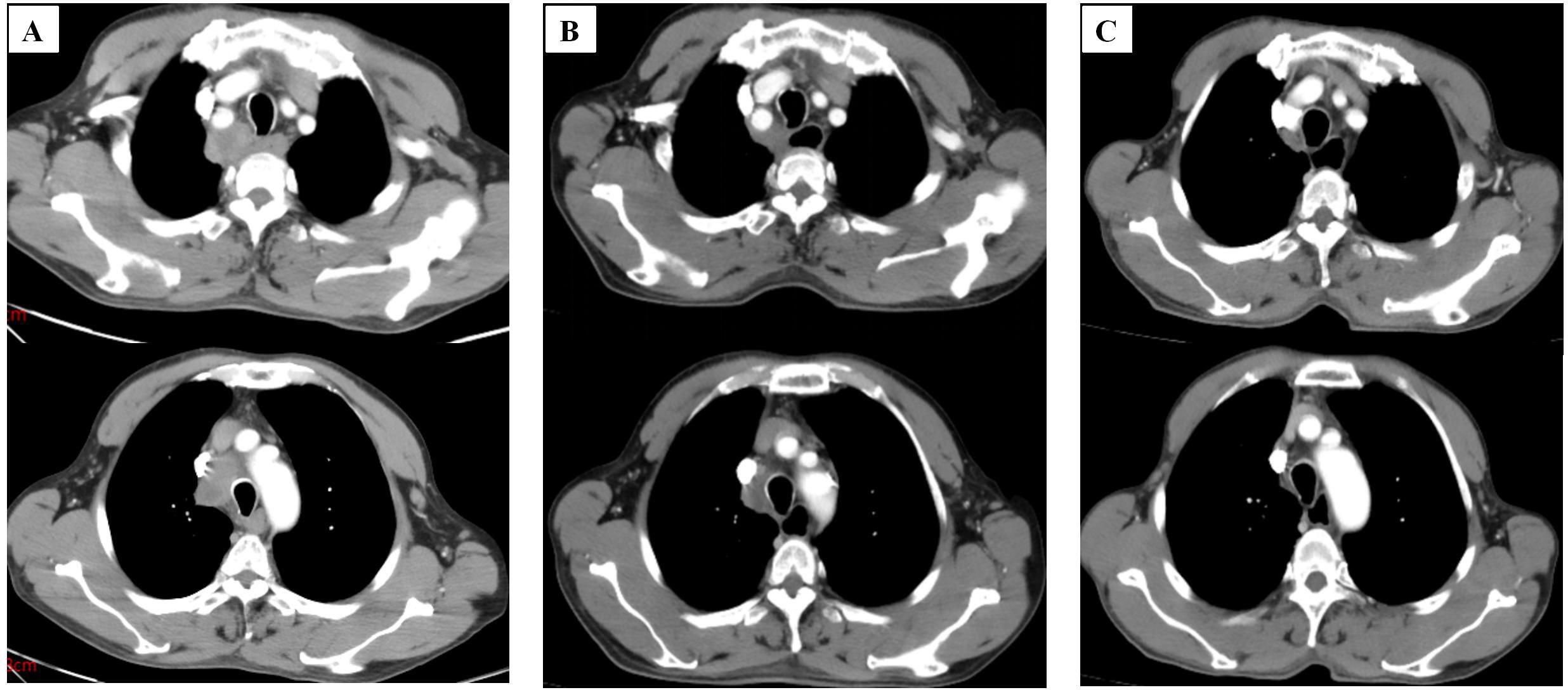
Figure 3. Computed tomography images of the patient. (A) Computed tomography image shows enlarged lymph nodes in the mediastinum before chemotherapy (January 04, 2024). (B) Computed tomography images of patients after 2 cycles of chemotherapy(February 23, 2024). (C) Computed tomography images of patients after 4 cycles of chemotherapy (April 8, 2024) (slice thickness: 5 mm; window width: 360; window level: 60; arterial phase).
Discussion
This case highlights two salient clinical observations: (1) The exceptional metastatic pattern of ileal SCC to mediastinal lymph nodes, and (2) Differential chemosensitivity between fluoropyrimidine/oxaliplatin and taxane/platinum regimens in SCC histology.
Small bowel cancers are relatively rare compared to tumors in other gastrointestinal organs. They comprise a heterogeneous group of approximately 40 histological subtypes, the most common being adenocarcinomas, neuroendocrine tumors, mesenchymal tumors, and lymphomas, with SCCs exceptionally uncommon (2). In this case, HE staining confirmed SCC, and immunohistochemical analysis (CK7(-)) ruled out adenocarcinoma, while CK5/6(+), P63(+), and P40(+) supported the diagnosis of SCC. Small bowel SCC pathogenesis remains enigmatic, with proposed mechanisms including squamous metaplasia of adenomatous tissue or ectopic squamous epithelium (4). Our literature synthesis identified nine jejunoileal SCC cases (5–13) (Table 1), demonstrating male predominance (6:4) and variable survival (1–55 months). Notably, only two cases exhibited nodal metastasis, underscoring our patient’s unique dissemination pattern.
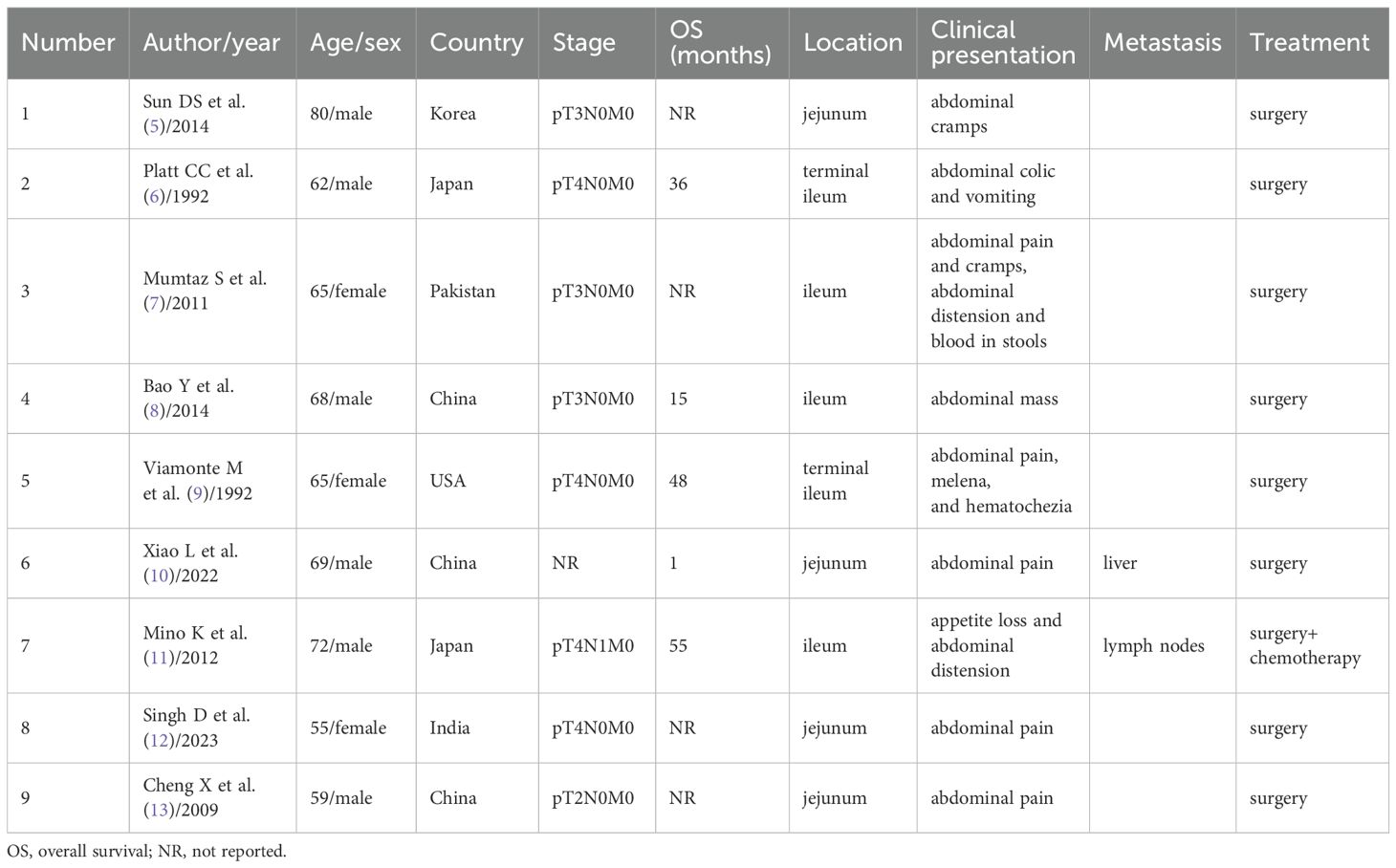
Table 1. Clinicopathologic features of primary jejunal and ileal squamous cell carcinoma reported in the literature.
Currently, only nine cases of colorectal cancer metastasizing solely to mediastinal lymph nodes without lung involvement have been reported in the English literature (14). The paradoxical mediastinal metastasis from lower abdominal malignancies challenges conventional lymphatic drainage paradigms. Proposed mechanisms include:1. Thoracic duct reflux secondary to lymphatic valve incompetence from tumor obstruction; 2. Aberrant trans diaphragmatic lymphatic connections; 3. Hematogenous seeding with nodal tropism. The El-Halabi et al.’s colorectal cancer case (15) with isolated mediastinal involvement parallels our observation, suggesting potential shared pathways in the retroperitoneal-to-mediastinal spread.
Therapeutic optimization for small bowel SCC remains undefined due to rarity. While NCCN guidelines recommend XELOX for intestinal adenocarcinomas, our experience and Mino et al.’s report (16) suggest superior SCC responsiveness to paclitaxel/cisplatin regimens. The paclitaxel/cisplatin is the most important component of lung SCC, esophageal SCC, and head and neck SCC recommended chemotherapy regimens in the NCCN guidelines (17–19). This highlights the imperative for histology-driven therapy in gastrointestinal malignancies.
Conclusion
This case expands the clinicopathological spectrum of small bowel SCC, emphasizing the diagnostic challenge of atypical metastatic presentations, the critical role of immunohistochemical profiling in tumor characterization, and the potential superiority of taxane-based regimens in SCC management. Further molecular characterization is warranted to establish evidence-based protocols for this orphan disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Liaocheng People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Conceptualization, Investigation, Resources, Writing – original draft. YY: Data curation, Writing – original draft. ZZ: Conceptualization, Data curation, Investigation, Resources, Writing – original draft. HL: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Shandong Province Medicine and Health Science and Technology Project (Health Care Project) (2023BJ000049).
Acknowledgments
We would like to thank the patient for his help in the process of writing and submitting the case. We are grateful to all the doctors and nurses who have taken care of the patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Vlachou E, Koffas A, Toumpanakis C, and Keuchel M. Updates in the diagnosis and management of small-bowel tumors. Best Pract Res Clin Gastroenterol. (2023) 64-65:101860. doi: 10.1016/j.bpg.2023.101860
3. Chiorean EG, Chiaro MD, Tempero MA, Malafa MP, Benson AB, Cardin DB, et al. Ampullary adenocarcinoma, version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2023) 21(7):753–82. doi: 10.6004/jnccn.2023.0034
4. Wang FD, Wang ZW, Xue HD, Wu HW, Zhang Y, Yu JC, et al. Primary squamous cell carcinoma of the small intestine: pathogenesis and clinical features. Chin Med J (Engl). (2016) 129(17):2131–3. doi: 10.4103/0366-6999.189067
5. Sun DS, Shin OR, Ku YM, Kim YS, and Seo KJ. Squamous cell carcinoma of the small bowel manifesting as a jejunal perforation: a case report. Int J Clin Exp Pathol. (2014) 7:6345–9. doi: 10.23880/mjccs-16000354
6. Platt CC, Haboubi NY, and Schofield PF. Primary squamous cell carcinoma of the terminal ileum. J Clin Pathol. (1991) 44:253–4. doi: 10.1136/jcp.44.3.253
7. Mumtaz S, Ahmad Z, Fatima S, and Qureshi A. Squamous cell carcinoma in the small intestine. BMJ Case Rep. (2011) 2011:bcr0120113762. doi: 10.1136/bcr.01.2011.3762
8. Bao Y, Zhong ZX, and Yu YW. Squamous cell carcinoma of small intestine: a case report. Chin Med Sci J. (2014) 29:239–41. doi: 10.1016/s1001-9294(14)60078-x
9. Viamonte M and Viamonte M. Primary squamous-cell carcinoma of the small bowel. Report of a case. Dis Colon Rectum. (1992) 35:806–9. doi: 10.1007/BF02050334
10. Xiao L, Sun L, Zhang JX, and Pan YS. Rare squamous cell carcinoma of the jejunum causing perforated peritonitis: A case report. World J Gastrointest Oncol. (2022) 14:2295–301. doi: 10.4251/wjgo.v14.i11.2295
11. Mino K, Kamii N, Kawanishi N, Okada T, and Todo S. Recurrence of primary squamous cell carcinoma of the ileum diagnosed by elevation of serum SCC: report of a case. Clin J Gastroenterol. (2012) 5:239–44. doi: 10.1007/s12328-012-0309-2
12. Singh D, Kumar A, Gowda V, Sahai R, Kumar S, and Kishore S. An adding up of an exceptionally rare case report: Primary Squamous cell carcinoma of Jejunum from North India. J Cancer Res Ther. (2023) 19:S451–3. doi: 10.4103/jcrt.JCRT_1333_20
13. Cheng X and Wang ZG. Primary squamous carcinoma of intestine: report of a case. Zhonghua Bing Li Xue Za Zhi. (2009) 38:350–1. doi: 10.3760/cma.j.issn.0529-5807.2009.05.018
14. Watanabe Y, Suzuki R, Kinoshita M, and Hirota M. Solitary anterior mediastinal lymph node metastasis with pericardial invasion from colon cancer: A case report. Mol Clin Oncol. (2022) 17:128. doi: 10.3892/mco.2022.2561
15. El-Halabi MM, Chaaban SA, Meouchy J, Page S, and Salyers WJ Jr. Colon cancer metastasis to mediastinal lymph nodes without liver or lung involvement: A case report. Oncol Lett. (2014) 8:2221–4. doi: 10.3892/ol.2014.2426
16. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Small bowel adenocarcinoma, version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2019) 17(9):1109–33. doi: 10.6004/jnccn.2019.0043
17. Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-small cell lung cancer, version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2024) 22(4):249–74. doi: 10.6004/jnccn.2204.0023
18. Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2023) 21(4):393–422. doi: 10.6004/jnccn.2023.0019
Keywords: squamous cell carcinoma, mediastinal lymph nodes, ileum, case report, metastasis
Citation: Zhang Y, Yue Y, Zhao Z and Liu H (2025) Metastatic squamous cell carcinoma of mediastinal lymph nodes from the ileum: a case report and literature review. Front. Oncol. 15:1579029. doi: 10.3389/fonc.2025.1579029
Received: 18 February 2025; Accepted: 08 July 2025;
Published: 29 July 2025.
Edited by:
Yunjie Lu, The First Affiliated Hospital of Soochow University, ChinaReviewed by:
Xiaotao Geng, Weifang People’s Hospital, ChinaPeng Zhang, Hubei University of Medicine, China
Copyright © 2025 Zhang, Yue, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Liu, bGNsaGYyMDE4QDE2My5jb20=
 Yanan Zhang1
Yanan Zhang1 Haifeng Liu
Haifeng Liu