- 1Department of Obstetrics and Gynecology, People’s Hospital of Leshan, Leshan, China
- 2Department of Proctology, People’s Hospital of Leshan, Leshan, China
- 3Department of Pathology, People’s Hospital of Leshan, Leshan, China
- 4Department of Traditional Chinese Medicine, People’s Hospital of Leshan, Leshan, China
Human papillomavirus (HPV) can cause tumors at specific anatomical sites in human body. Herein, we report the case of a 73-year-old female patient with a rare presentation of three distinct HPV-associated squamous cell carcinomas (anal, vulvar, and cervical), who had undergone kidney transplantation due to renal failure and was administered long-term cyclosporine, mycophenolate mofetil, and prednisone acetate for 20 years. Evidence of HPV16/59 infection was detected in Evidence of HPV16/59 infection was detected in formalin-fixed paraffin-embedded (FFPE) tissue samples obtained from the patient’s vulvar tumor and anal tumor via PCR-based assays, as well as from exfoliated cells of the cervix. This case confirms the potential of HPV to drive carcinogenesis across multiple ano-genital sites within the same patient. Immunosuppression in transplant patients significantly increases the risk of human papillomavirus (HPV)- associated malignancies; however, the synchronous or metachronous development of triple anogenital cancers in a single individual is exceptionally uncommon. This case highlights the aggressive potential of HPV oncogenesis in immunocompromised hosts and underscores the need for rigorous cancer surveillance in this population.
1 Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections. Persistent HPV infection can cause tumors at specific anatomical sites in the human body (1). According to published statistics, over 99% of cervical cancer cases are caused by HPV infection (2). Additionally, HPV can causes cancers of the external genitalia, vagina, anus, and oropharynx (3–7). The HPV virus can integrate into the host cell genome, induce mutations and alter gene expression patterns via E6/E7 gene expression, thereby affecting the integrity and expression of proteins involved in the progression of cancer (8). Integration of the HPV viral genome depends on the type of HPV and infected epithelium; of all sub-types, HPV type 16 is most likely to integrate into the host’s DNA (9). Herein, we report the case of an elderly female, post-renal transplant patient on long-term immunosuppressants, who was diagnosed with vulvar verrucous carcinoma, cervical cancer, and anal cancer. modern diagnostic and therapeutic procedures revealed evidence of HPV infection in all three lesions. The patient’s immunosuppressed state led to a lack of effective antiviral immune response (10), therefore increased the risk of persistent HPV infection and the incidence of HPV-associated cancers (11, 12). This may be the first case report of tumors in multiple regions within the same individual caused by HPV. This case highlights the aggressive potential of HPV oncogenesis in immunocompromised hosts and underscores the need for rigorous HPV infection and cancer surveillance in this population.
2 Case presentation
2.1 Relevant past interventions
In 2012, a 73-year-old female patient discovered a pea-sized vulvar tumor, that partially protruded from the skin, but was not associated with any symptoms such as redness, swelling, pain, itching, or ulceration. Laser therapy and cryo-therapy were performed on the tumor in 2012 and 2015 respectively. In 2016, the tumor recurred at the primary site, with a similar size and appearance as the initial presentation, but was not treated. In July 2021, the tumor had gradually proliferated, protruded outwards, and exhibited a faster growth rate. The patient purchased and used self-prescribed topical medication; however, the exact name of the medication could not be ascertained. There was no notable improvement in her symptoms, and the tumor surface gradually became ulcerated with minor bleeding; therefore, the patient visited our hospital on January 4, 2022.
2.2 Physical examination
Physical examination detected an irregular-shaped tumor on the left side of the external genitalia. The tumor measured 5 * 4cm in its widest diameter, and protruded considerably from the skin. Some parts of the tumor were covered by brown keratinous crusts, while other parts were covered by yellow or white keratinous tissues, with an incomplete epidermis. The color of the surrounding skin was normal, and there was no obvious lymphadenopathy in the groin.
During physical examination, we found a tumor in the anal region, that was not continuous with the vulvar tumor. The anal tumor was around the anus orifice and was brown in color, with cauliflower like changes, unclear boundaries, and surface erosion, accompanied by circular protrusion of hemorrhoids, and subcutaneous varicose veins. The anal tumor was hard and brittle, with poor mobility.
2.3 Treatment
On January 7, 2022, the patient underwent a wide local excision of the skin tumor on the vulva under general anesthesia. Postoperative pathological examination revealed verrucous carcinoma of the vulvar tumor (Figure 1). On January 10, 2022, a biopsy of the anal tumor was also performed, and histopathological evaluation revealed severe squamous epithelial hyperplasia and malignant transformation, accompanied by incomplete keratinization and hyperkeratosis. Therefore, on January 20, 2022, the anal tumor was successfully resected. Postoperative pathological examination suggested highly differentiated squamous cell carcinoma (Figure 2). On February 21, 2022, postoperative anal cancer radiotherapy was performed, followed by field reduction treatment. Due to a history of kidney transplantation and multiple underlying diseases, the patient and her family decided to abandon chemotherapy and undergo regular follow-up.
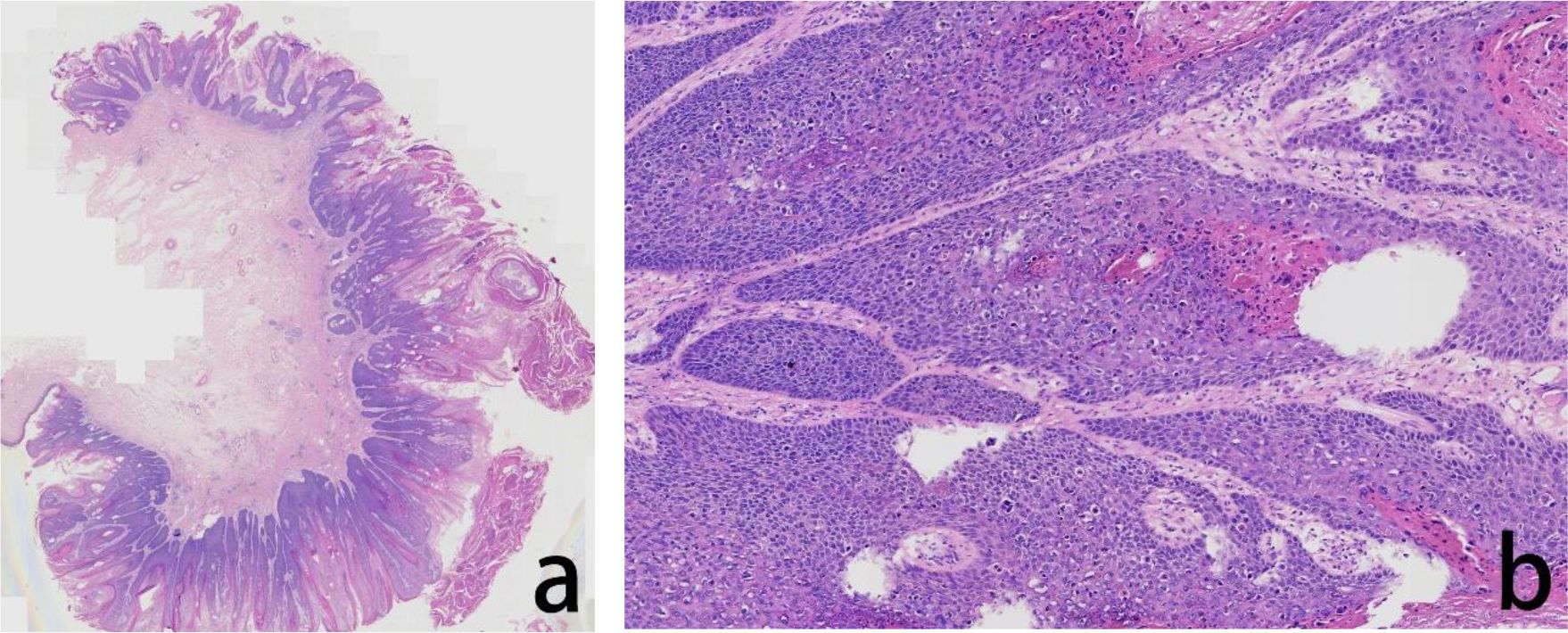
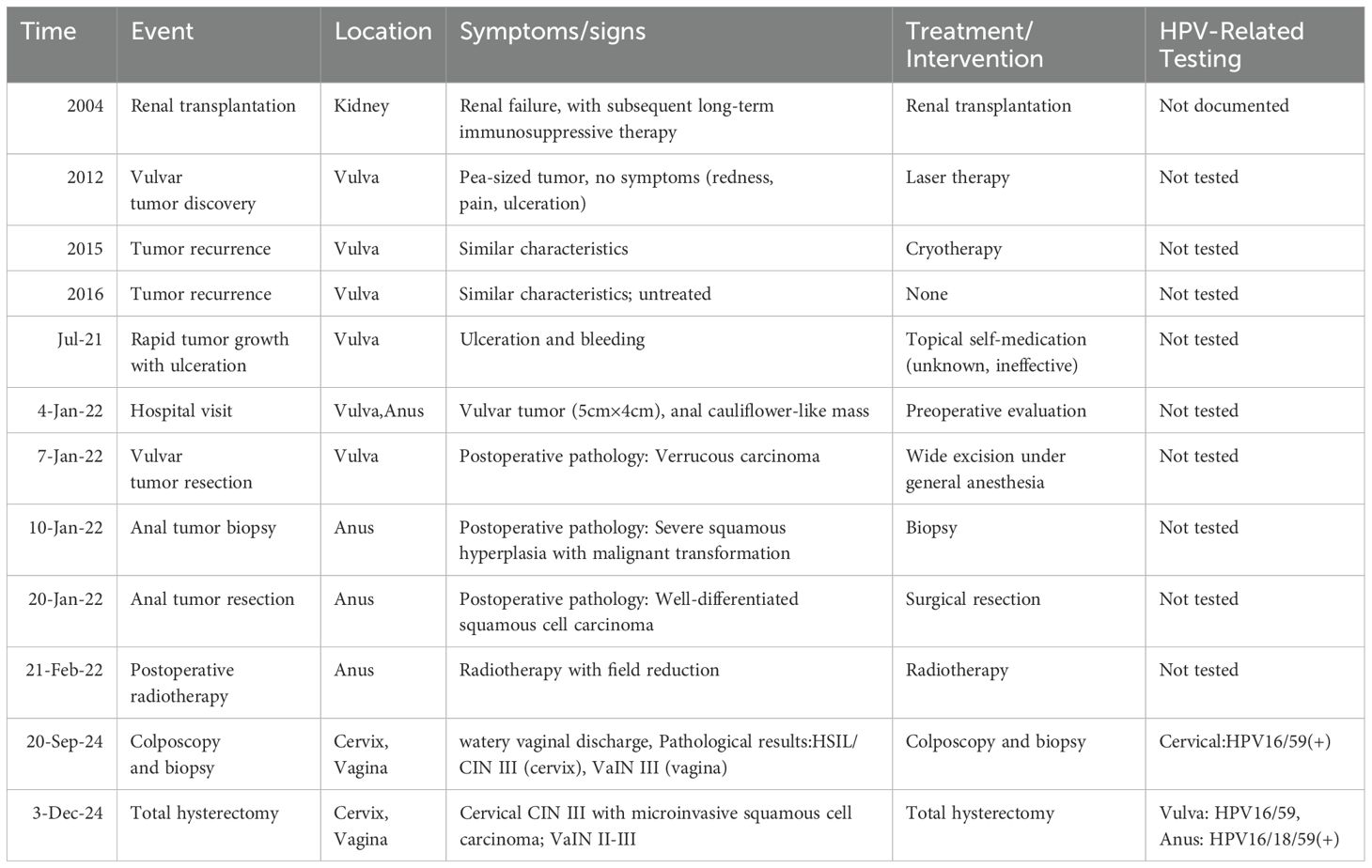
Figure 1. Assessment of the H&E stained vulvar tumor FFPE tissue. The histopathological evaluation in lower magnification, seen in the left panel, revealed exophytic, verrucous growth pattern with prominent hyperkeratosis, parakeratosis, and a ‘pushing’ border (a). In higher magnification, in the right panel, the tumor cells showed atypia, fragmented nuclei, and abnormal keratinization, hollowed out cells were visible (b).
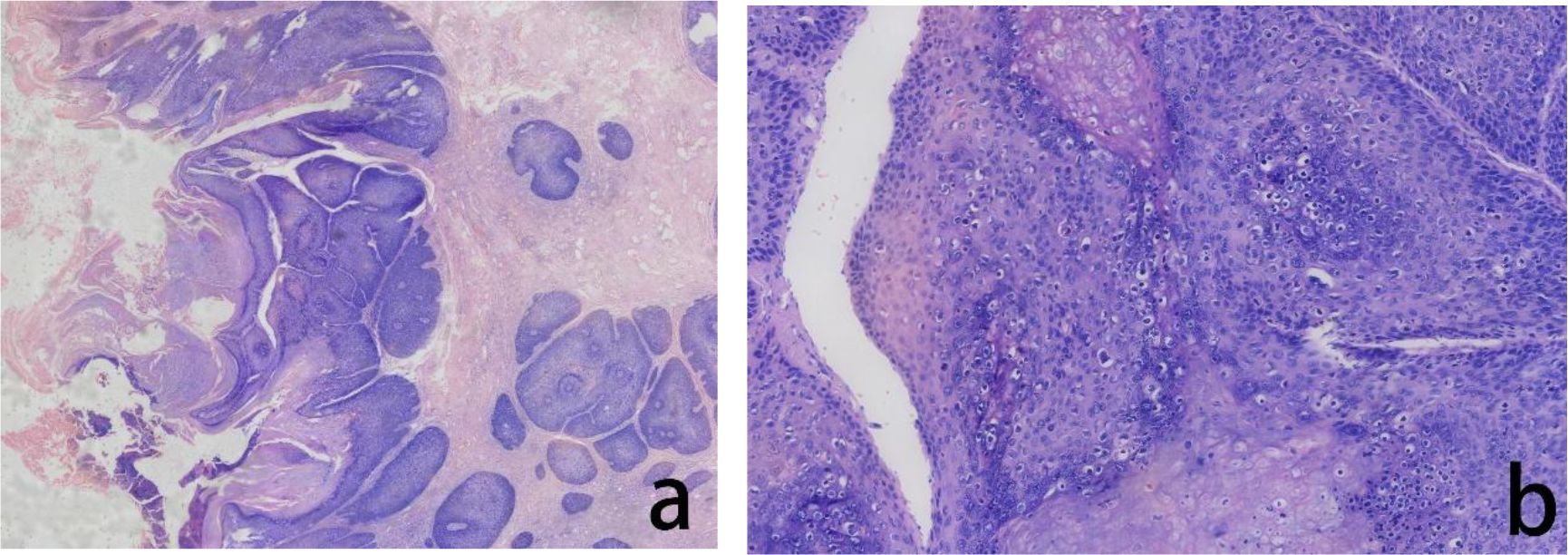
Figure 2. Assessment of the H&E stained anal tumor FFPE tissue. The histopathological evaluation in lower magnification, seen in the left panel, revealed severe squamous epithelial hyperplasia and malignant transformation, accompanied by incomplete keratinization and hyperkeratosis (a). In higher magnification, in the right panel, the tumor cells showed cellular atypia with increased nucleocytoplasmic ratio and visible hollowed out cells (b).
2.4 Follow-up and new lesions
Patient was followed up regularly, no tumors recurrence were detected, and no complications such as anal stenosis occurred at follow-up 2 years postoperatively. On September 20, 2024, the patient visited the gynecology department with a complain of watery vaginal discharge. Physical examination revealed congestion of the vaginal wall and cervix, and, significant contact bleeding from the vaginal wall, PCR-based assays of exfoliated cells of the cervix indicated HPV16/59 (+), Furthermore, liquid-based cytology indicated atypical squamous cells of undetermined significance (ASC-US). At our suggestion, the patients underwent cervical biopsy under colposcopy, which indicated high-grade squamous intraepithelial lesion (HSIL)/cervical intraepithelial neoplasia (CIN) III, but more severe lesions could not be ruled out (Figure 3). Biopsy of the vaginal wall indicated a vaginal intraepithelial neoplasia (VaIN) III (Figure 4). We explained two treatment options for the patient: Cervical Conization and Total Hysterectomy, while the patient requested treatment at a higher-level hospital and was appropriately referred. On December 3, 2024, the patient underwent a total hysterectomy at another hospital. Postoperative pathological examination revealed widespread cervical CIN III involving glandular tissue, localized microinvasive squamous cell carcinoma in the interstitium, and vaginal wall VaIN II-III. The patient is still on regular follow-up.
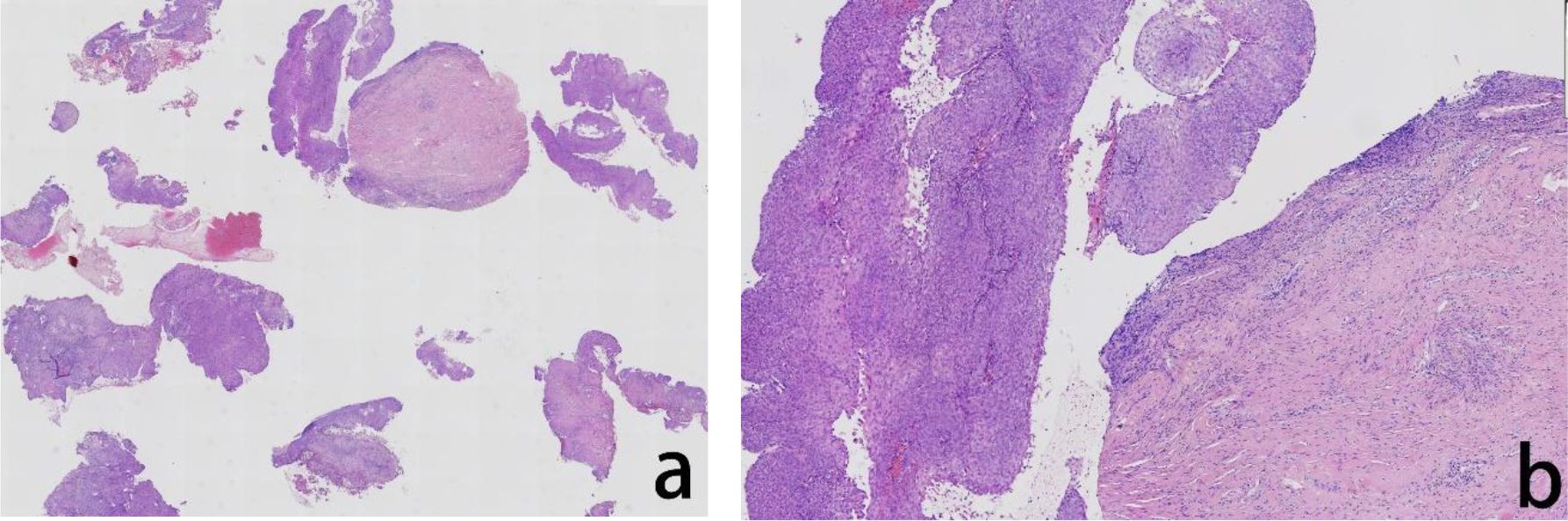
Figure 3. Assessment of the H&E stained cervical biopsied tissue The histopathological evaluation in lower magnification, seen in the left panel, revealed exophytic papillary growth and visible hollowed out cells, indicated high-grade squamous intraepithelial lesion (HSIL) (a) In higher magnification, in the right panel, tumor cells showed nuclear division and hollowed out cells were visible (b).
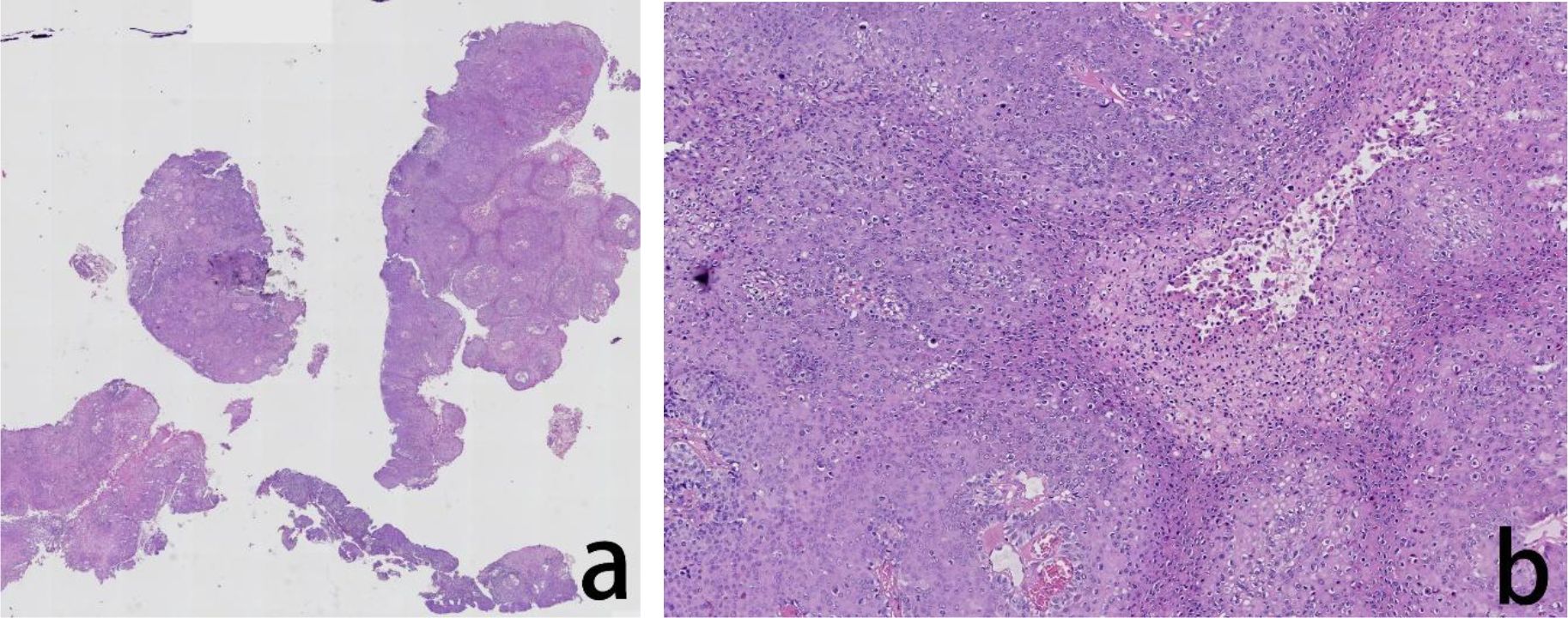
Figure 4. Assessment of the H&E stained vaginal biopsied tissue. The histopathological evaluation in lower magnification, seen in the left panel, revealed exophytic papillary growth and visible hollowed out cells, indicated cervical intraepithelial neoplasia (CIN) III (a) In higher magnification, in the right panel, tumor cells showed nuclear division and hollowed out cells were visible (b).
2.5 Medical history
In 2004, the patient underwent renal transplantation for renal failure at another hospital and was subsequently placed on long-term immunosuppressive therapy, including oral cyclosporine mycophenolate mofetil, and prednisone acetate. In 2014, the patient was diagnosed with hepatitis B virus (HBV) infection (10 years ago) and was prescribed long-term entecavir therapy. In April 2021, the patient developed herpes zoster on her the right lower extremity. In May 2021, she was diagnosed with immunocompromised host-associated pneumonia.
2.6 Further examination for HPV
PCR-based assays identified types 16 and 59 HPV in the patient’s exfoliated cervical cells. The FFPE tissue samples prepared from postoperative tumor tissue obtained from the patient’s vulva and anus were sent to a higher-level hospital wherein situ hybridization (RNA scope) testing was performed. Under light microscopy, positive spot and patchy signals were detected in tumor cells from the vulva and anus. Analysis of both sites confirmed the presence of transcriptionally active HPV at both locations. In Additionally, we conducted HPV typing tests (including 14 high-risk HPV types and 9 low-risk HPV types) on (FFPE) tissue samples prepared from two locations at a higher-level hospital, using polymerase chain reaction reverse dot hybridization as the detection method. The analysis revealed that the vulvar tissue was positive for HPV types 16 and 59, while the anal tissue was positive for HPV types 16,18, and 59. Case timeline and key data were described in detail in Table 1.
3 Discussion
Vulvar verrucous carcinoma is a unique, highly differentiated, low-grade malignant squamous cell carcinoma, that is generally considered as a rare subtype of squamous cell carcinoma unrelated to HPV infection (13, 14). However, some studies suggest that this condition may be related to type 6, 11, 16, and 18 of HPV. Evidence for HPV infection has been detected in some cases of vulvar verrucous carcinoma, especially in immunocompromised populations. For example, Rando R F et al. detected type 6 HPV in tissue from a patient with vulvar verrucous carcinoma (15), Cuesta K H et al. tested six cases of HIV- positive vulvar verrucous carcinoma; five patients were positive for HPV types 6, 11, 16, and 18 in the cancerous tissue (16). In our present case, we detected HPV types 16 and 59 in the vulvar tissue; these findings were consistent with previous literature. Our patient also had anal cancer; almost all cases of anal squamous cell carcinoma are caused by HPV and progresses from highly squamous intraepithelial lesions. The International Society of Anal Oncology has recommended high-risk HPV for the screening anal cancer (17). The risk factors for anal cancer include HPV infection, a history of anal or sexual intercourse; a history of cervical, vulvar, or vaginal cancer; immune suppression following solid organ transplantation or HIV infection; hematological malignancies; certain autoimmune diseases, and smoking (18). Our patient had multiple risk factors, including HPV infection, vulvar cancer, and a history of solid organ transplantation.
High-risk HPV testing has been validated alone as a primary screening method for cervical cancer detection. The US Health and Human Services recommends that for immunocompromised populations, the strategy for cervical cancer screening should comply with the screening strategy for patients with the human immunodeficiency virus (HIV) in that the interval between cervical cancer screenings should be reduced (19, 20), and vulvar inspection at the time of cervical cancer screening is also very necessary (21). The International Society for Anal Oncology (ISAO) endorses high-risk HPV testing as a standalone screening modality for anal cancer. For organ transplant recipients, it is recommended to commence screening for anal cancer 10 years after transplantation (17). A study from Australia showed that large reductions in cervical cancer incidence have been observed following the cytological and HPV testing programs (22). A cross-sectional study in kidney transplant recipients indicated that High-risk HPV and HSIL testing may identify kidney transplant recipients at higher risk of anal cancer (23). Unfortunately, our patient did not receive regular HPV testing from an early timepoint; thus, we are unable to determine when the patient was first infected with the HPV virus. If regular cervical and anal HPV testing can be implemented even earlier after kidney transplantation following the detection of anal and vulvar verrucous cancer, with regular review and follow-up, this practice may significantly reduce the probability of the occurrence of anal, cervical and vulvar cancers.
Currently, the HPV vaccine has been shown to be very effective in clinical trials and has been widely promoted and applied in China. A 10-year study conducted in Sweden and involving 1.7 million women aged between 10 and to 30 years, revealed that when compared to women who did not receive the HPV vaccine, vaccination before the age of 17 years reduced the incidence of cervical cancer by 88%. However, vaccination between the ages of 17 and 30 years reduced the incidence of cervical cancer by approximately 50% (24). Okunade K S reported that the HPV vaccine may reduce the incidence of vulvar cancer by approximately 50% and intraepithelial lesions of the lower genital tract by at least two-thirds (25). In view of the high HPV infection rate, and the incidence of anal and genital cancer in kidney transplant recipients, Chin-Hong, Peter V suggested that vaccination should be carried out before kidney transplantation in recipients of suitable age (26).
Immunosuppression is a high-risk factor for HPV infection and persistent infection, considerably increasing the incidence of anal, genital, and oral cancers in immunocompromised patients (27). A previous meta-analysis of patients undergoing large-scale organ transplantation revealed that the standardized incidence rate of HPV-related anal and genital cancers was elevated in these patients (28). It has been suggested that one of the mechanisms by which HPV causes cervical lesions in immunosuppressed patients is the immune system’s inability to eradicate the virus, leading to its persistence. If this hypothesis is correct, immunosuppressed individuals exposed to carcinogenic viruses would have an increased risk of persistent HPV infection and tumors development (29). A meta-analysis shows that Immunosuppression permits uncontrolled replication of oncogenic HPV subtypes (e.g., HPV16/18), which disrupt tumor suppressor pathways (p53/Rb) via E6/E7 proteins. Coinfection with multiple HPV subtypes may accelerate multifocal lesions (30). Our patient had a history of kidney transplantation, had been taking immunosuppressants and glucocorticoids for an extended period, and also had hepatitis B, requiring long-term antiviral treatment. Furthermore, as an elderly female with immunosuppression, she was at high- risk of persistent HPV infection and tumor development.
The findings of this study derived from a single patient case, which inherently restricts the ability to extrapolate conclusions to broader populations. The unique interplay of host factors, immunosuppressive regimens, and HPV-related oncogenesis in this case may not fully capture the heterogeneity observed in other post-transplant patients. While the clinical management and short-term outcomes are described, the absence of extended follow-up limits insights into long-term recurrence risks, immune reconstitution effects, or secondary malignancies. This gap hinders a comprehensive understanding of the dynamic relationship between sustained immunosuppression and HPV-driven carcinogenesis.
4 Conclusion
In summary, our experience with this case indicates that HPV can cause multiple HPV-related tumors in the anogenital region in the single individual, in immunocompromised populations. Thus, in this population, when diagnosing an HPV-related tumor, it is imperative to remain vigilant for other potential HPV- related tumors. HPV testing and follow-up should begin as early as possible, with timely intervention when lesions are detected. Additionally, promoting HPV vaccines is of great importance. These strategies may have important implications for reducing the incidence of HPV related tumors in such populations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Leshan People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XF: Writing – original draft, Investigation. Q-BS: Writing – original draft. KY: Writing – review & editing, Investigation. YM: Writing – review & editing. XW: Writing – review & editing. A-PM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to acknowledge pathology department of West China women’s and children’s Hospital for they help in providing assistance in pathological examination.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Scott-Wittenborn N and Fakhry C. Epidemiology of HPV related Malignancies. Semin Radiat Oncol. (2021) 31:286–96. doi: 10.1016/j.semradonc.2021.04.001
2. Monsonego J. Global challenges of cervical cancer prevention. Clin Exp Obstet Gynecol. (2001) 28:5–13.
3. Sinno AK, Saraiya M, Thompson TD, Hernandez BY, Goodman MT, Steinau M, et al. Human papillomavirus genotype prevalence in invasive vaginal cancer from a registry-based population. Obstet Gynecol. (2014) 123:817–21. doi: 10.1097/AOG.0000000000000171
4. Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, et al. Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Elsevier. (2019) 20:145–58. doi: 10.1016/S1470-2045(18)30682-X
5. Villa A and Hanna GJ. Human papillomavirus and oropharyngeal cancer. Curr Probl Cancer. (2018) 62:466–75. doi: 10.1016/j.currproblcancer.2018.06.005
6. Cruz CMDV, Cruz L M M D V, Pinheiro JDJV, Aragao JIVD, Pinheiro HHC, Fuzii HT, et al. prevalence of human papillomavirus in laryngeal cancer specimens. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 129:e179. doi: 10.1016/j.oooo.2019.06.758
7. Beckmann AM, Daling JR, Sherman KJ, Maden C, Miller BA, Coates RJ, et al. Human papillomavirus infection and anal cancer. Int J Cancer. (1989) 43:1042–9. doi: 10.1002/ijc.2910430615
8. Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Rosshandler L, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. (2014) 506:371–5. doi: 10.1038/nature12881
9. Catalán-Castorena O, Garibay-Cerdenares OL, Illades-Aguiar B, Rodríguez-Ruiz HA, ZubillagaGuerrero M, Leyva-Vázquez MA, et al. The role of HR-HPV integration in the progression of premalignant lesions into different cancer types. Heliyon. (2024) . 10:e34999. doi: 10.1016/j.heliyon.2024.e34999
10. Viac J, Chardonnet Y, Euvrard S, Chignol MC, and Thivolet J. Langerhans cells, inflammation markers and human papillomavirus infections in benign and Malignant epithelial tumors from transplant recipients. J Dermatol. (1992) 19:67–77. doi: 10.1111/j.1346-8138.1992.tb03183.x
11. Hewavisenti RV, Arena J, Ahlenstiel CL, and Sasson SC. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front Immunol. (2023) .137:1112513. doi: 10.3389/fimmu.2023.1112513
12. Eleutério J, Cavalcante LR, Gonçalves AKS, Eleutério RMN, and Giraldo PC. Prevalence of high-risk HPV and atypia in liquid-based cytology of cervical and intra-anal specimens from kidney-transplanted women. Diagn Cytopathol. (2019) 47:783–7. doi: 10.1002/dc.24180
13. Pilotti S, Donghi R, D'Amato L, Giarola M, Longoni A, Della TG, et al. HPV detection and p53 alteration in squamous cell verrucous Malignancies of the lower genital tract. Diagn Mol Pathol. (1993) 2:248–56. doi: 10.1097/00019606-199312000-00004
14. Akbari A, Pinto A, Amemiya Y, Seth A, Mirkovic J, Parra-Herran C, et al. Differentiated exophytic vulvar intraepithelial lesion: Clinicopathologic and molecular analysis documenting its relationship with verrucous carcinoma of the vulva. Mod Pathol. (2020) 33:2011–8. doi: 10.1038/s41379-020-0573-5
15. Rando RF, Sedlacek TV, Hunt J, Jenson AB, Kurman RJ, Lancaster WD, et al. Verrucous carcinoma of the vulva associated with an unusual type 6 human papillomavirus. Obstet Gynecol. (1986) 67:70S–5S. doi: 10.1097/00006250-198603001-00021
16. Cuesta KH, Palazzo JP, and Mittal KR. Detection of human papillomavirus in verrucous carcinoma from HIV-seropositive patients. J Cutan Pathol. (2010) 25:165–70. doi: 10.1111/j.1600-0560.1998.tb01710.x
17. Stier EA, Clarke MA, Deshmukh, Wentzensen N, Liu YX, Poynten MI, et al. International Anal Neoplasia Society's consensus guidelines for anal cancer screening. Int J Cancer. (2024) 154:1694–702. doi: 10.1002/ijc.v154.10
18. Engstrom PF, Arnoletti JP, Benson AB, Berlin JD, Berry JM, Chen YJ, et al. NCCN clinical practice guidelines in oncology. Anal Carcinoma J Natl Compr Cancer Netw. (2010) 8:106–20. doi: 10.6004/jnccn.2010.0007
19. US Department of Health and Human Services. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV [EB/OL. 2019-11-25]. Available online at: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/343/human-papillomavirus (Accessed June 21, 2025).
20. Moscicki AB, Flowers L, Huchko MJ, Long ME, MacLaughlin KL, Murphy J, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Genit Tract Dis. (2019) 29:168–79. doi: 10.1097/LGT.0000000000000866
21. Preti M, Lewis F, Carcopino X, Bevilacqua F, Ellis LB, Halonen P, et al. Vulvar inspection at the time of cervical cancer screening: European Society of Gynaecological Oncology (ESGO), International Society for the Study of Vulvovaginal Disease (ISSVD), European College for the Study of Vulval Disease (ECSVD), and European Federation for Colposcopy (EFC) consensus statements. Int J Gynecol Cancer. (2025) 35:100007. doi: 10.1016/j.ijgc.2024.100007
22. Lew JB, Simms KT, Smith MA, Hall M, Kang YJ, Xu XM, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health. (2017) 2:e96–e107. doi: 10.1016/S2468-2667(17)30007-5
23. Rosales BM, Langton-Lockton J, Hedley J, Cornall AM, Roberts JM, Garland SM, et al. Prevalence of anal cytological abnormalities and high-risk human papillomavirus prevalence in kidney transplant recipients: a cross-sectional study. Clin Transplant. (2021) 35:e14476. doi: 10.1111/ctr.v35.12
24. Printz C. Lower Cervical Cancer Risk Associated with HPV Vaccine. Cancer. (2021) 127(8):1171. doi: 10.1002/cncr.33577
25. Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynecol: J Institute Obstet Gynecol. (2019) 1:1–7. doi: 10.1080/01443615.2019.1634030
26. Chin-Hong PV. Human papillomavirus in kidney transplant recipients. Semin Nephrol. (2016) 36:397–404. doi: 10.1016/j.semnephrol.2016.05.016
27. Wieland U, Kreuter A, and Pfister H. Human papillomavirus and immunosuppression. Curr Probl Dermatol. (2014) 45:154–65. doi: 10.1159/000357907
28. Wan XL, Wang X, Feng ZP, Zhou XL, Han ZW, Wu JM, et al. Analysis of risk factors for intraoperative bleeding in the surgical treatment of cesarean scar pregnancy and development of predictive models. J Multidiscip Healthc. (2024) 17:2021–30. doi: 10.2147/JMDH.S458968
29. Downey G, Emery V, and Walker P. A longitudinal study of human papillomavirus 16 positivity in the development of lower genital intraepithelial neoplasia in immunosuppressed women. J Lower Genit Tract Dis. (1999) 3:163–70. doi: 10.1046/j.1526-0976.1999.08110.x
Keywords: human papillomavirus, kidney transplantation, vulvar verrucous cancer, anal cancer, cervical cancer
Citation: Feng X, Shen Q-b, Yuan K, Mao Y, Wang X and Min A-p (2025) Human papillomavirus-associated anal cancer, vulvar verrucous cancer, and cervical cancer in a post-renal transplant patient-a case report. Front. Oncol. 15:1579795. doi: 10.3389/fonc.2025.1579795
Received: 19 February 2025; Accepted: 16 June 2025;
Published: 04 July 2025.
Edited by:
Marcelo A. Soares, National Cancer Institute (INCA), BrazilReviewed by:
Yara Lucia Furtado, Federal University of Rio de Janeiro, BrazilKritika Srinivasan Rajsri, New York University, United States
Copyright © 2025 Feng, Shen, Yuan, Mao, Wang and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing-bo Shen, MTM3NjgxOTAyQHFxLmNvbQ==
 Xin Feng
Xin Feng Qing-bo Shen
Qing-bo Shen Ke Yuan
Ke Yuan Yu Mao
Yu Mao Xia Wang
Xia Wang Ai-ping Min
Ai-ping Min