- Department of Pediatrics, Blood and Marrow Transplantation and Cellular Therapy, King Hussein Cancer Center, Amman, Jordan
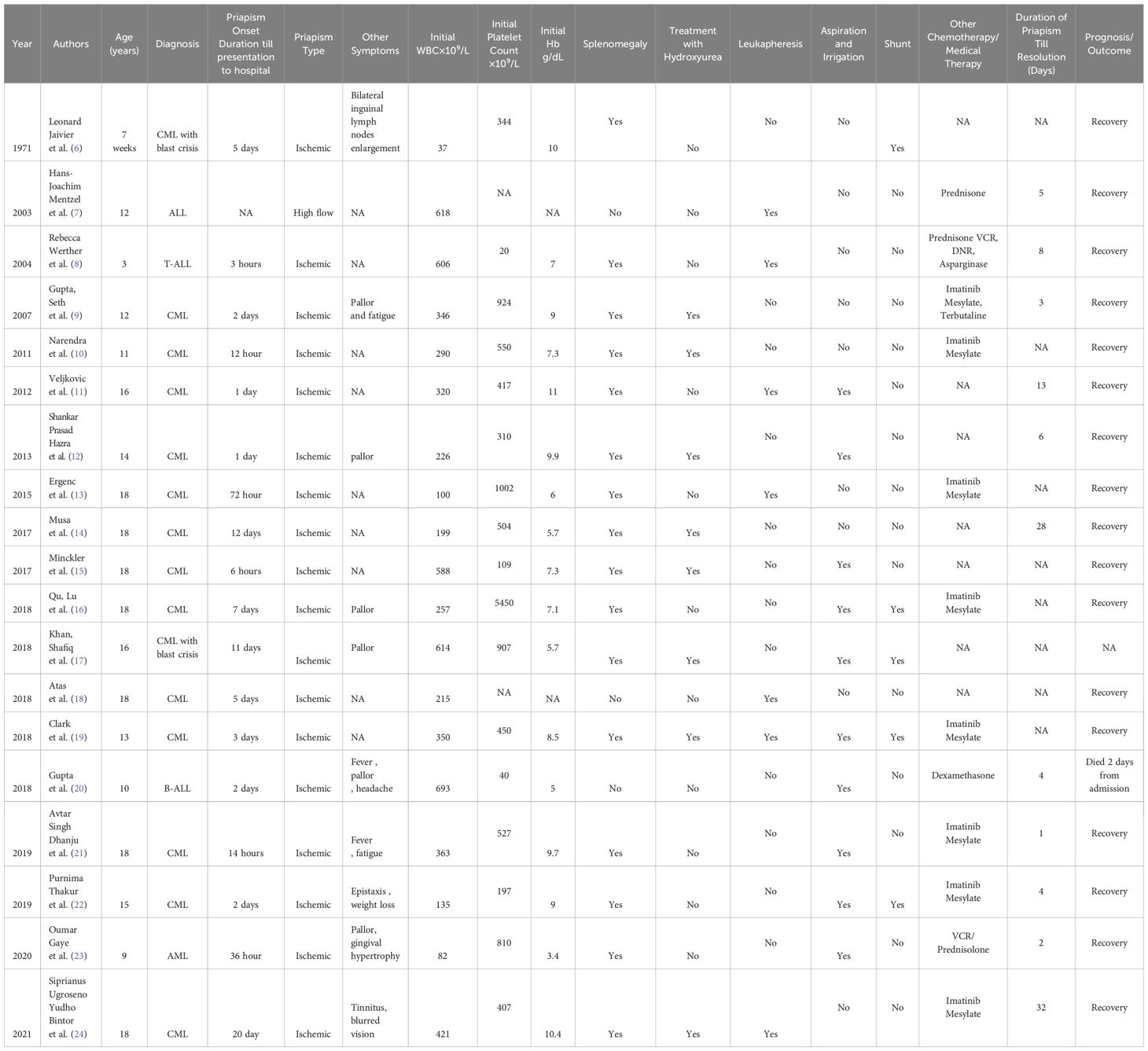
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by uncontrolled myeloid cell proliferation and is primarily caused by a reciprocal chromosomal translocation [t(9;22)(q34;q11.2)]. Typical manifestations of CML include nonspecific constitutional symptoms such as fatigue, weight loss, night sweats, and abdominal discomfort due to hepatosplenomegaly. Although priapism is a rare but recognized complication of CML, it more often occurs in adults than in children. This case report describes an 11-year-old patient who experienced persistent priapism and hyperleukocytosis and ultimately received a CML diagnosis. Priapism in pediatric CML is a serious medical emergency requiring prompt medical and surgical intervention to prevent long-term complications, including the loss of erectile function. A literature review identified 19 pediatric cases of priapism associated with leukemia, 15 of which were attributed to CML. The cases varied in clinical presentation, treatment approaches, and outcomes, with management often involving a combination of aspiration, irrigation, leukapheresis, and chemotherapy. In most cases, priapism was resolved with these interventions, but some required additional measures, including shunt surgery. This review emphasizes the importance of early recognition and intervention to prevent complications in children with CML-associated priapism.
Introduction
Chronic myeloid leukemia (CML) in childhood is uncommon, constituting 2–3% of leukemias in children under 15 years of age and 9% in adolescents 15–19 years of age. The hallmark of CML is the Philadelphia chromosome, which results from a translocation between chromosomes 9 and 22 (1). The BCR-ABL1 oncoprotein, which causes clonal expansion of affected hematopoietic cells, is central to the pathogenesis of CML (2). Children, especially in the chronic phase of CML, typically present with hyperleukocytosis, splenomegaly, and other general symptoms such as fatigue, weight loss, night sweats, left upper quadrant abdominal discomfort, and thrombosis or bleeding events (3). Priapism is a urological emergency defined as a prolonged pe erection persisting beyond 4 hours, irrespective of sexual arousal. This condition can arise from an imbalance in penile blood dynamics and is categorized as low-flow (ischemic) or high-flow (non-ischemic) (4). CML-associated priapism, particularly in pediatric patients, is rare but primarily ischemic due to a hyperleukocytosis-associated increase in blood viscosity (5).
Case report
A previously healthy 11-year-old male patient entered the emergency room with a painful, 3-day penile erection that was neither sexual nor trauma-induced. No similar episodes were encountered previously. He also experienced fever and night sweats for 1 week before hospital admission.
During the initial encounter in the emergency room, the patient was pale and in severe pain. A physical examination identified pallor and a severely swollen, erythematous, erect, and tender to touch penis (Figure 1). There were no signs of infection of the overlying scrotal or penile skin, and there was no abnormal urethral discharge or inguinal lymph node enlargement. The patient also had massive splenomegaly that reached the umbilicus.
Laboratory tests showed a hemoglobin level of 8.9 g/dL, a white blood cell (WBC) count of 360 × 103/µL (neutrophils 90.3%, lymphocytes 1.7%, basophils 1.9%, eosinophils 2.9%, platelets 932 × 103/µL), and lactate dehydrogenase of 1294 U/L. A blood film analysis identified leukocytosis with absolute basophilia, and serum chemistry was unremarkable.
The patient was admitted to the pediatric intensive care unit for further evaluation, symptom management, and observation. He received vigorous hydration, morphine sulfate continuous infusion, and IV antibiotics. He also received 20 mg/kg hydroxycarbamide and frequent monitoring of laboratory features. Cytogenetics showed that 94% of peripheral blood cells were positive for the BCR-ABL1 t(9;22)(q34;q11.2) rearrangement (Figure 2). Fluorescence in situ hybridization also identified the BCR-ABL1 t(9;22)(q34;q11.2) rearrangement. Flow cytometry analysis identified myeloblasts that were positive for CD34, CD117, and HLA-DR.
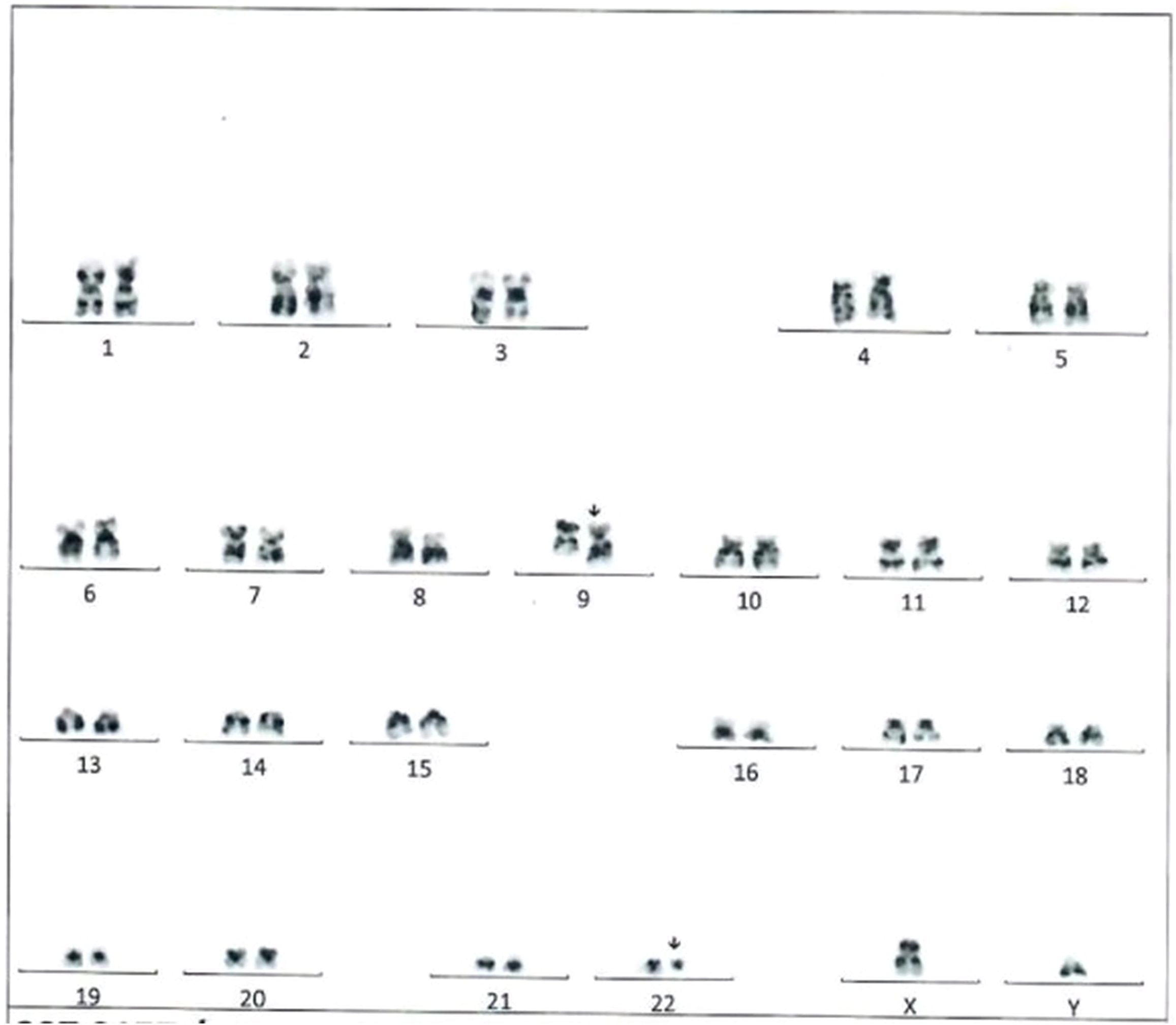
Figure 2. Karyotype analysis in the reported patient indicating the translocation t(9,22){q34;q11.2}.
On the second day of treatment, the patient’s WBC count dropped to 293 × 103/µL and he started receiving imatinib mesylate (340 mg/m2/day). The urology team was involved early during therapy, but their evaluation was consistent with corpora cavernosal tissue damage due to the duration of symptoms. Insertion of a 23-gauge butterfly needle at 3 and 9 o’clock enabled the aspiration of 3–4 ml of clotted blood, followed by irrigation with normal saline. Further aspiration resulted in partial detumescence. The patient received an intracorporal injection of 15 µg of adrenaline to decrease pain and relieve the erection, after which the pain slightly improved but the penis remained rigid. Penile blood gas measurements showed severe acidosis.
The care team conducted continuous monitoring and frequent clinical examinations to assess clinical response and determine the next steps in surgical management, such as possible distal shunting if the erection persisted. The patient received enoxaparin sodium and was treated with leukapheresis. The next day, he developed external edema at the aspiration site, but the erection was softer. Foley’s catheter was inserted to bypass any possible obstruction or urine retention.
The leukocyte count after leukapheresis was 278 × 103/µl, so the patient underwent a second leukapheresis session. His leukocyte count remained high after the second session (242 × 103/µL), so the patient received low-dose cytarabine as cytoreductive (100 mg/m2 twice daily for 4 days). Meanwhile, conservative management was continued, including cold compresses at the root of the penis and pain control by continuous infusion of morphine sulfate and oral administration of gabapentin. No further surgical interventions were needed.
After the administration of low-dose cytarabine, the patient’s leukocyte count decreased gradually to 120 × 103/µl. He continued to have a swollen penis, but the erection and rigidity improved substantially with the combination of cytarabine, imatinib mesylate, and hydroxyurea, along with cold compresses.
Discussion
In this report, we present a rare case of priapism in a pediatric patient with CML. The marked leukocytosis and significant splenomegaly indicated CML, which caused priapism. This report also reviews previous cases of priapism in pediatric patients (under 18 years of age) with CML (Table 1).
CML is a recognized cause of ischemic priapism, primarily due to hyperleukocytosis and the resulting leukocyte aggregation within the sinusoids of the corpora cavernosa. This process causes sinusoidal engorgement and an erection that obstructs venous outflow through the emissary veins (25). An enlarged spleen may also mechanically compress abdominal veins, further contributing to venous congestion in the corpora cavernosa (26).
Hematologic disorders, particularly sickle cell anemia and leukemia, are the predominant causes of priapism in pediatric patients (26). Leukemic priapism is typically of the low-flow, ischemic type triggered by hyperleukocytosis (12). Although CML often presents with nonspecific symptoms, priapism is an exceptionally rare initial manifestation in pediatric cases. Given the rarity of cases, medical professionals have limited clinical experience with pediatric CML involving priapism.
Common features of CML, including leukocytosis, often lead to complications such as pulmonary leukostasis, which can result in acute respiratory distress (27). Additional symptoms may include neurological manifestations such as headache, dizziness, and visual or auditory disturbances (28, 29).
Few reports describe priapism as an initial presentation of pediatric CML (30), but some describe its occurrence during therapy for CML (1, 26, 28). Most pediatric patients with CML involving priapism initially had a median hemoglobin of 11.1 g/dL, a median WBC count of 250 × 103/µL (range, 8 × 103–800 × 103/µL), and a mean platelet count of 500 × 103/µL (range, 40 × 103–2 × 106/µL), along with notable splenomegaly.
Among cases reviewed in the literature, priapism in pediatric CML was observed at a median age of 13 years (range, 9–53), with an average duration of 36 hours (range, 18 hours to 7 days) (5).
Priapism is a urological emergency that may have a poor prognosis, with a 50% risk of impotence despite appropriate management (30). Reports consistently emphasize the emergent nature of this condition in hematologic disease (26, 30), highlighting the need for prompt and accurate diagnosis. This underscores the importance of thorough history -taking, clinical examination, laboratory tests, and imaging studies (24).
Management of priapism typically involves a combination of aggressive supportive care and systemic treatments (26). As ischemic priapism is a compartment syndrome, early interventions such as hydration, pain relief, cytoreductive therapies, and leukapheresis are crucial. Pain associated with priapism can be severe and may not respond to high-dose analgesics. In cases of refractory pain, leukapheresis can alleviate symptoms by rapidly reducing leukocyte counts (30).
Several case series have reported successful outcomes with therapeutic leukapheresis for priapism (30), often in conjunction with cytotoxic therapy (11). Commonly used local interventions include the aspiration of blood from the corpora cavernosa followed by saline irrigation to clear sludged blood (31). If these measures are ineffective, a vasoconstrictive agent such as phenylephrine (100–200 µg/mL) can be administered in 5-minute intervals until complete detumescence is achieved. In resistant cases, surgical shunting may be considered, as recommended by the American Urological Association. This procedure creates a shunt between the corpus cavernosum and the glans penis, corpus spongiosum, or a vein to bypass the obstructed veno-occlusive mechanism (31) (“How I manage priapism in chronic myeloid leukaemia patients - PubMed,” n.d.).
Few case reports specifically describe the management of pediatric priapism associated with CML (24). Some groups reported the resolution of priapism after aspiration, irrigation, or shunt surgery, whereas others noted successful outcomes with CML-specific treatments, including leukapheresis and chemotherapy (24).
In eight cases of CML-associated priapism, complete resolution was achieved by using priapism-specific interventions, including either aspiration and irrigation or shunting. Shankar and Hazra (12) reported successful resolution of priapism in a 14-year-old male who presented with pallor, leukocytosis (WBC 226 × 103/µL), and a 1-day history of priapism. Aspiration and irrigation resolved the priapism after 6 days. Similarly, Khan et al. (17) described a 16-year-old male with CML in blast crisis and an initial WBC of 614 × 10⁹/L, platelet count of 907 × 10⁹/L, and hemoglobin level of 5.7 g/dL. His priapism resolved within 2 days after aspiration, irrigation, and shunt surgery.
Graiver et al. (6) reported a unique case of infantile priapism in a 7-week-old with CML in blast crisis and an initial WBC of 37 × 10⁹/L. This patient required both aspiration and irrigation and shunt surgery. Minckler et al. (15) described an 18-year-old male with an initial WBC of 588 × 10⁹/L whose priapism was resolved through aspiration and irrigation approximately 1 day after presentation.
Thakur et al. (22) reported on a 15-year-old with CML who had a 2-day history of painful priapism, epistaxis, and weight loss. With aspiration, irrigation, and shunt surgery, the patient achieved detumescence 4 days after priapism onset.
Nerdana et al. (10) described an 11-year-old male with CML and an initial WBC of 290 × 10⁹/L whose priapism was resolved through supportive measures. Avtar et al. (21) described an 18-year-old who had 14 hours of priapism, generalized fatigue, fever, and a WBC count of 363 × 10⁹/L. After aspiration and irrigation, his priapism resolved 1 day after initiation of CML-specific therapy.
Qu Lu et al. (16) described an 18-year-old male presenting with priapism and pallor lasting 7 days, with a hemoglobin level of 7.1 g/dL, platelet count of 5450 × 10⁹/L, and a WBC count of 257 × 10⁹/L. Initial aspiration and irrigation did not resolve the priapism, so shunt surgery was required.
We identified seven pediatric cases requiring leukapheresis for priapism management, with some requiring multiple sessions (range, 2 to 8) as did the patient in our study. Werther et al. (8) reported a 3-year-old with acute T-cell lymphoblastic leukemia who presented with a 3-hour history of painful priapism and a WBC count of 606 × 10⁹/L. This case required leukapheresis and chemotherapy, resulting in detumescence after 8 days of treatment. In the reported cases, leukapheresis effectively reduced blast cell numbers but was insufficient on its own due to the risk of blast cell rebound, making systemic treatment essential to address the underlying condition (32).
After priapism resolution, patients should receive leukemia- or CML-specific therapies, including tyrosine kinase inhibitors (TKIs) (3, 32). Pediatric patients with confirmed CML and priapism at initial presentation require a combination of medical therapies such as hydroxycarbamide, prednisolone, dexamethasone, vincristine, TKIs, cytarabine, and anticoagulants (28), along with surgical interventions to achieve priapism resolution (1).
Reversing priapism through medical therapy generally takes longer than other methods such as aspiration, leukapheresis, irrigation, and shunting (26). Previous studies show that priapism in CML presents with variable clinical manifestations, duration, and treatment responses (31). Management strategies are diverse and depend on the initial presentation and clinical response, with all approaches aiming for complete resolution of priapism and control of the underlying disease.
In adults, priapism is also uncommon but occurs more frequently than in children, with a bimodal incidence peak between ages 5 and 10 in children and 20 and 50 in adults (3). Adults may also present with symptoms such as unprovoked priapism, abdominal pain, bruising, bleeding, and weakness. The standard first-line treatments for acute ischemic priapism, which involve surgical blood drainage, are often unsuccessful in preserving erectile function in adults (31).
This report has several limitations, including the limited number of reported pediatric patients with priapism, which restricts the generalizability of our findings. Additionally, the long-term assessment of sexual function in survivors was not feasible.
This review emphasizes that early clinical recognition and intervention are key to the successful treatment and prevention of late complications of priapism. Systemic intervention combined with local therapy improves the likelihood of successful outcomes.
Conclusion
Although priapism rarely occurs during the initial presentation of CML in children, this condition requires immediate medical and surgical attention. Early interventions targeting the underlying cause, coupled with localized treatment, are essential to reduce the risk of long-term sequelae such as erectile dysfunction.
Data availability statement
All relevant data is contained within the article: The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study of human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients or patients legal guardian was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
RB: Writing – original draft, Writing – review & editing. LB: Writing – original draft, Writing – review & editing. RR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Athale U, Hijiya N, Patterson BC, Bergsagel J, Andolina JR, Bittencourt H, et al. Management of chronic myeloid leukemia in children and adolescents: Recommendations from the Children’s Oncology Group CML Working Group. Pediatr Blood Cancer. (2019) 66:e27827. doi: 10.1002/pbc.27827
2. Minciacchi VR, Kumar R, and Krause DS. Chronic myeloid leukemia: A model disease of the past, present and future. Cells. (2021) 10:117. doi: 10.3390/cells10010117
3. Pizzo & Poplack’s pediatric oncology . Available online at: https://www.wolterskluwer.com/en/solutions/ovid/pizzo–poplacks-pediatric-oncology-833 (Accessed April 25, 2024).
4. Levey HR, Kutlu O, and Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl. (2012) 14:156–63. doi: 10.1038/aja.2011.114
5. Ali E, Soliman A, De Sanctis V, Nussbaumer D, and Yassin M. Priapism in patients with chronic myeloid leukemia (CML): A systematic review. Acta BioMed. (2021) 92:e2021193. doi: 10.23750/abm.v92i3.10796
6. Graivier L, Gran G, Rhoades RB, Reynolds RC, and Windmiller J. Priapism in a 7-week-old infant with chronic granulocytic leukemia. J Urol. (1971) 105:137–9. doi: 10.1016/s0022-5347(17)61479-4
7. Mentzel H-J, Kentouche K, Doerfel C, Vogt S, Zintl F, and Kaiser WA. High-flow priapism in acute lymphatic leukaemia. Pediatr Radiol. (2004) 34(7):560–3. doi: 10.1007/s00247-003-1124-1
8. Werther R, Oakley E, and Heath JA. Priapism as a presentation of T-cell acute lymphoblastic leukaemia in a child. Emerg Med Australas. (2004) 16:425–7. doi: 10.1111/j.1742-6723.2004.00645.x
9. Gupta A, Seth T, and Gupta A. Successful use of terbutaline in persistent priapism in a 12-year-old boy with chronic myeloid leukemia. Pediatr Hematol Oncol. (2009) 26(1):70–3. doi: 10.1080/08880010802435146
10. Narendra R. Priapism in teenager chronic myelogenous leukemia: A rare occurrence. Asian J Pharm Health Sci. (2011) 1(4).
11. Veljkovic D, Kuzmanovic M, Micic D, and Šerbic-Nonkovic O. Leukapheresis in management hyperleucocytosis induced complications in two pediatric patients with chronic myelogenous leukemia. Transfus Apher Sci. (2012) 46(3). doi: 10.1016/j.transci.2012.03.012
12. Hazra S, Priyadarshi V, Gogoi D, Sharma PK, Pal DK, and Chakraborty SC. Pediatric priapism: a rare first manifestation of leukemia. APSP J Case Rep. (2013) 4(3):39.
13. Ergenc H, Varim C, Karacaer C, and Çekdemir D. Chronic myeloid leukemia presented with priapism: Effective management with prompt leukapheresis. Niger J Clin Pract. (2015) 18(6):828–30. doi: 10.4103/1119-3077.163282
14. Musa A, Ndakotsu M, Abubakar S, and Agwu P. Chronic myeloid leukemia with an initial presentation as ischemic priapism: a case report and review of literature. Arch Int Surg. (2017) 7(2):68–72.
15. Minckler MR, Conser E, Figueroa JJ, Scott AJ, Gaither J, and Amini R. The semantics of priapism and the first sign of chronic myeloid leukemia. Case Rep Emerg Med. (2017) 2017:2656203. doi: 10.1155/2017/2656203
16. Qu M, Lu X, Wang L, Liu Z, Sun Y, and Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: Case report. Asian J Urol. (2019) 6(4):373–6. doi: 10.1016/j.ajur.2018.12.004
17. Khan A, Shafiq I, Shah MH, Khan S, Shahid G, and Arabdin M. Chronic myeloid leukaemia presenting as priapism: A case report from Khyber Pakhtunkhwa. J Pak Med Assoc. (2018) 68:942–4.
18. Atas A, Meydanal YE, Iltar U, Ulas T, Salim O, and Undar L. Priapism-a rare presentation of chronic myeloid leukaemia. J Clin Diagnostic Res. (2019), 13.
19. Clark AJ, Hsu P, Darves-Bornoz A, Tanaka ST, Mason EF, and Katzenstein HM. Case 3: priapism in a 13-year-old boy. Pediatr Rev. (2018) 39(12):617–9. doi: 10.1542/pir.2017-0051
20. Gupta G, Kumar D, and Trivedi M. Acute lymphoblastic leukemia in a child presenting primarily with priapism. J Indian Assoc Pediatr Surg. (2020) 25(1):52–4. doi: 10.4103/jiaps.JIAPS_214_18
21. Dhanju A, Tyagi P, Dhaliwal S, Paul S, Singh R, Singh J, et al. Priapism: a rare presentation in chronic myeloid leukemia. Int J Adv Med. (2019) 6:1937. doi: 10.18203/2349-3933.ijam20195253
22. Thakur P, Verma V, Fotedar V, and Singh K. Priapism in a pediatric chronic myeloid leukaemia patient: Unusual presentation of a rare disease in children. Clin Cancer Invest J. (2019) 8:76–8. doi: 10.4103/ccij.ccij_3_19
23. Gaye O, Thiam NM, Cassell A, Gueye SM, Sow Y, Fall B, et al. Unusual presentation of priapism associated with acute and chronic myeloid leukemia in two patients: emergency management. Case Rep Urol. (2020) 2020:4982432. doi: 10.1155/2020/4982432
24. Bintoro U, Zaky Romadhon P, Suryantoro S, Aminy R, Windradi C, and Widiyastuti K. Case Report: Priapism as the clinical presentation of chronic myeloid leukemia in accordance with reports created during last twenty years: a case report and literature review. F1000Research. (2021) 10:571. doi: 10.12688/f1000research.53365.1
25. Jameel T and Mehmood K. Priapism – an unusual presentation in chronic myeloid leukaemia: case report and review of the literature. Biomedica. (2009) 25:197–9.
26. Becerra-Pedrazaa LC, Jiménez-Martínez LE, Pena-Morfin I, Nava-Esquivel R, and Villegas-Martínez JA. Priapism as the initial sign in hematologic disease: Case report and literature review. ScienceDirect. (2018). doi: 10.1016/j.ijscr.2017.12.038
27. Porcu P, Cripe LD, Ng EW, Bhatia S, Danileson CM, Orazi A, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leukemia and Lymphoma. (2009) 39:1–18. doi: 10.3109/10428190009053534
28. Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, and Lux S. Nathan and oski’s hematology and oncology of infancy and childhood. 8th Edition. Saunders: Elsevier Shop. (2014). Available at: https://shop.elsevier.com/books/nathan-and-oskis-hematology-and-oncology-of-infancy-and-childhood-2-volume-set/orkin/978-1-4557-5414-4. 2-Volume Set.
29. Osman AEG and Deininger MW. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. (2021) 49:100825. doi: 10.1016/j.blre.2021.100825
30. van der Velde MGAM, Tiellemans SMB, de Lil H, and Nieuwenhuizen L. The value of leukapheresis for treatment of priapism as presenting feature of chronic myeloid leukemia-Case report and review of literature. EJHaem. (2022) 3:1100–15. doi: 10.1002/jha2.545
31. Cherian J, Rao AR, Thwaini A, Kapasi F, Shergill IS, and Samman R. Medical and surgical management of priapism. Postgrad Med J. (2006) 82:89–94. doi: 10.1136/pgmj.2005.037291
Keywords: ALL, acute lymphoblastic leukemia, B-ALL, B-cell acute lymphoblastic leukemia, CML, chronic myeloid leukemia, T-ALL, T-Cell acute lymphoblastic leukemia
Citation: Budair R, Baqain L and Rihani R (2025) Rare manifestations of pediatric chronic myeloid leukemia: a case report on priapism and a literature Review. Front. Oncol. 15:1579981. doi: 10.3389/fonc.2025.1579981
Received: 19 February 2025; Accepted: 15 May 2025;
Published: 19 June 2025.
Edited by:
Joanna Zawitkowska, Medical University of Lublin, PolandReviewed by:
Shinsuke Takagi, Toranomon Hospital, JapanWojciech Czogała, Jagiellonian University Medical College, Poland
Copyright © 2025 Budair, Baqain and Rihani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rawad Rihani, cnJpaGFuaUBraGNjLmpv
 Rawan Budair
Rawan Budair Laith Baqain
Laith Baqain Rawad Rihani
Rawad Rihani