- 1Department of Radiation Oncology, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, China
- 2Department of Oncology, Fuzhou Pulmonary Hospital of Fujian, Fuzhou, China
Background: Esophageal cancer is among the leading causes of cancer-related mortality in males. This study aimed to evaluate the efficacy and safety of nedaplatin (NDP) in comparison to other platinum-based (OPB) agents combined with paclitaxel and concurrent neoadjuvant radiotherapy for locally advanced thoracic segmental esophageal squamous cell carcinoma (ESCC).
Methods: This single-center, retrospective cohort study was conducted in China. The primary endpoints of this study were safety and efficacy assessments. Unpaired t-tests, chi-squared tests, and Fisher’s exact tests were used to compare intergroup differences, as appropriate. Multivariate logistic regression models were used to explore the associations between postoperative outcomes and the two treatment groups. Kaplan–Meier survival curves and Cox proportional hazards regression models based on OS and PFS were used to compare the efficacy between the two groups.
Results: A total of 212 patients were enrolled in this retrospective cohort study, including 79 who received NDP and 133 who received OPB (82 were treated with cisplatin, 20 with carboplatin, 19 with lobaplatin, and 12 with oxaliplatin) agents. The incidences of grade 3–4 acute radiotherapy-associated esophagitis, pneumonitis, and leukemia were significantly lower in the NDP group than in the OPB group (p = 0.02, p < 0.001, and p = 0.002, respectively). All grades of acute gastrointestinal reactions, including nausea, vomiting, anorexia, and diarrhea, were significantly more frequent in the OPB group than in the NPD group (p < 0.001, p = 0.032, p < 0.001, and p = 0.002, respectively). The Kaplan–Meier curves for overall survival (OS) and progression-free survival (PFS) showed similar results for both groups.
Conclusions: The safety profile of nedaplatin may be superior to those of other platinum-based agents in terms of acute radiotherapy toxicity and postoperative side effects; however, there was no difference in the efficacy between the two groups regarding short-term prognostic tumor regression grades or long-term OS and PFS.
1 Introduction
Esophageal cancer is among the lea ding causes of cancer-related mortality in males, ranking tenth in the United States, with an estimated 12,880 male deaths by 2024 (1). The 5-year survival rate for esophageal cancer is alarmingly low at approximately 22% (1, 2). In Asia, male predominance is striking, with squamous cell carcinoma accounting for approximately 90% of cases (3). The R0 resection rate was only 50%, and the early recurrence rate was notably high (4). Neoadjuvant radiotherapy combined with concurrent chemotherapy has been postulated to enhance the pathological response rate and mitigate adverse events in locally advanced esophageal squamous cell carcinoma (ESCC); however, its long-term benefits remain controversial (5). Despite these interventions, recurrence rates following neoadjuvant therapy can reach 33–48% (6, 7). Consequently, there is an urgent need to develop more effective and safer treatment strategies for ESCC.
Nedaplatin (NDP), a second-generation platinum analog, has shown promise in clinical trials, with a response rate similar to that of cisplatin, a gold-standard platinum-based agent (8–10). However, the durability of response and overall survival (OS) rates with NDP have been reported to be lower than those achieved with cisplatin (11–13). Previous studies have compared NDP with other platinum-based (OPB) chemotherapies for the treatment of malignancies including ESCC (12, 14–17). For instance, Ohnuma et al. previously reported that patients with stage IB to IV esophageal cancer receiving NDP-based chemoradiotherapy achieved an 82.1% complete response rate and exhibited median progression-free and overall survivals of 41.2 months (18). Furthermore, the choice of a concurrent chemotherapy regimen in combination with NDP has been debated, with variable results reported across different patient populations and geographical regions (19–22).
This study aimed to conduct a comprehensive safety and efficacy analysis of neoadjuvant radiotherapy combined with concurrent paclitaxel plus NDP compared with OPB chemotherapy regimens for the treatment of thoracic segmental ESCC. By evaluating the short- and long-term outcomes, we aimed to determine the potential advantages of this treatment approach in terms of pathological response rates, OS, and toxicity profiles. Additionally, these findings may contribute to a better understanding of the underlying mechanisms that govern the response to neoadjuvant therapy and the development of novel therapeutic strategies tailored to the specific molecular characteristics of ESCC.
2 Materials and methods
2.1 Study design and population
This single-center, retrospective cohort study was conducted at Fujian Cancer Hospital in China. Patients diagnosed with locally advanced ESCC between January 2006 and December 2022, who underwent neoadjuvant chemoradiotherapy (nCRT) and surgical resection, were included. Patients were considered for inclusion if they met the following criteria: (1) aged ≥ 18 years, (2) clinically staged as II/III/IVA with no distant metastases at the time of initial diagnosis, (3) received complete neoadjuvant radiotherapy, (4) received neoadjuvant chemotherapy with paclitaxel plus a platinum-based drug (NDP or OPB derivatives such as cisplatin, carboplatin, laboplatin, or oxaliplatin), (5) underwent resection of the primary esophageal lesion after nCRT, and (6) had complete pathological data, including tumor regression grades according to the NCCN and JSED criteria. The exclusion criteria were as follows: (1) cervical and gastroesophageal junction tumors; (2) neoadjuvant chemotherapy with drugs other than paclitaxel and platinum; (3) severe preexisting organ disease before treatment; (4) presence of multiple primary malignancies; and (5) missing or incomplete medical records or follow-up information.
2.2 Cohort assignment
This study grouped patients according to different treatment regimens as exposures. The specific grouping criteria were as follows: patients who received nCRT concurrent with paclitaxel plus NDP, followed by surgical resection and lymph node dissection (NDP group), and those who received nCRT concurrent with paclitaxel plus OPB drugs, followed by surgical resection and lymph node dissection (OPB group). Neoadjuvant radiotherapy consisted of 41.4~50.4 Gy/23~28 fractions delivered using three-dimensional conformal radiation therapy or intensity-modulated radiation therapy in computed tomography (CT) simulation and conformal treatment planning. The preoperative chemotherapy agents included paclitaxel plus NDP or OPB derivatives (including cisplatin, carboplatin, cisplatin, and oxaliplatin).
2.3 Endpoints
The primary endpoints of this study were safety and efficacy assessments. Safety assessments included acute toxic reactions during neoadjuvant treatment until the time of surgery and postoperative evaluation metrics. Acute toxic reactions included radiation-related dermatitis, esophagitis, and pneumonitis, in addition to gastrointestinal reactions such as nausea, vomiting, neurogenic anorexia, and diarrhea. The hematological toxic effects included leukopenia, hemoglobin changes, thrombocytopenia, and an increase in the alanine aminotransferase-to-bilirubin ratio. Postoperative indicator, textbook outcome (TO), was assessed using a composite index comprising the lymph node yield, R0 resection status, length of postoperative hospital stay (PLOS), occurrence of postoperative complications, 30-day mortality, and readmission rates. The pathological tumor regression grades (TRGs) were reported by two experienced pathologists: NCCN-TRG and JSED-TRG. OS and progression-free survival (PFS) were used to assess the efficacy. OS was defined as the date from the start of surgery to the last follow-up or death from any cause. PFS was defined as the date from the start of surgery to the last follow-up or disease progression, which was determined radiologically or pathologically.
2.4 Statistical analyses
All statistical analyses were performed using R software (version 4.3.2). Baseline characteristics are presented based on variable types. Continuous variables with a normal distribution were expressed as means ± standard deviation (SD), while those with a skewed distribution were reported as medians with interquartile ranges. Categorical and ordinal variables were presented as proportions. Unpaired t-tests, chi-squared tests, and Fisher’s exact tests were used to compare intergroup differences, as appropriate. Multivariate logistic regression models adjusted for confounding factors were used to explore the associations between postoperative outcomes (TO, NCCN-TRG, and JSED-TRG) and the two treatment groups. Kaplan–Meier survival curves based on OS and PFS were used to compare the efficacy between the two groups. Univariate and multivariate Cox proportional hazards regression models, along with their visualized forest plots, were used to identify subgroups within the two groups that demonstrated OS or PFS benefits. Statistical significance was set at p < 0.05.
3 Results
3.1 Baseline characteristics
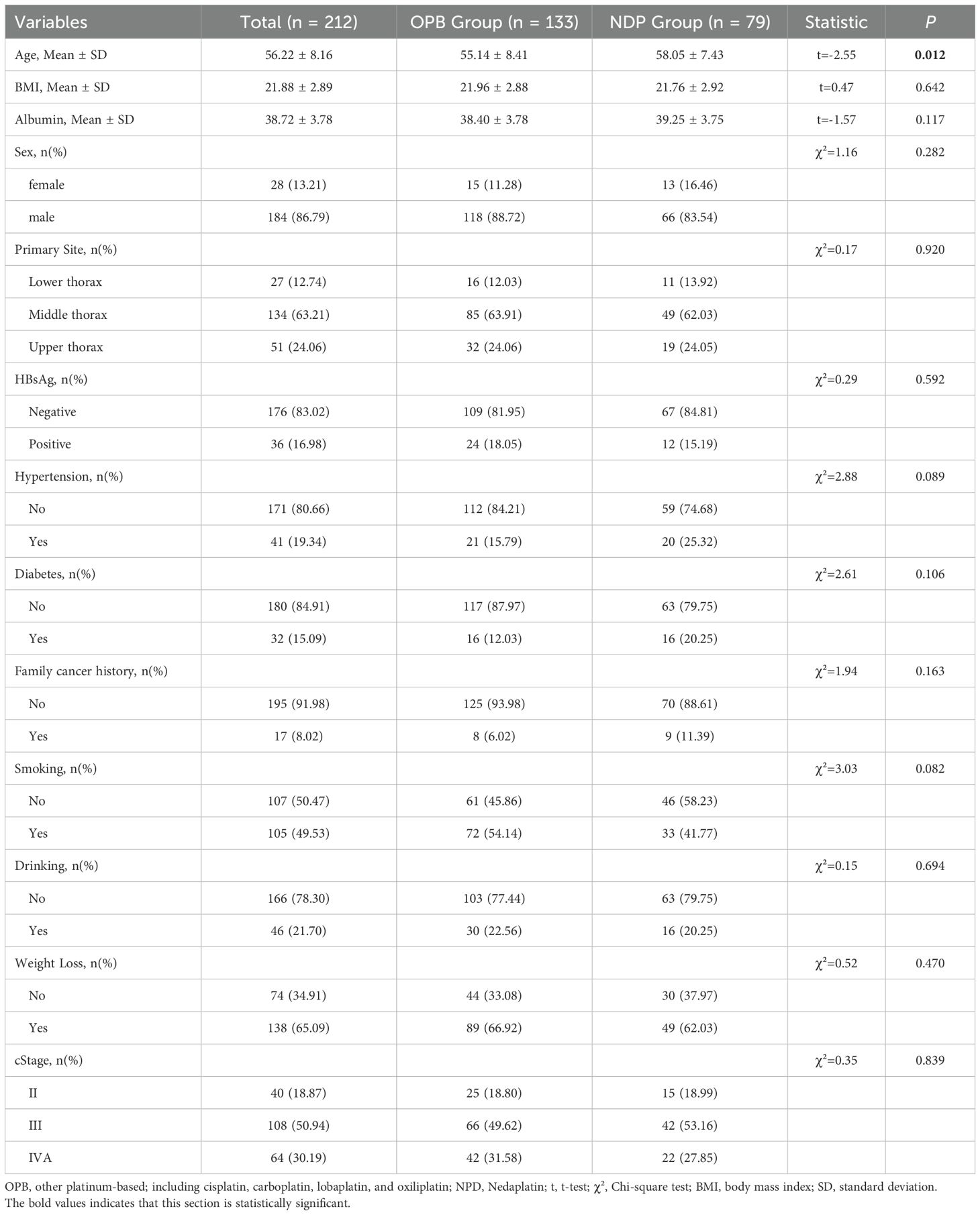
In this study, 357 patients with ESCC in the thoracic segment were included; 145 patients did not meet the inclusion criteria. Finally, 212 patients were included in the analysis: 79 in the NDP-based treatment group and 133 in the OPB treatment group. The baseline characteristics of the two groups and their intergroup differences are presented in Table 1. The mean age of the patients was 56.22 ± 8.16 years. The OPB group had a mean age of 55.14 ± 8.41 years, while the NDP group had a mean age of 58.05 ± 7.43 years (t = –2.55, p = 0.012). The sex distribution was similar in both groups, with females comprising 13.21% and 16.46% of the OPB and NDP groups, respectively, and males comprising 86.79% and 83.54% of the OPB and NDP groups, respectively (χ² = 1.16; p = 0.282). Furthermore, the body mass index (BMI) and albumin levels did not differ between the two groups (t = 0.47, p = 0.642; t = –1.57, p = 0.117, respectively). HBsAg status, primary site, hypertension, diabetes, family cancer history, smoking, drinking, weight loss, and tumor stage were similar in both groups.
3.2 Comparison of different treatment regimes
In the OPB treatment group, 82 were treated with cisplatin, 20 with carboplatin, 19 with lobaplatin, and 12 with oxaliplatin. The clinical assessments, treatments, and pathological staging of the different treatment groups are shown in Table 2. The interval between neoadjuvant therapy and surgery (N and S) was similar across all treatment groups, with a mean of 5.24 ± 1.70 days. The mean blood loss during surgery of the NDP group (160.38 ± 121.73 ml) was slightly lower than that in the OPB group; however, there was no significant difference between all groups (F = 0.38; p = 0.825). The preoperative clinical evaluation revealed similar responses across the treatment groups (p = 0.286). The number of chemotherapy cycles and radiation approaches used varied slightly across the treatment groups. In addition, the tumor stage at the time of pathological diagnosis did not differ significantly between the treatment groups, including pT, pN, and pStage (p = 0.171, 0.658, and 0.683, respectively).
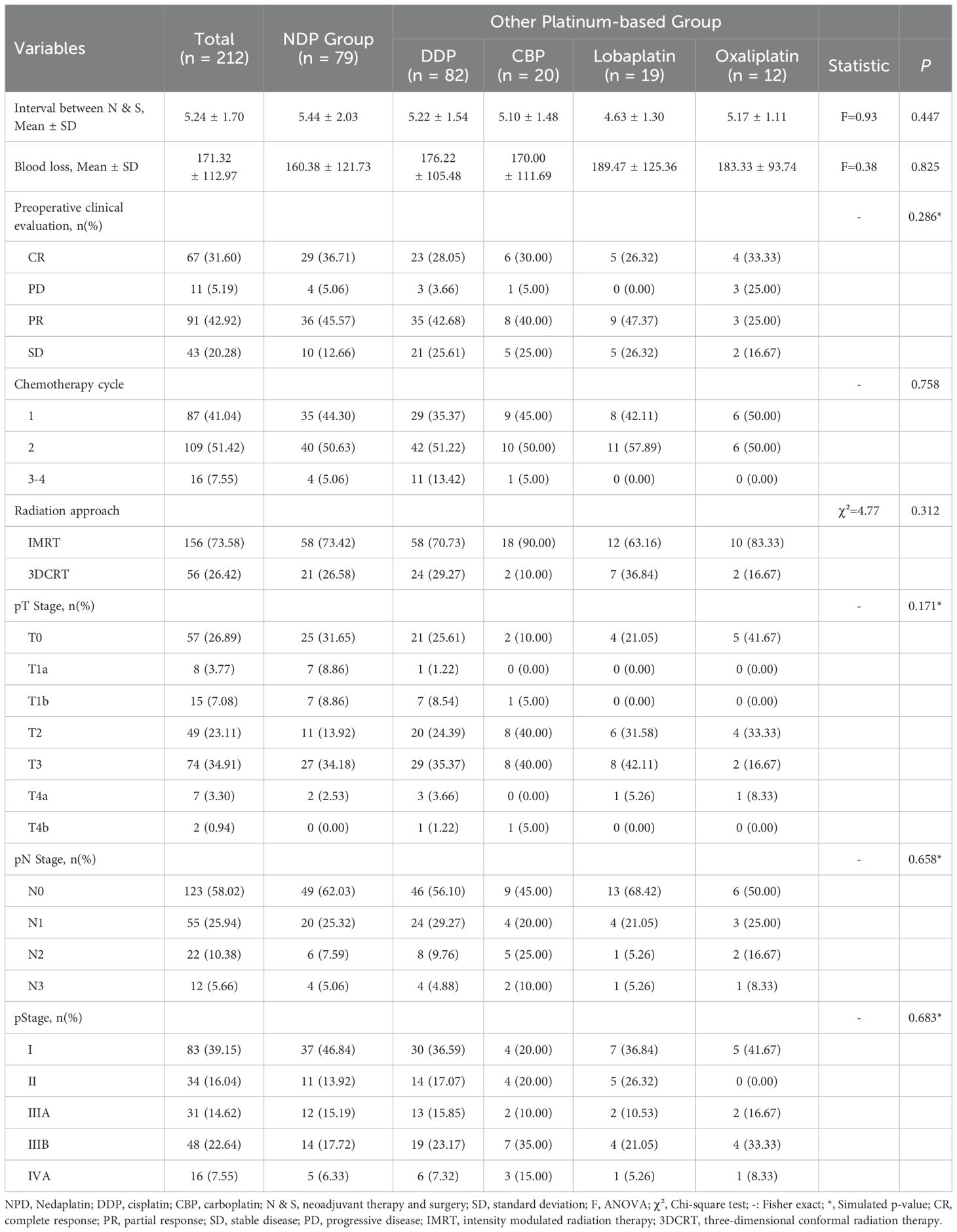
Table 2. Different interventions and clinical outcomes of locally advanced esophageal squamous carcinoma.
3.3 Acute toxic effects reported during neoadjuvant therapy or before surgery
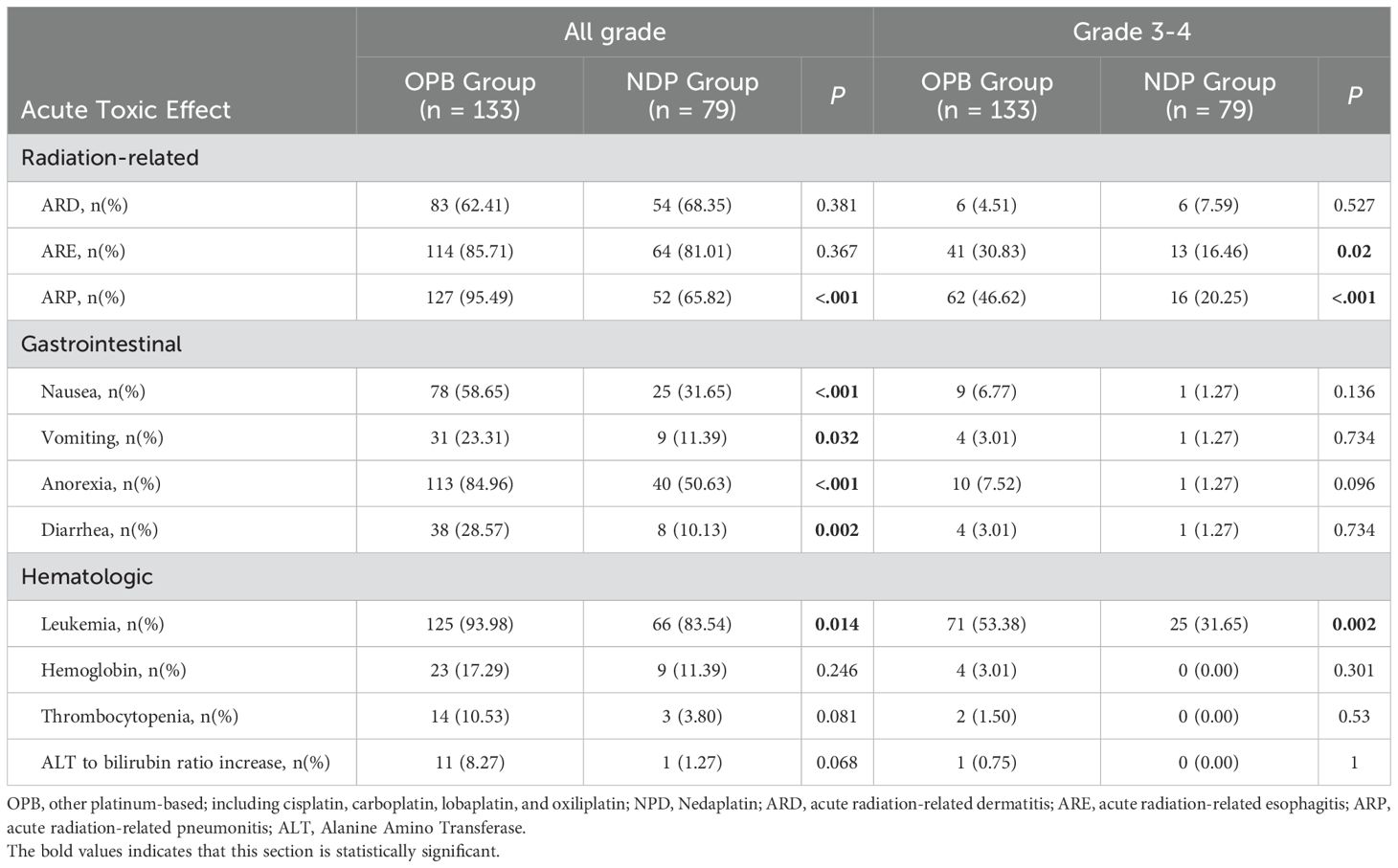
Radiation-related toxic effects were common among all patients who received neoadjuvant therapy (Table 3). The most frequently reported was acute radiation-related dermatitis (ARD), which occurred in 83% of patients in the OPB group and 54% in the NDP group, respectively, although this difference was not statistically significant (p = 0.381). However, acute radiation-related esophagitis (ARE) was more commonly reported in the OPB group (n = 114, 85.71%) than in the NDP group (n = 64, 81.01%); this difference was statistically significant (p = 0.367). Furthermore, acute radiation-related pneumonitis (ARP) was significantly more common in the OPB group (n = 127, 95.49%) than in the NDP group (n = 52, 65.82%); this difference was also statistically significant (p < 0.001).
Gastrointestinal toxicity was also common. Nausea was the most reported gastrointestinal symptom, with 78% of patients in the OPB group and 25% in the NDP group experiencing it, with a statistically significant difference between groups (p < 0.001). Vomiting was less common, with 31 patients (23.31%) in the OPB group and nine patients (11.39%) in the NDP group, with a difference that approached statistical significance (p = 0.032). Anorexia was significantly more common in the OPB group (n = 113, 84.96%) than in the NDP group (n = 40, 50.63%; p < 0.001). Furthermore, diarrhea was less common, with 38 patients (28.57%) in the OPB group and eight patients (10.13%) in the NDP group experiencing it, with a statistically significant difference (p = 0.002).
Hematological toxicity was also observed. Leukopenia, a decrease in the white blood cell count, was significantly more common in the OPB group (n = 125, 93.98%) than in the NDP group (n = 66, 83.54%; p = 0.014). The hemoglobin levels decreased in 23 patients (17.29%) in the OPB group and 9 patients (11.39%) in the NDP group, with no statistically significant difference between groups (p = 0.246). Thrombocytopenia, a decrease in the platelet count, occurred in 14 patients (10.53%) in the OPB group and three patients (3.80%) in the NDP group, with no statistically significant difference between groups (p = 0.081). Furthermore, an increase in the ALT-to-bilirubin ratio, an indicator of liver function abnormalities, was more common in the OPB group (n = 11, 8.27%) than in the NDP group (n = 1, 1.27%), although this difference was not statistically significant (p = 0.068).
3.4 Postoperative evaluation indicators
We first analyzed the differences in the surgical evaluation indicators and complications between the two groups (Table 4). The TO rate was higher in the OPB group (84.21%) compared to the NDP group (74.68%), although this difference was not statistically significant (χ² = 2.88; p = 0.089). However, the number of lymph node dissections (LNDs) with < 20 lymph nodes was significantly higher in the NDP group (38.35%) compared to the OPB group (51%; χ² = 3.78; p = 0.052). The R0 tumor margin rates were 90.98% in the OPB group and 98.73% in the NDP group (χ² = 3.92; p = 0.048). The PLOS was similar between the two groups, with 59.40% of patients in the OPB group and 46.58% in the NDP group having a PLOS of >14 days (χ² = 0.03; p = 0.867). Regarding postoperative complications, the OPB group had a higher rate of severe complications (17.29%) than the NDP group (8.86%), although the difference was not statistically significant (χ² = 2.90; p = 0.089). However, the rates of specific complications, such as hydrothorax, pneumonia, pyothorax, anastomotic fistula, anastomotic stenosis, and 30-day mortality, were similar between the two groups, and no statistically significant differences were observed.
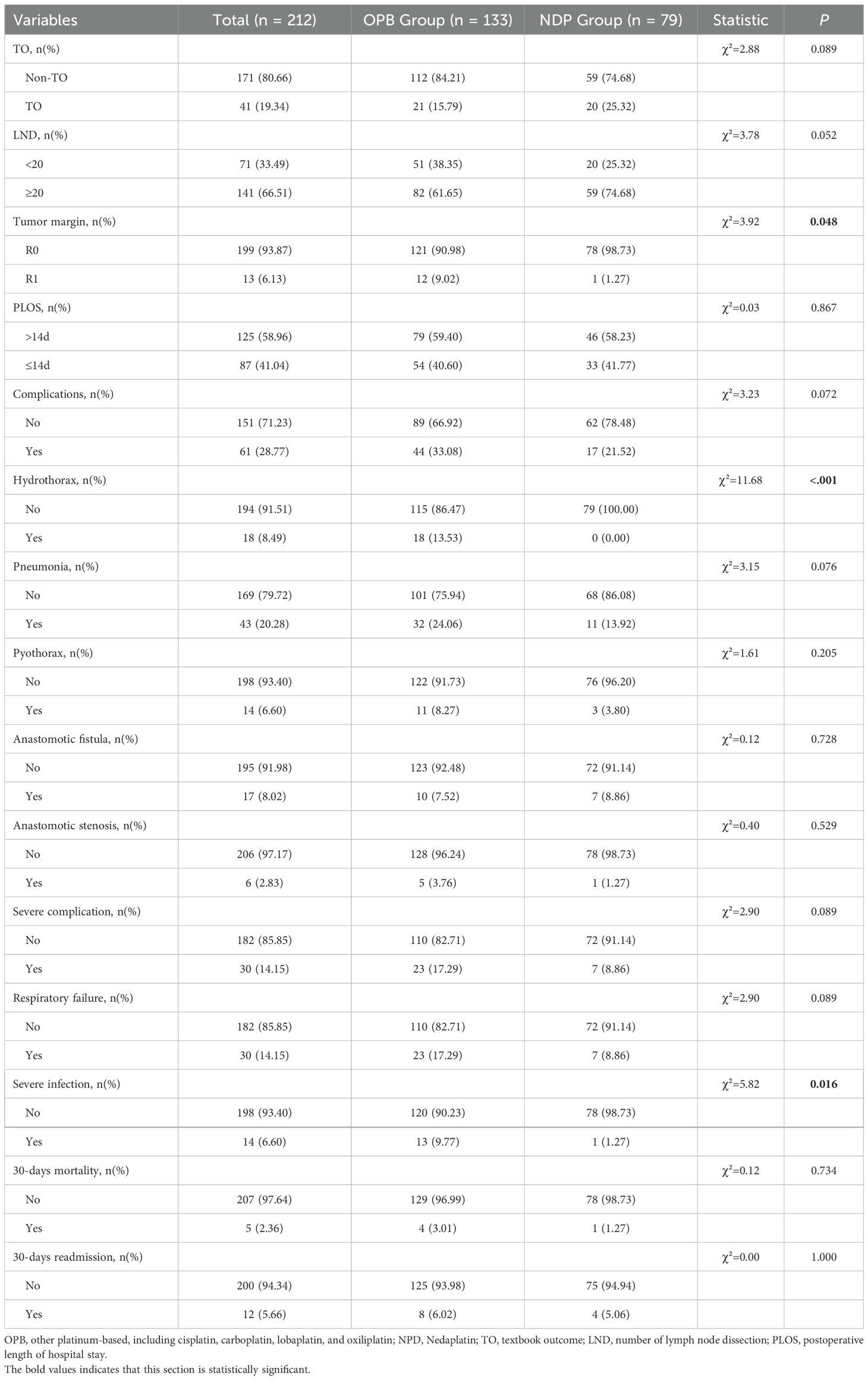
Table 4. Surgical evaluation indicators and complications of locally advanced esophageal squamous carcinoma.
We performed logistic regression to compare the differences in short-term prognostic evaluation indicators between the two groups with covariate adjustments. The results showed that none of the odds ratios (ORs) for the NDP group compared with the OPB group were statistically significant in any of the models, including the endpoints of TO, NCCN-TRG, and JSED-TRG (Table 5).
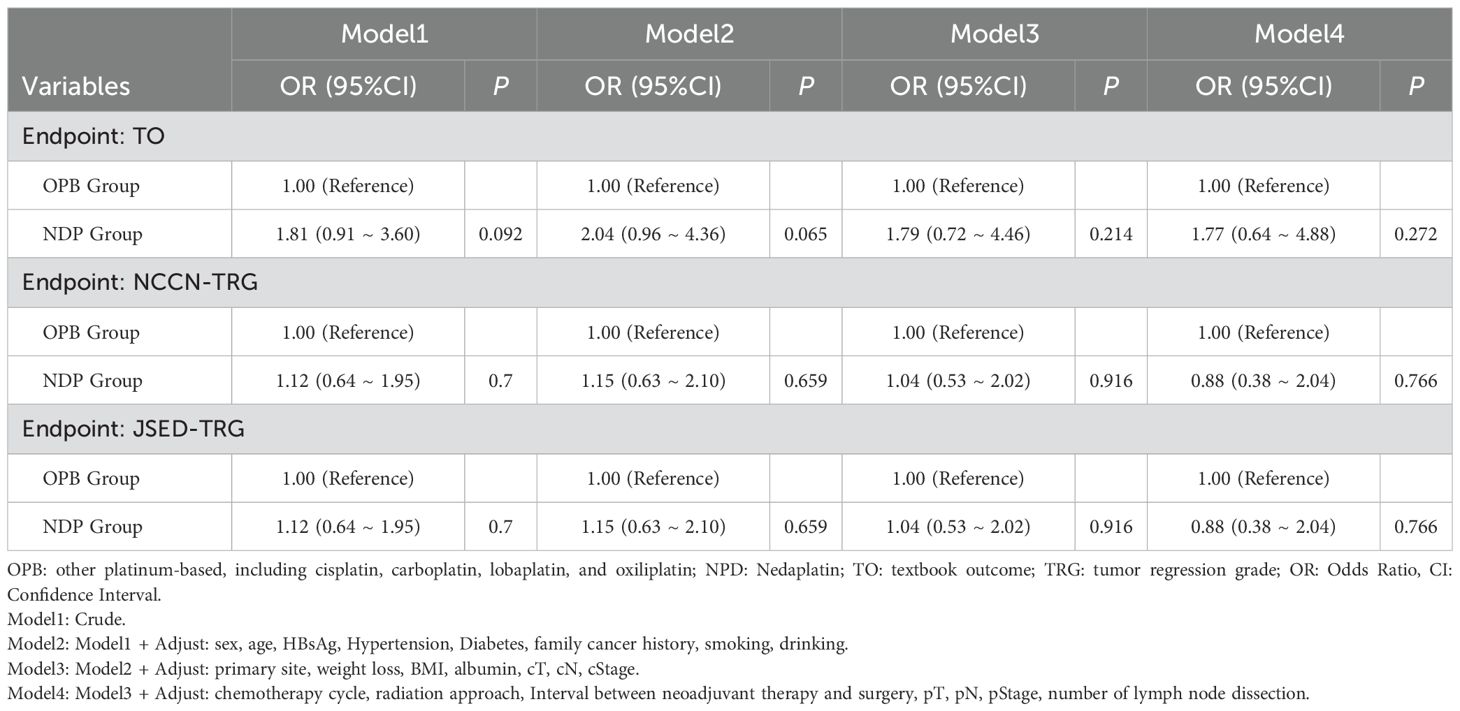
Table 5. Covariate adjusted logistic regression with OPB and NDP models in patients with locally advanced ESCC.
3.5 Comparison of long-term prognostic indicators
PFS and OS were used to assess long-term prognostic efficacy. First, the results of the Kaplan–Meier survival curves and the log-rank test showed that there were no statistical differences between the two groups for either OS (Figure 1A, hazard ratio [HR] = 0.795, 95% confidence interval [CI]: 0.577–1.094, p = 0.153) or PFS (Figure 1B, HR = 0.846, 95% CI: 0.626–1.144, p = 0.265). Subsequently, we performed univariate Cox proportional hazards regression analyses of each covariate for both groups and plotted the results as forest plots. The forest plots indicated that the OPB group had a worse OS in clinical stage III (Figure 2A, 56 deaths of 66 patients in the OPB group, 29 deaths of 42 patients in the NDP group, HR = 0.61, 95% CI: 0.39–0.96, p = 0.033), NCCN-TRG four (Figure 2A, 16 deaths of 16 patients in the OPB group, nine deaths of 10 patients in the NDP group, HR = 0.41, 95% CI: 0.17–1.00, p = 0.049), and patients undergoing 3D-CRT (Figure 2A, 33 deaths of 35 patients in the OPB group, 14 deaths of 21 patients in the NDP group, HR = 0.52, 95% CI: 0.28–0.98, p = 0.044), and a similarly significantly worse PFS in the population receiving 3D-CRT (Figure 2B, 34 of 35 patients in the OPB group, 16 of 21 patients in the NDP group, HR = 0.54, 95% CI: 0.29–0.98, p = 0.042). The p-values for the interactions for all subpopulations did not show statistical differences.
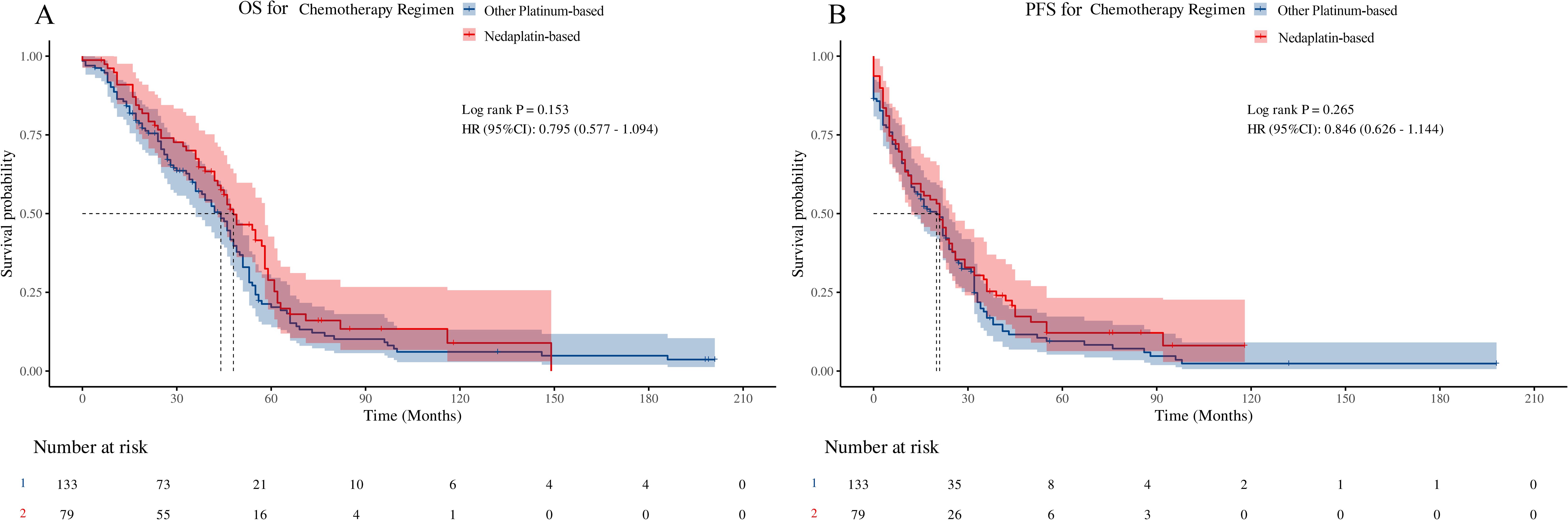
Figure 1. Kaplan-Meier curve and log-rank test based on overall survival (OS) and progression-free survival (PFS) for nedaplatin-based versus other platinumbased regimens. (A) OS for chemotherapy regimens; (B) PFS for chemotherapy regimens.
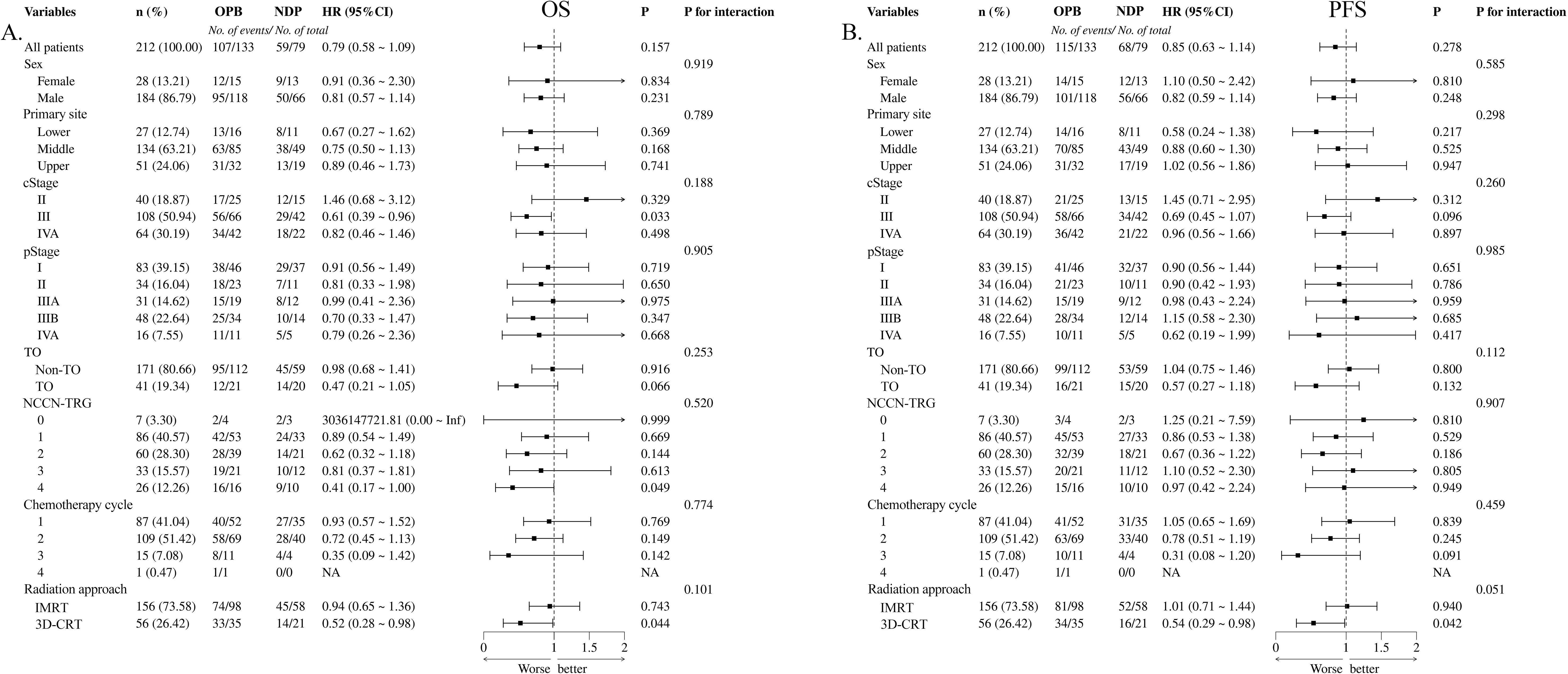
Figure 2. Forest plots based on overall survival (OS) and progression-free survival (PFS) of univariate Cox proportional hazard regression for nedaplatin-based versus other platinum-based regimens. (A) OS for chemotherapy regimens; (B) PFS for chemotherapy regimens.
4 Discussion
In the present study, we conducted a comprehensive safety and efficacy analysis of neoadjuvant radiotherapy combined with concurrent paclitaxel plus NDP compared with OPB chemotherapy regimens for thoracic segmental ESCC. The safety profile of nedaplatin may be superior to those of OPB agents in terms of acute radiotherapy toxicity and postoperative side effects. However, there was no difference in the efficacy between the two groups in terms of short-term prognostic TRG or long-term OS and PFS. These key findings are important indications for neoadjuvant therapy with NDP for locally advanced ESCC.
The primary focus of this study was the toxic effects and postoperative clinical outcomes of the two treatment regimens. This study found that the toxic effects of neoadjuvant radiotherapy combined with paclitaxel and NDP were generally less severe than those associated with OPB chemotherapy regimens. Specifically, the incidences of ARP and ARE, gastrointestinal reactions, and marrow suppression were significantly lower in the NDP group than in the OPB group. This finding aligns with previous research that highlighted the relatively milder toxicity profile of NDP compared to OPB drugs (23–26). Wang et al. reported 24.5% treatment-related adverse events of grade 3 or worse and Tang et al. observed a significant difference in the incidence of grade 3 and 4 auditory toxic effects favoring nedaplatin over cisplatin (17.7% vs 10.5%, p = 0.04) in patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy (16, 27). Several factors may have contributed to the reduced toxicity of NDP. NDP has a unique chemical structure that differentiates it from OPB drugs (28, 29). It contains a monofunctional linker that is believed to affect cellular uptake and intracellular pharmacodynamics, leading to reduced DNA binding and subsequent toxicity. This monofunctional linker may play a role in mitigating the toxic effects of NDP by reducing interactions between the drug and normal tissues (28). Second, the reduced toxicity of NDP may be attributed to its pharmacokinetic properties (29). Studies have shown that NDP has higher lipophilicity and slower renal excretion than OPB drugs, leading to a more sustained plasma concentration and potentially prolonged antitumor activity (27). Kawai et al. have previously indicated that the nephrotoxicity of nedaplatin is associated with its accumulation in the renal cortex, suggesting that while increased lipophilicity may enhance tumor tissue distribution, it could also lead to prolonged renal exposure and increased toxicity risk (30). Jin et al. reported 8.7% of grade 3 anemia leucopenia, 17.4% of grade 4 anemia leucopenia, and 19.6% of neutropenia, respectively, following NDP-based second-line chemotherapy for cisplatin-pretreated refractory metastatic/recurrent ESCC (31). This sustained exposure may allow for more targeted delivery of the drug to tumor cells, thereby reducing the exposure of normal tissues and associated toxicities (32, 33). Furthermore, previous studies suggested that the intracellular processing of NDP may differ from that of OPB drugs, leading to unique mechanisms of action and toxicity profiles (34). For instance, NDP is more susceptible to inactivation by thiol-containing compounds, which may modulate its toxicity and contribute to its milder side effect profile (32, 35). These factors collectively contribute to the favorable toxicity profile of NDP, making it a potentially attractive option for treating thoracic segmental ESCC.
Regarding the postoperative clinical outcomes, the study indicated that while the TO rate was higher in the OPB group than in the NDP group, the difference was not statistically significant. However, the NDP group showed a higher proportion of LNDs with < 20 lymph nodes, suggesting that NDP may have facilitated a more thorough lymph node evaluation. Moreover, R0 tumor margin rates were significantly higher in the NDP group, indicating a greater likelihood of achieving clear surgical margins and potentially better oncological outcomes. Ohnuma et al. also reported NDP-based nCRT had an R0 resection rate of 89.3% and a pathological complete response rate of 32% in patients with locally advanced ESCC (36). Higher lymph node dissection rates may suggest improved surgical thoroughness, potentially contributing to higher R0 resection rates and better oncological outcomes. Supporting this, Shang et al. have previously demonstrated that extensive lymph node dissection correlates positively with improved survival and reduced recurrence rates (37). The PLOS was similar between the two groups, with a higher proportion of patients in both groups having a PLOS of > 14 days. This suggests that NDP does not adversely affect the duration of the hospital stay after surgery. The OPB group exhibited a higher rate of severe postoperative complications than the NDP group, although this difference was not statistically significant. Specifically, the rates of hydrothorax, pneumonia, pyothorax, anastomotic fistula, anastomotic stenosis, and 30-day mortality were similar between the two groups, indicating that NDP did not increase the risk of these specific complications. The reduced toxicity associated with NDP may contribute to its potential benefits on postoperative outcomes, as it allows for better patient tolerance and recovery from chemotherapy and radiotherapy (38–41). Thus, the present study suggests that neoadjuvant radiotherapy combined with concurrent paclitaxel and NDP may offer some advantages in terms of postoperative evaluation indicators compared with OPB chemotherapy regimens.
In terms of short- and long-term prognosis, the findings suggest that, in the context of this study, the use of NDP in neoadjuvant chemotherapy does not lead to significant improvements in short-term prognostic evaluation indicators compared to OPB drugs. This may be due to the similar efficacy profiles of NDP and OPB in the treatment of thoracic segmental ESCC as well as the complex interplay of various factors that affect prognosis, such as tumor stage, lymph node status, and patient characteristics (42–45). In the long-term prognostic analysis, Kaplan–Meier survival curves and log-rank tests showed no significant differences in the OS or PFS between the two groups. However, the subgroup analysis revealed that the OPB group had a worse OS in patients with clinical stage III, NCCN-TRG four, and 3D-CRT. Similarly, the OPB group had a significantly worse PFS than the 3D-CRT group. Isohashi et al. previously presented that IMRT had better locoregional control, PFS, and 3-year OS rate than 3D-CRT (95% vs. 85%, 92% vs. 70%, 92% vs. 85%, respectively) in patients with cervical cancer receiving NDP-based treatment (46, 47). The lack of significant differences in the OS and PFS between the two groups may be due to the small sample size and the potential for residual confounding factors that could influence survival outcomes. It is also possible that the benefits of NDP in terms of reduced toxicity and improved patient tolerance, which may contribute to better adherence to the treatment regimen and overall treatment efficacy, are not fully reflected in the OS and PFS outcomes (48–50). Shukuya et al. observed significantly better OS in the NDP group (median 13·6 months, 95% CI 11·6-15·6) than in the cisplatin group (11·4 months,10·2-12·2; hazard ratio 0·81, 95% CI 0·65-1·02; p=0·037) with fewer grade 3 or worse nausea (3.95% vs. 14.29%), fatigue (3.39% vs. 11.43%), hyponatremia (13.56% vs. 30.29%), and hypokalemia (2.26% vs. 8.57%) in advanced squamous cell lung cancer (12). Besides, the significant difference in age between the two groups suggests that it may affect chemotherapy tolerance. Vilmi et al. have previously support using less toxic platinum-based regimens, such as nedaplatin, for elderly patients to minimize adverse events (51). Therefore, while NDP appears to have a favorable toxicity profile and may contribute to better postoperative outcomes, the study did not demonstrate significant differences in short- or long-term prognostic indicators when compared with OPB.
The limitations of this study include its retrospective design, which may introduce selection bias; a small sample size, particularly in the NDP group, which limits generalizability; and the lack of molecular profiling data, precluding a deeper understanding of the molecular mechanisms underlying treatment response and outcomes. Future work should include prospective randomized trials to confirm the efficacy and safety of neoadjuvant radiotherapy with NDP in a larger patient population, incorporate molecular profiling to elucidate the role of specific biomarkers in predicting the response to NDP-based neoadjuvant therapy, and further investigate long-term outcomes, including quality of life and survival, to fully assess the clinical utility of NDP in the treatment of thoracic segmental ESCC.
5 Conclusions
In conclusion, this retrospective study compared the safety and efficacy of neoadjuvant radiotherapy combined with paclitaxel plus NDP with those of OPB chemotherapy regimens for thoracic segmental ESCC. The findings revealed that NDP exhibited a favorable toxicity profile with reduced acute radiotherapy toxic effects and postoperative side effects compared with OPB drugs. However, the efficacy outcomes, including pathological response rates, OS, and PFS, did not differ significantly between the NDP and OPB chemotherapy groups. These findings contribute to a better understanding of the potential advantages and limitations of NDP in the context of neoadjuvant therapy for locally advanced ESCC and guide the development of personalized and effective treatment strategies tailored to the specific needs of patients with thoracic segmental ESCC.
Data availability statement
The data presented in this study are available on request from the corresponding author due to protection of patient privacy.
Ethics statement
The studies involving humans were approved by The Institutional Ethics Committee of Fujian Cancer Hospital (YKT2020- 017-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. HL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. LT: Conceptualization, Funding acquisition, Methodology, Writing – original draft. HZ: Data curation, Investigation, Writing – original draft. JCL: Writing – review & editing. JW: Funding acquisition, Validation, Writing – review & editing. JLL: Funding acquisition, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare (2022) (F2227R-LJ01-01), Joint Funds for the innovation of science and Technology, Fujian province (2021Y9202, 2023Y9450), the National Clinical Key Specialty Construction Program (2021).
Acknowledgments
We sincerely appreciate the visualization work of Huimin Zhan and her encouragement to our team. Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The authors state that the views expressed in the submitted article are their own and not an official position statement of the institution or funder.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: A Cancer J clinicians. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Duan H, Shao C, Pan M, Liu H, Dong X, Zhang Y, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE). Front Immunol. (2022) 13:849984. doi: 10.3389/fimmu.2022.849984
3. Zhang G, Yuan J, Pan C, Xu Q, Cui X, Zhang J, et al. Multi-omics analysis uncovers tumor ecosystem dynamics during neoadjuvant toripalimab plus nab-paclitaxel and S-1 for esophageal squamous cell carcinoma: a single-center, open-label, single-arm phase 2 trial. EBioMedicine. (2023) 90:104515. doi: 10.1016/j.ebiom.2023.104515
4. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg (London England). (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
5. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: A prospective, single-arm, phase 2 trial. Front Immunol. (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
6. Yang Y, Zhang J, Meng H, Ling X, Wang X, Xin Y, et al. Neoadjuvant camrelizumab combined with paclitaxel and nedaplatin for locally advanced esophageal squamous cell carcinoma: a single-arm phase 2 study (cohort study). Int J Surg (London England). (2024) 110:1430–40. doi: 10.1097/js9.0000000000000978
7. Gong Y, Ren L, Zhou L, Zhu J, Huang M, Zhou X, et al. Phase II evaluation of nedaplatin and paclitaxel in patients with metastatic esophageal carcinoma. Cancer chemotherapy Pharmacol. (2009) 64:327–33. doi: 10.1007/s00280-008-0874-8
8. Alberto ME, Lucas MF, Pavelka M, Russo N. The second-generation anticancer drug Nedaplatin: a theoretical investigation on the hydrolysis mechanism. J Phys Chem B. (2009) 113:14473–9. doi: 10.1021/jp9056835
9. Alberto ME, Butera V, Russo N. Which one among the Pt-containing anticancer drugs more easily forms monoadducts with G and A DNA bases? A comparative study among oxaliplatin, nedaplatin, and carboplatin. Inorganic Chem. (2011) 50:6965–71. doi: 10.1021/ic200148n
10. Aboeita NM, Fahmy SA, El-Sayed MMH, Azzazy HME, Shoeib T. Enhanced anticancer activity of nedaplatin loaded onto copper nanoparticles synthesized using red algae. Pharmaceutics. (2022) 14(2):418. doi: 10.3390/pharmaceutics14020418
11. Sekine I, Nokihara H, Horiike A, Yamamoto N, Kunitoh H, Ohe Y, et al. Phase I study of cisplatin analogue nedaplatin (254-S) and paclitaxel in patients with unresectable squamous cell carcinoma. Br J cancer. (2004) 90:1125–8. doi: 10.1038/sj.bjc.6601700
12. Shukuya T, Yamanaka T, Seto T, Daga H, Goto K, Saka H, et al. Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial. Lancet Oncology. (2015) 16:1630–8. doi: 10.1016/s1470-2045(15)00305-8
13. Cao W, Xu C, Lou G, Jiang J, Zhao S, Geng M, et al. A phase II study of paclitaxel and nedaplatin as first-line chemotherapy in patients with advanced esophageal cancer. Japanese J Clin oncology. (2009) 39:582–7. doi: 10.1093/jjco/hyp058
14. Lu S, Chen Z, Hu C, Zhang J, Chen Y, Song Y, et al. Nedaplatin plus docetaxel versus cisplatin plus docetaxel as first-line chemotherapy for advanced squamous cell carcinoma of the lung - A multicenter, open-label, randomized, phase III trial. J thoracic oncology: Off Publ Int Assoc Study Lung Cancer. (2018) 13:1743–9. doi: 10.1016/j.jtho.2018.07.006
15. Mabuchi S, Morishige K, Isohashi F, Yoshioka Y, Takeda T, Yamamoto T, et al. Postoperative concurrent nedaplatin-based chemoradiotherapy improves survival in early-stage cervical cancer patients with adverse risk factors. Gynecologic oncology. (2009) 115:482–7. doi: 10.1016/j.ygyno.2009.09.002
16. Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncology. (2018) 19:461–73. doi: 10.1016/s1470-2045(18)30104-9
17. Tang QN, Liu LT, Qi B, Guo SS, Luo DH, Sun R, et al. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: A 5-year follow-up secondary analysis of a randomized clinical trial. JAMA network Open. (2021) 4:e2138470. doi: 10.1001/jamanetworkopen.2021.38470
18. Ohnuma H, Sato Y, Hirakawa M, Okagawa Y, Osuga T, Hayashi T, et al. A phase 1/2 study of definitive chemoradiation therapy using docetaxel, nedaplatin, and 5-fluorouracil (DNF-R) for esophageal cancer. Int J Radiat oncology biology physics. (2015) 93:382–90. doi: 10.1016/j.ijrobp.2015.05.041
19. Zhang MQ, Liu SP, Wang XE. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: preliminary results of a phase II study. Int J Radiat oncology biology physics. (2010) 78:821–7. doi: 10.1016/j.ijrobp.2009.08.069
20. Xu J, He X, Cheng K, Guo W, Bian X, Jiang X, et al. Concurrent chemoradiotherapy with nedaplatin plus paclitaxel or fluorouracil for locoregionally advanced nasopharyngeal carcinoma: Survival and toxicity. Head neck. (2014) 36:1474–80. doi: 10.1002/hed.23487
21. Du J, Hu C, Zhang Y, Hu B, Wang F, Zhang Y. A retrospective study of paclitaxel combining nedaplatin chemotherapy for esophageal cancer. Anti-cancer Drugs. (2015) 26:101–5. doi: 10.1097/cad.0000000000000170
22. Miyazaki T, Sohda M, Tanaka N, Suzuki S, Ieta K, Sakai M, et al. Phase I dose-escalation study of docetaxel, nedaplatin, and 5-fluorouracil combination chemotherapy in patients with advanced esophageal cancer. Cancer chemotherapy Pharmacol. (2013) 71:853–7. doi: 10.1007/s00280-013-2076-2
23. Ohashi T, Ohnishi M, Tanahashi S, Murai M. Efficacy and toxicity of concurrent chemoradiotherapy with nedaplatin and S-1 for head and neck cancer. Japanese J Clin oncology. (2011) 41:348–52. doi: 10.1093/jjco/hyq196
24. Torres M, Khan S, Duplanty M, Lozano HC, Morris TJ, Nguyen T, et al. Raman and infrared studies of platinum-based drugs: cisplatin, carboplatin, oxaliplatin, nedaplatin, and heptaplatin. J Phys Chem A. (2018) 122:6934–52. doi: 10.1021/acs.jpca.8b04023
25. He YF, Ji CS, Hu B, Fan PS, Hu CL, Jiang FS, et al. A phase II study of paclitaxel and nedaplatin as front-line chemotherapy in Chinese patients with metastatic esophageal squamous cell carcinoma. World J gastroenterology. (2013) 19:5910–6. doi: 10.3748/wjg.v19.i35.5910
26. Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J cancer. (2022) 151:128–37. doi: 10.1002/ijc.33976
27. Wang ZQ, Wang DS, Wang FH, Ren C, Tan Q, Li YH. Recombinant human endostatin plus paclitaxel/nedaplatin for recurrent or metastatic advanced esophageal squamous cell carcinoma: a prospective, single-arm, open-label, phase II study. Investigational New Drugs. (2021) 39:516–23. doi: 10.1007/s10637-020-01021-1
28. Uehara T, Yamate J, Torii M, Maruyama T. Comparative nephrotoxicity of Cisplatin and nedaplatin: mechanisms and histopathological characteristics. J toxicologic pathology. (2011) 24:87–94. doi: 10.1293/tox.24.87
29. Wang Y, Lu H, Wang D, Li S, Sun K, Wan X, et al. Inhibition of glutathione synthesis eliminates the adaptive response of ascitic hepatoma 22 cells to nedaplatin that targets thioredoxin reductase. Toxicol Appl Pharmacol. (2012) 265:342–50. doi: 10.1016/j.taap.2012.09.001
30. Kawai Y, Taniuchi S, Okahara S, Nakamura M, Gemba M. Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol pharmaceutical bulletin. (2005) 28:1385–8. doi: 10.1248/bpb.28.1385
31. Jin J, Xu X, Wang F, Yan G, Liu J, Lu W, et al. Second-line combination chemotherapy with docetaxel and nedaplatin for Cisplatin-pretreated refractory metastatic/recurrent esophageal squamous cell carcinoma. J thoracic oncology: Off Publ Int Assoc Study Lung Cancer. (2009) 4:1017–21. doi: 10.1097/JTO.0b013e3181add9c7
32. El-Shafie S, Fahmy SA, Ziko L, Elzahed N, Shoeib T, Kakarougkas A. Encapsulation of nedaplatin in novel PEGylated liposomes increases its cytotoxicity and genotoxicity against A549 and U2OS human cancer cells. Pharmaceutics. (2020) 12(9):863. doi: 10.3390/pharmaceutics12090863
33. Fahmy SA, Ponte F, Abd El-Rahman MK, Russo N, Sicilia E, Shoeib T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochimica Acta Part A Mol biomolecular spectroscopy. (2018) 193:528–36. doi: 10.1016/j.saa.2017.12.070
34. Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu B, et al. Dihydromyricetin Enhances the Chemo-Sensitivity of Nedaplatin via Regulation of the p53/Bcl-2 Pathway in Hepatocellular Carcinoma Cells. PloS One. (2015) 10:e0124994. doi: 10.1371/journal.pone.0124994
35. Yamashita H, Haga A, Takenaka R, Kiritoshi T, Okuma K, Ohtomo K, et al. Efficacy and feasibility of ambulatory treatment-based monthly nedaplatin plus S-1 in definitive or salvage concurrent chemoradiotherapy for early, advanced, and relapsed esophageal cancer. Radiat Oncol (London England). (2016) 11:4. doi: 10.1186/s13014-016-0587-9
36. Ohnuma H, Sato Y, Hayasaka N, Matsuno T, Fujita C, Sato M, et al. Neoadjuvant chemotherapy with docetaxel, nedaplatin, and fluorouracil for resectable esophageal cancer: A phase II study. Cancer science. (2018) 109:3554–63. doi: 10.1111/cas.13772
37. Shang QX, Chen LQ, Hu WP, Deng HY, Yuan Y, Cai J. Three-field lymph node dissection in treating the esophageal cancer. J thoracic disease. (2016) 8:E1136–e49. doi: 10.21037/jtd.2016.10.20
38. Zhong LZ, Xu HY, Zhao ZM, Zhang GM, Lin FW. Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating Malignant pleural effusion. OncoTargets Ther. (2018) 11:5509–12. doi: 10.2147/ott.S168391
39. Zhang F, Wang Y, Wang ZQ, Sun P, Wang DS, Jiang YX, et al. Efficacy and safety of cisplatin-based versus nedaplatin-based regimens for the treatment of metastatic/recurrent and advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis. Dis esophagus: Off J Int Soc Dis Esophagus. (2017) 30:1–8. doi: 10.1111/dote.12490
40. Ueda H, Kawakami H, Nonagase Y, Takegawa N, Okuno T, Takahama T, et al. Phase II trial of 5-fluorouracil, docetaxel, and nedaplatin (UDON) combination therapy for recurrent or metastatic esophageal cancer. oncologist. (2019) 24:163–e76. doi: 10.1634/theoncologist.2018-0653
41. Jingu K, Matsushita H, Takeda K, Umezawa R, Takahashi C, Sugawara T, et al. Long-term results of radiotherapy combined with nedaplatin and 5-fluorouracil for postoperative loco-regional recurrent esophageal cancer: update on a phase II study. BMC cancer. (2012) 12:542. doi: 10.1186/1471-2407-12-542
42. Yamashita H, Omori M, Takenaka R, Okuma K, Kobayashi R, Ohtomo K, et al. Involved-field irradiation concurrently combined with nedaplatin/5-fluorouracil for inoperable esophageal cancer on basis of (18)FDG-PET scans: a phase II study. Radiotherapy oncology: J Eur Soc Ther Radiol Oncology. (2014) 113:182–7. doi: 10.1016/j.radonc.2014.11.004
43. Naito Y, Kubota K, Ohmatsu H, Goto K, Niho S, Yoh K, et al. Phase II study of nedaplatin and docetaxel in patients with advanced squamous cell carcinoma of the lung. Ann oncology: Off J Eur Soc Med Oncology. (2011) 22:2471–5. doi: 10.1093/annonc/mdq781
44. Li Y, Zhou A, Liu S, He M, Chen K, Tian Z, et al. Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized Phase II clinical trial: A randomized clinical trial of neoadjuvant therapy for ESCC. BMC medicine. (2023) 21:86. doi: 10.1186/s12916-023-02804-y
45. Miyazaki T, Ojima H, Fukuchi M, Sakai M, Sohda M, Tanaka N, et al. Phase II study of docetaxel, nedaplatin, and 5-fluorouracil combined chemotherapy for advanced esophageal cancer. Ann Surg oncology. (2015) 22:3653–8. doi: 10.1245/s10434-015-4440-4
46. Isohashi F, Yoshioka Y, Mabuchi S, Konishi K, Koizumi M, Takahashi Y, et al. Dose-volume histogram predictors of chronic gastrointestinal complications after radical hysterectomy and postoperative concurrent nedaplatin-based chemoradiation therapy for early-stage cervical cancer. Int J Radiat oncology biology physics. (2013) 85:728–34. doi: 10.1016/j.ijrobp.2012.05.021
47. Isohashi F, Mabuchi S, Yoshioka Y, Seo Y, Suzuki O, Tamari K, et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose-volume histogram parameters. Radiat Oncol (London England). (2015) 10:180. doi: 10.1186/s13014-015-0486-5
48. Takahashi M, Takahashi K, Muguruma K, Ohira M, Nagayama K. Establishment of nedaplatin safety dose formula based on renal function for combination chemotherapy with nedaplatin and 5-fluorouracil in patients with esophageal cancer. Int J Clin oncology. (2022) 27:348–53. doi: 10.1007/s10147-021-02057-w
49. Oshita F, Ohe M, Honda T, Murakami S, Kondo T, Saito H, et al. Phase II study of nedaplatin and irinotecan with concurrent thoracic radiotherapy in patients with locally advanced non-small-cell lung cancer. Br J cancer. (2010) 103:1325–30. doi: 10.1038/sj.bjc.6605875
50. Yamashita H, Abe O, Nakagawa K. Involved-field irradiation concurrently combined with nedaplatin/5-fluorouracil for inoperable esophageal cancer on basis of 18FDG-PET scans: A long follow-up results of phase II study. Radiotherapy oncology: J Eur Soc Ther Radiol Oncology. (2017) 123:488. doi: 10.1016/j.radonc.2017.04.002
Keywords: nedaplatin, platinum, neoadjuvant chemoradiotherapy, esophageal squamous cell carcinoma, safety analysis, efficacy analysis
Citation: Zhuang Q, Li H, Tang L, Zheng H, Li J, Wu J and Li J (2025) Safety and efficacy analysis of neoadjuvant radiotherapy combined with concurrent paclitaxel plus nedaplatin versus other platinum-based chemotherapy for thoracic segmental esophageal squamous cell carcinoma. Front. Oncol. 15:1582481. doi: 10.3389/fonc.2025.1582481
Received: 24 February 2025; Accepted: 18 April 2025;
Published: 13 May 2025.
Edited by:
Ji-Feng Feng, University of Chinese Academy of Sciences, ChinaReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyYang Yang, University of Chinese Academy of Sciences, China
Copyright © 2025 Zhuang, Li, Tang, Zheng, Li, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinluan Li, bGlqaW5sdWFuQGZqbXUuZWR1LmNu; Junxin Wu, anVueGlud3VmakBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Qingyang Zhuang
Qingyang Zhuang Hui Li
Hui Li Lirui Tang
Lirui Tang Hongying Zheng1,2†
Hongying Zheng1,2† Jiancheng Li
Jiancheng Li Junxin Wu
Junxin Wu Jinluan Li
Jinluan Li