- 1Department of Medical Oncology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Department of Medical Oncology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 3Breast Center, Central Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Department of Radiotherapy, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 5Department of Chemotherapy, Zibo Central Hospital, Zibo, Shandong, China
Introduction: In mainland China, trastuzumab deruxtecan was first authorized in February 2023 as a monotherapy for the treatment of patients with HER-2-positive adult breast cancer who were either unresectable or had metastasized after receiving one or more anti-HER-2 treatments. In July 2023, trastuzumab deruxtecan was approved for the treatment of patients with metastatic disease who had undergone at least one previous systemic therapy, as well as those with unresectable or metastatic adult breast cancer who had low expression of HER-2 and who had experienced a relapse within six months of finishing adjuvant chemotherapy.
Methods: The study included seven participants with HER-2 low expression breast cancer and eighteen participants with HER-2-positive advanced breast cancer from six study centers in Shandong Province, China. Efficacy and safety data on trastuzumab deruxtecan were also gathered. The study involved intravenous injection of trastuzumab deruxtecan at a dosage of 5.4 mg/kg every three weeks until the disease progressed or the drug's toxicity became unmanageable, whichever came first.
Results and Discussion: During a 8-month follow-up period, the disease control rate for patients with HER-positive breast cancer was 88.89% (16/18). The disease control rate for patients with HER-2 low-expressing breast cancer was 85.71% (6/7). The most common adverse reactions were gastrointestinal reactions, such as nausea, which occurred in 64.00% (16/25), and interstitial lung disease, which had a probability of occurring in 4.00% (1/25). In this real-world study, trastuzumab deruxtecan showed favorable efficacy and safety in both HER-2-positive breast cancer and HER-2 low-expressing breast cancer.
1 Introduction
HER-2, a receptor found in 25-30% of breast cancer cases, is often associated with poorer clinical outcomes (1, 2). Despite the advent of targeted therapies like trastuzumab (3), lapatinib (4), patuzumab (5) and Trastuzumab Emtansine(T-DM1) (6), many patients continue to experience disease progression, highlighting the urgent need for more effective treatments.
Trastuzumab Deruxtecan(T-DXd) is a novel antibody-drug conjugate (ADC) that pairs trastuzumab, a monoclonal antibody targeting HER-2, with deruxtecan, a potent agent that inhibits topoisomerase I (7). By binding to HER-2 receptors on tumor cells, T-DXd delivers the cytotoxic drug directly to the cancer cells, and it also exerts a ‘ bystander effect ‘, targeting surrounding HER-2-positive cells (8).
T-DXd received approval in mainland China in early 2023 to treat HER-2-positive breast cancer, especially in patients whose disease is metastatic or incurable. The treatment was initially offered to patients who had previously received anti-HER-2 treatments. By mid-2023, however, the indication was broadened to encompass patients with HER-2-low expression (IHC 1+ or IHC 2+/ISH-), including those who experienced a relapse within six months of adjuvant chemotherapy, and those who had received at least one round of systemic therapy during the metastatic phase. This wider approval represents a major advancement in the treatment of breast cancer by providing patients with few options with additional therapeutic options.As of right now, T-DXd is approved globally to treat a number of malignancies, including lung, stomach, breast, and gastroesophageal junction adenocarcinoma (9–13).
T-DXd has proven to be highly effective in several important clinical trials. The 2019 San Antonio Conference revealed that, despite participants having had an average of six previous lines of therapy, T-DXd achieved an objective response rate (ORR) of 60.9%, a disease control rate (DCR) of 97.3%, and a progression-free survival (PFS) of 16.4% (14). In 2020, follow-up data revealed even better results: overall survival (OS) was 24.6 months, PFS was 19.4 months, and ORR was 61.4%. Nonetheless, drug-related pneumonia or interstitial lung disease (ILD) affected 15.8% of patients, highlighting the necessity of respiratory health monitoring during treatment (15).
The DESTINY-Breast03 trial further validated T-DXd’s superiority over T-DM1 in patients with advanced HER-2-positive breast cancer who had previously received treatment with trastuzumab and taxanes. T-DXd showed a median PFS evaluated by the researchers was 29.0 months, while it was 7.2 months for the T-DM1 group. The median OS was 52.6 months and 42.7 months respectively (16, 17). These results played a pivotal role in T-DXd’s FDA approval for patients with prior anti-HER-2 treatments. Thrombocytopenia, anemia, and neutropenia were the most often reported serious adverse effects.
In patients who had previously had therapy with T-DM1, T-DXd outperformed the physician’s selected treatment (TPC) in the DESTINY-Breast02 trial. The median progression-free survival (PFS) for T-DXd was 16.7 months, which was much longer than the 5.5 months for TPC, and the ORR was 74.1%, as opposed to 27.2% for TPC. This trial further established T-DXd as the recommended treatment for individuals who had not responded to previous treatments such as T-DM1; the median overall survival (OS) was 35.7 months as opposed to 25.0 months for TPC (18, 19).
The approval of T-DXd for patients with low HER-2 expression (IHC 1+ or IHC 2+/ISH-), a previously underserved category, represents a revolutionary step. Low HER-2 expression is seen in 45–55% of breast cancer cases in China, highlighting a serious therapeutic gap. T-DXd was shown to be successful for these patients in the DESTINY-Breast04 study, with a median OS of 23.4 months versus 16.8 months and a median PFS of 9.9 months compared to 5.1 months with physician-choice therapies. These findings highlight T-DXd’s revolutionary impact in changing the therapeutic landscape for patients with low HER-2 malignancies and have increased treatment options for these individuals (20). Among Asian patients with hormone receptor-positive mBC, the median PFS of T-DXd and TPC were 10.9 and 5.3 months, respectively, and 10.9 and 4.6 months, respectively in the entire Asian population. Among these two populations, the median OS of patients with T-DXd was not achieved, and the median OS of patients with TPC was 19.9 months (21).In the recent DESTINY-Breast06study when comparing T-DXd with TPC(capecitabine/paclitaxel), the median PFS of T-DXd was significantly prolonged in patients with extremely low HER-2 expression (IHC 0+) (13.3 months vs 8.5 months). Support the FDA’s approval of T-DXd for the indication of breast cancer with ultra-low expression of HR+/HER-2- in January 2025 (22).
With an emphasis on patients with HER-2-positive and HER-2-low expressing breast cancer, this study attempts to further assess T-DXd’s safety and effectiveness in the Chinese population, building on the increasing body of evidence. T-DXd is in a strong position to meet the unmet requirements of patients with metastatic or incurable disease because of its growing indications and growing clinical data. This study aims to advance global knowledge of T-DXd’s potential in both HER-2-positive and HER-2-low subgroups by providing additional insights into its practical utilization.
2 Materials and methods
2.1 Study population
This observational study took place between November 2023 and October 2024 at eight medical institutes in Shandong Province, China. The study was approved by the ethical committee of Qilu Hospital, Shandong University. All participants provided informed consent after being fully apprised of the study’s purpose and procedures. The trial followed Good Clinical Practice (GCP) principles throughout.
The trial included adult patients with metastatic or inoperable breast cancer. Participants have to have HER-2 IHC 3+ or HER-2 IHC 2+ and a confirmed positive fluorescence in situ hybridization (FISH) result. Furthermore, patients with low HER-2 expression (IHC 1+ or IHC 2+/ISH-) who had relapsed within six months of adjuvant chemotherapy or had undergone at least one course of systemic therapy during the metastatic phase were eligible.
Eligibility criteria for participation included being 18 years or older, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Each participant had to have at least one measurable lesion according to the RECIST 1.1 criteria.
Patients needed to meet the following clinical parameters: left ventricular ejection fraction (LVEF) ≥50%, absolute neutrophil count ≥1500 cells/mm³, hemoglobin levels ≥90 g/L, platelet count ≥100,000 cells/mm³, and liver enzyme levels (AST and ALT) ≤2.5 times the upper limit of normal (ULN). Bilirubin levels were required to be ≤1.5×ULN.
Within three weeks of screening, patients could not have received anti-HER-2 antibody-drug conjugates (ADCs), chemotherapy, hormonal therapy, radiation, or surgery. Patients with symptomatic chronic heart failure, severe arrhythmias, substantial systemic illnesses, human immunodeficiency virus, hepatitis B infection, pregnancy, breastfeeding, known hypersensitivity to T-DXd, or other contraindications were excluded.
2.2 Treatment methods
During the first cycle, patients received an intravenous infusion of T-DXd at a rate of 5.4 mg/kg over 90 minutes. In following cycles, the infusion period was shortened to 30 minutes. Treatment was scheduled every three weeks until disease progression or severe adverse events developed, whichever came first. Patients who encountered severe adverse events that required treatment termination were classed as having uncontrolled toxicity.
To avoid or reduce infusion-related responses, premedication for each infusion consisted of a mix of analgesics, antipyretics, and antihistamines. According to the study design, palliative care, ondansetron, and palonosetron were among the additional supportive medications that were given as needed.
2.3 Efficacy and toxicity
Evaluations were carried out utilizing the RECIST 1.1 criteria in order to gauge the tumor response (23). Twelve-week intervals were allotted for these exams, with more frequent evaluations occurring if there was indication of accelerated illness progression.
The disease control rate (DCR) and objective response rate (ORR) were the main indicators of efficacy in this study. The DCR represented the percentage of patients with an evaluable response who obtained either a complete response (CR), partial response (PR), or stable disease (SD). The percentage of CR and PR is reflected in the ORR. Complete Response (CR): This is defined as the total disappearance of all target lesions, no appearance of new lesions, and stable tumor markers for a minimum duration of 4 weeks.Partial Response (PR): A decrease of at least 30% in the largest diameter of the target lesions, sustained for a minimum of 4 weeks. Stable Disease (SD): A condition where the sum of the diameters of target lesions changes by no more than a 30% decrease or a 20% increase.
Furthermore, adverse events (AEs) were closely tracked and classified using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. A tiny percentage of patients on T-DXd may experience interstitial lung disease. We performed a baseline assessment before to treatment, which comprised a baseline imaging examination, a baseline pulmonary function test, a baseline laboratory test, and a collection of the respiratory system history. Frequent imaging monitoring was performed once every 6–8 weeks during the course of treatment. It is imperative that lung function tests be performed again very away if there are any new respiratory symptoms or if imaging changes are suspected.
2.4 Statistical analysis
For the analysis of efficacy outcomes, statistical comparisons were made between the HER-2-positive and HER-2-low expressing breast cancer subgroups. Categorical data were analyzed using either Chi-square tests or Fisher’s exact tests, depending on the characteristics of the data. The Kaplan-Meier test was used to estimate PFS. The statistical and graphical software used was SPSS software (version 24.0), Graphpad, and the statistical significance threshold was set at p≤ 0.05.
3 Results
3.1 Attributes of patients
A total of 25 participants were included in the study, comprising 18 individuals with HER-2-positive breast cancer and 7 with HER-2-low expressing breast cancer. The participants’ ages ranged from 33 to 73 years, with a median age of 55. Among the group, 60% (15/25) were hormone receptor-positive. Every subject had distant metastases; 40% (10/25) had brain involvement, and 92% (23/25) had visceral metastases. Before enrolling in the study, every patient had received at least one prior line of systemic treatment. Table 1 lists all of the individuals’ baseline characteristics in detail.
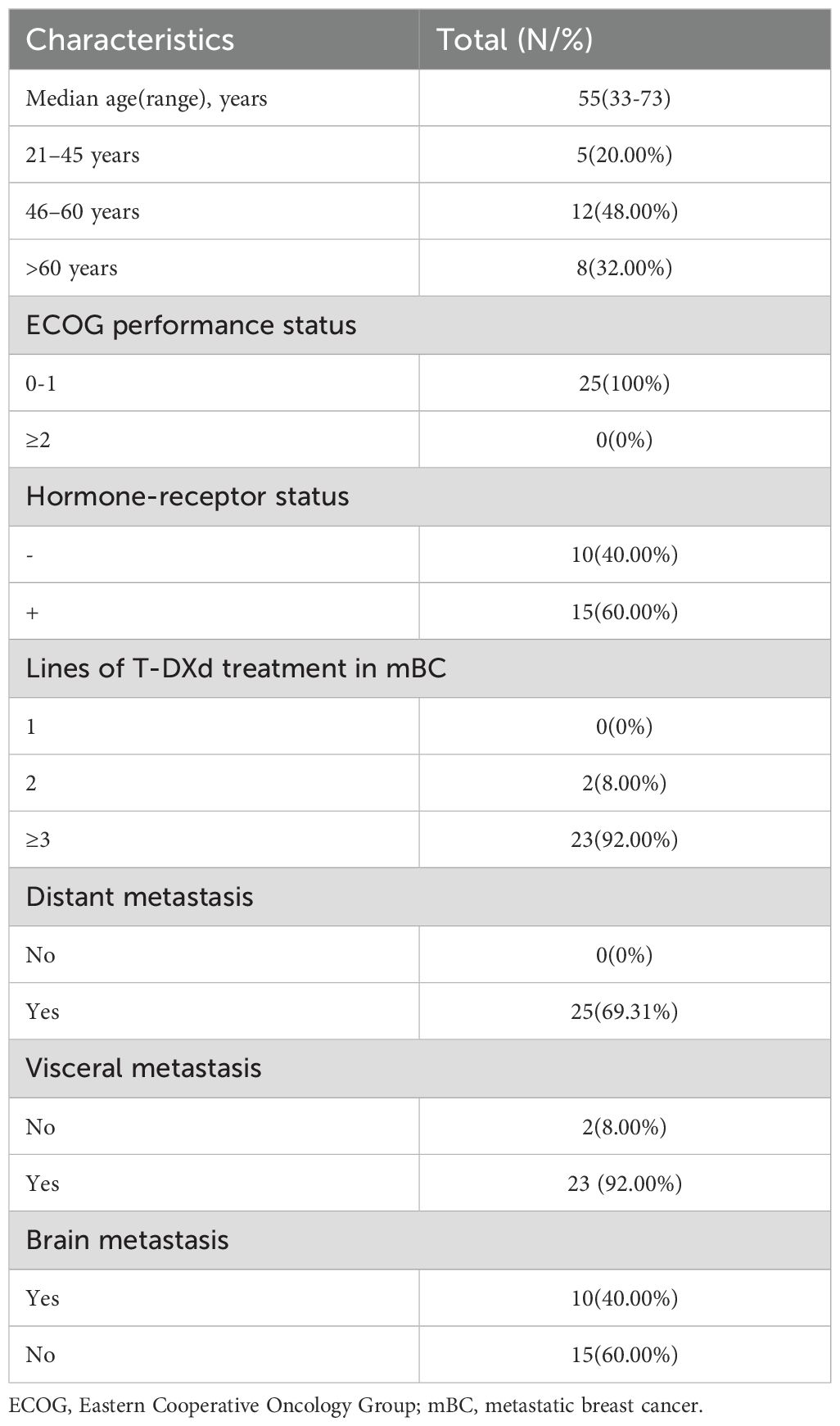
Table 1. Baseline clinicopathological and disease characteristics of 25 breast cancer patients enrolled in the study.
3.2 Treatment administration
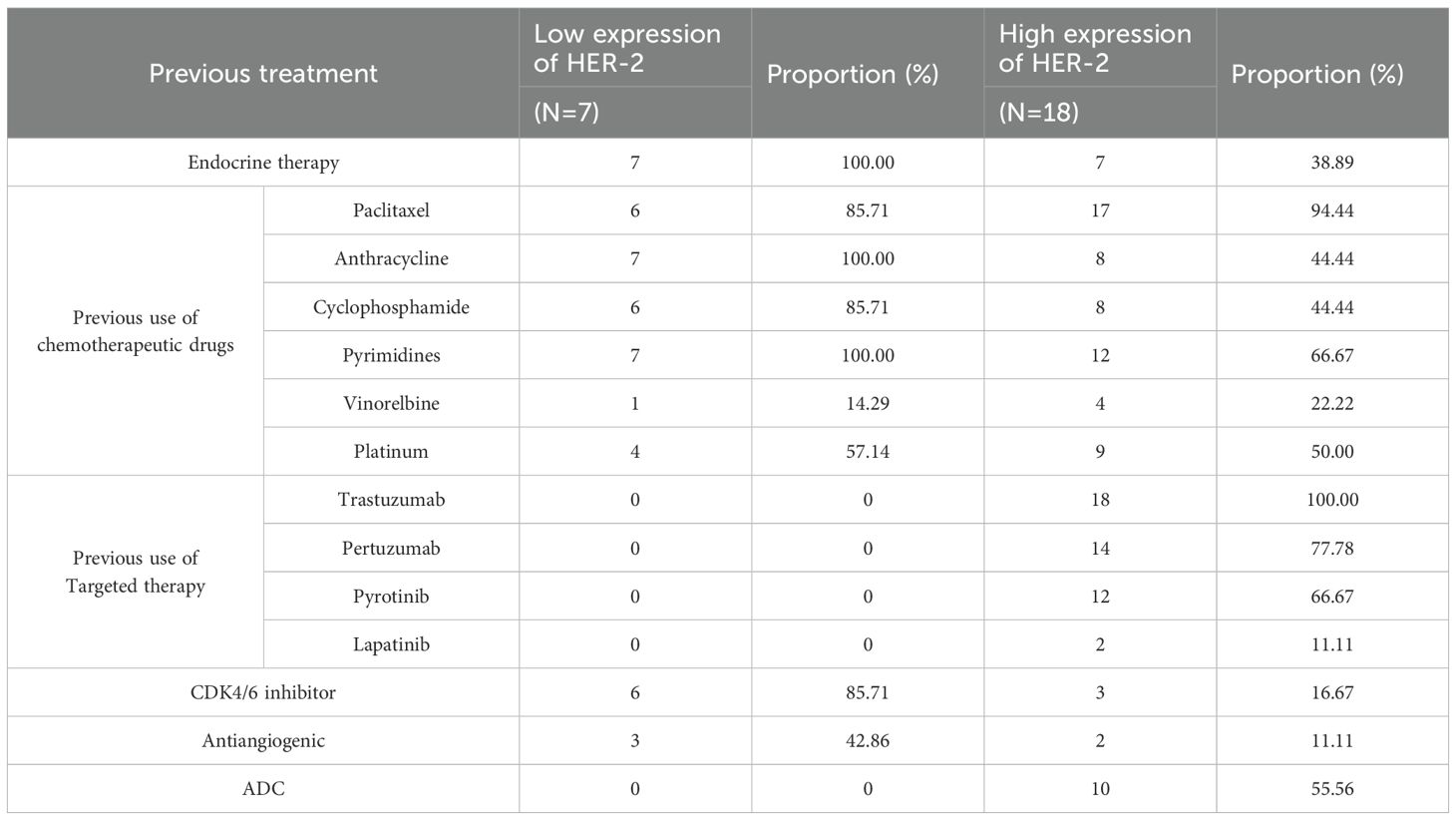
Participants have received a variety of treatment plans prior to enrollment. Table 2 provides an overview of their past medical history and treatment measures. The median follow-up time was 9.1 months.
3.3 Clinical efficacy
Key outcomes such ORR, DCR, and the median progression-free survival (mPFS) for both HER-2-positive and HER-2-low expressing subsets of breast cancer were used to evaluate the therapeutic efficacy of T-DXd. The mPFS was 6.3 months for patients with low HER-2 expression and 8.7 months for the high-expression group. As detailed in Table 3 and Figure 1. There was no statistically significant difference in ORR (p=0.899 95%CI 0.19 ~ 4.38) DCR (p=0.270 95%CI 0.22 ~ 1.53) and mPFS (p= 0.24 95%CI 0.65 -5.40) compared to patients with high and low HER-2 expression. There were also no statistically significant differences observed between subgroups when comparing their clinical outcomes.
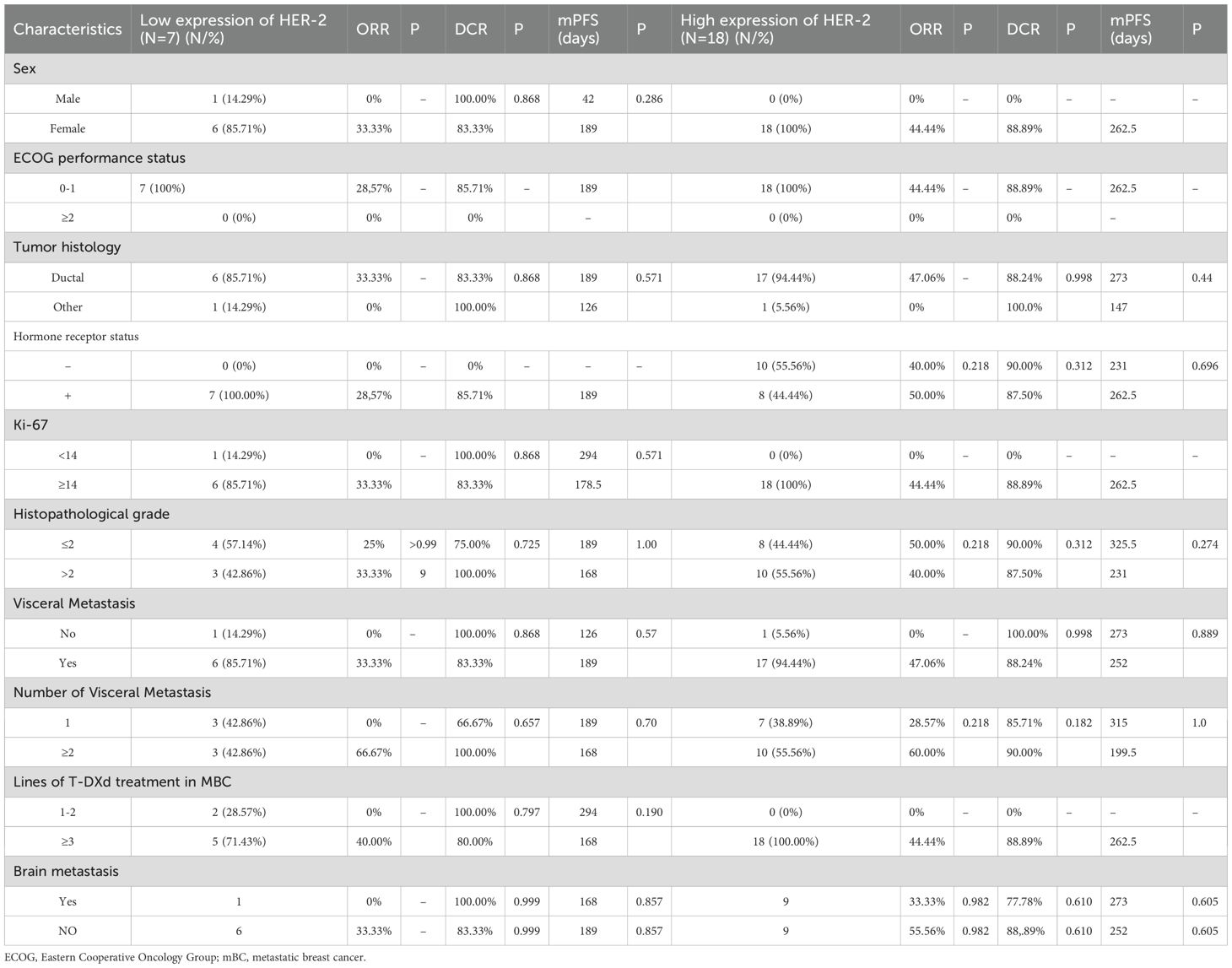
Table 3. Evaluation of efficacy in patients with different clinicopathologic and disease characteristics.
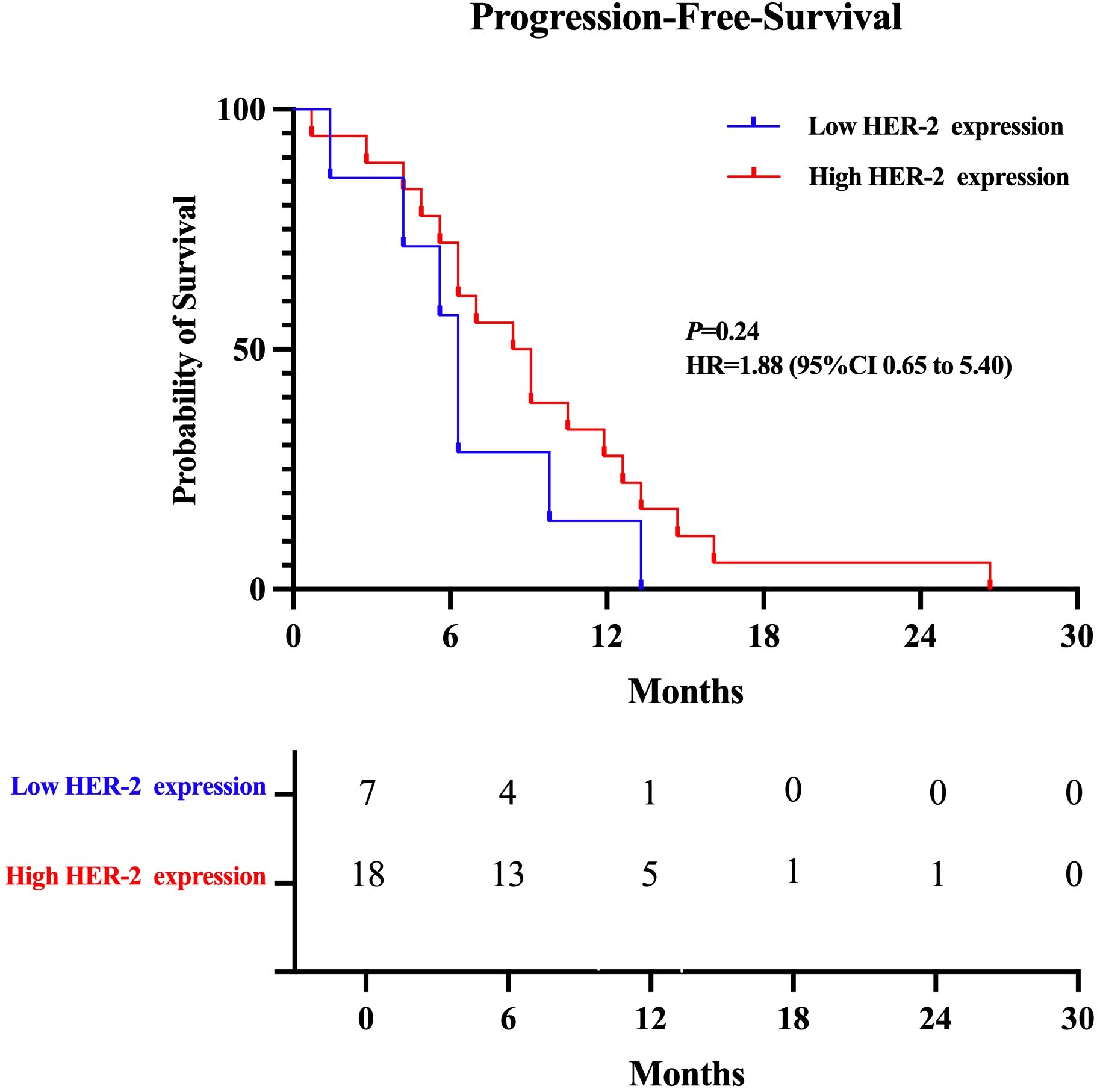
Figure 1. Kaplan-Meier plot of the progression-free survival (PFS) of T-Dxd in patients with low and high HER-2 expression.
3.4 Safety
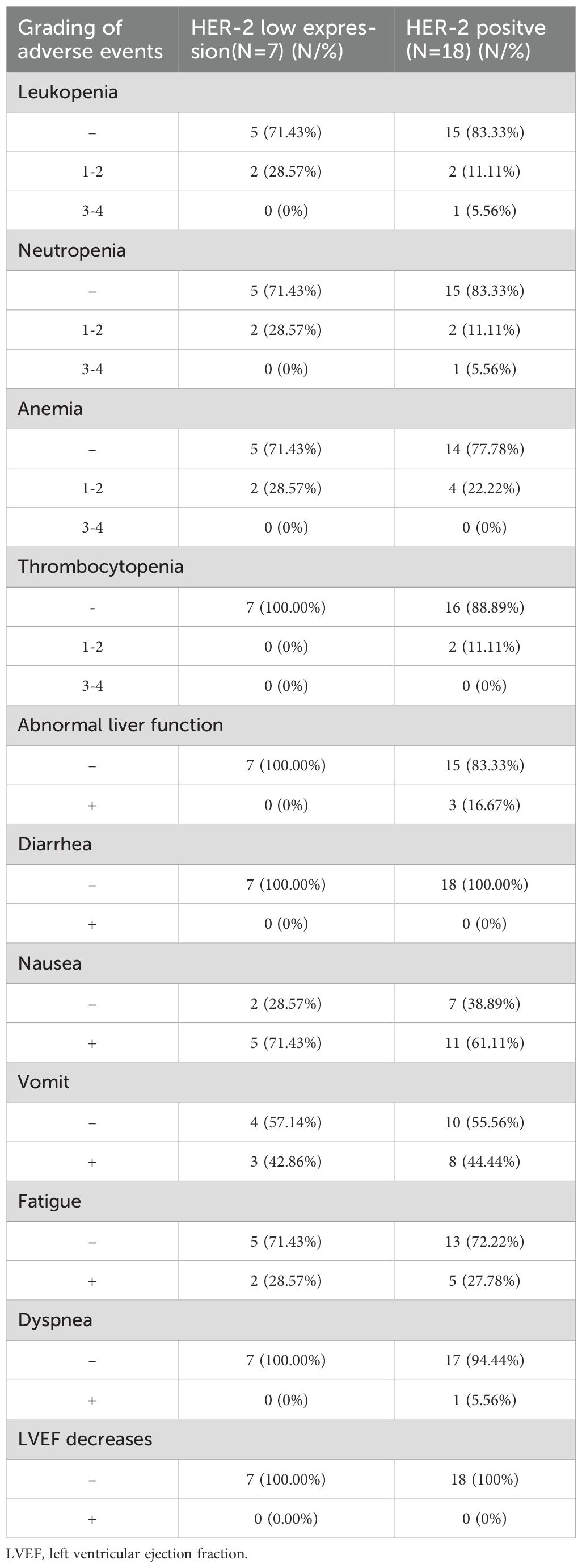
A total of 25 patients were monitored for treatment-related adverse events (AEs). In total, 61.1% (11/18) of the HER-2-positive group and 71.43% (5/7) of the HER-2-low expressing group experienced treatment-related adverse events. Gastrointestinal side effects were the most frequently reported. 71.43% (5/7) of the patients with low HER-2 expression and 61.1% (11/18) of the patients with HER-2-positive expressed nausea. Vomiting occurred in 44.44% (8/18) of HER-2-positive patients and 42.86% (3/7) of the HER-2-low expressing patients. 28.57% (2/7) of patients with HER-2-low expression and 27.78% (5/18) of patients with HER-2-positive expressed fatigue. However, all of these reactions were grade 1-2.
Other adverse events included anemia in 24% (6/25) of the total cohort, and neutropenia in 20% (5/25) of patients. Other adverse reactions included anemia in 24% (6/25) of patients and neutropenia in 20% (5/25), with one case rated grade 3. Hematologic and gastrointestinal toxicities were most common, but most were grade 1-2. Of note, one (1/25) patient experienced dyspnea, which resolved completely after two days of oxygen therapy and steroid medication. This adverse reaction was rated as grade 3.
A full list of the adverse events is provided in Table 4. Overall, the adverse events were manageable, and symptomatic treatments such as Aprepitant and ondansetron were used to alleviate discomfort, enabling most patients to continue their treatment with T-DXd.
4 Discussion
T-DXd has emerged as a significant therapeutic option for metastatic breast cancer overexpressing HER-2, demonstrating robust clinical efficacy in recent studies. Typically, initial treatment for HER-2-positive breast cancer consists of a combination of monoclonal antibodies targeting HER-2—pertuzumab and trastuzumab—alongside chemotherapy based on taxanes (5, 24, 25).
However, as shown by the results of the EMILIA trial, T-DM1 is frequently regarded as the next line of treatment for patients whose condition worsens following first-line medications (6).
Recent studies have highlighted the better PFS reported with T-DXd. In particular, 75.8% of patients treated with T-DXd were progression-free and alive at 12 months, compared to only 34.1% in the T-DM1 cohort. Furthermore, an astonishing 96.6% of patients who received T-DXd had disease control. These findings were consistent across all subgroups, including patients with varying hormone receptor statuses, those who had previously received pertuzumab, and those with visceral or brain metastases (26).
T-DXd showed good efficacy in both HER-2-positive and HER-2 low expression metastatic BC patients (16, 20). Our work supports these findings by demonstrating encouraging results in both HER-2-positive and HER-2-low expressing breast cancer patients.
One significant feature of T-DXd is its ability to efficiently target tumors with low levels of HER-2. The DESTINY-Breast04 trial reported that the median PFS of patients with low HER-2 expression was 9.9 months and the DCR was 87.9%. In our study, while the DCR for the HER-2-low subgroup was slightly lower at 85.71% and the median PFS was 6.3 months, these results still highlight the potential of T-DXd for this previously underrepresented group of patients (20). Several factors could explain the minor discrepancies found in our investigation. Specifically, the HER-2-low subgroup was tiny, with only seven patients. Furthermore, a large proportion of patients got T-DXd as a third-line treatment or later, which could have impacted the clinical results seen.
Brain metastases continue to be a significant difficulty in treating metastatic HER-2-positive breast cancer. Animal studies have shown that T-DXd can slow tumor progression and enhance survival outcomes in both HER-2-positive and HER-2-negative brain metastases (27), We found no statistically significant difference in PFS between patients with and without brain metastases in our cohort. This lack of significance is most likely owing to the small sample size, which reduced the statistical power of our analysis. Nonetheless, our findings support the notion that T-DXd could be a viable treatment option for individuals with brain metastases, though bigger trials are required to confirm these promising findings.
In terms of safety, the adverse event profile of T-DXd corresponds with that of T-DM1, albeit the frequency of major side effects such as interstitial lung disease (ILD) or pneumonia was significantly greater in some studies, including 10.5% of patients in the T-DXd group against 1.9% for T-DM1 (26). In the Chinese population, common gastrointestinal issues like nausea, vomiting, and fatigue were reported. In the HER-2-low category, 52.6% had grade 3 or higher adverse events, while 12.1% developed ILD or pneumonia (20) Our findings revealed a lower occurrence of grade 3 or higher adverse events, with the majority of side effects being moderate (grades 1 or 2). This could be linked to the proactive use of antiemetic medications to alleviate the intensity of gastrointestinal symptoms.
While the adverse effects were usually treatable, problems such as ILD remain significant concerns. Symptoms such as shortness of breath, cough, and fatigue can worsen in severe cases, sometimes leading to respiratory distress (28, 29) The precise processes behind these toxicities remain unknown, and more research is needed to better understand the dangers associated with these side effects (30, 31).
The median time to first ILD/pneumonia was 5.5 months. Most of the T-DXd-related ILD/pneumonia adverse effects were of modest grade (32). Several studies have reported factors that may be associated with T-DXd-associated ILD/pneumonia, including T-DXd dose, baseline oxygen saturation (SpO2), moderate/severe renal impairment, and the presence of certain pulmonary comorbidities (32, 33). Only one patient developed dyspnea in this study, which may be due to the small number of cases and the fact that follow-up did not reach the median time for ILD/pneumonia.
This retrospective study has some limitations. Firstly, since T-DXd is not included in China’s medical insurance, the actual number of patients using it is relatively small, which may lead to result deviations. In addition, the follow-up time of the patients was relatively short. Therefore, the long-term efficacy of T-DXd in different types of populations, such as PFS or OS, requires further verification through large prospective cohort studies.
This real-world study provides solid evidence for T-DXd’s therapeutic benefits in treating metastatic breast cancer, both HER-2-positive and HER-2-low expressing subtypes. While the small number of patients and short duration of follow-up are acknowledged limitations, the findings indicate that T-DXd is a potential therapeutic option for people with metastatic breast cancer. Additional large-scale and long-term investigations are required to validate and expand on these favorable findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Qilu Hospital, Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because patients’ informed consent was exempted after review by this ethics committee.
Author contributions
MH: Data curation, Formal Analysis, Methodology, Writing – original draft. XL: Data curation, Writing – review & editing. YZ: Data curation, Writing – review & editing. ZS: Data curation, Writing – review & editing. TS: Data curation, Writing – review & editing. YH: Conceptualization, Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant number: 81600092), the Natural Science Foundation of Shandong Province (No. ZR2023QH292) and the horizontal project Molecular Characterization of Breast Cancer with ESR1 Gene Mutation and Relevance to Treatment (260101120023BL).
Acknowledgments
Thanks to all the researchers who participated in the data collection and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, and McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the her-2/neu oncogene. Sci (New York NY). (1987) 235:177–82. doi: 10.1126/science.3798106
2. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the her-2/neu proto-oncogene in human breast and ovarian cancer. Sci (New York NY). (1989) 244:707–12. doi: 10.1126/science.2470152
3. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. New Engl J Med. (2001) 344:783–92. doi: 10.1056/nejm200103153441101
4. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for her2-positive advanced breast cancer. New Engl J Med. (2006) 355:2733–43. doi: 10.1056/NEJMoa064320
5. Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. New Engl J Med. (2012) 366:109–19. doi: 10.1056/NEJMoa1113216
6. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for her2-positive advanced breast cancer. New Engl J Med. (2012) 367:1783–91. doi: 10.1056/NEJMoa1209124
7. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. Ds-8201a, a novel her2-targeting adc with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-dm1. Clin Cancer Res: An Off J Am Assoc Cancer Res. (2016) 22:5097–108. doi: 10.1158/1078-0432.Ccr-15-2822
8. Ogitani Y, Hagihara K, Oitate M, Naito H, and Agatsuma T. Bystander killing effect of ds-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. (2016) 107:1039–46. doi: 10.1111/cas.12966
9. Mauricio D, Bellone S, Mutlu L, McNamara B, Manavella DD, Demirkiran C, et al. Trastuzumab deruxtecan (Ds-8201a), a her2-targeting antibody-drug conjugate with topoisomerase I inhibitor payload, shows antitumor activity in uterine and ovarian carcinosarcoma with her2/neu expression. Gynecol Oncol. (2023) 170:38–45. doi: 10.1016/j.ygyno.2022.12.018
10. Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (Ds-8201), a her2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. (2017) 18:1512–22. doi: 10.1016/s1470-2045(17)30604-6
11. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated her2-positive gastric cancer. New Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
12. Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and europe with her2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (Destiny-gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. (2023) 24:744–56. doi: 10.1016/s1470-2045(23)00215-2
13. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with her2-expressing solid tumors: primary results from the destiny-pantumor02 phase ii trial. J Clin Oncol: Off J Am Soc Clin Oncol. (2024) 42:47–58. doi: 10.1200/jco.23.02005
14. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated her2-positive breast cancer. New Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
15. Saura C, Modi S, Krop I, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated patients with her2-positive metastatic breast cancer: updated survival results from a phase ii trial (Destiny-breast01). Ann Oncol: Off J Eur Soc Med Oncol. (2024) 35:302–7. doi: 10.1016/j.annonc.2023.12.001
16. Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with her2-positive metastatic breast cancer: updated results from destiny-breast03, a randomised, open-label, phase 3 trial. Lancet (London England). (2023) 401:105–17. doi: 10.1016/s0140-6736(22)02420-5
17. Cortés J, Hurvitz SA, Im SA, Iwata H, Curigliano G, Kim SB, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in her2-positive metastatic breast cancer: long-term survival analysis of the destiny-breast03 trial. Nat Med. (2024) 30:2208–15. doi: 10.1038/s41591-024-03021-7
18. André F, Hee Park Y, Kim SB, Takano T, Im SA, Borges G, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with her2-positive metastatic breast cancer (Destiny-breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet (London England). (2023) 401:1773–85. doi: 10.1016/s0140-6736(23)00725-0
19. Fehm T, Cottone F, Dunton K, André F, Krop I, Park YH, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with her2-positive metastatic breast cancer (Destiny-breast02): patient-reported outcomes from a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. (2024) 25:614–25. doi: 10.1016/s1470-2045(24)00128-1
20. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated her2-low advanced breast cancer. New Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
21. Yamashita T, Sohn JH, Tokunaga E, Niikura N, Park YH, Lee KS, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in previously treated asian patients with her2-low unresectable/metastatic breast cancer: subgroup analysis of the destiny-breast04 study. Breast Cancer (Tokyo Japan). (2024) 31:858–68. doi: 10.1007/s12282-024-01600-7
22. Wang F and Yuan P. Destiny-breast06 continues to explore hr(+), her2-negative metastatic breast cancer beyond destiny-breast04. Trans Breast Cancer Res: J Focusing Trans Res Breast Cancer. (2025) 6:11. doi: 10.21037/tbcr-24-51
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised recist guideline (Version 1.1). Eur J Cancer (Oxford England: 1990). (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
24. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th eso-esmo international consensus guidelines for advanced breast cancer (Abc 5). Ann Oncol: Off J Eur Soc Med Oncol. (2020) 31:1623–49. doi: 10.1016/j.annonc.2020.09.010
25. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in her2-positive metastatic breast cancer. New Engl J Med. (2015) 372:724–34. doi: 10.1056/NEJMoa1413513
26. Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. New Engl J Med. (2022) 386:1143–54. doi: 10.1056/NEJMoa2115022
27. Kabraji S, Ni J, Sammons S, Li T, Van Swearingen AED, Wang Y, et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res: An Off J Am Assoc Cancer Res. (2023) 29:174–82. doi: 10.1158/1078-0432.Ccr-22-1138
28. Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, et al. Chest ct diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the fleischner society. Chest. (2021) 159:1107–25. doi: 10.1016/j.chest.2020.11.027
29. Kubo K, Azuma A, Kanazawa M, Kameda H, Kusumoto M, Genma A, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Invest. (2013) 51:260–77. doi: 10.1016/j.resinv.2013.09.001
30. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. (2012) 13:39. doi: 10.1186/1465-9921-13-39
31. Kumagai K, Aida T, Tsuchiya Y, Kishino Y, Kai K, and Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting ab-drug conjugate, in monkeys. Cancer Sci. (2020) 111:4636–45. doi: 10.1111/cas.14686
32. Soares LR, Vilbert M, Rosa VDL, Oliveira JL, Deus MM, and Freitas-Junior R. Incidence of interstitial lung disease and cardiotoxicity with trastuzumab deruxtecan in breast cancer patients: A systematic review and single-arm meta-analysis. ESMO Open. (2023) 8:101613. doi: 10.1016/j.esmoop.2023.101613
Keywords: trastuzumab deruxtecan, antibody-drug conjugate, DCR, ILD, breast cancer
Citation: He M, Liu X, Zhang Y, Shao Z, Sun T and Hu Y (2025) The efficacy of trastuzumab deruxtecan in Chinese breast cancer patients: a real-world multicenter study. Front. Oncol. 15:1582498. doi: 10.3389/fonc.2025.1582498
Received: 24 February 2025; Accepted: 26 July 2025;
Published: 18 August 2025.
Edited by:
Vivek Podder, Mount Sinai Medical Center, United StatesReviewed by:
Jan Trøst Jørgensen, Dx-Rx Institute, DenmarkRyan Varghese, Saint Joseph’s University, United States
Copyright © 2025 He, Liu, Zhang, Shao, Sun and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Hu, aHV5dXNkdUAxNjMuY29t
 Miao He
Miao He Xiaohui Liu3
Xiaohui Liu3 Yu Hu
Yu Hu