- 1Department of Neurology, Wroclaw Medical University, Wrocław, Poland
- 2Department of Pulmonology and Lung Oncology, Wroclaw Medical University, Wrocław, Poland
Introduction: Brain metastases are common and devastating complication of the lung cancer (LC) but predictive biomarkers for their risk are still lacking.
Objectives: To analyze the relationships between complete blood count (CBC)-based and selected biochemical indices and occurrence of brain metastases in patients diagnosed with LC.
Patients and methods: The study was based on retrospective analysis of medical records of 217 patients diagnosed with LC and undergoing follow-up in one specialist center. Clinical and laboratory data on admission were determined, including: neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), neutrophil-to-platelet ratio (NPR) and red cell distribution width-to-platelet ratio (RPR) and selected biochemical parameters. Relationships were evaluated between these parameters and occurrence of brain metastases, other distant metastases and death within 12 months of follow-up.
Results: 168 patients had the follow-up data for 6 months, and 128 - for 12 months. Brain metastases were detected in 41 patients and 1-year mortality rate was 17.61%. Patients who developed brain metastases during 12 months had significantly higher baseline NLR (4.66 vs 2.75, p<0.001), PLR (170.83 vs 142.42, p=0.03) and lower LMR (1.61 vs 2.33, p=0.008).In univariate analysis, higher leukocyte count (HR 1.08, p=0.016), neutrophil count (HR 1.11, p=0.0036), NLR (HR 1.09, p=0.005), d-dimer levels (HR 1.0002, p=0.0043), and lower LMR (HR 0.67, p=0.018) were significantly associated with the risk of developing brain metastases. Liver metastases were associated with lower LMR (1.69 vs 2.29, p=0.04), while metastases to the other lung – with lower PLR (126.52 vs 161.8, p=0.02) and higher LMR (2.51 vs 1.96, p=0.02) and RPR (0.184 vs 0.154, p=0.03). No significant relationships were found between CBC-based indices and mortality.
Conclusions: CBC-based indices could be useful and easily accessible predictive markers of brain metastases in the patients with lung cancer.
1 Introduction
Lung cancer is the most common cause of metastases to the central nervous system (CNS) and accounts for about 39-56% of all brain metastases (1). Due to raised intracranial pressure and focal symptoms of neurological deficit, metastatic brain tumors are associated with devastating impact on the patient’s condition as well as with poor prognosis. The overall median survival time since the time of diagnosis of brain metastases was estimated at 15.2 months for adenocarcinoma and 9.2 months for other types of non-small-cell lung cancer (NSCLC) (2). Thus, early identification of risk factors for neoplastic dissemination to CNS seems crucial for monitoring of the patients and optimal therapeutic decisions, aimed at the improvement of prognosis.
The inflammatory process is one of the relevant mechanisms of tumor progression and dissemination (3), therefore markers of the inflammatory response have been intensively studied for their predictive value in lung cancer. Particular attention has been paid to indices based on complete blood count (CBC), especially because of their accessibility and ease of identification. The best-known indicator with proven usefulness is the NLR (neutrophil-to-lymphocyte ratio), considered a prognostic factor in patients with solid tumors (4). In NSCLC, NLR was shown to correlate negatively with mean survival time (5), correspond with the prognosis after curative surgical resection (6) and response to chemotherapy (7) or immunotherapy (8). Other potentially prognostic markers that have been assessed in patients with lung cancer include PLR (platelet-to-lymphocyte ratio) (9) and LMR (lymphocyte-to-monocyte ratio) (10). Prognostic value in several malignancies was also suggested for other CBC indices such as red cell distribution width-to-platelet ratio (RPR) (11, 12) or neutrophil-to-platelet ratio (NPR), also considered indicators of an acute inflammatory response (13). These indices can be applied as single markers or as components of diagnostic/prognostic panels, e.g. advanced lung cancer inflammation index (ALI), which includes: body mass index, serum concentration of albumin and NLR, and has been used as a predictor of response to treatment with PD-L1 inhibitors (14). However, there is little evidence for the predictive value of these indices with regard to the occurrence of brain metastases in lung cancer (15, 16), as they have not been consistently investigated in this field.
2 Aim
The aim of the study was to analyze the relationships between CBC – based and selected biochemical indices and the occurrence of brain metastases in patients diagnosed with lung cancer and to evaluate predictive usefulness of these indices.
3 Patients and methods
3.1 Patients
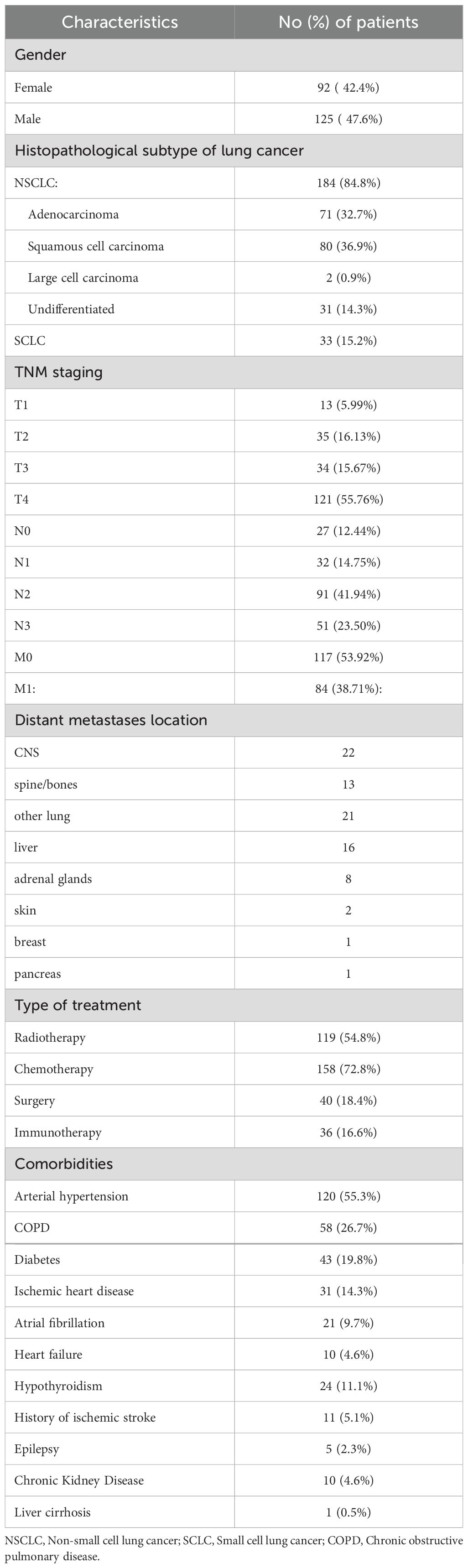
The study was based on the retrospective analysis of the medical records from the Department of Pulmunology and Lung Oncology of the Lower Silesian Centre of Oncology, Lung Diseases and Haematology (Wroclaw, Poland). Patients diagnosed with lung cancer between 2016 and 2022, with documented follow-up for at least 3 months (unless they died before reaching this time point), were included in the study group. Exclusion criteria comprised active infection, concomitant inflammatory/autoimmune disorders, other malignancies on admission and current corticosteroid use, as well as lack of brain imaging results (computed tomography - CT or magnetic resonance imaging - MRI) in the documented follow-up. The final cohort consisted of 217 patients, aged 41–91 years. The diagnosis of lung cancer in all patients was confirmed with histopathological examination.
Age, sex, clinical and laboratory findings on admission were extracted from each patient’s medical records and anonymized/coded for further analysis. The following laboratory findings were determined: CBC with automated differential counts, serum level of C-reactive protein (CRP), d-dimers, glucose, electrolytes, parameters of kidney and liver function. On the basis of CBC, the following indices were calculated: NLR, PLR, LMR, NPR and RPR. Clinical data included histopathological subtype of lung cancer (small-cell lung cancer – SCLC, and NSCLC: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, undifferentiated), TNM Classification of Malignant Tumours staging at the time of diagnosis, and comorbidities (Table 1). Relationships were evaluated between CBC-based indices (and other initial laboratory findings) and occurrence of brain metastases within 6 and 12 months of follow-up. Other outcomes of the lung cancer included in the analysis were metastases to other organs and death.
All the procedures were performed in accordance with the Declaration of Helsinki and its further amendments. Informed consent was not required for this study as it was conducted retrospectively and involved the analysis of previously collected data. The project of the study was approved by the Wroclaw Medical University Bioethics Committee (approval no. KB-918/2021).
3.2 Statistical analysis
Categorical variables were presented as numerical and percentage values and continuous ones – as the mean and standard deviation (SD) or median and interquartile range. The χ2 test, Student’s t-test and Pearson’s correlation coefficient were used for normally distributed data, and non-parametric tests (Mann–Whitney U test and Spearman’s correlation coefficient) were used for other variables.
After establishing differences between analyzed groups, Cox proportional hazards regression was used to evaluate the association between potentially significant laboratory variables and occurrence of brain metastases during one year of follow up. Time-to-event was measured in months, defined as the duration from the enrollment to the event of interest (death or the diagnosis of metastases) or censoring. All variables were assessed in univariable Cox models. Hazard ratios (HRs), 95% confidence intervals (CIs), and corresponding p-values were reported for each predictor. Model assumptions, including the proportional hazards assumption, were assessed using Schoenfeld residuals. The linearity assumption for continuous variables was evaluated through Martingale residual plots.
To determine suggested threshold values for continuous predictors identified as significant in the Cox regression, receiver operating characteristic (ROC) curve analysis was performed. The area under the curve (AUC) was reported, and cut-off values for high risk of developing brain metastases were selected based on the Youden index (calculated as: specificity+sensitivity – 1).The p-values <0.05 were considered statistically significant. The analysis was performed with STATISTICA v. 13.0 software (StatSoft Polska, Cracow, Poland).
4 Results
4.1 Clinical outcomes
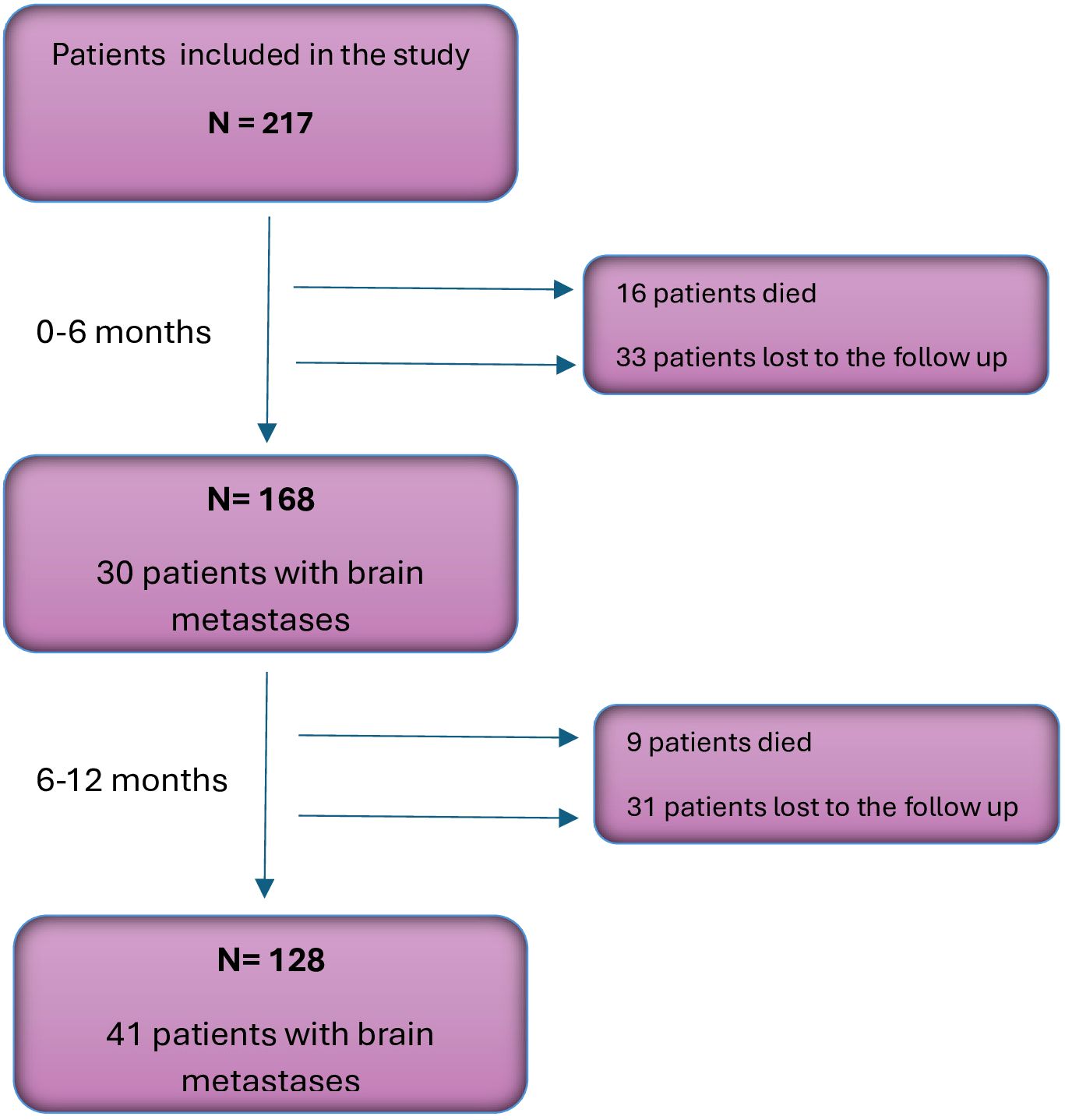
Of the 217 patients included in the study, 168 had the follow-up data for 6 months, and 128 patients - for 12 months. During the analyzed follow-up period, CNS metastases were detected in 41 patients (32.03%). Other sites of metastases included: spine/bones (n=26, 20.31%), the other lung (n=23, 17.97%), liver (n=19, 14.84%), adrenal glands (n=11, 8.59%), skin (n=3, 1.38%), breast (n=1, 0.46%) and pancreas (n=1, 0.46%). Figure 1 shows changes in the structure of the study group during the follow-up.
4.2 Baseline laboratory findings and occurrence of brain metastases
Values of the baseline laboratory tests were compared between the patients in whom brain metastases were or were not detected during the follow-up period. The results of the comparison with regard to the time of the metastasis occurrence are presented in Table 2. At baseline, patients who developed brain metastases during subsequent 12 months, had significantly higher median total leukocyte and neutrophil counts, red cell distribution width (RDW) and lower median eosinophil count, as well as higher alanine aminotransferase (ALT), d-dimer, CRP and lactate dehydrogenase (LDH) serum levels. Although patients with brain metastases revealed during first 6 months had significantly lower lymphocyte counts and mean platelet volume (MPV) at baseline, this difference did not reach statistical significance with regard to the assessment after 12 months. Analysis of CBC-based indices revealed significantly higher values of NLR, PLR and lower LMR in those patients who developed brain metastases at any time point during the follow-up.
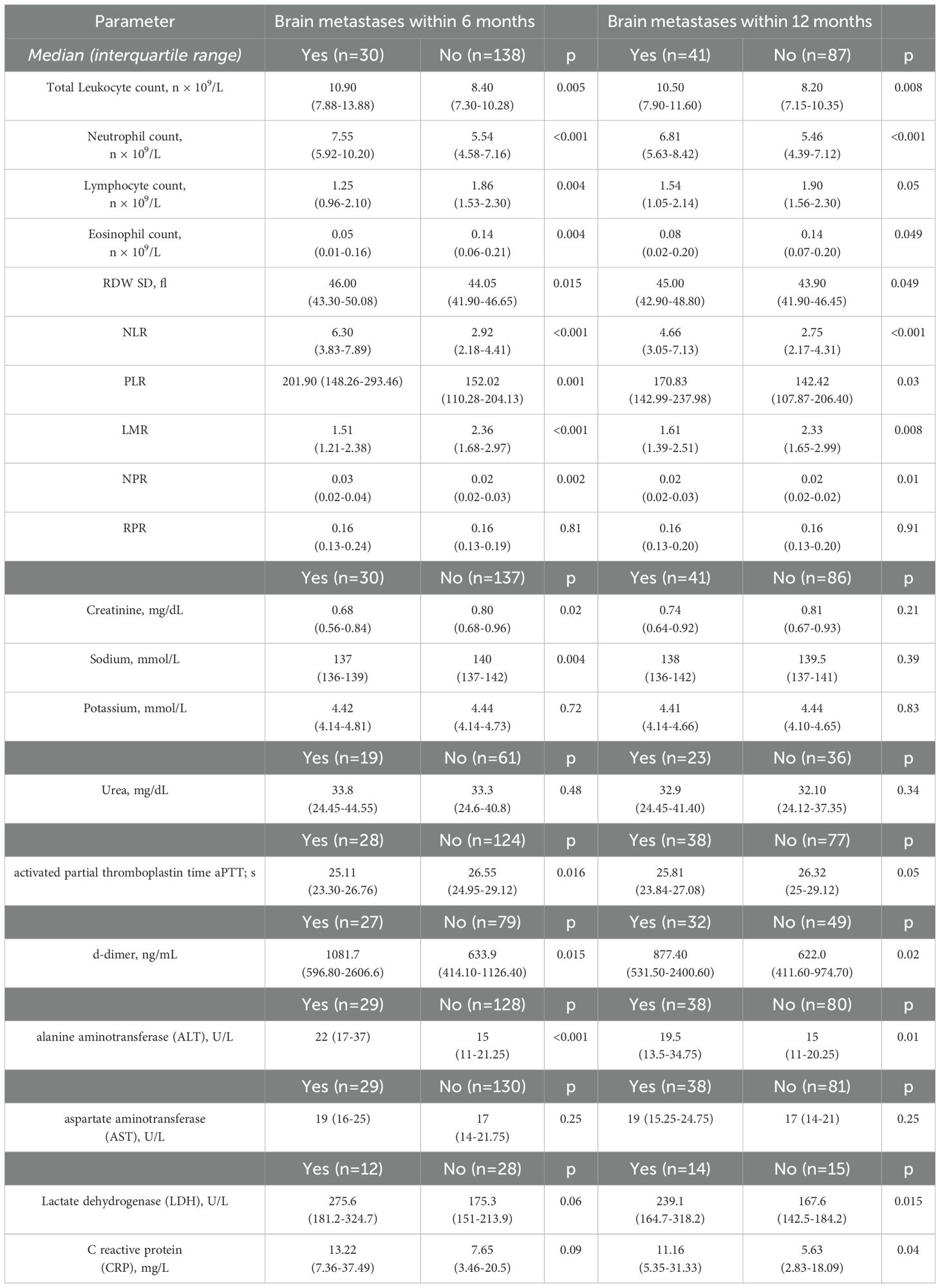
Table 2. Baseline blood- based indices regarding a subsequent occurrence of brain metastases during the follow-up.
Variables for which significant differences were detected at baseline were included in further analysis as potential predictors of brain metastases.
The univariate analysis revealed significant associations between the occurrence of brain metastases and total leukocyte count (HR 1.08, 95% CI 1.02-1.15, p=0.016), neutrophil count (HR 1.11, 95% CI 1.03-1.19, p=0.0036), NLR (HR 1.09, 95% CI 1.03-1.19, p=0.005), LMR (HR 0.67, 95% CI 0.48-0.93, p=0.018) and d-dimer (HR 1.0002, 95% CI 1.0001-1.0004, p=0.0043) levels.
To determine the optimal thresholds for predicting higher risk of brain metastases, receiver operating characteristic (ROC) curve analysis was performed for aforementioned variables. The area under the ROC curve (AUC) was highest for NLR (0.70, 95% CI: 0.60–0.80), indicating good discriminatory ability. The optimal cut-off value for NLR, identified using the Youden Index, was 4.56, corresponding to a sensitivity of 54% and a specificity of 80% (Youden Index = 0.34).
The results of Cox proportional hazards regression and ROC/AUC analysis are summarized in Table 3.
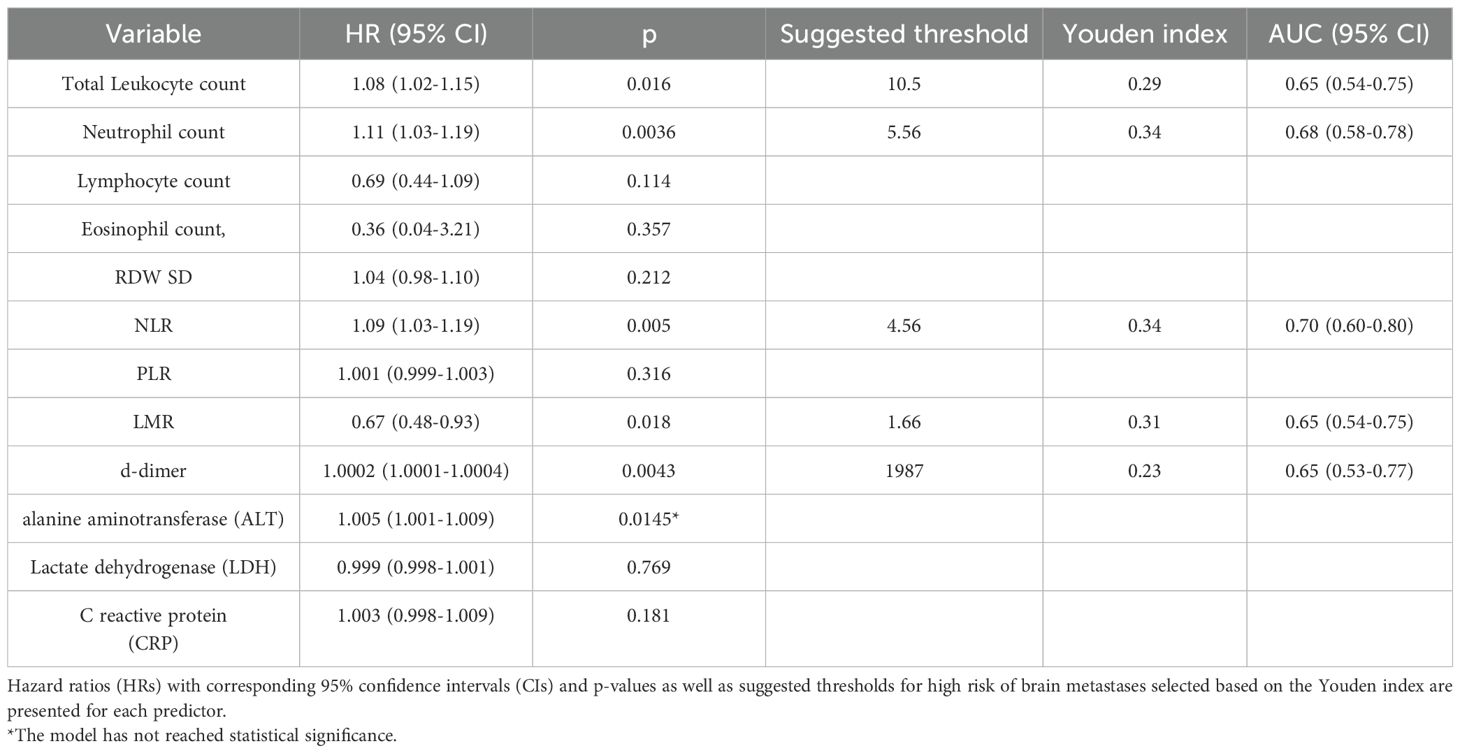
Table 3. Results of univariable Cox regression analyses for CBC indices and other laboratory predictors of occurrence of brain metastases.
4.3 Baseline laboratory findings and other localization of metastases
An analysis of baseline laboratory findings was also performed with regard to the occurrence of metastases to other organs within 12 months of the follow-up. Liver metastases were associated with higher baseline level of CRP and urea, and lower LMR.
Patients with metastases to the other lung had lower PLR and platelet count as well as higher LMR and RDW/platelet ratio. There were no significant differences in baseline laboratory findings between patients with or without bone metastases.
4.4 Mortality
Mortality rate in the study group during one year of follow-up was 17.61% (n=25). Patients who died during the follow-up, presented at baseline with significantly lower median eosinophil count and serum sodium level and higher median CRP level, while no statistically significant differences were found for CBC indices.
The baseline CBC parameters with regard to the occurrence of particular metastases and death during the follow-up are summarized in Table 4.
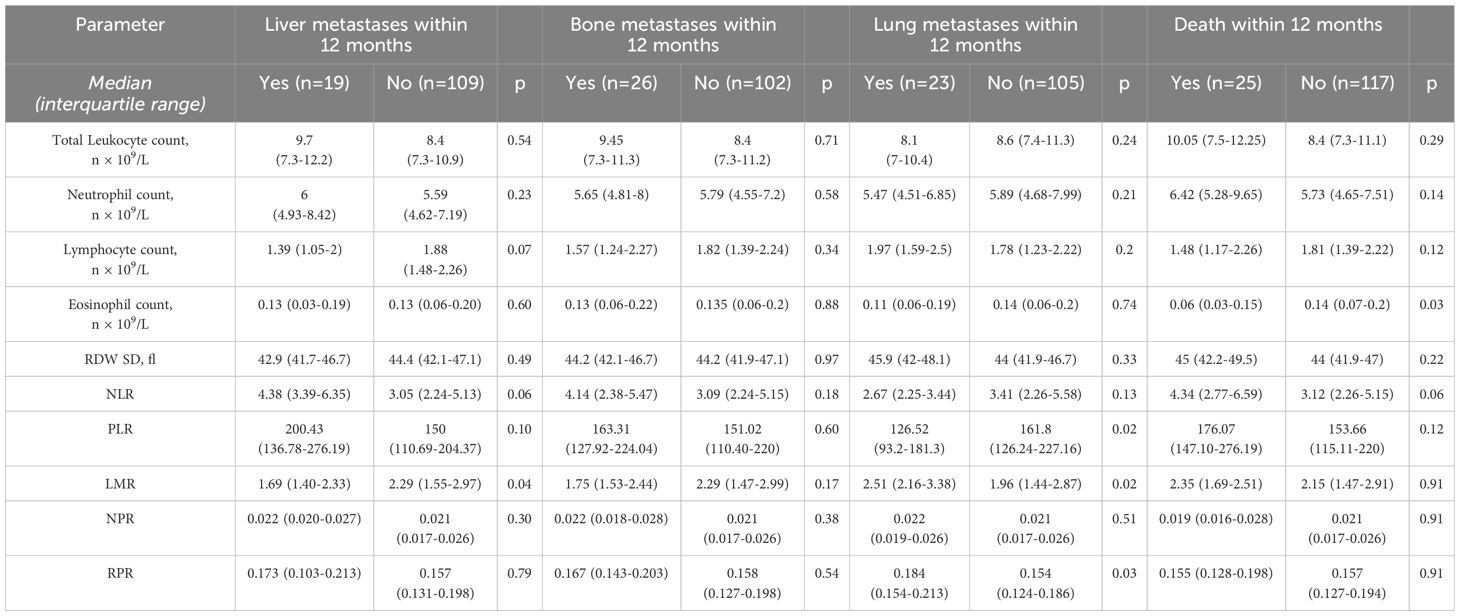
Table 4. Analysis of baseline CBC-based indices regarding a subsequent occurrence of other metastases and death during 12 months of follow-up.
5 Discussion
The aim of the current study was to identify parameters based on easily available peripheral blood tests with potential predictive value for brain metastases in patients with lung cancer. We found significant association between developing brain metastases within 6 and 12 months of follow-up and baseline leukocyte subpopulation counts and blood cell subsets ratios, as well as selected biochemical parameters.
Increased number of neutrophils and lowered number of lymphocytes in the patients with brain metastases seem consistent with the current views on the role of neutrophils in the progression of the neoplasm. Neutrophils - especially their population defined as tumor associated neutrophils (TANs) - take part in many processes related to the development of neoplasms, including systemic immunosuppression (by inhibiting T-cells activity and proliferation) and production of specific cytokines, chemokines and proteases, which modulate tumor microenvironment (TME), contribute to local angiogenesis and dissemination of neoplastic cells (17, 18). Interestingly, TANs can adopt either anti-tumor (N1) or pro-tumor (N2) phenotypes. This bilateral functionality depends on various factors within the TME, and pro-inflammatory factors, particularly tumor growth factor β (TGF- β), are considered to promote the latter (19). One of possible functions of TANs is the remodeling of the extracellular matrix through the secretion of proteases such as neutrophil elastase, matrix metalloproteinases and cathepsin G as well as promoting epithelial–mesenchymal transition via the release of reactive oxygen species (ROS) and cytokines such as IL-8, enhancing tumor cells invasiveness (20). Another possible mechanism enhancing metastatic activity is by aggregating with circulating tumor cells (CTCs) and protecting them from immune detection while in circulation. In TME, neutrophil extracellular traps (NETs) can trap CTCs and provide a vector for their adhesion to endothelial surfaces, promoting extravasation and colonization. NET formation has been associated with microvascular damage and enhanced metastatic burden in preclinical models (20). Moreover, TANs also contribute to the formation of the pre-metastatic niche by releasing pro-inflammatory mediators (such as IL-1β, TNF-α) and promoting vascular permeability, which primes target tissues for colonization (21).
This mechanism of action may be highly relevant in the context of brain metastases, where the integrity of the blood-brain barrier (BBB) is a critical limiting factor. In the setting of systemic inflammation and tumor progression, TANs and associated cytokines play a key role in increasing BBB permeability. Several studies have demonstrated that pro-inflammatory cytokines disrupt tight junction proteins such as occludin and claudin-5 in brain microvascular endothelial cells (22). This disruption leads to increased paracellular permeability, facilitating the migration of tumor cells across the BBB. Additionally, TAN-derived ROS and nitric oxide further compromise endothelial barrier function.
In turn, a lower number of lymphocytes, responsible for the specific immune response, has been found to correlate with a worse overall survival (OS) and progression free survival (PFS) in solid tumors, including lung cancer (23, 24). Another parameter associated with brain metastases in the study group was an increased baseline RDW, an indicator of the variability of red blood cell size. This may further strengthen the hypothesis of the inflammatory background of metastatic activity, as RDW was reported to correlate positively with established inflammatory markers (25). Furthermore, RDW was also considered as a negative prognostic marker in NSCLC (26). Song et al. found significantly higher values of RDW in patients with NSCLC compared with healthy individuals, as well as a positive correlation between RDW and TNM stage. Additionally, in the patients who developed brain metastases, we observed lower baseline eosinophil counts. This finding seems particularly interesting in view of other reports on lung cancer, which indicate a role of eosinophil level as a predictor of response to therapy with immune checkpoint inhibitors (27, 28).
Indices based on CBC have been extensively studied and encouraged for their use in the assessment of the intensity of systemic inflammatory processes. Their diagnostic and prognostic utility has been confirmed in various autoimmune diseases, such as systemic lupus erythematosus (29), Sjogren syndrome (30) or ulcerative colitis (31), but also in several types of cancer (32–35). The most popular among these indices, NLR, reflects a disproportion between leukocyte subpopulations. Increased NLR values have already been associated with a worse prognosis and poor response to treatment in lung cancer (5, 36).
Some authors reported correlation of other indices (mainly PLR and MLR) with survival in patients with lung cancer (9, 10) and in patients with metastatic brain tumors in general (37, 38). However, data on prognostic utility of these indices for CNS metastases from the lung cancer is scarce.
Indeed, we found that patients with occurrence of brain metastases within a year since lung cancer was diagnosed, had a significantly higher baseline NLR value than patients who did not develop metastases. Similar association was observed for lower baseline values of LMR and (with lower but still relevant level of significance) higher baseline PLR.
Limited evidence on the investigated relationships is available in the literature. Multivariate analysis performed by Koh et al. (16) in patients with stage IV NSCLC revealed that patients with high NLR at diagnosis had higher cumulative incidence of subsequent brain metastases, with a threshold value of NLR ≥4.95 as their significant independent predictor. In another study, that included patients with lung adenocarcinoma (15), elevated levels of NLR were independently associated with an increased risk of the presence of brain metastases on admission. Similar findings were reported for patients with lung adenocarcinoma treated with radical surgery, with higher baseline PLR value related to increased risk of brain metastases during the follow-up (median - 30 months) (39).
Apart from CBC-based indices, we also investigated basic biochemical parameters. Significantly increased baseline level of CRP and LDH in the patients with subsequent brain metastases seem consistent with putative inflammatory/immune-mediated background for dissemination of neoplasm. CRP is a model inflammatory protein, and lactate dehydrogenase has been recently viewed as a pivotal modulator of immunogenicity and metabolic activity of various neoplasms (40). A significantly higher baseline level of d-dimers in these patients is primarily associated with dysfunction of the coagulation system during cancer progression and has been also associated with a worse OS and PFS in patients with lung cancer (41). We have also noted a significantly higher median levels of alanine aminotransferase in patients with subsequent brain metastases, which might reflect early concomitant hepatic cells destruction. However, the difference in baseline levels of aspartate aminotransferase between the subgroups of patients did not reach the statistical significance.
In this exploratory analysis, we identified several parameters that showed significant associations with the risk of developing brain metastases, including total leukocyte count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and d-dimer levels. Among these markers, NLR demonstrated the highest discriminatory ability in ROC curve analysis (AUC = 0.70), with an optimal cut-off of 4.56 providing moderate sensitivity (54%) and relatively high specificity (80%).
However, while these results suggest a possible predictive utility of inflammatory markers - particularly NLR - in identifying patients at elevated risk of brain involvement, due to the retrospective and single-center design of this study, the overall discriminative performance may be not sufficient for clinical application at this stage. Therefore the cut-off thresholds should be interpreted cautiously. We believe the findings may be valuable as hypothesis-generating, and further validation in larger, prospective cohorts is necessary before drawing conclusions or recommending integration into clinical decision-making.
It seems interesting that unlike CNS involvement, metastases to the other organs in the study group did not show such relevant relationships with baseline CBC indices. For those with or without liver metastases, only the difference in baseline LMR values reached statistical significance (insignificant trend was noticeable for total lymphocyte count and NLR). Furthermore, lower baseline eosinophil count was the only parameter significantly associated with mortality. Interestingly, among 25 patients who died during the follow-up, only 6 had been diagnosed with brain metastases. Relatively smaller number of patients with metastases to other organs compared to the group of patients with brain metastases as well as the number of patients lost to follow-up should be also taken into account; perhaps the results would be more relevant with a larger and more homogenous group of patients. However, our findings may suggest a greater impact of systemic inflammatory processes and immune dysfunction upon formation of brain metastases in comparison to their other locations.
While the systemic pro-inflammatory state is known to facilitate metastasis in general (42), organ-specific factors may influence metastatic patterns. In physiological conditions, the blood-brain barrier (BBB) limits the migration of cells and macromolecules. However, inflammation-induced disruption of the BBB - mediated by TANs and cytokines (e.g. IL-1β, TNF-α, and IL-6) - can increase vascular permeability and create a permissive environment for tumor cell infiltration into the CNS (22, 43, 44). In contrast, other organ sites may not require such inflammatory priming for metastatic colonization. This could explain the stronger correlation observed between inflammatory markers and brain metastases. Further research is needed to understand the mechanisms driving site-specific metastatic patterns and their interaction with systemic inflammation.The strengths of this study are associated with focusing on brain metastases from the lung cancer, which seem underexplored, but crucial complications of the disease. The wide and diverse range of parameters was considered in the analysis, including complete blood count and biochemical markers. The use of easily accessible and routinely measured laboratory parameters as potential prognostic markers makes this approach highly applicable to real-world settings. These findings have the potential to encourage more frequent use of these markers in clinical practice, aimed at identification of patients with greater risk of CNS involvement and undertaking individualized management strategies.
Several limitations of the study include relatively small sample size (further limited by those who were lost to follow-up) which impeded stratification by histopathological type of lung cancer. The single-center and retrospective character of the analysis may introduce selection bias and limit the generalizability of the results to more diverse populations. Also, due to the modest sample size, cross-validation methods were not applied. Therefore, the results should be interpreted with caution and considered exploratory in nature. Validation in larger, multi-center cohorts is necessary to confirm the observed associations and to establish the broader applicability of the proposed markers. An additional bias might have been caused by unrecognized brain metastases in some of the deceased patients. Due to retrospective mode of study, some data potentially relevant for their prognostic value were not available for analysis (e.g. genetic profiling of the patients, lifestyle factors including smoking). Oncogenic driver mutations, particularly EGFR and ALK, are known to increase the risk of brain metastases in non-small cell lung cancer (NSCLC) patients (45). Moreover, targeted therapies such as EGFR- and ALK-tyrosine kinase inhibitors (TKIs) are capable of crossing the blood-brain barrier and have been shown to effectively treat asymptomatic brain metastases and prolong brain-specific progression-free survival (46, 47). These factors likely influence both the incidence and timing of the metastatic burden, and their absence in our study may confound the findings. In future studies, incorporating molecular profiling and treatment stratification will be important for accurately assessing the prognostic value of systemic inflammatory markers in patients receiving targeted therapies.
6 Conclusions
Significant relationships were found between CBC – based indices in the patients diagnosed with lung cancer and subsequent occurrence of brain metastases. These findings may suggest the importance of inflammatory process in neoplastic activity leading to CNS involvement. CBC - based indices may be easily accessible and useful markers in identification of patients with increased risk of brain metastases, contributing to individualized diagnostic and therapeutic approach for optimal outcomes of the disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Wroclaw Medical University Bioethics Committee (approval no. KB-918/2021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KM: Writing – original draft. MK: Writing – review & editing. AP: Writing – review & editing. AB: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by Wroclaw Medical University SUBZ.A520.25.008.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nayak L, Lee EQ, and Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. (2012) 14:48–54. doi: 10.1007/s11912-011-0203-y
2. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. (2017) 3:827–31. doi: 10.1001/jamaoncol.2016.3834
3. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
4. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
5. Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. (2012) 14:864–9. doi: 10.1007/s12094-012-0872-5
6. Tomita M, Shimizu T, Ayabe T, Yonei A, and Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. (2011) 31:2995–8.
7. Yao Y, Yuan D, Liu H, Gu X, and Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. (2013) 62:471–9. doi: 10.1007/s00262-012-1347-9
8. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung cancer. (2017) 106:1–7. doi: 10.1016/j.lungcan.2017.01.013
9. Ding N, Pang Z, Shen H, Ni Y, Du J, and Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep. (2016) 6:34823. doi: 10.1038/srep34823
10. Jin J, Yang L, Liu D, and Li WM. Prognostic value of pretreatment lymphocyte-to-monocyte ratio in lung cancer: A systematic review and meta-analysis. Technol Cancer Res Treat. (2021) 20:1533033820983085. doi: 10.1177/1533033820983085
11. Bilgin B, Sendur MAN, Hizal M, Dede DS, Akinci MB, Kandil SU, et al. Prognostic effect of red cell distribution width-to-platelet ratio in colorectal cancer according to tumor stage and localization. J Cancer Res Ther. (2019) 15:54–60. doi: 10.4103/jcrt.JCRT_624_17
12. Lee HS, Jung EJ, Kim JM, Kim JY, Kim JR, Kim TH, et al. The usefulness of red blood cell distribution width and its ratio with platelet count in breast cancer after surgery and adjuvant treatment: a retrospective study. Gland Surg. (2022) 11:1864–73. doi: 10.21037/gs-22-410
13. Somaschini A, Cornara S, Demarchi A, Mandurino-Mirizzi A, Fortuni F, Crimiet G, et al. Neutrophil to platelet ratio: A novel prognostic biomarker in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Eur J Prev Cardiol. (2020) 27:2338–40. doi: 10.1177/2047487319894103
14. Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J, Samitaset K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non- small-cell lung cancer. ESMO Open. (2021) 6:100254. doi: 10.1016/j.esmoop.2021.100254
15. Hu C, Wu J, Liu Y, Zhou J, Wang W, Wanget X, et al. Relationship between neutrophil-to-lymphocyte ratio and brain metastasis in non- small cell lung cancer patients. Cancer Control. (2022) 29:10732748221076805. doi: 10.1177/10732748221076805
16. Koh YW, Choi JH, Ahn MS, Choi YW, and Lee HW. Baseline neutrophil-lymphocyte ratio is associated with baseline and subsequent presence of brain metastases in advanced non-small-cell lung cancer. Sci Rep. (2016) 6:38585. doi: 10.1038/srep38585
17. Rakic A, Beaudry P, and Mahoney DJ. The complex interplay between neutrophils and cancer. Cell Tissue Res. (2018) 371:517–29. doi: 10.1007/s00441-017-2777-7
18. Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W, et al. The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications. Mol Cancer. (2023) 22:148. doi: 10.1186/s12943-023-01843-6
19. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
20. Yan M, Zheng M, Niu R, Yang X, Tian S, Fanet L, et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol. (2022) 10:938289. doi: 10.3389/fcell.2022.938289
21. Masucci MT, Minopoli M, and Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. (2019) 9:1146. doi: 10.3389/fonc.2019.01146
22. Yang J, Ran M, Li H, Lin Y, Ma K, Yang Y, et al. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front Mol Neurosci. (2022) 15:1013933. doi: 10.3389/fnmol.2022.1013933
23. Zhang J, Huang SH, Li H, Li Y, Chen XL, Zhang WQ, et al. Preoperative lymphocyte count is a favorable prognostic factor of disease-free survival in non-small-cell lung cancer. Med Oncol. (2013) 30:352. doi: 10.1007/s12032-012-0352-3
24. Zhao J, Huang W, Wu Y, Luo Y, Wu B, Cheng J, et al. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis. Cancer Cell Int. (2020) 20:15. doi: 10.1186/s12935-020-1094-5
25. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. (2009) 133:628–32. doi: 10.5858/133.4.628
26. Song B, Shi P, Xiao J, Song Y, Zeng M, Cao Y, et al. Utility of red cell distribution width as a diagnostic and prognostic marker in non-small cell lung cancer. Sci Rep. (2020) 10:15717. doi: 10.1038/s41598-020-72585-4
27. Takeuchi E, Kondo K, Okano Y, Ichihara S, Kunishige M, Kadota N, et al. Pretreatment eosinophil counts as a predictive biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Thor Cancer. (2023) 14:3042–50. doi: 10.1111/1759-7714.15100
28. Caliman E, Fancelli S, Ottanelli C, Mazzoni F, Paglialunga L, Lavacchi D, et al. Absolute eosinophil count predicts clinical outcomes and toxicity in non-small cell lung cancer patients treated with immunotherapy. Cancer Treat Res Commun. (2022) 32:100603. doi: 10.1016/j.ctarc.2022.100603
29. Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. (2016) 26:372–6. doi: 10.3109/14397595.2015.1091136
30. Hu ZD, Sun Y, Guo J, Huang YL, Qin BD, Gao Q, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren’s syndrome. Clin Biochem. (2014) 47:287–90. doi: 10.1016/j.clinbiochem.2014.08.022
31. Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoyet S, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. (2013) 27:72–6. doi: 10.1002/jcla.2013.27.issue-1
32. Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. (2019) 2019:6036979. doi: 10.1155/2019/6036979
33. Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. (2020) 2020:9146042. doi: 10.1155/2020/9146042
34. Li YX, Chang JY, He MY, Wang HR, Luo DQ, Li FH, et al. Neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) predict clinical outcome in patients with stage IIB cervical cancer. J Oncol. (2021) 2021:2939162. doi: 10.1155/2021/2939162
35. Corbeau I, Jacot W, and Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: A systematic review. Cancers. (2020) 12:958. doi: 10.3390/cancers12040958
36. Liu N, Mao J, Tao P, Chi H, Jia W, and Dong C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Med (Baltimore). (2020) 101:e28617. doi: 10.1097/MD.0000000000028617
37. Starzer AM, Steindl A, Mair MJ, Deischinger C, Simonovska A, Widhalmet G, et al. Systemic inflammation scores correlate with survival prognosis in patients with newly diagnosed brain metastases. Br J Cancer. (2021) 124:1294–300. doi: 10.1038/s41416-020-01254-0
38. Schneider M, Schäfer N, Bode C, Borger V, Eichhorn L, Giordano FA, et al. Prognostic value of preoperative inflammatory markers in melanoma patients with brain metastases. J Clin Med. (2021) 10:634. doi: 10.3390/jcm10040634
39. Wang W, Bian C, Xia D, He JX, Hai P, Zhao R, et al. Combining carcinoembryonic antigen and platelet to lymphocyte ratio to predict brain metastasis of resected lung adenocarcinoma patients. BioMed Res Int. (2017) 2017:8076384. doi: 10.1155/2017/8076384
40. Claps G, Faouzi S, Quidville V, Chehade C, Shen S, Vagneret S, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. (2022) 19:749–62. doi: 10.1038/s41571-022-00686-2
41. Ma M, Cao R, Wang W, Wang B, Yang Y, Huang Y, et al. The D-dimer level predicts the prognosis in patients with lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. (2021) 16:243. doi: 10.1186/s13019-021-01618-4
42. Hibino S, Kawazoe T, Kasahara H, Itoh S, Ishimoto T, Sakata-Yanagimotoet M, et al. Inflammation-induced tumorigenesis and metastasis. Int J Mol Sci. (2021) 22:5421. doi: 10.3390/ijms22115421
43. Boire A, Brastianos PK, Garzia L, and Valiente M. Brain metastasis. Nat Rev Cancer. (2020) 20:4–11. doi: 10.1038/s41568-019-0220-y
44. Huang X, Hussain B, and Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. (2021) 27:36–47. doi: 10.1111/cns.13569
45. Guan J, Chen M, Xiao N, Li L, Zhang Y, Li Q, et al. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med Oncol. (2016) 33:1. doi: 10.1007/s12032-015-0714-8
46. Taslimi S, Brar K, Ellenbogen Y, Deng J, Hou W, Moraes FY, et al. Comparative efficacy of systemic agents for brain metastases from non-small-cell lung cancer with an EGFR mutation/ALK rearrangement: A systematic review and network meta-analysis. Front Oncol. (2021) 11:739765. doi: 10.3389/fonc.2021.739765
Keywords: lung cancer, NSCLC, complete blood count, NLR, brain metastases
Citation: Marschollek K, Kosacka M, Pokryszko-Dragan A and Brzecka-Bonnaud A (2025) Complete blood count parameters as potential predictive factors of brain metastases in lung cancer. Front. Oncol. 15:1582788. doi: 10.3389/fonc.2025.1582788
Received: 24 February 2025; Accepted: 05 May 2025;
Published: 26 May 2025.
Edited by:
Oraianthi Fiste, National and Kapodistrian University of Athens, GreeceCopyright © 2025 Marschollek, Kosacka, Pokryszko-Dragan and Brzecka-Bonnaud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karol Marschollek, a2Fyb2wubWFyc2Nob2xsZWtAZ21haWwuY29t
†These authors share senior authorship
 Karol Marschollek
Karol Marschollek Monika Kosacka2
Monika Kosacka2 Anna Pokryszko-Dragan
Anna Pokryszko-Dragan Anna Brzecka-Bonnaud
Anna Brzecka-Bonnaud