- 1Department of Urology, Singapore General Hospital, Singapore, Singapore
- 2Department of Diagnostic Radiology, Singapore General Hospital, Singapore, Singapore
- 3Department of Pathology, Singapore General Hospital, Singapore, Singapore
- 4Division of Radiation Oncology, National Cancer Centre Singapore, Singapore, Singapore
- 5Department of Urology, Sengkang General Hospital, Singapore, Singapore
Introduction: The Briganti 2019 nomogram stratifies risk of lymph node involvement (LNI) in prostate cancer, reducing unnecessary pelvic lymph node dissection (PLND) during radical prostatectomy (RP). However the applicability of the nomogram in diverse populations remains under-explored, with only one external validation study performed in an Asian population to date. We aim to evaluate the performance of the nomogram in a large tertiary Asian institution.
Methods: A retrospective cohort study was conducted, with analysis of the cancer registry in our tertiary institution of all patients who underwent RP with PLND between 1988 and 2023. The Briganti 2019 nomogram score was retrospectively calculated for each patient, and post-operative data was analyzed to determine rates of LNI in order to determine the performance of the nomogram in our cohort.
Results: 437 patients were included, with a median Briganti score of 11.2% (IQR 3.9–28.5%). The mean number of lymph nodes excised per patient was 15.1±12. 292 (66.8%) patients had a Briganti score greater than 7%, but only 8.6% were noted to harbor pN1 disease after RP. In our Asian cohort, the 2019 Briganti nomogram only had a moderate discriminatory ability with an area under the receiver operating characteristic curve (AUC) of 0.77. On multivariate analysis, independent predictors of LNI in our population included percentage of positive biopsy cores [Odds Ratio (OR) 1.02, 95%CI 1.01–1.04, p=0.01] and extraprostatic extension on MRI prostate (OR 3.00, 95%CI 1.20–7.56, p=0.02).
Conclusion: The Briganti 2019 nomogram, while effective in many settings, only had a moderate ability to identify patients with pN1 disease in our Asian cohort. With potential limitations in its generalizability to multiple populations, a re-evaluation of its thresholds and further calibration to other populations might be required.
1 Introduction
Globally, prostate cancer (PCa) lies among the top cancers diagnosed in men worldwide and the fifth leading cause of cancer death (1), and its prevalence and characteristics varies across different populations. Lymph node involvement (LNI) is a key factor for prognostication in PCa, and pelvic lymph node dissection (PLND) has been recommended for nodal staging in high-risk or selected intermediate-risk localized PCa (2). However, given the increased operative risks associated with PLND, current guidelines suggest the usage of nomograms for risk stratification of intermediate-risk patients prior to offering it (3).
Previously, the Briganti 2012, Briganti 2017, and the Memorial Sloan Kettering Cancer Center (MSKCC) nomograms have utilized clinical and pathological variables to aid in risk stratification for the risk of LNI (4–7). The latest iteration of the Briganti nomogram was released in 2019, and was based on multi-parametric MRI prostate findings and MRI-guided targeted biopsies for the risk stratification of patients, a feature which was unique to this nomogram. Accepting a threshold of 7% would spare 56% of unnecessary PLNDs, at the expense of missing 1.6% of patients with LNI (8) – and this threshold has since been accepted into international guidelines.
However, the main concern with nomograms is that they tend to be population-specific, and the generalizability of such nomograms in other diverse populations might be uncertain (9), especially since differences in the genetic makeup and environment of a population can significantly influence the prevalence, characteristics, and natural history of a disease (10). The Briganti 2019 nomogram was developed based on data from European institutions, where the majority of patients included were Caucasians; the majority of studies validating it were also inherently European in nature. Its applicability in diverse populations therefore remains under-explored, with only one study to date exploring its application in an Asian population (11). Our study therefore aims to evaluate the performance of the Briganti 2019 nomogram in a large tertiary Asian institution.
2 Methods
A retrospective cohort study was conducted at our tertiary institution, evaluating patients who underwent radical prostatectomy (RP) and PLND between 1988 and 2023. We included patients with localized PCa diagnosed with MRI prostate and an MRI-targeted biopsy, and subsequently underwent RP and PLND. Patients with incomplete data precluding calculation of the Briganti 2019 score were excluded from our study.
Data collected from our prospectively-maintained cancer registry included demographic information, pre-operative PSA levels, clinical stage, Gleason score (GS) on MRI-targeted biopsy, MRI lesion diameter, percentage of biopsy cores with clinically significant PCa at systematic biopsy, histological LNI, and postoperative outcomes. Biochemical recurrence (BCR) was defined as PSA ≥0.2 ng/mL with a second confirmatory level > 0.2 ng/mL after RP. The Briganti 2019 score was calculated for each patient based on these parameters. Patients were classified according to EAU risk classification for localized PCa, pathological nodal status (pN0 vs pN1), and Briganti 2019 score (above or below 7% risk threshold).
Excel version 16.87 and RStudio version 2024.04.2 + 764 were used for statistical analysis. The Mann-Whitney U and Chi-square tests were used to evaluate patient characteristics, while multivariate logistic regression was used to determine variables that significantly predicted post-operative LNI. The Area under the receiver-operating characteristic curve (AUC) was calculated to evaluate the discriminatory performance of the Briganti 2019 model in our cohort. Statistical significance was set at p<0.05.
3 Results
3.1 Patient baseline characteristics
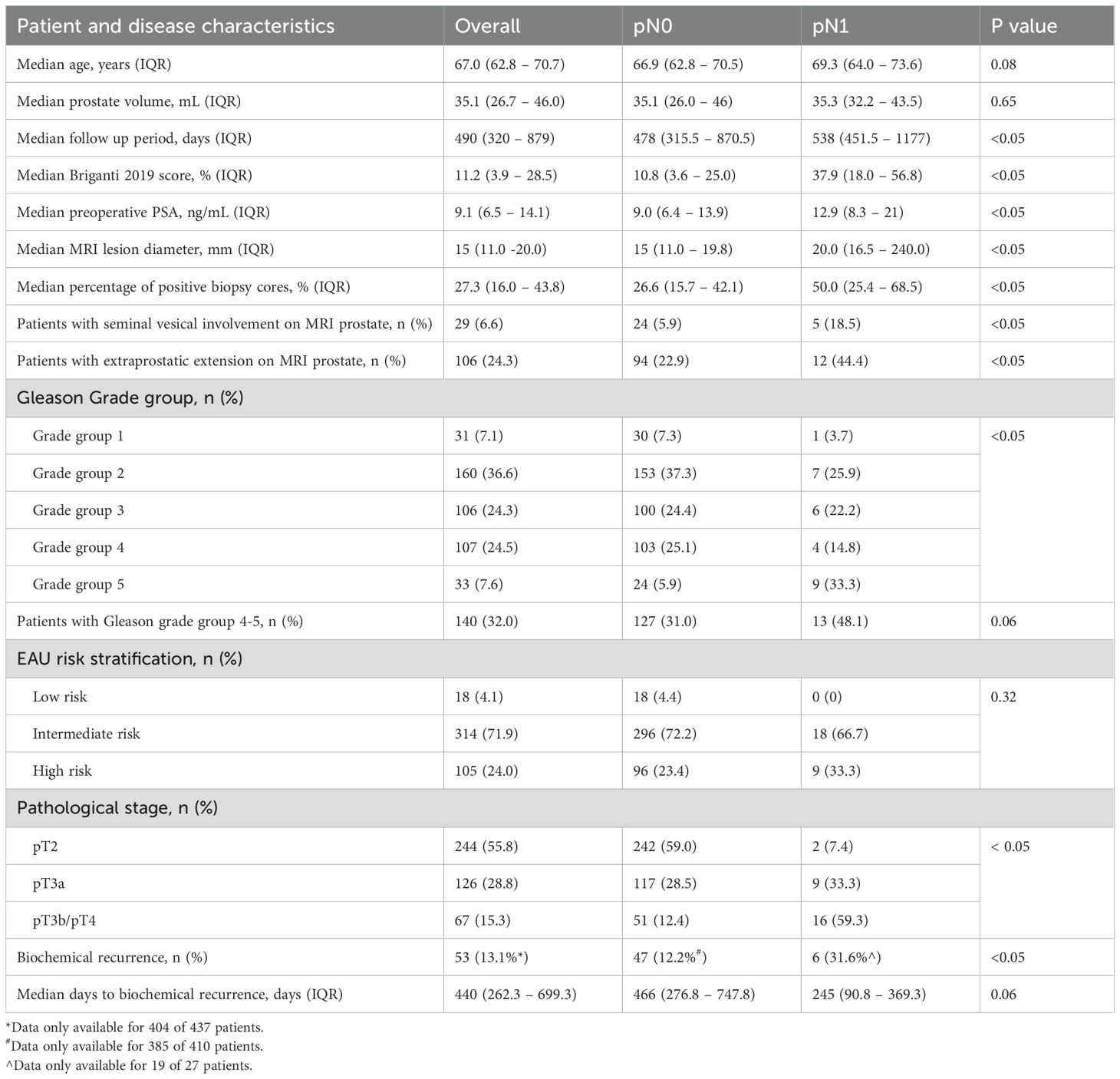
A total of 437 patients were included in this study, and all of them underwent robotic-assisted RP with PLND. Their characteristics are summarized in Table 1. Median age was 67 (62.8–70.7) years, median pre-operative PSA was 9.1 (5.4–14.1) ng/mL, and most patients had intermediate-risk disease (n = 314, 71.9%) (3). Mean operation time and post-operative hospital stay was 245±51 minutes 2.86±1.65 days respectively. Median post-operative follow-up was 20.7 (0.1–67.0) months.
Of the 437 patients, 27 had LNI (pN1) while 410 did not (pN0). Within our series, the mean number of lymph nodes(LN) dissected was 15.1±12.0. There was no significant difference between the mean number of LNs excised across patient who underwent RP and PLND from 2007–2014 compared to those from 2015 - 2023 (14.1 vs 15.1, p = 0.75). Notably, the pN1 cohort had a higher proportion of patients with high-risk disease compared to the pN0 cohort (33.3% vs 23.4%), as well as higher pre-operative PSA (12.9 vs 9.0), median MRI lesion diameter (20mm vs 15mm), and proportion of patients with MRI-defined extraprostatic extension (44.4% vs 22.9%) and seminal vesicle invasion (18.5 vs 5.9%).12.2% of patients in the pN0 cohort experienced biochemical recurrence compared to 31.6% of patients with pN1 disease. Biochemical recurrence occurred earlier in the pN1 cohort at a median of 245 (90.8-369.3) days compared to the pN0 cohort with a median of 466 (276.8 – 747.8) days, but there was not statistically significant.
The cohort had an overall median Briganti 2019 score of 11.2% (3.9 – 28.5%). The pN0 cohort had a significantly lower median Briganti score of 10.8% (3.6–25.0%) compared to the pN1 cohort 37.9% (18.0–56.8%). Most patients included in our present analysis had a Briganti score greater than 7% (N=292; 66.8%), but LNI was only noted in 8.6% (N=25).
3.2 Multivariate analysis
A multivariate analysis was performed to determine whether each individual clinical parameter included in the Briganti 2019 nomogram were independent predictors for LNI in our cohort (Table 2). The percentage of positive biopsy cores and the presence of extra-prostatic extension on MRI prostate were independent predictors for LNI, with an odds ratio (OR) of 1.02 (95%CI 1.01–1.04, p=0.01) and 3.00 (95%CI 1.20–7.56), p=0.02) respectively. The remaining clinical parameters including PSA, Gleason group 4-5, MRI lesion diameter, and seminal vesical involvement on MRI prostate were not predictors for LNI in our cohort.
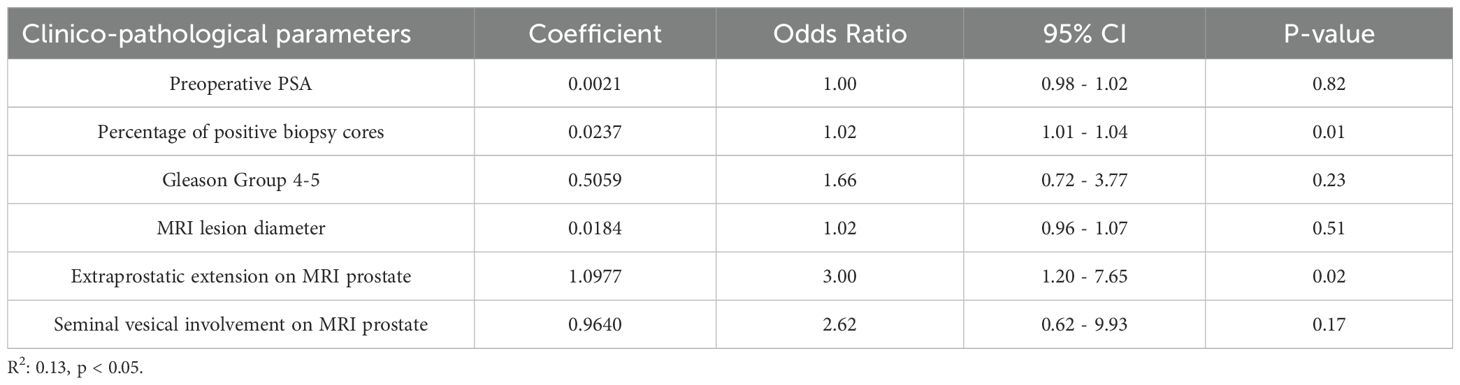
Table 2. Multivariate logistic regression analysis assessing the prediction of LNI based on Briganti 2019 nomogram clinical parameters.
3.3 Performance characteristics of the Briganti 2019 nomogram
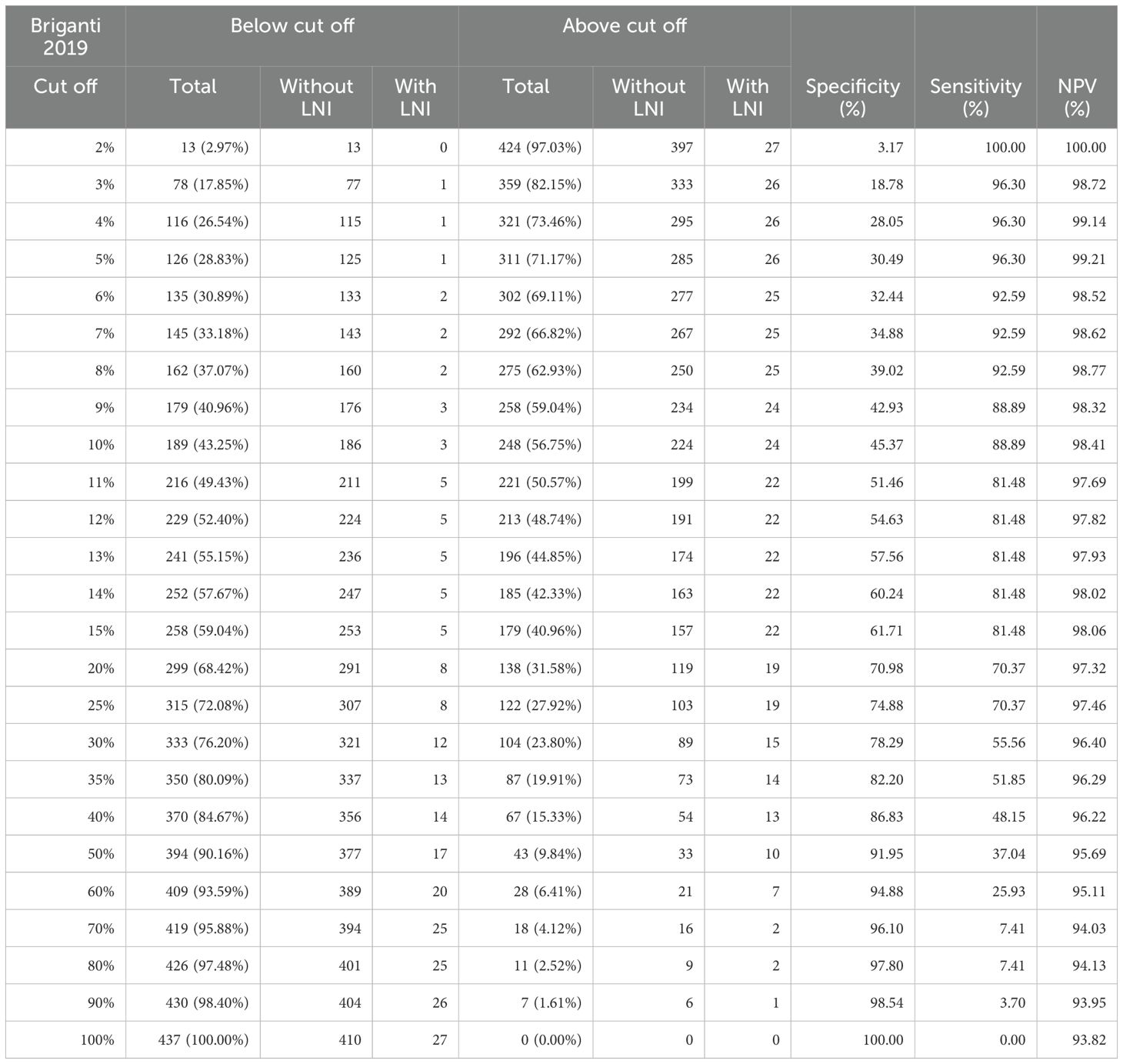
At the proposed Briganti 2019 nomogram threshold of 7%, there were 292 patients (66.8%) with a score greater than 7%, where 25 patients (8.6%) had LNI while 267 (91.4%) did not. 145 patients (33.2%) had a calculated Briganti 2019 score below 7%, and 2 patients (1.4%) had LNI, while the 143 (98.6%) did not. If the threshold of 7% was similarly adhered to in our Asian cohort, we would only spare 33.2% of patients an unnecessary PLND at the expense of missing 1.4% of patients with LN disease. Sensitivity, Specificity, and negative predictive value (NPV) for identification of LNI at a threshold of 7% was 92.59%, 34.88%, and 98.62% respectively (32).
When changing the threshold for LNI to 8%, the specificity improves slightly from 34.88% to 39.02%, with minimal impact on sensitivity (92.59%) and NPV (98.62%). Beyond an 8% cut off, sensitivity begins to drop significantly with a greater number of LNI being missed (Table 3).
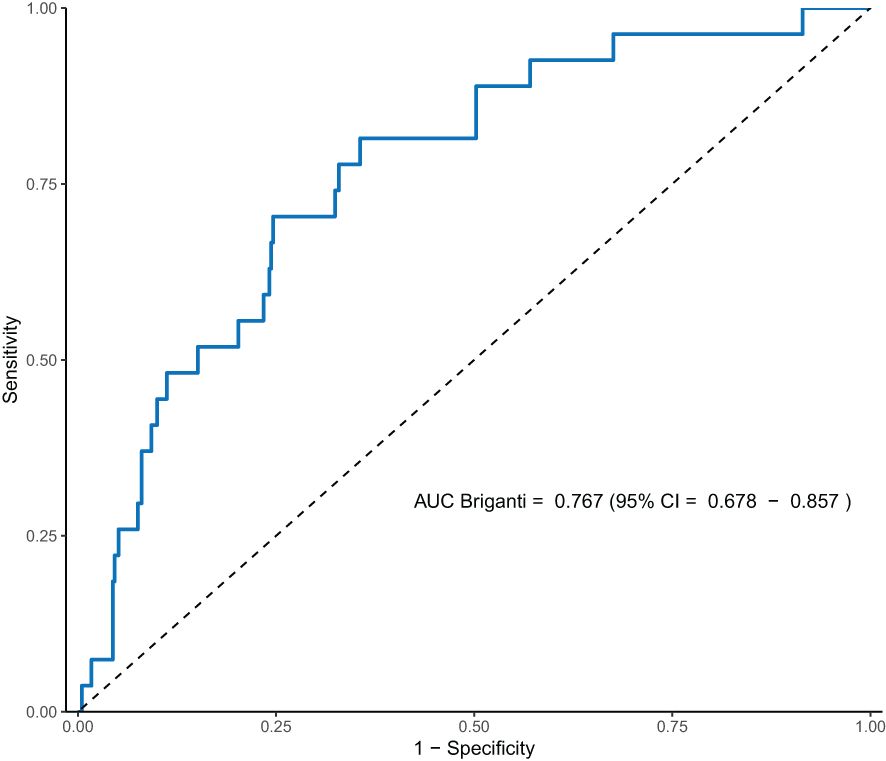
A Receiver-Operating Characteristic (ROC) curve was plotted based on the performance of the Briganti 2019 model in our cohort (Figure 1), and the calculated AUC was 0.77 (95% CI 0.68–0.86). The threshold at which AUC is maximized is at 15.8%, with a sensitivity of 81.5% and a specificity of 64.4%.
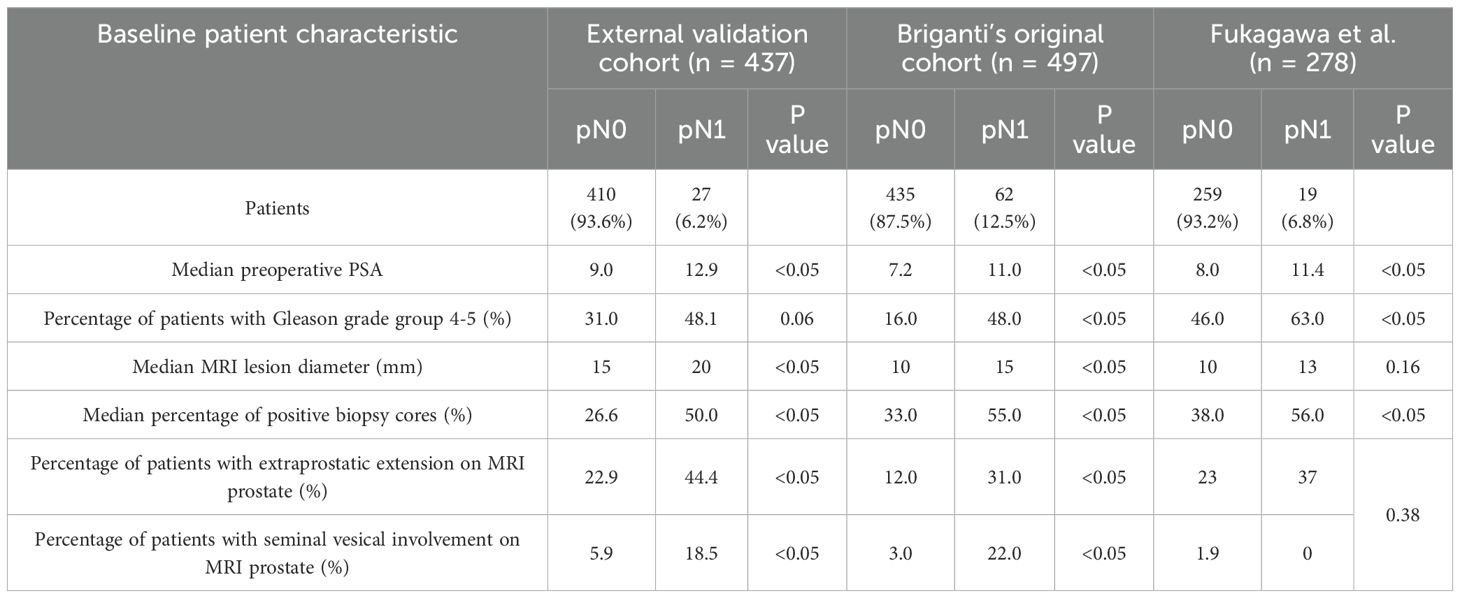
We also evaluated the performance of the Briganti 2019 across different cohorts, presented in Table 4, by incorporating 2 additional external validations performed to the table initially presented by Fukagawa et al, and compared it to the AUC derived from our cohort. Remarkably, the Briganti 2019 nomogram had a poorer discriminatory performance in our cohort and Fukugawa’s Japanese cohort (AUC 0.71), compared to the original population of patients and most other western external validation cohorts.
When comparing baseline characteristics, our cohort is considered higher risk pre-operatively for LNI across most parameters included in the nomogram compared to Briganti’s original cohort (Table 5). Of note, our pN0 cohort had greater high-risk features in all parameters except for percentage of positive biopsy cores, compared to Briganti’s cohort, similar to Fukagawa’s findings.
4 Discussion
4.1 Comparing external validation studies of the Briganti 2019 nomogram
The Briganti 2019 nomogram has been externally validated by multiple studies in primarily Caucasian populations, with findings consistent with Briganti’s own observations (12–16). However, the validity of Briganti’s model remains understudied in non-European populations, with only one study to date examining the nomogram’s validity in a primarily Asian population (11).
At the proposed 7% cut off, given the high Briganti 2019 scores of our patients, the number of unnecessary PLNDs that we would spare is low compared to Briganti’s original cohort. Even as we increase the cut off, the number of unnecessary PLNDs spared remains lower than Briganti’s observations with minimal change in sensitivity, specificity, and negative predictive value (Table 3). These findings are similar to Fukagawa’s observations, where they report a relatively higher score in their validation cohort compared to Briganti’s original cohort, yet with lower rates of LNI suggesting that the pre-operative evaluation of their cohort tended to be overestimated (11).
When evaluating the AUC of the Briganti 2019 nomogram in various populations, Fukagawa et al. found that the AUC in their own Japan-based cohort was lower compared to the three other existing external validation reports at time of publication, which were all based on a European cohort (11). Although the model did perform better in our cohort compared to Fukagawa’s cohort, we found that our AUC was lower compared to the AUC calculated in the original and other external validation cohorts (Table 4). This suggests a possible poorer fit of the model in the Asian population.
4.2 Prostate cancer characteristics amongst different demographics
This difference in patient characteristics between our cohort and Briganti’s original cohort could account for the poor fit of the nomogram in predicting LNI in our cohort compared to other external validation studies. Our multivariate logistic regression analysis showed that many of the clinical parameters included in the nomogram were not significant independent predictors for LNI. The only two parameters that were significant independent predictors were percentage of positive biopsy cores on MRI targeted prostate biopsy (OR 1.02, 95% CI 1.01 – 1.04, p = 0.01), and extraprostatic extension on MRI prostate (OR 3.00, 95% CI 1.20 – 7.56, p = 0.02). This suggests a possible difference in not only disease characteristics, but also in progression and tumor biology.
Differences in PCa characteristics and disease progression has been observed across various ethnic groups. Notably, Asian men have been observed to have more favorable survival rates despite poorer prognostic profiles (17). There have been various theories suggesting reasons for the discordance of disease progression between racial groups. Endogenous testosterone level has been shown to increase PCa risk, and in the presence of PCa, has been found to correlate with increased risk of disease progression (18). Asian men have been found to have lower testosterone levels compared with other racial groups, with a suggested role of genetics and lifestyle factors, such as dietary intake (19–21). Furthermore, lifestyle affecting intraprostatic microbiome and gut microbiome has potential effects on the risk and progression of PCa (22). Tumor genomics have also shown variability across different racial groups, and likely contributes to differences in disease characteristics and progression in various racial groups (23).
It is important to acknowledge, however, that with increased globalization and continuous shifts in sociodemographic and cultural trends, extrinsic factors such as environmental or lifestyle factors are dynamic and may lead to future changes in the observed patterns of disease progression (24). Other factors to consider include variations in access to healthcare, screening practices, and common surgical procedures across different countries. Therefore, although some countries may have similar racial profiles, differences in practice may add to differences in disease profile and risk of progression across different populations (25).
Given the differences in tumor characteristics and disease progression between populations, existing nomograms are limited to the populations in which they are created for, and we should approach the application of these nomograms to other population groups with caution. Moving forward, creating population specific nomograms will allow for more precise risk stratification, to aid decision making (9). Furthermore, the incorporation of new imaging modalities such as PSMA PET/CT and genetic biomarkers will allow for better characterization of disease and further improve our predictive tools (26–29).
4.3 Limitations
There are a few limitations of this study to note. Firstly, given that it is not routine practice for all patients with PCa to undergo PLND, there is a selection bias within our patient cohort in which patients who are higher risk for LN disease are more likely to be offered PLND. Therefore amongst low risk patients who are less likely to have undergone PLND, there are potentially patients with missed nodal disease.
Secondly, MRI reporting and MRI targeted biopsy techniques are subjective and clinician dependent. In Briganti’s cohort, although clinicians were advised to take a minimum of 2 targeted cores for each suspicious lesion and at least 6 random cores outside the MRI-targeted biopsy area, the final decision for number of targeted and systematic cores taken was still dependent on the judgement of each treating physician (8). Within our institution, there is no standardized practice for number of cores to take in a MRI-targeted biopsy, which could potentially affect rates of false negatives. Furthermore, reporting of multiparametric MRI prostate is reporter-dependent, and can vary between institutions.
Lastly, given the extended time frame of our data, there is a lack of data regarding PLND templates and surgical technique. Although the mean number of nodes dissected (n = 15.1) is comparable with Briganti’s’ original cohort, suggesting adequate dissection, we are unable to comment on the extent of PLND in our patient cohort. We therefore have to interpret our data with caution, recognizing the challenge of direct application of our findings on other institutions due to possible differences in practice.
5 Conclusion
The Briganti 2019 nomogram, while effective in many settings, shows poorer performance in our PCa Asian cohort with high-risk features, necessitating a re-evaluation of its thresholds and clinical parameters included in the model. Population-specific adjustments and the incorporation of additional predictive factors may improve the predictive tools of LNI and further improve our selection of patients with PLND.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
RL: Writing – original draft, Writing – review & editing. HL: Writing – original draft, Writing – review & editing. KF: Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. YT: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. NN: Writing – original draft, Writing – review & editing. JT: Writing – original draft, Writing – review & editing. KT: Writing – original draft, Writing – review & editing. LL: Writing – original draft, Writing – review & editing. CC: Writing – original draft, Writing – review & editing. HH: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing. KC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Bader P, Burkhard FC, Markwalder R, and Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. (2003) 169:849–54.
3. Uroweb - European Association of Urology. EAU guidelines on prostate cancer (2024). Available online at: https://uroweb.org/guidelines/prostate-cancer/chapter/treatment (Accessed July 4, 2024).
4. Di Pierro GB, Salciccia S, Frisenda M, Tufano A, Sciarra A, Scarrone E, et al. Comparison of four validated nomograms (Memorial sloan kettering cancer center, Briganti 2012, 2017, and 2019) predicting lymph node invasion in patients with high-risk prostate cancer candidates for radical prostatectomy and extended pelvic lymph node dissection: clinical experience and review of the literature. Cancers. (2023) 15:1683. doi: 10.3390/cancers15061683
5. Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. (2012) 61:480–7. doi: 10.1016/j.eururo.2011.10.044
6. Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell’Oglio P, Bravi CA, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. (2017) 72:632–40. doi: 10.1016/j.eururo.2017.03.049
7. Prostate cancer nomograms: dynamic prostate cancer nomogram: coefficients | Memorial sloan kettering cancer center (2025). Available online at: https://www.mskcc.org/nomograms/prostate/pre_op/coefficients (Accessed February 14, 2025).
8. Gandaglia G, Ploussard G, Valerio M, Mattei A, Fiori C, Fossati N, et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol. (2019) 75:506–14. doi: 10.1016/j.eururo.2018.10.012
9. Panaiyadiyan S and Kumar R. Prostate cancer nomograms and their application in Asian men: a review. Prostate Int. (2024) 12:1–9. doi: 10.1016/j.prnil.2023.07.004
10. Mbemi A, Khanna S, Njiki S, Yedjou CG, and Tchounwou PB. Impact of gene–environment interactions on cancer development. Int J Environ Res Public Health. (2020) 17:8089. doi: 10.3390/ijerph17218089
11. Fukagawa E, Yamamoto S, Ohde S, Yoshitomi KK, Hamada K, Yoneoka Y, et al. External validation of the Briganti 2019 nomogram to identify candidates for extended pelvic lymph node dissection among patients with high-risk clinically localized prostate cancer. Int J Clin Oncol. (2021) 26:1736–44. doi: 10.1007/s10147-021-01954-4
12. Diamand R, Oderda M, Albisinni S, Fourcade A, Fournier G, Benamran D, et al. External validation of the Briganti nomogram predicting lymph node invasion in patients with intermediate and high-risk prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies: A European multicenter study. Urol Oncol Semin Orig Investig. (2020) 38:847.e9–847.e16. doi: 10.1016/j.urolonc.2020.04.011
13. Gandaglia G, Martini A, Ploussard G, Fossati N, Stabile A, De Visschere P, et al. External validation of the 2019 Briganti nomogram for the identification of prostate cancer patients who should be considered for an extended pelvic lymph node dissection. Eur Urol. (2020) 78:138–42. doi: 10.1016/j.eururo.2020.03.023
14. Małkiewicz B, Ptaszkowski K, Knecht K, Gurwin A, Wilk K, Kiełb P, et al. External validation of the Briganti nomogram to predict lymph node invasion in prostate cancer—Setting a new threshold value. Life. (2021) 11:479.
15. Frego N, Paciotti M, Buffi NM, Maffei D, Contieri R, Avolio PP, et al. External validation and comparison of two nomograms predicting the probability of lymph node involvement in patients subjected to robot-assisted radical prostatectomy and concomitant lymph node dissection: A single tertiary center experience in the MRI-era. Front Surg. (2022) 9:829515/full. doi: 10.3389/fsurg.2022.829515/full
16. Soeterik TFW, Hueting TA, Israel B, van Melick HHE, Dijksman LM, Stomps S, et al. External validation of the Memorial Sloan Kettering Cancer Centre and Briganti nomograms for the prediction of lymph node involvement of prostate cancer using clinical stage assessed by magnetic resonance imaging. BJU Int. (2021) 128:236–43. doi: 10.1111/bju.v128.2
17. Robbins AS, Koppie TM, Gomez SL, Parikh-Patel A, and Mills PK. Differences in prognostic factors and survival among white and Asian men with prostate cancer, California, 1995-2004. Cancer. (2007) 110:1255–63. doi: 10.1002/cncr.v110:6
18. Porcaro AB, Bianchi A, Gallina S, Ditonno F, Ornaghi PI, Serafin E, et al. Endogenous testosterone density associates with predictors of tumor upgrading and disease progression in the low through favorable intermediate prostate cancer risk categories: analysis of risk factors and clinical implications. Afr J Urol. (2023) 29:34. doi: 10.1186/s12301-023-00366-2
19. Wu AH, Whittemore AS, Kolonel LN, Stanczyk FZ, John EM, Gallagher RP, et al. Lifestyle determinants of 5alpha-reductase metabolites in older African-American, white, and Asian-American men. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2001) 10:533–8.
20. Xu L, Au Yeung SL, Kavikondala S, Leung GM, and Schooling CM. Testosterone concentrations in young healthy us versus Chinese men. Am J Hum Biol. (2014) 26:99–102. doi: 10.1002/ajhb.22482
21. Kurniawan AL, Hsu CY, Chao JCJ, Paramastri R, Lee HA, Lai PC, et al. Association of testosterone-related dietary pattern with testicular function among adult men: A cross-sectional health screening study in Taiwan. Nutrients. (2021) 13:259. doi: 10.3390/nu13010259
22. Miyake M, Tatsumi Y, Ohnishi K, Fujii T, Nakai Y, Tanaka N, et al. Prostate diseases and microbiome in the prostate, gut, and urine. Prostate Int. (2022) 10:96–107. doi: 10.1016/j.prnil.2022.03.004
23. Mahal BA, Alshalalfa M, Kensler KH, Chowdhury-Paulino I, Kantoff P, Mucci LA, et al. Racial differences in genomic profiling of prostate cancer. New Engl J Med. (2024) 383(11):1083–85. doi: 10.1056/NEJMc2000069
24. Cai Q, Chen Y, Zhang D, Pan J, Xie Z, Xu C, et al. Estimates of over-time trends in incidence and mortality of prostate cancer from 1990 to 2030. Transl Androl Urol. (2020) 9:196–209. doi: 10.21037/tau.2020.02.21
25. Hsing AW, Tsao L, and Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. (2000) 85:60–7. doi: 10.1002/(SICI)1097-0215(20000101)85:1<60::AID-IJC11>3.0.CO;2-B
26. Chow KM, So WZ, Lee HJ, Lee A, Yap DWT, Takwoingi Y, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: A systematic review and meta-analysis. Eur Urol. (2023) 84:36–48. doi: 10.1016/j.eururo.2023.03.001
27. Chin J, Tan YG, Lee A, Ng TK, Shi R, Tang CYL, et al. Seeing is believing”: additive utility of 68Ga-PSMA-11 PET/CT in prostate cancer diagnosis. Cancers. (2024) 16:1777. doi: 10.3390/cancers16091777
28. Meijer D, van Leeuwen PJ, Roberts MJ, Siriwardana AR, Morton A, Yaxley JW, et al. External validation and addition of prostate-specific membrane antigen positron emission tomography to the most frequently used nomograms for the prediction of pelvic lymph-node metastases: an international multicenter study. Eur Urol. (2021) 80:234–42. doi: 10.1016/j.eururo.2021.05.006
Keywords: prostate cancer, nomograms, lymph node involvement, normograms, radical prostatectomy, lymph node staging, pelvic lymph node dissection, Briganti nomogram
Citation: Lau R, Lee HJ, Fong KY, Lee AY, Tan Y G, Law YM, Ngo NT, Tuan J, Tay KJ, Lee LS, Cheng C, Ho H, Yuen J and Chen K (2025) Real world prevalence of pelvic lymph node involvement in prostate cancer in Asia: do we need a rethink on normograms? Front. Oncol. 15:1583806. doi: 10.3389/fonc.2025.1583806
Received: 26 February 2025; Accepted: 17 April 2025;
Published: 27 May 2025.
Edited by:
Bartosz Malkiewicz, Wroclaw Medical University, PolandReviewed by:
Jakub Karwacki, Wroclaw Medical University, PolandRafal Osiecki, Independent Public Hospital them. Prof. W. Orlowski, Poland
Copyright © 2025 Lau, Lee, Fong, Lee, Tan, Law, Ngo, Tuan, Tay, Lee, Cheng, Ho, Yuen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth Chen, a2VubmV0aC5jaGVuQHNpbmdoZWFsdGguY29tLnNn
 Rachel Lau
Rachel Lau Han Jie Lee
Han Jie Lee Khi Yung Fong1
Khi Yung Fong1 Alvin Yuanming Lee
Alvin Yuanming Lee Yu Guang Tan
Yu Guang Tan Kae Jack Tay
Kae Jack Tay John Yuen
John Yuen Kenneth Chen
Kenneth Chen