Abstract
Purpose:
Cancer-associated thromboembolism (CAT) is a major complication in gastric cancer, impacting patient outcomes. This study aimed to evaluate preoperative thrombocytopenia as a risk factor for CAT in gastric cancer patients undergoing gastrectomy.
Materials and methods:
A retrospective analysis was conducted on 610 gastric cancer patients who underwent D2 gastrectomy between 2005 and 2017. The incidence of CAT and its association with preoperative thrombocytopenia, cancer stage, and recurrence were analyzed. Prognostic factors for CAT and survival were assessed using Kaplan-Meier analysis and Cox regression models.
Results:
The median follow-up was 67.0 months. Among the study participants, 5.7% (n=35) developed CAT. Preoperative thrombocytopenia was present in 71 patients (11.6%) and older age (≥65 years) was noted in 308 patients (50.1%). The 5-year incidence rate of CAT was 16.3% in patients with preoperative thrombocytopenia, compared to 4.8% in those with normal platelet counts. Patients with preoperative thrombocytopenia had a higher incidence of CAT compared to those with normal platelet counts (HR = 3.180, 95% CI 1.527-6.623, p = 0.002). Multivariate analysis revealed that thrombocytopenia (HR = 2.202, 95% CI 1.029-4.711, p = 0.042), older age (HR 2.484, 95% CI 1.166-5.290, p = 0.018), stage IV (HR = 2.966, 95% CI 1.106-8.466, p = 0.038) were independent poor prognostic factors for preoperative CAT in gastric cancer patients. Additionally, 29.7% of patients experienced cancer relapse, and 26.1% died during post-treatment follow-up. the patients who developed thromboembolisms had a significantly shorter 5-year OS rate compared with those who did not (56.6% vs. 76.1%, HR = 2.277, 95% CI = 1.376–3.767, p = 0.001).
Conclusions:
Preoperative thrombocytopenia is a significant predictor of CAT in gastric cancer patients. Along with advanced stage and older age, it increases thromboembolic risk and worsens survival. Further studies are needed to refine predictive models and optimize management strategies.
Introduction
Gastric cancer is a prevalent malignancy characterized by high morbidity and the fourth-highest mortality rate worldwide (1). Among the complications associated with gastric cancer, the thromboembolism is a significant concern due to its potential impact on patient outcomes and survival.
The incidence of cancer-associated venous thromboembolism, including pulmonary embolism (PE) and deep vein thrombosis (DVT), has been closely associated with survival outcomes in cancer patients (2, 3). Cancer-associated thromboembolism has been linked to various risk factors. Among these factors, the primary site of the tumor has emerged as a significant determinant of cancer-associated thromboembolism. Gastric cancer, in particular, has been identified as one of the most thrombogenic tumor types (4).
Many studies have demonstrated that cancer-associated thromboembolism is an independent prognostic factor for poor survival in patients with cancer. This correlation has been observed in gastric cancer (5) and other gastrointestinal malignancies, such as colon (6, 7), pancreatic (8), and lung cancer (9). Therefore, it is crucial to identify the risk factors associated with thromboembolism related to gastric cancer.
Cancer patients with thrombocytopenia are risk for developing thrombosis, a complication that poses significant clinical challenges (10). While low platelet counts typically increase the risk of bleeding, in the context of malignancy, various factors such as pro-coagulant activity of tumor cells, chemotherapy, and inflammation can lead to thrombotic events despite thrombocytopenia. This thrombosis, often associated with poor prognosis, necessitates careful clinical evaluation and management. Early recognition and appropriate treatment are essential to mitigate the risk of life-threatening complications in these patients, even before definitive diagnostic confirmation.
Therefore, this study investigated the occurrence of cancer-associated thromboembolism in a cohort of patients with gastric cancer who underwent gastrectomy as part of their treatment. The study aimed to identify the prognostic factors associated with the incidence of thromboembolism in patients with gastric cancer post-gastrectomy.
Materials and methods
Patient cohort
A total of 610 patients with primary gastric cancer who had undergone D2 radical gastrectomy at the Chung-Ang University Hospital (Seoul, Korea) between January 2005 and December 2017 were enrolled in this study. The Chung-Ang University Hospital’s pathologist histologically examined all the cases. Cancer variables, including stage and grade, were retrospectively collected. The diagnosis of gastric cancer was confirmed by pathological staining. The cancer staging was performed according to the American Joint Committee on Cancer, 7th edition (11). This study was approved by the Institutional Review Board of Chung-Ang University Hospital (IRB number: 2212-017-19450).
Study outcomes
The study’s primary outcome is to indentify risk factors for cancer-associated thromboembolism; the secondary outcome is to idenfify risk factors for patients’ disease free survival(DFS) and overall survival (OS).
The primary outcome measure was the incidence of cancer-associated thromboembolism, including symptomatic or incidentally found PE, DVT of the upper or lower limbs, and visceral venous thrombosis of patients who had undergone gastronomy for gastric cancer. The thromboembolism was confirmed by computed tomography. The patients’ time of death, cancer progression time, or time to develop cancer-associated thromboembolism post-gastrectomy were determined for all cases. In our study, thrombocytopenia was defined as a platelet count of less than 150,000/mL.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 26.0 (IBM Statistical Software™, IBM Corp., Armonk, NY, USA), including the patients’ baseline characteristics and prognostic factors. The correlation between prognostic factors involved thrombocytopenia, stage and older age and the CAT was examined using Cox proportional hazards regression modeling The correlation between death and the CAT was examined using Cox proportional hazards regression modeling and the Kaplan–Meier survival curve. A p value < 0.05 was considered statistically significant. GraphPad Prism 9.0 (GraphPad Inc., La Jolla, CA, USA) was used to generate the survival curves.
Results
Patients’ baseline characteristics
A total of 610 patients were included in this study, among whom 35 (5.7%) developed cancer-associated thromboembolism (CAT). The median age of the cohort was 65 years (range: 28–90), with a significantly higher median age observed in the CAT-positive group (68 years) compared to the CAT-negative group (64 years, P = 0.009). Patients aged ≥65 years were more likely to develop CAT (71.4% vs. 49.2%, P = 0.011). No significant difference was observed in sex distribution between the two groups (P = 0.598) (Table 1).
Table 1
| Characteristics | Total (n=610) | CAT (–) (n=575) | CAT (+) (n=35) | P value |
|---|---|---|---|---|
| Demographic Value | ||||
| Age - years | ||||
| median | 65 | 64 | 68 | 0.009 |
| range | 28-90 | 28-90 | 50-86 | |
| Age ≥65 years | 308 (50.5%) | 283 (49.2%) | 25 (71.4%) | 0.011 |
| Sex | ||||
| Male | 411 (67.4%) | 386 (67.1%) | 25 (71.4%) | 0.598 |
| Female | 199 (32.6%) | 189 (32.9%) | 10 (28.6%) | |
| Height (cm), mean ± SD | 161.9 ± 9.0 | 162.0 ± 9.0 | 1604 ± 10.2 | 0.445 |
| Weight (kg), mean ± SD | 62.2 ± 11.9 | 62.3 ± 11.9 | 60.6 ± 3.1 | 0.810 |
| BMI, kg/m2, mean ± SD | 23.6 ± 3.5 | 23.6 ± 3.5 | 23.4 ± 3.1 | 0.281 |
| Hematologic Value | ||||
| Platelet < 150,000/mL | 71 (11.6%) | 61 (10.6%) | 10 (28.6%) | 0.001 |
| Cancer specific variables | ||||
| Gastric cancer stage | ||||
| Stage I | 360 (59.0%) | 340 (59.1%) | 22 (62.9%) | 0.003 |
| Stage II | 98 (16.1%) | 95 (16.5%) | 3 (8.6%) | |
| Stage III | 121 (19.8%) | 117 (20.3%) | 4 (11.4%) | |
| Stage IV | 29 (4.8%) | 23 (4.0%) | 6 (17.1%) | |
| Adjuvant chemotherapy | ||||
| No | 370 (60.7%) | 348 (60.5%) | 22 (62.9%) | 0.784 |
| Yes | 240 (39.3%) | 227 (39.5%) | 13 (37.1%) | |
| Khorana Score variables | ||||
| BMI ≥ 35 kg/m2 | 1 (0.2%) | 1 (0.2%) | 0 (0%) | 0.805 |
| Hemoglobine < 10 g/dL | 84 (13.8%) | 80 (13.9%) | 4 (11.4%) | 0.679 |
| WBC ≥ 11000/mL | 258 (42.3%) | 244 (42.4%) | 14 (40.0%) | 0.777 |
| Platelet ≥ 350,000/mL | 48 (4.9%) | 47 (8.2%) | 1 (2.9%) | 0.257 |
| Khorana score Intermediate (≤2) | 269 (44.1%) | 252 (43.8%) | 17 (48.6%) | 0.583 |
| Khorana score High (≥3) | 341 (55.9%) | 323 (56.2%) | 18 (51.4%) | |
Patients’ baseline characteristics.
CAT. Cancer associated thromboembolism, BMI, body mass index; SD, standard deviation; WBC, white blood cell count.
Regarding hematologic parameters, thrombocytopenia (platelet <150,000/mL) was more prevalent in the CAT-positive group (28.6% vs. 10.6%, P = 0.001). Cancer-specific variables showed a significant association with CAT, particularly in patients with advanced-stage gastric cancer. The incidence of CAT was highest in stage IV patients (20.7%), followed by stage I (6.1%), stage III (3.3%), and stage II (3.1%) (P = 0.002). Adjuvant chemotherapy was not significantly associated with CAT occurrence (P = 0.784).
For Khorana score components, no significant differences were observed between the CAT-positive and CAT-negative groups in terms of BMI ≥35 kg/m² (P = 0.805), hemoglobin <10 g/dL (P = 0.679), WBC ≥11,000/mL (P = 0.777), and platelet ≥350,000/mL (P = 0.257).
Incidence of cancer-associated thromboembolism
The incidence of cancer-associated thromboembolism in the 610-patient cohort post- gastrectomy was 5.7% (35 patients). Among these cases, two patients presented with extremity DVT, while 19 patients were diagnosed with splanchnic vein thrombosis (SVT). Additionally, 10 patients experienced PE, and four exhibited other embolic events, including three cases of brain infarction and one of myocardial infarction.
The cumulative incidence of venous thromboembolism during the follow-up period (median 67.0 months) reached 5.7%. After gastrectomy. The 5-year incidence rate of cancer associated thrombosis was 16.3% in patients with preoperative thrombocytopenia, compared to 4.8% in those with normal platelet counts. Patients with preoperative thrombocytopenia had a higher incidence of cancer-associated thrombosis compared to those with normal platelet counts (HR = 3.180, 95% CI 1.527-6.623, p = 0.002) (Figure 1).
Figure 1
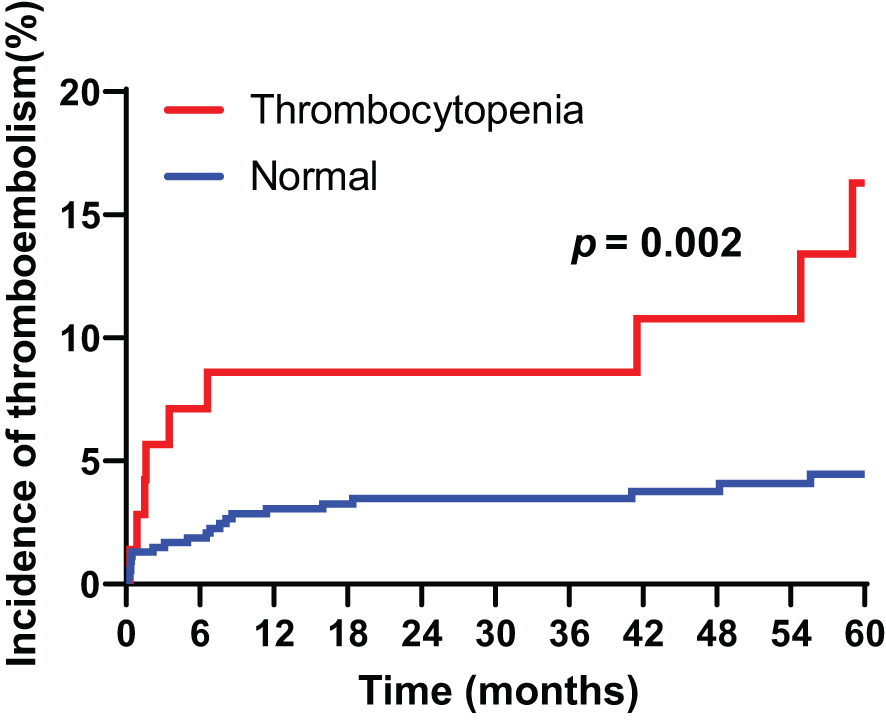
Cumulative incidence of cancer-associated thromboembolism in patients with gastric cancer according to thrombocytopenia.
Furthermore, the cumulative incidence of venous thromboembolism was 1.8%, 2.3%, and 2.8% at 12, 24, and 36 months, respectively. The cumulative incidence of thromboembolism according to cancer stage was 6.1%, 3.1%, 3.3%, and 20.7% in stages I, II, III, and IV, respectively; these differences were statistically significant (p = 0.002). The results indicated no statistically significant differences in cumulative incidences of thromboembolism between cancer stage groups as follows: stage I, p = 0.723; stage II, p = 0.973; stage III, p = 0.795; and stage IV, p = 0.063.
Multivariate analysis revealed that both thrombocytopenia (HR = 2.202, 95% CI 1.029-4.711, p = 0.042), older age (HR 2.484, 95% CI 1.166-5.290, p = 0.018) and stage IV (HR = 2.996, 95% CI 1.106-8.466, p = 0.038) were independent poor prognostic factors for preoperative CAT in gastric cancer patients (Table 2).
Table 2
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Demographic Value | ||||||
| Age ≥ 65 | 2.580 | 1.217-5.468 | 0.013 | 2.484 | 1.166-5.290 | 0.018 |
| Male | 0.817 | 0.384-1.736 | 0.599 | |||
| Hematologic variable | ||||||
| Platelet < 150,000/mL | 3.370 | 1.545-7.352 | 0.002 | 2.202 | 1.029-4.711 | 0.042 |
| Cancer specific variables | ||||||
| Gastric cancer stage | 0.003 | 0.011 | ||||
| Stage I | Reference | |||||
| Stage II | 0.501 | 0.150-1.673 | 0.261 | 0.422 | 0.124-1.433 | 0.167 |
| Stage III | 0.685 | 0.235-1.995 | 0.448 | 0.425 | 0.130-1.342 | 0.145 |
| Stage IV | 6.109 | 2.408-15.498 | <0.001 | 2.996 | 1.106-8.466 | 0.038 |
| Adjuvant chemotherapy | 0.943 | 0.475-1.872 | 0.866 | |||
| Khorana Score variables | ||||||
| BMI ≥ 35 kg/m2 | 0.050 | 0.000-5.567E+16 | 0.887 | |||
| Hemoglobin < 10 g/dL | 0.798 | 0.274-2.322 | 0.679 | |||
| WBC ≥ 11000/mL | 0.883 | 0.449-1.736 | 0.718 | |||
| Platelet ≥ 350,000/mL | 0.368 | 0.050-2.688 | 0.324 | |||
Univariate and multivariate analysis of the incidence of cancer-associated thromboembolism (CAT) by risk factor.
BMI, body mass index; CI, confidence interval; HR, hazard ratio; WBC, white blood cell count.
Survival
The study’s cutoff for analyses was January 2005, and the median follow-up duration was 67.0 months (range 0.1–155.0 months). The 5-year OS rate was 75.0%, and the 5-year disease-free survival (DFS) rate was 70.9%. Of the 610 patients, 181 (29.7%) had cancer relapse after treatment, and 159 (26.1%) died. The patients who developed thromboembolism had a significantly shorter 5-year DFS rate compared with those who did not (50.8% vs. 72.0%, HR = 2.155, 95% CI = 1.323–3.508, p = 0.002). In addition, the patients who developed thromboembolisms had a significantly shorter 5-year OS rate compared with those who did not (56.6% vs. 76.1%, HR = 2.277, 95% CI = 1.376–3.767, p = 0.001) (Figure 2).
Figure 2
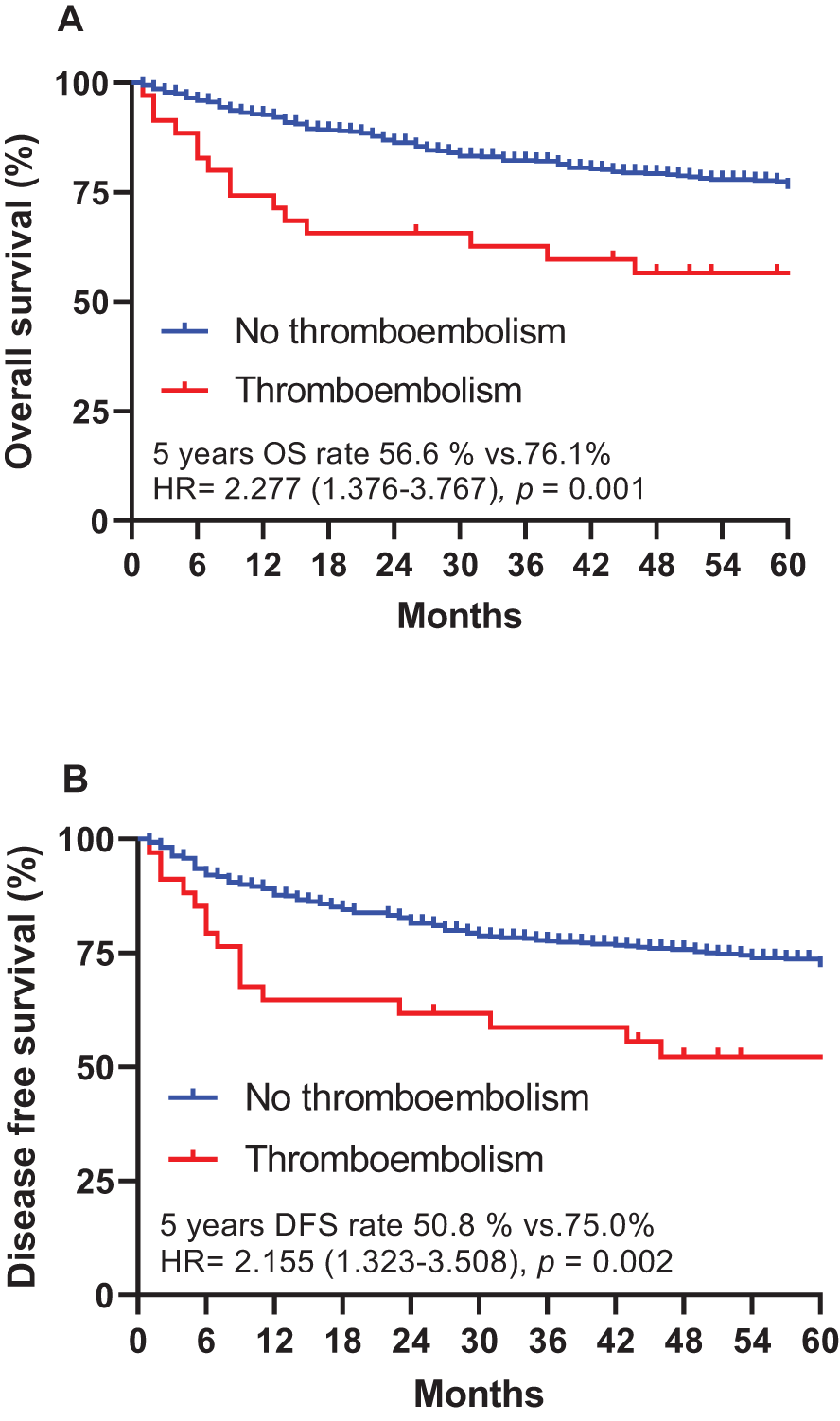
Overall survival (A) and disease-free survival (B), stratified by cancer-associated thromboembolism (CAT) in patients with gastric cancer. DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
In univariate analysis, age ≥65 years was significantly associated with poor overall survival (HR: 2.251, 95% CI: 1.618–3.133, P < 0.001), which remained significant in multivariate analysis (HR: 2.094, 95% CI: 1.494–2.934, P < 0.001). Advanced gastric cancer stage was a strong predictor of worse survival outcomes, with stage IV patients having the highest mortality risk (HR: 21.839, 95% CI: 13.007–36.850, P < 0.001 in univariate analysis; HR: 20.278, 95% CI: 11.433–35.966, P < 0.001 in multivariate analysis).
Cancer-associated thromboembolism was associated with increased mortality risk in univariate analysis (HR: 2.277, 95% CI: 1.376–3.767, P = 0.001), but this association did not reach statistical significance in multivariate analysis (HR: 1.684, 95% CI: 0.956–2.966, P = 0.071). Other factors, such as hemoglobin <10 g/dL and WBC ≥11,000/mL, showed statistical significance in univariate analysis but were not significant predictors in multivariate analysis (Table 3).
Table 3
| Risk factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Demographic Value | ||||||
| Age ≥ 65 | 2.251 | 1.618-3.133 | <0.001 | 2.094 | 1.494-2.934 | <0.001 |
| Male | 0.935 | 0.673-1.301 | 0.691 | |||
| Hematologic variables | ||||||
| Platelet < 150,000/mL | 1.250 | 0.797-1.960 | 0.332 | |||
| Cancer specific variables | ||||||
| Gastric cancer stage | <0.001 | <0.001 | ||||
| Stage I | Reference | Reference | ||||
| Stage II | 1.923 | 1.108-3.335 | 0.020 | 1.928 | 1.059-3.513 | 0.032 |
| Stage III | 9.492 | 6.425-14.024 | <0.001 | 9.843 | 6.136-15.789 | <0.001 |
| Stage IV | 21.839 | 13.007-36.850 | <0.001 | 20.278 | 11.433-35.966 | <0.001 |
| Cancer thromboembolism | 2.277 | 1.376-3.767 | 0.001 | 1.684 | 0.956-2.966 | 0.071 |
| Khorana Score variables | ||||||
| BMI ≥ 35 kg/m2 | 0.050 | 0-16993705 | 0.765 | |||
| Hemoglobin < 10 g/dL | 2.223 | 1.531-3.227 | <0.001 | 0.976 | 0.656-1.452 | 0.903 |
| WBC ≥ 11000/mL | 0.397 | 0.272-0.564 | <0.001 | 0.992 | 0.645-1.526 | 0.971 |
| Platelet ≥ 350,000/mL | 1.550 | 0.937-2.564 | 0.088 | 0.781 | 0.456-1.338 | 0.368 |
Univariate and multivariate analysis of the overall survival.
BMI, body mass index; CI, confidence interval; HR, hazard ratio; WBC, white blood cell count.
Discussion
This study investigated the incidence and risk factors of cancer-associated thromboembolism (CAT) in a cohort of gastric cancer patients who underwent gastrectomy. The overall incidence of CAT was 5.7%, with a significantly higher occurrence in patients with preoperative thrombocytopenia. This finding reinforces the role of thrombocytopenia as a potential predictor of thromboembolic complications in gastric cancer patients.
Thrombocytopenia is a common condition in cancer patients due to multiple factors, including disease burden and chemotherapy-induced toxicity (12). It has frequently been reported in conjunction with CAT events, and our study further supports its association with increased thromboembolic risk. The 5-year incidence rate of CAT in patients with preoperative thrombocytopenia was markedly higher at 16.3% compared to 4.8% in those with normal platelet counts. Multivariate analysis confirmed thrombocytopenia as an independent risk factor for CAT, along with older age and stage IV disease. These findings align with prior studies suggesting that thrombocytopenia may paradoxically contribute to a hypercoagulable state, possibly due to increased circulating procoagulant microparticles from platelet destruction, enhanced thrombopoiesis, or tumor-driven inflammation (13).
Although thrombocytopenia is typically associated with bleeding risk, several biological mechanisms may explain its contribution to thrombotic susceptibility in cancer. Platelet fragmentation can release microparticles that serve as catalytic surfaces for thrombin generation (14). In response to low platelet counts, the bone marrow may generate immature, hyperreactive platelets. Additionally, tumor-secreted cytokines can activate coagulation pathways independently of platelet numbers (15).
This study also examined the impact of gastric cancer stage on CAT incidence. A statistically significant difference was observed across cancer stages, with stage IV patients exhibiting the highest thromboembolic incidence at 20.7%. This underscores the importance of considering cancer stage when assessing thromboembolic risk and highlights the necessity for proactive risk management strategies, particularly in patients with advanced disease.
Regarding the impact of CAT on patient survival, our results revealed a significantly shorter 5-year overall survival (OS) rate in patients with thromboembolism compared to those without. This highlights the detrimental impact of CAT on long-term prognosis and underscores the need for early identification and intervention to improve patient outcomes.
Notably, a portion of patients within our study population developed arterial thrombotic complications, including strokes and heart attacks, even in the context of low platelet counts. This observation suggests that in gastric cancer, thrombocytopenia may reflect underlying platelet hyperactivity or a prothrombotic state, rather than simply indicating a bleeding predisposition. In these individuals, the use of antiplatelet therapy alongside low molecular weight heparin (LMWH) may hold therapeutic promise (16). Nonetheless, due to the elevated hemorrhagic risk associated with gastric tumors—particularly those with mucosal involvement—such dual-agent regimens should be carefully tailored to each patient (17). Further prospective trials are essential to clarify the safety and potential benefits of these combined interventions in this challenging clinical scenario.
Despite its valuable insights, this study has limitations. As a single-center study, potential selection bias cannot be excluded, and the findings may not be fully generalizable to broader populations. We acknowledge that external validation is necessary to strengthen the robustness of our findings. Multicenter, prospective studies are warranted to validate our predictive model and ensure its applicability across diverse clinical settings.
Furthermore, while we identify thrombocytopenia as a significant risk factor, the study was not designed to investigate underlying biological mechanisms. Future translational studies are needed to elucidate the molecular and cellular pathways that link thrombocytopenia to thrombosis in gastric cancer.
In conclusion, this study identifies key predictive factors for CAT in gastric cancer patients post-gastrectomy, including preoperative thrombocytopenia, advanced cancer stage and older age. These findings support the need for personalized risk stratification and targeted preventive strategies to reduce the burden of CAT in this population. Furthermore, careful consideration of antithrombotic strategies—balancing thrombotic and bleeding risks—is essential, particularly in patients with arterial events and thrombocytopenia. Further research is essential to optimize thromboprophylactic approaches and improve clinical outcomes in gastric cancer patients at risk for thromboembolism.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Chung-Ang University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived due to the retrospective nature of the study and minimal risk to participants.
Author contributions
SP: Writing – original draft, Writing – review & editing. MJ: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. JK: Writing – original draft, Writing – review & editing. IH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2
Anderson LA Moore SC Gridley G Stone BJ Landgren O . Concomitant and antecedent deep venous thrombosis and cancer survival in male US veterans. Leuk Lymphoma. (2011) 52:764–70. doi: 10.3109/10428194.2010.551572
3
Agnelli G Becattini C Meyer G Munoz A Huisman MV Connors JM et al . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. (2020) 382:1599–607. doi: 10.1056/NEJMoa1915103
4
Levitan N Dowlati A Remick SC Tahsildar HI Sivinski LD Beyth R et al . Rates of initial and recurrent thromboembolic disease among patients with Malignancy versus those without Malignancy. Risk analysis using Medicare claims data. Med (Baltimore). (1999) 78:285–91. doi: 10.1097/00005792-199909000-00001
5
Fuentes HE Oramas DM Paz LH Wang Y Andrade XA Tafur AJ . Venous thromboembolism is an independent predictor of mortality among patients with gastric cancer. J Gastrointest Cancer. (2018) 49:415–21. doi: 10.1007/s12029-017-9981-2 , PMID:
6
Mandala M Barni S Floriani I Isa L Fornarini G Marangolo M et al . Incidence and clinical implications of venous thromboembolism in advanced colorectal cancer patients: the ‘GISCAD-alternating schedule’ study findings. Eur J Cancer. (2009) 45:65–73. doi: 10.1016/j.ejca.2008.09.005
7
Alcalay A Wun T Khatri V Chew HK Harvey D Zhou H et al . Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. (2006) 24:1112–8. doi: 10.1200/JCO.2005.04.2150
8
Mandala M Reni M Cascinu S Barni S Floriani I Cereda S et al . Venous thromboembolism predicts poor prognosis in irresectable pancreatic cancer patients. Ann Oncol. (2007) 18:1660–5. doi: 10.1093/annonc/mdm284
9
Mansfield AS Tafur AJ Wang CE Kourelis TV Wysokinska EM Yang P . Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. (2016) 14:1773–8. doi: 10.1111/jth.13378
10
Carney BJ Wang TF Ren S George G Al Homssi A Gaddh M et al . Anticoagulation in cancer-associated thromboembolism with thrombocytopenia: a prospective, multicenter cohort study. Blood Adv. (2021) 5:5546–53. doi: 10.1182/bloodadvances.2021005966
11
Sobin LH Compton CC . TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. (2010) 116:5336–9. doi: 10.1002/cncr.25537
12
Liebman HA . Thrombocytopenia in cancer patients. Thromb Res. (2014) 133 Suppl 2:S63–9. doi: 10.1016/S0049-3848(14)50011-4
13
Hsu C Patell R Zwicker JI . The prevalence of thrombocytopenia in patients with acute cancer-associated thrombosis. Blood Adv. (2023) 7:4721–7. doi: 10.1182/bloodadvances.2022008644
14
Freyssinet JM Toti F . Formation of procoagulant microparticles and properties. Thromb Res. (2010) 125 Suppl 1:S46–8. doi: 10.1016/j.thromres.2010.01.036
15
Sharma D Brummel-Ziedins KE Bouchard BA Holmes CE . Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. (2014) 229:1005–15. doi: 10.1002/jcp.24539
16
Frere C Font C Esposito F Crichi B Girard P Janus N . Incidence, risk factors, and management of bleeding in patients receiving anticoagulants for the treatment of cancer-associated thrombosis. Support Care Cancer. (2022) 30:2919–31. doi: 10.1007/s00520-021-06598-8
17
Abbas U MacKenzie R Khan U Fatima R Wang TF Luo R et al . Anticoagulant management of cancer-associated thrombosis and thrombocytopenia: a retrospective chart review. Res Pract Thromb Haemost. (2025) 9:102684. doi: 10.1016/j.rpth.2025.102684
Summary
Keywords
thrombocytopenia, thromboembolism, gastric cancer, gastrectomy, prognosis
Citation
Jung M, Park SE, Choi JH, Kim JE and Hwang IG (2025) Preoperative thrombocytopenia as a predictor of cancer-associated thromboembolism in gastric cancer patients undergoing gastrectomy. Front. Oncol. 15:1585201. doi: 10.3389/fonc.2025.1585201
Received
28 February 2025
Accepted
30 June 2025
Published
16 July 2025
Volume
15 - 2025
Edited by
Marco Materazzo, Policlinico Tor Vergata, Italy
Reviewed by
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), Italy
Xin Tang, Hangzhou Wuyunshan Hospital, China
Updates
Copyright
© 2025 Jung, Park, Choi, Kim and Hwang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In Gyu Hwang, oncology@cau.ac.kr
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.