- 1Department of Pediatrics, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Baylor College of Medicine, Children’s Hospital of San Antonio, San Antonio, TX, United States
- 3Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 4Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York, NY, United States
- 5Department of Pathology and Laboratory Medicine, Northwell Health Northern Westchester Hospital, Mount Kisco, NY, United States
- 6Clinical Sciences, Global Development, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, United States
Dias–Logan syndrome (DLS) is a rare condition caused by heterozygous germline BCL11A pathogenic variants associated with global developmental delay, distinctive facial features, and asymptomatic persistence of fetal hemoglobin. There has been no evidence of an association between DLS and increased risk of cancer. We report the first instance of a child with DLS diagnosed with cancer, a Wilms tumor (WT), who is notably much older than the typical onset. Although this case alone is insufficient to warrant routine WT screening in DLS, given the extreme rarity, we cannot rule out an association with DLS and WT predisposition.
Introduction
Dias–Logan syndrome (DLS), also known as BCL11A-related intellectual disability (BCL11A-ID), is caused by germline heterozygous pathogenic variants in BCL11A, which is located on chromosome 2p16.1 (1). DLS/BCL11A-ID is inherited in an autosomal dominant manner, most often caused by a de novo mutation. This is a very rare event, and only 75 individuals have been reported with germline pathogenic/likely pathogenic variants in BCL11A, with a median age at molecular diagnosis of 7 years (range 1–19 years). Patients, usually children, with DLS/BCL11A-ID may present with a variety of clinical manifestations including global developmental delay/intellectual disability of variable degree, neonatal hypotonia, microcephaly, non-specific dysmorphic facial features, behavior problems, autism spectrum disorder, and asymptomatic persistence of fetal hemoglobin. Some patients have also been reported to have growth delay, seizures, and gastrointestinal and musculoskeletal problems (2). Notably, there are no reports of individuals with DLS/BCL11A-ID diagnosed with either solid or hematologic malignancies, although there was one patient with a benign (and relatively common) osteochondroma (3).
We describe the first case of a child with DLS/BCL11A-ID diagnosed with Wilms tumor (WT), a rare pediatric embryonal tumor of the kidney with approximately 500 cases per year diagnosed in the United States (4–6). The mean age at diagnosis is 44 months for unilateral cases and 31 months for bilateral cases of Wilms tumor, and 75% of patients are diagnosed before 5 years of age (5). Although most cases occur apparently sporadically, 10%–20% of children with WT are noted to have an established cancer predisposition (7, 8).
Case description
This case report describes a girl born to a non-consanguineous couple of Northern and Eastern European descent who was noted as an infant to have hypotonia and global developmental delays, including speech apraxia. Genetic testing was pursued including a chromosomal microarray, expanded neurodevelopmental gene panel, and trio exome sequencing. The microarray showed a copy number gain on 9q31.1 that was found to be maternally inherited and therefore reclassified as benign. Exome sequencing identified a de novo heterozygous likely pathogenic germline variant denoted BCL11A c.55 + 1G>A. The genomic and clinical history was consistent with DLS/BCL11A-ID.
At 11 years old, she developed increasing episodes of headache and associated eye pain along with nausea and vomiting that awoke her from sleep. The left side of her abdomen was tender and distended. She was directed to a local emergency room, where an abdominal ultrasound identified a large left-sided renal lesion with mass effect on the infrarenal aorta. A CT scan of her chest, abdomen, and pelvis identified a 14-cm heterogeneous left renal mass without evidence of distant metastatic spread (Figures 1A, B). She underwent upfront left complete nephrectomy, and surgical pathology was consistent with a favorable histology Wilms tumor, predominantly blastemal in appearance, local stage III due to lympho-vascular invasion of the tumor with multiple regional lymph nodes demonstrating evidence of tumor (Figures 1C, D). The possibility of an additional condition resulting in a cancer predisposition was evaluated using germline Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), which analyzed 90 cancer predisposition genes, as well as dedicated testing for Beckwith–Wiedemann syndrome (BWS), including methylation and copy number analysis of 11p15.5 and CDKN1C sequencing. No established cancer predisposition syndrome was identified through these tests. There were no potential environmental exposures that we could identify as contributors to cancer development, such as certain maternal exposures to carcinogens during pregnancy. The patient does not have a family history of childhood cancer and has multiple healthy siblings.
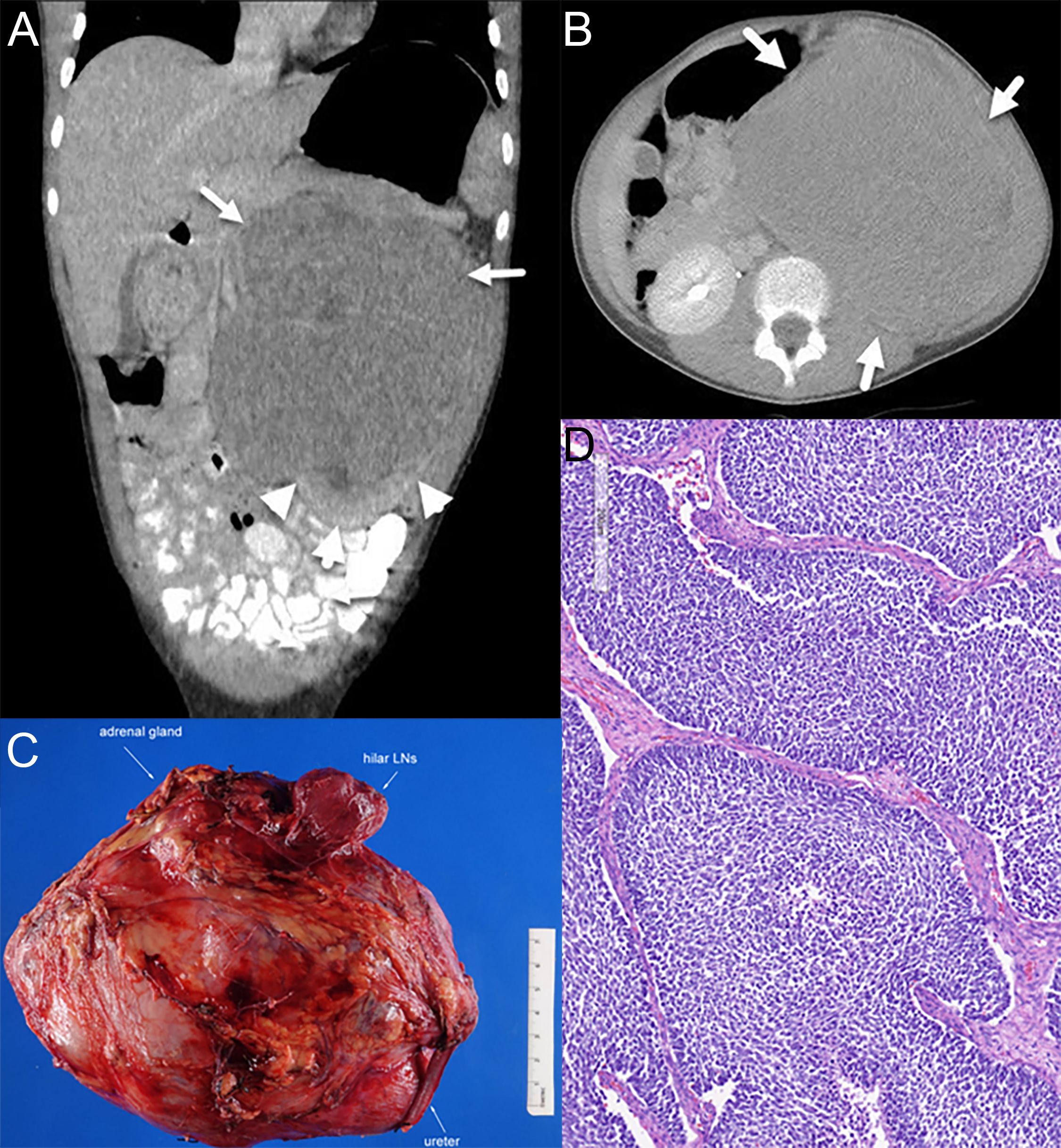
Figure 1. Radiology and pathology of the patient’s tumor. (A) Coronal view on CT scan at diagnosis. Arrows indicate tumor, and arrowheads show normal kidney and claw sign, demonstrating the renal origin. (B) Axial view on CT scan at diagnosis. (C) Gross appearance exhibiting a large renal mass. (D) Representative histology of the Wilms tumor with predominantly blastemal elements.
Cytogenetic testing of the tumor was positive for loss of heterozygosity (LOH) of chromosome 1p, but negative for LOH of chromosome 16q and negative for copy number gain of chromosome 1q. On Children’s Oncology Group (COG) Study AREN0532, the presence of LOH 1p and positive lymph node status was noted to be an adverse prognostic finding with a 4-year event-free survival of 73.8% compared to 88% for the stage III study cohort as a whole and 96.7% for stage III patients without LOH 1p nor 16q and no lymph node involvement (9). Additionally, her older age of onset along with somatic DROSHA miRNA microprocessing defect was further concerning (10, 11). Using shared decision making with the family based on these results, the decision was made to augment chemotherapeutic treatment from Regimen DD4A to Regimen M, thereby incorporating cyclophosphamide and etoposide onto her existing vincristine, dactinomycin, and doxorubicin backbone as per the COG Study AREN0533. She finished treatment, including standard dose flank radiation therapy, without any unexpected adverse events, and is now more than 3 years from completion of therapy with no evidence of disease recurrence.
Discussion
This is the first report of a child with DLS/BCL11A-ID diagnosed with a malignancy, in this case, Wilms tumor, a rare pediatric kidney tumor. Currently, there is no evidence that individuals with DLS/BCL11A-ID are at an increased risk of developing cancer. However, as there are so few cases reported, and at such young average ages, it is possible that a low-penetrant cancer predisposition may be as-yet unknown (3). Notably, while there are no known reports of WTs in individuals with germline deletions or duplications involving BCL11A (2p15p16.1 deletion or duplication syndrome), renal anomalies such as multicystic kidneys and hydronephrosis have been reported in this related condition, suggesting that perhaps inactivation of BCL11A may be involved in physiologic renal development, although these syndromes include many additional adjacent genes, in addition to BCL11A, which may instead be contributory (12).
The established function of BCL11A is that it forms a key component of the mammalian SWI/SNF complex, a chromatin remodeling apparatus that has been implicated in 20% of all human tumors and notably aberrant in several other pediatric kidney tumors such as malignant rhabdoid tumor of the kidney and renal medullary carcinoma, both of which are characterized by biallelic inactivation of SMARCB1 (13); indeed, somatic alterations of BCL11A are noted in many different, generally adult-onset, cancers (14). While we are unaware of genetic somatic inactivation of BCL11A as a driver of Wilms tumors, BCL11A is indeed one of the most highly downregulated transcripts in SIX1-mutant WT, which notably form a related subset of WT characterized by a pre-induction metanephric mesenchyme gene expression profile and often associated with DROSHA mutations, as seen in this case (10, 15). Along with other key epigenomic drivers of nephrogenesis, including other SWI/SNF complex constituents or downstream PRC2 complex components, BCL11A appears to play a role in the differentiation program of nephron progenitor cells, further supporting its potential as a tumor suppressor candidate (16).
In children diagnosed with WT predisposition conditions, surveillance typically involves renal ultrasonography every 3–4 months until age 7 (17). The patient described in this case was diagnosed at 11 years of age, so she would not have been identified earlier with conventional screening strategies for established WT cancer predisposition syndromes. In the most common WT predisposition condition, BWS, WT screening has been shown to result in an earlier stage at diagnosis of tumors. Given the importance of BCL11A in cancer, and this case report, it will be important to determine over time if other individuals with DLS/BCL11A-ID are diagnosed with WT or other cancer types, if screening is necessary, and what age range of screening would be most appropriate (18). As such, we do feel that this very rare constellation of possibly related conditions is worth describing. Indeed, as selective knockdown of BCL11A has become a novel therapeutic strategy to treat sickle cell disease, a further understanding of this gene will become more important beyond rare cases of children with DLS/BCL11A-ID (19).
In conclusion, we describe an 11-year-old female patient with DLS/BCL11A-ID who developed WT, and we raise the possibility that DLS/BCL11A-ID could be associated with WT predisposition, although further observations are needed to better characterize this association, if any. At this point, there are inadequate data to warrant screening for WT in patients with DLS/BCL11A-ID, but future research should focus on what tumor spectrum, if any, is associated with this condition in order to design an appropriate screening strategy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
AT: Conceptualization, Writing – original draft, Writing – review & editing, Data curation. EF: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. TS: Writing – review & editing. AP: Visualization, Writing – review & editing. JMM: Writing – review & editing. JM: Writing – review & editing. MG: Writing – review & editing. MW: Conceptualization, Writing – review & editing. MO: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors at MSK acknowledge support from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) Cancer Center Support Grant P30 CA008748. M.O. acknowledges active research support from the Children’s Cancer Research Fund; Pediatric Cancer Foundation; Rally Foundation for Childhood Cancer Research; Infinite Love for Kids Fighting Cancer; The Jed Ian Taxel Foundation for Rare Cancer Research; Cannonball Kids’ cancer; Cookies for Kids’ Cancer; the Starr Cancer Consortium; Conquer Cancer, the ASCO Foundation; Cycle for Survival; Handstand Walk for Kids; the Serra Family and the Bianco Family Foundation; Debbie and Kevin Bhatt; and NCI/NIH via U01 CA263967.
Acknowledgments
We thank Joseph Olechnowicz for the editorial assistance.
Conflict of interest
Author MW was employed by the company Regeneron Pharmaceuticals, Inc. MO has received institutional research support for clinical and laboratory research from Chugai, Amgen, Karyopharm, Saol, and Bayer, which are unrelated to the above case report.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BCL11A-ID, BCL11A-related intellectual disability; BWS, Beckwith–Wiedemann syndrome; COG, Children’s Oncology Group; DLS, Dias–Logan syndrome; LOH, loss of heterozygosity; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; WT, Wilms tumor.
References
1. Dias C, Estruch SB, Graham SA, McRae J, Sawiak SJ, Hurst JA, et al. BCL11A haploinsufficiency causes an intellectual disability syndrome and dysregulates transcription. Am J Hum Genet. (2016) 99:253–74. doi: 10.1016/j.ajhg.2016.05.030
2. Peron A, Bradbury K, Viskochil DH, and Dias C. BCL11A-related intellectual disability. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, and Amemiya A, editors. GeneReviews((R)). Seattle (WA (1993).
3. Peron A, D’Arco F, Aldinger KA, Smith-Hicks C, Zweier C, Gradek GA, et al. BCL11A intellectual developmental disorder: defining the clinical spectrum and genotype-phenotype correlations. Eur J Hum Genet. (2024) 33:312–24. doi: 10.1038/s41431-024-01701-z
4. Bhutani N, Kajal P, and Sharma U. Many faces of Wilms Tumor: Recent advances and future directions. Ann Med Surg (Lond). (2021) 64:102202. doi: 10.1016/j.amsu.2021.102202
5. Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, et al. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/csr/1975_2018/ (Accessed February 1, 2025).
6. Spreafico F, Fernandez CV, Brok J, Nakata K, Vujanic G, Geller JI, et al. Wilms tumour. Nat Rev Dis Primers. (2021) 7:75. doi: 10.1038/s41572-021-00308-8
7. Liu EK and Suson KD. Syndromic Wilms tumor: a review of predisposing conditions, surveillance and treatment. Transl Androl Urol. (2020) 9:2370–81. doi: 10.21037/tau.2020.03.27
8. Balis F, Green DM, Anderson C, Cook S, Dhillon J, Gow K, et al. Wilms tumor (Nephroblastoma), version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:945–77. doi: 10.6004/jnccn.2021.0037
9. Fernandez CV, Mullen EA, Chi YY, Ehrlich PF, Perlman EJ, Kalapurakal JA, et al. Outcome and prognostic factors in stage III favorable-histology wilms tumor: A report from the children’s oncology group study AREN0532. J Clin Oncol. (2018) 36:254–61. doi: 10.1200/JCO.2017.73.7999
10. Walz AL, Ooms A, Gadd S, Gerhard DS, Smith MA, Guidry Auvil JM, et al. Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell. (2015) 27:286–97. doi: 10.1016/j.ccell.2015.01.003
11. Wegert J, Ishaque N, Vardapour R, Georg C, Gu Z, Bieg M, et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell. (2015) 27:298–311. doi: 10.1016/j.ccell.2015.01.002
12. Rajcan-Separovic E, Harvard C, Liu X, McGillivray B, Hall JG, Qiao Y, et al. Clinical and molecular cytogenetic characterisation of a newly recognised microdeletion syndrome involving 2p15-16. 1. J Med Genet. (2007) 44:269–76. doi: 10.1136/jmg.2006.045013
13. Perotti D, O’Sullivan MJ, Walz AL, Davick J, Al-Saadi R, Benedetti DJ, et al. Hallmark discoveries in the biology of non-Wilms tumour childhood kidney cancers. Nat Rev Urol. (2025). doi: 10.1038/s41585-024-00993-6
14. Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human Malignancy. Nat Genet. (2013) 45:592–601. doi: 10.1038/ng.2628
15. Gadd S, Huff V, Walz AL, Ooms A, Armstrong AE, Gerhard DS, et al. A Children’s Oncology Group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nat Genet. (2017) 49:1487–94. doi: 10.1038/ng.3940
16. Liu H, Hilliard S, Kelly E, Chen CH, Saifudeen Z, and El-Dahr SS. The polycomb proteins EZH1 and EZH2 co-regulate chromatin accessibility and nephron progenitor cell lifespan in mice. J Biol Chem. (2020) 295:11542–58. doi: 10.1074/jbc.RA120.013348
17. Scott RH, Walker L, Olsen OE, Levitt G, Kenney I, Maher E, et al. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child. (2006) 91:995–9. doi: 10.1136/adc.2006.101295
18. Mussa A, Duffy KA, Carli D, Griff JR, Fagiano R, Kupa J, et al. The effectiveness of Wilms tumor screening in Beckwith-Wiedemann spectrum. J Cancer Res Clin Oncol. (2019) 145:3115–23. doi: 10.1007/s00432-019-03038-3
Keywords: case report, Wilms tumor, genetics, BCL11A, Dias–Logan syndrome
Citation: Troullioud Lucas AG, Fiala E, Razeq A, Sauerhaft T, Price AP, Mosquera JM, Miyauchi J, Gao M, Walsh MF and Ortiz MV (2025) Case Report: First report of a Wilms tumor in an individual with Dias–Logan syndrome (BCL11A-related intellectual disability). Front. Oncol. 15:1585492. doi: 10.3389/fonc.2025.1585492
Received: 28 February 2025; Accepted: 08 May 2025;
Published: 23 July 2025.
Edited by:
Li Chen, National Cancer Institute at Frederick (NIH), United StatesReviewed by:
Talita Aguiar, Columbia University Irving Medical Center, United StatesSrikrishna Narava, National Cancer Institute at Frederick (NIH), United States
Copyright © 2025 Troullioud Lucas, Fiala, Razeq, Sauerhaft, Price, Mosquera, Miyauchi, Gao, Walsh and Ortiz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael V. Ortiz, b3J0aXptMkBtc2tjYy5vcmc=
 Alexandre G. Troullioud Lucas
Alexandre G. Troullioud Lucas Elise Fiala1
Elise Fiala1 Juan Miguel Mosquera
Juan Miguel Mosquera Michael V. Ortiz
Michael V. Ortiz