- 1Department of Radiology and Imaging Sciences, Division of Interventional Radiology and Image-Guided Medicine, Emory School of Medicine, Atlanta, GA, United States
- 2Vanderbilt University School of Medicine, Nashville, TN, United States
Tumor-related lymphatic obstruction can cause malignant chylothorax, which can be debilitating. Conventional management includes dietary modifications, percutaneous drainage, and medical management (octreotide), most of which prove refractory in high-output chylothorax cases. Lymphangiogram and embolization in such cases offers a minimally-invasive alternative; however, its use in non-iatrogenic malignant chylothorax is underreported. We present three cases of malignant chylothorax managed with lymphangiogram followed by therapeutic embolization. Case 1: A 70-year-old female with relapsed angioimmunoblastic T-cell lymphoma presents with bilateral chylous effusions refractory to conventional management. Following thoracic duct embolization (TDE) drainage output decreased from over 600 mL/day to less than 200 mL/day, permitting resumption of systemic therapy and subsequent autologous stem cell transplantation. Case 2: A 28-year-old female with ALK-positive non–small cell lung cancer presents with severe respiratory compromise due to extensive mediastinal disease and high-output chylothorax (>1 L/day) refractory to conventional therapy. TDE reduced drainage to less than 150 mL/day, allowing for continued targeted therapy. Case 3: A 70-year-old female with HER2-positive, ER-/PR– breast cancer presents with recurrent right-sided chylothorax despite prior surgical lymphatic ligations. Direct lymphatic leak embolization resulted in marked reduction of chylous output and significant symptom relief. Lymphangiogram with embolization is a safe and effective intervention for malignant chylothorax, regardless of surgical history. Early intervention can alleviate chyle leaks, facilitate ongoing cancer therapy, and improve patient outcomes, making it an important option in multidisciplinary oncology care.
Highlights
● Novel Application: Lymphangiogram and embolization, traditionally used for postoperative chylothorax, can effectively treat malignant chylothorax arising from tumor-induced lymphatic obstruction.
● Clinical Benefit: Rapid reduction of high-output chyle leaks via lymphangiogram and embolization enables uninterrupted systemic therapy or provides significant symptom relief in advanced malignancies.
● Practice Implication: Early referral for lymphangiogram and embolization in malignant chylothorax may reduce morbidity, shorten hospital stays, and improve overall quality of life in oncology patients.
Introduction
Chylothorax, the accumulation of chyle in the pleural space, is most commonly associated with traumatic or postoperative injury to the lymphatic channels (1). Malignant chylothorax, however, can result from sequalae of tumor invasion or extrinsic lymphatic obstruction, and is frequently seen in advanced lymphoma, accounting for as much as 70% of cases (2, 3). Chyle leaks can result in severe nutritional deficits, immunosuppression, and significant respiratory distress, all of which can delay oncologic treatment and adversely affect prognosis (4, 5). While initial management includes dietary modifications, medical management with octreotide, and percutaneous drainage, high-output leaks such as those with greater than 1,500 mL/day, are at the threshold for which surgical intervention is recommended.
Lymphangiogram and embolization, including thoracic duct embolization (TDE), is a minimally invasive means by which a lymphatic system is accessed for deployment of coils or liquid embolic agents such as glue. Most commonly utilized in postoperative chylothorax, TDE has demonstrated high technical and clinical success rates in traumatic settings as high as 90% (6). However, its application in malignant chylothorax without recent surgical injury remains less frequently reported, and with variable success rates between 27% and 68% (7–9). The following case series focuses on lymphangiogram-directed embolization performed in three oncology patients with malignant chylothorax, underscoring its potential for rapid symptom relief and support of oncologic care (Tables 1, 2).
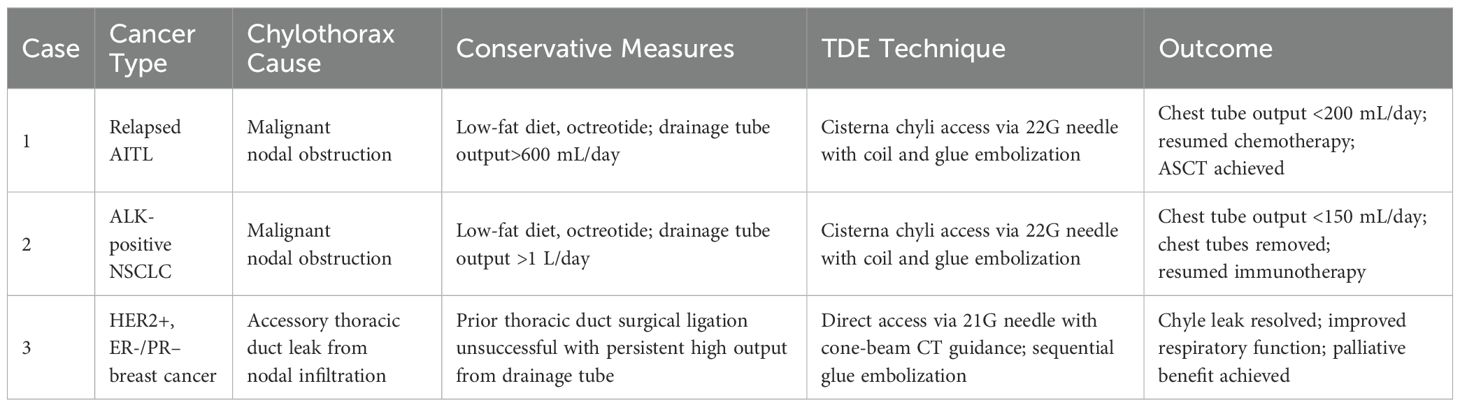
Table 1. Clinical Characteristics, lymphangiogram and embolization technique, and post-procedural outcomes.
Our standard thoracic duct embolization technique
After obtaining informed consent, the patient is brought to the Interventional Radiology suite, and bilateral groin lymph nodes are accessed with 25 gauge spinal needles with ultrasound guidance. Lipiodol is then injected, and the progression of lipiodol passage is observed under fluoroscopy until visualization of the cisterna chyli. Next, a 22-gauge Chiba needle is advanced through trans-abdominal approach under fluoroscopic guidance. A V18 wire was then advanced through the needle into the thoracic duct. The needle was then exchanged over the wire for a 2.4 French Microcatheter. A small amount of Omnipaque-300 contrast was injected for better opacification of the thoracic duct with visualization of lymphatic leaks where present. Thereafter, a combination of a 5 mm coil is then deployed into the thoracic duct, followed by additional embolization with 1:1 glue:lipiodol mixture. In particularly challenging cases, cone-beam CT guidance allows direct access to the leak site via a 25 gauge spinal needle when a microwire cannot be advanced from the traditional cisterna chyli access.
Case presentations
Case 1: Relapsed angioimmunoblastic t-cell lymphoma
A 70-year-old female with a history of AITL in remission following chemoradiation a decade prior, presents with cervical and supraclavicular lymphadenopathy and constitutional B- symptoms. Imaging demonstrated extensive lymphadenopathy with thoracentesis-confirmed bilateral chylous effusions, and biopsy-confirmed relapse of AITL. Specifically, chylous etiology was confirmed with definitive laboratory evidence: grossly milky yellow appearance, markedly elevated triglyceride levels (801 mg/dL, well above the diagnostic threshold of 110 mg/dL), and lymphocyte-predominant (77%) cellular composition. The patient was placed on a low-fat diet and a left pleural pigtail catheter was placed to drain the left pleural collection. With greater than 600mL of output daily, the decision to proceed with thoracic duct embolization was made with Interventional Radiology as per our standard TDE technique described above with coil and glue embolization (Figure 1A). The patient’s output decreased to less than 200 mL daily within 48 hours following 5mm coil and n-butyl cyanoacrylate (NBCA) glue embolization and the pigtail was eventually removed on post-procedural day 2 (Figures 1B, C). The patient subsequently resumed chemotherapy and went on to receive autologous stem cell transplantation. She did not develop any complications from the procedure.
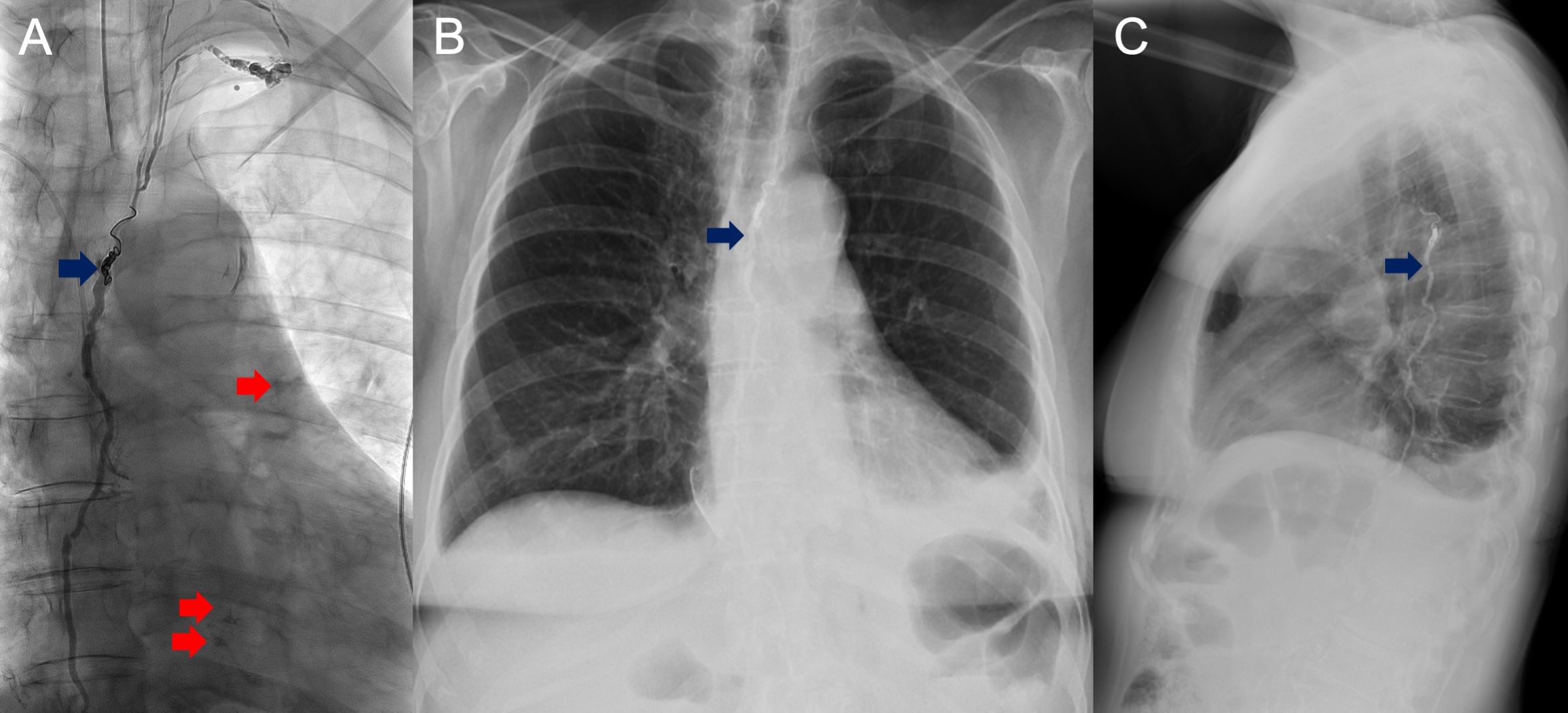
Figure 1. Anteroposterior (AP) orientation fluoroscopic images of Patient 1 with lipiodol-filled thoracic duct with areas of extravasation (red) visualized over the left lower hemithorax status post glue and coil embolization (blue) (A). Removal of left sided pigtail catheter at POD2, and AP (B) and lateral (C) chest radiograph obtained on POD7 demonstrating residual left sided effusion.
Case 2: ALK-positive non–small cell lung cancer
A 28-year-old female with ALK-positive NSCLC initially presents with right neck swelling, cough, and signs of respiratory distress. Subsequent imaging revealed extensive mediastinal lymphadenopathy, pericardial effusion with features of cardiac tamponade, and left sided effusion (Figure 2A). Following emergent pericardiocentesis, V-V ECMO support, and placement of a Y-tracheal stent due to severe tracheal narrowing, she was initiated on lorlatinib, but she soon developed bilateral chylothorax with chest tube outputs exceeding 1 L/day. This did not improve with a low-fat diet. With refractory and persistent chylothorax, TDE was performed with 5mm coil and glue (Figures 2B–D) following the our institutional TDE technique as described above. Following the procedure, chest tube output decreased to less than <150 mL/day. She did not develop any complications from the procedure.

Figure 2. Pre-procedural images of Patient 2 show contrast-enhanced axial CT reformat (A) demonstrating large pericardial and moderate left pleural effusion. Intraprocedural AP fluoroscopic images demonstrating difficult groin nodal access (B) with eventual microwire and microcatheter crossing the cisterna chylii (C) and lymphangiogram demonstrating lipiodol extravasation in the region overlying the right upper and left upper hemithorax (D).
Case 3: HER2-positive, ER-/PR– breast cancer
A 70-year-old woman with a history of HER2-positive, ER-/PR– breast cancer status post chemoradiation and bilateral mastectomies, presented for recurrent right-sided chylothorax. Low-fat diet and prior surgical thoracic duct ligation had failed to control the leak, and the patient continued to experience high output. Lymphangiogram did not opacify the thoracic duct, but it identified a leak overlying the right lower hemithorax and subsequent direct access with a 21-gauge x 20 cm long needle and embolization was performed with glue injection (1:1 dilution with lipiodol) through the needle, and confirmed on CBCT. Subsequent chylous output resolved, providing significant palliative benefit for the patient (Figures 3A–C). She did not develop any complications from the lymphatic embolization procedure.
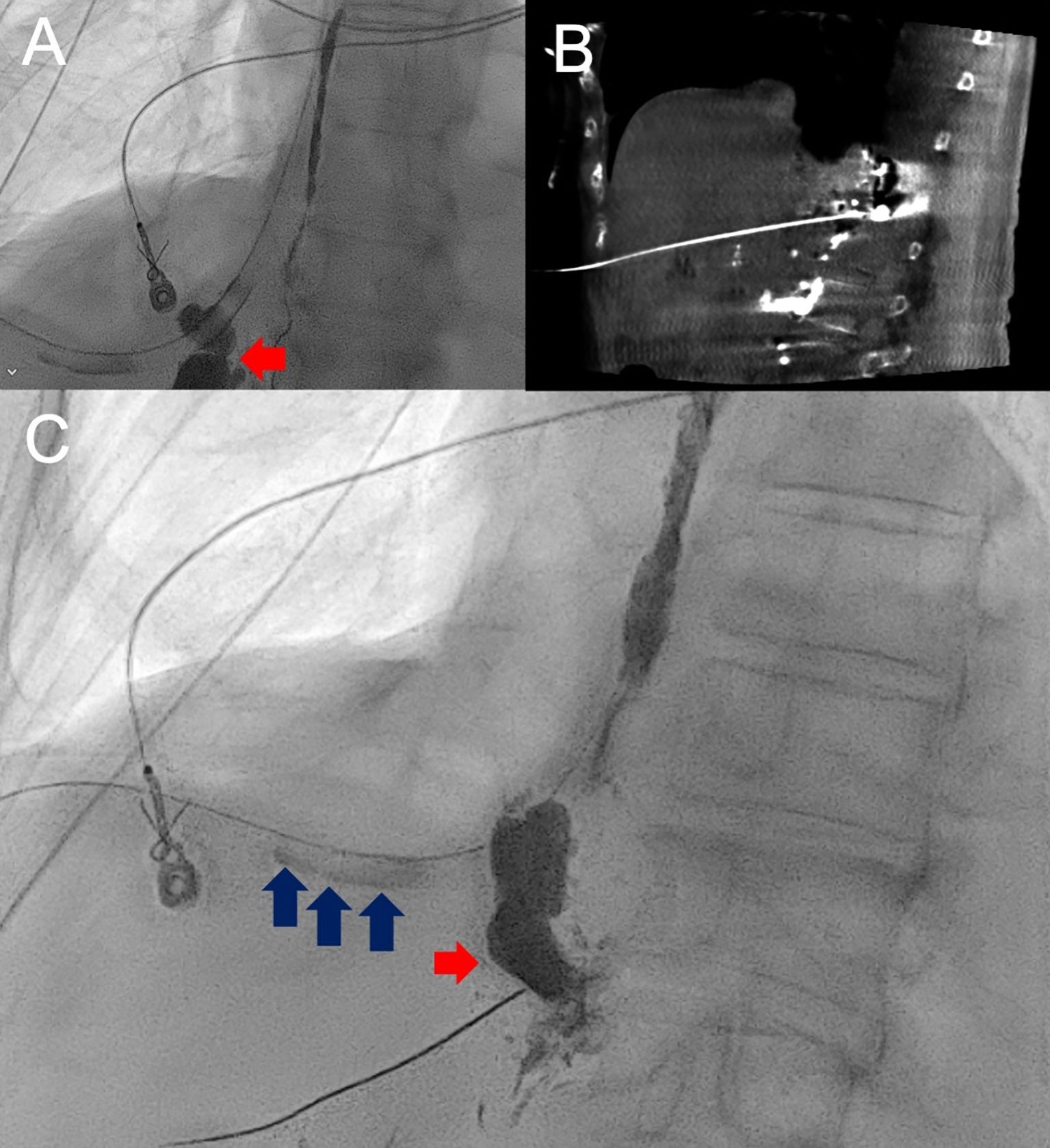
Figure 3. AP fluoroscopic image and lymphangiogram of Patient 3 with lipiodol opacified thoracic duct and extravasation overlying the right lower hemithorax (red arrow) (A). Following unsuccessful catheter and wire manipulation to the known site of leak, cone-beam CT-guided,anterior-approach transhepatic direct stick (B) of the leak with subsequent lipiodol injection confirmed leak with visualization of lipiodol passage through the right-sided chest tube (blue arrows) followed by glue embolization (C).
Discussion
Malignant chylothorax can lead to rapid nutritional depletion, immunosuppression, and respiratory compromise that can delay cancer therapy and worsen quality of life for patients. Conventional therapies are often inadequate for high-output chylous effusions. And, while surgical ligation has been the standard for refractory chylothorax, it is often contraindicated in cancer patients due to poor functional status, associated complication rates reported as high as 38.3%, and associated mortality as high as 25% (10, 11). In contrast, lymphangiogram with possible embolization, including thoracic duct embolization, offers a minimally invasive option with lower complication rates (3%) and no reported lymphangiogram-directed embolization-related deaths (7–9). However, in cases of non-iatrogenic chylothorax, accessing the lymphatic system is more technically challenging with as much as 30% of cases precluded secondary to inaccessibility (12).
In this case series, we demonstrate effective management of malignant chylothorax via lymphangiogram-directed embolization. In Case 1, a patient with relapsed AITL achieved a prompt reduction in chyle leak after TDE, which supported patient stabilization for further treatment with chemotherapy and autologous stem cell transplantation. This outcome is critical, as delaying lymphoma treatment may worsen prognosis. In case 2, the patient experienced a significant decrease in chyle output, allowing the continuation of targeted therapy (13). And, in Case 3, lymphangiogram-directed glue embolization proved as an effective therapy for resolving chylous output and improved patient quality of life where surgical ligation was insufficient.
The general technique follows traditional inguinal intranodal injection of lipiodol, followed by needle and microwire access into the cisternal chyli and advancement to the region of leakage (14). Embolization is often accomplished by NBCA glue given its ability to polymerize in the absence of clotting factors in the lymphatic space while the optional addition of coils may be complementary as it can serve as a backstop to prevent glue from advancing into the venous system. Although there have been cases reported where the lymphangiogram alone was therapeutic, our practice cannot attest to this phenomenon (8). Although no immediate complications were not observed in this cohort of patients, the most challenging part of the procedure is getting access to the lymphatic systems and complications can arise from access site bleeding or peritonitis (15). Additionally, embolization of glue into the pulmonary artery has been a reported complication and the intent of the addition of coiling serves to mitigate the risk of glue advancement. Regarding access, our approach consistently utilizes bilateral inguinal lymph node access for initial lymphangiography, followed by direct cisterna chyli puncture for thoracic duct catheterization while alternative approaches via the subclavian vein have been described.
Our case series underscores the importance of early referral to interventional radiology for lymphangiogram with possible embolization when conservative measures fail, that is when there is persistent high output chylothorax over several days. With early intervention, we may offer patients a shortened course of nutritional deficiency, symptomatic respiratory distress in those without indwelling tubes, and stabilization for continued cancer-related treatments.
The novelty of our case series lies in its focus on malignant chylothorax unrelated to surgical injury, an area with limited prior reports. By demonstrating that lymphangiogram-directed embolization can be effectively used across different oncologic contexts (lymphoma, lung cancer, and breast cancer), we add to the growing evidence that minimally invasive management can transform the care of such patients.
Conclusion
Lymphangiogram-directed embolization is a safe, minimally invasive, and effective treatment option for patients with malignant chylothorax. The above case series demonstrates the clinical and palliative benefit that lymphangiogram-directed embolization can offer for non-iatrogenic etiologies of malignant chylothorax by not only decreasing chylous output but improving nutritional status and clinical condition to be permissive of continued cancer treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JM: Data curation, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. HL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. OA: Writing – original draft, Writing – review & editing. NS: Conceptualization, Data curation, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J. (1997) 10:1157–62. doi: 10.1183/09031936.97.10051157
2. Doerr CH, Allen MS, Nichols FC, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. (2005) 80:867–70. doi: 10.4065/80.7.867
3. Bhatnagar M, Fisher A, Ramsaroop S, Carter A, Pippard B. Chylothorax: pathophysiology, diagnosis, and management-a comprehensive review. J Thorac. Dis. (2024) 16:1645–61. doi: 10.21037/jtd-23-1636
4. Agrawal A, Chaddha U, Kaul V, Desai A, Gillaspie E, Maldonado F. Multidisciplinary management of chylothorax. Chest. (2022) 162:1402–12. doi: 10.1016/j.chest.2022.06.012
5. Rudrappa M, Paul M. Chylothorax. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2025).
6. Ahn HY, H. I. Non-conservative management of chylothorax. J Chest Surg. (2021) 54:325–9. doi: 10.5090/jcs.21.056
7. Juszczyk K, Waugh R, Sandroussi C. Lymphangiography as therapeutic management of chylothorax. J Med Imaging Radiat Oncol. (2013) 57:460–1. doi: 10.1111/j.1754-9485.2012.02452.x
8. Matsumoto T, Yamagami T, Kato T, Hirota T, Yoshimatsu R, Masunami T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. (2009) 82(976):286–90. doi: 10.1259/bjr/64849421
9. Schild HH, Pieper C. Chylothorax: current therapeutic options. Zentralblatt fur Chirurgie. (2019) 144(S 01):S24–30. doi: 10.1055/a-0831-2649
10. Tenny BC, Madjarov J, Shipe T. Surgical intervention in a complicated persistent chyle leak. Int J Surg Case Rep. (2018) 42:7–9. doi: 10.1016/j.ijscr.2017.11.031
11. Yang RF, Liu TT, Wang P, Zhang RQ, Li C, Han B, et al. Ligation of thoracic duct during thoracoscopic esophagectomy can lead to decrease of T lymphocyte. J Cancer Res Ther. (2018) 14(7):1535–9. doi: 10.4103/jcrt.JCRT_596_17
12. Nadolski GJ, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest. (2013) 143:158–63. doi: 10.1378/chest.12-0526
13. Pospiskova J, Smolej L, Belada D, Simkovic M, Motyckova M, Sykorova A, et al. Experiences in the treatment of refractory chylothorax associated with lymphoproliferative disorders. Orphanet J Rare Dis. (2019) 14(1):9. doi: 10.1186/s13023-018-0991-3
14. Benjamin J, O’Leary C, Hur S, Gurevich A, Klein WM, Itkin M. Imaging and interventions for lymphatic and lymphatic-related disorders. Radiology. (2023) 307:e220231. doi: 10.1148/radiol.220231
Keywords: lymphatic embolization, lymphatic, embolization (therapeutic), coil embolisation, glue embolisation
Citation: Moon JT, Li H, Abdalla O and Swilley N (2025) Case Report: Lymphangiogram and embolization for malignant chylothorax in cancer patients. Front. Oncol. 15:1586047. doi: 10.3389/fonc.2025.1586047
Received: 01 March 2025; Accepted: 14 April 2025;
Published: 12 May 2025.
Edited by:
François Montagne, Valenciennes Hospital Center, FranceReviewed by:
Mathilde Vermersch, Centre Hospitalier de Valenciennes, FranceMarc Haberlay, Centre Hospitalier de Valenciennes, France
Copyright © 2025 Moon, Li, Abdalla and Swilley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John T. Moon, anRtb29uQGVtb3J5LmVkdQ==
 John T. Moon
John T. Moon Hanzhou Li
Hanzhou Li Omar Abdalla2
Omar Abdalla2 Nicholas Swilley
Nicholas Swilley