- Department of Pathology, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
Fumarate hydratase deficiency-associated renal cell carcinoma (FH-RCC) is a rare, aggressive subtype of renal cell carcinoma (RCC) driven by FH gene mutations. Its non-specific imaging features and heterogeneous pathological morphology complicate early diagnosis. This case report describes the cytological evaluation of peritoneal fluid from a 35-year-old male with FH-RCC, highlighting distinct cellular features within the effusion. These findings provide insights into the clinicopathological differential diagnosis of serous cavity effusions, potentially enhancing the recognition and management of this uncommon malignancy.
1 Introduction
Fumarate hydratase deficiency-associated renal cell carcinoma (FH-RCC) is an infrequent subtype of renal cell carcinoma (RCC) resulting from germline or somatic mutations in the FH gene (1). Germline mutations are linked to hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC), an autosomal dominant condition characterized by renal malignancies, uterine leiomyomas, and cutaneous leiomyomas (2). Compared to common RCC subtypes, such as clear cell RCC (ccRCC), FH-RCC exhibits earlier onset, aggressive behavior, and a propensity for early metastasis (1). However, its rarity limits comprehensive understanding of its multi-omics profile (3), including genomics, epigenomics, and immune microenvironment in metastatic settings. Non-specific imaging and clinical presentations further challenge timely diagnosis. This report presents a cytological analysis of peritoneal fluid in a patient with FH-RCC, aiming to delineate characteristic morphological features that may facilitate early detection and inform clinical management.
2 Case presentation
A 35-year-old male presented with significant peritoneal effusion, thoracolumbar vertebral destruction, and hypodense liver lesions on imaging. Twenty-two months prior, he underwent radical nephrectomy for a renal tumor, with postoperative pathology confirming HLRCC-associated FH-RCC. Post-surgery, he received tiragolumab and cabozantinib combination therapy. Subsequent bone and lung metastases prompted treatment with bevacizumab, lenvatinib, and toripalimab. Six months later, massive peritoneal effusion developed, necessitating aspiration. Liquid-based cytology and cell block sections were analyzed. This study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University.
Pathological Findings: Liquid-based cytology revealed abundant tumor cells arranged in papillary formations, with areas of solid, nested, or dispersed single cells (Figures 1A, B). Tumor cells were large, with indistinct borders, eosinophilic or clear cytoplasm, and occasional biphasic staining. Cytoplasmic vacuoles were observed in some cells (Figures 1C–F). Nuclei displayed variable size and shape, irregular contours, prominent eosinophilic nucleoli, and occasional perinucleolar halos (Figures 1E, F). Phagocytosis of similar cells and eosinophilic globular material was noted in some tumor cells (Figures 2A–D). Immunophenotype (Peritoneal Fluid): 2SC (+), Ber-EP4 (+), FH (+), CK (+), Ki-67 (30% in hotspots), MOC-31 (+), Pax-8 (+), P53 (wild-type), CAIX (+), CR (-), Desmin (-), WT-1 (-), CK20 (-), CK7 (-), P40 (-) (Figures 2E, F). Post-Nephrectomy Pathology: The primary tumor exhibited papillary, nested, and solid architecture with prominent nucleoli (Figures 3A, B). Tumor emboli were identified in blood vessels and the renal vein. Immunophenotype (Primary Tumor): 2SC (+), CAIX (+), CD10 (-), CK (+), EMA (+), FH (+), Ki-67 (30% in hotspots), TFE3 (-), CK20 (-). Diagnosis: Fumarate hydratase-deficient renal cell carcinoma (FH-RCC).
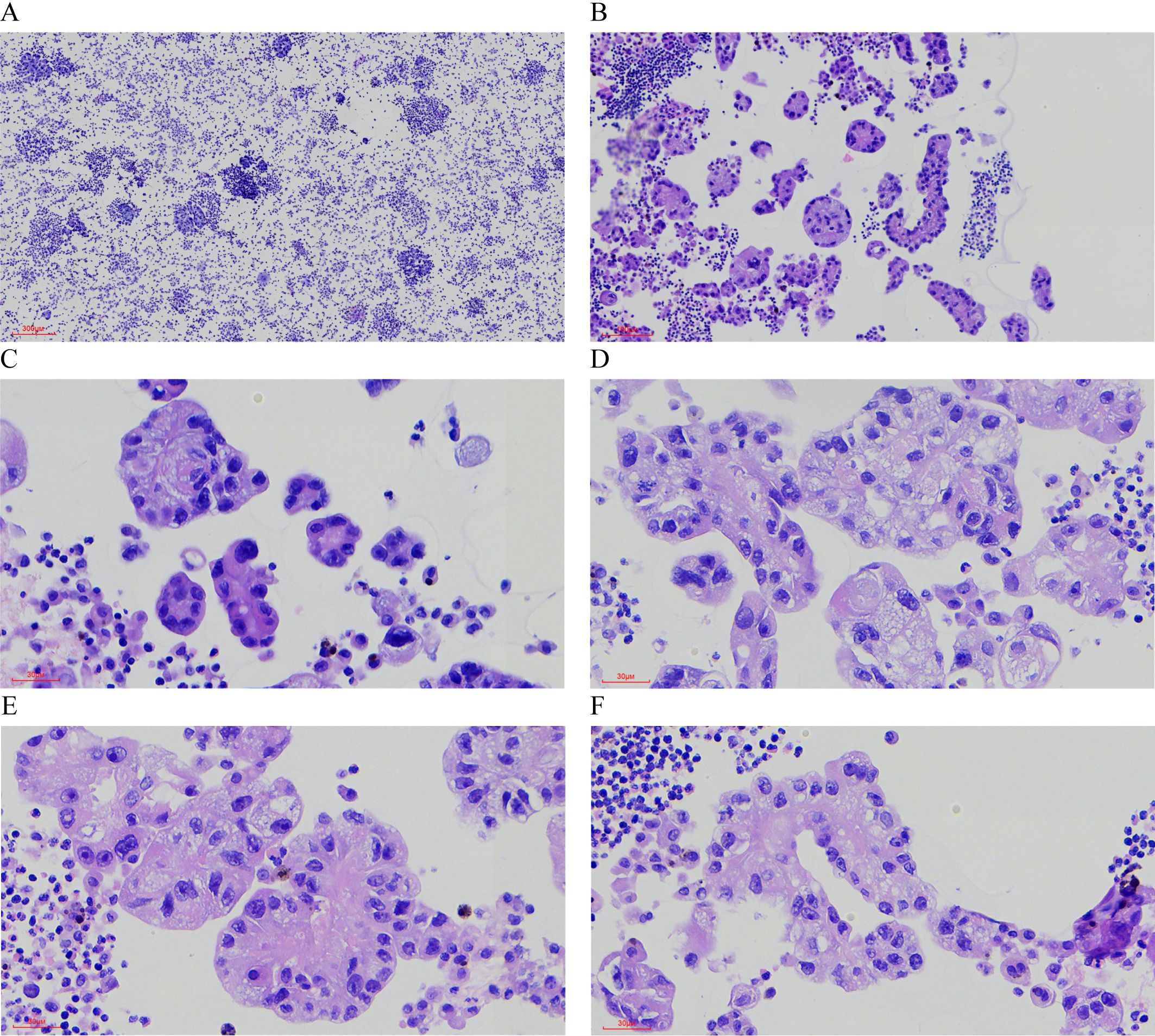
Figure 1. Hematoxylin-Eosin (HE) Staining of Tumor Cells in Ascitic Fluid. (A) Tumor cells are numerous and densely packed in the ascitic fluid. (×40) (B) The tumor cells exhibit a variety of growth patterns, including papillary, solid, and nested structures. (×100) (C-F) The cytoplasm is abundant, eosinophilic or transparent, with some cells showing amphophilic staining or cytoplasmic vacuoles. (×400) (E, F) Prominent eosinophilic nucleoli are visible, some surrounded by a distinct halo (×400).
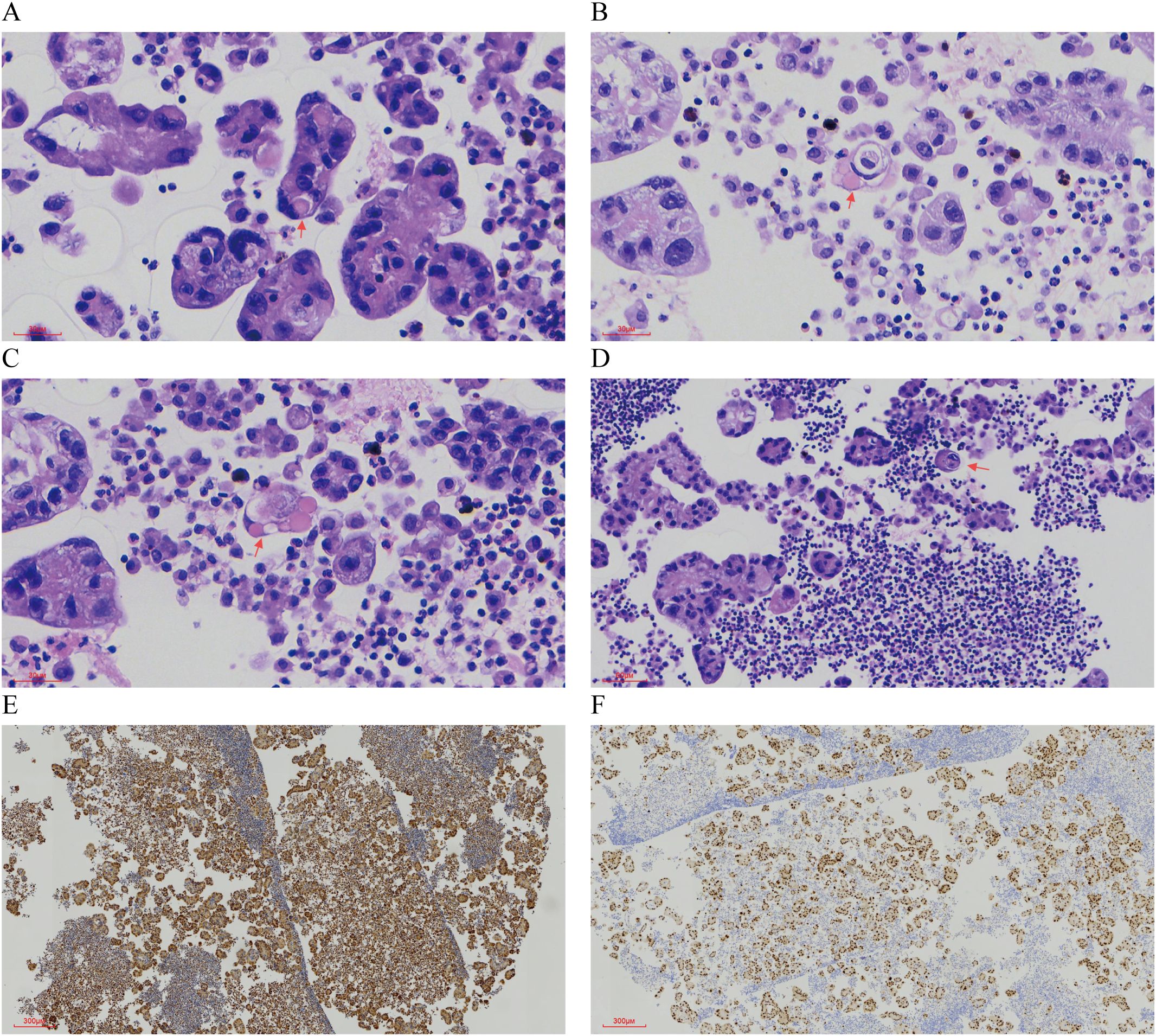
Figure 2. Characteristic Morphology and Immunohistochemical Features in Ascitic Fluid. (A-C) Eosinophilic spherules are observed. (×400) (D) Tumor cells exhibit phagocytosis. (×200) (E) Immunohistochemical staining for FH is positive. (×40) (F) Immunohistochemical staining for PAX8 is positive (×40).
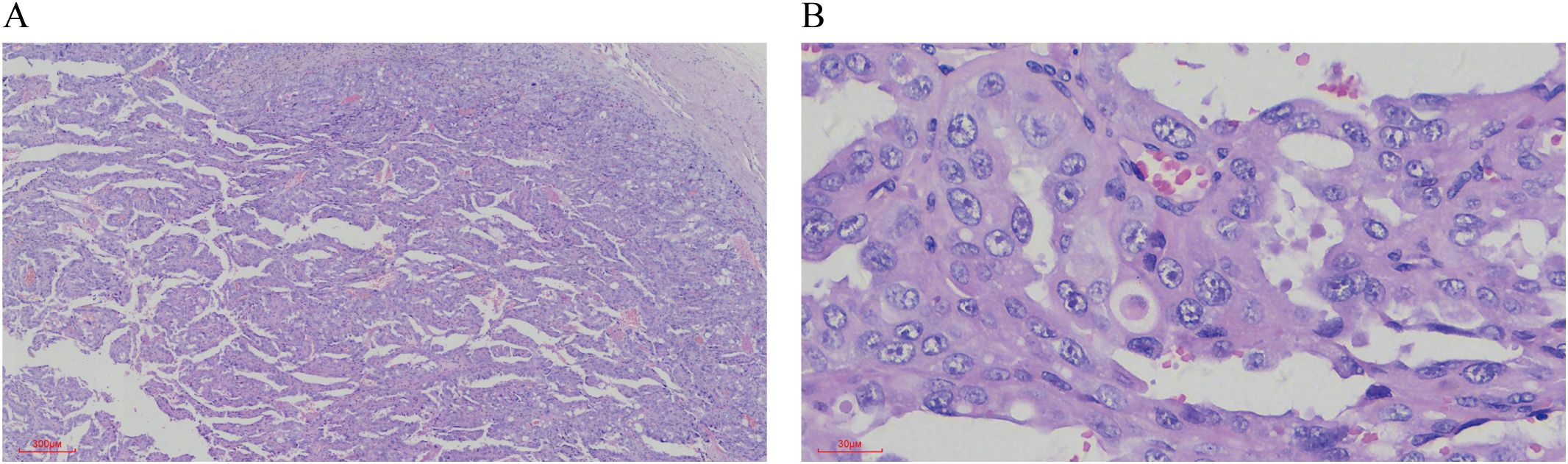
Figure 3. HE staining of Postoperative Renal Tumor Features. (A) The tumor tissue displays a mixture of papillary, nested, and solid growth patterns. (×40) (B) The tumor cells are large, with either eosinophilic or vacuolated cytoplasm. The nuclei vary in size, and prominent eosinophilic nucleoli are evident (×400).
Treatment Course: Three months post-nephrectomy, the patient began tiragolumab and cabozantinib. One year later, bone and lung metastases emerged, leading to a regimen of bevacizumab, lenvatinib, and toripalimab. Six months into this treatment, significant peritoneal effusion developed.
3 Discussion
Fumarate hydratase-deficient renal cell carcinoma (FH-RCC), first described by Launonen et al. in 2001, arises from FH gene mutations or inactivation on chromosome 1q42.3-43 (2, 4). This leads to fumarate accumulation, stabilizing hypoxia-inducible factor 1-alpha (HIF1α) and driving metabolic dysregulation and tumorigenesis. Classified under HLRCC in the 2016 WHO renal tumor classification (4th edition), FH-RCC was redefined in the 2022 WHO update to encompass all RCCs with FH alterations (5, 6). According to the 5th edition of the WHO classification, the required diagnostic criteria for fumarate hydratase-deficient renal cell carcinoma (FH-RCC) include immunohistochemical evidence of FH germline or somatic mutation, or FH deficiency, defined by loss of FH protein expression and/or positive 2SC staining. The supportive diagnostic features include the presence of multiple, admixed architectural patterns with at least focal macronucleoli, and a personal or family history of cutaneous and uterine leiomyomas, which may indicate an association with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome (7).
FH-RCC typically affects younger patients and presents as a solitary renal mass with non-specific symptoms (8). Its aggressive nature predisposes to early metastasis, commonly to lymph nodes, bones, liver, and lungs (9). In this case, a 35-year-old male developed bone and lung metastases 15 months post-nephrectomy, followed by peritoneal effusion and liver metastasis at 22 months.
Cytologically, FH-RCC is characterized by prominent eosinophilic nucleoli with perinucleolar halos (8). In this patient’ s peritoneal effusion, eosinophilic globular material was also observed, potentially reflecting fibrin or peroxisomal complexes linked to autophagic defects, as suggested by prior studies (10). Similar globules have been reported in papillary and clear cell RCC subtypes, distinguishable by their distinct cytoplasmic boundaries (11, 12).
A review of FH-RCC cases with eosinophilic globules (Table 1) revealed: (1) no gender predilection (female: male= 4:3); (2) mean onset age of 38 years (range: 11–50); (3) frequent FH mutations (e.g., G354R, p.K80fs); (4) association with HLRCC features in some cases; (5) variable tumor sizes (0.2–21 cm) and growth patterns (solid, tubular, cystic); (6) consistent FH negativity and 2SC positivity; and (7) variable outcomes, with some patients disease-free post-surgery and others experiencing recurrence or metastasis.
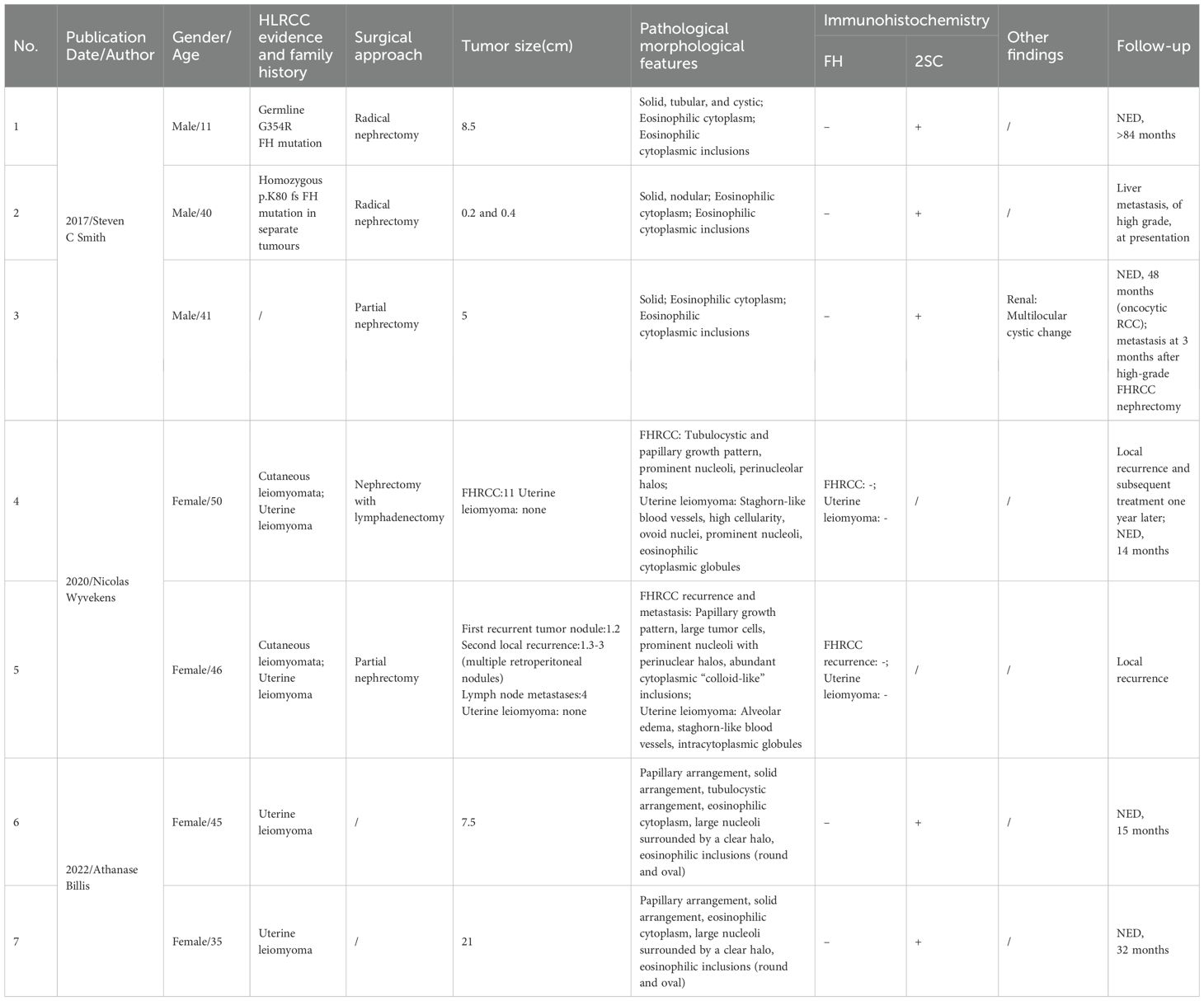
Table 1. Summary of clinical and pathological features of cases with eosinophilic globules in fumarate hydratase-deficient renal cell carcinoma.
Differential diagnosis of peritoneal effusion includes: (1) Ovarian Cancer: Features irregular clusters or glandular structures, often with calcified psammoma bodies in serous carcinoma, contrasting with FH-RCC’s eosinophilic nucleoli and “owl-eye” appearance (13–15). (2) Gastrointestinal Adenocarcinoma: Exhibits glandular or signet-ring morphology with PAS-positive vacuoles, distinguishable by negative CDX-2 and villin in FH-RCC. (3) Mesothelioma: Shows scattered or clustered cells with basophilic cytoplasm and BAP1 loss in ~60% of cases, unlike FH-RCC’s eosinophilic features (16).
The presence of eosinophilic globules in this case, alongside characteristic nucleoli and immunoprofile (FH loss, 2SC positivity), strongly supports FH-RCC diagnosis. Absent FH IHC staining in tumor cells is highly specific for FH-RCC, but its sensitivity is limited. In contrast, diffuse expression of 2-succinocysteine (2SC), resulting from aberrant cysteine modification due to fumarate accumulation, is highly sensitive but lacks specificity. Additionally, a recently described pattern of heterogeneous FH expression—characterized by patchy positive staining—has been associated with FHRCC. This phenomenon is thought to result from certain missense mutations that impair FH enzymatic activity while allowing for variable protein expression (17–21). In this case, immunohistochemical staining showed diffuse and strong positivity for 2SC, whereas FH demonstrated a patchy immunoreactivity pattern.
Therapeutically, FH-RCC lacks standardized guidelines. Bevacizumab with erlotinib has shown partial efficacy in HLRCC-associated RCC, while immune checkpoint inhibitors (ICIs) with anti-angiogenic agents yield objective response rates (ORR) of 27–44% in retrospective studies (22–24). In this patient, despite sequential therapies (tiragolumab/cabozantinib, then bevacizumab/lenvatinib/toripalimab), disease progression persisted, highlighting therapeutic challenges in advanced FH-RCC.
4 Conclusion
This report presents the first cytological description of FH-RCC in peritoneal effusion, emphasizing distinctive features—eosinophilic nucleoli, perinucleolar halos, and globular material—that aid differential diagnosis. Despite multimodal therapy, disease progression underscores the aggressive nature and poor prognosis of some FH-RCC cases, necessitating further research into effective treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YD: Conceptualization, Writing – original draft, Writing – review & editing. YS: Resources, Writing – original draft, Writing – review & editing. CS: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Liaoning Provincial Scientific and Technological Plan Project(2023010789-JH3/108).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rare Kidney Cancer Collaborative Group, Genitourinary Cancer Committee, and China Anti-Cancer Association. Consensus on clinical diagnosis and treatment of fumarate hydratase-deficient renal cell carcinoma. Zhonghua Wai Ke Za Zhi. (2022) 60:961–8. doi: 10.3760/cma.j.cn112139-20220729-00328
2. Patel VM, Handler MZ, Schwartz RA, and Lambert WC. Hereditary leiomyomatosis and renal cell cancer syndrome: an update and review. J Am Acad Dermatol. (2017) 77:149–58. doi: 10.1016/j.jaad.2017.01.023
3. Board PDQCGE. Hereditary leiomyomatosis and renal cell cancer (Pdq®): health professional version. In: Pdq Cancer Information Summaries. National Cancer Institute (US, Bethesda (MD (2002).
4. Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U.S.A. (2001) 98:3387–92. doi: 10.1073/pnas.051633798
5. Inamura K. Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 who classification. Int J Mol Sci. (2017) 18(10):2195. doi: 10.3390/ijms18102195
6. Goswami PR, Singh G, Patel T, and Dave R. The who 2022 classification of renal neoplasms (5th edition): salient updates. Cureus. (2024) 16:e58470. doi: 10.7759/cureus.58470
7. Hes O, Gill AJ, Gupta S, Jimenez RE, Smith SC, and Trpkov K. Fumarate hydratase-deficient renal cell carcinoma. In: WHO Classification of Tumours: Urinary and Male Genital Tumours. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC) (2022). pp. 78–79.
8. Kuroda N, Tsutsui M, Iguchi M, Nobuoka E, Uehara T, Sonobe Y, et al. Fumarate hydratase-deficient renal cell carcinoma: A clinicopathological study of seven cases including hereditary and sporadic forms. Ann Diagn Pathol. (2020) 49:151599. doi: 10.1016/j.anndiagpath.2020.151599
9. Paschall AK, Nikpanah M, Farhadi F, Jones EC, Wakim PG, Dwyer AJ, et al. Hereditary leiomyomatosis and renal cell carcinoma (Hlrcc) syndrome: spectrum of imaging findings. Clin Imaging. (2020) 68:14–9. doi: 10.1016/j.clinimag.2020.06.010
10. Danbara N, Shikata N, Kawamura H, Shintaku M, and Tsubura A. Eosinophilic cytoplasmic inclusions in papillary renal cell carcinoma. Med Mol Morphol. (2005) 38:262–6. doi: 10.1007/s00795-005-0286-3
11. Yu Z, Ma J, Li X, Liu Y, Li M, Wang L, et al. Autophagy defects and related genetic variations in renal cell carcinoma with eosinophilic cytoplasmic inclusions. Sci Rep. (2018) 8:9972. doi: 10.1038/s41598-018-28369-y
12. Ungari M, Trombatore M, Ferrero G, Gusolfino MD, Manotti L, Tanzi G, et al. Eosinophilic cytoplasmic inclusions in type 2 papillary renal cell carcinoma. Pathologica. (2019) 111:369–74. doi: 10.32074/1591-951x-28-19
13. Li J, Zhang Y, Nan P, Liu Y, and Sui Y. Significance of ascites cytological features combined with serum CA-125, HE4, and imaging findings in the diagnosis of ovarian cancer. J Diagn Pathol. (2021) 28:532–5. doi: 10.3969/j.issn.1007-8096.2021.07.005
14. Chang Y, Zou B, Cai Y, Yang S, Zhang Y, Liang J, et al. Cytological features of peritoneal effusion in the diagnosis of ovarian serous carcinoma. Chin J Oncol. (2023) 45:424–32. doi: 10.3760/cma.j.cn112152-20211201-00889
15. Billis A, Assis-Mendonça GR, Tavares TF, Parreira K, Costa LBE, Barreto IS, et al. Fumarate hydratase-deficient renal cell carcinoma: A tumor with diverse morphology including cannibalism, lymphocytic emperipolesis, and defective autophagy. Ann Diagn Pathol. (2022) 56:151844. doi: 10.1016/j.anndiagpath.2021.151844
16. Lucà S, Pignata G, Cioce A, Salzillo C, De Cecio R, Ferrara G, et al. Diagnostic challenges in the pathological approach to pleural mesothelioma. Cancers (Basel). (2025) 17(3):481. doi: 10.3390/cancers17030481
17. Lau HD, Chan E, Fan AC, Kunder CA, Williamson SR, Zhou M, et al. A clinicopathologic and molecular analysis of fumarate hydratase-deficient renal cell carcinoma in 32 patients. Am J Surg Pathol. (2020) 44:98–110. doi: 10.1097/PAS.0000000000001372
18. Chen YB, Brannon AR, Toubaji A, Dudas ME, Won HH, Al-Ahmadie HA, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. (2014) 38:627–37. doi: 10.1097/PAS.0000000000000163
19. Trpkov K, Hes O, Agaimy A, Bonert M, Martinek P, Magi-Galluzzi C, et al. Fumarate hydratase-deficient renal cell carcinoma is strongly correlated with fumarate hydratase mutation and hereditary leiomyomatosis and renal cell carcinoma syndrome. Am J Surg Pathol. (2016) 40:865–75. doi: 10.1097/PAS.0000000000000617
20. Muller M, Guillaud-Bataille M, Salleron J, Genestie C, Deveaux S, Slama A, et al. Pattern multiplicity and fumarate hydratase (FH)/S-(2-succino)-cysteine (2SC) staining but not eosinophilic nucleoli with perinucleolar halos differentiate hereditary leiomyomatosis and renal cell carcinoma-associated renal cell carcinomas from kidney tumors without FH gene alteration. Mod Pathol. (2018) 31:974–83. doi: 10.1038/s41379-018-0017-7
21. Anderson WJ, Tsai HK, Sholl LM, and Hirsch MS. A clinicopathological and molecular analysis of fumarate hydratase (FH)-deficient renal cell carcinomas with heterogeneous loss of FH expression. Int J Surg Pathol. (2022) 30:606–15. doi: 10.1177/10668969221074600
22. Srinivasan R, Su D, Stamatakis L, Siddiqui MM, Singer EA, Shuch B, et al. 5 mechanism based targeted therapy for hereditary leiomyomatosis and renal cell cancer (Hlrcc) and sporadic papillary renal cell carcinoma: interim results from a phase 2 study of bevacizumab and erlotinib. Eur J Cancer. (2014) 50:8. doi: 10.1016/S0959-8049(14)70131-5
23. Xu Y, Kong W, Cao M, Wang J, Wang Z, Zheng L, et al. Genomic profiling and response to immune checkpoint inhibition plus tyrosine kinase inhibition in fh-deficient renal cell carcinoma. Eur Urol. (2023) 83:163–72. doi: 10.1016/j.eururo.2022.05.029
Keywords: peritoneal effusion, fumarate hydratase deficiency, renal cell carcinoma, cytological diagnosis, cell block (CB)
Citation: Du Y, Shang Y and Shi C (2025) Morphological analysis of peritoneal fluid in fumarate hydratase deficiency-associated renal cell carcinoma: a case report. Front. Oncol. 15:1586283. doi: 10.3389/fonc.2025.1586283
Received: 02 March 2025; Accepted: 19 June 2025;
Published: 03 July 2025.
Edited by:
Derek Allison, University of Kentucky, United StatesReviewed by:
Cherry Bansal, Tantia University, IndiaTreeva Jassim, Tufts Medical Center, United States
Copyright © 2025 Du, Shang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Shi, NTg1NzAzMDNAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Yingying Du
Yingying Du Yu Shang
Yu Shang Chang Shi
Chang Shi