- 1Department of Nephrology, Lianyungang Clinical College of Nanjing Medical University, Lianyungang, China
- 2Department of Nephrology, The First People’s Hospital of Lianyungang, Lianyungang, China
- 3Department of Nephrology, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China
Postrenal obstruction is a rare but reversible cause of acute kidney injury (AKI), often underrecognized when hydronephrosis is mild or absent. We present a 55-year-old woman with gastric cancer who developed severe AKI requiring hemodialysis. Initial non-contrast abdominal CT revealed only mild bilateral hydronephrosis without obvious ureteral obstruction. Given these subtle radiologic findings and a history of chemotherapy and NSAID exposure. Initially, a multidisciplinary team attributed the AKI to intrinsic renal causes. Subsequent renal biopsy revealed only minimal glomerular changes, insufficient to explain the degree of renal dysfunction. Despite supportive care, her renal function continued to decline. Further urological evaluation led to the placement of bilateral ureteral stents, which resulted in a prompt increase in urine output and improvement in serum creatinine. However, rapid restenosis occurred within four days, necessitating percutaneous nephrostomy. This two-step intervention restored renal function and improved short-term prognosis. This case underscores the diagnostic challenge of postrenal AKI in malignancy, particularly when imaging findings are subtle. Peritoneal carcinomatosis may cause ureteral encasement through mechanisms such as inflammation, fibrosis, and lymphatic disruption, often without significant collecting system dilation. Timely urologic intervention, guided by clinical judgment and supported by multidisciplinary collaboration, is critical to improving outcomes in such atypical presentations.
1 Introduction
Postrenal AKI is caused by urinary tract obstruction, accounts for approximately 5%–10% of AKI cases and is typically reversible if identified early (1, 2). However, diagnosis becomes challenging when imaging reveals only mild hydronephrosis or no overt signs of obstruction. In patients with gastric cancer, AKI is more frequently attributed to chemotherapy-induced nephrotoxicity, sepsis, or hemodynamic instability (3–6). Obstructive etiologies, particularly those related to peritoneal metastasis (PM), are rare and often underrecognized (7). Previous reports have more commonly linked malignant urinary tract obstruction to direct invasion or metastasis involving the bladder (8) or cervix (9, 10). Recent studies indicate that in malignancy-associated obstruction—especially when caused by extrinsic ureteral compression due to peritoneal spread or fibrosis—the collecting system may not dilate significantly. This obstructive urinary tract lesion with minimal dilation of the collection system is associated with delayed diagnosis and poor renal prognosis (11–13). Here, we present a rare case of postrenal AKI in a patient with gastric cancer, in whom peritoneal metastasis was considered. The initial imaging demonstrated only mild bilateral hydronephrosis. Despite the delay in diagnosis, subsequent urological interventions led to full recovery of renal function. This case underscores the importance of maintaining a high index of suspicion and re-evaluating the diagnosis in patients with malignancy and AKI.
2 Case presentation
2.1 Initial presentation
A 55-year-old woman presented with nausea, back pain, and oliguria. One year prior, she had undergone total gastrectomy for gastric adenocarcinoma (T4N2M0, stage IIIA), followed by eight cycles of adjuvant chemotherapy with oxaliplatin and capecitabine, which concluded six months earlier. She had no other significant medical history. Five days before admission, she experienced recurrent flank pain and sought emergency care. Abdominal ultrasonography revealed bilateral hydronephrosis with proximal ureteral dilation and no residual bladder urine (There is separation of the echogenic band in the renal collecting systems, measuring approximately 17 mm on the right side and 11 mm on the left side.). Non-contrast abdominal CT scan showed mild bilateral hydronephrosis, perihepatic and perisplenic fluid accumulation, and localized intestinal distension, without evidence of soft tissue masses in the abdomen or retroperitoneum (Figure 1). Oral analgesics provided symptomatic relief, but the patient declined further diagnostic workup.
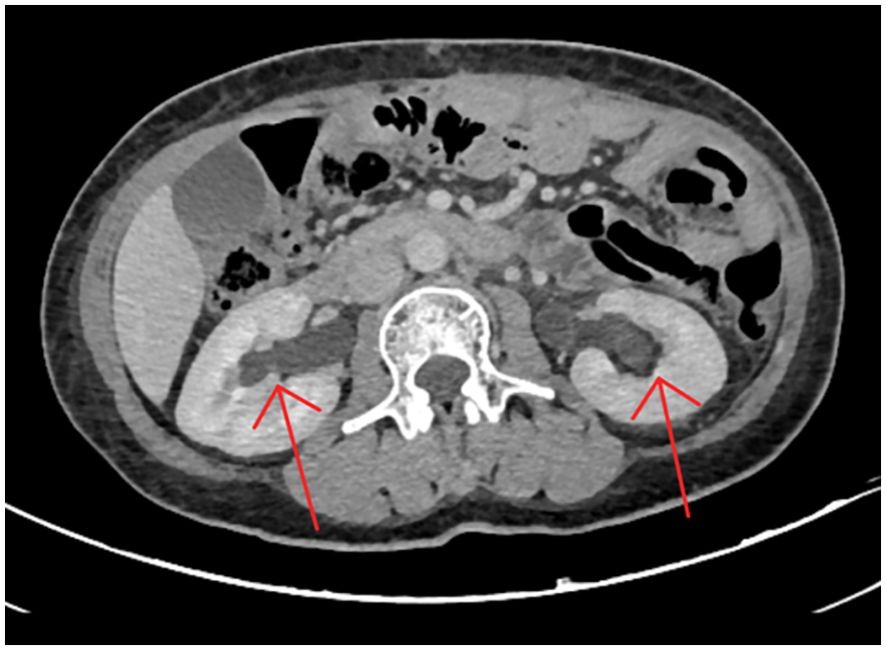
Figure 1. Non-contrast abdominal CT scan revealing minimal bilateral hydronephrosis. The scan showed mild dilation of the bilateral renal pelvis and collecting systems, without visible ureteral stones, wall thickening, strictures, or other obstructive lesions. These subtle findings likely contributed to the initial underestimation of the severity of urinary tract obstruction.
2.2 Diagnostic workup
On admission, her vital signs were stable: T 36.5°C, P 86 beats/min, R 16 breaths/min, BP 124/78 mmHg. Physical examination revealed mild pitting edema in both lower extremities. Laboratory findings revealed stage 3 AKI according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, accompanied by hyperkalemia: serum creatinine 1099.9 μ mol/L (baseline: 42.9 μ mol/L), and potassium 6.85 mmol/L. On admission, the patient presented with anuria; urine analysis was deferred due to anuria. Emergency hemodialysis was initiated via a temporary femoral venous catheter, along with bladder catheterization. Post-dialysis, nausea improved, potassium normalized, and serum creatinine decreased to 998.9 μ mol/L. Due to persistent anuria and modest improvement in renal function, additional hemodialysis was performed on days 3 and 5.
2.3 Interventions and management
On day 7, a multidisciplinary team (nephrology, urology, oncology, and radiology) convened to review the case. Given the mild hydronephrosis and history of nephrotoxic chemotherapy, intrinsic AKI was initially prioritized. Obstruction was reconsidered only after biopsy excluded tubular injury. Corticosteroids (40 mg prednisone daily) were started to reduce inflammation and optimize biopsy safety. On day 9, ultrasound-guided renal biopsy was performed without complications. Despite ongoing hemodialysis and symptomatic treatment, on day 13, the renal biopsy pathology showed only minor glomerular abnormalities (Figure 2). Repeat imaging revealed worsening bilateral hydronephrosis (There is separation of the echogenic band in the renal collecting systems, measuring approximately 25 mm on the right side and 24 mm on the left side), prompting a urology consultation. On day 14, retrograde ureteral stenting (RUS) was performed. Within 24 hours, urine output increased to 4850 mL, lower extremity edema improved. Dialysis was discontinued on day 16 following renal recovery (serum creatinine: 155.6 μ mol/L), and the catheter was scheduled for removal. On day 17, oliguria recurred and furosemide administration had no effect. A plain abdominal X-ray confirmed proper stent placement, but persistent hydronephrosis suggested ongoing obstruction. On day 21, percutaneous nephrostomy (PCN) was performed under CT guidance, yielding 3000 mL of urine output within 24 hours. Over the next few days, renal function, serum creatinine, and electrolytes normalized. The femoral catheter was removed on day 24, and the patient was discharged in stable condition on day 26.
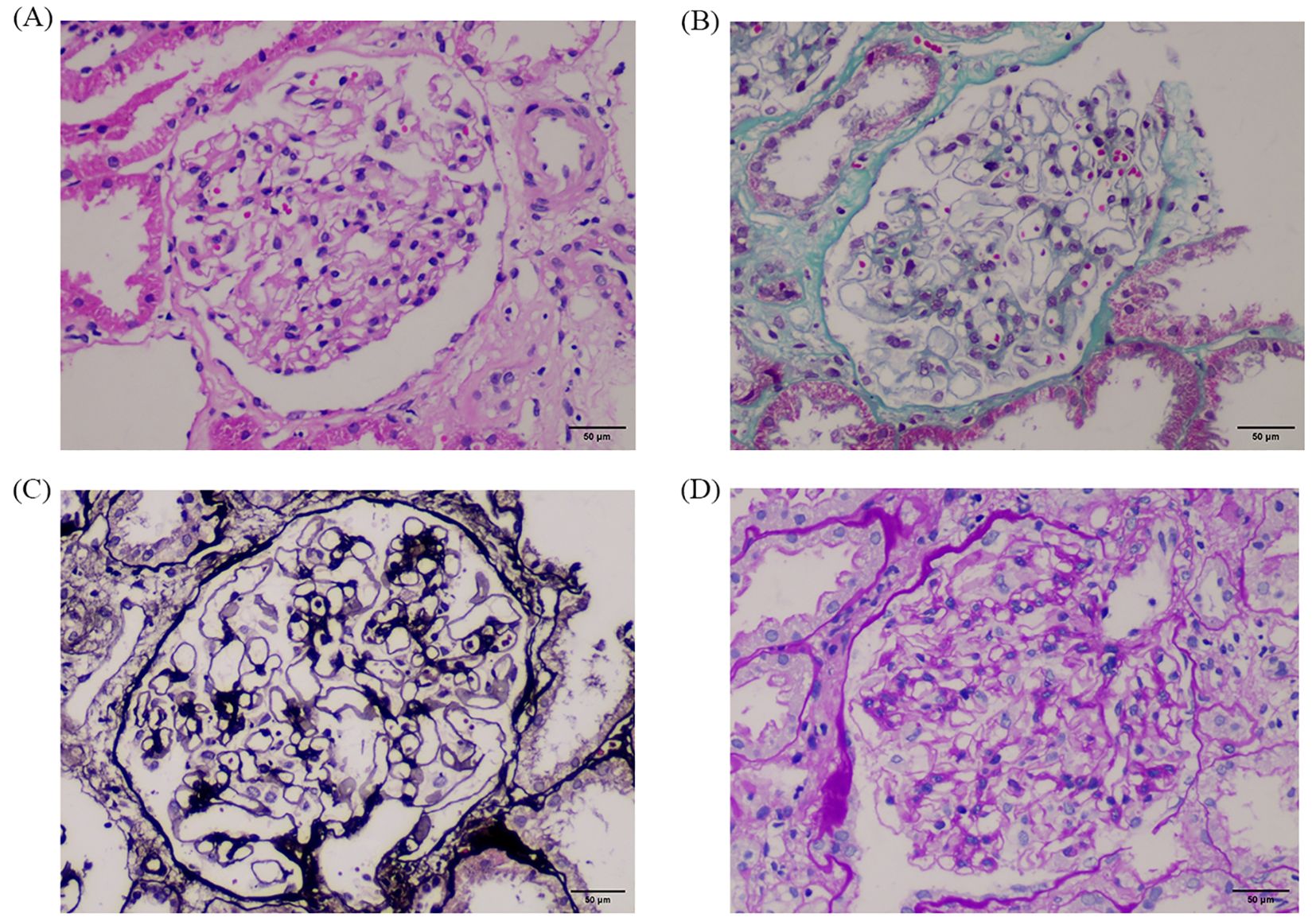
Figure 2. Renal biopsy findings at 400× magnification. Panels show results of different staining techniques: (A) H.E, (B) Masson, (C) P.A.S.M, and (D) P.A.S. The glomeruli exhibit only minimal pathological changes, with no segmental sclerosis, crescents, or necrosis. Tubules show no evidence of necrosis or cast formation. These findings support a diagnosis of minimal glomerular lesion without intrinsic renal injury, consistent with a postrenal cause of acute kidney injury. A representative 50 μm scalebar was added using ImageJ based on a 400× magnification and standard field-of-view estimation.
2.4 Follow-up
The patient underwent cancer-specific follow-up in the oncology department every week after discharge. Twenty-one days after discharge, the patient came to our hospital to reinsert the double-J tube due to the loss of the left nephrostomy tube. During the 2-month follow-up period, the patient’s stent remained unobstructed without any signs of failure (creatinine was within the normal range, and imaging showed that hydronephrosis was resolved). Unfortunately, approximately two months after discharge, the patient visited another hospital due to persistent abdominal pain, abdominal distension and other symptoms. During the surgical treatment of intestinal obstruction, multiple metastatic lesions in the abdominal and pelvic cavities were found, marking significant tumor progression. She refused further diagnosis or treatment procedures and chose to relieve her symptoms with sustained-release oxycodone (5 milligrams every 12 hours). Reviewing the case, the diagnosis of peritoneal metastasis in the patient was based on reasonable clinical suspicion (Imaging suggests indirect signs of peritoneal metastasis), multidisciplinary team (MDT) discussions, and subsequent disease evolution.
We have created a timeline illustrating the patient’s clinical course and management during hospitalization (Figure 3).
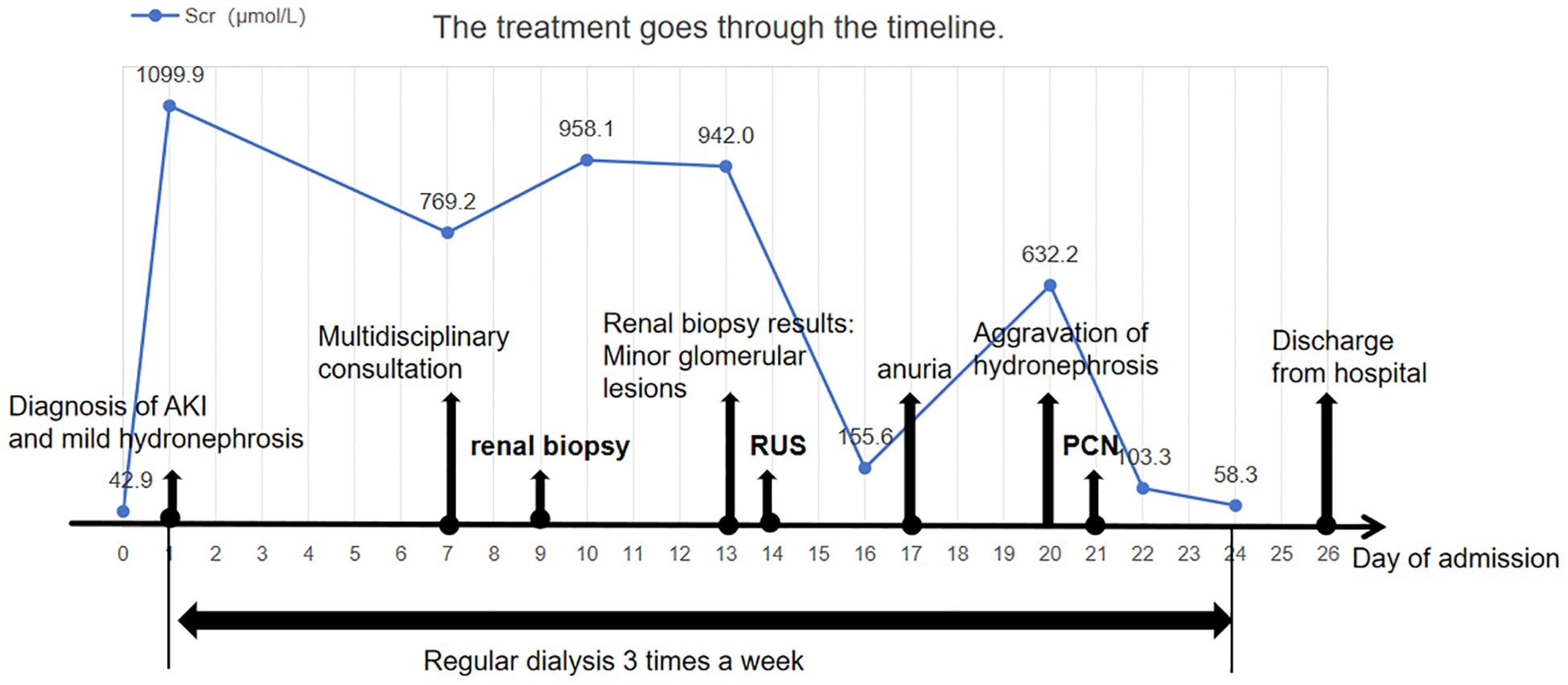
Figure 3. Clinical timeline of the patient’s presentation, diagnosis, and management. Timeline summarizing key clinical events, including serial serum creatinine levels, timing of major interventions (renal biopsy, retrograde ureteral stenting, and percutaneous nephrostomy), dialysis sessions, and clinical milestones such as onset of anuria and hospital discharge. The figure illustrates how initially subtle imaging findings contributed to a delay in diagnosis. Early recognition and prompt relief of obstruction may improve short-term outcomes in patients with malignancy-associated obstructive AKI.
2.5 Patient’s point of view
The patient reported significant anxiety during the period of diagnostic uncertainty. After inviting a multidisciplinary consultation and engaging in thorough communication, she adopted a positive attitude towards renal biopsy. Once the obstruction was confirmed, we informed her of the likelihood of persistent renal dysfunction and potential disease progression. She and her family clearly expressed a desire for active but minimally invasive management to preserve kidney function, avoid ICU-level care, and maintain quality of life for as long as possible. She expressed gratitude for the multidisciplinary care she received but stated her wish for earlier detection of obstruction in similar cases.
3 Discussion
3.1 Mechanisms of peritoneal metastasis-associated obstructive nephropathy
In cancer patients, uric acid crystals in tumor lysis syndrome, light chain casts in multiple myeloma, or the crystallization of certain drugs such as platinum-based chemotherapy agents can lead to tubular obstruction in the kidneys (7, 14, 15). Tumor cells may also directly extend into and invade both ureters or renal parenchyma, causing local tissue destruction (6, 16). In rare cases, treatment-related fibrosis from radiotherapy or chemotherapy (17–19), as well as peritoneal metastasis (9), can result in external compression of the urinary tract, as seen in our patient, contributing to the development of acute kidney injury.
Peritoneal metastasis is a common complication in patients with gastric cancer, observed in approximately 10%-20% of cases at the time of initial diagnosis (20–22). This proportion may be even higher in surgical and observational studies (23, 24). Cancer cells can spread into the abdominal cavity through direct invasion or hematogenous dissemination, implanting on the peritoneal surface and forming peritoneal metastases (25). As metastases progress, tumor cells can exert pressure on nearby structures, like the ureter, due to local growth and expansion. This pressure can impair urinary flow and lead to obstruction. Molecular drivers play pivotal roles in ureteral compression from gastric cancer-related PM. Metastatic lesions release abundant proinflammatory cytokines, especially interleukin-6 (IL-6), which activates the JAK/STAT3 pathway and recruits macrophages and neutrophils. This process fosters a proinflammatory milieu that promotes intra-abdominal adhesions and sets the stage for subsequent fibrosis (26–28). Both tumor cells and infiltrating macrophages secrete high levels of transforming growth factor-β (TGF-β), which bind TGFBR1/2 and triggers phosphorylation of Smad2/3. The Smad2/3-Smad4 complex then translocates to the nucleus to initiate transcription of profibrotic genes such as COL1A1, fibronectin (FN1), and connective tissue growth factor (CTGF), leading to excessive extracellular matrix (ECM) deposition around the ureters and the development of peritoneal adhesions (27, 29–31). Vascular endothelial growth factor-A (VEGF-A), produced by tumor cells, binds VEGFR2 on endothelial cells, thereby activating the PI3K/Akt and ERK1/2 pathways to promote aberrant angiogenesis. Additionally, VEGF induces phosphorylation of VE-cadherin, which disrupts tight junctions and increases vascular permeability. The resulting vascular leakage, combined with localized edema and tissue congestion, further intensifies pressure on surrounding structures including the ureters (32–35). Moreover, metastasized peritoneal tumor cells can disrupt lymphatic drainage in the abdominal cavity, which contributes to increased intra-abdominal pressure and aggravates urinary tract obstruction (21, 36–39). Recent studies have discovered several biomarkers closely related to peritoneal metastasis of gastric cancer. CLDN18.2 (40), highly expressed in gastric cancer cells with peritoneal metastasis, facilitates tumor cell adhesion and invasion within the peritoneal cavity. MMP-9 (41), frequently elevated in gastric cancer patients with peritoneal metastasis, degrades the extracellular matrix, promoting tumor cell migration and metastasis. These biomarkers not only serve as potential tools for early detection of peritoneal metastasis but also offer promising therapeutic targets.
3.2 Epidemiological background
Urinary tract obstruction, as an initial manifestation of peritoneal metastasis, is underreported in clinical practice (9, 42). We believe the possible reasons are as follows: 1. Limitations of Imaging: CT is the primary tool for detecting peritoneal metastases (43), but early-stage disease often lacks clear radiological signs, with a reported miss rate of approximately 16% (36, 44, 45). PET/CT or diffusion-weighted MRI have demonstrated superior sensitivity and specificity (46), but our patients refused for personal reasons and economic issues. 2. Low Clinical Suspicion: Typical PM manifestations include ascites, bowel obstruction, and cachexia, which may mask subtle signs of urinary obstruction (22). 3. Epidemiological Data Gaps: Urinary tract obstruction is more common in gynecologic, colorectal, and lymphoid cancers (47). Moreover, most studies on AKI in gastric cancer lack mechanistic stratification (prerenal/renal/postrenal), and hydronephrosis severity is rarely graded or reported, leaving no precise epidemiological data. Using an estimated AKI incidence of 13.9% in gastric cancer and a 5%–10% post-renal cause estimate (48), we calculate post-renal AKI at 0.7%–1.39%. Importantly, the majority of reported gastric cancer-related obstructive uropathy involves distant metastases (e.g., to bladder (8), cervix (9, 10) rather than peritoneal disease causing ureteral compression. Our case likely represents a rare subset within this already small group, consistent with its unreported status.
Analogous cases have been sporadically documented. In a case reported by Elfert et al., a patient was admitted with anuria and bilateral hydronephrosis; CT revealed gastric cancer with PM, and renal function improved after DJ stent insertion (49). Similarly, Boubaker et al. described a patient with PM-induced bilateral ureteral compression and severe hydronephrosis who responded well to DJ stenting (7). We emphasize that although analogous cases of gastric cancer with peritoneal metastasis causing urinary obstruction have been reported, our case is distinct in that only trace hydronephrosis was present initially, leading to clinical underestimation and delayed diagnosis.
3.3 Diagnostic pitfalls in minimal hydronephrosis
Hydronephrosis is a sensitive indicator of urinary tract obstruction, reflecting the dilation of the collecting system (2, 50, 51). Ultrasound and CT are key imaging modalities for assessment (52). However, in cases of mild dilation, their sensitivity drops significantly—ultrasound sensitivity can be as low as 6% (7), contributing to diagnostic delays.
Our patient presented with established AKI, but had multiple potential nephrotoxic factors (NSAIDs, cancer-related nephropathy) (2, 5, 16, 53–55), and imaging showed only minimal hydronephrosis. A multidisciplinary team (nephrology, urology, oncology and radiology) reviewed the case and initially ruled out obstruction. After extensive discussion, the patient consented to renal biopsy, which revealed only mild glomerular changes—insufficient to explain the degree of renal dysfunction. This prompted a crucial reassessment: even minimal hydronephrosis can lead to significant AKI.
Prior reports have described a rare syndrome—obstructive nephropathy with minimal collecting system dilation (7, 56). These patients exhibit significantly elevated creatinine levels, often accompanied by symptoms such as oliguria/anuria, abdominal pain, and nausea/vomiting, but imaging reveals little to no hydronephrosis. This syndrome comprises 5% of all urinary tract obstruction cases (57). The pathophysiology of this syndrome is likely multifactorial. External compression or fibrosis may impair ureteral peristalsis and disrupt urine flow, leading to renal pelvic pressure and functional decline (58, 59). Yet, in the absence of complete obstruction, the collecting system often lacks significant dilation. Some theories suggest that urine may instead drain via the renal sinus or be rerouted through lymphatic or venous pathways, thereby reducing pelvic pressure and minimizing detectable hydronephrosis on imaging (60, 61). Notably, 60%-66% of these cases result from extrinsic ureteral compression by retroperitoneal fibrosis (RPF) or metastatic malignancy (62, 63). Among them, 86% of AKI cases with negative initial imaging were eventually diagnosed as obstructive through urography (62). Several reported cases well illustrate this phenomenon: Shahzad et al. reported a breast cancer patient with PM presenting with anuric AKI and no visible hydronephrosis (56). AKI was initially thought to be secondary to drug-induced acute tubular necrosis. Due to the application of anticoagulants for deep vein thrombosis, renal biopsy could not be performed. Later, after re-evaluation with pyelography, she was diagnosed with postrenal AKI. Similarly, Onuigbo et al. documented a bladder cancer case where unilateral hydronephrosis was evident, but contralateral obstruction with minimal dilation was only revealed via imaging-guided intervention (64). In other limited reports, obstructive nephropathy with minimal collection system dilation has also been described in colon cancer (65) and uterine cancer (12). All patients had their obstructions relieved through RUS or PCN, resulting in the recovery of renal function. By contrast, our patient had a primary gastric cancer lesion—rarely reported in this context—making this case particularly instructive. These cases remind us that in high-risk populations, early urography should be considered to confirm obstruction, even in the absence of overt imaging findings. Based on these insights, we propose a five-step diagnostic algorithm for postrenal AKI in cancer patients (Figure 4).
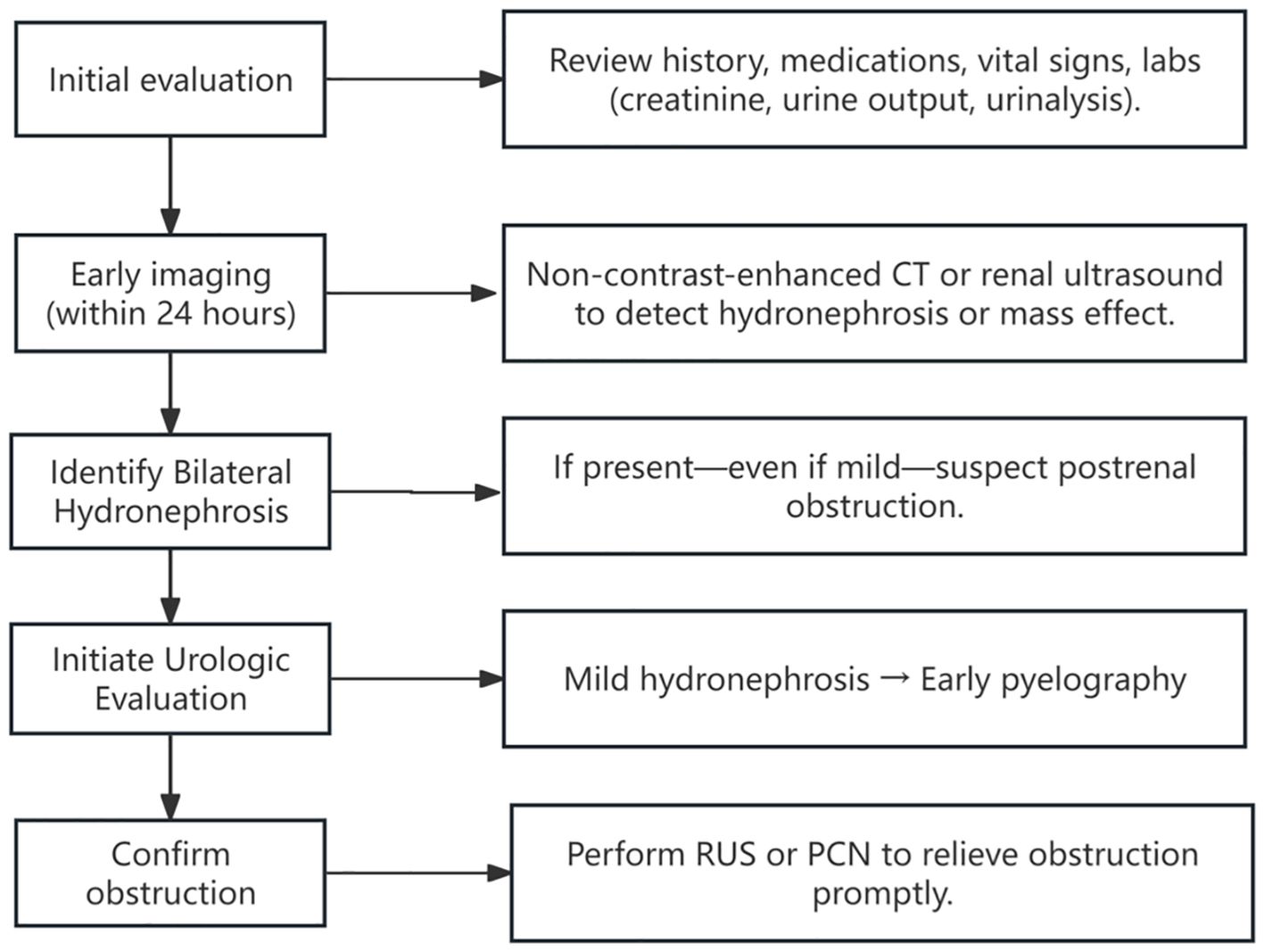
Figure 4. Clinical timeline of the patient’s presentation, diagnosis, and management. Timeline summarizing key clinical events, including serial serum creatinine levels, timing of major interventions (renal biopsy, retrograde ureteral stenting, and percutaneous nephrostomy), dialysis sessions, and clinical milestones such as onset of anuria and hospital discharge. The figure illustrates how initially subtle imaging findings contributed to a delay in diagnosis. Early recognition and prompt relief of obstruction may improve short-term outcomes in patients with malignancy-associated obstructive AKI.
3.4 Therapeutic challenges and multidisciplinary management
The treatment of cancer-related postrenal AKI should emphasize a “rapid assessment–prompt decompression” strategy. In this case, the patient met the KDIGO criteria for stage 3 AKI (66), with acute anuria and hyperkalemia requiring emergent dialysis. After renal biopsy excluded intrinsic renal pathology, follow-up urinary tract ultrasound revealed progressive hydronephrosis, indicating worsening obstruction. Studies have shown that kidney injury due to urinary tract obstruction is partially reversible, and renal prognosis depends primarily on the timely relief of obstruction (5, 67, 68).
After consulting with the urology team, urgent decompression was pursued. Retrograde ureteral stenting (RUS) was selected as the first choice due to its minimally invasive nature, low bleeding risk, minimal impact on daily life, and cost-effectiveness (69–71). In clinical practice, especially in cancer patients, it is crucial to monitor for complications such as obstruction recurrence, stent displacement, or crystallization after double-J stent placement (72–76). A retrospective study reported a 37% failure rate of stent placement in malignant ureteral obstruction, underscoring that RUS alone is often insufficient (77). In our case, immediate improvement in urine output and renal function following stent insertion further confirmed the obstructive etiology. However, the patient developed recurrent anuria four days later. Follow-up abdominal X-ray revealed no twisting or displacement of the stent, we opted for additional percutaneous nephrostomy (PCN). Although ultrasound-guided PCN is considered the gold standard for percutaneous access to the renal urinary system (78), it has limited success in non-dilated systems and carries higher risks of minor vascular injury and bleeding (79). CT, on the other hand, offers more precise visualization of the renal pelvis and calyces, allowing for quicker and safer insertion of the puncture needle (80–82). Thus, CT-guided PCN was performed. The patient, previously anuric, produced 3000 mL of urine within 24 hours post-PCN. Serum creatinine decreased from 632.2 µmol/L to 58.3 µmol/L within 72 hours and remained within the normal range thereafter.
This two-step intervention strategy—RUS followed by PCN—effectively managed the obstruction and highlights the importance of multidisciplinary collaboration and dynamic reassessment in the management of malignant obstructive AKI.
3.5 Follow up
Beyond acute management, long-term follow-up is critical to assess prognosis in patients with gastric cancer and peritoneal metastasis. The prognosis of patients with gastric cancer complicated with peritoneal metastasis is generally poor. The median survival period is 4 to 6 months (83), and the 5-year survival rate is usually less than 10% (84). Nevertheless, some patients may benefit from multimodal therapies, including systemic chemotherapy, intraperitoneal treatment, and cytoreductive surgery, along with effective symptom management, leading to extended survival and enhanced quality of life (85–87). Regrettably, two months after discharge, our patient was diagnosed with multiple metastatic lesions. Although an Eastern Cooperative Oncology Group (ECOG) score was not conducted, during this period, she exhibited progressive fatigue and limited activity, and refused further diagnostic assessment or therapeutic interventions, choosing to passively await disease progression.
4 Conclusion
In summary, this report describes a rare case of postrenal AKI caused by small dilated obstructive urinary tract lesions caused by gastric cancer involving the peritoneum. In the context of gastric cancer, AKI may act as an early warning signal, prompting a thorough evaluation of the underlying cause and personalized treatment strategies. Initially, the very small amount of hydronephrosis shown on imaging led to an underestimation of obstructive components, and we assumed that the patient’s AKI was attributable to chemotherapy, NSAIDS, and other factors. However, the small glomerular changes found by renal needle biopsy are nonspecific and insufficient to explain severe renal dysfunction. Subsequent recovery of urine volume and renal function after double J tube insertion confirmed obstruction as the main cause. Rapid restenosis (4 days after stent placement) and widespread peritoneal metastasis within 2 months reflect an aggressive clinical course of gastric cancer. Although specific molecular markers, such as HER2 status or MMR deficiency, were not assessed in this case, these features are associated with advanced disease progression in gastric cancer.
This case provides four critical insights for clinical practice and medical education: First, it demonstrates that even mild hydronephrosis may mask life-threatening postrenal AKI in patients with advanced malignancies, necessitating heightened clinical vigilance. Second, it serves as an important teaching example that subtle imaging findings must be carefully evaluated in cases of acute renal deterioration. Third, it highlights how early multidisciplinary collaboration enables timely intervention and may improve short-term renal outcomes. Finally, by challenging the conventional assumption that hydronephrosis severity correlates with degree of obstruction, this rare presentation of peritoneal carcinomatosis contributes valuable new evidence to our understanding of atypical obstructive nephropathy in oncological patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XX: Writing – original draft, Writing – review & editing. XW: Writing – original draft, Writing – review & editing. JM: Writing – review & editing. MS: Writing – review & editing. WC: Writing – review & editing. BZ: Writing – review & editing. YiZ: Writing – review & editing. JW: Writing – review & editing. LZ (9th Author): Writing – review & editing. YoZ: Writing – review & editing. TZ: Writing – review & editing. LZ (11th Author): Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to the medical, nursing, and pharmacy staff who were involved in the patient’s hospitalization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AKI, acute kidney injury; PM, peritoneal metastasis; MDT, multidisciplinary team; KDIGO, Kidney Disease: Improving Global Outcomes; RUS, retrograde ureteral stenting; PCN, percutaneous nephrostomy; ECGO, Eastern Cooperative Oncology Group; RPF, retroperitoneal fibrosis.
References
1. Yaxley J and Yaxley W. Obstructive uropathy – acute and chronic medical management. World J Nephrol. (2023) 12:1–9. doi: 10.5527/wjn.v12.i1.1
2. Pérez-Aizpurua X, Benavente RC, Serrano GB, Peral JMA, Mañas BG-J, Jaumot J, et al. Obstructive uropathy: Overview of the pathogenesis, etiology and management of a prevalent cause of acute kidney injury. World J Nephrol. (2024) 13:93322. doi: 10.5527/wjn.v13.i2.93322
3. Bellomo R, Kellum JA, and Ronco C. Acute kidney injury. Lancet. (2012) 380:756–66. doi: 10.1016/S0140-6736(11)61454-2
4. Turgut F, Awad A, and Abdel-Rahman E. Acute kidney injury: medical causes and pathogenesis. J Clin Med. (2023) 12:375. doi: 10.3390/jcm12010375
5. Liu S, Zhao J, and Wang F. Acute kidney injury in cancer patients. Clin Exp Nephrol. (2022) 26:103–12. doi: 10.1007/s10157-021-02131-7
6. Rosner MH and Perazella MA. Acute kidney injury in the patient with cancer. Kidney Res Clin Pract. (2019) 38:295–308. doi: 10.23876/j.krcp.19.042
7. Boubaker K, Alkadi M, Fitouri O, Rahil AIA, and Malki HA. An unusual case of acute kidney injury caused by obstructive uropathy revealing gastric cancer. Qatar Med J. (2022) 1:15. doi: 10.5339/qmj.2022.15
8. Rosenblum H, Mha YB-D, Dovrish Z, Lew S, Weisenberg N, Neumann A, et al. The endless differential diagnosis of acute obstructive renal failure: unusual challenges for the sharp-sighted clinician. Isr Med Assoc J. (2010) 12:280–2.
9. Treszezamsky A, Altuna S, Diaz L, Vighi S, and Sardi J. Metastases to the uterine cervix from a gastric carcinoma presenting with obstructive renal failure: a case report. Int J Gynecol Cancer. (2003) 13:555–7. doi: 10.1046/j.1525-1438.2003.13043.x
10. Bahall V, De Barry L, Barrow M, and Ramnarace R. Metastatic gastric adenocarcinoma of the uterine cervix – A case report and review of the literature. World J Surg Oncol. (2021) 20:177. doi: 10.21203/rs.3.rs-1051189/v1
11. Schattner A, Drahy Y, and Dubin I. The bladder ran dry: bilateral ureteral obstruction. BMJ Case Rep. (2017) 2017:bcr2016218173. doi: 10.1136/bcr-2016-218173. bcr-2016-218173.
12. Onuigbo MAC, Lawrence K, and Onuigbo NTC. Non-dilated obstructive uropathy – an unrecognized cause of acute renal failure in hospitalized US patients: three case reports seen over 6 months in a northwestern Wisconsin Nephrology practice. Renal Failure. (2010) 32:1226–9. doi: 10.3109/0886022X.2010.517343
13. El-Alali E, Moreno C, and Al Jaber E. Thinking beyond acute kidney injury. Case Rep Nephrol Dialysis. (2022) 12:16–21. doi: 10.1159/000522312
14. Yavuzsen T, Oztop I, Yilmaz U, Cavdar C, Sifil A, Sarioglu S, et al. Gastric cancer diagnosed in a patient with crescentic glomerulonephritis. Gastric Cancer. (2003) 6:267–9. doi: 10.1007/s10120-003-0259-y
15. Klimko A, Toma GA, Bejinariu N, Secareanu S-M, and Andreiana I. Acute kidney injury in a patient with cryoglobulinemia secondary to hepatic mucosa-associated lymphoid tissue lymphoma: case report and literature review. Cureus. (2020) 12:e10451. doi: 10.7759/cureus.10451
16. Wen Y-K and Chen M-L. Acute renal failure secondary to small cell lung cancer with tumor infiltration of the kidneys. Renal Failure. (2006) 28:261–4. doi: 10.1080/08860220600580423
17. Christiansen CF, Johansen MB, Langeberg WJ, Fryzek JP, and Sørensen HT. Incidence of acute kidney injury in cancer patients: A Danish population-based cohort study. Eur J Internal Med. (2011) 22:399–406. doi: 10.1016/j.ejim.2011.05.005
18. Nash K, Hafeez A, and Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. (2002) 39:930–6. doi: 10.1053/ajkd.2002.32766
19. Zhang J, Feng G, Yang Y, Zhang P, Pu C, and Zhao G. Acute kidney injury after radical gastrectomy: a single center study. Int Urol Nephrol. (2014) 46:973–7. doi: 10.1007/s11255-013-0618-5
20. Yonemura Y, Bandou E, Kawamura T, Endou Y, and Sasaki T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol (EJSO). (2006) 32:602–6. doi: 10.1016/j.ejso.2006.03.003
21. Thomassen I, Van Gestel YR, Van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer. (2014) 134:622–8. doi: 10.1002/ijc.28373
22. Koemans WJ, Lurvink RJ, Grootscholten C, Verhoeven RHA, De Hingh IH, and Van Sandick JW. Synchronous peritoneal metastases of gastric cancer origin: incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer. (2021) 24:800–9. doi: 10.1007/s10120-021-01160-1
23. Rijken A, Pape M, Simkens GA, De Hingh IHJT, Luyer MDP, Van Sandick JW, et al. Peritoneal metastases from gastric cancer in a nationwide cohort: Incidence, treatment and survival. Int J Cancer. (2024) 154:992–1002. doi: 10.1002/ijc.34780
24. Brandl A and Van Sandick JW. Treatment of gastric cancer peritoneal metastases: role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surgery. (2024) 111:znae149. doi: 10.1093/bjs/znae149
25. Li W, Ng JM-K, Wong CC, Ng EKW, and Yu J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. (2018) 37:4903–20. doi: 10.1038/s41388-018-0341-x
26. Wu H, Xiang Z, Huang G, He Q, Song J, Dou R, et al. BGN/FAP/STAT3 positive feedback loop mediated mutual interaction between tumor cells and mesothelial cells contributes to peritoneal metastasis of gastric cancer. Int J Biol Sci. (2023) 19:465–83. doi: 10.7150/ijbs.72218
27. Zhang F, Yan Y, Cao X, Guo C, Wang K, and Lv S. TGF-beta-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer. Cell Death Dis. (2024) 15:218. doi: 10.1038/s41419-024-06594-w
28. Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, et al. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. (2016) 35:1787–95. doi: 10.3892/or.2016.4544
29. Lv Z-D, Na D, Liu F-N, Du Z-M, Sun Z, Li Z, et al. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res. (2010) 29:139. doi: 10.1186/1756-9966-29-139
30. Wilson RB, Archid R, and Reymond MA. Reprogramming of mesothelial-mesenchymal transition in chronic peritoneal diseases by estrogen receptor modulation and TGF-β1 inhibition. Int J Mol Sci. (2020) 21:4158. doi: 10.3390/ijms21114158
31. Kinashi H, Ito Y, Sun T, Katsuno T, and Takei Y. Roles of the TGF-beta(-)VEGF-C pathway in fibrosis-related lymphangiogenesis. Int J Mol Sci. (2018) 19:2487. doi: 10.3390/ijms19092487
32. Wei H, Xu Z, Chen L, Wei Q, Huang Z, Liu G, et al. Long non-coding RNA PAARH promotes hepatocellular carcinoma progression and angiogenesis via upregulating HOTTIP and activating HIF-1α/VEGF signaling. Cell Death Dis. (2022) 13:102. doi: 10.1038/s41419-022-04505-5
33. Natsume M, Shimura T, Iwasaki H, Okuda Y, Hayashi K, Takahashi S, et al. Omental adipocytes promote peritoneal metastasis of gastric cancer through the CXCL2-VEGFA axis. Br J Cancer. (2020) 123:459–70. doi: 10.1038/s41416-020-0898-3
34. Wang X, Che X, Yu Y, Cheng Y, Bai M, Yang Z, et al. Hypoxia-autophagy axis induces VEGFA by peritoneal mesothelial cells to promote gastric cancer peritoneal metastasis through an integrin alpha5-fibronectin pathway. J Exp Clin Cancer Res. (2020) 39:221. doi: 10.1186/s13046-020-01703-x
35. Kariya T, Nishimura H, Mizuno M, Suzuki Y, Matsukawa Y, Sakata F, et al. TGF-beta1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol. (2018) 314:F167–F80. doi: 10.1152/ajprenal.00052.2017
36. Coccolini F. Peritoneal carcinomatosis. World J Gastroenterol. (2013) 19:6979. doi: 10.3748/wjg.v19.i41.6979
37. Green BL and Davis JL. Gastric adenocarcinoma peritoneal carcinomatosis: a narrative review. Digest Med Res. (2022) 5:37–. doi: 10.21037/dmr-21-94
38. Yarema R, Ohorchak M, Hyrya P, Kovalchuk Y, Safiyan V, Karelin I, et al. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J Gastrointest Oncol. (2020) 12:569–81. doi: 10.4251/wjgo.v12.i5.569
39. Kim D-W, Jee YS, Kim CH, Kim J-J, Park S, Choi SI, et al. Multicenter retrospective analysis of intraperitoneal paclitaxel and systemic chemotherapy for advanced gastric cancer with peritoneal metastasis. World J Surg Oncol. (2020) 20:50–9. doi: 10.5230/jgc.2020.20.e6
40. Liu S, Zhang Z, Jiang L, Zhang M, Zhang C, and Shen L. Claudin-18.2 mediated interaction of gastric Cancer cells and Cancer-associated fibroblasts drives tumor progression. Cell Commun Signaling. (2024) 22:27. doi: 10.1186/s12964-023-01406-8
41. Sun Y, Zhang H, Shi DB, and Gao P. SP-1-activated LINC01016 overexpression promotes gastric cancer invasion and metastasis through inhibiting EIF4A3-mediated MMP9 mRNA decay. Cell Death Dis. (2025) 16:54. doi: 10.1038/s41419-024-07250-z
42. Migita K, Watanabe A, Samma S, Ohyama T, Ishikawa H, and Kagebayashi Y. Clinical outcome and management of ureteral obstruction secondary to gastric cancer. World J Surg. (2011) 35:1035–41. doi: 10.1007/s00268-011-1016-8
43. Dong D, Tang L, Li Z-Y, Fang M-J, Gao J-B, Shan X-H, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. (2019) 30:431–8. doi: 10.1093/annonc/mdz001
44. Huang Z, Liu D, Chen X, Yu P, Wu J, Song B, et al. Retrospective imaging studies of gastric cancer: Study protocol clinical trial (SPIRIT Compliant). Medicine. (2020) 99:e19157. doi: 10.1097/MD.0000000000019157
45. Soussan M, Des Guetz G, Barrau V, Aflalo-Hazan V, Pop G, Mehanna Z, et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal Malignancy. Eur Radiol. (2012) 22:1479–87. doi: 10.1007/s00330-012-2397-2
46. Liu S, He J, Liu S, Ji C, Guan W, Chen L, et al. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur Radiol. (2019) 30:239–46. doi: 10.1007/s00330-019-06368-5
47. Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, et al. Acute kidney injury in patients receiving systemic treatment for cancer: A population-based cohort study. JNCI: J Natl Cancer Inst. (2019) 111:727–36. doi: 10.1093/jnci/djy167
48. Li Y, Chen X, Shen Z, Wang Y, Hu J, Zhang Y, et al. Prediction models for acute kidney injury in patients with gastrointestinal cancers: a real-world study based on Bayesian networks. Renal Failure. (2020) 42:869–76. doi: 10.1080/0886022x.2020.1810068
49. Khaled Elfert BT, Xheni D, Ali R, and Beran AA. S3599 Gastric cancer presenting with bilateral ureteral obstruction. Am J Gastroenterol. (2022) 117:2257–8. doi: 10.14309/01.ajg.0000871036.17830.80
50. Yap E, Salifu M, Ahmad T, Sanusi A, Joseph A, and Mallappallil M. Atypical causes of urinary tract obstruction. Case Rep Nephrol. (2019) 2019:1–5. doi: 10.1155/2019/4903693
51. Patel K and Batura D. An overview of hydronephrosis in adults. Br J Hosp Med. (2020) 81:1–8. doi: 10.12968/hmed.2019.0274
52. Nuraj P and Hyseni N. The diagnosis of obstructive hydronephrosis with color doppler ultrasound. Acta Inform Med. (2017) 25:178–81. doi: 10.5455/aim.2017.25.178-181
53. Bonomi M, Nortilli R, Molino A, Sava T, Santo A, Caldara A, et al. Renal toxicity and osteonecrosis of the jaw in cancer patients treated with bisphosphonates: a long-term retrospective analysis. Med Oncol. (2010) 27:224–9. doi: 10.1007/s12032-009-9195-y
54. Su Y-Q, Yu Y-Y, Shen B, Yang F, and Nie Y-X. Management of acute kidney injury in gastrointestinal tumor: An overview. World J Clin Cases. (2021) 9:10746–64. doi: 10.12998/wjcc.v9.i35.10746
55. Uchida M, Kondo Y, Suzuki S, and Hosohata K. Evaluation of acute kidney injury associated with anticancer drugs used in gastric cancer in the Japanese adverse drug event report database. Ann Pharmacother. (2019) 53:1200–6. doi: 10.1177/1060028019865870
56. Shahzad MA, Baxi PV, and Rodby RA. The challenges of diagnosing nondilated obstructive uropathy: A case report. Can J Kidney Health Dis. (2022) 9:20543581221086683. doi: 10.1177/20543581221086683
57. Charasse C, Carnus C, Darnault P, and Guill E. Acute nondilated anuric obstructive nephropathy on echography: Difficult diagnosis in the intensive care unit. Intensive Care Med. (1991) 17:387–91. doi: 10.1007/BF01720675
58. Kalayeh K, Fowlkes JB, Xie H, Schultz WW, and Sack BS. Peristalsis prevents ureteral dilation. Neurourol Urodynamics. (2024) 43:258–66. doi: 10.1002/nau.25332
59. Chen F. Genetic and developmental basis for urinary tract obstruction. Pediatr Nephrol. (2009) 24:1621–32. doi: 10.1007/s00467-008-1072-y
60. Chong BH, Trew P, Meng L, and Pitney WR. Anuric renal failure due to encasement of the ureters by lymphoma—Ureteric obstruction without dilatation. Aust New Z J Med. (1981) 11:542–4. doi: 10.1111/j.1445-5994.1981.tb04628.x
61. Lam AQ and Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol. (2012) 7:1692–700. doi: 10.2215/CJN.03140312
62. Feliciangeli V, Noce A, Montalto G, Germani S, Miano R, and Asimakopoulos AD. Non-dilated obstructive nephropathy. Clin Kidney J. (2024) 17:sfae249. doi: 10.1093/ckj/sfae249
63. Leong WS, Sells H, and Moretti KL. Anuric, obstructive uropathy in the absence of obvious radiological evidence of obstruction. ANZ J Surgery. (2004) 74:611–3. doi: 10.1111/j.1445-2197.2004.03001.x
64. Onuigbo MA. Symptomatic uraemia from bilateral obstructive uropathy secondary to metastatic urinary bladder cancer showing only unilateral hydronephrosis: a case report. NDT Plus. (2009) 2:387–9. doi: 10.1093/ndtplus/sfp093
65. Kulkarni S, Jayachandran M, Davies A, Mamoun W, and Al-Akraa M. Non-dilated obstructed pelvicalyceal system: Non-Dilated Obstructed Pelvicalyceal System. Int J Clin Pract. (2005) 59:992–4. doi: 10.1111/j.1368-5031.2005.00507.x
66. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. (2024) 105:S117–S314. doi: 10.1016/s0085-2538(24)00110-8
67. Nishimura K, Takenouchi A, Komatsu S, Kawaguchi Y, Kudo W, Takiguchi S, et al. Effect of emergent nephrostomy on long-term total and split renal function in patients with upper urinary tract obstruction due to pelvic Malignant tumors. Pediatr Surg Int. (2024) 40:234. doi: 10.1007/s00383-024-05810-0
68. Yang J, Sun BG, Min H-J, Son Y-B, Kim TB, Lee J, et al. Impact of acute kidney injury on long-term adverse outcomes in obstructive uropathy. Sci Rep. (2021) 11:23639. doi: 10.1038/s41598-021-03033-0
69. Ganatra AM and Loughlin KR. The management of Malignant ureteral obstruction treated with ureteral stents. J Urol. (2005) 174:2125–8. doi: 10.1097/01.ju.0000181807.56114.b7
70. Ucuzal M and Serçe P. Ureteral stents: impact on quality of life. Holistic Nurs Pract. (2017) 31:126–32. doi: 10.1097/HNP.0000000000000200
71. Cozma C, Georgescu D, Popescu R, Geavlete B, and Geavlete P. Double-J stent versus percutaneous nephrostomy for emergency upper urinary tract decompression. J Med Life. (2023) 16:663–7. doi: 10.25122/jml-2022-0334
72. Heo JE, Jeon DY, Lee J, Ham WS, Choi YD, and Jang WS. Clinical outcomes after urinary diversion for Malignant ureteral obstruction secondary to non-urologic cancer: an analysis of 778 cases. Ann Surg Oncol. (2021) 28:2367–73. doi: 10.1245/s10434-020-09423-4
73. Docimo SG and Dewolf WC. High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at 2 institutions. J Urol. (1989) 142:277–9. doi: 10.1016/S0022-5347(17)38729-3
74. Wu KJ, Chen YZ, Chen M, and Chen Y-H. Clinical factors predicting ureteral stent failure in patients with external ureteral compression. Open Med. (2021) 16:1299–305. doi: 10.1515/med-2021-0345
75. Ohtaka M, Kawahara T, Takamoto D, Mochizuki T, Hattori Y, Teranishi J-i, et al. Gastrointestinal cancer and bilateral hydronephrosis resulted in a high risk of ureteral stent failure. BMC Urol. (2018) 18:35. doi: 10.1186/s12894-018-0346-3
76. Ong K, Chen J, Kong J, and Kuan M. Malignant ureteral obstruction: comparison of metallic, 8 French and 6 French ureteric stents after failure of initial ureteric stent. World J Urol. (2024) 42:92. doi: 10.1007/s00345-024-04803-x
77. Feng MI, Bellman GC, and Shapiro CE. Management of ureteral obstruction secondary to pelvic Malignancies. J Endourol. (1999) 13:521–4. doi: 10.1089/end.1999.13.521
78. Zhang H, Chen Y, Liu P, Zhang L, and Cao J. Evaluation of the safety and efficiency of color Doppler ultrasound-guided percutaneous nephrolithotomy in clinical practice: results from a retrospective study. Renal Failure. (2023) 45:2275714. doi: 10.1080/0886022X.2023.2275714
79. Severova G, Karanfilovski V, Nikolov I, Dzekova-Vidimliski P, Rambabova-Bushljetik I, Dohcev S, et al. Percutaneous nephrostomy in the treatment of hydronephrosis in renal transplant patients – case report. PRILOZI. (2022) 43:55–60. doi: 10.2478/prilozi-2022-0036
80. Baby A, Kesav P, Kumar P, and Madhusudhan KS. CT-guided percutaneous nephrostomy in an obstructed pelvic pancake kidney: a report of a novel transiliopsoas approach. BMJ Case Rep. (2019) 12:e232665. doi: 10.1136/bcr-2019-232665
81. Sommer CM, Huber J, Radeleff BA, Hosch W, Stampfl U, Loenard BM, et al. Combined CT- and fluoroscopy-guided nephrostomy in patients with non-obstructive uropathy due to urine leaks in cases of failed ultrasound-guided procedures. Eur J Radiol. (2011) 80:686–91. doi: 10.1016/j.ejrad.2010.09.035
82. Brandt MP, Lehnert T, Czilwik T, Borgmann H, Gruber-Rouh T, Thalhammer A, et al. CT-guided nephrostomy–An expedient tool for complex clinical scenarios. Eur J Radiol. (2019) 110:142–7. doi: 10.1016/j.ejrad.2018.11.028
83. Prabhu A, Mishra D, Brandl A, and Yonemura Y. Gastric cancer with peritoneal metastasis—A comprehensive review of current intraperitoneal treatment modalities. Front Oncol. (2022) 12:864647. doi: 10.3389/fonc.2022.864647
84. Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtröder C, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. (2019) 23:11–22. doi: 10.1007/s10120-019-00978-0
85. Ji ZH, Zhao QD, and Li Y. The effects of HIPEC on survival of gastric cancer patients with peritoneal metastasis. J Surg Oncol. (2024) 130:1190–5. doi: 10.1002/jso.27877
86. Kang X, Li W, Liu W, Liang H, Deng J, Wong CC, et al. LIMK1 promotes peritoneal metastasis of gastric cancer and is a therapeutic target. Oncogene. (2021) 40:3422–33. doi: 10.1038/s41388-021-01656-1
Keywords: acute kidney injury, gastric cancer, mild hydronephrosis, obstructive nephropathy, peritoneal metastasis 1
Citation: Xiang X, Wei X, Miao J, Sun M, Cao W, Zhao B, Zhang Y, Wei J, Zhu L, Zhang Y, Zhang T and Zhang L (2025) Case Report: Acute kidney injury due to minimal dilated obstructive nephropathy in the context of gastric cancer. Front. Oncol. 15:1586443. doi: 10.3389/fonc.2025.1586443
Received: 02 March 2025; Accepted: 07 August 2025;
Published: 01 September 2025.
Edited by:
Georgia Damoraki, National and Kapodistrian University of Athens, GreeceReviewed by:
Michela Giulii Capponi, Santo Spirito in Sassia Hospital, ItalySpyros Foutadakis, Biomedical Research Foundation of the Academy of Athens (BRFAA), Greece
Copyright © 2025 Xiang, Wei, Miao, Sun, Cao, Zhao, Zhang, Wei, Zhu, Zhang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyuan Zhang, emx5bHlneXlAMTYzLmNvbQ==
†These authors share first authorship
 Xinyu Xiang
Xinyu Xiang Xiaobao Wei2†
Xiaobao Wei2† Wei Cao
Wei Cao