- 1Department of Neurosurgery, The Third Affiliated Hospital of Chengdu Medical College, Chengdu Pidu District People’s Hospital, Chengdu, China
- 2School of Clinical Medicine, Chengdu Medical College, Chengdu, China
- 3Department of Pathology and Pathophysiology, Chengdu Medical College, Chengdu, China
- 4Graduate School, Chengdu Medical College, Chengdu, China
Background: Glioma, a prevalent brain tumor originating from glial cells, exhibits rapid growth, high recurrence, and significant invasiveness. Standard treatments include surgery, radiotherapy, and chemotherapy, yet their effectiveness remains unsatisfactory. Recent studies implicate oxidative stress in promoting glioma cell proliferation and migration, as well as enhancing survival rates, suggesting antioxidant therapy as a potential tumor treatment strategy.
Objective: The aim of this review is to summarize the research hotspots on antioxidant treatment options for gliomas in the last twelve years and analyze the future trends through bibliometric analysis.
Method: We collected articles on oxidative stress in gliomas published between January 1, 2013, and April 5, 2025, using the Web of Science (WOS) database. We also visualized and analyzed annual publications, countries, and journals using VOSviewer, Citespace, and pajek.
Result: The search yielded a total of 1020 publications. Visual analyses show that the number of articles on this topic has increased annually over the last twelve years. Most of the studies came from China, followed by the United States. The three most cited journals were International Journal of Molecular Sciences, Cancer and Frontiers in Oncology. The author who published the most articles on this topic was Wang HD.
Conclusion: Through a systematic analysis, we found that current research hotspots mainly focus on the dose of reactive oxygen species (ROS) and tumor proliferation, inflammatory response, apoptosis, etc. in relation to oxidative stress. In addition, we analyzed the direction of future research: a possible focus on the treatment of gliomas via ‘tumor microenvironment’, ‘blood-brain barrier’, ‘anti-inflammatory’ and ‘ ferroptosis induction ‘ routes.
1 Introduction
Gliomas are primary malignant tumors of the central nervous system (CNS) and constitute the most prevalent type of adult CNS tumors, accounting for 81% of all malignant brain tumors (1). They are traditionally classified into four grades based on morphological changes. Grade I gliomas are benign, non-invasive tumors with a favorable prognosis and infrequent occurrence. Grade II gliomas are infiltrative and low proliferative, with a tendency to recur. Grade III gliomas represent highly malignant types, such as anaplastic astrocytoma. Grade IV gliomas are recognized as glioblastoma (GBM) (2), the most invasive and prevalent brain cancer in adults, accounting for approximately 60% of all cases (1).
GBM is an immunosuppressive deadly brain cancer containing glioblastoma stem-like cells. GBM progresses rapidly, exhibits strong resistance to treatment, and has a high recurrence rate, attributed to factors such as rapid growth, molecular heterogeneity, invasive potential into critical brain structures, and regenerative capabilities of drug-resistant cancer stem cells. Clinical management of gliomas includes surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy (3).
The management of glioblastoma remains a profound challenge for researchers and clinicians, yet recent breakthroughs in oxidative stress research have offered novel therapeutic paradigms for long-term glioma intervention (2). The reduction of molecular oxygen in mitochondria generates ROS as part of normal cellular metabolism. Oxidative stress arises from the ongoing imbalance between the production of ROS/free radicals and the cellular antioxidant defenses that neutralize them (4). Mitochondria serve as the primary loci of ROS generation, accounting for approximately 90% of cellular ROS production. Under hypoxic or bioenergetic stress, dysfunction in the electron transport chain leads to electron leakage from Complex I (NADH dehydrogenase) and Complex II (succinate dehydrogenase), facilitating the reduction of molecular oxygen to superoxide anions, the precursor of downstream ROS species (5). Current evidence underscores the dual role of ROS in gliomagenesis: physiologic ROS levels act as signaling molecules to promote tumor cell proliferation and survival through mitogenic pathways, whereas excessive ROS induces cytotoxicity via DNA/RNA strand breaks, protein carbonylation, and lipid peroxidation, thereby regulating glioma cell cycle progression and apoptotic cell death (6, 7). Beyond these effects, ROS modulate key hallmarks of tumor malignancy, including invasive migration, neovascularization, and therapeutic resistance, establishing their centrality in glioma progression and treatment response (5).
Consequently, contemporary therapeutic strategies predominantly aim to exploit oxidative stress for inducing glioma cell apoptosis (8).This is achieved through various strategies, such as enhancing ROS levels by targeted mitochondrial interventions, activating NADPH oxidase (NOX) to elevate ROS levels in tumor cells, and inhibiting TrxR activity to accumulate ROS in vivo (9). Once ROS levels surpass the cellular tolerance threshold of tumor cells, oxidative damage ensues, triggering mitochondria-mediated apoptotic cascades via intrinsic signaling pathways (10).
To systematically characterize the current landscape and emerging trajectories of oxidative stress research in glioma biology, we employed bibliometric methodologies to conduct quantitative analysis and visual mapping of scholarly literature in this domain. As a widely adopted framework for integrative analysis of research outputs, bibliometrics enables qualitative and quantitative assessment of contributions from countries, institutions, and collaborative networks (11, 12). By deciphering the knowledge architecture and identifying focal research areas, this analytical tool provides a structured foundation for informing future investigative directions (13, 14).
2 Materials and methods
2.1 Data collection
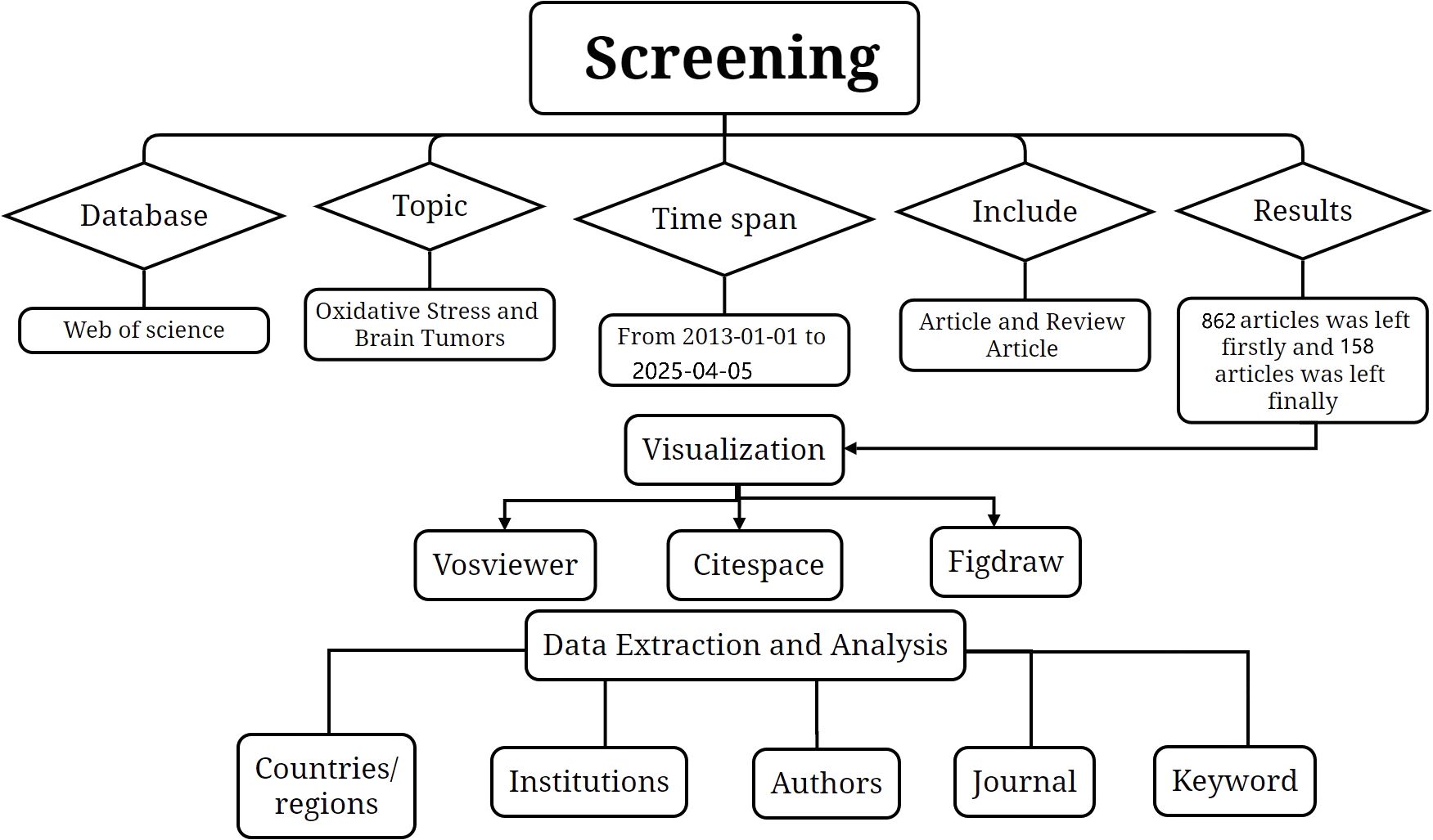
The database of this study was collected from WOS, which is known to be a high-quality database of digital bibliographic resources. Most researchers consider it as the most appropriate database for bibliometric analyses, with more than 12,000 high-quality journals. Based on the literature regarding oxidative stress and glioma, we expanded his keywords first and then searched the authors who published the most on this topic and by this we collected the free words about the topic. By respectively expanding the free words for glioma and oxidative stress and then intersecting the two, we came up with the search formula. The search strategy is shown in Supplementary Material 1 (S1). Only the English-language papers and review papers published between 1 January 2013 and 5 April 2025 were selected. The truncation symbol “*” was used with the aim of preventing the omission of valid keywords. By setting the time, article type, keyword expansion and reading screening, 1020 English papers and review papers were finally identified as the research objects to start the topic discussion (Figure 1).
2.2 Data analysis and visualization
Quantitative analysis and knowledge domain visualization were performed using two prominent bibliometric software packages:
VOSviewer 1.6.19 was utilized to construct co-citation and co-occurrence networks, generating visual maps for authors, journals, and keywords based on bibliographic coupling or term co-occurrence data (15). This tool enabled the visualization of collaborative patterns and thematic clusters through density- and similarity-based mapping.
CiteSpace 6.2.R3 (developed by Prof. Chaomei Chen) was employed for temporal trend analysis and critical node identification, leveraging citation burst detection and time-zone visualization to uncover emerging research frontiers and pivotal milestones in the oxidative stress–glioma field.
For descriptive statistical analysis, Microsoft Excel 2019 was used to tabulate essential bibliometric indicators, including annual publication trends, author productivity, country/region and institutional contributions, journal distributions, citation counts, and keyword co-occurrence frequencies. To systematically evaluate research impact, H-indices for leading authors, countries, and institutions were extracted, alongside 2022 journal metrics—specifically, impact factors and Journal Citation Reports quartile rankings—for the top 10 journals by publication output in this domain.
Mechanistic schematics depicting the role of oxidative stress in glioma biology, including molecular pathways and therapeutic targets, were visualized using Figdraw. These diagrams synthesized key findings from the literature analysis to illustrate the bidirectional relationship between redox homeostasis and glioma progression, enhancing the interpretability of complex biological interactions.
3 Results
3.1 Publication and citation analysis
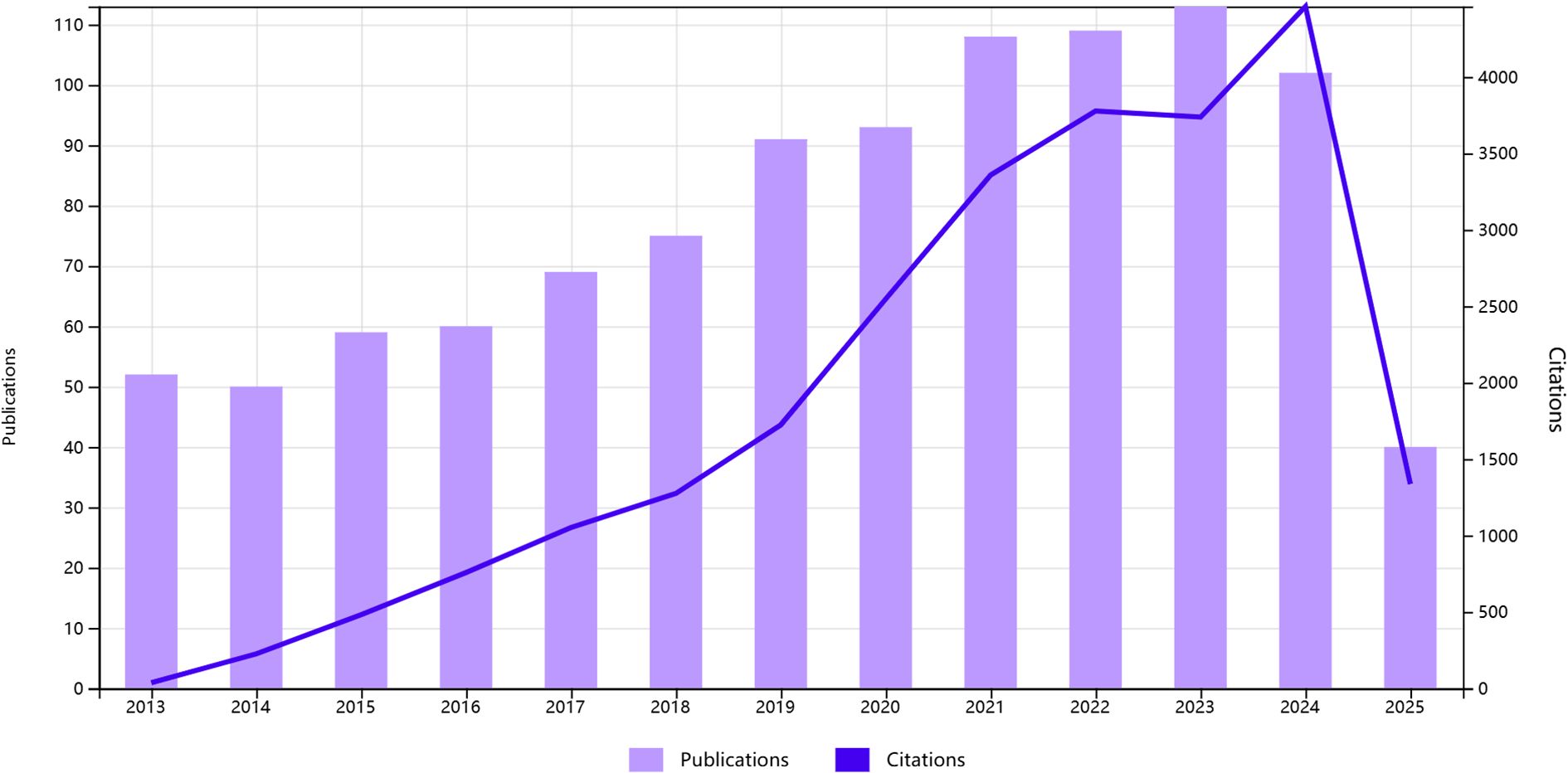
1020 English-language articles on oxidative stress and gliomas obtained in our search, 158 reviews and 862 papers were included. The total number of citations among them was around 18,000, excluding the number of self-citations about 17,000. The average number of citations for publications was 21.59 As shown in Figure 2, overall trend of the number of publications showed a gradual increase. Analyzing the data from the papers shows that the number of annual publications has gradually increased from 52 in 2013 to 119 in 2023. From 2013 to 2017 the number of publications was more stable, fluctuating around 60. It indicates that there is not much research progress during that period. From 2017 to 2025, the number of publications increased year by year, and surpassed 100 in 2021, indicating a breakthrough in research on oxidative stress. It is predictable that oxidative stress will be a major hotspot for glioma treatment in the future.
3.2 Country analyses
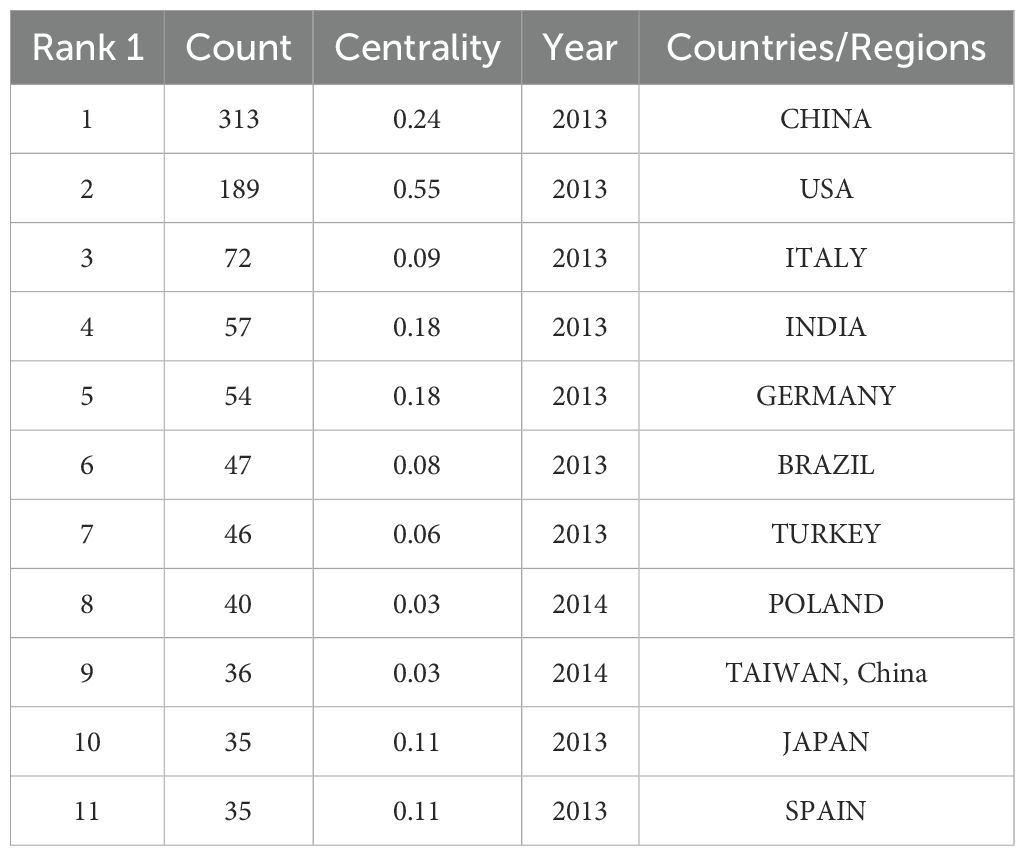
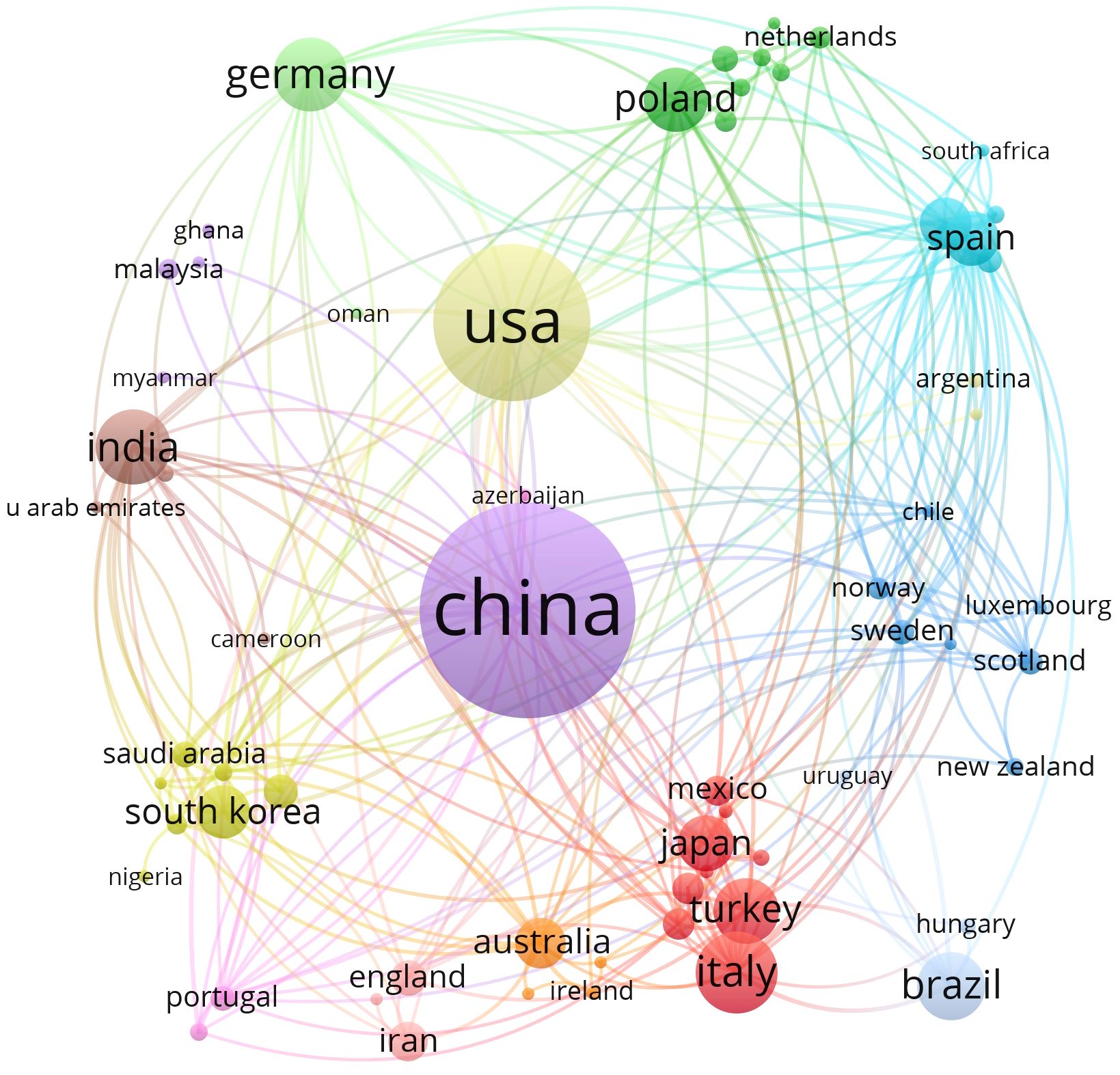
A total of 71 countries were involved in the study of oxidative stress in gliomas from 1 January 2013 to 5 April 2025, Table 1 lists the top ten countries with the highest number of publications, with the highest number of publications in China (count = 313) followed by the United States (count = 189) and Italy (count = 72) In terms of centrality, the United States ranked as the world’s first (centrality = 0.55) followed by China, (centrality = 0.24). Both countries have a centrality degree greater than 0.2, indicating that both countries have a strong academic influence in this field. VOSviewer was used to display the 71 countries mentioned above in the field of international cooperation, as shown in Figure 3. Circles and labels form an element, the size of the element depends on the degree of the node, the strength of the linkage, the number of citations, etc., and the colors of the element represent the cluster it belongs to, and different clusters are indicated by different colors. This indicates that China and the United States are the most connected in this area.
3.3 Author analysis
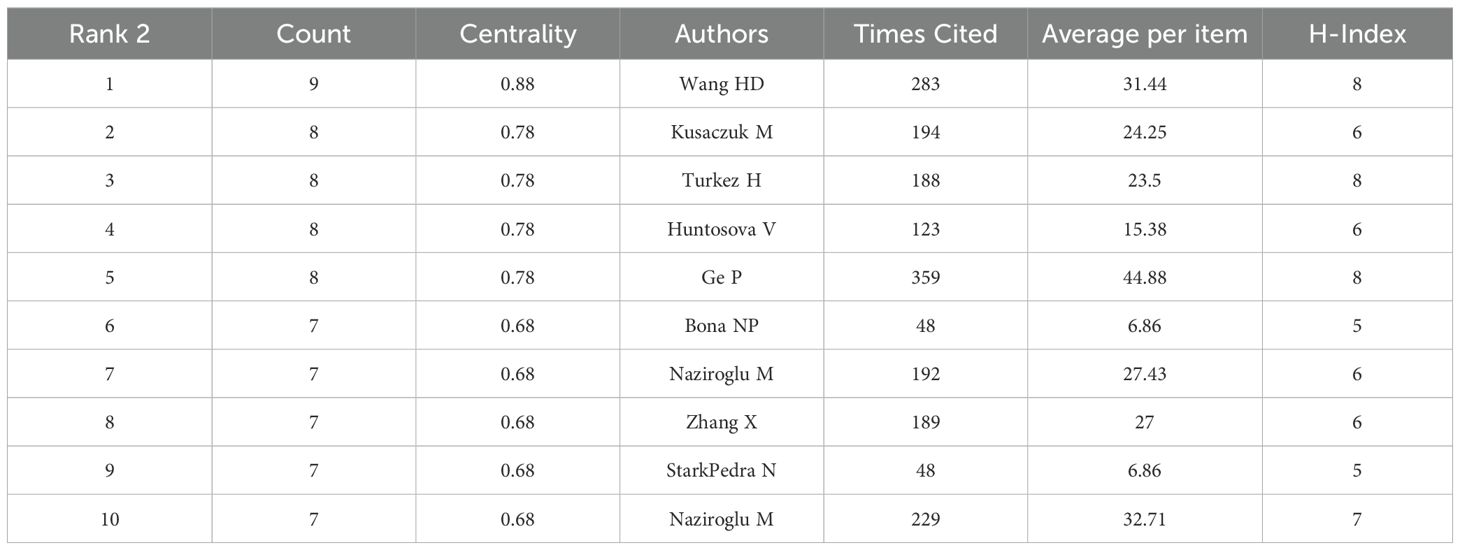
In our analysis of oxidative stress in gliomas, the top 10 most prolific authors based on publication count were listed in Table 2. Among them, Wang, HD. emerged as the most productive author with 9 publications and a total citation count of 283, marking a high level of both output and impact. A comparative review of authors’ total citations (Table 3) revealed that most of the top 10 publishing authors had citation counts exceeding 100; however, two authors exhibited a discrepancy between productivity and influence, with citation counts as low as 48.
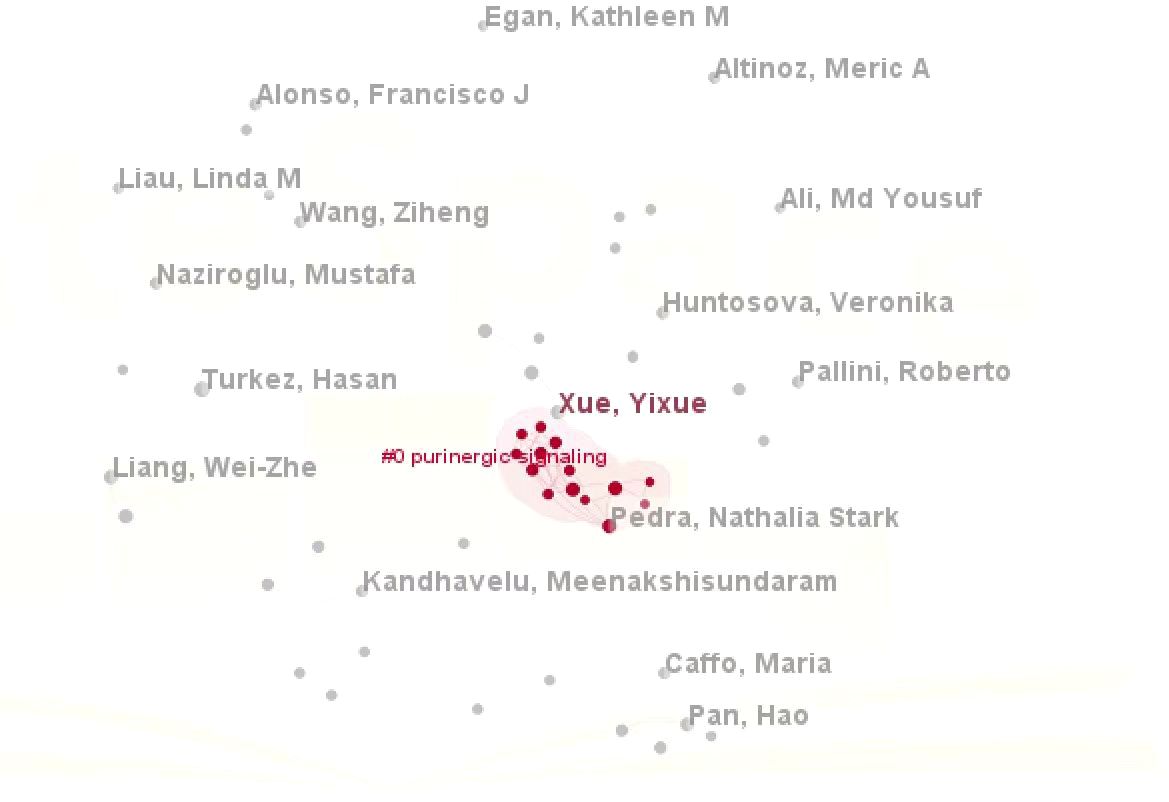
Notably, Ge, P stood out with a total citation count of 359, which was significantly higher than that of all other authors, solidifying their status as the most influential contributor in this field. As can be seen from the density plot of VOSviewer, the area with the brightest color (e.g. yellow) represents the highest co-citation density, in which the authors (e.g. Zhou Y, Wang HD, Wang F, Liu Y, etc.) are at the core of the academic network, suggesting that their research results on glioma and oxidative stress have been widely cited, and have a high impact in this field(Figure 4). Complementary analysis using Citespace, where nodes represent author clusters, further illustrated a fragmented network: clusters were dispersed across the map, demonstrating minimal interconnectivity between different groups (Figure 5). These findings highlight the need for enhanced interdisciplinary and international collaboration to foster breakthroughs in mechanistic research and therapeutic development for gliomas.
3.4 Journal analysis
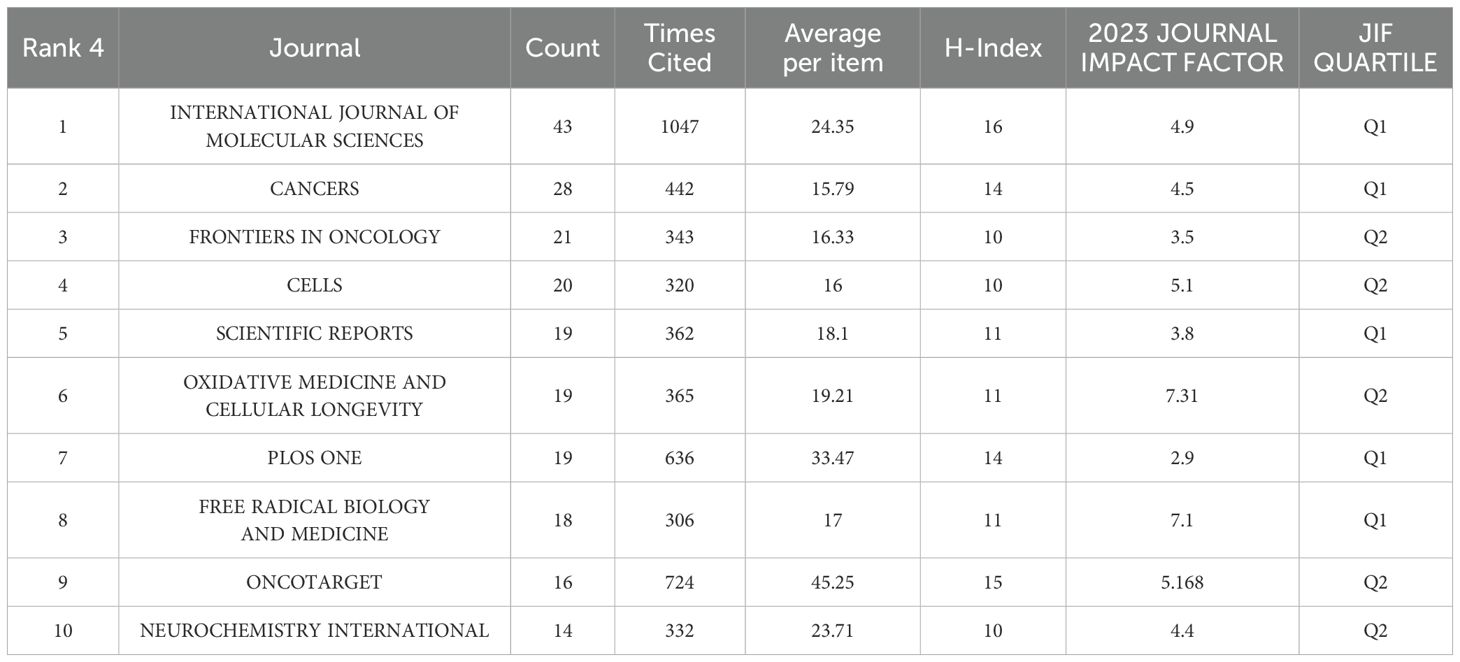
There were about 396 journals in total and the journal with the highest number of publications was INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES with 43 articles published and the 2023 JOURNAL IMPACT FACTOR is 4.9. The number of citations was 1047 and the average number of citations was 24.35. The next highest number of publications was CANCERS with 28 publications, cited 442 times with an average of 15.79 citations. The impact factor (IF) of journals in 2023 is shown in Table 4. The top 10 journals with the highest number of articles published are shown in Table 4. The top 10 high-productivity journals are divided equally between Q2 and Q1. In addition, we find that the journal with the highest impact factor is MEDICINE AND CELLULAR.
3.5 Keywords analysis
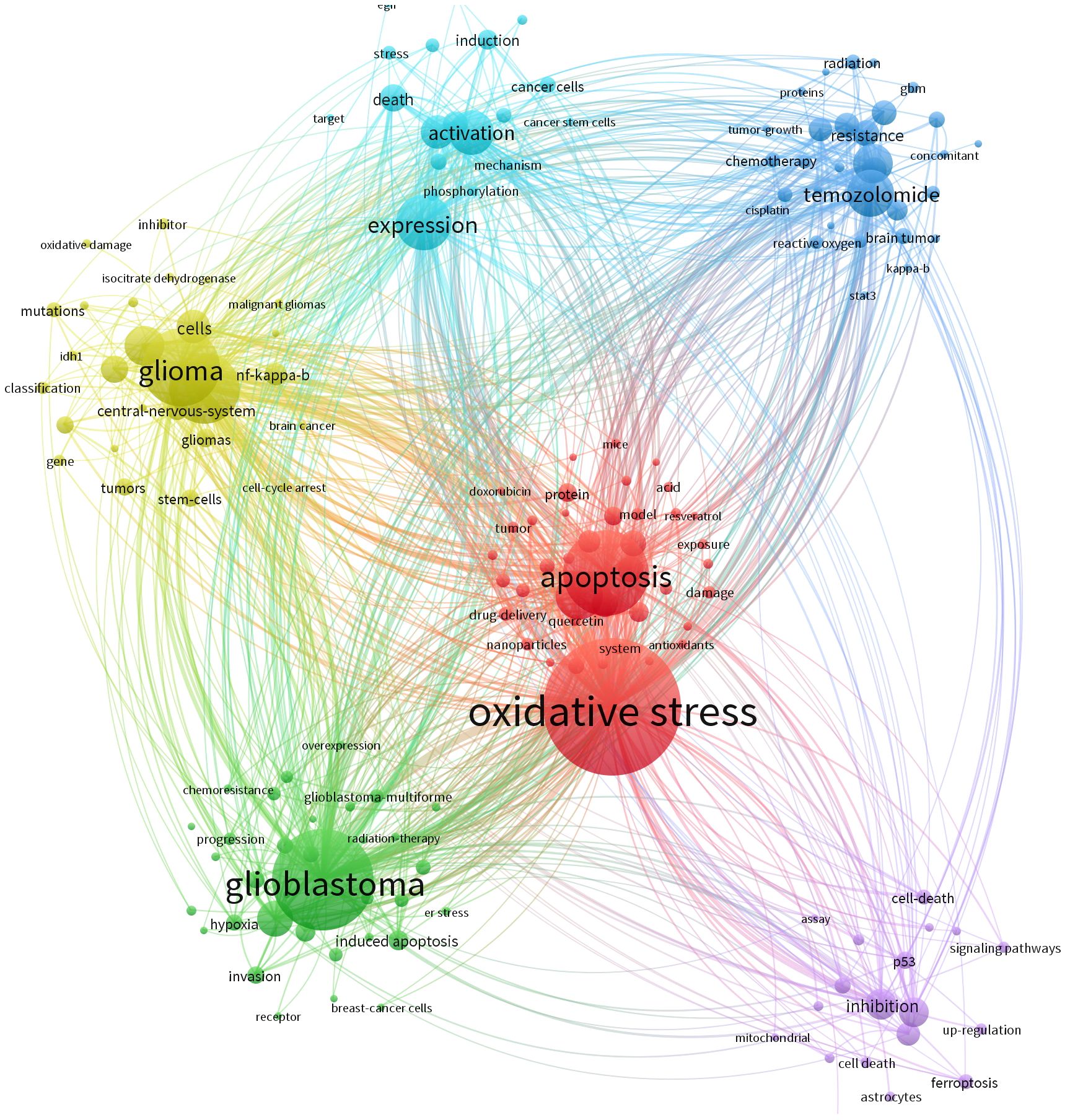
Keywords typically reflect the thematic focus of scholarly publications. Using VOSviewer, we extracted 4,838 keywords from an initial corpus of 1,020 articles for co-occurrence analysis and clustering, yielding the six-cluster co-occurrence network depicted in Figure 6. Circular nodes represent keywords, with size indicating frequency of occurrence, larger nodes denote more representative and frequently appearing hotspots, while connecting lines signify association strength, where thicker lines indicate higher co-occurrence frequency within the same documents. Node colors demarcate distinct clusters corresponding to unique research themes.
The blue cluster focuses on mechanisms of cancer cell activation and target discovery under oxidative stress in glioma, incorporating keywords such as “induction,” “activation,” “expression,” “cancer cells,” and “cancer stem cells.” This theme centers on the activation processes of cancerous and cancer stem cells under oxidative stress conditions, examining molecular mechanisms like phosphorylation and investigating targets linked to cell death to elucidate molecular activity patterns and potential intervention points in glioma cells experiencing oxidative stress. The light-blue cluster, featuring keywords including “radiation,” “tumor growth,” “resistance,” “reactive oxygen,” “kappa-b,” and “signal transducer and activator of transcription 3,” addresses glioma responses to radiotherapy, chemotherapy, and agents like temozolomide within oxidative stress contexts, exploring tumor growth dynamics and resistance mechanisms mediated by these factors.
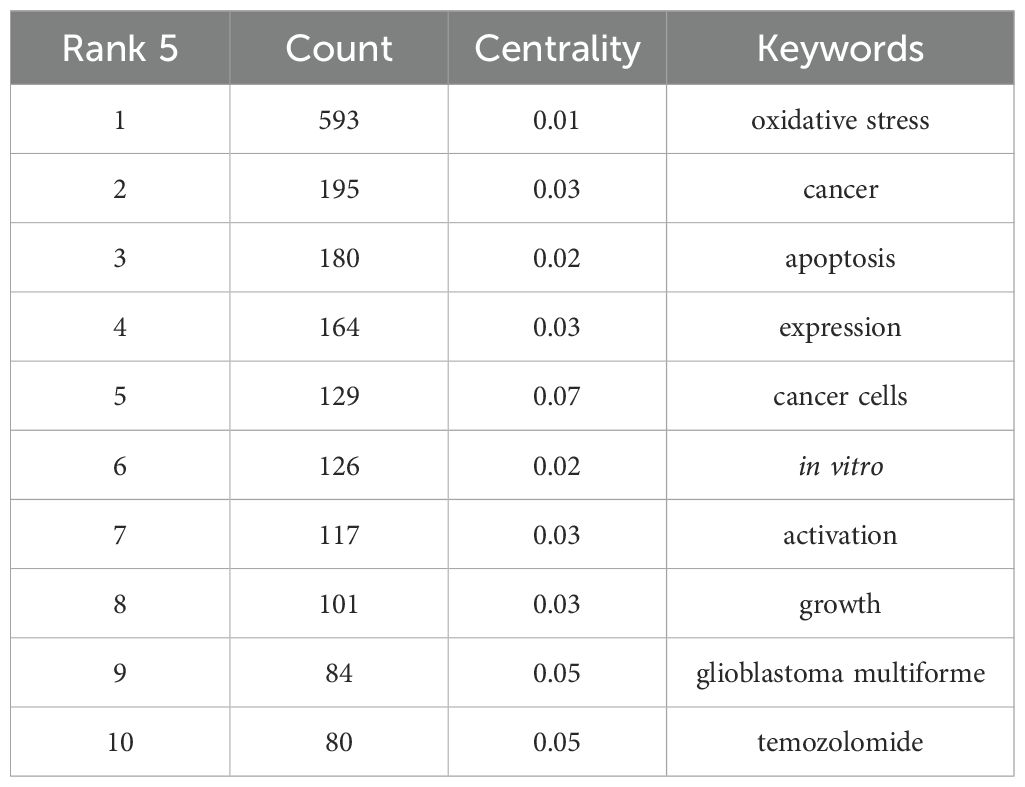
The purple cluster, characterized by “signaling pathways,” “ferroptosis,” and “inhibition,” is dedicated to uncovering how oxidative stress modulates glioma-related signaling pathways to regulate cell death processes such as ferroptosis. The green cluster revolves around treatment resistance and disease progression in glioblastoma, with core keywords “glioblastoma,” “chemoresistance,” and “progression,” dissecting how oxidative stress influences therapeutic responses and tumor advancement in this aggressive glioma subtype. The yellow cluster emphasizes intrinsic glioma properties, including “glioma,” “mutations,” and “stem cells,” focusing on molecular alterations like genetic mutations and stem cell characteristics that may interact bidirectionally with oxidative stress environments. Lastly, the red cluster, anchored by “oxidative stress” and “apoptosis,” directly addresses the regulatory relationship between oxidative stress and apoptosis, investigating how oxidative stress impacts apoptotic pathways and their functional interplay in glioma biology. Following keyword collation, the ten most frequently occurring keywords are listed in order as: oxidative stress, cancer, apoptosis, expression, cancer cells, in vitro, activation, growth, glioblastoma multiforme, and temozolomide. Among these, “cancer cells” exhibits the highest centrality (Table 5), underscoring its pivotal role in contextualizing discussions around oxidative stress.
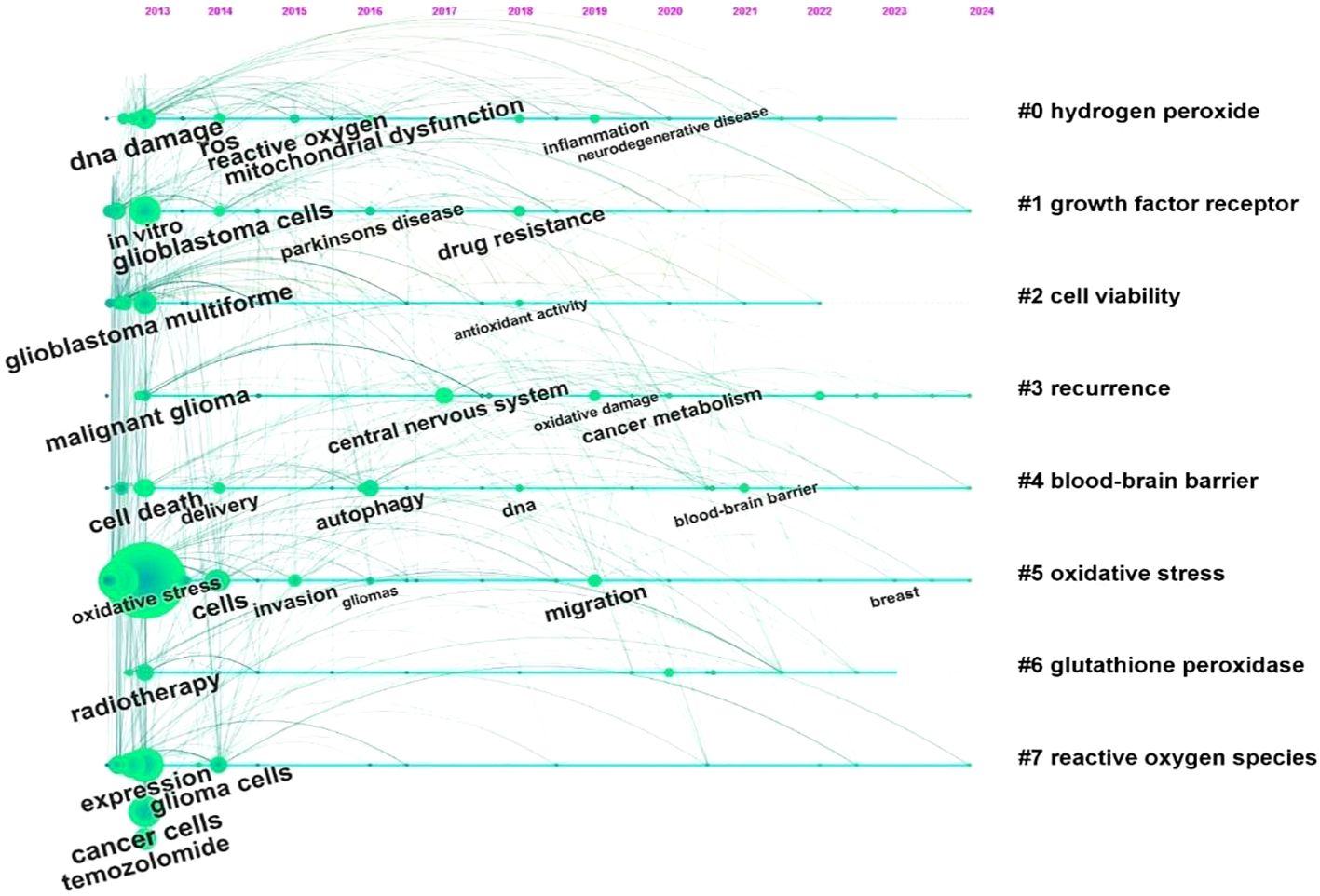
Using CiteSpace, we analyzed the burst strength of keywords and identified the top 25 with significant temporal dynamics (Figure 7). The parameters “strength,” “start,” and “end” denote the intensity of the burst, its initiation year, and termination year, respectively, while red highlights the duration of the burst period. Integrating these data with the timeline graph (Figure 8) reveals that “inflammation” and “tumor microenvironment” have shown the highest citation burst intensities in recent years, reflecting sustained research momentum. Conversely, “blood brain barrier (BBB)” and “ferroptosis” have emerged as emerging hotspots, indicating rising investigative focus in these areas.
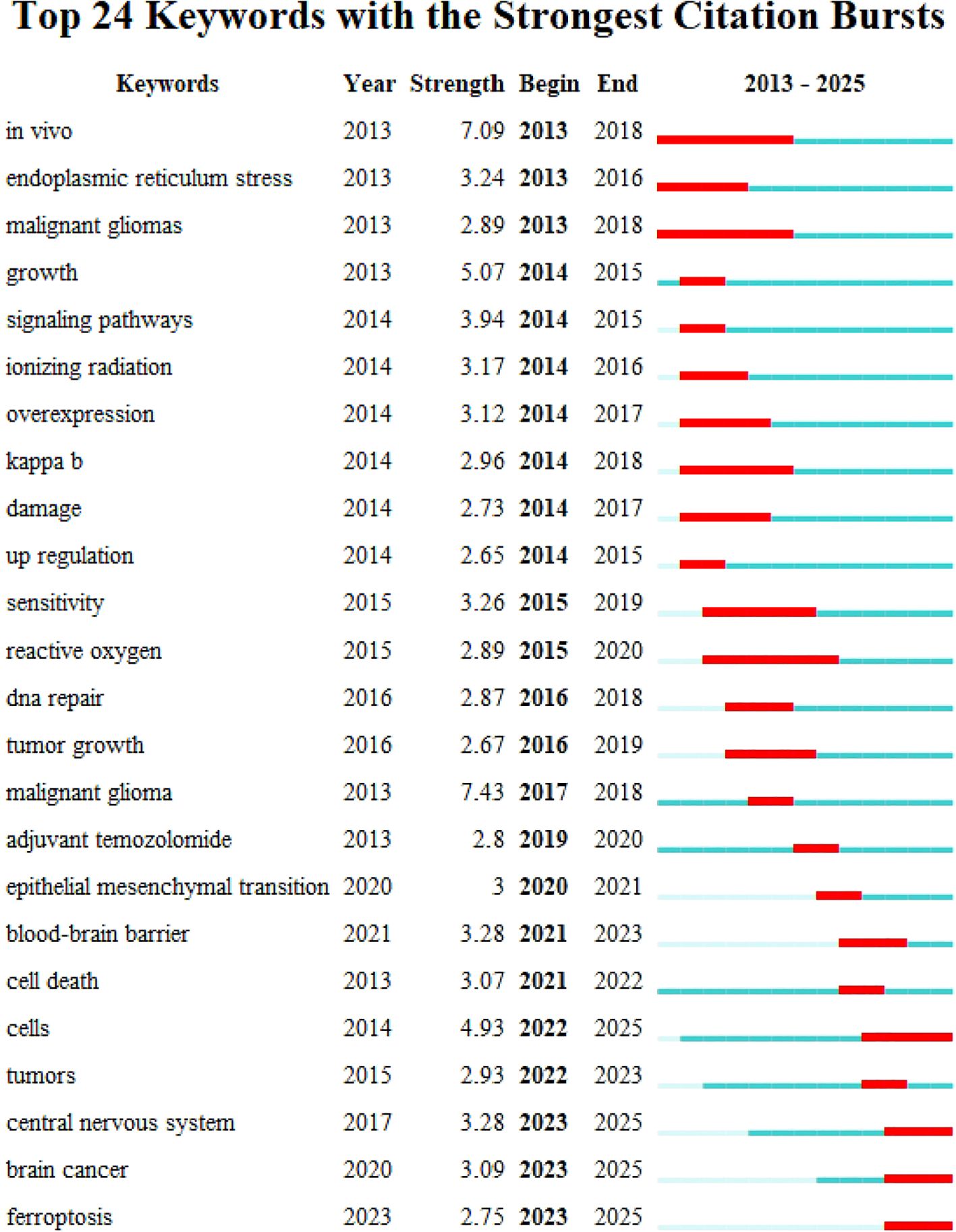
Figure 7. Keyword emergence map for topics related to glioma and oxidative stress: the top 25 keywords with the strongest citation explosion are mainly shown.
3.6 Institution analysis
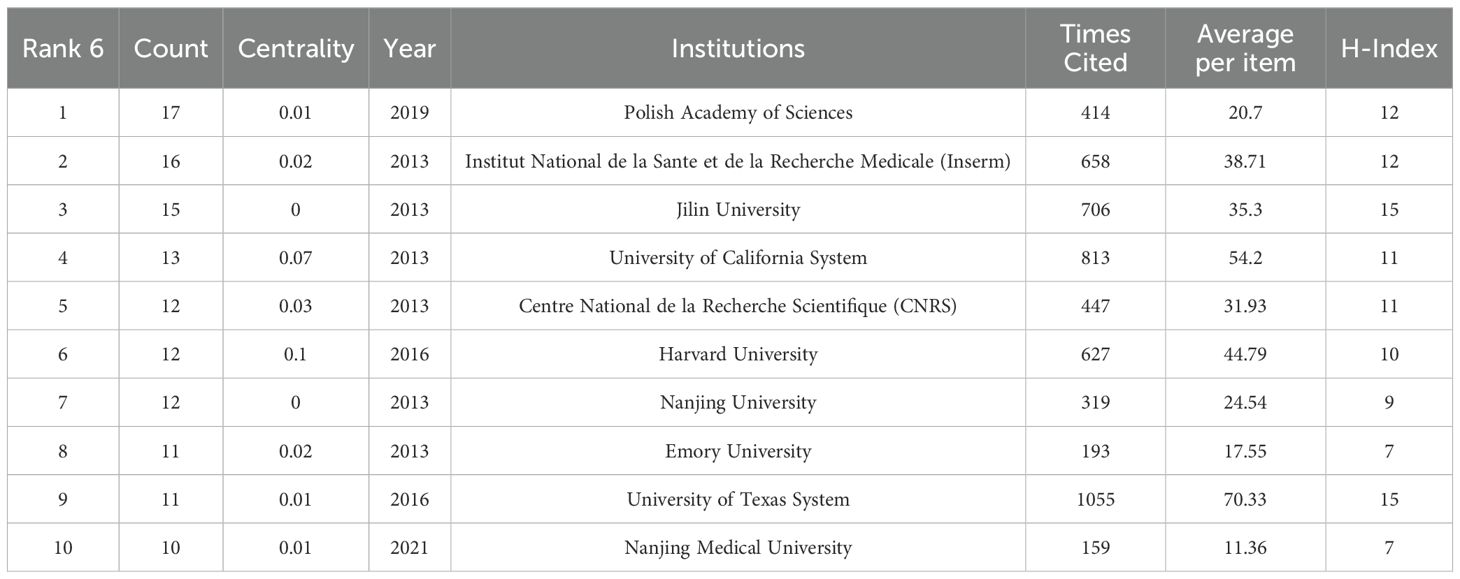
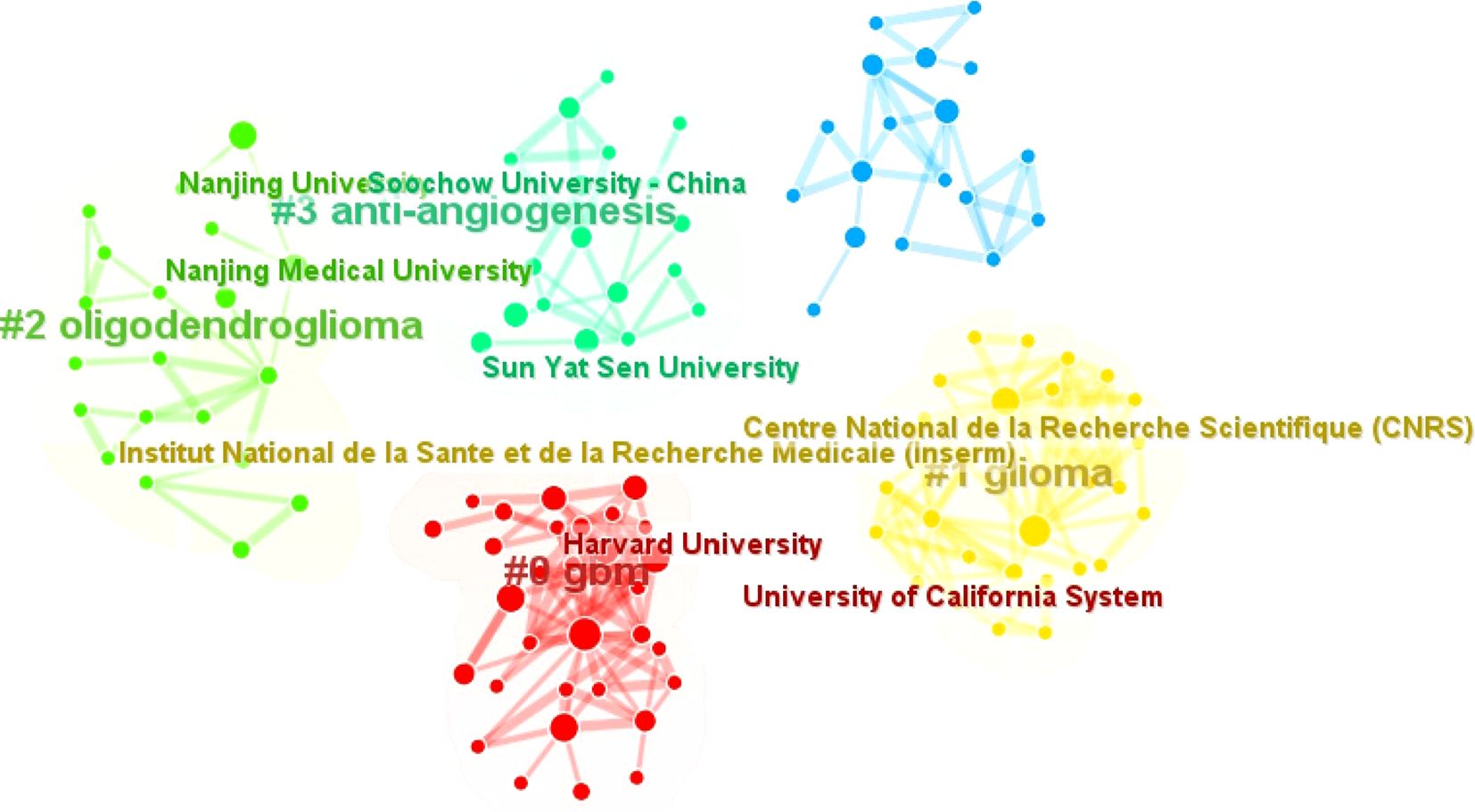
As shown in Table 6, we collected the top 10 institutions from the WOS database. The geographical distribution reveals that four institutions are from the United States, three from China, two from France, and one from Poland. The University of Texas System stands out with the highest citation frequency (1,055 times) and the highest average citations per article (70.33), underscoring its significant influence in the field. The Polish Academy of Sciences has the largest publication output, contributing 17 articles, while Harvard University exhibits the highest centrality score (0.1), indicating its pivotal role in connecting research networks.
Among the three Chinese institutions, Jilin University has the most publications (n=15), reflecting its active research engagement. The CiteSpace visualization classifies these institutions into five thematic clusters based on the thematic trends of their publications, with consistent colors denoting shared research focuses (Figure 9). Notably, Harvard University and the University of California System are grouped in the same cluster, with their research primarily centered on the analysis of glioblastoma.
Collectively, these top 10 institutions have contributed 129 articles, making substantial contributions to advancing the field through their research on glioma biology, oxidative stress mechanisms, and translational applications.
4 Discussion
To the best of my knowledge, this is the first bibliometric study to systematically characterize research trends at the interface of oxidative stress and gliomas over the 2013–2025 period. As illustrated in Figure 2, there has been a consistent upward trend in scholarly publications addressing the relationship between oxidative stress and gliomas. This observed growth can be attributed to both heightened scientific interest in the health implications of oxidative mechanisms and ongoing advancements in optimizing therapeutic strategies for glioma treatment. Through quantitative and visual analyses of 1,020 scholarly documents, our study provides a comprehensive overview of hotspots in this domain, identifying contributions from 71 countries and regions where the USA and China lead in both publication output and collaborative research networks.
Despite advancements in surgical resection, radiotherapy, and chemotherapy for gliomas, the disease remains highly lethal due to incomplete understanding of its molecular pathogenesis and limitations of emerging therapies such as immunotherapy, whose efficacy is substantially constrained by the blood-brain barrier and immunosuppressive tumor microenvironment (16). These unmet clinical needs have spurred increasing interest in oxidative stress pathways as critical therapeutic targets. By analyzing keyword frequencies, burst dynamics, and temporal trends, our study reveals that current research intensively explores the roles of ROS in glioma proliferation, invasion, and resistance to therapy, alongside investigations into redox-modulating agents and their interactions with inflammatory processes.
Building on these insights, future research is poised to focus on deciphering the bidirectional relationship between inflammation and oxidative stress, such as the crosstalk between ROS mediated signaling and inflammatory pathways like NF-κB, in the context of glioma progression, as well as developing oxidative therapies that target both the blood-brain barrier to enhance drug delivery and the tumor microenvironment to exploit its redox vulnerabilities. Additionally, leveraging molecular heterogeneity defined by The Cancer Genome Atlas glioblastoma subtypes (proneural, neural, classical, mesenchymal), studies may aim to design precision interventions that capitalize on subtype-specific redox signatures, such as augmenting ROS levels in mesenchymal glioblastoma cells with elevated antioxidant capacities (17).
To ensure translational relevance, our work integrates genetic factors and environmental influences that modulate redox homeostasis, offering a mechanistic framework for understanding how inflammation and oxidative stress collectively impact glioma treatment responses. By synthesizing bibliometric evidence with molecular insights, this study underscores the centrality of oxidative stress in glioma biology and highlights potential targets for overcoming therapeutic resistance, thereby informing the development of innovative, redox based strategies to improve clinical outcomes for patients with these aggressive brain tumors.
4.1 Oxidative stress and tumor proliferation
Mitochondria are the primary sites of ROS production. ROS can be generated through mitochondrial electron transport chains, endogenous pathways (such as cell signaling, metabolic processes, and inflammation), or exogenous pathways (including X-rays, gamma rays, alpha particles, oxidants, or ultraviolet light) (18). Research indicates that elevated ROS concentrations in cancer cells arise from multiple mechanisms: heightened cellular metabolism, mitochondrial dysfunction induced by hypoxia or autophagy, peroxisome activation, dysregulation of growth factor mediated signaling pathways, oncogene activation, and increased activity of ROS-generating enzymes like xanthine oxidase and peroxidases. In contrast, normal cells maintain redox homeostasis through a sophisticated antioxidant system, which is critical for preventing oxidative damage (19, 20).
For example, in the central nervous system, the glutathione system composed of reduced and oxidized forms of glutathione plays a crucial role in protecting astrocytes and neurons from oxidative damage (21). However, when levels of hydrogen peroxide and other ROS increase, glioblastoma cells respond robustly by activating antioxidant defense mechanisms (22). Research indicates that under conditions of elevated ROS levels, glioma cells invoke antioxidant defense mechanisms including manganese superoxide dismutase (MnSOD), catalase, and glutathione peroxidase (GPx) to combat oxidative stress and ensure cell survival (23, 24). MnSOD, located within mitochondria, catalyzes the breakdown of superoxide radicals into oxygen and hydrogen peroxide, thereby alleviating oxidative damage at the mitochondrial level (25). Catalase, predominantly found in peroxisomes, effectively decomposes hydrogen peroxide into water and oxygen, thereby inhibiting the spread of ROS-induced cell damage (26). GPx, a selenium-dependent enzyme, clears lipid hydroperoxides and H2O2 while utilizing reduced glutathione (GSH) as a cofactor, thereby enhancing cellular defenses against oxidative damage (27). This coordinated activation of MnSOD, catalase, and GPx enables glioblastoma cells to tolerate high ROS microenvironments, facilitating survival in metabolically stressed or inflamed tumor niches. Concurrently, oxidative stress influences glioma malignancy through multifaceted mechanisms: inhibiting apoptosis, modulating tumor-associated gene expression (e.g., redox-sensitive transcription factors like Nrf2 and HIF-1α), and perturbing energy metabolism—processes that collectively enhance invasive and metastatic potential (28).
In the pathogenesis of gliomas, the inhibition of apoptosis is a defining feature that promotes tumor cell survival, a trait markedly distinct from normal cellular homeostasis (8). Malignant transformation involves dysregulation of apoptotic signaling pathways, enabling tumor cells to evade both intrinsic and extrinsic programmed cell death stimuli (29), which underlies the uncontrolled proliferation and invasive phenotype of glioblastomas. A key resistance mechanism involves disruption of both death receptor–mediated extrinsic and mitochondria-driven intrinsic apoptotic pathways (30). Within the extrinsic pathway, the tumor necrosis factor receptor superfamily member Fas (Apol/CD95) is central to apoptotic regulation (31). Its trimeric ligand, Fas ligand, targets cytotoxic T lymphocytes and mediates apoptosis by binding to Fas receptors on target cells (32). Dysregulation of the Fas-Fas ligand axis in gliomas is recognized as a mechanism that promotes tumor cell survival by blocking apoptotic signaling pathways. Furthermore, mitochondria serve as key initiators of the intrinsic apoptotic pathway under conditions such as DNA damage, hypoxia, and metabolic disturbances (33). In this cascade, the Bcl-2 protein family plays a crucial regulatory role by modulating mitochondrial membrane permeability and controlling the release of apoptotic factors (34). Notably, oxidative stress is a hallmark feature of the glioma microenvironment. During oxidative stress, ROS inhibit pro-apoptotic proteins (such as p53, Bcl-2, and caspase-9), thereby intervening in apoptotic regulation (35). This inhibition is mediated through activation of the phosphoinositide 3-kinase (PI3K) and Akt signaling pathways, collectively hindering apoptosis in tumor cells and granting survival advantage under adverse conditions (36). The intricate interactions among apoptotic regulatory mechanisms underscore the formidable challenge of combating gliomas, emphasizing the necessity for targeted interventions aimed at restoring apoptotic competence in tumor cells.
Notably, ROS-induced apoptosis may have a negative impact on health. Therefore, a new technology - using pyroelectric sensors made of polyvinylidene fluoride (37)- has been proposed, which can indirectly assess the health status of cells by detecting changes in their thermal properties (e.g., thermal resistance and heat capacity). For example, oxidative stress may lead to changes in cell membrane structure, protein denaturation, or metabolic changes that affect thermal conductivity and heat capacity (38, 39). If this thermoelectric analysis method is used before and after treatment, changes in these parameters can be monitored and thus the effectiveness of the treatment can be assessed. This technique, with its high sensitivity, low sample requirement and dynamic response (40), has promising applications in both basic research and clinical translation of oxidative stress for gliomas (40).
The transition from normal cellular physiology to malignant tumors triggers a series of events, including activation of the body’s immune response and subsequent inflammatory processes (41). Following cell transformation, various inflammatory cells, including macrophages and neutrophils, are recruited, creating an environment rich in ROS (16). These ROS act as critical secondary messengers, coordinating the activation of key signaling pathways such as mitogen-activated protein kinase, Ras-extracellular signal-regulated kinase, and activator protein-1 pathways. Collectively, these pathways profoundly impact the proliferation of glioblastoma cells and the dynamics of the cell cycle (Figure 10).
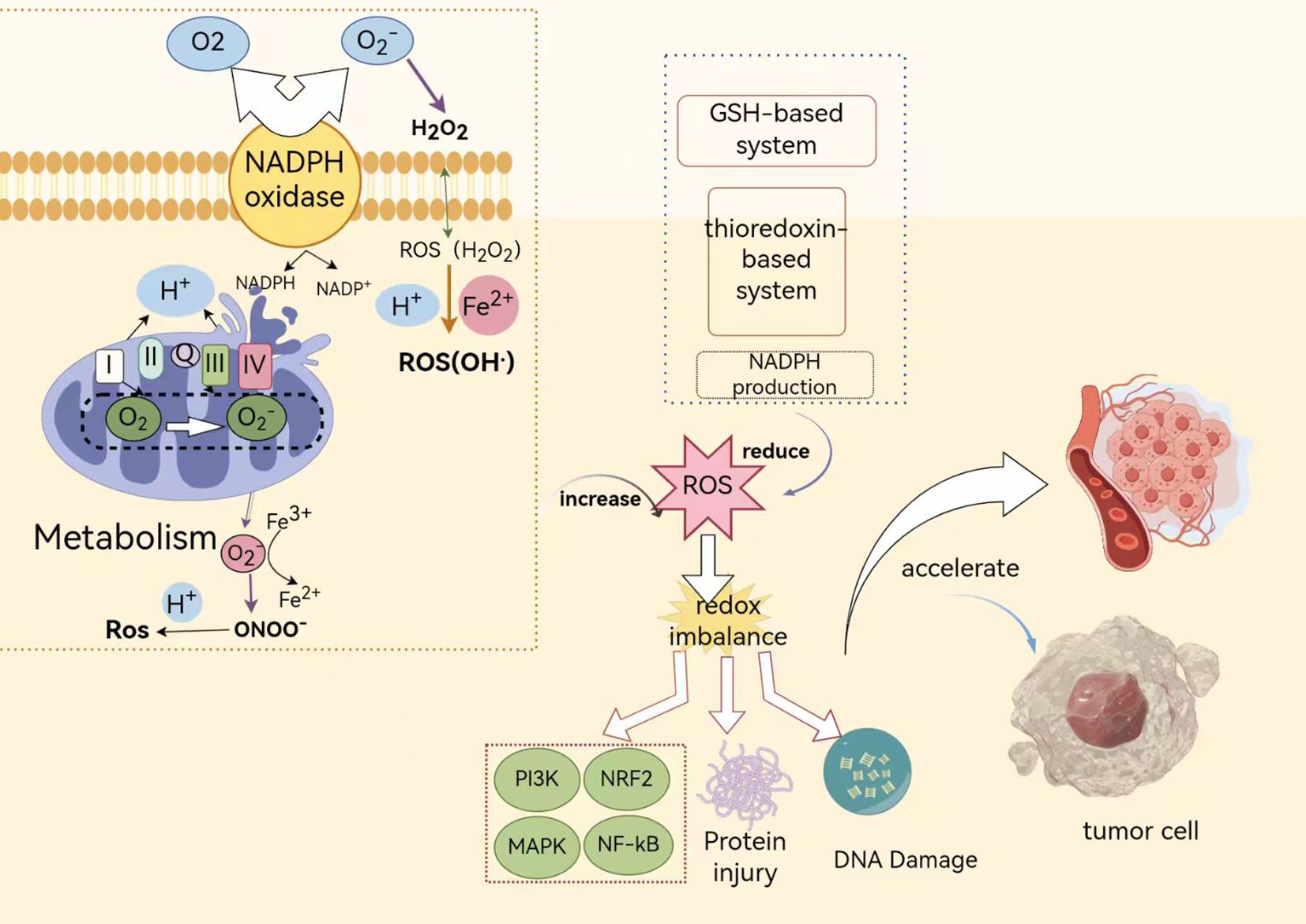
Figure 10. Illustrates the mechanisms by which oxidative stress regulates tumor development and progression. In this figure, the green boxes encompass the pathways of ROS production inside and outside the cell, while the blue dashed boxes represent pathways that antagonize ROS production. PI3K, Phosphoinositide 3-kinase; Nrf2, Nuclear factor erythroid 2-related factor 2; MAPK, Mitogen-activated protein kinase; NF-κB, Nuclear factor kappa B; GSH-based system: A biological system based on GSH; Thioredoxin-based system: A biological system centered around thioredoxin.
4.2 Oxidative stress and inflammatory responses
Persistent oxidative stress is an intrinsic mechanism triggering chronic inflammation, which in turn can mediate various related diseases such as cancer, diabetes, cardiovascular diseases, and neurological disorders. During the inflammatory process, mast cells and leukocytes are recruited to the damaged site, leading to increased release and accumulation of ROS due to enhanced oxygen uptake, resulting in a “respiratory burst” (42). Therefore, ROS not only serve as signaling molecules but also play a critical role as mediators in inflammation, where the generation of ROS and reactive nitrogen species drives the inflammatory process (43). In the treatment of GBM, induced inflammation promotes tumor cell apoptosis through ROS production. Thus, the intricate interactions between ROS, oxidative stress pathways, and inflammation significantly influence treatment resistance (44).
Two main inflammatory pathways, the NF-κB signaling pathway and the Nrf-2 pathway, significantly impact the relationship between oxidative stress and tumors (45). Numerous studies indicate that the NF-κB signaling pathway can promote tumor development. The basic NF-κB signaling pathway comprises receptor and receptor-proximal signal adapter proteins, IκB kinase complexes, IκB proteins, and NF-κB dimers. Upon various endogenous and exogenous stimuli, IκB kinase is activated, leading to the phosphorylation and ubiquitination of IκB proteins, followed by their degradation and the release of NF-κB dimers (46). These dimers translocate into the nucleus, producing pro-inflammatory cytokines (such as IL-6), inducible nitric oxide synthase (iNOS or NOS2), and cyclooxygenase-2 (COX-2) (47, 48), thereby promoting inflammation and signaling survival cues for tumors in a carcinogenic environment. Additionally, oxidative stress, inflammation, and other factors can activate the Nrf-2 pathway. The Nrf-2 pathway plays a dual role in tumorigenesis: it can prevent chemically induced carcinogenesis by activating detoxifying enzymes, yet its overexpression is associated with tumor development and drug resistance. In the treatment of GBM, Nrf-2 acts as a critical transcription factor regulating antioxidant responses to maintain redox balance, crucial in mitigating DNA damage induction and the effects of carcinogens (49).
Under oxidative stress conditions, Nrf-2 rapidly dissociates from the Keap1 complex and translocates to the nucleus (50). Within the nucleus, it forms heterodimers with Maf proteins, promoting the transcription of phase II detoxifying enzymes and antioxidant proteins such as SOD, GPx, GST, and HO-1, thereby serving as a defense mechanism against oxidative damage and exerting an anti-tumor effect. However, due to its dual role, increased levels of Nrf-2 can also promote tumor cell survival. Fan et al. reported that increased expression of Nrf-2 in gliomas, coupled with decreased expression of Keap1, significantly accelerates cell proliferation, promotes tumor formation, and impedes programmed cell death (51) (Figure 11).

Figure 11. Two pathways affecting the relationship between oxidative stress and tumors: the NF-κB signaling pathway and the Nrf-2 pathway.
However, due to genetic and environmental differences in the oxidative stress response, the therapeutic effects may vary in different individuals. Bigner et al. (1988) found that high expression of CYBB (NADPH oxidase subunit) was significantly correlated with copy number gain of chromosome 7, suggesting that it may be the genetic basis for oxidative stress dysregulation in mesenchymal glioblastoma (52). Chromosome 7 gain was accompanied by overexpression of oncogenes such as EGFR and MEOX2 and enhanced mitochondrial antioxidant capacity through activation of the CYBB/Nrf2/SOD2 axis, thereby mediating chemoresistance (53, 54). In addition, radiotherapy exacerbated DNA damage by increasing mitochondrial ROS levels, whereas glioma cells with high CYBB expression inhibited ROS accumulation by activating the Nrf2/SOD2 pathway, thereby enhancing resistance to radiotherapy (55).
4.3 Active oxygen-based anticancer drugs
In recent years, researchers have proposed targeting the upregulation of ROS to induce mitochondrial release of apoptotic factors. Additionally, NOX are proteins involved in electron transfer across biological membranes, crucial for various physiological processes but implicated in diseases such as tumors, diabetes, hypertension, and heart disease when overexpressed (56). Zhu Daqian’s team suggested using NOX inhibitors to suppress H2O2 and O2-mediated tumor cell proliferation (57). Boyi Niu et al. proposed enhancing ROS levels by depleting GSH for cancer treatment. Elevated GSH levels in cancer cells can scavenge excessive ROS, promoting tumorigenesis. Using GSH inhibitors enhances oxidative stress responses, thus inducing tumor cell apoptosis (58).
5 Future trend
5.1 Tumor microenvironment dynamics -based therapies
Gliomas feature a unique microenvironment including hypoxia, acidic pH, and oxidative stress. Within the tumor microenvironment (TME), glioma-associated microglia/macrophages play a crucial role. However, tumor-released anti-inflammatory factors promote M2 polarization of macrophages. This shift stimulates the release of growth factors like vascular endothelial growth factor and promotes tumor proliferation, invasion, and metastasis (59, 60). In the TME, ROS is generated through various pathways, including the mitochondrial electron transport chain, Nox transmembrane protein catalysis, and the Fenton reaction. Studies indicate that overexpression of the mitochondrial membrane protein Romo1 enhances ROS production via mTORC1 signaling, significantly inhibiting the TME. Furthermore, a prognosis model assessing GBM and ROS levels found that high expression of ROS related genes, such as HSPB1, LSP1, and PTX3, correlates not only with the presence of M2 macrophages but also with shortened survival in GBM patients (61).
These findings underscore the potential of ROS related genes as therapeutic targets for GBM. Strategies aimed at inhibiting M2 macrophage polarization may thus benefit GBM treatment. Additionally, myeloid derived suppressor cells (MDSCs) constitute a significant proportion (30%-50%) in the solid glioma, exerting profound immunosuppressive effects in the TME (62). MDSCs inhibit T cell activation and promote tumor immune evasion through upregulation of ARG-1 expression, secretion of immunosuppressive factors, and expression of inhibitory ligands (63). Moreover, MDSCs enhance angiogenesis by releasing vascular endothelial growth factors and IL-10, thus facilitating tumor growth and invasion (64). MDSCs thrive in environments rich in ROS, with elevated Nrf-2 expression promoting their survival. ROS also maintains MDSCs in an undifferentiated state, sustaining expression of immunosuppressive molecules (65). Targeting MDSCs represents a promising strategy for glioma therapy.
5.2 BBB based therapies
The BBB consists of endothelial cells, astrocyte end-feet, and pericytes, collectively protecting the brain from systemic circulation, shielding it from harmful substances, and regulating transport of crucial molecules to maintain a stable microenvironment (66). However, the BBB hinders intravenous administration, with chemotherapy drugs achieving penetration rates in the brain of only 1-2%. This results in extensive dispersion of drugs into healthy tissues and organs, causing severe toxicity. (67) Temozolomide, a first line alkylating agent for gliomas, represents a notable exception due to its near-100% BBB permeability and broad antitumor activity (68). It induces DNA methylation mediated strand breaks, thereby inhibiting tumor cell proliferation, arresting the cell cycle, and promoting apoptosis mechanisms that have been clinically validated to slow glioblastoma progression and extend patient survival.
In recent years, scientists have proposed integrating nanotechnology into the BBB as a novel treatment strategy. Advances in nanotechnology have facilitated the production of nanoparticles capable of crossing or bypassing the BBB, revolutionizing glioma therapy. Surface modification of nanoparticles with BBB targeting peptides or cell penetrating peptides enhances their penetration across the BBB, facilitating precise targeted therapy for tumor cells. Researchers have pioneered ROS based nanomedicine technologies for cancer treatment, including photodynamic therapy, photothermal therapy, chemotherapy, sonodynamic therapy, radiotherapy, and other modalities (57, 69). These methods utilize nano sensitizers and exogenous/endogenous stimuli such as light, ultrasound, X-rays, and hydrogen peroxide to induce ROS production in tumor cells. The disruption of redox balance has been shown to effectively eliminate tumor cells (70).
By comparing nanocarriers such as NanoTherm®, NU-0129, Onyvide® (polyethylene glycolated irinotecan liposomes), and Caelyx® (polyethylene glycolated adriamycin liposomes), which are currently being used in clinical trials for patients with recurrent GBM, academic Juan Aparicio Blanco found that NanoTherm®, administered via intratumoral delivery and combined with alternating magnetic field thermal ablation, resulted in a median overall survival of 13.4 months, which was significantly better than that of the historical control group of 6.2 months. The technology received CE Mark approval as a medical device for GBM treatment in 2013, a strong indication that nanotechnology has made great strides in the treatment of gliomas (71).
5.3 Active oxygen-based anti-inflammatory therapies
Inflammation regulated by NF-κB and Nrf-2 signaling pathways is a pivotal contributor to carcinogenesis and disease progression, driving growing interest in antioxidants as potential cancer therapeutics. These molecules mitigate cancer risk by scavenging free radicals, with molecular hydrogen (H2), a novel antioxidant notable for its small molecular size and high bioavailability, exhibiting unique intracellular interactions that exert antioxidant, anti-inflammatory, and anti-apoptotic effects (72). Preclinical and clinical evidence highlights H2 as a promising adjunct for GBM therapy, demonstrating tumor growth inhibition through dual mechanisms: direct ROS scavenging and modulation of gene transcription involved in cell survival and proliferation, which may extend patient survival (73). Elevated ROS levels are associated with disease progression and drug resistance, highlighting their importance as therapeutic targets. Under specific conditions, H2 and various H2 donors effectively reduce oxidative stress and exhibit anti-inflammatory properties, offering a new treatment strategy for cancer therapy. Administration methods for H2 include inhalation, drinking hydrogen-rich water, or injection of hydrogen saline (73, 74). Despite the therapeutic potential of anti-inflammatory agents, their clinical application is hindered by significant limitations. For instance, cyclooxygenase-2 inhibitors have demonstrated efficacy in preclinical and clinical studies for cancer treatment, yet their use is associated with an elevated risk of cardiovascular events. To address this, strategies such as intermittent dosing of celecoxib are being explored to determine whether this approach can maintain antitumor activity while reducing cardiotoxicity. As a precaution, these drugs are contraindicated in patients with a history of or high risk for cardiovascular disease (75).
5.4 Oxidative stress detection based on temperature/SAR monitoring techniques
The non thermal biological effects of electromagnetic radiation (EMR), such as oxidative stress induction, represent a critical frontier in current research. Existing literature indicates that prolonged exposure to electromagnetic fields (EMFs) emitted by wireless networks may disrupt cellular homeostasis via oxidative stress pathways, despite radiation intensities under established safety guidelines generally falling below thermal threshold levels (76). To quantify such risks, the present study developed a real-time monitoring system integrating synthetic aperture radar (SAR) and infrared thermometry sensors to dynamically track energy absorption profiles and temperature changes (ΔT) in human tissues during electromagnetic exposure.
Elevated temperatures, even minor increases of 1–2°C, can induce free radical generation through thermally activated biochemical reactions, while localized SAR spikes from high-frequency EMFs (e.g., 27 GHz) may interfere with mitochondrial electron transport chain function, exacerbating oxidative damage. For instance, the localized heating induced by high-frequency electromagnetic waves has been shown to disrupt mitochondrial ETC complexes, leading to increased ROS production. Glioma cells, characterized by high metabolic activity, exhibit heightened sensitivity to such oxidative perturbations, making them particularly vulnerable to EMR-induced redox imbalance. The developed system provides quantitative SAR/ΔT correlation data, offering real-time exposure metrics to support research on oxidative stress biomarkers (e.g., ROS, 8-OHdG) and their clinical relevance in EMR-associated pathologies (77).
5.5 Oxidative stress treatment based on iron death
In recent years, investigations into the regulatory mechanisms of ferroptosis have yielded novel targeting strategies for glioma treatment. Studies demonstrate that dihydroartemisinin (DHA) exerts anti-glioma effects by inducing iron-dependent oxidative stress, characterized by ROS accumulation and lipid peroxidation (78). However, this process concurrently triggers endoplasmic reticulum stress and activates the PERK-ATF4-HSPA5 signaling axis, leading to compensatory upregulation of glutathione peroxidase 4 (GPX4)—an enzyme that neutralizes lipid peroxides and attenuates ferroptotic cell death (79). Conversely, genetic or pharmacological inhibition of HSPA5 disrupts this compensatory pathway, significantly enhancing DHA-induced tumor cell demise (80). On the mechanistic front, activation of SIRT1 promotes ATF3 nuclear translocation via a NAD+-dependent mechanism, which in turn suppresses the expression of SLC7A11 and GPX4. This leads to impaired cystine uptake, glutathione depletion, and iron overload, ultimately potentiating RSL3-induced lipid peroxidation–driven ferroptosis (81). Collectively, these findings reveal the bidirectional plasticity of the ferroptosis regulatory network: targeted inhibition of pro-survival compensatory pathways (e.g., the PERK-ATF4-HSPA5 axis) or activation of pro-death signaling (e.g., the SIRT1-ATF3 axis) can overwhelm tumor cell antioxidant defenses by amplifying oxidative stress, offering a dual intervention strategy of “enhancing pro-death signals” and “blocking negative feedback” to optimize ferroptosis-based glioma therapies.
6 Limitations and expectations
This study is subject to several limitations stemming from objective factors. Firstly, the literature included in this study was sourced exclusively from the Web of Science database, potentially leading to the omission of relevant publications. Furthermore, the dynamic nature of the Web of Science database, continually updated for bibliometric analyses, may result in findings that lag behind current research progress. Future research could draw on well-established physical-mathematical modelling ideas in fields such as environmental engineering to further advance the precision of oxidative stress treatments (82).
To address these gaps, future studies could adopt a multi-database approach (incorporating platforms like PubMed, Scopus, and Embase) to minimize selection bias and enhance data diversity. Moreover, integrating well-established physical-mathematical modeling frameworks from disciplines such as environmental engineering and systems biology—including kinetic simulations of redox signaling pathways or computational models of tumor microenvironment interactions—holds promise for advancing the precision of oxidative stress-targeted therapies. Such interdisciplinary methodologies may help identify context-specific therapeutic windows and patient-specific redox signatures, thereby facilitating the development of personalized interventions that account for interindividual variability in treatment response.
7 Conclusion
Through a comprehensive bibliometric analysis, this study systematically examined 1,020 academic articles on oxidative stress in glioma, identifying research hotspots and emerging trends in this interdisciplinary field. By synthesizing data from measurement tools, we characterized key thematic clusters centered around terms such as “inflammation,” “oxidative stress,” “glioma,” “apoptosis,” “glioblastoma,” and “neuroglioma” — these terms dominate the current research landscape, reflecting a sustained interest in the molecular interactions between redox imbalance and glioma pathogenesis.
Future research trajectories are anticipated to focus on developing precise therapeutic approaches, leveraging four interconnected axes: anti - inflammatory interventions to disrupt pro -tumoral immune microenvironments, BBB targeted drug delivery systems to enhance treatment of brain tumors, ferroptosis, and TME modulation strategies that exploit the dependence of glioma on oxidative stress adaptation. These trends highlight a shift toward translational approaches, which integrate mechanistic insights into redox signaling with clinical needs, such as overcoming BBB impermeability and reversing immunosuppression.
Author contributions
YZ: Visualization, Writing – original draft. XL: Visualization, Writing – original draft. YW: Writing – original draft. JH: Writing – review & editing. LZ: Writing – review & editing, Funding acquisition, Supervision. HC: Supervision, Writing – review & editing. LC: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Open Project of Development and Regeneration Sichuan Provincial Key Laboratory (23LHPDZZD05), the Chinese Ministry of Education Cooperative Education Project (231100882305626), and the Sichuan Provincial College Student Innovation and Entrepreneurship Training Program Projects (S202313705089, S202413705092). The person in charge of the above funds is Zhang Lushun, and the person in charge of the funds for the National College Student Innovation and Entrepreneurship Training Program Project (202313705029) is Li Cheng.
Conflict of interest
The researchers affirm that the study was carried out without any business or monetary affiliations that could be interpreted as a possible clash of interests.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1586515/full#supplementary-material
References
1. Ostrowski RP and Pucko EB. Harnessing oxidative stress for anti-glioma therapy. Neurochem Int. (2022) 154:425–50. doi: 10.1016/j.neuint.2022.105281
2. Li TZ, Li JF, Chen Z, Zhang SH, Li SL, Wageh S, et al. Glioma diagnosis and therapy: Current challenges and nanomaterial-based solutions. J Controlled Release. (2022) 352:338–70. doi: 10.1016/j.jconrel.2022.09.065
3. Chen WL, Lei CX, Liu PH, Liu YF, Guo XP, Kong ZR, et al. Progress and prospects of recurrent glioma: A recent scientometric analysis of the web of science in 2019. World Neurosurgery. (2020) 134:E387–99. doi: 10.1016/j.wneu.2019.10.078
4. Liu SY, Dong LH, Shi WY, Zheng ZZ, Liu ZJ, Meng LB, et al. Potential targets and treatments affect oxidative stress in gliomas: An overview of molecular mechanisms. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.921070
5. Tong S, Xia MQ, Xu Y, Sun Q, Ye LG, Yuan FE, et al. Identification and validation of a novel prognostic signature based on mitochondria and oxidative stress related genes for glioblastoma. J Trans Med. (2023) 21:136. doi: 10.1186/s12967-023-03970-6
6. Olivier C, Oliver L, Lalier L, and Vallette FM. Drug resistance in glioblastoma: the two faces of oxidative stress. Front Mol Biosci. (2021) 7. doi: 10.3389/fmolb.2020.620677
7. Liu WC, Xu JT, Pi ZS, Chen YD, Jiang GY, Wan Y, et al. Untangling the web of intratumor microbiota in lung cancer. Biochim Et Biophys Acta-Reviews Cancer. (2023) 1878:189025. doi: 10.1016/j.bbcan.2023.189025
8. Zhang K, Hong R, Kaping L, Xu F, Xia W, Qin G, et al. CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Lett. (2019) 447:130–40. doi: 10.1016/j.canlet.2019.01.005
9. Chen TC, Chuang JY, Ko CY, Kao TJ, Yang PY, Yu CH, et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. (2020) 30:101413. doi: 10.1016/j.redox.2019.101413
10. Stupp R, Mason WP, Bent MJVD, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
11. Moed HF. New developments in the use of citation analysis in research evaluation. Archivum Immunologiae Et Therapiae Experimentalis. (2009) 57:13–8. doi: 10.1007/s00005-009-0001-5
12. Qin YF, Ren SH, Shao B, Qin H, Wang HD, Li GM, et al. The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.931783
13. Gao Y, Wang YR, Zhai X, He YF, Chen R, Zhou JJ, et al. Publication trends of research on diabetes mellitus and T cells (1997-2016): A 20-year bibliometric study. PloS One. (2017) 12:e0184869. doi: 10.1371/journal.pone.0184869
14. Tang W, Yang Y, Yang L, Tang M, Chen Y, and Li C. Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states. Asian J Pharmaceutical Sci. (2021) 16:459–70. doi: 10.1016/j.ajps.2021.03.005
15. van Eck NJ and Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
16. George A, Sahin I, Carneiro BA, Dizon DS, Safran HP, and El-Deiry WS. Strategies to sensitize cancer cells to immunotherapy. Hum Vaccines Immunotherapeutics. (2021) 17:2595–601. doi: 10.1080/21645515.2021.1891817
17. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. (2010) 17:98–110. doi: 10.1016/j.ccr.2009.12.020
18. Lerner A, Kornweitz H, Zilbermann I, Yardeni G, Saphier M, Bar Ziv R, et al. Radicals in ‘biologically relevant’ concentrations behave differently: Uncovering new radical reactions following the reaction of hydroxyl radicals with DMSO. Free Radic Biol Med. (2021) 162:555–60. doi: 10.1016/j.freeradbiomed.2020.11.012
19. Veal E, Jackson T, and Latimer H. Role/s of ‘Antioxidant’ Enzymes in ageing. Subcell Biochem. (2018) 90:425–50. doi: 10.1007/978-981-13-2835-0_14
20. Reczek CR and Chandel NS. The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol. (2017) 1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808
21. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. (2000) 62:649–71. doi: 10.1016/S0301-0082(99)00060-X
22. Fukazawa T, Maeda Y, Sladek FM, Al E, Aacr CT, Fukazawa T, et al. Development of a cancer-targeted tissue-specific promoter system. Cancer Research. (2004) 64:363–9. doi: 10.1158/0008-5472.CAN-03–2507
23. Oberley LW and Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. (1979) 39:1141–9.
24. Trachootham D, Lu W, Ogasawara MA, Valle NR-D, and Huang P. Redox regulation of cell survival. Antioxidants Redox Signaling. (2008) 10:1343–74. doi: 10.1089/ars.2007.1957
25. FRIDOVICH and Trans. Superoxide radical and superoxide dismutase. Biochemical Society Transactions. (1973) 1:48–50. doi: 10.1042/bst0010048
26. Chance B, Boveris A, Nakase Y, and Sies H. Hydroperoxide metabolism: an overview. Life Sciences. (1978) 95–106. doi: 10.1007/978-3-642-67132-6_12
27. Flohé L and Günzler WA. Assays of glutathione peroxidase. Enzymol JM. (1984) 105:114–21. doi: 10.1016/s0076-6879(84)05015-1
28. Cheng P, Ni Z, Dai X, Wang B, Ding W, Smith AR, et al. The novel BH-3 mimetic apogossypolone induces Beclin-1- and ROS-mediated autophagy in human hepatocellular carcinoma cells. Cell Death & Disease (2013) 4:489. doi: 10.1038/cddis.2013.17
29. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. J Cell. (2011) 144:646. doi: 10.1016/j.cell.2011.02.013
30. Frank T, Tuppi M, Hugle M, Dötsch V, van Wijk SJL, and Fulda S. Cell cycle arrest in mitosis promotes interferon-induced necroptosis. Cell Death Differ. (2019) 26:2046–60. doi: 10.1038/s41418-019-0298-5
31. Angulo JC and Shapiro O. The changing therapeutic landscape of metastatic renal cancer. Cancers (Basel). (2019) 11:1227. doi: 10.3390/cancers11091227
32. Ashkenazi A and Dixit VM. Death receptors: signaling and modulation. J Sci. (1998) 281:1305–8. doi: 10.1126/science.281.5381.1305
33. Schoenichen C, Bode C, and Duerschmied D. Role of platelet serotonin in innate immune cell recruitment. Front Biosci (Landmark Ed). (2019) 24:514–26. doi: 10.2741/4732
34. Adams JM and Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. JCD Differentiation. (2018) 25:27–36. doi: 10.1038/cdd.2017.161
35. Bergwik J, Kristiansson A, Welinder C, Göransson O, Hansson SR, Gram M, et al. Knockout of the radical scavenger α(1)-microglobulin in mice results in defective bikunin synthesis, endoplasmic reticulum stress and increased body weight. Free Radic Biol Med. (2021) 162:160–70. doi: 10.1016/j.freeradbiomed.2020.02.019
36. Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, and Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. J Genes Dev. (2008) 22:436–48. doi: 10.1101/gad.1627008
37. Pullano SA, Greco M, Corigliano DM, Foti DP, Brunetti A, Fiorillo AS, et al. Cell-line characterization by infrared-induced pyroelectric effect. J Biosens Bioelectron. (2019) 140:111338. doi: 10.1016/j.bios.2019.111338
38. Qiao J, Mu X, and Qi L. Construction of fluorescent polymeric nano-thermometers for intracellular temperature imaging: A review. JB Bioelectronics. (2016) 85:403–13. doi: 10.1016/j.bios.2016.04.070
39. Park BK, Woo Y, Jeong D, Park J, Choi T-Y, Simmons DP, et al. Thermal conductivity of biological cells at cellular level and correlation with disease state. J Appl Physics. (2016) 119:224701. doi: 10.1063/1.4953679
40. Pullano SA, Greco M, Oliva G, Laganà F, Islam SK, and Fiorillo AS. Thermoelectrical analysis of cell lines with a pyroelectric sensor. Sensors Microsystems. (2025) 1334:245–9. doi: 10.1007/978-3-031-82076-2_34
41. Lin F, Zheng Y, Pan L, and Zuo Z. Attenuation of noisy environment-induced neuroinflammation and dysfunction of learning and memory by minocycline during perioperative period in mice. Brain Res Bull. (2020) 159:16–24. doi: 10.1016/j.brainresbull.2020.03.004
42. Wong EA, Evans S, Kraus CR, Engelman KD, Maiello P, Flores WJ, et al. IL-10 Impairs Local Immune Response in Lung Granulomas and Lymph Nodes during Early Mycobacterium tuberculosis Infection. J Immunol. (2020) 204:644–59. doi: 10.4049/jimmunol.1901211
43. Conti A, Gulì C, La Torre D, Tomasello C, Angileri FF, and Aguennouz M. Role of inflammation and oxidative stress mediators in gliomas. Cancers (Basel). (2010) 2:693–712. doi: 10.3390/cancers2020693
44. Mittal M, Siddiqui MR, Tran K, Reddy SP, and Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. doi: 10.1089/ars.2012.5149
45. Keszler A, Lindemer B, Weihrauch D, Jones D, Hogg N, and Lohr NL. Corrigendum to “Red/near infrared light stimulates release of an endothelium dependent vasodilator and rescues vascular dysfunction in a diabetes model” [Free Radic. Biol. Med. 113:157-164. Free Radic Biol Med. (2019) 131:443. doi: 10.1016/j.freeradbiomed.2018.12.007
46. Nikolaou PE, Efentakis P, Abu Qourah F, Femminò S, Makridakis M, Kanaki Z, et al. Chronic empagliflozin treatment reduces myocardial infarct size in nondiabetic mice through STAT-3-mediated protection on microvascular endothelial cells and reduction of oxidative stress. Antioxid Redox Signal. (2021) 34:551–71. doi: 10.1089/ars.2019.7923
47. Conti A, Miscusi M, Cardali S, Germanò A, Suzuki H, Cuzzocrea S, et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. (2007) 54:205–18. doi: 10.1016/j.brainresrev.2007.01.013
48. Grivennikov SI, Greten FR, and Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
49. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. (2013) 53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320
50. Ahmed SM, Luo L, Namani A, Wang XJ, and Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:585–97. doi: 10.1016/j.bbadis.2016.11.005
51. Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M, et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. (2017) 6:e371. doi: 10.1038/oncsis.2017.65
52. Bigner SH, Mark J, Burger PC, Mahaley MS Jr., Bullard DE, Muhlbaier LH, et al. Specific chromosomal abnormalities in Malignant human gliomas. Acta Neuropathol. (1988) 48:405–11.
53. Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou M-F, et al. Stem cell–related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. (2008) 26:3015–24. doi: 10.1200/JCO.2007.15.7164
54. Vital AL, Tabernero MD, Castrillo A, Rebelo O, Tão H, Gomes F, et al. Gene expression profiles of human glioblastomas are associated with both tumor cytogenetics and histopathology. Neuro-Oncology. (2010) 12:991–1003. doi: 10.1093/neuonc/noq050
55. An Z, Yu J-R, and Park W-Y. Rosiglitazone enhances radiosensitivity by inhibiting repair of DNA damage in cervical cancer cells. JR biophysics e. (2017) 56:89–98. doi: 10.1007/s00411-016-0679-9
56. Taylor JT, Ellison S, Pandele A, Wood S, Nathan E, Forte G, et al. Actinomycin D downregulates Sox2 and improves survival in preclinical models of recurrent glioblastoma. Neuro Oncol. (2020) 22:1289–301. doi: 10.1093/neuonc/noaa051
57. Wang J, Liu W, Luo G, Li Z, Zhao C, Zhang H, et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy & Environmental Science (2018) 11:3375–9. doi: 10.1039/C8EE02656D
58. Niu B, Liao K, Zhou Y, Wen T, and Wu C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. J Biomaterials. (2021) 7):121110. doi: 10.1016/j.biomaterials.2021.121110
59. Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, et al. Contents – eur. J. Immunol. 1/2007. Eur J Immunol. (2006) 37:4–7. doi: 10.1002/eji.200790033
60. Narendra BL, Reddy KE, Shantikumar S, and Ramakrishna S. Immune system: a double-edged sword in cancer. JIR. (2013) 62:823–34. doi: 10.1007/s00011-013-0645-9
61. Yang YC, Zhu Y, Sun SJ, Zhao CJ, Bai Y, Wang J, et al. ROS regulation in gliomas: implications for treatment strategies. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1259797
62. Salemizadeh Parizi M, Salemizadeh Parizi F, Abdolhosseini S, Vanaei S, Manzouri A, and Ebrahimzadeh F. Myeloid-derived suppressor cells (MDSCs) in brain cancer: challenges and therapeutic strategies. Inflammopharmacology. (2021) 29:1613–24. doi: 10.1007/s10787-021-00878-9
63. García-Navas R, Munder M, and Mollinedo F. Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy. (2012) 8:1557–76. doi: 10.4161/auto.21315
64. Ding XC, Wang LL, Zhang XD, Xu JL, Li PF, Liang H, et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol Oncol. (2021) 14:92(2021). doi: 10.1186/s13045-021-01102-5
65. Sinha P, Clements VK, Bunt SK, Albelda SM, and Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. (2007) 179:977–83. doi: 10.4049/jimmunol.179.2.977
66. Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherited Metab Dis. (2013) 36:437–49. doi: 10.1007/s10545-013-9608-0
67. Golden PL and Pollack GM. Blood-brain barrier efflux transport. J Pharm Sci. (2003) 92:1739–53. doi: 10.1002/jps.10424
68. Li G, Qin Z, Chen Z, Xie L, Wang R, and Zhao H. Tumor microenvironment in treatment of glioma. Open Med (Wars). (2017) 12:247–51. doi: 10.1515/med-2017-0035
69. de Sá Junior PL, Câmara DAD, Porcacchia AS, Fonseca PMM, Jorge SD, Araldi RP, et al. The roles of ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev. (2017) 2017:2467940. doi: 10.1155/2017/2467940
70. Borhan A, Herea DD, Gherca D, Stavila C, Minuti AE, Grigoras M, et al. Flash-cooling assisted sol-gel self-ignited synthesis of magnetic carbon dots-based heterostructure with antitumor properties. Mater Sci Eng C Mater Biol Appl. (2020) 117:111288. doi: 10.1016/j.msec.2020.111288
71. Aparicio-Blanco J, Sanz-Arriazu L, Lorenzoni R, and Blanco-Prieto MJ. Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: Nanomedicine approaches to improve their efficacy. Int J Pharmaceutics. (2020) 581:119283. doi: 10.1016/j.ijpharm.2020.119283
72. Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. (2020) 130:6151–7. doi: 10.1172/JCI141374
73. Rochette L, Dogon G, Zeller M, Cottin Y, and Vergely C. Antitumoral activity of molecular hydrogen and proton in the treatment of glioblastoma: an atypical pharmacology? Brain Sci. (2023) 13:1168. doi: 10.3390/brainsci13081168
74. Hashem NM, Gonzalez-Bulnes A, and Simal-Gandara J. Polyphenols in farm animals: source of reproductive gain or waste? Antioxidants (Basel). (2020) 9:1023. doi: 10.3390/antiox9101023
75. Mohammed A, Yarla NS, Madka V, and Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. Int J Mol Sci. (2018) 19:2332. doi: 10.3390/ijms19082332
76. Babajani A, Eftekharinasab A, Bekeschus S, Mehdian H, Vakhshiteh F, and Madjd Z. Reactive oxygen species from non-thermal gas plasma (CAP): implication for targeting cancer stem cells. JCCI. (2024) 24:344. doi: 10.1186/s12935-024-03523-x
77. Laganà F, Bibbò L, Calcagno S, De Carlo D, Pullano SA, Pratticò D, et al. Smart electronic device-based monitoring of SAR and temperature variations in indoor human tissue interaction. Applied Sciences. (2025) 15:2439. doi: 10.3390/app15052439
78. Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J of Experimental & Clin Can Res volume (2019) 38:1–16. doi: 10.1186/s13046-019-1413-7
79. Maiorino M, Conrad M, and Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. JA Signaling r. (2018) 29:61–74. doi: 10.1089/ars.2017.7115
80. Pyrko P, Schönthal AH, Hofman FM, Chen TC, and Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in Malignant gliomas. JCr. (2007) 67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625
81. Wang Z, Li C, Zhang Z, Lu S, Wang X, Liang Q, et al. SIRT1 activated by AROS sensitizes glioma cells to ferroptosis via induction of NAD+ depletion-dependent activation of ATF3. Redox Biology. (2024) 69:103030. doi: 10.1016/j.redox.2024.103030
Keywords: oxidative stress, gliomas, bibliometrics, Citespace, VOSviewer, visualization
Citation: Zeng Y, Lu X, Wang Y, He J, Cao H, Zhang L and Cheng L (2025) Recent status and trends regarding oxidative stress in gliomas (2013 - 2025): a systematic review and bibliometric analysis. Front. Oncol. 15:1586515. doi: 10.3389/fonc.2025.1586515
Received: 03 March 2025; Accepted: 22 April 2025;
Published: 16 May 2025.
Edited by:
Mario Versaci, Mediterranea University of Reggio Calabria, ItalyReviewed by:
Filippo Laganà, University Magna Graecia of Catanzaro, ItalyGiuseppe Oliva, Magna Græcia University, Italy
Domenico De Carlo, Mediterranea University of Reggio Calabria, Italy
Copyright © 2025 Zeng, Lu, Wang, He, Cao, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lushun Zhang, emhhbmdsczIwMTJAY21jLmVkdS5jbg==; Li Cheng, MTAwMDE1MUBjbWMuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Lushun Zhang, orcid.org/0000-0001-6094-2460
 Yijun Zeng1†
Yijun Zeng1† Hui Cao
Hui Cao Lushun Zhang
Lushun Zhang