- 1Teaching and Research Section of Clinical Nursing, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Pulmonology, Children’s Hospital, National Clinical Research Center For Child Health, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of General Surgery, Xiangya Hospital, Central South University, Changsha, China
- 4International Joint Research Center of Minimally Invasive Endoscopic Technology Equipment & Standards, Xiangya Hospital, Changsha, China
- 5National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: Liver cancer is one of the major causes of cancer-related death in the world. As a breakthrough therapy, immunotherapy had significantly improved the prognosis of patients. However, the current research status and research hotspots in the field of liver cancer immunotherapy still lack systematic review. Based on the bibliometric analysis of highly cited papers, this study intended to reveal the current research status, research hotspots and future research trends in this field.
Objective: The purpose of this study was to analyze the national/regional contributions, authors and institutions cooperation network, keywords clustering and keywords burst analysis of highly cited papers on liver cancer immunotherapy through bibliometrics, so as to clarify the research frontier and development direction, and provide objective data support for future research direction and clinical practice.
Methods: The highly cited papers on liver cancer immunotherapy from the Web of Science core collection up to February 23, 2025 were retrieved, and 232 studies were included. CiteSpace was used to build a knowledge map, analyze the distribution of years, countries, authors, institutions and cooperation networks, and identify research hotspots and emerging trends through keyword clustering and burst detection.
Results: The number of highly cited papers continued to increase from 2014 and reached a peak in 2022. China and the United States had the highest number of publications and the centrality of cooperation networks. The author with the highest number of papers was Llovet, Josep M, whose research direction mainly focused on immune checkpoint inhibitor combination therapy and molecular typing. The author with the highest cooperation network centrality was Duda, Dan G, whose research team focused on tumor microenvironment regulation. Harvard University and the University of Barcelona played an important central role in the institutional collaboration. Keywords analysis showed that immune checkpoint inhibitors, tumor microenvironment and combination therapy were the core of liver cancer immunotherapy. Burst keywords such as cell lung cancer, pembrolizumab, advanced melanoma, blockade, lymphocytes, etc. had revealed the research frontier of liver cancer immunotherapy research.
Conclusion: The research on liver cancer immunotherapy had made multi-dimensional progress, with China and the United States leading the global cooperation. The main research directions were the combination strategy of immunization, the regulation of tumor microenvironment and the exploration of novel targets. In the future, it is necessary to optimize treatment resistance solutions, integrate interdisciplinary resources, and promote the development of precision and personalized treatment.
1 Introduction
Liver cancer is a cancer that originates in the liver and was an aggressive tumor that often occurs in the context of chronic liver disease and cirrhosis (1). Hepatocellular Carcinoma (HCC) accounts for 75% to 85% of primary liver cancers (2), and was the fifth most common cancer in men and the seventh most common cancer in women, and the third leading cause of cancer-related death worldwide (3). Traditional treatment methods, such as surgical resection, liver transplantation, local ablation and chemotherapy, had achieved certain efficacy in early stage liver cancer, but the effect was limited for advanced patients (4).
In recent years, immunotherapy, especially the application of Immune Checkpoint Inhibitors (ICIS), had brought new hope for the treatment of hepatocellular carcinoma (5). The rise of immunotherapy stemmed from a deeper understanding of tumor immune escape mechanisms. The discovery of programmed death receptor-1 (PD-1) and its ligand, PD-L1, revealed the mechanism by which tumor cells evaded immune surveillance by inhibiting T cell function (6). Based on this mechanism, PD-1/PD-L1 inhibitors had shown significant clinical efficacy in a variety of solid tumors (7–9). In addition, the research on new immune checkpoints such as FGL1, CTLA-4, TIM-3, and LAG-3 were also constantly advancing, providing more possibilities for liver cancer immunotherapy (10–14).
With the wide application of immunotherapy in liver cancer, the number of related research papers had increased exponentially. However, how to identify high-impact research results from the massive papers and reveal the research hotspots and development trends in this field had become an important topic in the academic circle and clinical practice. As a tool for quantitative analysis of paper data, Bibliometrics can systematically reveal the dynamic changes, research hotspots and knowledge networks in specific research fields (15). CiteSpace can extract important knowledge points, development trends and research frameworks in the field through time series analysis, co-word analysis and co-citation analysis of a large number of papers, helping researchers to comprehensively understand the research status and future development direction of this field (16).
Highly cited papers refer to research results that was cited significantly more frequently than similar papers within a certain period of time. Highly cited papers usually represent a milestone achievement in a certain field or research with important academic value, and its analysis can reveal the distribution characteristics of key theoretical breakthroughs, technical paths and academic influence. Therefore, in this study, CiteSpace, a bibliometric analysis tool, was intended to be used to analyze highly cited papers in the research field of liver cancer immunotherapy, so as to identify core countries, authors and institutions, reveal potential research gaps, hot spots and frontier issues, and provide theoretical support and methodological guidance for future research.
2 Data and methods
2.1 Data sources
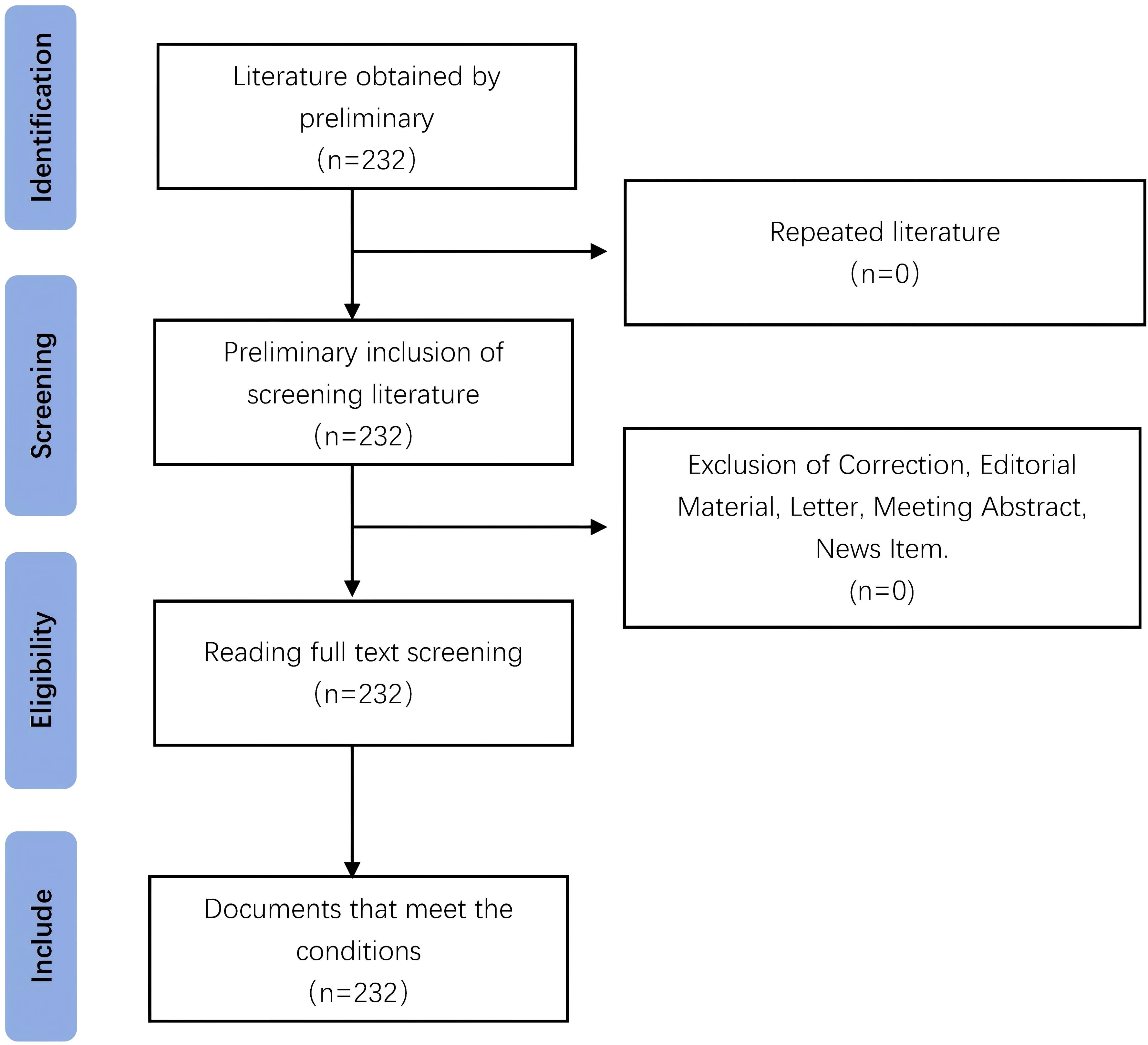
The advanced search function was used to construct search strategy and search in the WOS database. The time range was set from the establishment of the database to February 23, 2025. The retrieval strategy for WOS core collections was: ((((((((((((((((((((TS=(Hepatic Neoplasms)) OR TS=(Hepatic Neoplasm)) OR TS=(Neoplasm, Hepatic)) OR TS=(Neoplasms, Hepatic)) OR TS=(Neoplasms, Liver)) OR TS=(Liver Neoplasm)) OR TS=(Neoplasm, Liver)) OR TS=(Cancer of Liver)) OR TS=(Liver Cancer)) OR TS=(Cancer, Liver)) OR TS=(Cancers, Liver)) OR TS=(Liver Cancers)) OR TS=(Hepatocellular Cancer)) OR TS=(Cancers, Hepatocellular)) OR TS=(Cancer of the Liver)) OR TS=(Hepatocellular Cancers)) OR TS=(Cancer, Hepatocellular)) OR TS=(Hepatic Cancer)) OR TS=(Cancer, Hepatic)) OR TS=(Cancers, Hepatic)) OR TS=(Hepatic Cancers) AND TS=(immune therapy). Articles and reviews in highly cited papers were selected. The highly cited papers analyzed in this study were directly screened and exported from WOS database, and a total of 232 highly cited papers were retrieved. By reading the title and abstract of the papers, and reading the full text, when necessary. 232 studies were finally included after excluding conference papers, paper call notices, irrelevant topics and duplicate papers. See Figure 1 for the specific papers screening process.
2.2 Methods
Developed by Dr. Chaomei Chen’s team, CiteSpace is a Java-based information visualization tool that can be used to reveal the structure and development trends of scientific knowledge (17). By constructing a scientific knowledge map, the software can analyze research hotspots, frontiers, key papers, core authors and institutions, and predict the development direction of the field (18). This study imported the information of 232 highly cited papers into CiteSpace, set the time span from the establishment of the database to February 23, 2025, and the time partition length was 1 year. The “Top N” strategy was adopted for analysis, Pathfinder was selected by Pruning. The annual distribution of highly cited papers, the distribution and cooperation of countries, authors and institutions, keyword distribution and clustering and emergence analysis will be carried out based on the above strategies. Among them, Top N refers to the nodes whose occurrence frequency is greater than or equal to N every year. Pathfinder is a network pruning algorithm, which is mainly used to remove redundant edges in the network and make the knowledge graph clearer and more concise.Cluster analysis can be used to identify the core subject of a discipline, and Burst keywords can reveal the academic frontier in a specific period. Burst keywords refer to terms or keywords that suddenly and significantly increase in frequency in a specific period of time, usually reflecting emerging trends, hot topics or major breakthroughs in the research field (19–21). In the knowledge map generated by CiteSpace (22), N stands for the number of network nodes, E stands for the number of connections, Density stands for the network density, and Centrality reflects the influence and connectivity of a certain node in the knowledge map, such as country, author, institution, keyword, etc., in the network. The quality of the atlas can be evaluated through Modularity Q (Q value) and Silhouette (S value). Q>0.3 indicates significant cluster structure, S>0.5 indicates reasonable clustering and high homogeneity. The knowledge map uses citation rings to represent the influence of corresponding nodes, and the frequency and line thickness reflect the co-occurrence relationship between nodes.
3 Results
3.1 Analysis of annual publications
The highly cited papers analyzed in this study were mainly distributed from 2014 to 2024, with an annual publication volume of 1, 6, 10, 8, 16, 19, 24, 25, 52, 47, and 24, respectively, with the highest number of highly cited papers in 2022. No highly cited papers had been detected in 2025, and the reason may be related to our search time.
3.2 Countries or regions distribution
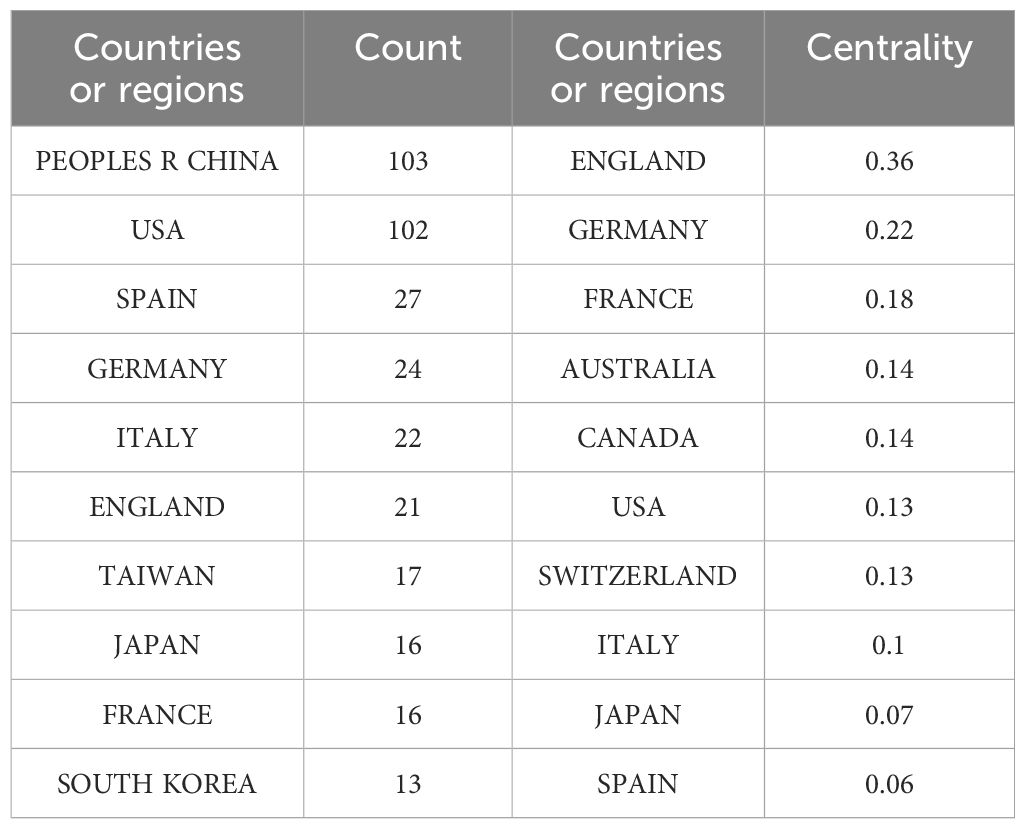
China topped the list with 103 papers, followed by the United States with 102 papers. Other countries or regions with high volumes of publications include Spain, Germany and France. The specific results were shown in Table 1. In terms of centrality, China leaded with a centrality value of 0.45, demonstrating its centrality in the field. The centrality of the United States was also high, reaching 0.36, followed by the United Kingdom and Germany, the specific results were shown in Table 1. The low centrality of countries or regions such as Spain, Italy and Taiwan of China suggested that while their publishing output was significant, their connectivity in global research networks was relatively weak.
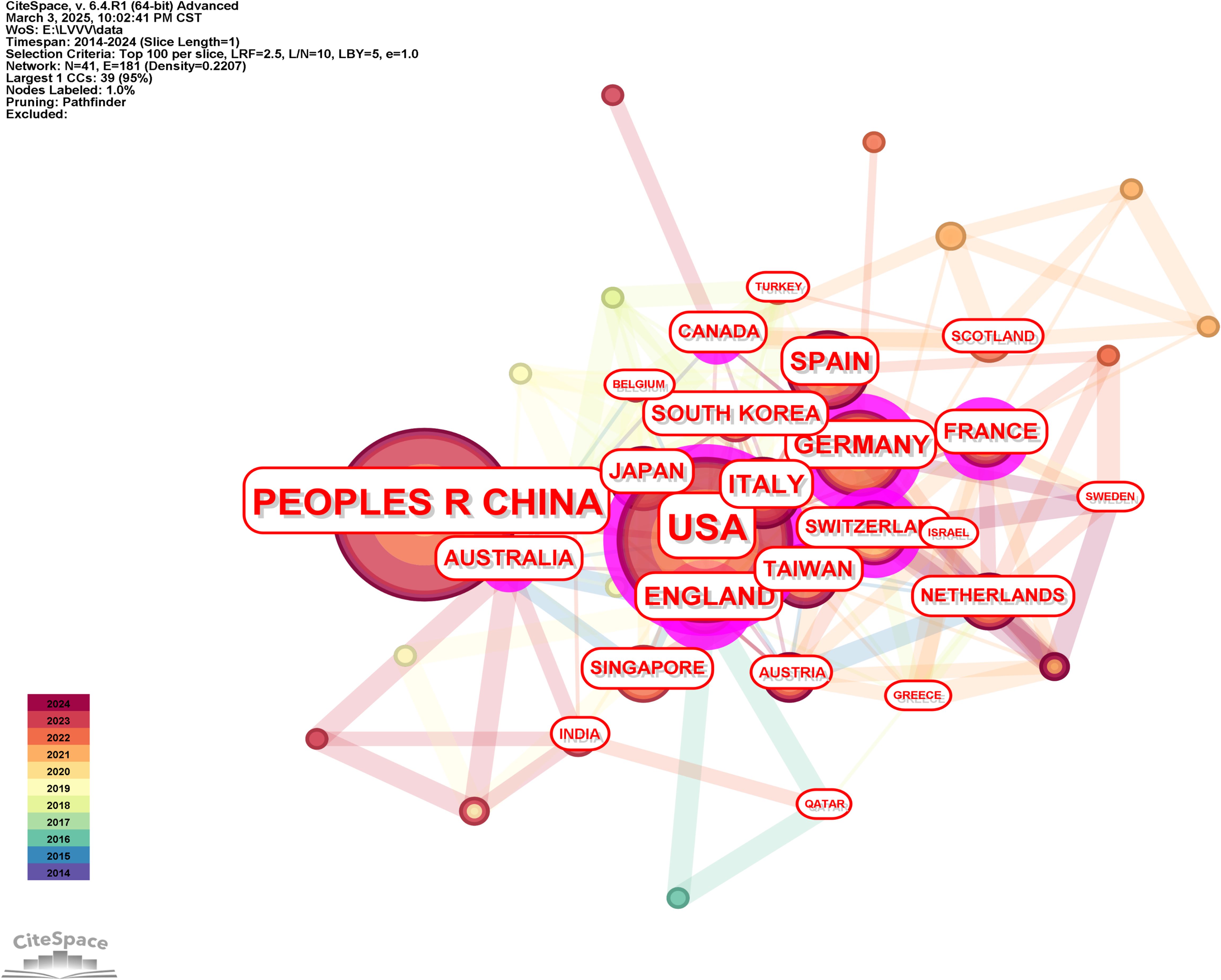
We generated the knowledge map of the national cooperative network through the following strategies (see Figure 2 for the results): The node selected the country, the Top N selected 100, the Timespan selected 2014-2024, and the Pruning selected the Pathfinder. The results in Figure 2 show strong collaboration among China, the United States, and European countries or regions such as Germany, Spain, and France, which form an active research core. It was not difficult to see from the knowledge map that countries or regions with higher outputs, particularly China and the United States, also had higher centrality, indicating that they were key players in global collaboration and research dissemination in the field of liver cancer immunotherapy.
3.3 Authors distribution
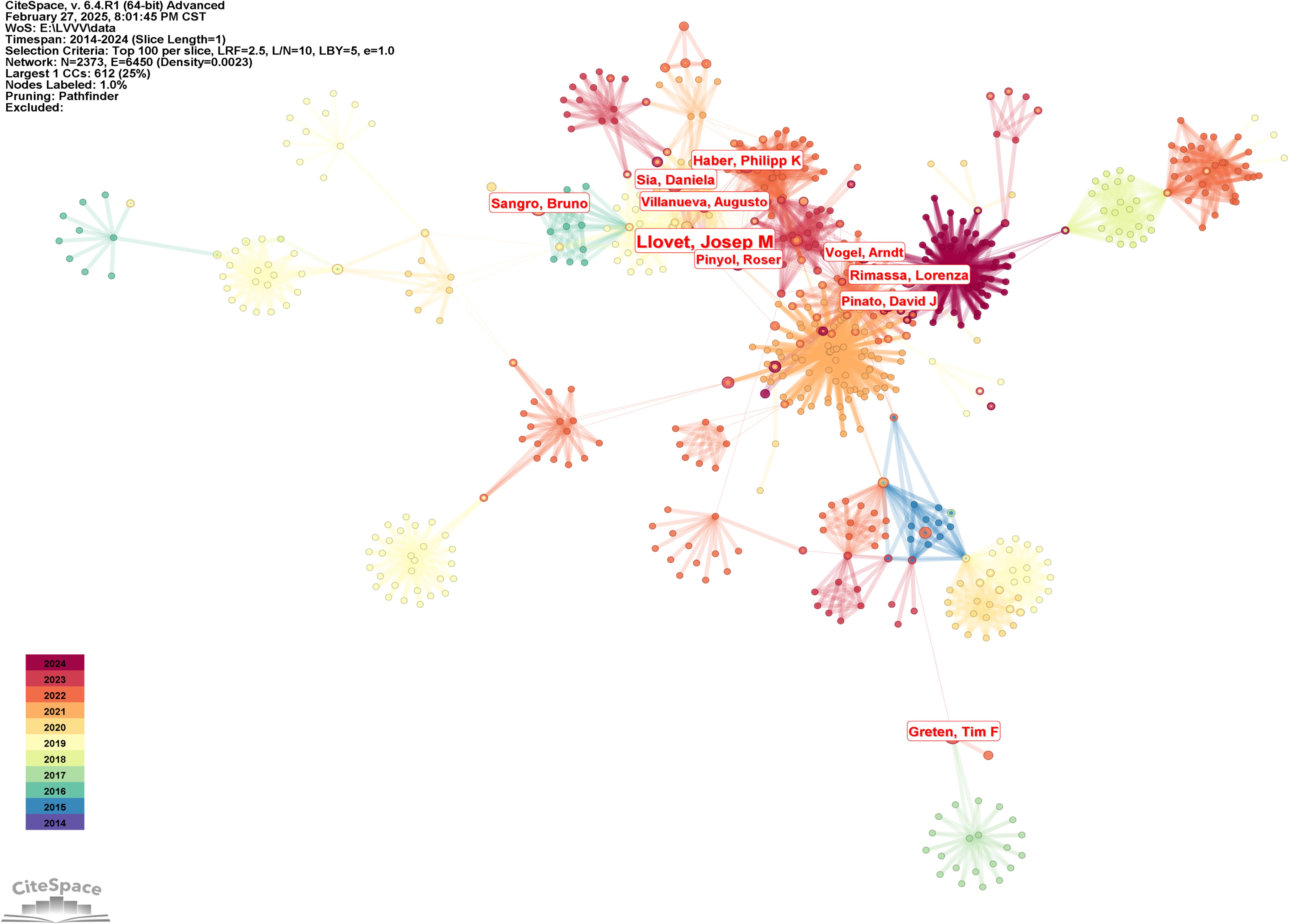
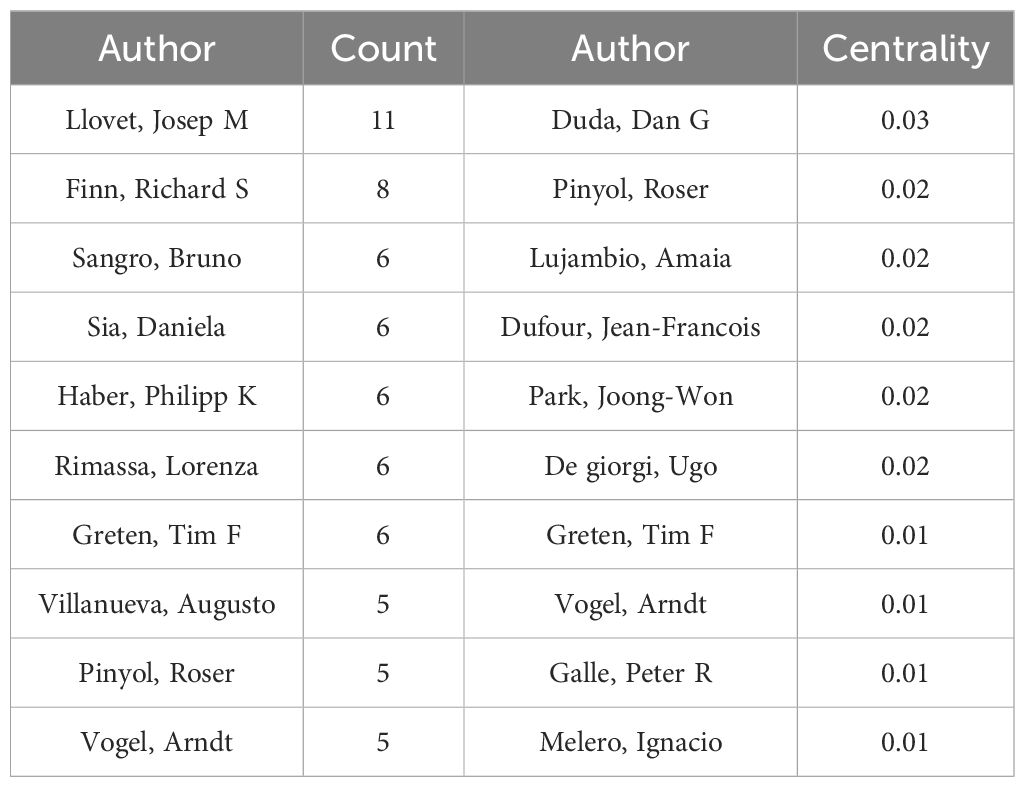
The author cooperation network knowledge map was generated by the following strategies (the results were shown in Figure 3): The node selected the author, the Top N selected 100, the Timespan selected 2014-2024, and the Pruning selected the Pathfinder. The result in Figure 3 shows the collaboration among different authors. The knowledge map reveals multiple groups of authors with a high degree of collaboration, of which Llovet, Josep M and Pinato, David J were central figures in the field, working closely with multiple authors. Other important core authors included Sangro, Bruno, Greten, Tim F, Rimassa, Lorenza, Vogel, Arndt and Haber, Philipp K, among others, with a close network of collaborations among these authors. Among the highly cited authors, Llovet, Josep M, had the highest number of papers with 11, followed by Finn, Richard S, Sangro, Bruno, Sia, Daniela, Haber, Philipp K, Rimassa, Lorenza, Greten, Tim F, Villanueva, Augusto, Pinyol, Roser, Vogel, Arndt. Duda, Dan G had the highest centrality (0.03), followed by Pinyol, Roser, Lujambio, Amaia, Dufour, Jean-Francois, Park, Joong-Won, De giorgi, Ugo, Greten, Tim F, Vogel, Arndt, Galle, Peter R, Melero, Ignacio, the number of authors’ publications and the results of centrality were shown in Table 2.
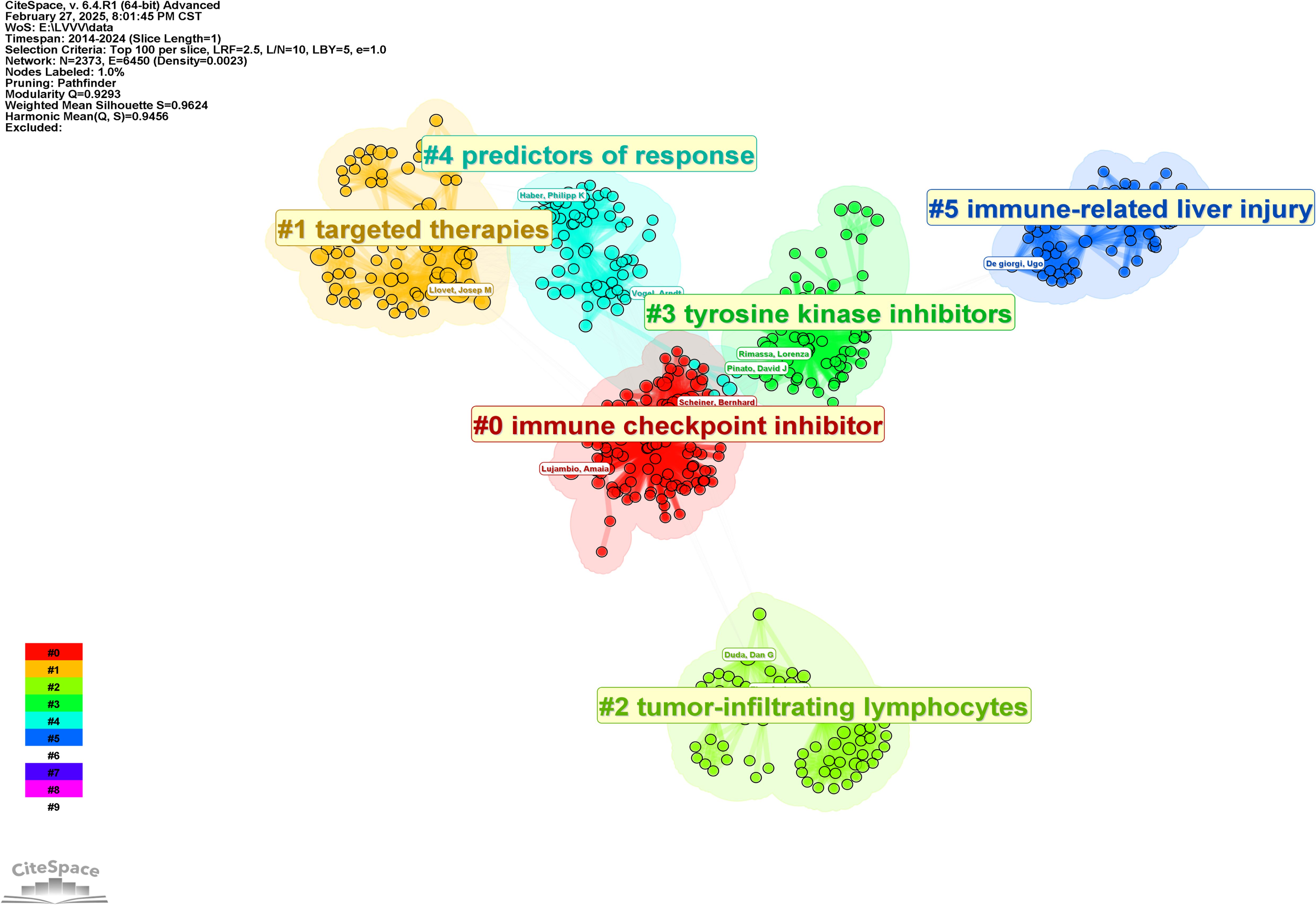
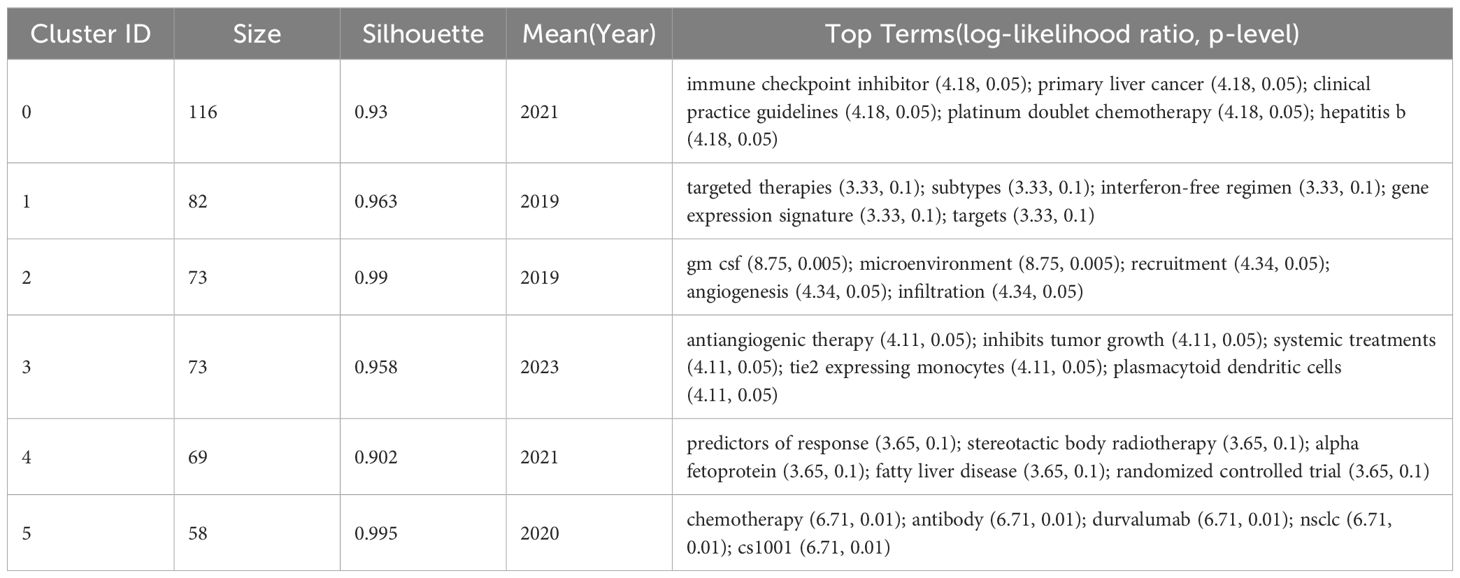
In order to further analyze the research direction of the collaboration between the authors, we conducted cluster analysis for the keywords based on Figure 3. The result was shown in Figure 4. The Q value is 0.9293 and the S value is 0.9624, indicating a good clustering effect. A total of 6 thematic clusters were obtained. The thematic clustering labels were immune checkpoint inhibitor, targeted therapies, tumor-infiltrating lymphocytes and tyrosine kinase inhibitors, predictors of response and immune-related liver injury, which indicated that the research directions of the authors’ cooperation mainly focused on the above 6 aspects, and the specific clustering information was shown in Table 3.
3.4 Institutions distribution
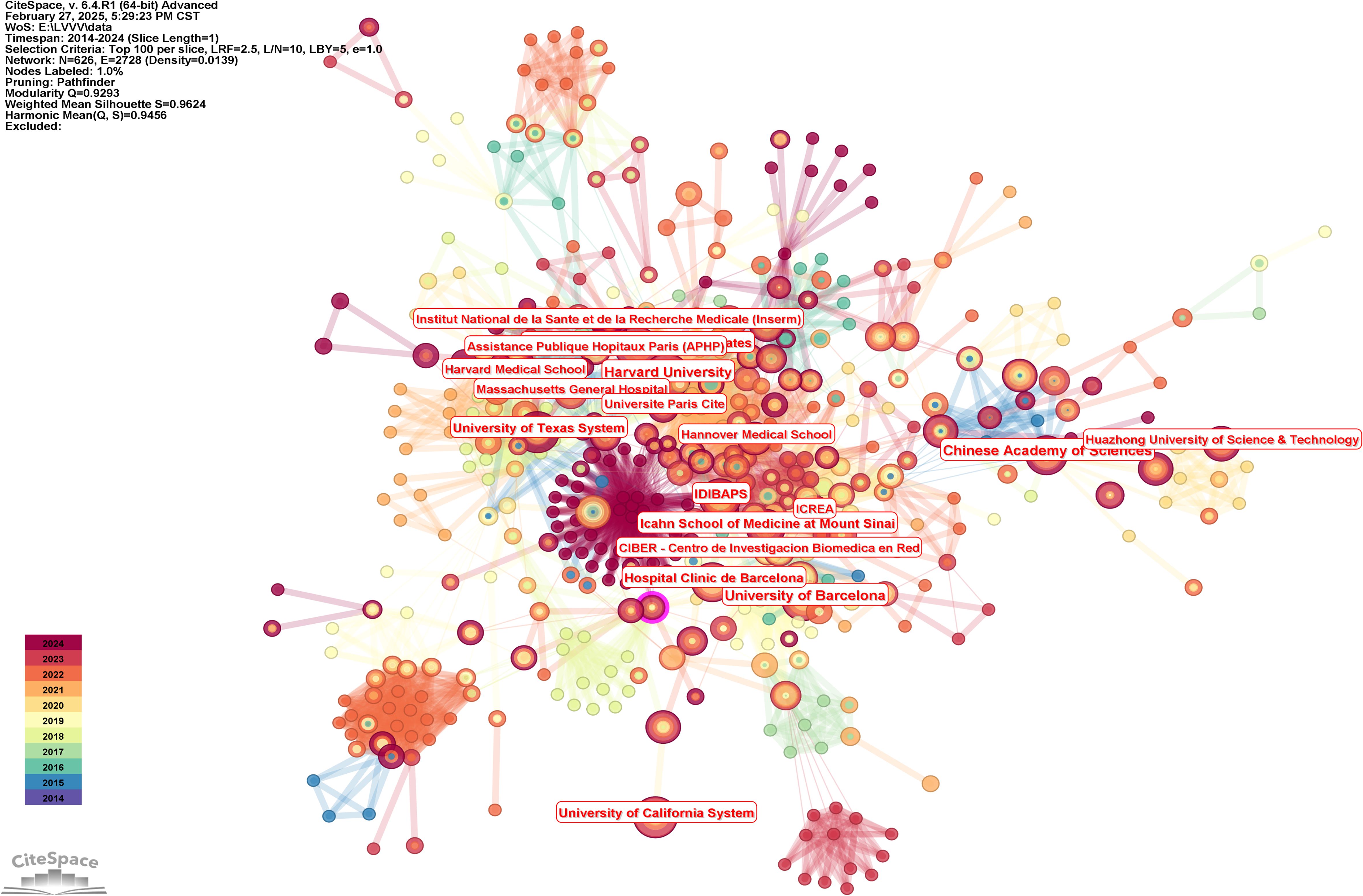
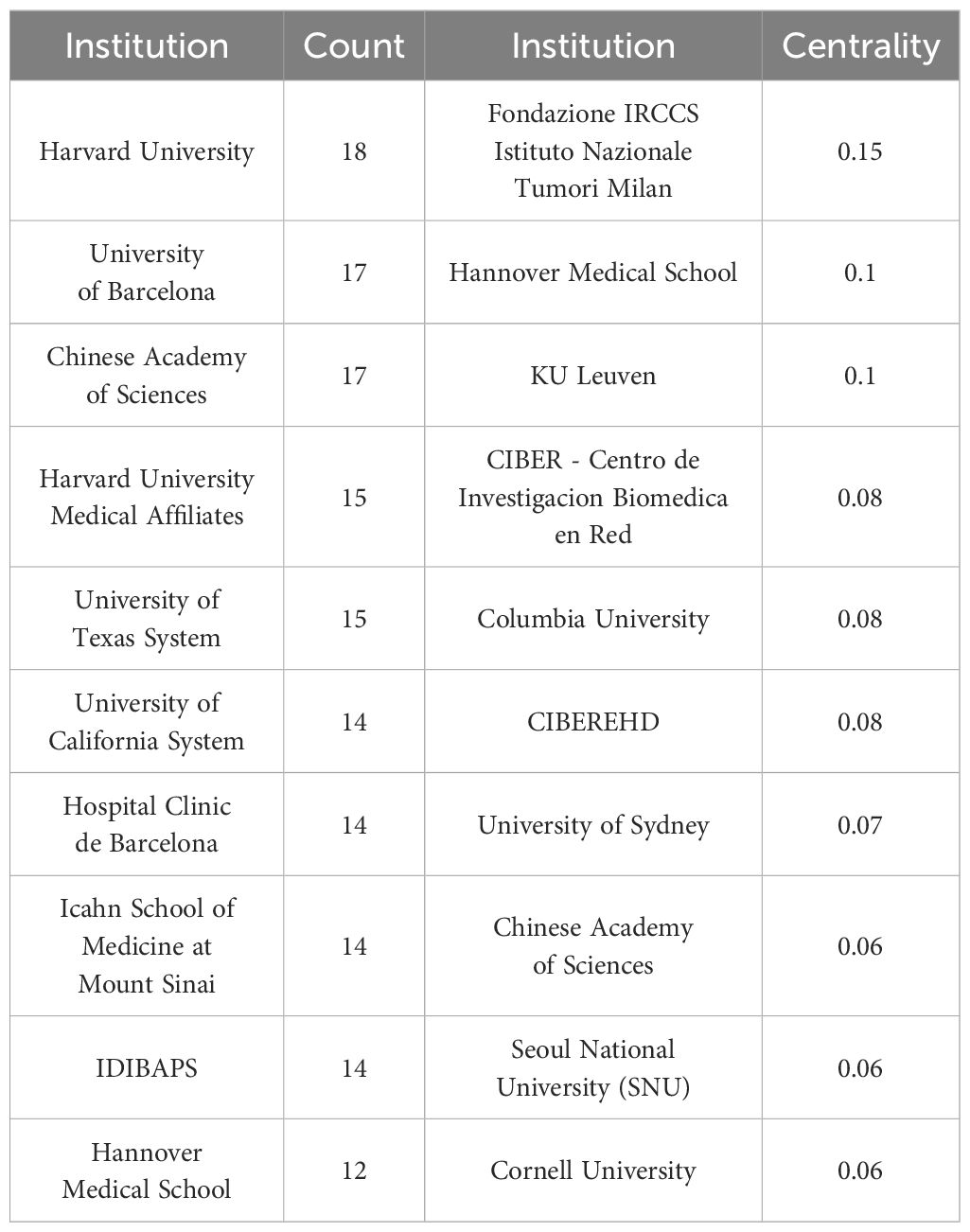
We generated the knowledge map of the institutional cooperation network through the following strategies (see Figure 5 for the results): the node selected institution, the Top N selected 100, the Timespan selected 2014-2024, and the Pruning selected the Pathfinder. In general, the knowledge map center of institutional cooperation network shows a centralized trend, and scattered cooperative groups can be seen around it. Among them, Harvard University, University of Barcelona and Chinese Academy of Sciences occupy the core positions in the cooperation network, demonstrating their importance in the global cooperation network. In terms of publication volume, Harvard University ranked first with 18 papers, followed by the University of Barcelona and the Chinese Academy of Sciences, both with 17 papers. Other institutions with high volume of publications included the Harvard University Medical Affiliate, the University of Texas System, and the University of California System. In terms of centrality, Harvard University’s centrality was 0.2, showing its leading role in the field of liver cancer immunotherapy. Other institutions with high centrality included Fondazione IRCCS Istituto Nazionale Tumori Milan and Hannover Medical School. The University of Sydney and Seoul National University had relatively low centrality. The number of documents issued by institutions and the results of centrality were shown in Table 4.
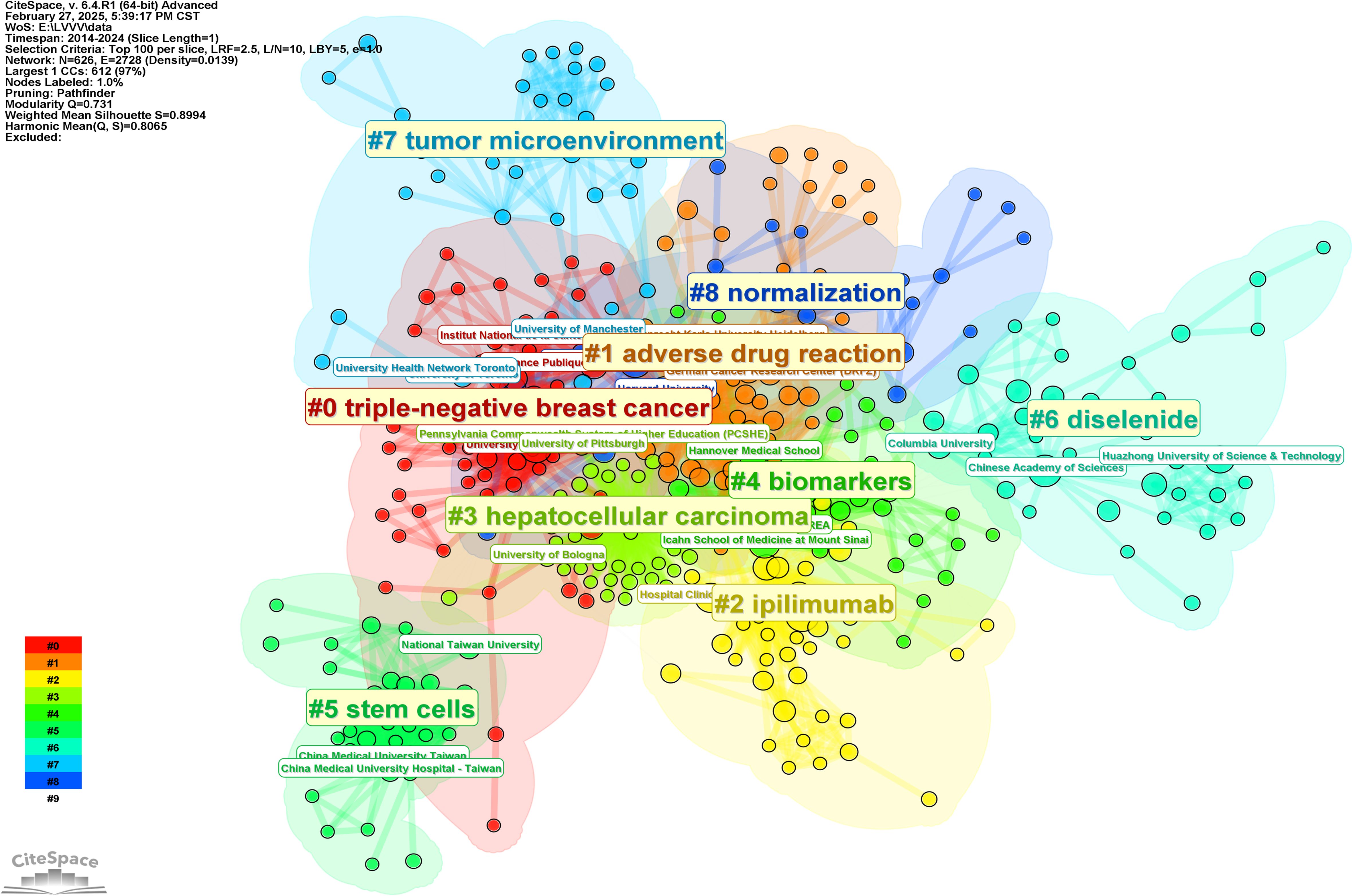
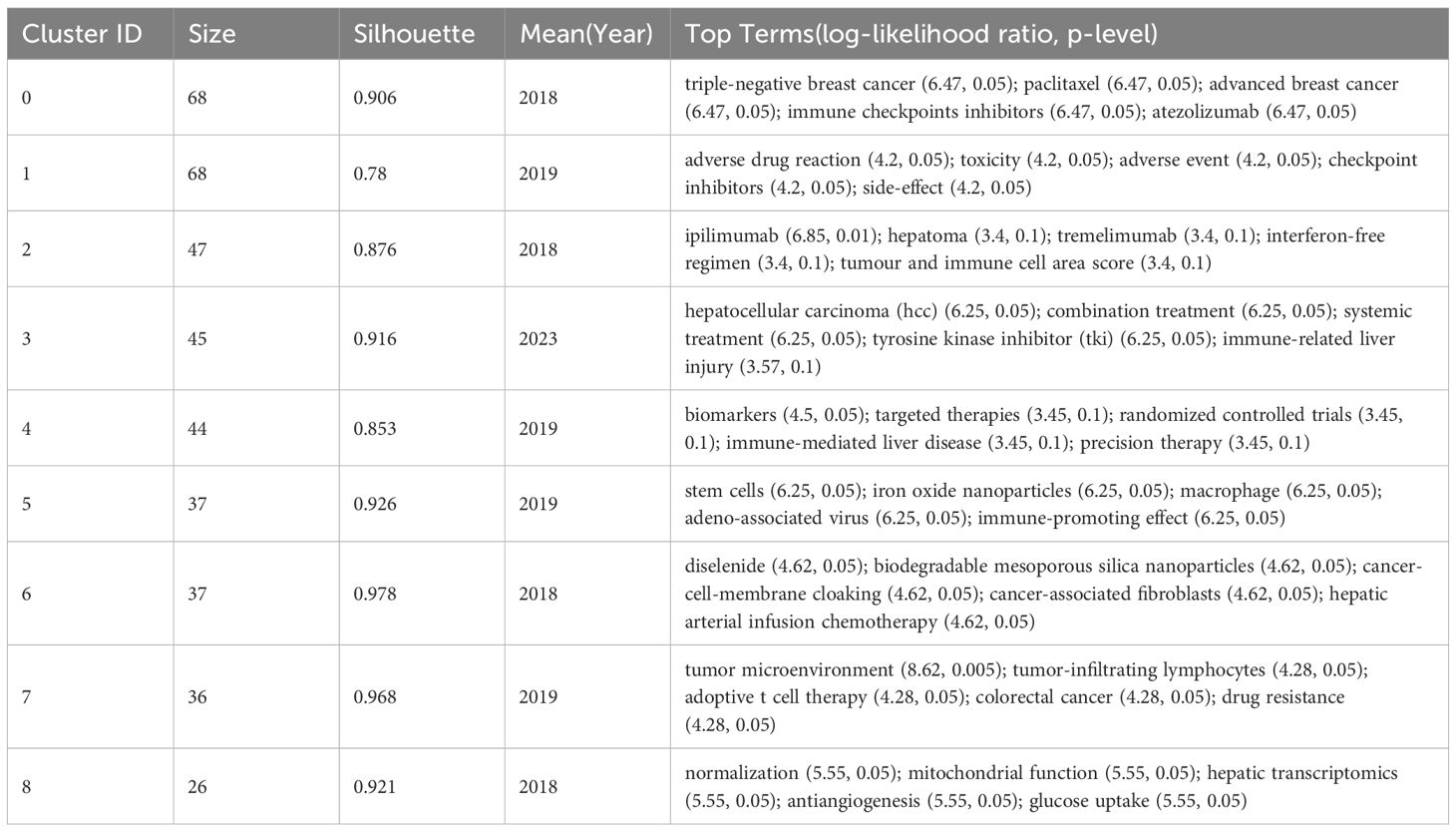
In order to further analyze the research direction of the cooperation among the institutions, we conducted cluster analysis for the keywords based on Figure 5. The result was shown in Figure 6. The Q value of the map is 0.731 and the S value is 0.8994, indicating a good clustering effect. A total of 9 thematic clusters were obtained. The thematic cluster labels were triple-negative breast cancer, adverse drug reaction, ipilimumab, hepatocellular carcinoma, biomarkers and stem cells, diselenide, tumor microenvironment, and normalization showed that the research directions of cooperation among institutions mainly focus on the above 9 aspects. The specific information of clustering was shown in Table 5.
3.5 Keywords distribution
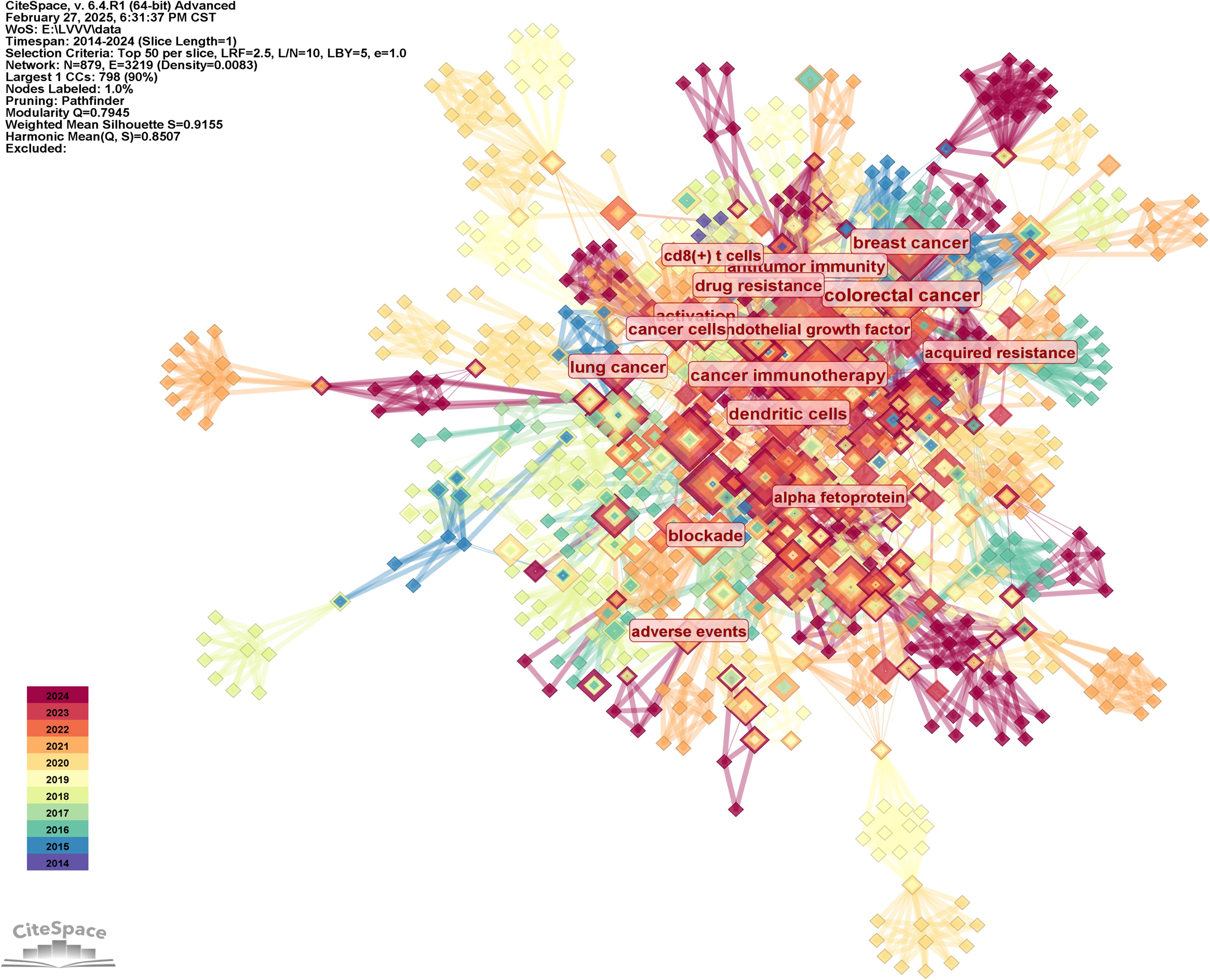
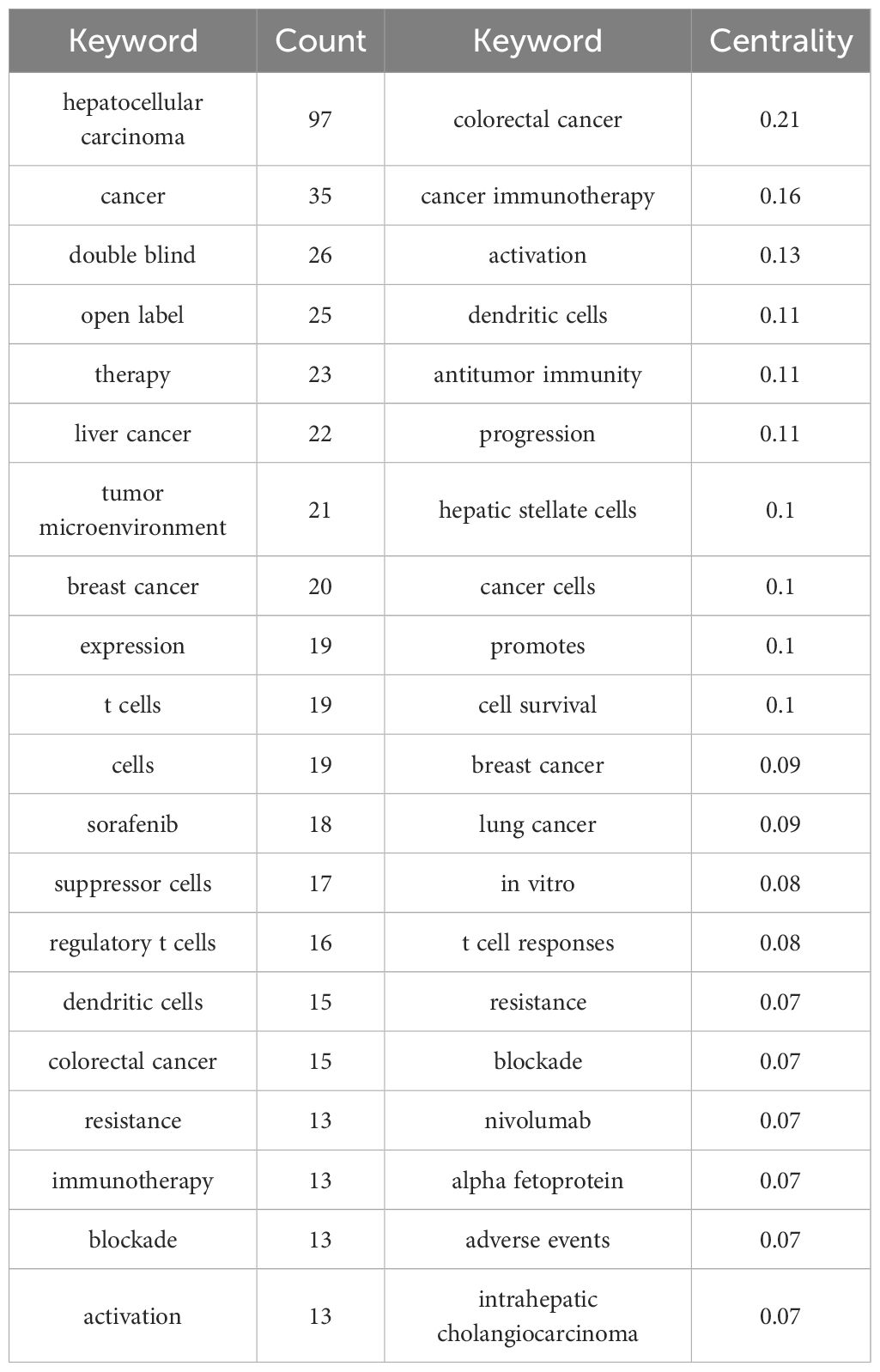
We generated the keyword distribution knowledge map by following strategies (the results were shown in Figure 7): the node selected keyword, the Top N selected 50, the Timespan selected 2014-2024, and the Pruning selected the Pathfinder. It was not difficult to see from Figure 7 that the distribution of keywords was centered, which indicated that the research directions of highly cited authors analyzed in this study were similar. They were working together on some important research topics. The most frequently used keywords were hepatocellular carcinoma (97 times), followed by cancer, double blind, open label, therapy, liver cancer, tumor microenvironment, breast cancer, expression, t cells, etc. The keyword with the highest centrality was colorectal cancer, with a centrality of 0.21, followed by cancer immunotherapy, activation, dendritic cells, antitumor immunity, progression, hepatic stellate cells, cancer cells, promotes. The frequency and centrality of cells, promotes, and keywords were shown in Table 6.
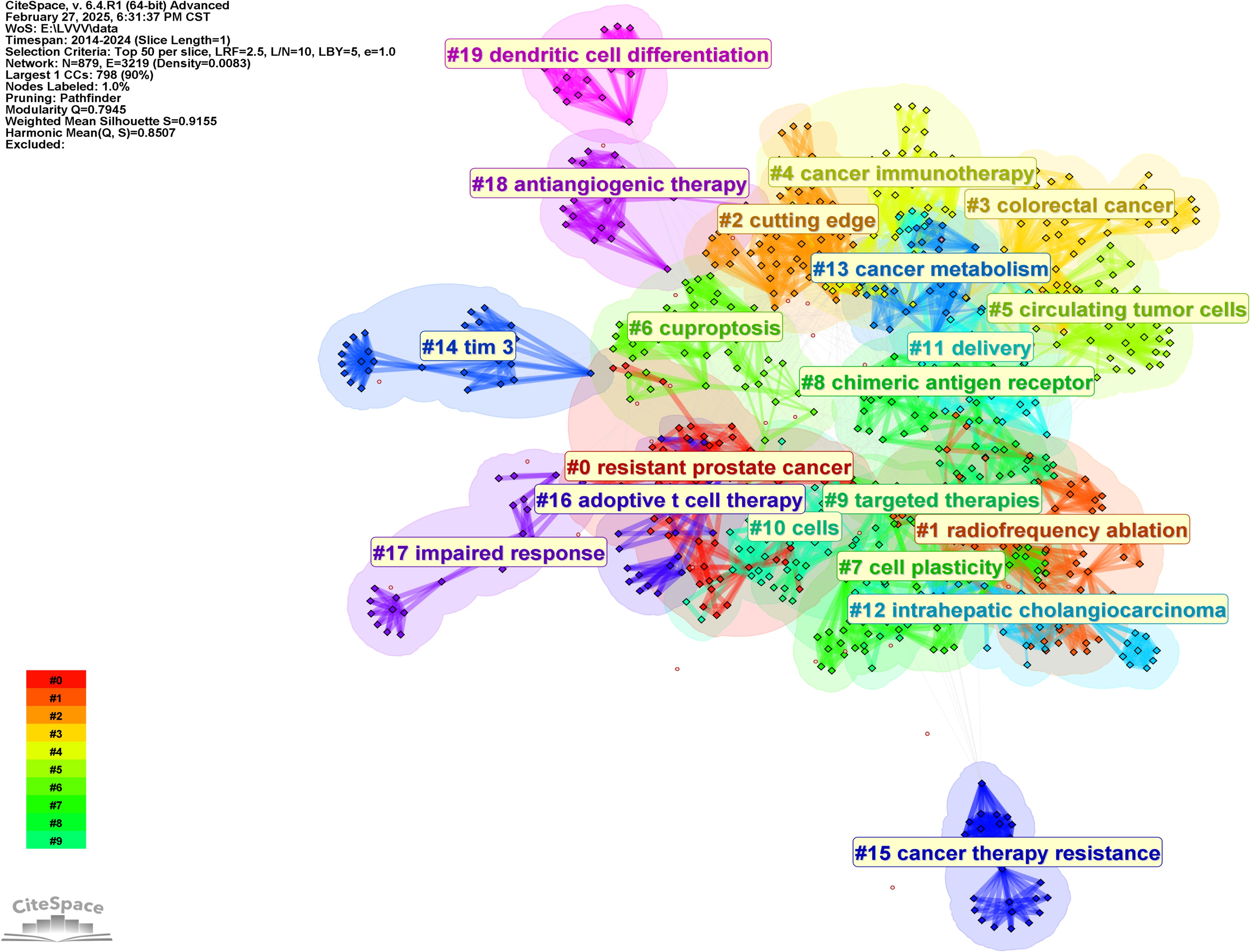
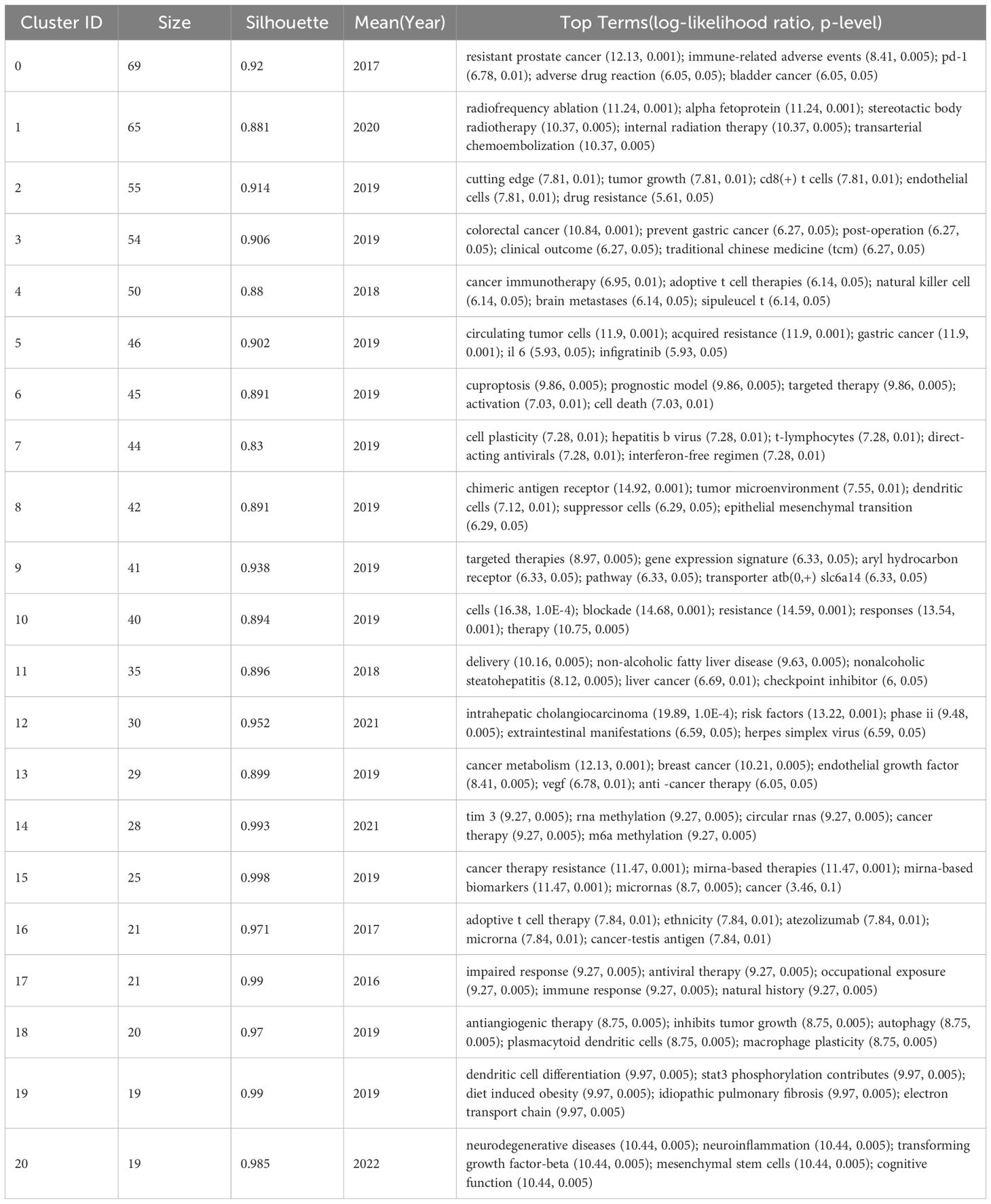
The keywords in Table 6, to a certain extent, represent the research direction of the highly cited authors analyzed in this study. In order to further analyze their research direction, we performed cluster analysis on the keywords based on Figure 7. The result was shown in Figure 8. The Q value of the map is 0.7945 and the S value is 0.9155, indicating good clustering effect. The thematic cluster labels were resistant prostate cancer, radiofrequency ablation, cutting edge, colorectal cancer and cancer immunotherapy, circulating tumor cells, cuproptosis, cell plasticity, chimeric antigen receptor, targeted therapies, cells, delivery, intrahepatic cholangiocarcinoma, cancer metabolism, tim 3, cancer therapy resistance, adoptive t cell therapy, impaired response, antiangiogenic therapy, dendritic cell differentiation, neurodegenerative diseases, and specific results were shown in Table 7.
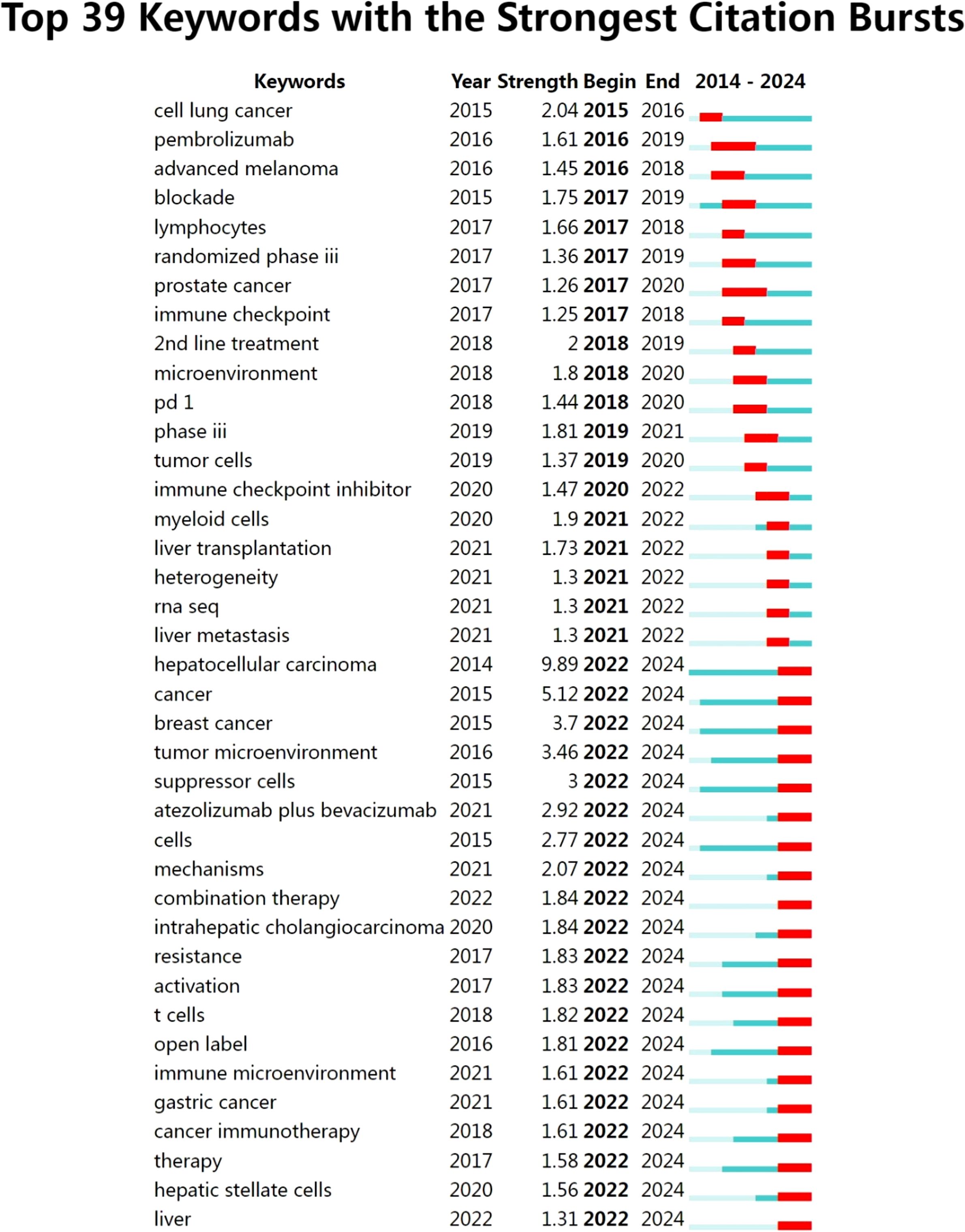
In order to further analyze the research frontiers of highly cited authors analyzed in this study, we conducted keywords burst analysis of keywords, and a total of 39 burst keywords were obtained. They were cell lung cancer, pembrolizumab, advanced melanoma, blockade, lymphocytes, randomized phase iii, prostate cancer, and immune checkpoint, 2nd line treatment, microenvironment, PD-1, phase iii, tumor cells, immune checkpoint inhibitor, myeloid cells, liver transplantation, heterogeneity, rna seq, liver metastasis, hepatocellular carcinoma, cancer, breast cancer, tumor microenvironment, suppressor cells, atezolizumab plus bevacizumab, cells, mechanisms, combination therapy, intrahepatic cholangiocarcinoma, resistance, activation, t cells, open label, immune microenvironment, gastric cancer, cancer immunotherapy, therapy, hepatic stellate cells, liver, and the specific results were shown in Figure 9.
4 Discussion
4.1 Analysis of annual publications
The number of highly cited papers in the field of liver cancer immunotherapy increased year by year since 2014, especially reaching the highest number of published papers in 2022. This trend reflects that liver cancer immunotherapy as an emerging research field had gained increasing attention in recent years. The peak of papers output in 2022 may be related to the publication of several key research results in this field, as well as the clinical application of new immunotherapy approaches such as immune checkpoint inhibitors. With the continuous progress of liver cancer immunotherapy technology, related research results continue to emerge, which promotes the academic community’s attention and investment in this field.
4.2 Analysis of Courtiers or regions cooperation
In this study, it was found that China and the United States ranked first in the number of highly cited papers publications in the field of liver cancer immunotherapy. This was mainly due to China’s rich clinical sample resources and rapid development of immunotherapy research system, as well as the long-term advantages of the United States in basic scientific research accumulation, technological innovation and international cooperation network. China, in particular, with its unique clinical samples and advantages in immunotherapy research, had become one of the leading countries in the field globally. The United States, with its long-term research accumulation and strong research and development base, occupied a core position in the global research network. At the same time, countries such as Spain, Germany and France also played an important role in liver cancer immunotherapy research. In addition, from the perspective of centrality analysis, China’s centrality value was 0.45, which was significantly ahead of other countries, indicating its centrality in the global research network. The United States followed closely with a centrality value of 0.36, while the United Kingdom and Germany had a lower centrality, indicating that these countries’ research activities in the field of liver cancer immunotherapy, while important, were less influential in the global collaborative network compared to China and the United States. Through the construction of the knowledge map of the national cooperation network, we further revealed the cooperation model of the world in the field of liver cancer immunotherapy. For example, China had formed close cooperation with the United States and some countries in Europe, which had an important impact on promoting the research and clinical application of liver cancer immunotherapy worldwide.
4.3 Research direction analysis of important authors and institutions
The author of the most published paper was Llovet, Josep M. His research mainly focused on the development and application of molecular targeted therapy and immunotherapy for liver cancer. His research team had participated in a number of studies on systematic treatment of liver cancer, especially the clinical trials of targeted drugs and immune checkpoint inhibitors for advanced liver cancer (23). The combination treatment of atizumab (anti-PD-L1) and bevacizumab (anti-VEGF) significantly improved the survival rate of patients in the IMbrave150 trial, becoming a new standard of first-line treatment for liver cancer (9). Llovet, Josep M’s research team was also involved in the molecular classification of liver cancer and precision medicine. Through genomic and transcriptomic analysis, they revealed the molecular heterogeneity of liver cancer and proposed a classification system for liver cancer based on molecular characteristics. For example, one of their studies showed that liver cancer can be divided into different subtypes according to its molecular characteristics, and these subtypes had different responses to different therapeutic strategies (such as targeted therapy and immunotherapy) (24), which provided a theoretical basis for the individualized treatment of liver cancer (25). In addition, the research team of Llovet, Josep M also investigated the combination of local treatments (such as radiofrequency ablation, transarterial chemoembolization TACE) and systemic treatment strategies. One of their studies showed that local treatment can induce the release of tumor antigen and enhance the anti-tumor response of the immune system, thus improving the efficacy of systemic treatment (26). TACE combined with PD-1 inhibitor had shown good efficacy in patients with liver cancer, especially in patients with advanced liver cancer (27).
The author with the highest centrality value was Duda, Dan G. His research direction mainly focused on the interaction between tumor microenvironment and immunotherapy. His research team revealed the heterogeneity of immune cells in TME and its role in immunotherapy through single-cell RNA sequencing and spatial transcriptomics technology (28). Another study of them also showed that the expression levels of immunosuppressive cells (such as myelogenic suppressor cells MDSCs) and immune checkpoint molecules in TME were closely related to the efficacy of immunotherapy (29). The research of the Duda, Dan G team also involved the combination of immunotherapy and targeted therapy strategies for liver cancer. They validated the efficacy of multiple combination treatment strategies through preclinical models and clinical trials. For example, the combination of PD-1 inhibitors with anti-angiogenic agents (such as bevacizumab) had shown significant survival benefits in patients with liver cancer (9). In addition, they investigated the combination of other targeted drugs (such as FGFR inhibitors) with immunotherapy and found that this combination therapy can significantly improve the treatment outcome in patients with liver cancer (30).
Harvard University’s research in the field of liver cancer immunotherapy had formed a global cooperative network, and had established close cooperative relations with many authoritative institutions such as the National Institutes of Health (NIH), the European Association for the Study of Liver Diseases (EASL) and Zhongshan Hospital affiliated to Fudan University in China. Its research scope covered the whole chain from basic mechanism to clinical transformation, and it had made breakthroughs in the following core directions: optimization of immune checkpoint inhibitors (31, 32), interaction regulation of metabolism and immunity (32–35), and development of novel cell therapies (36, 37). Specifically, in terms of immune microenvironment regulation, the Harvard research team had made important discoveries. They revealed that liver cancer cells inhibit T cell function by hijacking glucose metabolism (such as lactic acid accumulation), and that interferon alpha (IFNα) combined with PD-1 inhibitors can reverse this phenomenon, significantly improving the mitochondrial activity and anti-tumor efficacy of CD8+ T cells (38). More interestingly, the team also found that iron death inducers can enhance T cell infiltration by regulating glutathione metabolism, but the risk of hepatotoxicity needs to be carefully balanced (39). In addition, targeting the PPAR-γ pathway reduced the accumulation of myelo-derived suppressor cells (MDSCs), thereby reshaping the immunosuppressive microenvironment (40). In terms of technological innovation, the Harvard team also showed strong research and development strength. For example, nitric oxide nanocarriers (NanoNO) developed at Harvard Medical School were able to consistently release NO and efficiently deliver it to hepatocellular carcinoma. Low-dose NanoNO can not only normalize tumor blood vessels, but also significantly improved the delivery and effectiveness of chemotherapy drugs related to tumor necrosis factor, apoptosis induction, and ligand-based therapy in primary tumors and metastases (41).In addition, the Harvard team also made a breakthrough in the exploration of novel immune checkpoint targets. They revealed that fibrinin-as-protein 1 (FGL1) was the main functional ligand of the immunosuppressive receptor LAG-3. FGL1, which was secreted at low levels in normal liver but was abnormally high expressed in a variety of cancers, inhibits T cell activation and promotes tumor immune escape by binding LAG-3. Blocking FGL1-LAG-3 interaction can significantly enhance anti-tumor T cell responded and produced synergistic effects with anti-PD-1 therapy, providing a potential target for the development of novel cancer immunotherapies (42). At the same time, the Harvard team also delved into the mechanisms of 4-1BB (CD137) as a target for cancer immunotherapy. They summarized the anti-tumor function of urelumab through activation of T cells and NK cells, analyzed the efficacy and hepatotoxicity challenges of urelumab and utomilumab in clinical trials, and proposed strategies such as local administration, bisspecific antibodies and masking techniques to optimize efficacy and reduce systemic toxicity (43). In response to immunotherapy-induced hepatotoxicity, the Harvard team found through single-cell sequencing and animal models that CXCR3+CD8+ effector memory T cells and type 1 conventional dendritic cells (cDC1) were key promoters of response, while myeloid inhibitory subsets were associated with toxicity. Targeting TNFR2 selectively enhanced the anti-tumor immune response while avoiding irAEs exacerbation, which provided a new strategy for the development of safer combination immunotherapies (44). Finally, the Harvard team also systematically summarized the mechanism of action of RING finger ubiquitin ligase (E3s) in cancer and the bidirectional effects of gene variants. By regulating key processes such as cell cycle, DNA repair, signal transduction, and hypoxia response, these enzymes can both promote tumor development as oncogenes (e.g. MDM2) and inhibit cancer as tumor suppressor genes (e.g. BRCA1, VHL). Its dysfunction was closely related to the occurrence of cancer, and had become the focus of targeted therapy (such as small molecule inhibitors), but the clinical efficacy still needs to be further verified (45).
4.4 Important research hotspots and frontiers
Based on the cooperative knowledge map and specific clustering information of authors and institutions, and through reading specific papers, we found that the current cooperative research directions in the field of liver cancer immunotherapy mainly focus on the following directions: First of all, the combined treatment strategy of immune checkpoint inhibitors (ICIs) was the core direction. For example, atizumab combined with bevacizumab (IMbrave150 test) significantly extended the overall survival of patients and became the new standard of first-line treatment for liver cancer (9). The combination of PD-1 inhibitors and CTLA-4 inhibitors (such as nabuliumab combined with ipilimumab) had shown a synergistic effect in advanced liver cancer (23). Secondly, the regulatory mechanism of tumor microenvironment (TME) had attracted much attention. Studies had revealed the immunosuppressive effect of myeloid suppressor cells (MDSCs) and immune checkpoint molecules (such as PD-L1) in TME. And reshaped the immunosuppressive environment by targeting metabolic reprogramming (such as the PPAR-γ pathway) or inhibiting angiogenic factors (such as VEGF) (29, 46). Thirdly, the exploration of novel immunotherapy targets had promoted the development of personalized therapy, such as the preclinical studies of LAG-3/FGL1 axis and 4-1BB agonists showing that they can enhance the antitumor activity of T cells (42, 43). Fourthly, the combination of local therapy and systemic immunotherapy had become a hot spot. Radiofrf ablation or TACE combined with PD-1 inhibitors can enhance the systemic immune response by releasing tumor antigens (7), while radiotherapy combined with immunotherapy can improve the therapeutic effect by inducing immunogenic cell death (47). In addition, the development of biomarkers and precision therapy were the key directions of cooperation. Researchers had identified CXCR3+CD8+T cells and CD11c+ antigen-presenting cells in TME through single-cell sequencing and genomic analysis, and explored the application of circulating tumor DNA (ctDNA) in efficacy monitoring (48, 49). Future studies need to further optimize joint strategies, address immune resistance and establish interdisciplinary cooperation networks to promote liver cancer immunotherapy towards precision and individualization.
In the highly cited papers analyzed in this study, Cancer, Immunotherapy, Tumor microenvironment appeared frequently, which may further emphasize the immune escape mechanism and the influence of microenvironment on the immunotherapy effect of liver cancer (50–53). In addition, Double blind and Open label were also high-frequency words, which indicated that randomized controlled trials (RCTs) and open label studies were common research methods for liver cancer immunotherapy at present. However, high-frequency keywords such as Therapy and Sorafenib may indicated that targeted therapeutic drugs played an important role in the research of liver cancer immunotherapy (54). High-frequency keywords T cells, Dendritic cells, Regulatory T cells, etc. may indicated the potential role of these cells in immunotherapy (55–57), especially in immune escape and immune tolerance of liver cancer. The Resistance, Blockade may reflect the challenge of immunotherapy. Keywords Breast cancer and Colorectal cancer also appeared in multiple papers, indicating that liver cancer immunotherapy was closely linked to other cancers (58, 59).
The keywords with high centrality not only reflected the core direction of liver cancer immunotherapy research, but also revealed the hot topics in the current field. Among the high-citation centers analyzed in this study. Colorectal cancer had the highest centrality value, which may be related to the metastatic relationship among colorectal cancer and liver cancer and similar immune escape mechanisms (60, 61). In addition, the centrality of Breast cancer, Lung cancer, and Intrahepatic cholangiocarcinoma was also high, indicating that studies on liver cancer often overlap with immunotherapy studies on these cancer types (28, 62). Tumor microenvironment, Hepatic stellate cells, Regulatory T cells, and Suppressor cells may be involved in the study of tumor microenvironment and immune escape. Cancer immunotherapy, Activation, Blockade, Immunotherapy, Antitumor immunity, T cell responses, Nivolumab mainly reflected the key mechanisms and therapies of immunotherapy (32, 63–65), especially immune checkpoint inhibition, T cell activation, immune escape and drug resistance. Alpha fetoprotein, Resistance, promotion and Cell survival may be closely related to biomarkers and drug resistance in liver cancer immunotherapy. In vitro and Cells may involve the design of experiments and the application of research methods in immunotherapy.
Based on the results of keyword distribution and cluster analysis, and subsequent reading of specific papers, we found that the main research directions of liver cancer immunotherapy include the efficacy and mechanism of immune checkpoint inhibitors (ICI) in liver cancer (23, 66, 67), synergistic effect of local ablation and immunotherapy (47, 68), non-alcoholic steatohepatitis (NASH) and immunotherapy resistance to liver cancer (34, 55, 69), molecular typing and targeted therapy of intrahepatic cholangiocarcinoma (iCCA) (24, 70), Ferroptosis and treatment of liver cancer (56, 71), gut microbiome and liver cancer immunotherapy response (72), management of immune-related adverse events (irAEs) (73–75), clinical application of novel biomarkers (such as CRAFITY score) (67, 76), exploration of CAR-T cell therapy in liver cancer (73, 77, 78), regulation of myeloid suppressor cells (MDSC) in the tumor microenvironment (29, 40), tumor metabolic reprogramming and immunotherapy response (involving IDH mutations and glycometabolic pathways) (38, 49, 55), synergistic effects of anti-angiogenic therapy and immunotherapy (e.g., Renvastinib combined with pabolizumab) (7, 23, 79), Application of immunotherapy in liver transplantation patients (80, 81), clinical exploration of neoadjuvant immunotherapy for liver cancer (25, 82), mechanism of T cell depletion and immune checkpoint resistance (44, 48), preclinical study of oncolytic virus combined immunotherapy (41, 83), the immunomodulatory role of tumor-associated fibroblasts (CAF) (84, 85).
Burst keywords that persisted until 2024 included hepatocellular carcinoma, cancer, breast cancer, tumor microenvironment, suppressor cells, atezolizumab plus bevacizumab, cells, mechanisms, combination therapy, intrahepatic cholangiocarcinoma, resistance, activation, t cells, open label, immune microenvironment, gastric cancer, cancer immunotherapy, therapy, hepatic stellate cells, liver. These keywords represented that the research of liver cancer immunotherapy is also developing in the direction of personalized treatment, microenvironment regulation and multi-mode combination therapy, which was also the frontier hot spot of liver cancer immunotherapy. Subsequent studies can further promote the research and development of liver cancer immunotherapy based on these key contents.
Taking the above content into account, it was not difficult to see that the research on liver cancer immunotherapy showed a multidimensional progress trend, covering many aspects such as immunotherapy strategy, tumor microenvironment, cancer types, drug resistance and experimental methods. Immunotherapy research not only focused on the unique immune escape mechanism of liver cancer, but also drawed on the experience of other cancer types to explore how to overcome drug resistance, improve treatment effectiveness, and further delved into the regulation of tumor microenvironment. These research directions provided theoretical support for the development of liver cancer immunotherapy and pointed out the focus of future research.
5 Conclusion
In this paper, the bibliometrics method and CiteSpace software were systematically used to comprehensively analyze the highly cited papers in the field of liver cancer immunotherapy, and revealed the research hotspots, international cooperation pattern and future trend in this field. By constructing a collaborative network map of countries, authors and institutions, the central position of China and the United States in global research was clarified, and key authors and their research directions were identified. In addition, through keyword clustering and burst analysis, this paper accurately captured cutting-edge research directions such as immune checkpoint inhibitor combination therapy and tumor microenvironment regulation, providing a new perspective and theoretical support for future research. This multi-dimensional analysis method not only had filled the gap of systematic research in the field of liver cancer immunotherapy, but also provided an important reference for the academic community and clinical practice.
6 Limitations
Only highly cited papers related to liver cancer immunotherapy in the Web of Science database were included in this study, and data from other databases were not included at present. The reason for this was that Citespace software can currently only import data from a single database. However, compared with other databases, this software was more efficient in analyzing data in Web of Science (86, 87), which was also the reason why we choose data in Web of Science database for analysis. But to address this limitation, future research could explore integrating data from multiple databases to obtain a more comprehensive and diverse view of research. At the same time, researchers can also consider using other analytical tools or methods to process data from different databases to ensure the comprehensiveness and reliability of research results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
PS: Data curation, Formal analysis, Investigation, Software, Writing – original draft. YQH: Data curation, Formal analysis, Writing – review & editing. JY: Data curation, Formal analysis, Writing – review & editing. YH: Funding acquisition, Supervision, Writing – review & editing. YX: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Zhejiang Province (LQN25H160049), Natural Science Foundation of Hunan Province (2024JJ4095), Research Project of Hunan Provincial Science and Technology Department (2022ZK4067), Education and Teaching Reform Research Project of Central South University (2023jy085 and 2024JGB153). The authors declare that they have no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The handling editor MW declared a shared affiliation with the author(s) PS, YH, JY, YX at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. (2018) 27:205–12. doi: 10.1097/CEJ.0000000000000428
3. Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. (2015) 5:a021535. doi: 10.1101/cshperspect.a021535
4. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
5. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2015) 12:681–700. doi: 10.1038/nrgastro.2015.173
6. Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. (2022) 13:1070961. doi: 10.3389/fimmu.2022.1070961
7. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
8. Stella L, Hollande C, Merabet YB, Fakhouri H, Leclerc V, Ponziani FR, et al. Promising PD-1 antagonists for liver cancer: an evaluation of phase II and III results. Expert Opin Emerg Drugs. (2024) 29:369–82. doi: 10.1080/14728214.2024.2430493
9. Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. (2023) 79:506–15. doi: 10.1016/j.jhep.2023.03.003
10. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
11. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
12. Zhao L, Yu G, Han Q, Cui C, Zhang B. TIM-3: An emerging target in the liver diseases. Scand J Immunol. (2020) 91:e12825. doi: 10.1111/sji.12825
13. Qian W, Zhao M, Wang R, Li H. Fibrinogen-like protein 1 (FGL1): the next immune checkpoint target. J Hematol Oncol. (2021) 14:147. doi: 10.1186/s13045-021-01161-8
14. Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. (2020) 11:167. doi: 10.3389/fimmu.2020.00167
15. Cheng P, Tang H, Lin F, Kong X. Bibliometrics of the nexus between food security and carbon emissions: hotspots and trends. Environ Sci pollut Res Int. (2023) 30:25981–98. doi: 10.1007/s11356-022-23970-1
16. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
17. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PloS One. (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
18. Chen C. CiteSpace: A Practical Guide for Mapping Scientific Literature. Beijing: Nova Science Publishers Press (2016) p. 20.
19. Li J, Chen CM. CiteSpace: Text Mining and Visualization in Scientific Literature. 2nd ed. Chengdu: Capital University of Economics and Business Press (2017). p. 301.
20. Luo H, Cai Z, Huang Y, Song J, Ma Q, Yang X, et al. Study on pain catastrophizing from 2010 to 2020: A bibliometric analysis via citeSpace. Front Psychol. (2021) 12:759347. doi: 10.3389/fpsyg.2021.759347
21. Alonso-Betanzos A, Bolon-Canedo V. Big-data analysis, cluster analysis, and machine-learning approaches. Adv Exp Med Biol. (2018) 1065:607–26. doi: 10.1007/978-3-319-77932-4_37
22. Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 3):57. doi: 10.1002/asi.20317
23. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
24. Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabró R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. (2020) 73:315–27. doi: 10.1016/j.jhep.2020.03.008
25. Llovet JM, Pinyol R, Yarchoan M, Singal AG, Marron TU, Schwartz M, et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol. (2024) 21:294–311. doi: 10.1038/s41571-024-00868-0
26. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:293–313. doi: 10.1038/s41575-020-00395-0
27. Zhu X-D, Huang C, Shen Y-H, Ji Y, Ge N-L, Qu X-D, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. (2021) 10:320–9. doi: 10.1159/000514313
28. Ma L, Heinrich S, Wang L, Keggenhoff FL, Khatib S, Forgues M, et al. Multiregional single-cell dissection of tumor and immune cells reveals stable lock-and-key features in liver cancer. Nat Commun. (2022) 13:7533. doi: 10.1038/s41467-022-35291-5
29. Chiu DK-C, Tse AP-W, Xu IM-J, Di Cui J, Lai RK-H, Li LL, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. (2017) 8:517. doi: 10.1038/s41467-017-00530-7
30. Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, et al. Cholangiocarcinoma — novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. (2023) 20:470–86. doi: 10.1038/s41571-023-00770-1
31. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. (2021) 75:960–74. doi: 10.1016/j.jhep.2021.07.004
32. Wang X, He Q, Shen H, Xia A, Tian W, Yu W, et al. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol. (2019) 71:731–41. doi: 10.1016/j.jhep.2019.05.015
33. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. (2023) 78:770–82. doi: 10.1016/j.jhep.2023.01.011
34. Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey JC, Kabat J, et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. (2022) 77:748–60. doi: 10.1016/j.jhep.2022.03.010
35. Gong F, Xu J, Liu B, Yang N, Cheng L, Huang P, et al. Nanoscale CaH2 materials for synergistic hydrogen-immune cancer therapy. Chem. (2022) 8:268–86. doi: 10.1016/j.chempr.2021.11.020
36. Shen K-Y, Zhu Y, Xie S-Z, Qin L-X. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives. J Hematol Oncol. (2024) 17:25. doi: 10.1186/s13045-024-01549-2
37. Dai H, Zhu C, Huai Q, Xu W, Zhu J, Zhang X, et al. Chimeric antigen receptor-modified macrophages ameliorate liver fibrosis in preclinical models. J Hepatol. (2024) 80:913–27. doi: 10.1016/j.jhep.2024.01.034
38. Hu B, Yu M, Ma X, Sun J, Liu C, Wang C, et al. IFNα Potentiates anti–PD-1 efficacy by remodeling glucose metabolism in the hepatocellular carcinoma microenvironment. Cancer Discovery. (2022) 12:1718–41. doi: 10.1158/2159-8290.CD-21-1022
39. Liu J, Kang R, Tang D. Adverse effects of ferroptotic therapy: mechanisms and management. Trends Cancer. (2024) 10:417–29. doi: 10.1016/j.trecan.2024.01.002
40. Xiong Z, Chan SL, Zhou J, Vong JSL, Kwong TT, Zeng X, et al. Targeting PPAR-gamma counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut. (2023) 72:1758–73. doi: 10.1136/gutjnl-2022-328364
41. Sung Y-C, Jin P-R, Chu L-A, Hsu F-F, Wang M-R, Chang C-C, et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat Nanotechnology. (2019) 14:1160–9. doi: 10.1038/s41565-019-0570-3
42. Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. (2019) 176:334–347.e12. doi: 10.1016/j.cell.2018.11.010
43. Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. (2018) 131:49–57. doi: 10.1182/blood-2017-06-741041
44. Chuah S, Lee J, Song Y, Kim H-D, Wasser M, Kaya NA, et al. Uncoupling immune trajectories of response and adverse events from anti-PD-1 immunotherapy in hepatocellular carcinoma. J Hepatol. (2022) 77:683–94. doi: 10.1016/j.jhep.2022.03.039
45. Lipkowitz S, Weissman AM. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer. (2011) 11:629–43. doi: 10.1038/nrc3120
46. Xiong Z, Chan S, Zhou J, Vong J, Kwong T, Zeng X, et al. Targeting PPAR-gamma counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut. (2023) 72:736–48. doi: 10.1136/gutjnl-2022-328364
47. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. (2017) 23:1388–96. doi: 10.1158/1078-0432.CCR-16-1432
48. Barsch M, Salié H, Schlaak AE, Zhang Z, Hess M, Mayer LS, et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J Hepatol. (2022) 77:397–409. doi: 10.1016/j.jhep.2022.02.032
49. Ma L, Wang L, Khatib SA, Chang C-W, Heinrich S, Dominguez DA, et al. Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. (2021) 75:1397–408. doi: 10.1016/j.jhep.2021.06.028
50. Zhao B, Li H, Xia Y, Wang Y, Wang Y, Shi Y, et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. (2022) 15:153. doi: 10.1186/s13045-022-01364-7
51. Lu J, Luo Y, Rao D, Wang T, Lei Z, Chen X, et al. Myeloid-derived suppressor cells in cancer: therapeutic targets to overcome tumor immune evasion. Exp Hematol Oncol. (2024) 13:24. doi: 10.1186/s40164-024-00505-7
52. Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med. (2016) 38:3–15. doi: 10.3892/ijmm.2016.2620
53. Huang H, Wang Z, Zhang Y, Ra B. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. (2021) 40(6):656–73.e7. doi: 10.1016/j.ccell.2022.04.011
54. Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. (2015) 61:1591–602. doi: 10.1002/hep.27665
55. Pfister D, Núez NG, Pinyol R, Govaere O, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0
56. Cheu WS, Lee D, Li Q, Goh CC, Bao HR, Yuen WH, et al. Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell Mol Gastroenterol Hepatol. (2023) 16:133–59. doi: 10.1016/j.jcmgh.2023.03.001
57. Ling ZN, Jiang YF, Ru JN, Lu JH, Ding B, Wu J. Amino acid metabolism in health and disease. Signal Transduction Targeted Ther. (2023) 010):008. doi: 10.1038/s41392-023-01569-3
58. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer - ScienceDirect. Ann Oncol. (2021) 32(8):994–1004. doi: 10.1016/j.annonc.2021.05.801
59. Bhat AA, Nisar S, Singh M, Ashraf B, Masoodi T, Prasad CP, et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Newsletter. (2022) 008):042. doi: 10.1002/cac2.12295
60. Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. (2021) 22(16):8470. doi: 10.3390/ijms22168470
61. Tauriello D, Batlle E. 8 TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. ESMO Open. (2018) 554(7693):538–43. doi: 10.1038/nature25492
62. Parvaresh H, Roozitalab G, Golandam F, Behzadi P, Jabbarzadeh Kaboli P. Unraveling the potential of ALK-targeted therapies in non-small cell lung cancer: comprehensive insights and future directions. Biomedicines. (2024) 12:297. doi: 10.3390/biomedicines12020297
63. Du S, Chen G, Yang P, Chen Y, Hu Y, Zhao Q, et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int. J. Radiat. Oncol. Biol. Phys. (2022) 112(5):1243–55. doi: 10.1016/j.ijrobp.2021.12.162
64. Chuah S, Lee J, Song Y, H.-D. K, Wasser M, Kaya NA, et al. Uncoupling immune trajectories of response and adverse events from anti-PD-1 immunotherapy in hepatocellular carcinoma. J Hepatology: J Eur Assoc Study Liver. (2022) 3):77. doi: 10.1016/j.jhep.2022.03.039
65. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. (2019) 4:611–21. doi: 10.1016/S2468-1253(19)30086-X
66. Yau T, Hsu C, Kim T-Y, Choo S-P, Kang Y-K, Hou M-M, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol. (2019) 71:543–52. doi: 10.1016/j.jhep.2019.05.014
67. Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy &x2013; development and validation of the CRAFITY score. J Hepatol. (2022) 76:353–63. doi: 10.1016/j.jhep.2021.09.035
68. Du S-S, Chen G-W, Yang P, Chen Y-X, Hu Y, Zhao Q-Q, et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int J Radiat Oncology Biology Phys. (2022) 112:1243–55. doi: 10.1016/j.ijrobp.2021.12.162
69. Leslie J, Mackey JBG, Jamieson T, Ramon-Gil E, Drake TM, Fercoq F, et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. (2022) 71:2093–106. doi: 10.1136/gutjnl-2021-326259
70. Greten TF, Schwabe R, Bardeesy N, Ma L, Goyal L, Kelley RK, et al. Immunology and immunotherapy of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. (2023) 20:349–65. doi: 10.1038/s41575-022-00741-4
71. Conche C, Finkelmeier F, Pesic M, Nicolas AM, Bottger TW, Kennel KB, et al. Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut. (2023) 72:1774–82. doi: 10.1136/gutjnl-2022-327909
72. Schwabe RF, Greten TF. Gut microbiome in HCC &x2013; Mechanisms, diagnosis and therapy. J Hepatol. (2020) 72:230–8. doi: 10.1016/j.jhep.2019.08.016
73. Donne R, Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. (2023) 77:1773–96. doi: 10.1002/hep.32740
74. Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. (2016) p:210–25. doi: 10.1016/j.ejca.2016.02.024
75. Celsa C, Cabibbo G, Fulgenzi CAM, Scheiner B, D’Alessio A, Manfredi GF, et al. Characteristics and outcomes of immunotherapy-related liver injury in patients with hepatocellular carcinoma versus other advanced solid tumours. J Hepatol. (2024) 80:431–42. doi: 10.1016/j.jhep.2023.10.040
76. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. (2023) 12:13. doi: 10.21037/hbsn-22-469
77. Liu Z, Zhou Z, Dang Q, Xu H, Lv J, Li H, et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. (2022) 12:6273–90. doi: 10.7150/thno.76854
78. Yang S, Xu J, Dai Y, Jin S, Sun Y, Li J, et al. Neutrophil activation and clonal CAR-T re-expansion underpinning cytokine release syndrome during ciltacabtagene autoleucel therapy in multiple myeloma. Nat Commun. (2024) 15:360. doi: 10.1038/s41467-023-44648-3
79. Cheng A-L, Hsu C, Chan SL, Choo S-P, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. (2020) 72:307–19. doi: 10.1016/j.jhep.2019.09.025
80. Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng A-L, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. (2020) 73:1460–9. doi: 10.1016/j.jhep.2020.07.026
81. Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. (2016) 65:719–26. doi: 10.1016/j.jhep.2016.04.008
82. Topalian SL, Forde PM, Emens LA, Yarchoan M, Smith KN, Pardoll DM. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell. (2023) 41:1551–66. doi: 10.1016/j.ccell.2023.07.011
83. Xiao Y, Chen J, Zhou H, Zeng X, Ruan Z, Pu Z, et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat Commun. (2022) 13:758. doi: 10.1038/s41467-022-28279-8
84. Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease – novel insights into cellular communication circuits. J Hepatol. (2022) 77:1136–60. doi: 10.1016/j.jhep.2022.06.012
85. Martin-Serrano MA, Kepecs B, Torres-Martin M, Bramel ER, Haber PK, Merritt E, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut. (2022) 72:736–48. doi: 10.1136/gutjnl-2021-326514
86. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. (2008) 22:338–42. doi: 10.1096/fj.07-9492LSF
Keywords: liver cancer (LC), immunotherapy, bibliometrics, CiteSpace, hotspot, frontier
Citation: Su P, Han Y, Yi J, Hou Y and Xiao Y (2025) Research status and frontiers in liver cancer immunotherapy: a bibliometric perspective on highly cited literature. Front. Oncol. 15:1587252. doi: 10.3389/fonc.2025.1587252
Received: 04 March 2025; Accepted: 14 March 2025;
Published: 10 April 2025.
Edited by:
Mingyuan Wang, Department of Geriatrics, Central South University, ChinaReviewed by:
Guangquan Zhang, Sun Yat-sen University, ChinaQingfei K, Harbin Medical University, China
Copyright © 2025 Su, Han, Yi, Hou and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Hou, aG91eXVAemp1LmVkdS5jbg==
 Pan Su1
Pan Su1 Yao Xiao
Yao Xiao