- 1Markey Cancer Center, University of Kentucky, Lexington, KY, United States
- 2College of Pharmacy, University of Kentucky, Lexington, KY, United States
- 3College of Medicine, University of Kentucky, Lexington, KY, United States
- 4Molecular Science Liaison Group, Caris Life Sciences, Phoenix, AZ, United States
- 5Department of Neurosurgery, University of Kentucky, Lexington, KY, United States
- 6Department of Pathology and Laboratory Medicine, University of Kentucky, Lexington, KY, United States
Meningiomas are the most common primary tumor in the central nervous system, yet an effective systemic treatment remains a challenge. We present a grade 2 meningioma that resulted in a positive and prolonged response to pembrolizumab. Our case had polybromo-1 (PBRM1) and BAP1 functional loss, tumor mutational burden of 4 Muts/Mb, stable microsatellite status, and a PD-L1 tumor proportion score of <1%. We add to the limited literature regarding PBRM1 mutations in meningiomas. We discuss our findings in relation to the ongoing investigation of immune checkpoint inhibitor therapy in treating higher-grade refractory meningiomas.
Introduction
Meningioma incidence is increasing in the US population. Meningioma is one of the rare tumors whose incidence continues to rise with advancing age (1, 2). They arise from arachnoid cells of the leptomeninges and are the most common primary tumor in the central nervous system (CNS) (1, 3). Although there are widespread asymptomatic cases in 1%–2% of the general adult population, nearly all are non-malignant grade 1 tumors (1, 4, 5). In 2016, the World Health Organization defined grade 2 meningiomas as atypical, exhibiting mitotic rates of 4–19 per 10 high power fields (HPFs), brain invasion, or at least three of five defined histological features (necrosis, sheet-like growth, prominent nucleoli, high cellularity, or high nuclear:cytoplasmic ratio within cells). Grade 3 meningiomas were considered anaplastic or malignant and were described as having mitotic rates >20 per 10 HPFs or papillary or rhabdoid histological features (6, 7). More recently, the 2021 WHO guidelines emphasize that, regardless of any underlying pathologic characteristics, atypical and anaplastic meningiomas should be classified as grade 2 and grade 3, respectively (5). Additionally, where rhabdoid and papillary features previously would be automatically classified as a grade 3 meningioma, the WHO CNS5 now recommends that meningiomas be classified based on criteria outside of those cytologic features (5). There are several molecular biomarkers that can be utilized in the classification of meningiomas. BAP1 is associated with the rhabdoid and papillary subtypes, SMARCE1 is consistent with the clear cell subtype, and KLF4/TRAF7 mutations are associated with the secretory subtype. Furthermore, meningiomas with CDKN2A/B homozygous deletions and/or TERT promoter mutations are classified as grade 3. Prognosis can be estimated through methylome profiling, and some mutations (H2K27me3 loss of nuclear expression) may be associated with poorer prognoses (5). From surgically resected cases, higher-grade meningiomas remain a minority: atypical or borderline malignant grade 2 tumors occur in 5%–15% and malignant grade 3 tumors in 1%–3% of cases (1, 4). The recurrence rates following surgery are low for grade 1 tumors but increase to 30% to 40% for grade 2 and 50% to 80% for grade 3 (1, 4).
Regarding immune access, recent anatomical discoveries demonstrate that the central nervous system is no longer considered a strictly immune-privileged organ (8). Lymphatic vessels, adjacent to the blood vascular system, are the primary means by which bodily tissues can eliminate excess fluid and proteins (9). Tissues with higher metabolic rates typically contain denser lymphatic systems. Interestingly, despite the high rate of metabolic byproduct formation, the brain and spinal cord do not contain a lymphatic tree (9, 10). Instead, waste products from the CNS are removed through the exchange of cerebrospinal fluid (CSF) and interstitial fluid (ISF) within the para-arterial interstitial space (9, 11). ISF then drains out of the CNS into the subarachnoid lymphatic-like membrane (SLYM). This recently identified structure present under the dura separates the subarachnoid space into outer and inner compartments and limits the exchange of most peptides and proteins between the two subarachnoid compartments. The recent discovery of the SLYM adds to the suggestion that CSF transport is more sophisticated than previously acknowledged (12).
We report one of the first pathologically proven cases of meningioma having a significant and prolonged response to a single agent pembrolizumab. This patient’s tumor had a truncation in the polybromo-1 (PBRM1) gene, which is a tumor suppressor gene involved in the control of the cell cycle, the promotion of genomic stability, and centromeric cohesion (8). Overall, PBRM1 is mutated in nearly 40% of all clear cell renal cell carcinoma (RCC) occurrences, as well as some papillary RCCs and bladder carcinoma (13). PBRM1 mutations are relatively uncommon in meningiomas, but when present, they are associated with papillary subtypes and often have overlapping BAP1 mutations (14).
The occurrence of meningiomas has undergone only limited formal investigation in regard to therapies, and they currently remain among the few relatively common tumors without a Food and Drug Administration (FDA)-approved therapy. Meningiomas are chemotherapy resistant, and both targeted and immune-based therapies have been actively investigated (15–18). As discussed previously, there is evidence of an immune-based role in higher-grade meningiomas, including containing a significantly greater intra-tumoral T-cell infiltrate, inducing known local and systemic immunosuppression, a recent case of possible immune-mediated abscopal effect from radiation therapy, and several case reports of activity for immune checkpoint inhibitors (ICIs) (19, 20). With our case study, we have begun to further explore the occurrence of PBRM1 mutations and subsequent outcomes in patients with meningiomas.
Caris genomics study
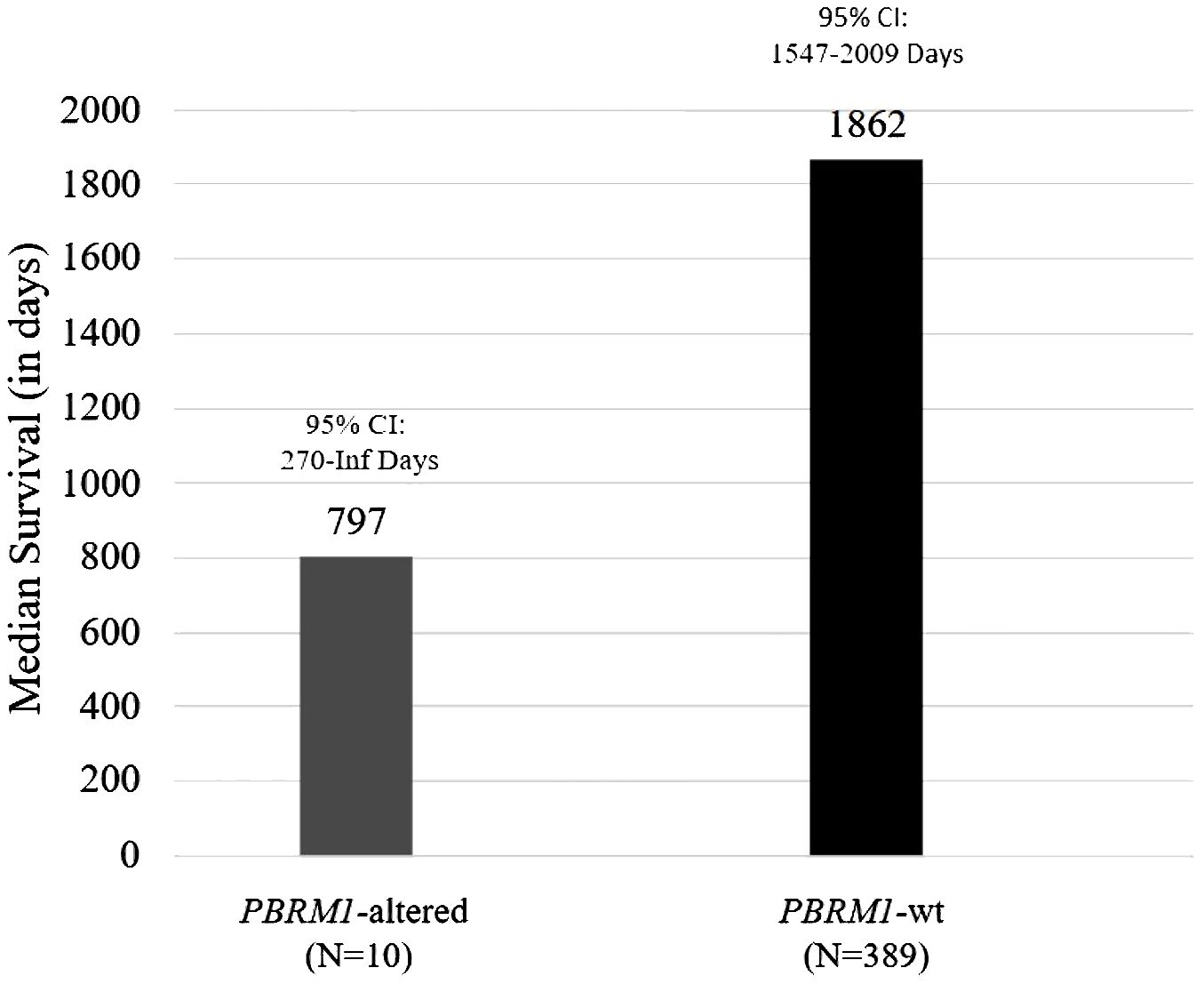
Genomics data from patient tumors that were sent to Caris Life Sciences for next-generation sequencing were utilized for this analysis. A total of 399 patients with meningiomas were identified, and 2.5% (n = 10) had alterations in PBRM1. Of the 10 patients with PBRM1 alterations, one patient had a known pathogenic point mutation variant (R1027X). Overall survival (OS) was estimated using the Kaplan–Meier method. The median OS was 797 days (95% CI 270 to ∞ days) for patients with PBRM1 alteration and 1,862 days for those without (95% CI 1,547–2,009 days) (Figure 1). Of note, the patient having the PBRM1 R1027X mutation had a survival of 797 days. The Kaplan–Meier plots and p-values were not generated due to a low sample size of PBRM1 alterations.
Clinical case
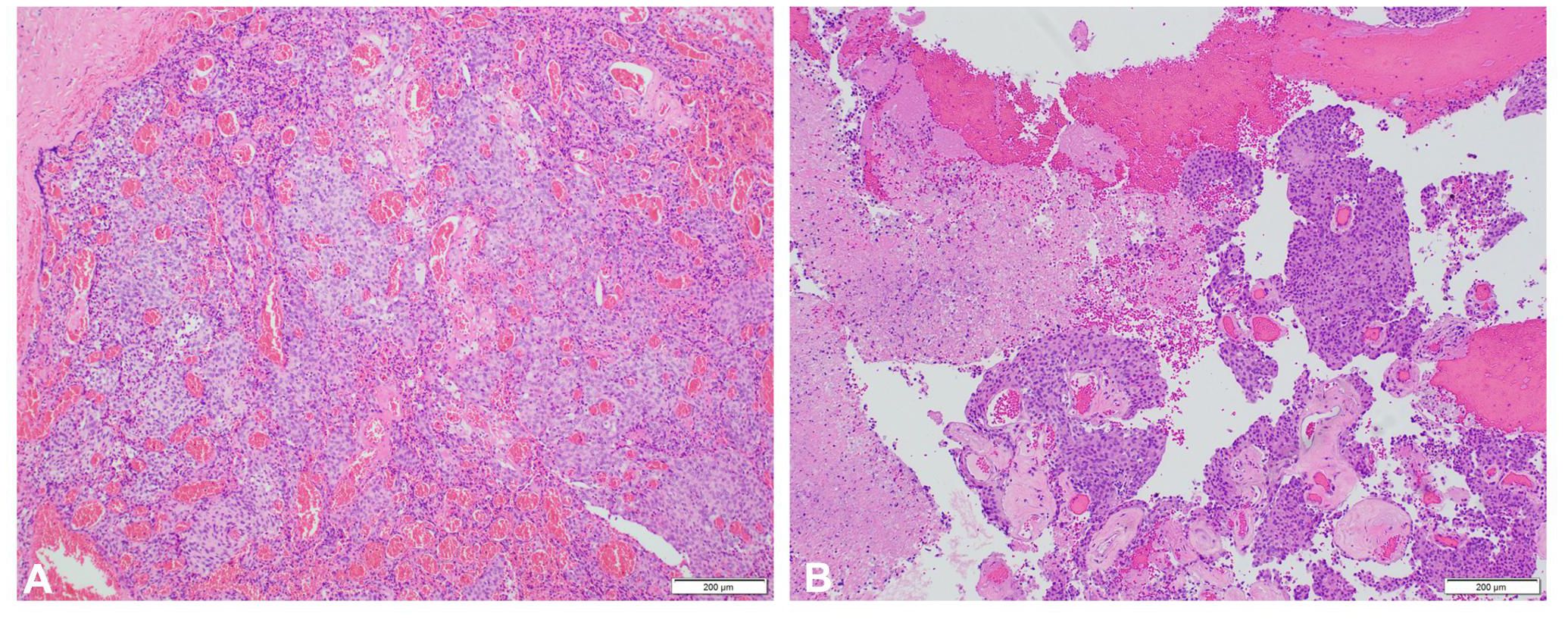
In 1993, at age 19, our patient was diagnosed with atypical meningioma, grade 2, located around the right mastoid region. Her treatment plan included two closely spaced surgeries and proton therapy, followed by surveillance for more than a decade. She lacked a family history of neurofibromatosis 2 (NF2) or cancer, as well as clinical or imaging evidence of NF2. Referral for genetic counseling was refused. In March 2017, at age 43, she developed progressive headaches, and imaging demonstrated an enhancing right upper neck mass with erosion of C1–C2 and extension into the posterior fossa (Figure 2A). Her first surgical resection (R1) following recurrence was performed in April 2017, and pathology demonstrated atypical meningioma lacking immune infiltrates. The post-surgery MRI and histopathology (H&E) images are depicted in Figures 2B, 3A, respectively.
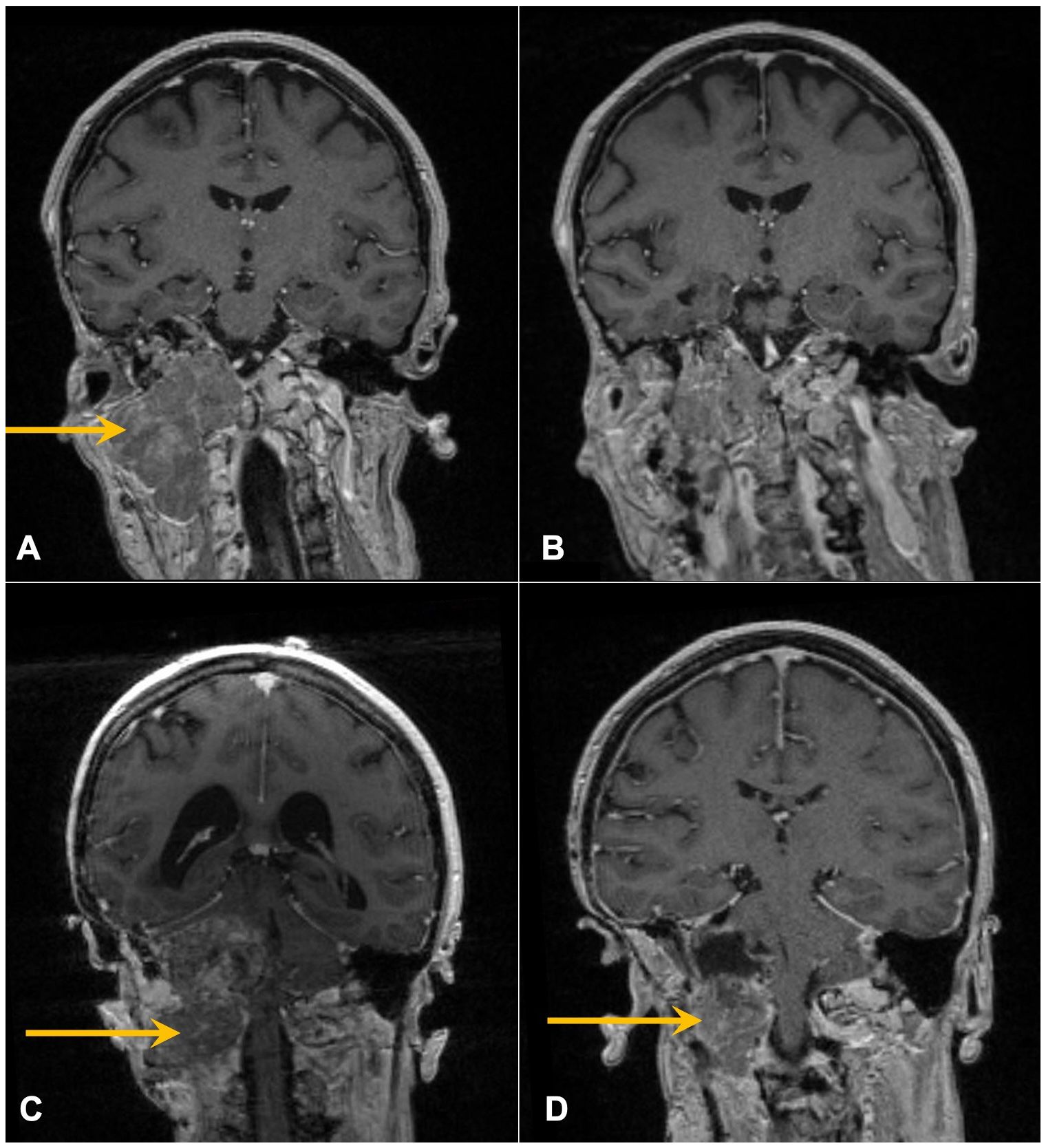
Figure 2. Contrast-enhanced coronal T1 MRI of the brain following recent surgeries. Arrows mark enhancing skull base mass prior to R1 (A) and R2 (C) and resulting post-operative images following R1 (B) and R2 (D).
She initiated somatostatin analog injections in July 2017, which she continued monthly for 22 months until imaging in November 2018 demonstrated progression (21–23). She also developed mild headaches, tearing in her right eye, and decreased movement in the right side of her face. The decision was made to undertake a large cancer-based surgery, R2 (Figures 2C, D), in April 2019. Pathology from this surgery was similar to that of the R1 specimen (Figure 3B).
After recovery (June 2019), she enrolled in a phase I clinical trial of BXQ-350, a synthetic form of the human glycoprotein saposin C (NCT02859857). Unfortunately, she soon progressed in August 2019 with the growth of the right skull base tumor and began to have increased symptoms of headaches, dysphagia with liquids, and weight loss. To identify possible targeted therapies, next-generation sequencing (NGS) testing was performed on the R2 specimen (right cerebellar tumor) with FoundationOne. This demonstrated a non-elevated tumor mutational burden (TMB) of 4 Muts/Mb, stable microsatellite status, and no recommended therapies or trials. There were alterations including FBXW7 G419, BAP1 loss, and PBRM1 loss of exons 2 to 12. The tumor was also found to have a PD-L1 tumor proportion score of <1% on PD-L1 22C3 IHC testing.
Having the PBRM1 mutation, a mutation possibly associated with immune therapy response in RCC, pembrolizumab was administered at standard flat dosing of 200 mg every 3 weeks starting in September 2019 (30 months from R1). The patient soon reported improvement in her symptoms and, after three cycles, returned to full-time work. She experienced no significant adverse effects from therapy. Repeat imaging demonstrated a reduction in size with near resolution of mass effect (Figures 4A, B), and she continued ICI therapy. At the time of manuscript writing, the patient had completed 66 cycles of pembrolizumab 200 mg every 3 weeks since R2. She continues to work and enjoys high performance status and has no evidence of disease on her most recent imaging (Figures 4C, D), approximately 3 years 9 months from the initiation of ICI therapy and 50 months from R2.
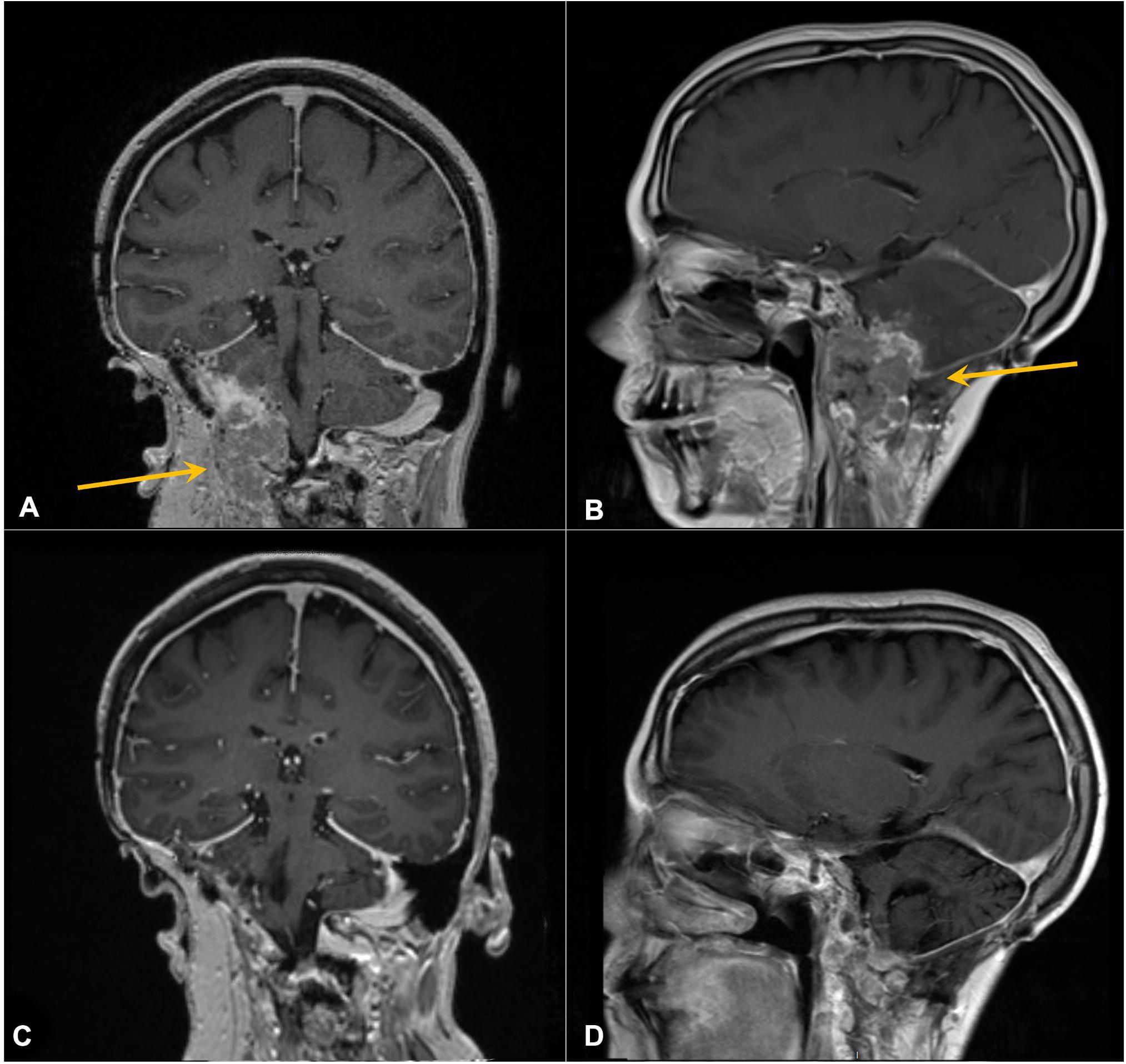
Figure 4. Contrast-enhanced coronal (A, C) and sagittal (B, D) T1 MRI of the brain. Arrows in panels A and B mark enhancing right cerebellar/skull base prior to pembrolizumab. (C, D) Following six cycles of therapy, demonstrating response.
Other cases benefiting from ICI therapy include an advanced lung cancer patient co-diagnosed with imaging-based meningioma (lacking tissue confirmation) in which both tumors continued growing on standard chemotherapy prior to seeing a positive response to nivolumab (24). Another case with atypical meningioma (grade 2)—with mismatch repair deficiency and disease extending extra-axially from a frontal convexity tumor to involving the scalp—had prior treatment with bevacizumab, temozolomide, two radiosurgeries, and seven surgical debulking procedures, but exhibited benefit from nivolumab (25). In a 1997 study, six patients with unresectable or malignant meningiomas were treated with interferon alpha-2B, with five patients showing a positive response to treatment. Of those five patients, four experienced tumor stabilization with a range of 6 to 14 months (26, 27). Furthermore, a study published in 2022 documented a slight trend in increased PD-L1 expression correlating with better outcomes and growth stabilization in pembrolizumab-treated meningioma patients (20). Twelve patients achieved a median progression-free survival (PFS) of 7.6 months, which was a favorable comparison to previous trials that had only reported PFS of 4–26 weeks (20, 28). The findings of this study also suggested that T-cell or myeloid-cell phenotypic dynamics, as well as the level of histological aggression, may dictate whether a clinical benefit or disease response is achieved from ICI therapy (20). Although limited in scope, these cases collectively support the exploration of immunotherapy as an option for the treatment of advanced meningiomas.
Discussion
PBRM1 is a tumor suppressor gene that codes for BAF180, a component of the chromatin remodeling complex (29). Thus, its loss of function impacts chromatin structure and downstream transcriptional and DNA repair processes (30, 31). In vivo experiments have demonstrated increased tumorigenesis in mice with downregulated PBRM1, with the greatest difference in gene expression being seen in the chemokine/chemokine receptor interaction pathway, suggesting a possible mechanism by which PBRM1 alters cell cycle progression and proliferation (32). Other recent studies have shown that a lack of PBRM1 subsequently results in DNA damage and dynamic chromosome instability (33).
In patients with clear cell RCC, loss-of-function mutations in PBRM1 are common and are associated with clinical benefit from immune checkpoint inhibitors (13, 34). Braun and colleagues reported consistent results: in 189 patients with metastatic clear cell RCC receiving nivolumab or everolimus as part of a clinical trial, 55 patients had a PBRM1 mutation, which was associated with both clinical benefit and longer PFS in nivolumab-treated patients. There was no effect noted in those treated with everolimus only.
In contrast, in a retrospective analysis conducted at three Chinese institutions, presumably in Asian patients, PBRM1 mutations were infrequent in patients with non-small cell lung cancer (84/2,767, 3%). This analysis demonstrated that PBRM1 may be potentially associated with poorer survival in patients treated with immunotherapy, despite previous reports suggesting a correlation between PBRM1 mutations and increased neoantigens (35, 36).
PBRM1 genetic alterations are infrequent (2.8%) in meningiomas, and alterations are usually associated with high-grade meningiomas (37). Unfortunately, the genomics data we obtained from Caris Life Sciences did not contain information regarding the tumor grade of the included patients. However, in a recent case series of 850 patients with meningiomas that were grade 1 (220/850, 26%), grade 2 (441/850, 52%), and grade 3 (176/850, 20%) (13 cases were not graded due to inadequate specimens), only 16 had an inactivating mutation in PBRM1 (1.9%) (14). The majority of the 16 PBRM1 meningioma cases (11) had papillary histologic features that were higher grade (2/16 grade 1, 8/16 grade 2, and 6/16 grade 3), all were microsatellite stable and had a low median TMB of 2.1 Muts/Mb, and five cases had an overlap mutation with BAP1. Our analyses of 399 meningioma patients undergoing NGS testing demonstrated that patients with PBRM1 alterations had likely lower overall survival. The frequency (2.5%) of PBRM1 alterations in meningiomas in our analysis matches published literature. Despite PBRM1 mutations rarely occurring in meningioma, this represents a potential therapeutic investigation.
BAP1 was originally identified as a BRCA1-interacting protein and encodes a de-ubiquitinating enzyme that is involved in many processes (38). BAP1 can act as a subunit of the Polycomb Repressive De-ubiquitinase complex (PR-DUB), which reverses the ubiquitinating activity of Polycomb Repressive Complex 1 (PRC1); one key PR-DUB substrate is histone H2A ubiquitinated at lysine 119, so BAP1 normally acts to modulate chromatin structure and cellular epigenetic status (39, 40). Thus, loss of BAP1 function is thought to affect DNA repair and transcription processes that are affected by chromatin state. Mutations in BAP1 have been reported to correlate with positive response to immunotherapy (41), perhaps by similar mechanisms as for PBRM1, but BAP1 alterations are even rarer (<1%) (37).
This report details our experiences with a patient with advanced meningiomas and illustrates the challenges associated with treating these malignancies. Our patient received proton therapy and aggressive multi-team surgery, underwent a first-in-human early-phase clinical trial, and was treated with a somatostatin analog for many years. Our patient’s meningioma demonstrated stable microsatellite status and PD-L1 negativity, but with a TMB of 4 Muts/Mb, which is higher than reported for atypical meningioma (mean 1.8 Muts/Mb) but lower than TMBs in other tumors (melanomas, many lung cancers, and microsatellite instability (MSI)-high cancers) (25) for which ICIs have FDA labeling. Thus, it seems unlikely that PD-L1 or TMB levels explain the positive response to immunotherapy.
The genomics report demonstrated probable loss-of-function alterations (large deletions) in the tumor suppressor gene PBRM1 on chromosome 3p21. Our patient had a PBRM1 deletion involving exons 2 through 12. Missense mutations in the bromodomain regions have been shown to result in the tumor suppressor activity of PBRM1, especially in the bromodomain 2 (42). Further, the bromodomains have also been found to be essential in the chromatin complex interaction (42, 43). Even though it is tempting to suggest that loss of function of PBRM1 and/or BAP1 plays a role in the positive response of our patient to pembrolizumab, the role of mutations in PBRM1 has yet to be well-characterized. Our genomics analysis on meningioma patients demonstrated 90% (9/10) patients having PBRM1 mutations with unknown oncogenic significance. The patient in our case report had a reported exon loss in PBRM1, which may result in truncating mutations leading to a loss of function as a tumor suppressor. Truncating or splice site mutations appear to be the majority of reported PBRM1 oncogenic alterations based on the cBioPortal database (44, 45). This suggests that despite PBRM1 being a potential biomarker for immunotherapy, heterogeneity in tumors may present challenges when validating biomarkers as a response to immunotherapy.
The current therapeutic landscape remains limited in meningioma, but treatments targeted to actionable mutations are promising. In addition to immunotherapy, ongoing clinical trials are under investigation involving FAK inhibition in patients based on preclinical synthetic lethality seen with NF2 loss and FAK inhibition (46). Despite evidence of PBRM1 loss contributing to genomic instability or neoantigen production, a majority of the reported literature is preclinical in nature, and the concept requires further research to validate PBRM1 as a marker for immunotherapy response. Our experience with immunotherapy in treating meningioma patients mirrors that observed in patients with other malignancies—i.e., while a substantial percentage of patients may have a positive or even exceptional response, others may not respond even though their tumors may possess a marker that would potentially predict a positive response. Our findings expand this paradigm to aggressive meningiomas from the positive outcome of our patient case, which adds to the limited previous literature demonstrating positive responses of these malignancies to immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ER: Formal analysis, Validation, Writing – original draft, Writing – review & editing. KP: Formal analysis, Validation, Writing – review & editing. RM: Validation, Writing – review & editing. HM: Validation, Writing – review & editing. DO: Supervision, Validation, Writing – review & editing. SR: Data curation, Validation, Writing – review & editing. TP: Validation, Writing – review & editing. JN: Validation, Writing – review & editing. JK: Validation, Writing – review & editing. JV: Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The Research Communications Office of the University of Kentucky Markey Cancer Center (P30CA177558) supported the publication of the manuscript but did not contribute to the writing of the manuscript.
Acknowledgments
We would like to thank our patient.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis D, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee W, et al. WHO classification of tumours of the central nervous system. 4th edition. Lyon: International Agency For Research On Cancer (2016).
2. Saraf S, McCarthy BJ, and Villano JL. Update on meningiomas. Oncologist. (2011) 16:1604–13. doi: 10.1634/theoncologist.2011-0193
3. Nakasu S, Hirano A, Shimura T, and Llena J. Incidental meningiomas in autopsy study. Surg Neurol. (1987) 27:319–22. doi: 10.1016/0090-3019(87)90005-X
4. Perry A, Stafford SL, Scheithauer BW, Suman V, and Lohse C. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. (1997) 21:1455–65. doi: 10.1097/00000478-199712000-00008
5. Louis DN, Perry A, Wesseling P, Brat D, Cree I, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
6. Maggio I, Franceschi E, Tosoni A, Di Nunno V, Gatto L, Lodi R, et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. (2021) 10:Cns72. doi: 10.2217/cns-2021-0003
7. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee W, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
8. Gao W, Li W, Xiao T, Liu X, and Kaelin W Jr. Inactivation of the PBRM1 tumor suppressor gene amplifies the HIF-response in VHL-/- clear cell renal carcinoma. Proc Natl Acad Sci U.S.A. (2017) 114:1027–32. doi: 10.1073/pnas.1619726114
9. Nedergaard M. Neuroscience. Garbage truck of the brain. Science. (2013) 340:1529–1530. doi: 10.1126/science.1240514
10. Loukas M, Bellary SS, Kuklinski M, Ferrauiola J, Yadav A, Shoja M, et al. The lymphatic system: a historical perspective. Clin Anat. (2011) 24:807–16. doi: 10.1002/ca.21194
11. Nagelhus EA, Mathiisen TM, and Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4. 1. Neurosci. (2004) 129:905–13. doi: 10.1016/j.neuroscience.2004.08.053
12. Mollgard K, Beinlich FRM, Kusk P, Miyakoshi L, Delle C, Pla V, et al. A mesothelium divides the subarachnoid space into functional compartments. Science. (2023) 379:84–8. doi: 10.1126/science.adc8810
13. Braun DA, Ishii Y, Walsh AM, Van Allen E, Wu C, Shukla S, et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol. (2019) 5:1631–3. doi: 10.1001/jamaoncol.2019.3158
14. Caruso G, Ferrarotto R, Curcio A, Metro L, Pasqualetti F, Gaviani P, et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. (2020) 140:89–93. doi: 10.1007/s00401-020-02161-7
15. Nayak L, Iwamoto FM, Rudnick JD, Norden A, Lee E, Drappatz J, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. (2012) 109:187–93. doi: 10.1007/s11060-012-0886-4
16. Norden AD, Raizer JJ, Abrey LE, Lamborn K, Lassman A, Chang S, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. (2010) 96:211–7. doi: 10.1007/s11060-009-9948-7
17. Dunn IF, Du Z, Touat M, Sisti M, Wen P, Umeton R, et al. Mismatch repair deficiency in high-grade meningioma: a rare but recurrent event associated with dramatic immune activation and clinical response to PD-1 blockade. JCO Precis Oncol. (2018) 2018. doi: 10.1200/PO.18.00190
18. Wen PY, Yung WK, Lamborn KR, Norden A, Cloughesy T, Abney L, et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08). Neuro Oncol. (2009) 11:853–60. doi: 10.1215/15228517-2009-010
19. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, and Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. (2017) 127:3210–9. doi: 10.1172/JCI90603
20. Brastianos PK, Kim AE, Giobbie-Hurder A, Lee E, Wang N, Eichler A, et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat Commun. (2022) 13:1325. doi: 10.1038/s41467-022-29052-7
21. Chamberlain MC, Glantz MJ, and Fadul CE. Recurrent meningioma. Salvage Ther long-acting somatostatin analogue. (2007) 69:969–73. doi: 10.1212/01.wnl.0000271382.62776.b7
22. Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon D, Kaley T, et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. (2015) 84:280–6. doi: 10.1212/WNL.0000000000001153
23. Schulz C, Mathieu R, Kunz U, and Mauer U. Treatment of unresectable skull base meningiomas with somatostatin analogs. Neurosurg Focus. (2011) 30:E11. doi: 10.3171/2011.1.FOCUS111
24. Garzon-Muvdi T, Bailey D, Pernik M, and Pan E. Regression of intracranial meningioma following treatment with nivolumab: Case report and review of the literature. J Clin Neurosci. (2017) 37:51–3. doi: 10.1016/j.jocn.2016.11.011
25. Bi WL, Greenwald NF, Abedalthagafi M, Wala J, Gibson W, Agarwalla P, et al. Genomic landscape of high-grade meningiomas. NPJ Genom Med. (2017) 2:15. doi: 10.1038/s41525-017-0014-7
26. Sioka C and Kyritsis AP. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J Neuro-Oncology. (2009) 92:1–6. doi: 10.1007/s11060-008-9734-y
27. Kaba SE, DeMonte F, Bruner JM, Kyritsis A, Jaeckle K, Levin V, et al. The treatment of recurrent unresectable and Malignant meningiomas with interferon alpha-2B. Neurosurgery. (1997) 40:271–5. doi: 10.1097/00006123-199702000-00007
28. Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. (2014) 16:829–40. doi: 10.1093/neuonc/not330
29. Gerlinger M, Horswell S, Larkin J, Rowan A, Salm M, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. (2014) 46:225–33. doi: 10.1038/ng.2891
30. Hodges C, Kirkland JG, and Crabtree GR. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb Perspect Med. (2016) 6. doi: 10.1101/cshperspect.a026930
31. Hopson S and Thompson MJ. BAF180: its roles in DNA repair and consequences in cancer. ACS Chem Biol. (2017) 12:2482–90. doi: 10.1021/acschembio.7b00541
32. Wang H, Qu Y, Dai B, Zhu Y, Shi G, Zhu Y, et al. PBRM1 regulates proliferation and the cell cycle in renal cell carcinoma through a chemokine/chemokine receptor interaction pathway. PLoS One. (2017) 12:e0180862. doi: 10.1371/journal.pone.0180862
33. Miao D, Margolis CA, Gao W, Voss M, Li W, Martini D, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. (2018) 359:801–6. doi: 10.1126/science.aan5951
34. Carril-Ajuria L, Santos M, Roldán-Romero JM, Rodriguez-Antona C, and de Velasco G. Prognostic and predictive value of PBRM1 in clear cell renal cell carcinoma. Cancers (Basel). (2019) 12. doi: 10.3390/cancers12010016
35. Zhou H, Liu J, Zhang Y, Huang Y, Shen J, Yang Y, et al. PBRM1 mutation and preliminary response to immune checkpoint blockade treatment in non-small cell lung cancer. NPJ Precis Oncol. (2020) 4:6. doi: 10.1038/s41698-020-0112-3
36. Park LC, Chang S, Ko T, Rhee K, Anker J, Bhave M, et al. P1.04–01 impact of chromatin remodeling genes including SMARCA2 and PBRM1 on neoantigen and immune landscape of NSCLC. J Thorac Oncol. (2018) 13:S525. doi: 10.1016/j.jtho.2018.08.716
37. Birzu C, Peyre M, and Sahm F. Molecular alterations in meningioma: prognostic and therapeutic perspectives. Curr Opin Oncol. (2020) 32:613–22. doi: 10.1097/CCO.0000000000000687
38. Louie BH and Kurzrock R. BAP1: Not just a BRCA1-associated protein. Cancer Treat Rev. (2020) 90:102091. doi: 10.1016/j.ctrv.2020.102091
39. Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty R, Fraterman S, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. (2010) 465:243–7. doi: 10.1038/nature08966
40. Campagne A, Lee M-K, Zielinski D, Michaud A, Le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun. (2019) 10:348. doi: 10.1038/s41467-018-08255-x
41. Ladanyi M, Sanchez Vega F, and Zauderer M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med. (2019) 11:18. doi: 10.1186/s13073-019-0631-0
42. Porter EG and Dykhuizen EC. Individual bromodomains of polybromo-1 contribute to chromatin association and tumor suppression in clear cell renal carcinoma. J Biol Chem. (2017) 292:2601–10. doi: 10.1074/jbc.M116.746875
43. Slaughter MJ, Shanle EK, McFadden AW, Hollis E, Suttle L, Strahl B, et al. PBRM1 bromodomains variably influence nucleosome interactions and cellular function. J Biol Chem. (2018) 293:13592–603. doi: 10.1074/jbc.RA118.003381
44. Cerami E, Gao J, Dogrusoz U, Gross B, Sumer S, Aksoy B, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. (2012) 2:401–4. doi: 10.1158/2159-8290.CD-12-0095
45. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer S, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. (2013) 6:pl1. doi: 10.1126/scisignal.2004088
Keywords: atypical meningioma, immunotherapy, PBRM1, grade 2 meningioma, PD-1/L1
Citation: Reusch E, Peh KH, Morgan R, Momo H, Orren D, Rock S, Pittman T, Neltner J, Kolesar J and Villano J (2025) Case Report: Advanced grade 2 meningioma with PBRM1 inactivation with prolonged response to immunotherapy. Front. Oncol. 15:1587752. doi: 10.3389/fonc.2025.1587752
Received: 04 March 2025; Accepted: 04 August 2025;
Published: 22 October 2025.
Edited by:
Giovanna Damia, Mario Negri Institute for Pharmacological Research (IRCCS), ItalyReviewed by:
Omkar Ijare, Houston Methodist Research Institute, United StatesYoung Zoon Kim, Sungkyunkwan University, Republic of Korea
Copyright © 2025 Reusch, Peh, Morgan, Momo, Orren, Rock, Pittman, Neltner, Kolesar and Villano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Villano, amx2aWxsYW5vQHVreS5lZHU=
 Ellen Reusch
Ellen Reusch Keng Hee Peh1,2
Keng Hee Peh1,2 Rachael Morgan
Rachael Morgan David Orren
David Orren Jill Kolesar
Jill Kolesar John Villano
John Villano