Abstract
Lymphangioleiomyomatosis (LAM) is a rare disorder that primarily affects women of childbearing age. It is characterized by the abnormal growth of smooth muscle-like cells. While LAM typically occurs in the lungs, it can also be found in the retroperitoneum and pelvis. However, cases originating in the mediastinum are extremely rare. This report discusses an unusual case of mediastinal LAM in a male patient with no abnormal clinical symptoms. The patient, a 70-year-old man, the chest computed tomography (CT) scan revealed an irregular hypodense mass in the left side of the anterior superior mediastinum. Interestingly, the mass did not exhibit significant enhancement in the arterial phase. Instead, it showed striated enhancement in the central area during the venous phase, with no abnormalities observed in the marginal area. To further understand this condition, we conducted a comprehensive review of relevant literature, focusing on the imaging characteristics of mediastinal LAM and the pathogenesis and therapeutic prognosis of LAM. By sharing this information, we aim to enhance understanding and knowledge of this disease.
Introduction
Lymphangioleiomyomatosis (LAM) is a rare systemic disease classified under the family of PEComas, characterized by the abnormal proliferation of smooth muscle-like cells and cystic lesions (1, 2). The most common site of LAM is the lung, accounting for about 90% of all cases. Extrapulmonary LAM is a rare disease that often occurs concurrently with pulmonary LAM. The primary sites include the pancreas, retroperitoneum, and pelvis, with mediastinal LAM being much rarer (3). LAM primarily affects women of childbearing age, with very few reported cases in males (4). The prevalence of LAM is extremely low, in women, with only 28.7 cases per 1 million persons reported in the literature. While in men, the prevalence of LAM is even lower, at 0.8 per 1 million persons (5). In this study, we conducted a literature review of case reports published in indexed journals from January 1998 to July 2023, focusing on mediastinal LAM. The search was performed using keywords “mediastinal lymphangioleiomyomatosis” in PubMed and CNKI databases. Only five cases of mediastinal LAM were identified in the literature, primarily discussing the pathological manifestations. In this report, we present a case of mediastinal LAM in an elderly man, describe the findings from chest computed tomography (CT), and provide a review of the relevant literature.
To the best of our knowledge, this case study presents the first case of a primary solitary extrapulmonary form of LAM in the mediastinum occurring in a male. We present the following case in accordance with the CARE reporting checklist.
Case report
A 72-year-old man presented with an anterior mediastinal mass eight years ago and was recently admitted to the hospital due to an increase in the size of the mass. The patient did not exhibit any abnormal clinical manifestations, family history, medical history, physical examination findings, or abnormal laboratory test results. He had no clinical features suggestive of tuberous sclerosis complex (TSC). Specifically, he did not have central nervous, hydrocephalus, mental retardation. He did not have dermatologic manifestations. He did not have a family member who was diagnosed with TSC. A plain chest computed tomography (CT) scan revealed an irregular hypodense mass measuring 3.5 cm × 3.0 cm × 1.9 cm on the left side of the anterior superior mediastinum. The mass had a CT value of 45.3 HU and displayed clear borders. Punctate calcification was observed at the edge of the lesion (Figure 1A). In the arterial phase, the mass did not exhibit significant enhancement, with a CT value of 46.1 HU (Figure 1B). However, during the venous phase, the mass showed striated enhancement in the central area, while the marginal area displayed no abnormalities. The CT values in the venous phase were 94.6 HU in the central area and 46.3 HU in the marginal area (Figure 1C). No enlarged lymph nodes were observed in the hilum, and the mass did not exert pressure on surrounding tissues or blood vessels. There was also no evidence of infiltration, and the lung window displayed clear lung texture without cystic changes (Figure 1D).
Figure 1

(A) This axial CT image shows an irregular hypodense mass in the left side of the anterior superior mediastinum, with clear boundaries (green arrow). Punctate calcification is observed at the edge of the lesion (red arrow). (B) This CT image in the arterial phase demonstrates that the mass does not exhibit significant enhancement, with a CT value of 46.1 HU. (C) In the venous phase CT image, striated enhancement is observed in the central area of the mass, while no abnormalities are seen in the marginal area (yellow arrow). The CT values are 94.6 HU and 46.3 HU, respectively. (D) The lung window image shows a clear texture in both lungs, with no evidence of cystic changes. (E, F) Coronal and sagittal reformations further illustrate the striated enhancement in the central area of the mass (yellow arrow), as well as the presence of punctate calcification at the edge of the lesion (red arrow). The mass does not exert any obvious pressure on the surrounding tissues and blood vessels, and there is no evidence of infiltration.
Based on a preoperative diagnosis of thymoma, the patient underwent thoracoscopic extended thymic body resection and thoracoscopic thoracic adhesion release. During the surgery, a cystic tumor measuring 3.5 cm × 3.0 cm × 2.0 cm was found in the upper left region of the thymus. The tumor had a regular shape with intact borders. The mediastinal mass, including the thymus gland and bilateral anterior mediastinal fat, was resected while preserving all vital structures.
Macroscopic examination of the resected specimen revealed a grayish-pink colored mass with clusters of thickened tubular structures in the local region of the incision (Figure 2A, blue arrow). Histological examination showed an endothelial-lined sinusoid cavity surrounded by papillary proliferative spindle-shaped smooth muscle-like cells (Figure 2B). Immunohistochemical analysis revealed positive staining for Desmin (Figures 2C, D), SMA (Figure 2E), CD31 (Figures 2F, G, H), and CD34 (Figure 2I), while staining for D2-40, HMB-45, and MelanA was negative. After correlating the chest CT findings, with these pathological and immunohistological findings, the tumor was diagnosed as mediastinal lymphangioleiomyomatosis (LAM).
Figure 2
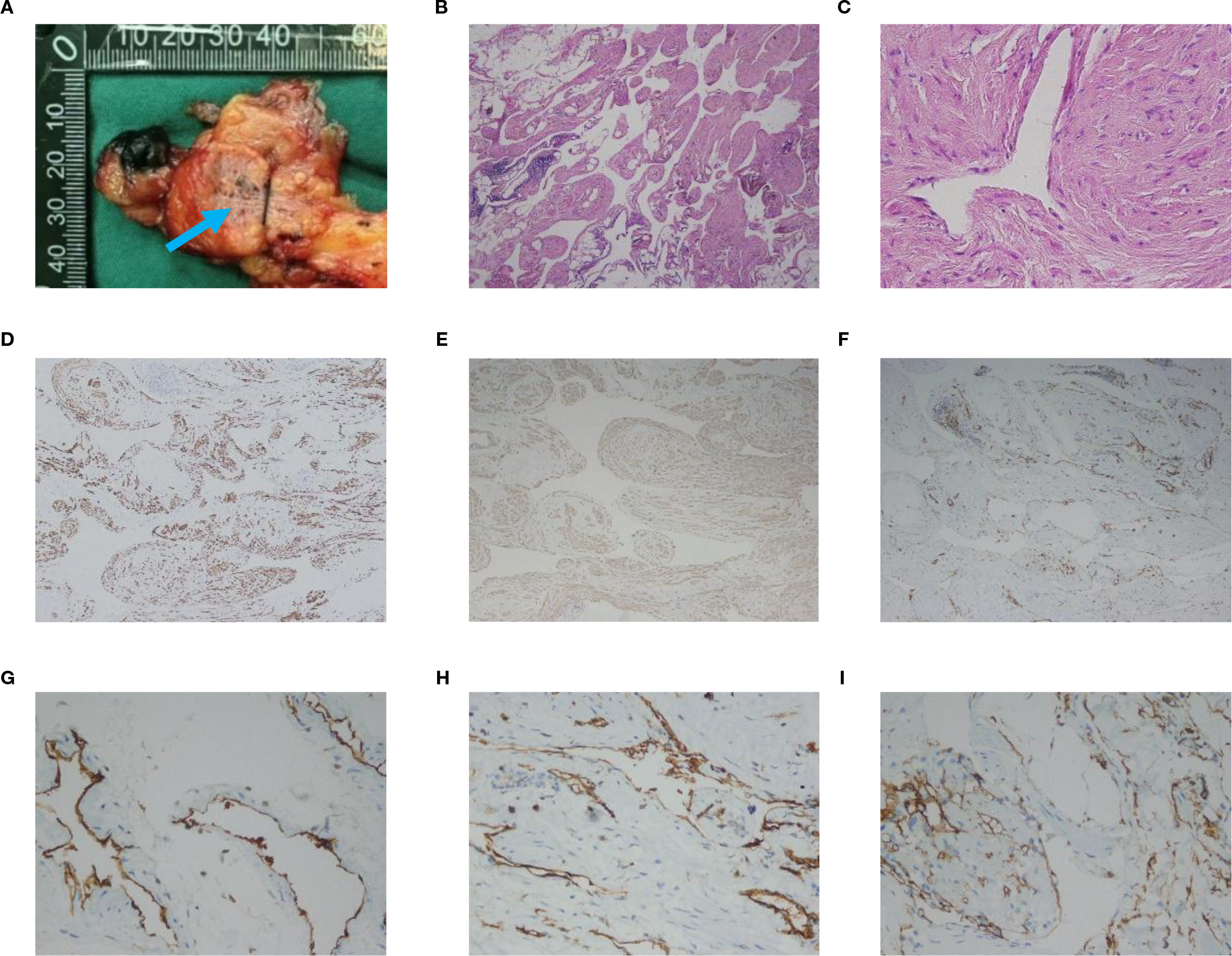
(A) Macroscopic examination of the resected specimen reveals clusters of thickened tubular tubules in the local region of the incision (blue arrow). (B, C) Histological image shows an endothelial-lined sinusoid cavity surrounded by papillary proliferative spindle-shaped smooth muscle-like cells. HE; (B) is original magnification×40; (C) is 400× magnification. (D) Immunohistochemical staining shows positive staining for Desmin (magnification ×100). (E) Immunohistochemical staining shows positive staining for SMA (magnification ×100). (F) Immunohistochemical staining shows positive staining for CD31 (magnification ×100). (G, H) Immunohistochemical staining shows positive staining for CD31 (magnification ×400). (I) Immunohistochemical staining shows positive staining for CD34 (magnification ×400).
The patient had an uncomplicated postoperative recovery and was discharged after seven days. As of one year post-surgery, the patient remains in good health without any pulmonary issues or recurrences. Unfortunately, long-term follow-up imaging beyond one year is lacking.
Discussion
LAM is a slowly progressive, low-grade, metastasizing neoplasm of women, characterized by infiltration of the lung parenchyma with abnormal smooth muscle-like cells, resulting in cystic lung destruction (1). LAM can also occur outside the lungs, although this is even rarer, with the retroperitoneum and pelvis being the most common sites of occurrence (6–8). The symptoms of LAM are non-specific, with progressive dyspnea, recurrent pneumothoraxes, and chylous effusions being common manifestations in the lungs (9). In the abdominopelvic cavity, LAM typically presents as a painless mass or abdominal distension (10). Due to the rare occurrence of extrapulmonary LAM and its atypical location, extrapulmonary LAM is often difficult to diagnose prior to surgery.
Histopathology is an important criterion of diagnosing LAM. LAM cells are spindle shaped or epithelioid with few mitoses and a bland appearance. These cells have features of smooth muscle that can be identified by antibodies, including HMB-45 antibody (11). LAM tumor cells express estrogen and progesterone receptors. Desmin is myogenic markers for smooth muscle-like cells, while D2–40 and CD34 are marker of lymphatic endothelial cells (12, 13). Immunostaining for HMB-45 is diagnostically useful, although in rare cases LAM lesions do not express this marker (14, 15). Possible causes are technical and biological factors. The test can be combined with other indicators (SMA, ER/PR). Besides, The American Thoracic Society and Japanese Respiratory Society clinical practice guidelines recommend VEGF-D ≥ 800 pg/ml as the threshold for diagnosing LAM (16).
LAM is a multisystem genetic disease caused by germline mutations in TSC genes, TSC1 or TSC2 (17). It occurs in two forms: a sporadic form and a TSC form. The former is a somatic mutation in the TSC gene, and the latter is a germ cell mutation. Statistically, approximately 80% of patients with LAM have a germ cell mutation in either TSC1 or TSC2, and only 15-20% have a disseminated mutation in the TSC gene (18). However, this result is reversed, more than 80% of patients with LAM in pulmonary clinics, registries, and trials have sporadic mutations (19). The reason for this may be the difference in severity between sporadic LAM and TSC LAM, as well as insufficient attention to the pulmonary manifestations of patients with TSC. It has been reported that LAM can be a substantial cause of mortality in patients with TSC. There is a need to intensify genetic analysis to identify TSC mutations in patients with LAM in the future.
LAM has been considered a female-specific disease. However, there are a few LAM cases reported in males. Based on a review of the literature on male LAM cases. There was no significant difference in clinical presentation between TSC-LAM patients and sporadic LAM. TSC-LAM patients were more likely to have renal Angiomyolipoma (AML) and thin-walled pneumothorax in both lungs. One article reviewed the literature of all published male LAM cases from April 1986 to October 2021 and statistically showed that the positivity rates of HMB-45, SMA, Desmin, and estrogen receptor in immunohistochemistry were approximately 40%, 76%, 89%, and 16%, respectively (20).
We report a case of male LAM occurring in the mediastinum. Immunohistochemical analysis revealed positive staining for Desmin, SMA, while staining for HMB-45 was negative. It is suspected that in male LAM, the positivity of SMA and Desmin is higher, while the positivity of HMB-45 is relatively low. Our patient did not receive genetic testing, but he had no history of TSC and no family history. The chest CT revealed no abnormalities in both lungs. Therefore, it is more likely that the patient has sporadic LAM.
Through a review of the literature on LAM reported in indexed journals such as PubMed and CNKI, it was found that there were 19 cases of LAM occurring outside the lungs. Among these cases, 13 occurred in the abdominopelvic cavity, 5 in the mediastinum, and 1 in the head. We summarized five cases of mediastinal LAM (Table 1) (6, 21–24).
Table 1
| Case report | Age & sex | Location | Imaging Findings | Treatment | Prognosis |
|---|---|---|---|---|---|
| Derweduwen et al. (6) | 32-year-old woman | upper anterior mediastinum | CT: cystic-solid mass and clear borders | thoracoscopic resection | metastasis to the neck after 2 years |
| Kataria et al. (21) | 23-month-old boy | superior mediastinum | CT: fluid-containing multiloculated cystic mass | surgical excision | survived |
| Ota et al. (22) | 70-year-old woman | anterior mediastinum | CT: round, homogeneous mass with low attenuation, regular margin; MRI: isosignal intensity on T1, high signal intensity on T2, and multiloculated heterogeneous intensity on Gd-enhanced T1-weighted images |
surgical excision | survived |
| Raghuprakash et al. (23) | 7-year-old girl | superior mediastinum | MRI: heterogenous T2 hyperintense multicystic lesion | surgical excision | survived |
| Che et al. (24) | 32-year-old woman | Inferior mediastinum | CT: multiple fat density areas | sirolimus | survived |
5 cases of mediastinal LAM.
Although there have been studies focusing on the pathological manifestations of mediastinal LAM, the imaging features have not been well-described. Based on a review of the literature on LAM, the imaging features of extrapulmonary LAM can be summarized as follows:
-
The diameter of the mass can vary from 1 cm to 20 cm, and larger lesions (diameter > 3 cm) tend to exhibit obvious cystic changes and may contain chylothorax.
-
CT imaging typically shows a cystic or cystic-solid mass with an irregular or lobulated shape and clear borders. A few reports state that there may be segregation within the mass of abdominal LAM, and the wall of the mass may have varying thickness. During contrast-enhanced scans, there is typically no enhancement in the cystic part of the mass, while the solid part may show uneven enhancement.
-
Lymph node involvement can be seen with LAM, and CT scans may reveal solid nodules with uniform density in the peripheral lymph nodes (25).
-
The masses may exert pressure on adjacent tissues and blood vessels but typically does not infiltrate them.
In our present case, the CT scan showed an irregular hypodense mass with clear borders and punctate calcification at the edge. This is consistent with previous findings reported in the literature (19). The mass did not demonstrate significant enhancement in the arterial phase, while in the venous phase, it showed striated enhancement in the central area and no abnormalities in the marginal area. The central enhancement in the venous phase may be associated with clusters of thickened tubular structures in the center of the mass. When LAM lesions contain solid components, the venous phase of CT may demonstrate mild to moderate enhancement. Pathologically, this is related to the presence of proliferating smooth muscle cells, fibrous tissue, and possible neovascularization within the solid components. The neovascularization may allow the solid components to absorb contrast agents during the CT venous phase, thereby exhibiting enhancement. In this case, the mass did not exert any obvious pressure on the surrounding tissues or blood vessels and did not manifest infiltration. The higher CT value observed on the plain chest CT in this case may be attributed to the high protein content within the lesion.
Mediastinal LAM needs to be differentiated from other mediastinal masses, including thymoma, teratoma, cystic lymphangioma, and lymphoma.
Thymoma: Thymomas are more common in individuals aged 30 to 50, with a higher incidence in men. They are typically located in the anterior and middle superior mediastinum. On CT scans, thymomas appear as irregular masses with uniform soft tissue density (26). Larger tumors may exhibit liquefaction necrosis or calcification (27). Contrast-enhanced CT shows inhomogeneous enhancement. Thymomas often locally invade the pericardium and pleura (28).
Teratoma: Teratomas are more prevalent in females aged 20 to 35 or in their early years. They are usually located in the anterior mediastinum. On CT scans, teratomas present as well-demarcated round unilocular or multilocular cystic lesions with an irregular wall of varying thickness. Punctate calcifications may be present inside the tumor (29). The density of the tumor is uneven, and it may contain calcification, ossification, or fat. Contrast-enhanced CT shows contrast enhancement of the wall and solid components.
Cystic lymphangioma: Cystic lymphangiomas are more common in males and can occur at any age (30). They are typically located in the anterior or middle superior mediastinum. On CT scans, these lesions appear as round multilocular cystic masses with sharp demarcation (31). They usually have a homogeneous water-like density and rarely show calcification (32). On contrast-enhanced CT, the cystic wall and septum show moderate enhancement (33). These tumors are characterized by spreading along the vascular space.
Lymphoma: Lymphomas primarily occur in young adults, with an average age of 30 years. They are mostly located in the anterior mediastinum and commonly present as primary mediastinal diffuse large B-cell lymphomas. On CT, lymphomas appear as irregular or lobulated masses. Larger tumors may contain hypodense necrosis in the center (34). Contrast-enhanced CT shows mild-to-moderate enhancement. Lymphomas often invade adjacent mediastinal structures, such as blood vessels, pleura, and lungs. Pleural and pericardial effusions can be observed in approximately half of the cases (35).
Currently, the rarity of patients with extrapulmonary LAM limits therapeutic measures. Considering the limited understanding of extrapulmonary LAM disease, traditional surgical resection is generally preferred when imaging shows an equivocal diagnosis, However, in patients with milder symptoms, surgical resection may be unacceptable and unnecessary. mTOR inhibitors, such as Sirolimus and Everolimus, are the primary clinical treatment for LAM (36). The American Thoracic Society/Japan Respiratory Society (ATS/JRS) guidelines state that sirolimus improves lung function and quality of life, and it is recommended for patients with LAM who have abnormal or decreased lung function (16). However, sirolimus does not reverse cystic changes or eliminate LAM tumors and may have potential side effects including nausea, diarrhea, mucositis, acne, lower extremity swelling, and hyperlipidemia (37, 38). The combination of surgery and sirolimus may be a more effective strategy for slowing the progression of LAM.
In conclusion, we have reported a rare case of mediastinal LAM with nonspecific clinical presentation and benign histologic characteristics. LAM is a slowly progressive disease that can involve multiple organs simultaneously or sequentially, highlighting the importance of early diagnosis. On CT imaging, mediastinal LAM appears as an irregular hypodense mass with clear borders. During the enhancement scan, the mass does not exhibit significant enhancement in the arterial phase but shows striated enhancement in the central area in the venous phase, with no abnormalities in the marginal area. It should be noted that this conclusion was derived from a single case, and its generalizability is limited. When mediastinal LAM is suspected, radiologists should recommend a chest CT examination. The presence of typical pulmonary LAM imaging features, such as multiple thin-walled cysts of uniform size randomly distributed in both lungs, can be helpful in suggesting the diagnosis of mediastinal LAM. Radiologists should be familiar with the imaging characteristics of mediastinal LAM to consider it as a possibility in the differential diagnosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Writing – original draft. QW: Writing – original draft. LS: Writing – review & editing. LZ: Writing – review & editing. YD: Writing – review & editing. FH: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Inner Mongolia Autonomous Region Natural Science Foundation (2020MS08051), Inner Mongolia Autonomous Region Education Science Research “13th Five-Year Plan” Project (NGJGH2020295), Inner Mongolia Medical University (YKD2022QN019), Inner Mongolia Medical University Joint Project (YKD2022LH020), Clinical Medical Research and Clinical New Technology Promotion Program of Inner Mongolia Autonomous Region Physicians Association (YSXH2024KYF042), Innovation and Entrepreneurship Project of Inner Mongolia Medical University(S202410132010, S202410132014), Youth Program of Affiliated Hospital of Inner Mongolia Medical University (2024GLLH0267).
Acknowledgments
The authors are grateful to the patient and his family for their kind cooperation.
Conflict of interest
Author YD was employed by company Siemens Medical Systems Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
McCarthy C Gupta N Johnson SR Yu JJ McCormack FX . Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med. (2021) 9:1313–27. doi: 10.1016/S2213-2600(21)00228-9
2
Sbaraglia M Bellan E Dei Tos AP . The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. (2021) 113:70–84. doi: 10.32074/1591-951X-213
3
Yalikun K Abudula M Aisha M Fu Q Sang W Kadeer K et al . A histopathologic diagnosis of brain lymphangiomyoma, clinically misdiagnosed as simple angiomyxoma: case report. Int J Clin Exp Pathol. (2019) 12:2753–7.
4
Wakida K Watanabe Y Kumasaka T Seyama K Mitani K Hiraki T et al . Lymphangioleiomyomatosis in a male. Ann Thorac Surg. (2015) 100:1105–7. doi: 10.1016/j.athoracsur.2014.11.069
5
Kimura Y Jo T Hashimoto Y Kumazawa R Ishimaru M Matsui H et al . Epidemiology of patients with lymphangioleiomyomatosis: A descriptive study using the national database of health insurance claims and specific health checkups of Japan. Respir Investig. (2024) 62:494–502. doi: 10.1016/j.resinv.2024.03.010
6
Derweduwen AM Verbeken E Stas M Verschakelen J Coolen J Verleden G et al . Extrapulmonary lymphangioleiomyomatosis: a wolf in sheep’s clothing. Thorax. (2013) 68:111–3. doi: 10.1136/thoraxjnl-2012-201973
7
Xiao S Chen Y Tang Q Xu L Zhao L Wang Z et al . Pelvic lymph node lymphangiomyomatosis found during surgery for gynecological fallopian tube cancer: A case report and literature review. Front Med. (2022) 9:917628. doi: 10.3389/fmed.2022.917628
8
Kebria M Black D Borelli C Modica I Hensley M Chi DS . Primary retroperitoneal lymphangioleiomyomatosis in a postmenopausal woman: a case report and review of the literature. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2007) 17:528–32. doi: 10.1111/j.1525-1438.2007.00785.x
9
Korzeniewska-Koseła M Maziarka D Wesołowski S Langfort R Słodkowska J Bestry I et al . Pulmonary lymphangioleiomyomatosis: presentation and results of treatment. Pneumonol Alergol Pol. (2001) 69:626–34.
10
Han JM Lee KH Kim SJ Rhim CC Park YH Kang JB et al . A case of lymphangioleiomyomatosis originated in the pelvic cavity. J Gynecol Oncol. (2008) 19:195–8. doi: 10.3802/jgo.2008.19.3.195
11
Johnson SR Taveira-DaSilva AM Moss J . Lymphangioleiomyomatosis. Clin Chest Med. (2016) 37:389–403. doi: 10.1016/j.ccm.2016.04.002
12
Suzuki K Nagasaka K Oda K Abe H Maeda D Matsumoto Y et al . A case of lymphangioleiomyomatosis associated with endometrial cancer and severe systemic lupus erythematosus. BMC Cancer. (2016) 16:390. doi: 10.1186/s12885-016-2413-z
13
Fu W Li Y Li H Yang P Xing X . Solitary extrapulmonary lymphangioleiomyomatosis of the liver: A case report and literature review. Exp Ther Med. (2016) 12:1499–502. doi: 10.3892/etm.2016.3502
14
Matsui K Tatsuguchi A Valencia J Yu ZX Bechtle J Beasley MB et al . Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. (2000) 31:1242–8. doi: 10.1053/hupa.2000.18500
15
Rolim I Makupson M Lovrenski A Farver C . Cathepsin K is superior to HMB45 for the diagnosis of pulmonary lymphangioleiomyomatosis. Appl Immunohistochem Mol Morphol AIMM. (2022) 30:108–12. doi: 10.1097/PAI.0000000000000968
16
Gupta N Finlay GA Kotloff RM Strange C Wilson KC Young LR et al . Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An official american thoracic society/Japanese respiratory society clinical practice guideline. Am J Respir Crit Care Med. (2017) 196:1337–48. doi: 10.1164/rccm.201709-1965ST
17
Carsillo T Astrinidis A Henske EP . Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. (2000) 97:6085–90. doi: 10.1073/pnas.97.11.6085
18
Gibbons E Minor BMN Hammes SR . Lymphangioleiomyomatosis: where endocrinology, immunology and tumor biology meet. Endocr Relat Cancer. (2023) 30:e230102. doi: 10.1530/ERC-23-0102
19
Tobino K Johkoh T Fujimoto K Sakai F Arakawa H Kurihara M et al . Computed tomographic features of lymphangioleiomyomatosis: evaluation in 138 patients. Eur J Radiol. (2015) 84:534–41. doi: 10.1016/j.ejrad.2014.12.008
20
Zhang H Hu Z Wang S Wu K Yang Q Song X . Clinical features and outcomes of male patients with lymphangioleiomyomatosis: A review. Med (Baltimore). (2022) 101:e32492. doi: 10.1097/MD.0000000000032492
21
Kataria R Bhatnagar V Gupta SD Mitra DK . Mediastinal lymphangiomyoma in a child: report of a case. Surg Today. (1998) 28:1084–6. doi: 10.1007/BF02483968
22
Ota H Kimura Y Kawai H Ogawa JI . Mediastinal lymphangiomyoma in an adult: Report of a case. Surg Today. (2010) 40:365–8. doi: 10.1007/s00595-009-4074-x
23
Raghuprakash S Ramakrishnan P Chittimuri C Agasty S Arava S Choudhary SK . Successful surgical correction of recurrent primary chylopericardium due to mediastinal lymphangiomyoma by total pericardiectomy and diaphragmatic fenestration. Indian J Thorac Cardiovasc Surg. (2022) 38:545–8. doi: 10.1007/s12055-022-01356-9
24
Chen HB Xu XH Yu CG Wan MT Feng CL Zhao ZY et al . Tuberous sclerosis complex-lymphangioleiomyomatosis involving several visceral organs: A case report. World J Clin Cases. (2021) 9:7085–91. doi: 10.12998/wjcc.v9.i24.7085
25
Avila NA Kelly JA Chu SC Dwyer AJ Moss J . Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. (2000) 216:147–53. doi: 10.1148/radiology.216.1.r00jl42147
26
Tomaszek S Wigle DA Keshavjee S Fischer S . Thymomas: review of current clinical practice. Ann Thorac Surg. (2009) 87:1973–80. doi: 10.1016/j.athoracsur.2008.12.095
27
Qu YJ Liu GB Shi HS Liao MY Yang GF Tian ZX . Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol. (2013) 20:66–72. doi: 10.1016/j.acra.2012.08.002
28
Chen JL Weisbrod GL Herman SJ . Computed tomography and pathologic correlations of thymic lesions. J Thorac Imaging. (1988) 3:61–5. doi: 10.1097/00005382-198801000-00010
29
Takeda S Miyoshi S Ohta M Minami M Masaoka A Matsuda H . Primary germ cell tumors in the mediastinum. Cancer. (2003) 97:367–76. doi: 10.1002/cncr.11068
30
Konen O Rathaus V Dlugy E Freud E Kessler A Shapiro M et al . Childhood abdominal cystic lymphangioma. Pediatr Radiol. (2002) 32:88–94. doi: 10.1007/s00247-001-0612-4
31
Davidson AJ Hartman DS . Lymphangioma of the retroperitoneum: CT and sonographic characteristic. Radiology. (1990) 175:507–10. doi: 10.1148/radiology.175.2.2183287
32
Yang DM Jung DH Kim H Kang JH Kim SH Kim JH et al . Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. RadioGraphics. (2004) 24:1353–65. doi: 10.1148/rg.245045017
33
Bonhomme A Broeders A Oyen RH Stas M De Wever I Baert AL . Cystic lymphangioma of the retroperitoneum. Clin Radiol. (2001) 56:156–8. doi: 10.1053/crad.2000.0162
34
Totanarungroj K Watcharaporn C Muangman N . Helpful CT findings for giving specific diagnosis of anterior mediastinal tumors. J Med Assoc Thail Chotmaihet Thangphaet. (2010) 93:489–96.
35
Takahashi K Al-Janabi NJ . Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging JMRI. (2010) 32:1325–39. doi: 10.1002/jmri.22377
36
Czarnecka AM Skoczylas J Bartnik E Świtaj T Rutkowski P . Management strategies for adults with locally advanced, unresectable or metastatic Malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manag Res. (2023) 15:615–23. doi: 10.2147/CMAR.S351284
37
O’Malley D Gupta N McCarthy C . Current concepts in the pathogenesis and clinical management of lymphangioleiomyomatosis. Curr Opin Pulm Med. (2025) 31:494–503. doi: 10.1097/MCP.0000000000001185
38
Interstitial Lung Disease Group, Chinese Thoracic Society, Chinese Medical Association, Expert Consensus Group of Lymphangioleiomyomatosis, Rare Diseases Research Center, Chinese Academy of Medical Sciences, Rare Diseases Society et al Consensus Statement: sirolimus (rapamycin) as therapy for lymphangioleiomyomatosis (2018). Chin J Tuberc Respir Dis. (2019) 42:92–7. doi: 10.3760/cma.j.issn.1001-0939.2019.02.002
Summary
Keywords
lymphangioleiomyomatosis (LAM), mediastinum, extrapulmonary, computed tomography (CT), mediastinal imaging, differential diagnosis
Citation
Wang Y, Wang Q, Shi L, Zhao L, Dou Y and Hao F (2025) Mediastinal lymphangioleiomyomatosis: a case report and literature review. Front. Oncol. 15:1588165. doi: 10.3389/fonc.2025.1588165
Received
05 March 2025
Accepted
31 July 2025
Published
09 September 2025
Volume
15 - 2025
Edited by
Jindong Xie, Sun Yat-sen University Cancer Center (SYSUCC), China
Reviewed by
Bingyu Li, Tongji University, China
Xuejiao Wu, Fudan University, China
Updates
Copyright
© 2025 Wang, Wang, Shi, Zhao, Dou and Hao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fene Hao, hfe1022@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.