- 1Department of Pediatric Medicine, Division of Critical Care Medicine, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 2Department of Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 3Pediatric Intensive Care Unit, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
- 4Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 5Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 6Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN, United States
- 7Division of Pediatric Critical Care Medicine, St. Louis Children’s Hospital, Washington University School of Medicine, St. Louis, MO, United States
- 8Institute for Informatics, Washington University School of Medicine, St. Louis, MO, United States
- 9Department of Critical Care, Le Bonheur Children’s Hospital, Memphis, TN, United States
- 10Extracorporeal Therapies Service Line, Le Bonheur Children’s Hospital, Memphis, TN, United States
Extra Corporeal Life Support (ECLS) for pediatric oncology and stem cell transplant patients over the past two decades has made progress. Substantial improvements in ECLS, Continuous Renal Replacement Therapy (CRRT), and mechanical ventilation techniques, along with enhanced anticoagulation management and infection control, have contributed to better patient outcomes. Additionally, advancements in HLA matching, donor selection, and the management of chemotherapy and transplant complications have further improved survival rates. The authors propose establishing an expert team and a standardized process to evaluate ECLS candidacy, addressing past controversies and optimizing outcomes for this vulnerable population. The criteria for candidacy have evolved significantly, necessitating expert evaluation.
1 Introduction
Survival of pediatric cancer has substantially improved over the last 2 decades, with a 5-year overall survival of ~ 80% largely due to enhanced diagnostics, novel treatment regimens, targeted therapies with reduced toxicities, and improved supportive care and management of treatment-related complications, including those that occur after hematopoietic cell transplantation (HCT) (1). Despite these advances, the survival of pediatric patients with cancer requiring critical care support remains lower than that of the general pediatric intensive care unit (PICU) patients (2). Nevertheless, recent advances in critical care management, such as early PICU admissions, careful use of noninvasive ventilation, lung-protective mechanical ventilation for intubated patients, and timely diagnostics for infections, have improved survival for PICU patients, including immunocompromised patients (2, 3). Additionally, advances in extracorporeal life support (ECLS) circuity and anticoagulation strategies in the general PICU population are improving ECLS outcomes and mitigating associated morbidity and mortality. Considering this current landscape in onco-critical care, we explored the evolving role of ECLS in caring for pediatric oncology and HCT patients, a population in which this intervention was once considered too high risk. Additionally, we share our current protocol for evaluating patient candidacy and facilitating referrals for ECLS.
2 Improving outcomes of children with cancer
Registry data from the American Cancer Society reveals that although the incidence of childhood cancer has increased, cancer-related deaths among children have decreased almost 70%: from 6.3 per 100,000 in 1970 to 1.9 per 100,000 in 2020 (4) A similar reduction occurred in adolescents (aged 13– <18 years), with cancer-related deaths declining 64% from 7.2 to 2.6 per 100,000 (4). Likely factors contributing to these improvements include large cooperative trials refining intensive multimodal therapies and advances in molecular profiling, which have enhanced prognostication and risk-adapted treatments (5). Additional scientific advances have facilitated the integration of targeted therapies, which have improved outcomes in cases where traditional approaches were ineffective (6–8). Furthermore, immunotherapy has become common for many high-risk or relapsed malignancies [e.g., dinutuximab for neuroblastoma and blinatumomab for B-cell acute lymphoblastic leukemia] and has greatly improved overall survival (6, 9–12). Despite these advances, treatment-related mortality remains a major cause of cancer mortality in children. More than 20% of childhood cancer deaths in high-income countries in the past 20 years have been attributed to treatment complications (13, 14). This finding underscores the ongoing need for better supportive care and cancer-directed treatments.
3 Improving outcomes of children after hematopoietic cell transplantation
The number of HCTs performed in the U.S. has nearly doubled over the past decade, reaching ~23,000 annually (15). Of those, ~1,300 are performed in children and young adults, with a modest annual increase (15, 16). Short- and long-term survival of children undergoing allogeneic HCT has improved concurrently: short-term (100 days post-HCT) survival rose from 86% (2000–2009) to 93% (2020–2019), while long-term (3-year) survival increased from 60% to 74%(15). These results are attributed to advances in managing disease relapse and acute and chronic graft-versus-host disease (GVHD); preventing infections; and better supportive care during the peri-transplant period. During the first 100 days post-HCT, organ failure, infection, and relapse are the leading causes of death in pediatric patients, especially in those who receive transplants from haploidentical or unrelated donors (15). Beyond that period, relapse remains the predominant cause of death.
Although acute organ failure caused by chemotherapy-related toxicity or genetic predisposition can be challenging, interventions can be tailored to improve outcomes. Mortality rates for pediatric HCT patients requiring critical care support have dramatically decreased from nearly 80% in the late 1990s and early 2000s, to 20% overall (and 50% for those requiring mechanical ventilation) since 2010 (16–22). These improvements likely result from early intensive care unit (ICU) referrals and enhanced multidisciplinary treatment strategies.
GVHD poses major risks for treatment-related morbidity and mortality after HCT. However, over the past decade, the frequency and severity of GVHD has decreased, and HCT outcomes have improved. Enhanced donor/recipient matching using high-resolution HLA (human leukocyte antigen) typing increased the use of reduced-intensity regimens, and T-cell–depleted grafts or post-HCT cyclophosphamide have all contributed to decreasing transplant-related mortality (23–25).
Several strategies have also been employed to reduce the risk of relapse after HCT. Using HLA-mismatched donors not only expands the donor pool but also enhances the graft-versus-leukemia effect. Strategies to achieve deeper remission before HCT, the application of cellular therapies, and optimizing conditioning regimens while minimizing toxicity have collectively reduced relapse rates (26–30). Although many of these strategies were initially developed for adult patients, similar trends are evident in pediatric populations (31–34).
4 Advances in managing challenges that affect outcomes of pediatric oncology and HCT patients
4.1 Infections
Patients undergoing HCT face a heightened risk of infection and associated morbidity (16). Infections and chemotherapy exposure can exacerbate organ dysfunction post-HCT, necessitating critical care support, mechanical ventilation, dialysis, and in rare cases ECLS (35). Prompt diagnosis and treatment of infections is crucial for ECLS success and overall outcomes (36). Traditional culture-based diagnosis of infection is often hindered by the patient’s prior antimicrobial exposure (37, 38). Furthermore, prophylactic antimicrobials can mitigate viral, bacterial, and fungal infections; however, their use also can lead to infections of multidrug-resistant organisms (39–42).
Recent advances in diagnostic tests and therapeutic interventions have enhanced our ability to detect and treat infections. For example, clinical molecular diagnostics can identify microbial pathogens, often surpassing conventional diagnostic methods (43–45). These tests expedite results, improve yield, and reduce hospitalization and antibiotic usage (46–49). Panels may include resistance genes, facilitating prompt targeted therapies (50). Such tests can be performed on various samples, including blood, respiratory tract specimens, stool, cerebrospinal fluid, and tissue (51). Metagenomics next-generation sequencing shows promise for advanced diagnosis, especially in complex cases [e.g., meningitis or encephalitis] (52).
Novel therapeutic agents, such as letermovir and maribavir for cytomegalovirus infections and novel beta-lactams combined with beta-lactamase inhibitors for multidrug-resistant bacterial infections, are increasingly utilized in cases where conventional treatments are limited by toxicity or resistance (53–55). Unfortunately, the current pipeline for clinical antibacterial drugs is limited in scope and innovation; thus, it inadequately addresses the growing threat of antibiotic-resistant bacteria (56–58). Nevertheless, advances continue to improve survival of these patients.
4.2 Acute kidney injury
Children with hematologic malignancies are susceptible to renal dysfunction, which is often described as acute kidney injury (AKI). Patients with tumor lysis syndrome, especially those with hyperphosphatemia, are at increased risk of AKI (59, 60). Severe tumor lysis syndrome is a relatively common indication necessitating ICU admission for frequent monitoring of renal function and metabolic status. In a cohort of 222 children with tumor lysis syndrome, 20 (9%) required CKRT, all of whom survived and regained renal function (60).
AKI is also prevalent in critically ill children after HCT; the incidence ranges from 21% to 84%, and severe AKI occurs in 12% (61). The etiology of AKI is often multifactorial, involving nephrotoxicity caused by medications (e.g., chemotherapy or antimicrobials), compromised renal perfusion due to capillary leak caused by hyperinflammatory states, sepsis, and transplant-related complications, such as sinusoidal obstruction syndrome and thrombotic microangiopathy (62).
Children with AKI have an increased risk of mortality. In a cohort of 484 post-HCT patients, AKI developed in 38%, and severe AKI developed in 42% (60). The relative risk of death is 4.6 times higher in patients with severe AKI, and ~30% of the severe AKI group required CKRT (61, 63).
Historical data indicate that survival of patients requiring CKRT is 26% to 33% (64). In a recent study of 68 pediatric HCT patients requiring CKRT, survival was 54% (65). In a cohort of children with severe sinusoidal obstruction syndrome post-HCT who underwent CKRT, 62% survived to ICU discharge (66). Enhanced survival is likely tied to better recognition of the adverse effects of fluid overload and the earlier initiation of CKRT when fluid overload exceeds 10%. Early detection and prevention of AKI progression are critical for improving outcomes. Tools such as electronic medical record alerts, renal injury risk–stratification systems (e.g., renal angina index), and biomarkers (e.g., cystatin C, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin) can aid in the early detection of AKI (62, 67).
4.3 Respiratory failure
Respiratory failure in the pediatric oncology and HCT population commonly results in PICU admission and contributes to morbidity and mortality (1, 22, 68, 69). Key treatment strategies for this complication include early recognition, lung-protective ventilation, and minimizing patient/ventilator asynchrony (70). Newer technologies, such as proportional assist ventilation (PAV), neurally adjusted ventilatory assist (NAVA), and airway pressure release ventilation (APRV), also improve invasive mechanical ventilation (71, 72). Informed decision-making based on a better understanding of physiological principles, underlying pathobiology, and treatment modalities has also improved treatment (73–76). Enhanced diagnostic capabilities, including Pediatric Early Warning Scores, and advanced tools, like computed tomography scans, bronchoalveolar lavage, and lung biopsies, have facilitated timely interventions, and comprehensive management strategies, such as the Pediatric Acute Lung Injury Consensus Conference version 2, have guided clinical practice (21, 77–88). Early ICU referrals enhanced multidisciplinary strategies, and more knowledge about the mechanisms underlying lung injury, including biotrauma and stress, have further informed clinical management (71, 72, 89–93).
Advanced monitoring, such as real-time measurements of volumetric CO2, dead space, and surrogates for work of breathing (e.g., esophageal manometry, airway occlusion pressure, and diaphragm thickness), have substantially enhanced our ability to optimize ventilatory support (94–99). Prone positioning has also emerged as a safe intervention in pediatrics and an essential intervention that improves oxygenation and overall outcome in adults (100–104). Ongoing multicenter trials, including the Protocol for the Prone and Oscillation Pediatric Clinical Trial [PROSpect; NCT: 03896763], are refining therapeutic strategies involving high-frequency oscillatory ventilation (HFOV) and prone positioning to further enhance patient care (105).
Ancillary therapies for both restrictive and obstructive respiratory failure in pediatric patients have been improved. A better understanding of fluid dynamics and clinical management strategies, including the use of diuretics and continuous kidney replacement therapy (CKRT), has been crucial in optimizing care (106–108). Additionally, therapeutic interventions, such as surfactant administration, inhaled nitric oxide, neuromuscular blockade, lung recruitment maneuvers, bronchodilators, immune modulation, corticosteroids, and ECLS, have expanded the therapeutic arsenal in pediatric patients with respiratory failure (88, 104, 109–115).
Looking ahead, the potential for personalized therapies is promising, with the integration of novel endothelial, genomic, and epigenetic biomarkers to assess the severity of respiratory failure (116–119). Research on molecular signaling pathways and fluid channel mechanisms may enhance our understanding of respiratory failure and identify new therapeutic targets (120–122). Additionally, mesenchymal cell–based therapies show promise for improving outcomes of pediatric respiratory failure (123). Leveraging these advances, the field is positioned to transform the landscape of pediatric respiratory failure, thereby paving the way for more precise, effective interventions to optimize care and outcomes of pediatric oncology and HCT patients.
5 Advances in ECLS for pediatric oncology and HCT patients
The improved approaches to treating the complications described above have rekindled interest in advanced life support, including ECLS, which was previously discouraged for pediatric oncology patients. Despite technological advances in ECLS pumps and circuits and improved anticoagulation strategies, evaluating ECLS candidacy in children with cancer remains complex (115, 124–126). This complexity arises from prognostic uncertainties related to the underlying malignancy, heightened risk of infection, and severe ECLS-related complications, such as bleeding and thrombosis, which are more severe in pediatric patients with cancer than in the general PICU population (127).
Current literature on ECLS candidacy among pediatric patients with cancer is limited and relies primarily on retrospective studies, case series, and anecdotal accounts (127). Many of those studies lack detailed data on the type of cancer, therapeutic exposures, and relevant variables crucial for clinical evaluations (128).
Over the past 2 decades, several pediatric oncology patients with cardiorespiratory failure that did not respond to comprehensive multimodal medical therapy have been supported by ECLS, yielding variable outcomes (129–135). A recent systematic review reported that the mortality rate of pediatric patients with cancer who underwent ECLS was comparable to that of the general PICU population who underwent ECLS: 60% vs. 55%, respectively, with inconsistent reporting of complications across studies (127). This elevated mortality can be attributed to the immunocompromised state, reduced organ reserve, and overall fragility of the patients with cancer. Additionally, studies have indicated a strong correlation among respiratory failure severity, mechanical ventilation duration, and mortality rates on ECLS (126, 136, 137).
Although bleeding complications are more prevalent in patients with cancer on ECLS, the incidence of nosocomial infections is similar to that in immunocompetent patients (138). Increased bleeding risk may arise from coagulopathy and thrombocytopenia associated with the underlying cancer and its treatment, compounded by systemic anticoagulation during ECLS (124, 133, 135). Alternatively, the prophylactic use of antimicrobials in immunocompromised patients with cancer may explain the comparable rates of nosocomial infections (138). Despite these findings, the lack of high-quality studies limits our ability to draw robust conclusions about the benefits of ECLS and its optimal timing in pediatric patients with cancer.
Patients undergoing HCT for malignant or nonmalignant disorders may also require critical care support. Over the last 2 decades, the probability of survival of HCT patients requiring PICU admission has improved to 48% to 75% (139, 140). This progress has renewed interest in using ECLS for HCT patients experiencing refractory cardiopulmonary failure (115).
Recent data indicate that survival of HCT patients supported by ECLS has gradually increased, though candidacy determination remains challenging (133–135, 141, 142). An analysis of the Extracorporeal Life Support Organization’s registry (1990–2020) revealed an increase in ECLS use among pediatric HCT patients and a substantial increase in survival to hospital discharge over the last decade (26% vs. 5%-10%) (142). Factors associated with higher mortality included the presence of malignancy, high peak pressures >30 during conventional mechanical ventilation before ECLS, and pulmonary or metabolic complications during ECLS (142). This increased survival is likely due to advances in HCT, critical care, and ECLS technology. For instance, adoptively transferring immune cells (e.g., virus-specific T cells) that target treatment-resistant viral infections during periods of immune reconstitution helps suppress life-threatening infections, and coating circuits and oxygenators with new anticoagulants (e.g., bivalirudin) helps mitigate bleeding risks (126, 143–145).
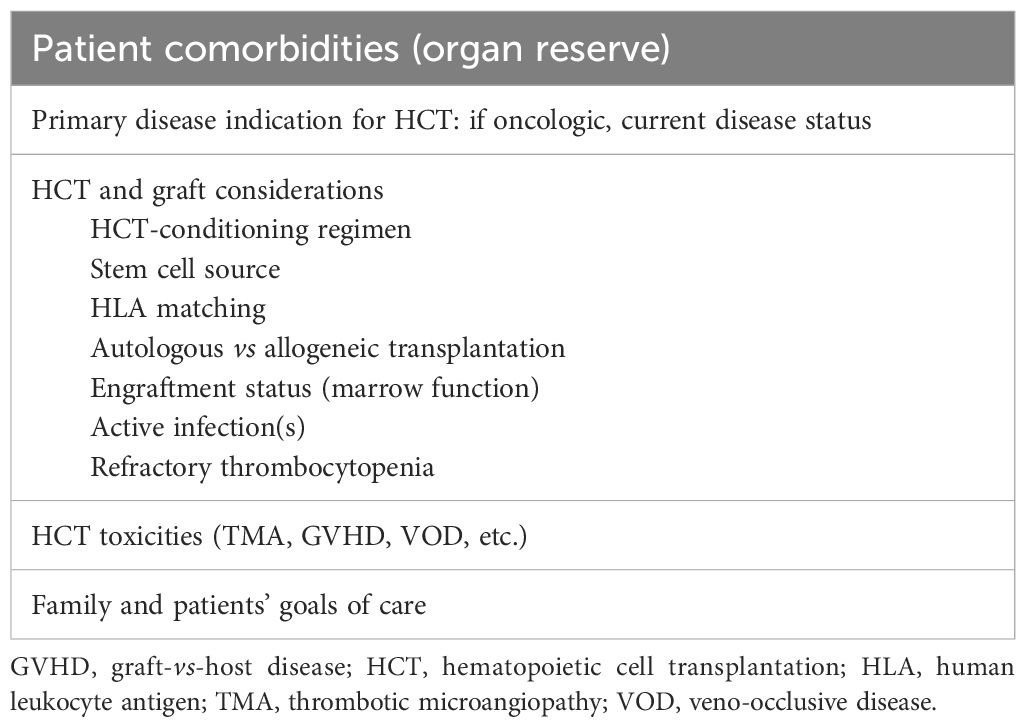
Recent guidelines indicate that ECLS may be considered for HCT patients with nonmalignant diseases or those with low risk of malignancy (re)occurrence at the time of ECLS evaluation (115). Additionally, the type of transplant (autologous vs. allogeneic) and the presence of active GVHD should be included in the ECLS candidacy evaluation (146–148).
During ECLS candidacy evaluations, clinicians must assess the reversibility of the critical illness, the extent of organ failure, transfusion dependence, bone marrow reserve, and the goals of care set by the patient and family (149–152). The importance of understanding improvements in cancer-directed and post-HCT care, as part of the ECLS candidacy decision and how it relates to the critical illness and the potential for recovery, is essential for ECLS success–the mortality risk due to ECLS-related complications increases by 1% to 3% per day of support (124, 153). In HCT patients, the number of organ failures and the need for invasive mechanical ventilation or renal replacement therapy are independent predictors of mortality that should be meticulously evaluated, as is the presence and response of GVHD to treatment (114, 149, 154).
5.1 Advances in managing anticoagulation during ECLS
Despite advances in ECLS technology since its inception in the 1970s, thrombotic and bleeding complications remain a substantial cause of morbidity and mortality (155). Managing anticoagulation in pediatric patients during ECLS poses a challenge–sufficient anticoagulation to prevent thrombosis must be balanced with coagulopathy to reduce bleeding. Historically, unfractionated heparin was the anticoagulant of choice (156). However, its reliance on antithrombin, the risk of heparin-induced thrombocytopenia, and unpredictable responses have spurred interest in using thrombin inhibitors as alternatives (157–160). Thrombin inhibitors show at least noninferior efficacy compared to unfractionated heparin without an increased risk of bleeding complications, and they offer simpler laboratory monitoring.
Additionally, literature on standardized blood product replacement thresholds that correlate with patient outcomes is lacking (161, 162). A recent systematic review highlighted these gaps, providing expert consensus recommendations, while identifying key areas for future research (163). The complexity of patient factors, underlying pathologies, and circuit variables in managing anticoagulation in pediatric patients during ECLS underscores the inadequacy of a one-size-fits-all approach. Biomedical informatics researchers are developing decision-support tools that incorporate multifaceted variables, thereby facilitating personalized, clinically applicable strategies to enhance patient outcomes.
5.2 Our process for assessing ECLS candidacy
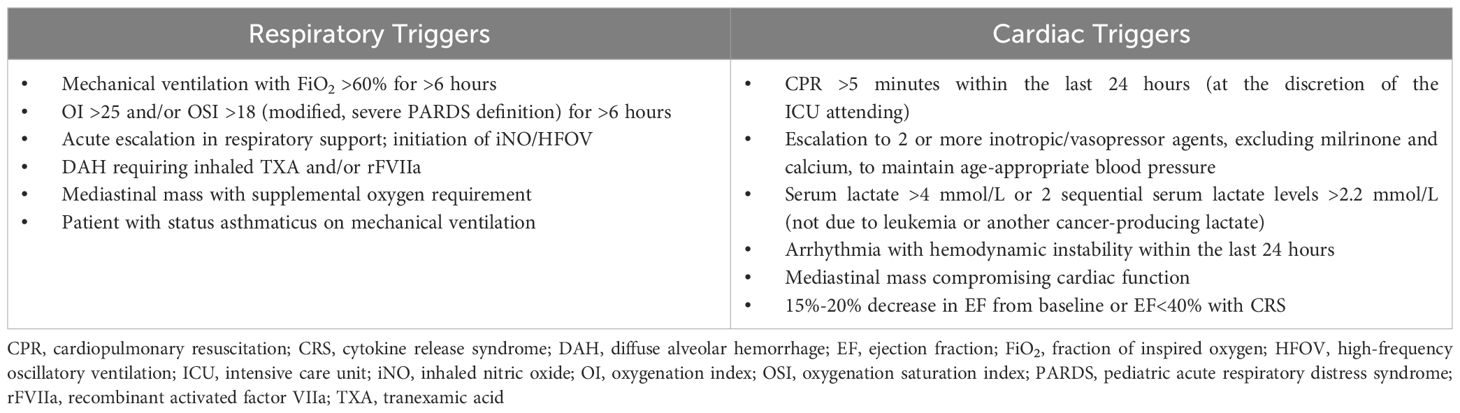
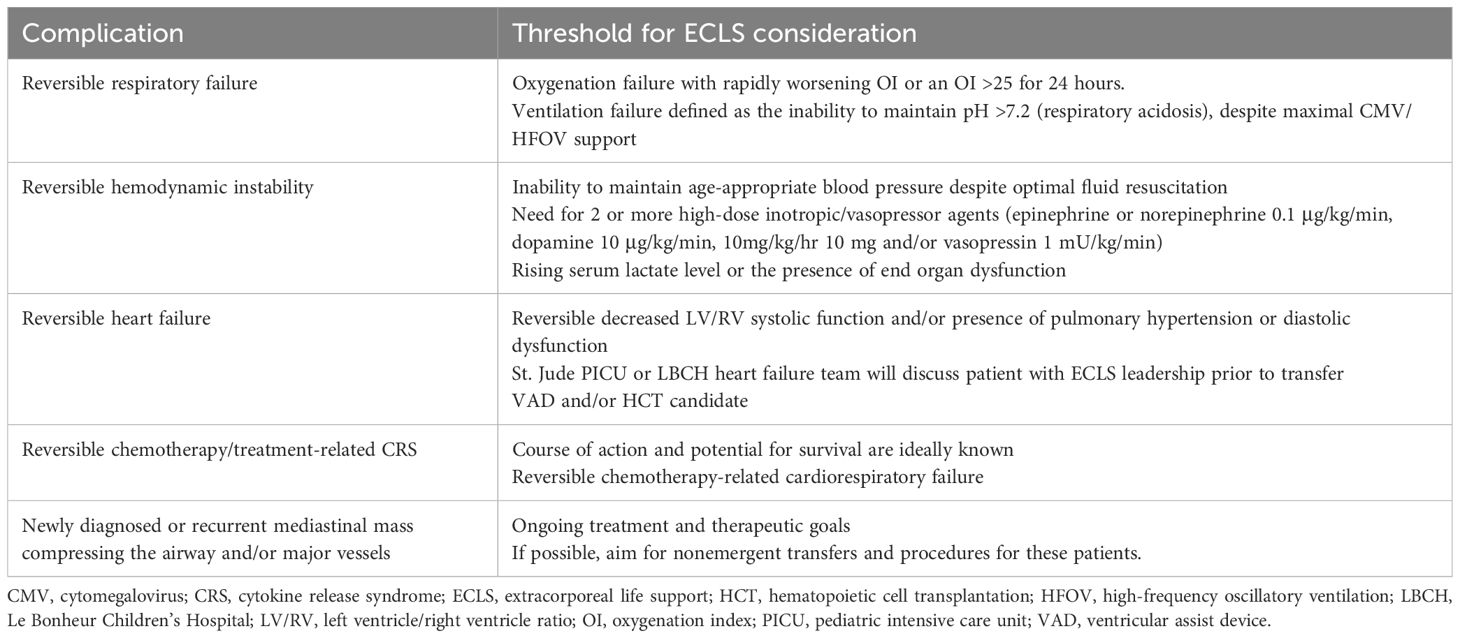
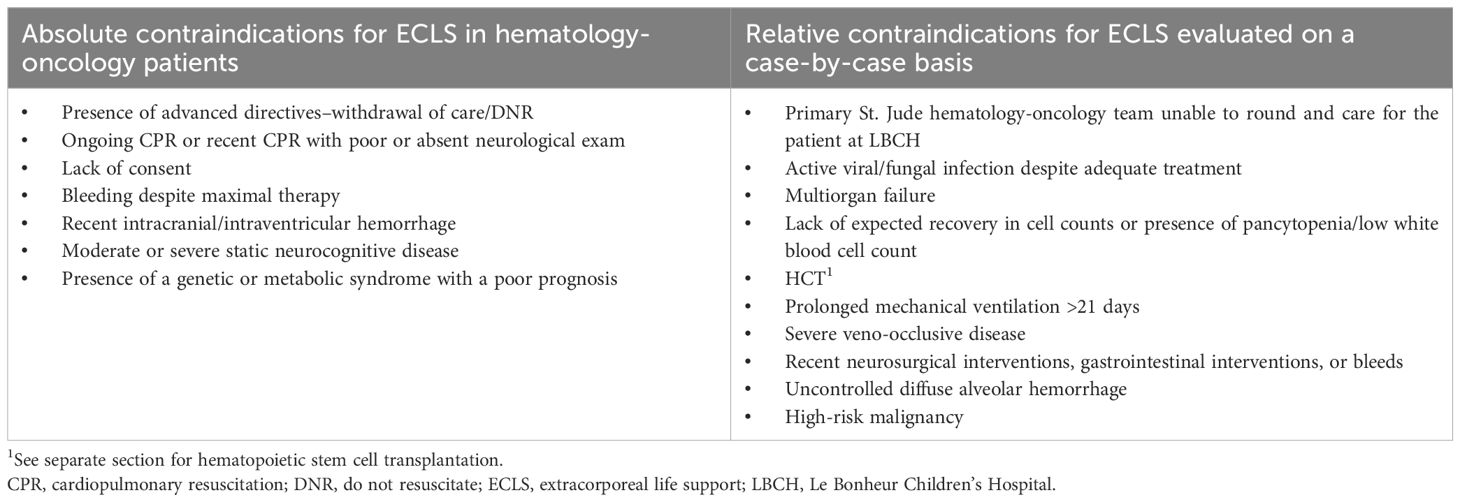
As a quaternary pediatric hematology/oncology center lacking on-site ECLS capabilities, we developed a standardized protocol for early identification and transfer of high-risk patients. A collaborative intensivist team from St. Jude and Le Bonheur Children’s Hospital (LBCH) evaluates ECLS candidacy using defined clinical and oncologic parameters. Patients are stratified as “ECLS-unsafe” (those with irreversible organ injury or prolonged instability) or “ECLS watchers” (high-risk patients meeting specific respiratory/cardiac criteria) (Table 1). Through multidisciplinary review, the team considers three key factors: disease status and prognosis, treatment phase and future therapies, and comorbid conditions. Each case culminates in one of three determinations: immediate transfer, deferred transfer with monitoring, or non-candidacy (Tables 2–4).
The transfer protocol emphasizes comprehensive preparation, beginning with early family counseling about ECLS indications. Clinical coordination includes direct physician-to-physician handoffs, complete medical record transmission with emphasis on medication reconciliation, and pre-transfer imaging review including vascular ultrasounds when indicated. Primary oncology teams maintain active involvement throughout the process. Absolute contraindications focus on irreversible organ damage, while relative contraindications incorporate disease-specific considerations for immunocompromised hosts.
Continuous process evaluation tracks multiple metrics: consultation frequency and outcomes, transfer timelines, ECLS utilization rates, and longitudinal patient outcomes. This systematic approach addresses the challenges of off-site ECLS availability while maintaining alignment with oncologic care priorities, demonstrating how specialized centers can develop effective partnerships to manage critical care needs for complex patient populations.
6 Conclusion
In recent years, advances in diagnostic and treatment capabilities have markedly improved outcomes for pediatric oncology and HCT patients. Enhanced infectious diseases management, anticancer treatments, HCT techniques, ventilation management, ECLS technology, precise anticoagulation, fluid management, and CKRT capabilities have all contributed to this progress. Improved understanding and clinical management of complications associated with chemotherapy and HCT have also played a crucial role. Consequently, outcomes for critically ill patients requiring ECLS have improved, making older data less relevant. However, determining ECLS candidacy for these patients remains a challenge.
We strongly advocate for the establishment of dedicated multidisciplinary teams comprising intensivists, ECLS directors, hematologist-oncologists, and infectious diseases specialists to make informed decisions about ECLS candidacy. These teams will benefit from cumulative experience, leading to continuous improvement in patient outcomes. St. Jude has witnessed substantial improvement in practice, understanding, and outcomes with the implementation of a formalized ECLS consultation team and process. Furthermore, collaboration between multidisciplinary teams from different institutions can facilitate the collection of granular prospective data, thereby advancing the standard of care for this vulnerable population and ensuring that the most critically ill pediatric patients receive the best-possible treatment. This collaborative approach is essential for advancing the field and improving the prognosis for pediatric oncology and HCT patients requiring ECLS. By identifying suitable candidates for ECLS, we can advance care and avoid futile measures.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CH: Writing – original draft, Writing – review & editing. MD: Writing – original draft, Writing – review & editing. MR: Writing – original draft, Writing – review & editing. DH: Writing – original draft, Writing – review & editing. AhS: Writing – original draft, Writing – review & editing. AkS: Writing – original draft, Writing – review & editing. LE: Writing – original draft, Writing – review & editing. MH: Writing – review & editing. JM: Writing – review & editing. HS: Writing – review & editing. SG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by the American Lebanese and Syrian Associated Charities (ALSAC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1588403/full#supplementary-material
Abbreviations
AKI, Acute kidney injury; CKRT, Continuous kidney replacement therapy; ECLS, Extracorporeal life support; GVHD, Graft-versus-host disease; HCT, Hematopoietic cell transplantation; HLA, Human leukocyte antigen; ICU, Intensive care unit; PICU, Pediatric intensive care unit.
References
1. Wosten-van Asperen RM, van Gestel JPJ, van Grotel M, Tschiedel E, Dohna-Schwake C, Valla FV, et al. PICU mortality of children with cancer admitted to pediatric intensive care unit a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2019) 142:153–63. doi: 10.1016/j.critrevonc.2019.07.014
2. Bhosale SJ, Joshi M, Patil VP, Kothekar AT, Myatra SN, Divatia JV, et al. Epidemiology and predictors of hospital outcomes of critically ill pediatric oncology patients: A retrospective study. Indian J Crit Care medicine: peer-reviewed Off Publ Indian Soc Crit Care Med. (2021) 25:1183–8. doi: 10.1182/blood-2019-132435
3. Lindell RB, Fitzgerald JC, Rowan CM, Flori HR, Di Nardo M, Napolitano N, et al. The use and duration of preintubation respiratory support is associated with increased mortality in immunocompromised children with acute respiratory failure. Crit Care Med. (2022) 50:1127–37. doi: 10.1097/CCM.0000000000005535
4. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. CA: Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
5. Forrest SJ, Geoerger B, and Janeway KA. Precision medicine in pediatric oncology. Curr Opin Pediatr. (2018) 30:17–24. doi: 10.1097/MOP.0000000000000570
6. Butler E, Ludwig K, Pacenta HL, Klesse LJ, Watt TC, and Laetsch TW. Recent progress in the treatment of cancer in children. CA: Cancer J Clin. (2021) 71:315–32. doi: 10.3322/caac.21665
7. Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. (2014) 28:1467–71. doi: 10.1038/leu.2014.30
8. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. (2020) 21:531–40. doi: 10.1016/S1470-2045(19)30856-3
9. Hutzen B, Paudel SN, Naeimi Kararoudi M, Cassady KA, Lee DA, and Cripe TP. Immunotherapies for pediatric cancer: current landscape and future perspectives. Cancer Metastasis Rev. (2019) 38:573–94. doi: 10.1007/s10555-019-09819-z
10. Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New Engl J Med. (2010) 363:1324–34. doi: 10.1056/NEJMoa0911123
11. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
12. Brown PA, Ji LY, Xu XX, Devidas M, Hogan L, Borowitz MJ, et al. A randomized phase 3 trial of blinatumomab vs. Chemotherapy as post-reinduction therapy in high and intermediate risk (HR/IR) first relapse of B-acute lymphoblastic leukemia (B-ALL) in children and adolescents/young adults (AYAs) demonstrates superior efficacy and tolerability of blinatumomab: A report from children’s oncology group study AALL1331. Blood. (2019) 134. doi: 10.1182/blood-2019-132435
13. Pole JD, Gibson P, Ethier MC, Lazor T, Johnston DL, Portwine C, et al. Evaluation of treatment-related mortality among paediatric cancer deaths: a population based analysis. Brit J Cancer. (2017) 116:540–5. doi: 10.1038/bjc.2016.443
14. Loeffen EAH, Knops RRG, Boerhof J, Feijen EAM, Merks JHM, Reedijk AMJ, et al. Treatment-related mortality in children with cancer: Prevalence and risk factors. Eur J Cancer. (2019) 121:113–22.
15. Phelan R, Chen M, Bupp C, Bolon YT, Broglie L, Brunner-Grady J, et al. Updated trends in hematopoietic cell transplantation in the United States with an additional focus on adolescent and young adult transplantation activity and outcomes. Transplant Cell Ther. (2022) 28:409 e1– e10. doi: 10.1016/j.jtct.2022.04.012
16. Tamburro RF, Barfield RC, Shaffer ML, Rajasekaran S, Woodard P, Morrison RR, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2008) 9:270–7.
17. Lamas A, Otheo E, Ros P, Vazquez JL, Maldonado MS, Munoz A, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. (2003) 29:91–6. doi: 10.1007/s00134-002-1549-2
18. Jacobe SJ, Hassan A, Veys P, and Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. (2003) 31:1299–305. doi: 10.1097/01.CCM.0000060011.88230.C8
19. Rowan CM, McArthur J, Hsing DD, Gertz SJ, Smith LS, Loomis A, et al. Acute respiratory failure in pediatric hematopoietic cell transplantation: A multicenter study. Crit Care Med. (2018) 46:e967–e74. doi: 10.1097/CCM.0000000000003277
20. Zinter MS, Dvorak CC, Spicer A, Cowan MJ, and Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. (2015) 43:1986–94. doi: 10.1097/CCM.0000000000001085
21. Zinter MS, Logan BR, Fretham C, Sapru A, Abraham A, Aljurf MD, et al. Comprehensive prognostication in critically ill pediatric hematopoietic cell transplant patients: results from merging the center for international blood and marrow transplant research (CIBMTR) and virtual pediatric systems (VPS) registries. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2020) 26:333–42.
22. Flerlage T, Fan K, Qin Y, Agulnik A, Arias AV, Cheng C, et al. Mortality risk factors in pediatric onco-critical care patients and machine learning derived early onco-critical care phenotypes in a retrospective cohort. Crit Care Explor. (2023) 5:e0976. doi: 10.1097/CCE.0000000000000976
23. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. New Engl J Med. (2010) 363:2091–101. doi: 10.1056/NEJMoa1004383
24. Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. New Engl J Med. (2023) 388:2338–48. doi: 10.1056/NEJMoa2215943
25. Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. (2012) 119:296–307.
26. Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin oncology: Off J Am Soc Clin Oncol. (2016) 34:329–36.
27. Bejanyan N, Zhang M, Bo-Subait K, Brunstein C, Wang H, Warlick ED, et al. Myeloablative Conditioning for Allogeneic Transplantation Results in Superior Disease-Free Survival for Acute Myelogenous Leukemia and Myelodysplastic Syndromes with Low/Intermediate but not High Disease Risk Index: A Center for International Blood and Marrow Transplant Research Study. Transplant Cell Ther. (2021) 27:68 e1– e9.
28. Scott BL, Pasquini MC, Fei M, Fraser R, Wu J, Devine SM, et al. Myeloablative versus reduced-intensity conditioning for hematopoietic cell transplantation in acute myelogenous leukemia and myelodysplastic syndromes-long-term follow-up of the BMT CTN 0901 clinical trial. Transplant Cell Ther. (2021) 27:483 e1– e6. doi: 10.1016/j.jtct.2021.02.031
29. Talleur AC, Qudeimat A, Metais JY, Langfitt D, Mamcarz E, Crawford JC, et al. Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv. (2022) 6:5737–49. doi: 10.1182/bloodadvances.2021006293
30. Percival ME, Wang HL, Zhang MJ, Saber W, de Lima M, Litzow M, et al. Impact of depth of clinical response on outcomes of acute myeloid leukemia patients in first complete remission who undergo allogeneic hematopoietic cell transplantation. Bone marrow transplantation. (2021) 56:2108–17. doi: 10.1038/s41409-021-01261-6
31. Pfeiffer T, Li Y, Ashcraft E, Karol SE, Rubnitz JE, Epperly R, et al. Venetoclax-based therapy as a bridge to allogeneic hematopoietic cell transplantation in children with relapsed/refractory AML. Bone marrow transplantation. (2023) 58:328–31.
32. Epperly R, Talleur AC, Li Y, Schell S, Tuggle M, Metais JY, et al. Sub-myeloablative Second Transplantations with Haploidentical Donors and Post-Transplant Cyclophosphamide have limited Anti-Leukemic Effects in Pediatric Patients. Transplant Cell Ther. (2022) 28:262 e1– e10. doi: 10.1016/j.jtct.2022.02.007
33. Sharma A, Huang S, Li Y, Brooke RJ, Ahmed I, Allewelt HB, et al. Outcomes of pediatric patients with therapy-related myeloid neoplasms. Bone marrow transplantation. (2021) 56:2997–3007.
34. Sharma A, Li Y, Huang S, Talleur AC, Suliman A, Qudeimat A, et al. Outcomes of pediatric patients who relapse after first HCT for acute leukemia or MDS. Bone marrow transplantation. (2021) 56:1866–75.
35. Faraci M, Bagnasco F, Giardino S, Conte M, Micalizzi C, Castagnola E, et al. Intensive care unit admission in children with Malignant or nonmalignant disease: incidence, outcome, and prognostic factors: a single-center experience. J Pediatr hematology/oncology. (2014) 36:e403–9. doi: 10.1097/MPH.0000000000000048
36. Kew AK, Couban S, Patrick W, Thompson K, and White D. Outcome of hematopoietic stem cell transplant recipients admitted to the intensive care unit. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2006) 12:301–5.
37. Dutton LK, Hinchcliff KM, Logli AL, Mallett KE, Suh GA, and Rizzo M. Preoperative antibiotics influence culture yield in the treatment of hand, wrist, and forearm infections. JB JS Open Access. (2022) 7. doi: 10.2106/JBJS.OA.21.00084
38. Grace CJ, Lieberman J, Pierce K, and Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis. (2001) 32:1651–5. doi: 10.1086/320527
39. McArdle JR. Critical care outcomes in the hematologic transplant recipient. Clin Chest Med. (2009) 30:155–67. doi: 10.1016/j.ccm.2008.10.002
40. Das S, Adler AL, Miles-Jay A, Kronman MP, Qin X, Weissman SJ, et al. Antibiotic prophylaxis is associated with subsequent resistant infections in children with an initial extended-spectrum-cephalosporin-resistant enterobacteriaceae infection. Antimicrob Agents Chemother. (2017) 61(5):e02656-16. doi: 10.1128/AAC.02656-16
41. Teillant A, Gandra S, Barter D, Morgan DJ, and Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis. (2015) 15:1429–37. doi: 10.1016/S1473-3099(15)00270-4
42. Zerr DM, Miles-Jay A, Kronman MP, Zhou C, Adler AL, Haaland W, et al. Previous Antibiotic Exposure Increases Risk of Infection with Extended-Spectrum-beta-Lactamase- and AmpC-Producing Escherichia coli and Klebsiella pneumoniae in Pediatric Patients. Antimicrob Agents Chemother. (2016) 60:4237–43. doi: 10.1128/AAC.00187-16
43. Liu Q, Jin X, Cheng J, Zhou H, Zhang Y, and Dai Y. Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens (Review). Mol Med Rep. (2023) 27(5):104. doi: 10.3892/mmr.2023.12991
44. Shin DJ, Andini N, Hsieh K, Yang S, and Wang TH. Emerging analytical techniques for rapid pathogen identification and susceptibility testing. Annu Rev Anal Chem (Palo Alto Calif). (2019) 12:41–67.
45. Wootton SH, Aguilera E, Salazar L, Hemmert AC, and Hasbun R. Enhancing pathogen identification in patients with meningitis and a negative Gram stain using the BioFire FilmArray((R)) Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob. (2016) 15:26. doi: 10.1186/s12941-016-0137-1
46. Clark TW, Lindsley K, Wigmosta TB, Bhagat A, Hemmert RB, Uyei J, et al. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: Results of a systematic review and meta-analysis. J Infect. (2023) 86:462–75. doi: 10.1016/j.jinf.2023.03.005
47. Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. (2015) 139:636–41. doi: 10.5858/arpa.2014-0257-OA
48. Subramony A, Zachariah P, Krones A, Whittier S, and Saiman L. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J pediatrics. (2016) 173:196–201 e2.
49. Xu M, Qin X, Astion ML, Rutledge JC, Simpson J, Jerome KR, et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol. (2013) 139:118–23. doi: 10.1309/AJCPH7X3NLYZPHBW
50. Banerjee R and Patel R. Molecular diagnostics for genotypic detection of antibiotic resistance: current landscape and future directions. JAC Antimicrob Resist. (2023) 5:dlad018.
51. Lewinski MA, Alby K, Babady NE, Butler-Wu SM, Bard JD, Greninger AL, et al. Exploring the utility of multiplex infectious disease panel testing for diagnosis of infection in different body sites: A joint report of the association for molecular pathology, american society for microbiology, infectious diseases society of america, and pan american society for clinical virology. J Mol Diagn. (2023) 25:857–75.
52. Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. New Engl J Med. (2019) 380:2327–40. doi: 10.1056/NEJMoa1803396
53. Imlay HN and Kaul DR. Letermovir and maribavir for the treatment and prevention of cytomegalovirus infection in solid organ and stem cell transplant recipients. Clin Infect Dis. (2021) 73:156–60. doi: 10.1093/cid/ciaa1713
54. Yahav D, Giske CG, Gramatniece A, Abodakpi H, Tam VH, and Leibovici L. New beta-lactam-beta-lactamase inhibitor combinations. Clin Microbiol Rev. (2020) 34(1).
55. Barbier F, Hraiech S, Kerneis S, Veluppillai N, Pajot O, Poissy J, et al. Rationale and evidence for the use of new beta-lactam/beta-lactamase inhibitor combinations and cefiderocol in critically ill patients. Ann Intensive Care. (2023) 13:65. doi: 10.1186/s13613-023-01153-6
56. Analysis of the antibacterial pipeline (2023). Available online at: https://www.who.int/observatories/global-observatory-on-health-research-and-development/analyses-and-syntheses/antimicrobial-resistance/analysis-of-the-antibacterial-pipeline (Accessed November 7, 2023).
57. Butler MS, Henderson IR, Capon RJ, and Blaskovich MAT. Antibiotics in the clinical pipeline as of December 2022. J Antibiot (Tokyo). (2023) 76:431–73.
58. Thomas D W. C. The state of innovation in antibacterial therapeutics. (2022). Available at: https://www.bio.org/sites/default/files/2022-02/The-State-of-Innovation-in-Antibacterial-Therapeutics.pdf (Accessed November 7, 2023).
59. Lemerle M, Schmidt A, Thepot-Seegers V, Kouatchet A, Moal V, Raimbault M, et al. Serum phosphate level and its kinetic as an early marker of acute kidney injury in tumor lysis syndrome. J Nephrol. (2022) 35:1627–36.
60. Anderson A, Shoulders L, James V, Ashcraft E, Cheng C, Ribeiro R, et al. Benefit of continuous kidney replacement therapy for managing tumor lysis syndrome in children with hematologic Malignancies. Front Oncol. (2023) 13:1234677. doi: 10.3389/fonc.2023.1234677
61. Raina R, Abu-Arja R, Sethi S, Dua R, Chakraborty R, Dibb JT, et al. Acute kidney injury in pediatric hematopoietic cell transplantation: critical appraisal and consensus. Pediatr Nephrol. (2022) 37:1179–203. doi: 10.1007/s00467-022-05448-x
62. James V, Angelo J, and Elbahlawan L. Kidney injury in children after hematopoietic stem cell transplant. Curr Oncol. (2023) 30:3329–43. doi: 10.3390/curroncol30030253
63. Bauer A, Carlin K, Schwartz SM, Srikanthan M, Thakar M, Burroughs LM, et al. Risk factors for severe acute kidney injury after pediatric hematopoietic cell transplantation. Pediatr Nephrol. (2023) 38:1365–72.
64. Elbahlawan L, Bissler J, and Morrison RR. Continuous renal replacement therapy: A review of use and application in pediatric hematopoietic stem cell transplant recipients. Front Oncol. (2021) 11:632263. doi: 10.3389/fonc.2021.632263
65. Raymakers-Janssen P, Lilien MR, Tibboel D, Kneyber MCJ, Dijkstra S, van Woensel JBM, et al. Epidemiology and outcome of critically ill pediatric cancer and hematopoietic stem cell transplant patients requiring continuous renal replacement therapy: A retrospective nationwide cohort study. Crit Care Med. (2019) 47:e893–901. doi: 10.1097/CCM.0000000000003973
66. Heyn M, Ashcraft E, Cheng C, Epperly R, and Elbahlawan L. Continuous kidney replacement therapy in children with sinusoidal obstruction syndrome after hematopoietic cell transplant: outcome and liberation. Pediatr Blood cancer. (2025) 72:e31473. doi: 10.1002/pbc.31473
68. Al Haj Moussa A, Maaz AUR, Faqih N, and Sundaram M. Critically ill pediatric oncology patients: what the intensivist needs to know? Pediatric critical care medicine. Indian J Crit Care medicine: peer-reviewed Off Publ Indian Soc Crit Care Med. (2020) 24:1256–63. doi: 10.5005/jp-journals-10071-23693
69. Rajapreyar PK and Randolph A. Acute Respiratory Failure and Management. In: Duncan CN, editor. Critical Care of the Pediatric Immunocompromised Hematology/Oncology Patient. Springer Cham (2018). p. 195–210.
70. Blokpoel RG, Burgerhof JG, Markhorst DG, and Kneyber MC. Patient-ventilator asynchrony during assisted ventilation in children. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2016) 17:e204–11. doi: 10.1097/PCC.0000000000000669
71. de Jager P, Kamp T, Dijkstra SK, Burgerhof JGM, Markhorst DG, Curley MAQ, et al. Feasibility of an alternative, physiologic, individualized open-lung approach to high-frequency oscillatory ventilation in children. Ann Intensive Care. (2019) 9:9. doi: 10.1186/s13613-019-0492-0
72. de Jager P, Koopman AA, Markhorst DG, and Kneyber MCJ. Lung behavior during a staircase high-frequency oscillatory ventilation recruitment maneuver. Intensive Care Med Exp. (2024) 12:42. doi: 10.1186/s40635-024-00623-w
73. Rowan C, Baloglu O, and McArthur J. Non-infectious pulmonary complications of hematopoietic stem cell transplantation. J Pediatr Intensive Care. (2014) 3:133–46. doi: 10.3233/PIC-14095
74. Williams KM. Noninfectious complications of hematopoietic cell transplantation. Hematol Am Soc Hematol Educ Program. (2021) 2021:578–86.
75. Fraebel J, Engelhardt BG, and Kim TK. Noninfectious pulmonary complications after hematopoietic stem cell transplantation. Transplant Cell Ther. (2023) 29:82–93. doi: 10.1016/j.jtct.2022.11.012
76. Fan K, McArthur J, Morrison RR, and Ghafoor S. Diffuse alveolar hemorrhage after pediatric hematopoietic stem cell transplantation. Front Oncol. (2020) 10:1757. doi: 10.3389/fonc.2020.01757
77. Soeteman M, Lekkerkerker CW, Kappen TH, Tissing WJ, Nieuwenhuis EE, and Wosten-van Asperen RM. The predictive performance and impact of pediatric early warning systems in hospitalized pediatric oncology patients-A systematic review. Pediatr Blood cancer. (2022) 69:e29636. doi: 10.1002/pbc.29636
78. Agulnik A, Gossett J, Carrillo AK, Kang G, and Morrison RR. Abnormal vital signs predict critical deterioration in hospitalized pediatric hematology-oncology and post-hematopoietic cell transplant patients. Front Oncol. (2020) 10:354. doi: 10.3389/fonc.2020.00354
79. Kornecki A and Shemie SD. Open lung biopsy in children with respiratory failure. Crit Care Med. (2001) 29:1247–50. doi: 10.1097/00003246-200106000-00035
80. Furuya ME, Ramirez-Figueroa JL, Vargas MH, Bernaldez-Rios R, Vazquez-Rosales JG, and Rodriguez-Velasco A. Diagnoses unveiled by early bronchoscopy in children with leukemia and pulmonary infiltrates. J Pediatr hematology/oncology. (2012) 34:596–600.
81. Elbahlawan LM, Avent Y, Montoya L, Wilder K, Pei D, Cheng C, et al. Safety and benefits of bronchoalveolar lavage and lung biopsy in the management of pulmonary infiltrates in children with leukemia. J Pediatr hematology/oncology. (2016) 38:597–601.
82. Ahmad AH, Brown BD, Andersen CR, Mahadeo KM, Petropolous D, Cortes JA, et al. Retrospective review of flexible bronchoscopy in pediatric cancer patients. Front Oncol. (2021) 11:770523. doi: 10.3389/fonc.2021.770523
83. Park JR, Fogarty S, and Brogan TV. Clinical utility of bronchoalveolar lavage in pediatric cancer patients. Med Pediatr Oncol. (2002) 39:175–80. doi: 10.1002/mpo.10130
84. Georgescu L, Rahrig AL, Montgomery G, and Rowan CM. Diagnostic yield of bronchoscopy in children with leukemia or post hematopoietic stem cell transplant. Pediatr Pulmonol. (2024) 59:129–36.
85. Papazian L, Doddoli C, Chetaille B, Gernez Y, Thirion X, Roch A, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. (2007) 35:755–62. doi: 10.1097/01.CCM.0000257325.88144.30
86. Ghafoor S, Fan K, Williams S, Brown A, Bowman S, Pettit KL, et al. Beginning restorative activities very early: implementation of an early mobility initiative in a pediatric onco-critical care unit. Front Oncol. (2021) 11:645716. doi: 10.3389/fonc.2021.645716
87. Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU up!: impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care medicine: J Soc Crit Care Med an World Fed Pediatr Intensive Crit Care Societies. (2016) 17:e559–e66. doi: 10.1097/PCC.0000000000000983
88. Emeriaud G, Lopez-Fernandez YM, Iyer NP, Bembea MM, Agulnik A, Barbaro RP, et al. Executive summary of the second international guidelines for the diagnosis and management of pediatric acute respiratory distress syndrome (PALICC-2). Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2023) 24:143–68.
89. Kneyber MC, Zhang H, and Slutsky AS. Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med. (2014) 190:258–65. doi: 10.1164/rccm.201401-0168CP
90. Shaikh FAR. Concept of stress and strain in pediatric mechanical ventilation. J Pediatr Crit Care. (2023) 10:139–44. doi: 10.4103/jpcc.jpcc_48_23
91. Kneyber MCJ, Khemani RG, Bhalla A, Blokpoel RGT, Cruces P, Dahmer MK, et al. Understanding clinical and biological heterogeneity to advance precision medicine in paediatric acute respiratory distress syndrome. Lancet Respir Med. (2023) 11:197–212. doi: 10.1016/S2213-2600(22)00483-0
92. Kneyber MCJ, de Luca D, Calderini E, Jarreau PH, Javouhey E, Lopez-Herce J, et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. (2017) 43:1764–80. doi: 10.1007/s00134-017-4920-z
93. Gattinoni L, Carlesso E, and Caironi P. Stress and strain within the lung. Curr Opin Crit Care. (2012) 18:42–7. doi: 10.1097/MCC.0b013e32834f17d9
94. Carteaux G, Parfait M, Combet M, Haudebourg AF, Tuffet S, and Mekontso Dessap A. Patient-self inflicted lung injury: A practical review. J Clin Med. (2021) 10(12). doi: 10.3390/jcm10122738
95. Telias I, Junhasavasdikul D, Rittayamai N, Piquilloud L, Chen L, Ferguson ND, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med. (2020) 201:1086–98. doi: 10.1164/rccm.201907-1425OC
96. Blumhof S, Wheeler D, Thomas K, McCool FD, and Mora J. Change in diaphragmatic thickness during the respiratory cycle predicts extubation success at various levels of pressure support ventilation. Lung. (2016) 194:519–25.
97. Nardi N, Mortamet G, Ducharme-Crevier L, Emeriaud G, and Jouvet P. Recent advances in pediatric ventilatory assistance. F1000Res. (2017) 6:290.
98. Bhalla AK, Rubin S, Newth CJ, Ross P, Morzov R, Soto-Campos G, et al. Monitoring dead space in mechanically ventilated children: volumetric capnography versus time-based capnography. Respir Care. (2015) 60:1548–55. doi: 10.4187/respcare.03892
99. Shimatani T, Kyogoku M, Ito Y, Takeuchi M, and Khemani RG. Fundamental concepts and the latest evidence for esophageal pressure monitoring. J Intensive Care. (2023) 11:22. doi: 10.1186/s40560-023-00671-6
100. Qin W, Mao L, Shen Y, and Zhao L. Prone position in the mechanical ventilation of acute respiratory distress syndrome children: a systematic review and meta-analysis. Front Pediatr. (2024) 12:1293453. doi: 10.3389/fped.2024.1293453
101. Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. New Engl J Med. (2013) 368:2159–68. doi: 10.1056/NEJMoa1214103
102. Johnson NJ, Luks AM, and Glenny RW. Gas exchange in the prone posture. Respir Care. (2017) 62:1097–110. doi: 10.4187/respcare.05512
103. Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. (2010) 36:585–99. doi: 10.1007/s00134-009-1748-1
104. Orloff KE, Turner DA, and Rehder KJ. The current state of pediatric acute respiratory distress syndrome. Pediatr Allergy Immunol Pulmonol. (2019) 32:35–44.
105. Kneyber MCJ, Cheifetz IM, Asaro LA, Graves TL, Viele K, Natarajan A, et al. Protocol for the prone and oscillation pediatric clinical trial (PROSpect). Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2024) 25:e385–e96. doi: 10.1097/PCC.0000000000003541
106. Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med. (2012) 40:2883–9. doi: 10.1097/CCM.0b013e31825bc54d
107. Michael M, Kuehnle I, and Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. (2004) 19:91–5.
108. Elbahlawan L, Srinivasan A, and Morrison RR. A critical care and transplantation-based approach to acute respiratory failure after hematopoietic stem cell transplantation in children. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2016) 22:617–26.
109. De Luca D, Cogo P, Kneyber MC, Biban P, Semple MG, Perez-Gil J, et al. Surfactant therapies for pediatric and neonatal ARDS: ESPNIC expert consensus opinion for future research steps. Crit Care. (2021) 25:75. doi: 10.1186/s13054-021-03489-6
110. Bhalla AK, Yehya N, Mack WJ, Wilson ML, Khemani RG, and Newth CJL. The association between inhaled nitric oxide treatment and ICU mortality and 28-day ventilator-free days in pediatric acute respiratory distress syndrome. Crit Care Med. (2018) 46:1803–10. doi: 10.1097/CCM.0000000000003312
111. Yehya N, Servaes S, Thomas NJ, Nadkarni VM, and Srinivasan V. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med. (2015) 41:1658–66. doi: 10.1007/s00134-015-3953-4
112. Meduri GU, Siemieniuk RAC, Ness RA, and Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care. (2018) 6:53. doi: 10.1186/s40560-018-0321-9
113. Thompson J, Yin Z, D’Souza A, Fenske T, Hamadani M, Hari P, et al. Etanercept and corticosteroid therapy for the treatment of late-onset idiopathic pneumonia syndrome. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2017) 23:1955–60.
114. Ghafoor S, Fan K, Di Nardo M, Talleur AC, Saini A, Potera RM, et al. Extracorporeal membrane oxygenation candidacy in pediatric patients treated with hematopoietic stem cell transplant and chimeric antigen receptor T-cell therapy: an international survey. Front Oncol. (2021) 11:798236. doi: 10.3389/fonc.2021.798236
115. Di Nardo M, Ahmad AH, Merli P, Zinter MS, Lehman LE, Rowan CM, et al. Extracorporeal membrane oxygenation in children receiving haematopoietic cell transplantation and immune effector cell therapy: an international and multidisciplinary consensus statement. Lancet Child Adolesc Health. (2022) 6:116–28. doi: 10.1016/S2352-4642(21)00336-9
116. Hernandez-Beeftink T, Guillen-Guio B, Villar J, and Flores C. Genomics and the acute respiratory distress syndrome: current and future directions. Int J Mol Sci. (2019) 20(16). doi: 10.3390/ijms20164004
117. Dahmer MK, Yang G, Zhang M, Quasney MW, Sapru A, Weeks HM, et al. Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med. (2022) 10:289–97. doi: 10.1016/S2213-2600(21)00382-9
118. Zheng F, Pan Y, Yang Y, Zeng C, Fang X, Shu Q, et al. Novel biomarkers for acute respiratory distress syndrome: genetics, epigenetics and transcriptomics. biomark Med. (2022) 16:217–31. doi: 10.2217/bmm-2021-0749
119. Flerlage T, Crawford JC, Allen EK, Severns D, Tan S, Surman S, et al. Single cell transcriptomics identifies distinct profiles in pediatric acute respiratory distress syndrome. Nat Commun. (2023) 14:3870. doi: 10.1038/s41467-023-39593-0
120. Huang Q, Le Y, Li S, and Bian Y. Signaling pathways and potential therapeutic targets in acute respiratory distress syndrome (ARDS). Respir Res. (2024) 25:30. doi: 10.1186/s12931-024-02678-5
121. Im D, Shi W, and Driscoll B. Pediatric acute respiratory distress syndrome: fibrosis versus repair. Front Pediatr. (2016) 4:28. doi: 10.3389/fped.2016.00028
122. Peters DM, Vadasz I, Wujak L, Wygrecka M, Olschewski A, Becker C, et al. TGF-beta directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci U S A. (2014) 111:E374–83.
123. Wang F, Li Y, Wang B, Li J, and Peng Z. The safety and efficacy of mesenchymal stromal cells in ARDS: a meta-analysis of randomized controlled trials. Crit Care. (2023) 27:31. doi: 10.1186/s13054-022-04287-4
124. Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. (2017) 196:762–71. doi: 10.1164/rccm.201609-1945OC
125. Soeteman M, Potratz J, Nielsen JSA, Willems J, Valla FV, Brierley J, et al. Research priorities in pediatric onco-critical care: an international Delphi consensus study. Intensive Care Med. (2019) 45:1681–3. doi: 10.1007/s00134-019-05706-x
126. Pollack BE, Kirsch R, Chapman R, Hyslop R, MacLaren G, and Barbaro RP. Extracorporeal membrane oxygenation then and now; broadening indications and availability. Crit Care Clin. (2023) 39:255–75. doi: 10.1016/j.ccc.2022.09.003
127. Slooff V, Hoogendoorn R, Nielsen JSA, Pappachan J, Amigoni A, Caramelli F, et al. Role of extracorporeal membrane oxygenation in pediatric cancer patients: a systematic review and meta-analysis of observational studies. Ann Intensive Care. (2022) 12:8. doi: 10.1186/s13613-022-00983-0
128. Leung KKY, Ray S, Chan GCF, and Hon KL. Functional outcomes at PICU discharge in hemato-oncology children at a tertiary oncology center in Hong Kong. Int J Clin Oncol. (2022) 27:1904–15. doi: 10.1007/s10147-022-02244-3
129. Linden V, Karlen J, Olsson M, Palmer K, Ehren H, Henter JI, et al. Successful extracorporeal membrane oxygenation in four children with Malignant disease and severe Pneumocystis carinii pneumonia. Med Pediatr Oncol. (1999) 32:25–31.
130. Meister B, Zelger B, Kropshofer G, Klein-Franke A, Crazzolara R, Fruhwirth M, et al. Extracorporeal membrane oxygenation as a rescue therapy for leukaemic children with pulmonary failure. Br J haematology. (2010) 148:126–31.
131. Cortina G, Neu N, Kropshofer G, Meister B, Klingkowski U, and Crazzolara R. Extracorporeal membrane oxygenation offers long-term survival in childhood leukemia and acute respiratory failure. Crit Care. (2018) 22:222. doi: 10.1186/s13054-018-2134-6
132. Maue DK, Hobson MJ, Friedman ML, Moser EA, and Rowan CM. Outcomes of pediatric oncology and hematopoietic cell transplant patients receiving extracorporeal membrane oxygenation. Perfusion. (2019) 34:598–604.
133. Steppan DA, Coleman RD, Viamonte HK, Hanson SJ, Carroll MK, Klein OR, et al. Outcomes of pediatric patients with oncologic disease or following hematopoietic stem cell transplant supported on extracorporeal membrane oxygenation: The PEDECOR experience. Pediatr Blood cancer. (2020) 67:e28403. doi: 10.1002/pbc.v67.10
134. Coleman RD, Goldman J, Moffett B, Guffey D, Loftis L, Thomas J, et al. Extracorporeal membrane oxygenation mortality in high-risk populations: an analysis of the pediatric health information system database. ASAIO J. (2020) 66:327–31. doi: 10.1097/MAT.0000000000001002
135. Bridges BC, Kilbaugh TJ, Barbaro RP, Bembea MM, Chima RS, Potera RM, et al. Veno-venous extracorporeal membrane oxygenation for children with cancer or hematopoietic cell transplant: A ten center cohort. ASAIO J. (2021) 67:923–9. doi: 10.1097/MAT.0000000000001336
136. Polito A, Dupuis-Lozeron E, Barbaro R, and Rimensberger PC. Ventilation parameters before extracorporeal membrane oxygenator and in-hospital mortality in children: A review of the ELSO registry. ASAIO J. (2022) 68:281–6. doi: 10.1097/MAT.0000000000001445
137. Ramanathan K, Yeo N, Alexander P, Raman L, Barbaro R, Tan CS, et al. Role of extracorporeal membrane oxygenation in children with sepsis: a systematic review and meta-analysis. Crit Care. (2020) 24:684. doi: 10.1186/s13054-020-03418-z
138. Suzuki Y, Mao RD, Shah NR, Schaeffer L, Deanda A, and Radhakrishnan RS. Prevalence and impact of infection during extracorporeal membrane oxygenation in oncologic patients: A retrospective analysis of the extracorporeal life support organization (ELSO) registry. J Intensive Care Med. (2023) 38:391–8. doi: 10.1177/08850666221128243
139. Balit CR, Horan R, Dorofaeff T, Frndova H, Doyle J, and Cox PN. Pediatric hematopoietic stem cell transplant and intensive care: have things changed? Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2016) 17:e109–16.
140. Brissot E, Rialland F, Cahu X, Strullu M, Corradini N, Thomas C, et al. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone marrow transplantation. (2016) 51:267–72. doi: 10.1038/bmt.2015.250
141. Di Nardo M, Locatelli F, Palmer K, Amodeo A, Lorusso R, Belliato M, et al. Extracorporeal membrane oxygenation in pediatric recipients of hematopoietic stem cell transplantation: an updated analysis of the Extracorporeal Life Support Organization experience. Intensive Care Med. (2014) 40:754–6. doi: 10.1007/s00134-014-3240-9
142. Olson TL, O’Neil ER, Kurtz KJ, MacLaren G, and Anders MM. Improving outcomes for children requiring extracorporeal membrane oxygenation therapy following hematopoietic stem cell transplantation. Crit Care Med. (2021) 49:e381–e93. doi: 10.1097/CCM.0000000000004850
143. Di Nardo M, Li Pira G, Amodeo A, Cecchetti C, Giorda E, Ceccarelli S, et al. Adoptive immunotherapy with antigen-specific T cells during extracorporeal membrane oxygenation (ECMO) for adenovirus-related respiratory failure in a child given haploidentical stem cell transplantation. Pediatr Blood cancer. (2014) 61:376–9.
144. Sheng D, Zhang L, Jia H, Guo B, Zhang X, and Li Y. Phosphorylcholine/heparin composite coatings on artificial lung membrane for enhanced hemo-compatibility. Langmuir. (2023) 39:9796–807.
145. Valdes CA, Sharaf OM, Bleiweis MS, Jacobs JP, Mumtaz M, Sharaf RM, et al. Heparin-based versus bivalirudin-based anticoagulation in pediatric extracorporeal membrane oxygenation: A systematic review. Front Med (Lausanne). (2023) 10:1137134. doi: 10.3389/fmed.2023.1137134
146. Holmqvist AS, Chen Y, Wu J, Battles K, Bhatia R, Francisco L, et al. Assessment of late mortality risk after allogeneic blood or marrow transplantation performed in childhood. JAMA Oncol. (2018) 4:e182453. doi: 10.1001/jamaoncol.2018.2453
147. Yen HJ, Eissa HM, Bhatt NS, Huang S, Ehrhardt MJ, Bhakta N, et al. Patient-reported outcomes in survivors of childhood hematologic Malignancies with hematopoietic stem cell transplant. Blood. (2020) 135:1847–58.
148. Rafiee M, Abbasi M, Rafieemehr H, Mirzaeian A, Barzegar M, Amiri V, et al. A concise review on factors influencing the hematopoietic stem cell transplantation main outcomes. Health Sci Rep. (2021) 4:e282. doi: 10.1002/hsr2.v4.2
149. Bailly DK, Reeder RW, Zabrocki LA, Hubbard AM, Wilkes J, Bratton SL, et al. Development and validation of a score to predict mortality in children undergoing extracorporeal membrane oxygenation for respiratory failure: pediatric pulmonary rescue with extracorporeal membrane oxygenation prediction score. Crit Care Med. (2017) 45:e58–66. doi: 10.1097/CCM.0000000000002019
150. Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, and Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. (2011) 39:364–70. doi: 10.1097/CCM.0b013e3181fb7b35
151. Neumann JL, Mau LW, Virani S, Denzen EM, Boyle DA, Boyle NJ, et al. Burnout, moral distress, work-life balance, and career satisfaction among hematopoietic cell transplantation professionals. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2018) 24:849–60.
152. Stephens AL and Bruce CR. Setting expectations for ECMO: improving communication between clinical teams and decision makers. Methodist Debakey Cardiovasc J. (2018) 14:120–5.
153. Jenks CL, Raman L, and Dalton HJ. Pediatric extracorporeal membrane oxygenation. Crit Care Clin. (2017) 33:825–41. doi: 10.1016/j.ccc.2017.06.005
154. Pichereau C, Lengline E, Valade S, Michonneau D, Ghrenassia E, Lemiale V, et al. Trajectories of acute graft-versus-host disease and mortality in critically ill allogeneic-hematopoietic stem cell recipients: the Allo-GRRR-OH score. Bone marrow transplantation. (2020) 55:1966–74. doi: 10.1038/s41409-020-0857-x
155. ECLS international summary of statistics (2023). Available online at: https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx. (Accessed July 22, 2024).
156. Neunert C, Chitlur M, and van Ommen CH. The changing landscape of anticoagulation in pediatric extracorporeal membrane oxygenation: use of the direct thrombin inhibitors. Front Med (Lausanne). (2022) 9:887199.
157. Cashen K, Saini A, Brandao LR, Le J, Monagle P, Moynihan KM, et al. Anticoagulant medications: the pediatric extracorporeal membrane oxygenation anticoagulation collaborativE consensus conference. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2024) 25:e7–e13. doi: 10.1097/PCC.0000000000003495
158. Kaushik S, Derespina KR, Chandhoke S, Shah DD, Cohen T, Shlomovich M, et al. Use of bivalirudin for anticoagulation in pediatric extracorporeal membrane oxygenation (ECMO). Perfusion. (2023) 38:58–65.
159. Lahart M and Said AS. Extracorporeal anticoagulation with bivalirudin; is the best still to come? ASAIO J. (2022) 68:e223.
160. Schill MR, Douds MT, Burns EL, Lahart MA, Said AS, and Abarbanell AM. Is anticoagulation with bivalirudin comparable to heparin for pediatric extracorporeal life support? Results from a high-volume center. Artif Organs. (2021) 45:15–21.
161. Nellis ME, Moynihan KM, Sloan SR, Delaney M, Kneyber MCJ, DiGeronimo R, et al. Prophylactic transfusion strategies in children supported by extracorporeal membrane oxygenation: the pediatric extracorporeal membrane oxygenation anticoagulation collaborativE consensus conference. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2024) 25:e25–34. doi: 10.1097/PCC.0000000000003493
162. Zantek ND, Steiner ME, Teruya J, Kreuziger LB, Raffini L, Muszynski JA, et al. Recommendations on monitoring and replacement of antithrombin, fibrinogen, and von willebrand factor in pediatric patients on extracorporeal membrane oxygenation: the pediatric extracorporeal membrane oxygenation anticoagulation collaborativE consensus conference. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2024) 25:e35–43. doi: 10.1097/PCC.0000000000003492
163. Alexander PMA, Bembea MM, Cashen K, Cheifetz IM, Dalton HJ, Himebauch AS, et al. Executive summary: the pediatric extracorporeal membrane oxygenation anticoagulation collaborativE (PEACE) consensus conference. Pediatr Crit Care medicine: J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Societies. (2024) 25:643–75.
Keywords: extracorporeal life support (ECLS), extracorporeal membrane oxygenation (ECMO), pediatric oncology, pediatric hematopoietic cell transplant, pediatric critical care
Citation: Hurley C, Di Nardo M, Rees M, Hijano DR, Said A, Sharma A, Elbahlawan L, Hines MR, McArthur JA, Sandhu H and Ghafoor S (2025) New perspectives on extracorporeal life support: expert teams and precise selection of candidates are transforming pediatric cancer and hematopoietic cell transplantation care. Front. Oncol. 15:1588403. doi: 10.3389/fonc.2025.1588403
Received: 05 March 2025; Accepted: 26 May 2025;
Published: 18 June 2025.
Edited by:
Brigid Garelik, Children’s Tumor Foundation, United StatesReviewed by:
Thomas Vincent Brogan, Seattle Children’s Hospital, United StatesSilvio Fabio Torres, Austral University, Argentina
Copyright © 2025 Hurley, Di Nardo, Rees, Hijano, Said, Sharma, Elbahlawan, Hines, McArthur, Sandhu and Ghafoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saad Ghafoor, c2FhZC5naGFmb29yQHN0anVkZS5vcmc=
 Caitlin Hurley
Caitlin Hurley Matteo Di Nardo
Matteo Di Nardo Matthew Rees
Matthew Rees Diego R. Hijano
Diego R. Hijano Ahmed Said
Ahmed Said Akshay Sharma
Akshay Sharma Lama Elbahlawan
Lama Elbahlawan Melissa R. Hines
Melissa R. Hines Jennifer A. McArthur
Jennifer A. McArthur Hitesh Sandhu
Hitesh Sandhu Saad Ghafoor
Saad Ghafoor