- 1Department of General Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of General Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Department of Ophthalmology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Retroperitoneal schwannoma, a rare mesenchymal-derived tumor originating from the retroperitoneum. Malignant retroperitoneal schwannoma carries a dismal prognosis. We present a case of a 19-year-old male who presented with left abdominal pain. Imaging examination revealed a large retroperitoneal mass (16×12×9 cm) in the left upper quadrant. Pathological examination following surgical resection confirmed the diagnosis of malignant schwannoma, with a notably high Ki-67 proliferation index of 50%. Despite radical resection, the tumor recurred with metastases to the ilium and sacrum within two years postoperatively. The patient ultimately discontinued treatment due to disease progression. This case underscores the aggressive nature of retroperitoneal malignant schwannoma, characterized by rapid local recurrence, distant metastasis, and resistance to surgical cure. These findings emphasize the urgent need for effective postoperative adjuvant therapies to improve outcomes in this highly malignant entity.
Background
Schwannoma, a mesenchymal-origin neoplasm, arises in any neural tissue containing Schwann cells, predominantly localized to anatomical regions with dense concentrations of both central and peripheral nervous system elements, particularly the craniofacial area. Schwannomas arising in the retroperitoneum represent a rare clinical entity, constituting merely 3% of all schwannoma cases (1), with the majority exhibiting benign biological behavior. Malignant retroperitoneal schwannomas are exceptionally uncommon, accounting for 1%-2% of total schwannoma presentations (2). Early-stage retroperitoneal schwannomas frequently manifest as asymptomatic lesions (2).
Anatomically, the retroperitoneal space extends cephalad from the diaphragmatic plane to caudally merge with the pelvic compartment. This potential space maintains structural continuity through defined anatomical conduits with both the peritoneal cavity and extraperitoneal pelvic regions. The posterior boundary is demarcated by the quadratus lumborum muscle, while maintaining continuity with the retroperitoneal adipose compartment. Consequently, schwannomas in this region demonstrate potential multidirectional expansion: posteriorly toward the posterior mediastinum, laterally along the abdominal wall musculature, and inferiorly into the extraperitoneal pelvis. Such expansive growth patterns typically result in significantly larger tumor volumes compared to schwannomas occurring in other anatomical regions (3).
Advanced disease progression often leads to mass effect-induced compression of adjacent viscera, precipitating symptom complexes secondary to mechanical displacement (4). Diagnostic challenges stem from the tumor’s nonspecific morphological features on non-invasive imaging, rendering it difficult to differentiate from colonic malignancies and mesenchymal tumors. Preoperative determination of malignancy remains elusive (5), potentially compromising therapeutic decision-making and prognostic evaluation. Notably, malignant variants demonstrate a 50%-60% postoperative recurrence rate despite initial surgical intervention achieving symptomatic relief (6).
Case presentation
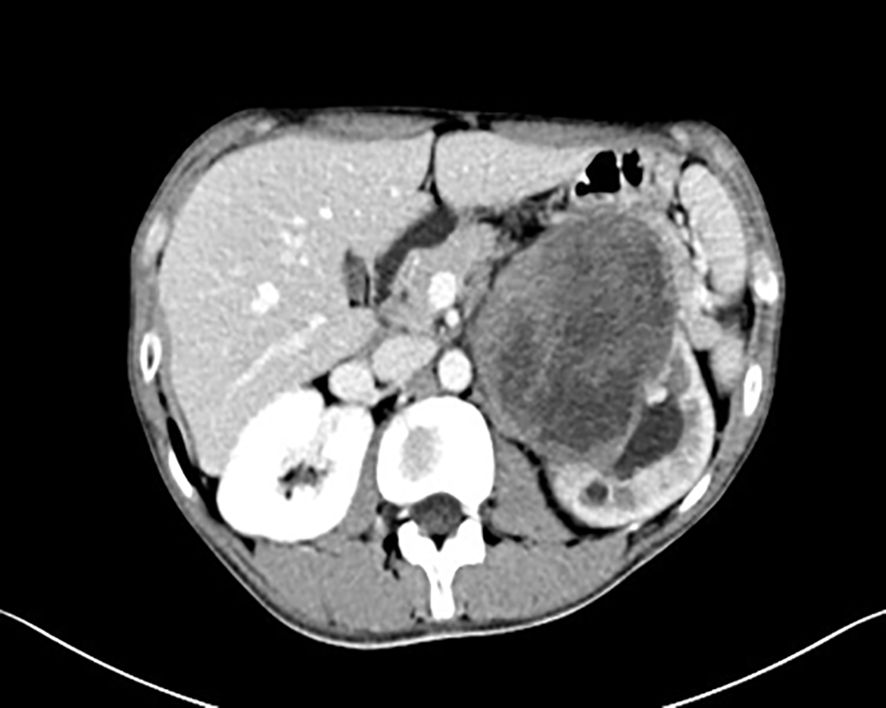
A 19-year-old male presented with a 1-year history of left flank pain exacerbated over the past week. No significant constitutional symptoms were reported. The patient denied family history of hereditary disorders or neoplastic syndromes. Physical examination revealed a palpable mass (7×8 cm) in the left upper quadrant demonstrating limited mobility with well-defined margins. CT(Computed Tomography) identified an intra-abdominal mass in the left upper quadrant, radiologically suspicious for stromal tumor (Figure 1). Percutaneous core needle biopsy histopathology demonstrated proliferating spindle-shaped cells with fascicular arrangement. Serum tumor marker analysis revealed markedly elevated CA19-9 (>1000 U/mL; normal <30 U/mL) and marginally increased CEA (7.3 ng/mL; normal <5 ng/mL).
Treatment
Following comprehensive preoperative evaluation excluding surgical contraindications, the patient underwent en bloc tumor resection under general anesthesia in December 2017. Intraoperative exploration identified a massive tumor (25×20×15 cm) in the left mid-lower abdomen with close anatomical relationships to the left hemicolon and the left Gerota’s fascia. We completely resected Gerota’s fascia during the operation, but complete separation from the colonic mesentery proved unfeasible. After multidisciplinary consultation, left hemicolectomy with end-to-end colorectal anastomosis was performed (operative duration: 224 minutes; EBL: 300 mL), requiring transfusion of 4 units SRBC (Suspended Red Blood Cells) and 425 mL FP (Frozen Plasma).Pathological examination revealed a 16×12×9 cm mass with firm, variegated cut surface. Immunohistochemical profile: CD34(+), CD117(-), DOG-1(-), Desmin(-), SMA(-/+), Calponin(-/+), S100(+), Ki-67 50%, MDM2(-), EMA(-). Final diagnosis favored malignant mesenchymal neoplasm with schwannomatous differentiation. All sampled lymph nodes (celiac:1, mesenteric:1, mesocolic:2) demonstrated reactive changes without malignancy. The patient recovered uneventfully and was advised to undergo periodic reexaminations and pursue aggressive treatment following the operation.
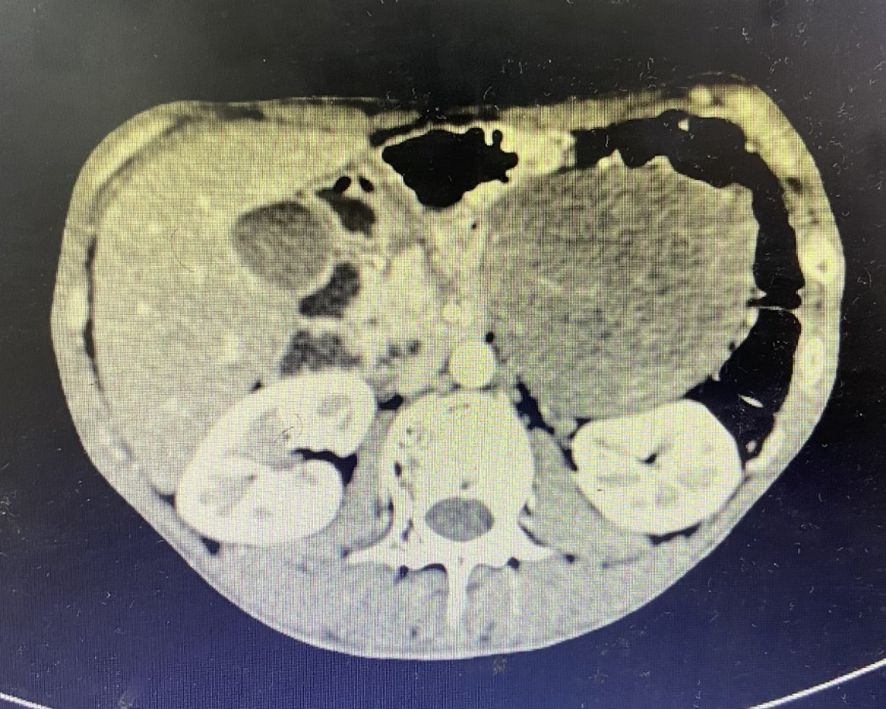
Nonadherence to follow-up ensued until February 2019 when abdominal CT revealed a 9.1×7.8 cm heterogeneously enhancing retroperitoneal mass adjacent to the left kidney (Figure 2). The patient underwent left retroperitoneal mass excision with nephrectomy at an external institution. Subsequent April 2019 surveillance CT confirmed post-nephrectomy status without residual disease.
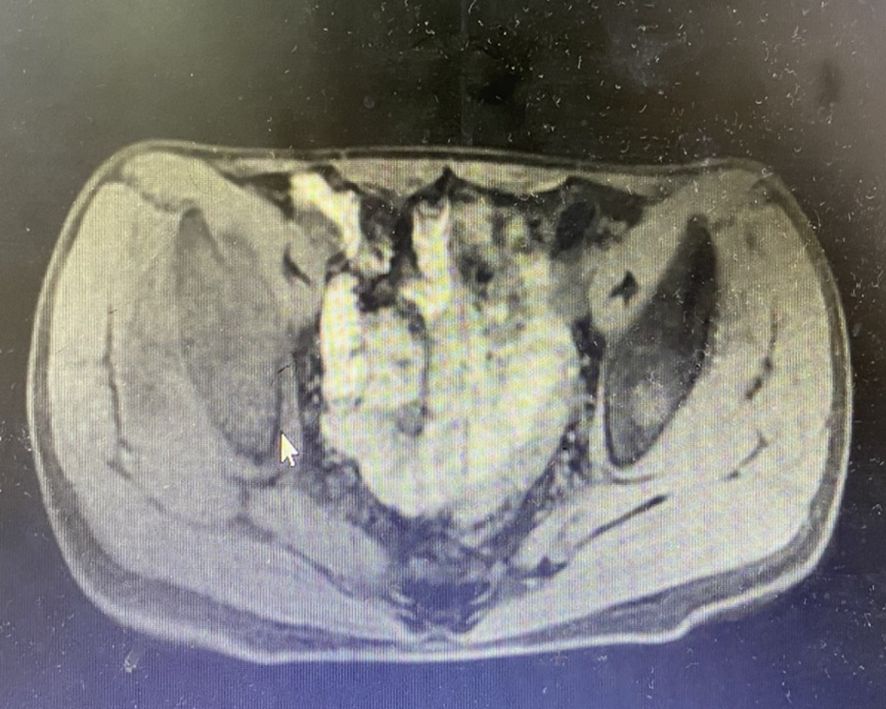
Figure 2. CT imaging characteristics. A 9.1×7.8 cm heterogeneously enhancing retroperitoneal mass adjacent to the left kidney.
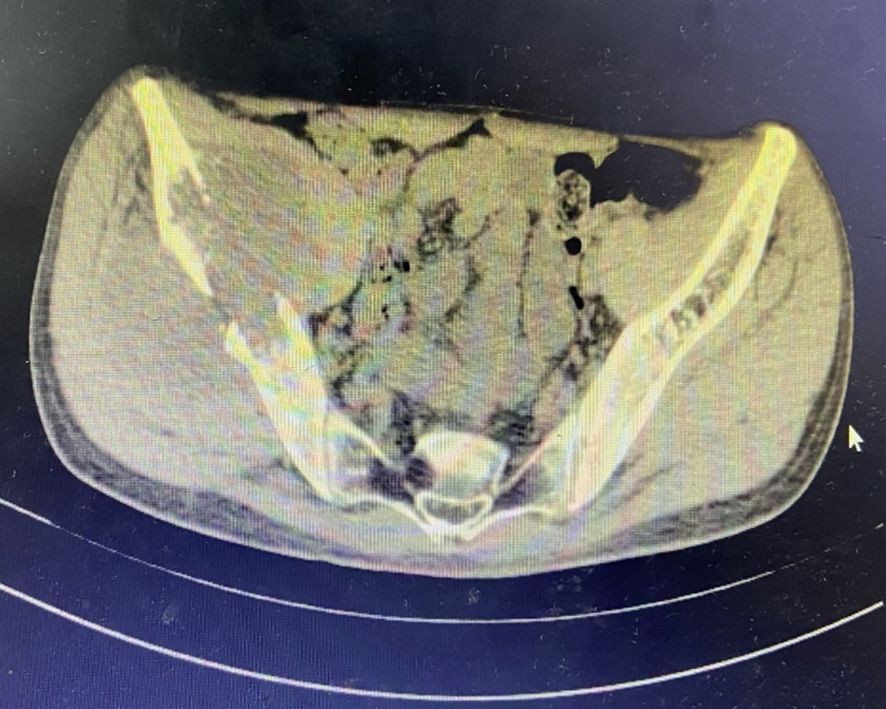
In June 2019, the patient presented with a 3-month history of right gluteal mass and ipsilateral lower extremity weakness (muscle strength 4+/5). Pelvic MRI(Magnetic Resonance Imaging) demonstrated right iliac bone destruction with a 76×58×67 mm T1-hypointense/T2-hyperintense mass, sacral and left iliac signal abnormalities suggesting osseous metastases, and a gluteal subcutaneous cystic nodule (Figures 3, 4). Upon the detection of bone metastases in this patient, a multidisciplinary team (MDT) comprising specialists from general surgery, orthopedics, interventional radiology, medical oncology, intensive care unit (ICU), radiotherapy, and pain rehabilitation was promptly assembled. The MDT focused on deliberating non-surgical treatment modalities, including chemotherapy, radiotherapy, immunotherapy, and also explored the feasibility of interventional procedures to ablate tumor tissues. Despite presenting a comprehensive array of subsequent treatment options, the patient and their family declined to proceed with further interventions, citing personal considerations.
Discussion
Retroperitoneal schwannoma, a rare neurogenic neoplasm originating from Schwann cells, demonstrates low clinical incidence with predominant benign biological behavior. Epidemiological studies reveal a significant female predilection (7). Anatomical distribution analysis indicates gastric schwannomas demonstrate fourfold higher incidence compared to intestinal counterparts, though malignant variants exhibit predilection for abdominal cavity and small intestine rather than gastric sites (2). Benign lesions typically follow an indolent clinical course due to slow growth kinetics, whereas malignant counterparts manifest rapid tumor enlargement, invasive growth patterns, and tissue destruction within short-term progression. Clinical presentations correlate with anatomical localization, exemplified by this case’s characteristic 1-year history of persistent flank pain. Radiologically, CT imaging reveals heterogeneous density with irregular enhancement patterns, pathologically corresponding to intratumoral necrosis, cystic degeneration, and hemorrhagic changes in malignant variants. Although MRI effectively delineates peritumoral soft tissue infiltration, its diagnostic specificity for malignancy remains limited. Although some experts have proposed the use of preoperative fine-needle aspiration biopsy (FNAB) for diagnosing the nature of tumors, studies on retroperitoneal schwannomas have shown that 0.4% to 2.0% of patients who underwent the biopsy experienced needle tract seeding metastasis (8, 9). This incidence is likely underestimated due to factors such as a short follow-up period, patient loss to follow-up, and the inherent difficulty in accurately assessing intra-abdominal seeding. Therefore, the routine application of preoperative needle biopsy remains highly controversial. For patients for whom surgical resection is the preferred treatment option, concerns regarding the potential oncological risk of seeding outweigh the diagnostic benefits of this examination (10).
Definitive diagnosis requires histopathological examination supplemented by immunohistochemical profiling, with hallmark features including coexistence of Antoni A areas (hypercellular palisading arrangements) and Antoni B regions (myxoid hypocellular stroma), coupled with strong diffuse S-100 protein immunoreactivity (11).Malignant differentiation necessitates combined morphological and molecular evaluation: benign lesions exhibit strong uniform S-100 expression, while malignant counterparts demonstrate attenuated immunopositivity accompanied by elevated Ki-67 index (>10%), aberrant p53 expression, and HE-staining features including marked cellular atypia, increased mitotic activity (≥5/10 HPF), and infiltrative growth patterns. Notably, intraoperative frozen section analysis demonstrates limited reliability in malignancy determination, and the diagnostic utility of preoperative biopsy remains controversial due to sampling limitations (11, 12).
Radical surgical resection with tumor-free margins constitutes the primary therapeutic intervention, though malignant variants demonstrate high propensity for local recurrence (40-60%) and distant metastasis (20-40%) (13). Prognostic determinants include tumor diameter (>5 cm), margin status (R0 vs R1/R2), and histological grade, with FNCLCC grading system validated as an independent prognostic factor (14). Adjuvant radiotherapy may reduce local recurrence rates (15-25% risk reduction), though no survival benefit has been established through randomized trials (15). Initially, adjuvant radiotherapy and other therapeutic options were contemplated for this patient. Nevertheless, contemporary clinical guidelines explicitly discourage the routine use of local radiotherapy for patients with completely resected tumors (16). A comprehensive assessment of the patient’s age, Karnofsky Performance Status (KPS), comorbidities, and physiological reserve, coupled with a risk-benefit analysis, indicated that radiotherapy would confer limited clinical benefit while potentially exposing the patient to unnecessary treatment-related toxicities. Consequently, this therapeutic modality was judiciously excluded from the treatment plan. In conclusion, this disease paradigm underscores the necessity for multimodal assessment protocols, particularly emphasizing long-term surveillance (minimum 10-year follow-up) for recurrence monitoring and outcome optimization.
Conclusion
Malignant retroperitoneal schwannoma is rare and highly invasive. Radical surgery is the only effective treatment, but the risks of recurrence and metastasis are high. This case indicates that early identification of malignant features, standardized postoperative management, and exploration of novel adjuvant therapies are the key directions for improving prognosis. Moreover, it is recommended that the multidisciplinary team (MDT) collaboration model be adopted in future diagnosis and treatment processes to optimize the surgical plan.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Second Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ZZ: Formal Analysis, Resources, Writing – original draft, Writing – review & editing. SF: Writing – original draft. DY: Writing – original draft. GW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xu J, Guo J, Yang HQ, Ji QL, Song RJ, Hou F, et al. Preoperative contrast-enhanced CT-based radiomics nomogram for differentiating benign and Malignant primary retroperitoneal tumors. Eur Radiol. (2023) 33:6781–93. doi: 10.1007/s00330-023-09686-x
2. Fan S, Wang H, Sun X, Gai C, Liang C, Wang G, et al. Comprehensive analysis of diagnosis and treatment in 99 cases of abdominal Schwannoma. Cancer Med. (2024) 13:e70140. doi: 10.1002/cam4.v13.16
3. Ishibashi H, Wakejima R, Takasaki C, and Okubo K. Successful excision of endobronchial cellular schwannoma with right lower sleeve lobectomy. Ann Thorac Surg. (2019) 107:e203–5. doi: 10.1016/j.athoracsur.2018.06.088
4. Peng C. Clinical diagnosis and treatment analysis of 109 cases of primary retroperitoneal schwannoma. Natl Med J China. (2015) 95:1755–8. doi: 10.3760/cma.j.issn.0376-2491.2015.22.011
5. Improta L, Tzanis D, Bouhadiba T, Abdelhafidh K, and Bonvalot S. Overview of primary adult retroperitoneal tumours. Eur J Surg Oncol. (2020) 46:1573–9. doi: 10.1016/j.ejso.2020.04.054
6. Daneshmand S, Youssefzadeh D, Chamie K, Boswell W, Wu N, Stein JP, et al. Benign retroperitoneal schwannoma: a case series and review of the literature. Urology. (2003) 62:993–7. doi: 10.1016/S0090-4295(03)00792-1
7. Wang CY, Yin HL, Lee KT, and Kuo KK. Extremely rare case of retroperitoneal “ancient” schwannoma with significant cystic change embedded in the psoas muscle. Kaohsiung J Med Sci. (2021) 37:632–3. doi: 10.1002/kjm2.12366
8. Van Houdt WJ, Schrijver AM, Cohen-Hallaleh RB, Memos N, Fotiadis N, Smith MJ, et al. Needle tract seeding following core biopsies in retroperitoneal sarcoma. Eur J Surg Oncol. (2017) 43:1740–5. doi: 10.1016/j.ejso.2017.06.009
9. Berger-Richardson D and Swallow CJ. Needle tract seeding after percutaneous biopsy of sarcoma: Risk/benefit considerations. Cancer. (2017) 123:560–7. doi: 10.1002/cncr.v123.4
10. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv51–67. doi: 10.1093/annonc/mdy096
11. Choudry HA, Nikfarjam M, Liang JJ, Kimchi ET, Conter R, Gusani NJ, et al. Diagnosis and management of retroperitoneal ancient schwannomas. World J Surg Oncol. (2009) 7:12. doi: 10.1186/1477-7819-7-12
12. Hirose T, Ishizawa K, Sakaki M, and Fujii Y. Retroperitoneal schwannoma is characterized by a high incidence of cellular type and GFAP-immunoreactivity. Pathol Int. (2012) 62:456–62. doi: 10.1111/j.1440-1827.2012.02822.x
13. Somatilaka BN, Sadek A, McKay RM, and Le LQ. Malignant peripheral nerve sheath tumor: models, biology, and translation. Oncogene. (2022) 41:2405–21. doi: 10.1038/s41388-022-02290-1
14. Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. (2006) 48:3–12. doi: 10.1111/j.1365-2559.2005.02284.x
15. Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. (2012) 19:878–85. doi: 10.1245/s10434-011-1978-7
Keywords: retroperitoneal schwannoma, malignant schwannoma, diagnosis, treatment, prognosis
Citation: Zhao Z, Fan S, Yu D and Wang G (2025) Malignant retroperitoneal schwannoma in a young adult: rapid recurrence, metastasis, and treatment reflections—a case report. Front. Oncol. 15:1588573. doi: 10.3389/fonc.2025.1588573
Received: 06 March 2025; Accepted: 09 May 2025;
Published: 30 May 2025.
Edited by:
Marco Caricato, Università Campus Bio-Medico di Roma, ItalyReviewed by:
Roberto Passa, Campus Bio-Medico University, ItalyChiara Pagnoni, Campus Bio-Medico University Hospital, Italy
Copyright © 2025 Zhao, Fan, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiying Wang, d2FuZ2d1aXlpbmdAaGVibXUuZWR1LmNu
 Zeming Zhao
Zeming Zhao Shaoqing Fan
Shaoqing Fan Donghao Yu
Donghao Yu Guiying Wang
Guiying Wang