Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, with proto-oncogene, receptor tyrosine kinase (c-kit), or PDGFRα mutations detected in around 85% of cases. GISTs without c-kit or platelet-derived growth factor receptor alpha (PDGFRα) mutations are considered wild-type (WT). Recently, some molecular alterations, including neurotrophic tyrosine receptor kinase (NTRK) fusions, have been reported in very few cases of WT GISTs. This novel finding opens the window for the use of tropomyosin receptor kinase (TRK) inhibitor therapy in these subtypes of GIST. In this case report, we present a rare NTRK3 fusion gastrointestinal stromal tumor (GIST) in a female patient with significant response to entrectinib. The patient was initially diagnosed with a giant gastric GIST (approximately 20.6cm×12.1cm×28.0m in size) showing classic immunohistochemical features (CD117+/DOG1+) on immunohistochemistry. After neoadjuvant imatinib therapy (400 mg/day), partial response was achieved with tumor shrinkage to 14.1cm×7.6cm×15.5cm, followed by radical surgery. Postoperative pathology confirmed high-risk GIST (ypT4N0), with genetic testing revealing a KIT exon11 deletion mutation (p.K558_V560del, VAF 63.80%). Continued oral imatinib adjuvant therapy was initiated. In 2024, disease progression was observed with residual KIT mutation (VAF 1.10%) and new-onset ETV6:NTRK3 fusion (VAF 35.29%) detected by circulating tumor DNA (ctDNA) analysis. Switching to entrectinib (600 mg/day) achieved partial imaging response within 4 weeks (tumor reduction of approximately 27%), with complete clearance of dual mutations observed in ctDNA after 3 months. The patient maintained sustained response without adverse events during final follow-up. This case highlights the breakthrough efficacy of TRK inhibitors in treating NTRK-fusion GIST and confirms the critical value of liquid biopsy in monitoring drug resistance mechanisms and guiding precision treatment.
Introduction
Gastrointestinal stromal tumor (GIST), the most common mesenchymal malignancy of the gastrointestinal tract, has been classified into five major molecularly subtypes through next-generation sequencing (NGS) including classical KIT/PDGFRA-mutant, SDH-deficient, NF1-assoicated, BRAF-mutant, and NTRK3-fusion GISTs (1). Notably, the NTRK3 fusion have emerged as rare but clinically significant molecular alternations defining a distinct subgroup of GISTs (2). Therefore, elucidating the clinicopathological characteristics, diagnostic approaches, and therapeutic implications regarding NTRK3 rearrangements hold critical value for advancing precision oncology. Herein, we present a case of a 60-year-old female diagnosed with an NTRK3 fusion, following we also include a comprehensive review of the current literature regarding this rare molecular entity.
Case description
A 60-year-old female presented with progressive abdominal distension lasting six months, culminating in admission to our institution on August, 2018. Initial evaluation at a local hospital on August, 2018, revealed a massive left abdominal mass (20.6 cm×12.1 cm×28.0 cm) on contrast-enhanced CT (Figure 1A), which showed poorly defined borders with adjacent organs (liver, stomach, pancreas, and bowel) and gastric architectural distortion. Radiological features suggested a malignant stromal tumor with extensive ulceration. Elevated CA-125 (161.60 U/mL) and gastroscopic findings further supported this suspicion, revealing a deep ulcerated lesion at the gastroesophageal junction with pseudodiverticular formation and mucosal irregularity. Biopsy pathology (No. 20185032) confirmed moderate chronic inflammation with acute exacerbation but lacked diagnostic clarity. On August 20, 2018, a subsequent CT-guided core needle biopsy was performed. Immunohistochemical analysis confirmed a GIST phenotypes: CD117(+), DOG1(+), CD34(+), SMA(+), Vimentin(+), Ki-67 (40%), CKpan (-), desmin (-), and S-100 (-) (Figures 1D–F). Because the gene sequencing technology for gastrointestinal stromal tumors was still in development at the time of the patient’s onset, the patient refused to undergo genetic testing after full communication. Following multidisciplinary tumor board consensus, neoadjuvant therapy with oral imatinib (400 mg daily) was initiated due to extensive tumor is large and involvement of the gastric wall, pancreas, and spleen. Two months of treatment resulted in volume reduction (19 cm×8.8 cm×23.5 cm) and improved margination (Figure 1B). Continued therapy-maintained disease control, with final follow-up on September 24, 2022, demonstrating sustained partial response (14.1 cm×7.6 cm×15.5 cm) and persistent gastrotumor communication (Figure 1C).
Figure 1

Imaging and pathological characteristics of NTRK3-fusion GIST case. (A) Baseline contrast-enhanced CT (August 2018) demonstrates a massive left abdominal mass (20.6 cm×12.1 cm×28.0 cm) with infiltrative borders (arrows) involving the gastric fundus and pancreatic tail; (B) Post-neoadjuvant therapy imaging (October 2018) reveals partial response with tumor reduction (19.0 cm×8.8 cm×23.5 cm) and improved margination (arrows); (C) Long-term follow-up CT (September 2022) confirms sustained partial response (14.1 cm×7.6 cm×15.5 cm) with persistent gastrotumor communication (dashed circle); (D-F) Representative immunohistochemical staining for CD117, CD34 and Ki-67.
In November 2022, the patient received a radical surgery at a tertiary hospital in Shaanxi province, involving proximal gastrectomy, distal pancreatectomy with splenectomy, and adhesiolysis. Histopathological evaluation of the resected specimen (proximal stomach, spleen, and pancreatic tail) demonstrated treatment-induced morphological changes, characterized by extensive hyaline degeneration and multifocal calcifications. The resected tumor measured 19 cm in maximum diameter with a mitotic count of 4/50 HPF, showing infiltrative growth into the splenic parenchyma and pancreatic capsule (R1 resection) (Figures 2A, B). Lymph node staging revealed no metastasis (0/25 nodes). The tumor was further classified as ypT4N0 (AJCC 8th), 3b (WHO) and high-risk NIH group. Postoperative immunohistochemical profiling confirmed classic GIST immunophenotype with CD117(+), DOG1(+), CD34(+), SDH-a(+), SDH-β(+), Desmin(-), H-cal desmon(-), S-100(-), SHA(-), and Ki-67 (4%) (Figures 2C–E). Importantly, NGS on the tumor samples identified a pathogenic KIT exon 11 deletion (p.K558_V560del) with 63.80% variant allele frequency (VAF) (Figure 2F). Based on multidisciplinary consensus, adjuvant imatinib therapy (400 mg daily) was recommended. However, the patient did not adhere to regular follow-up after 2022.
Figure 2
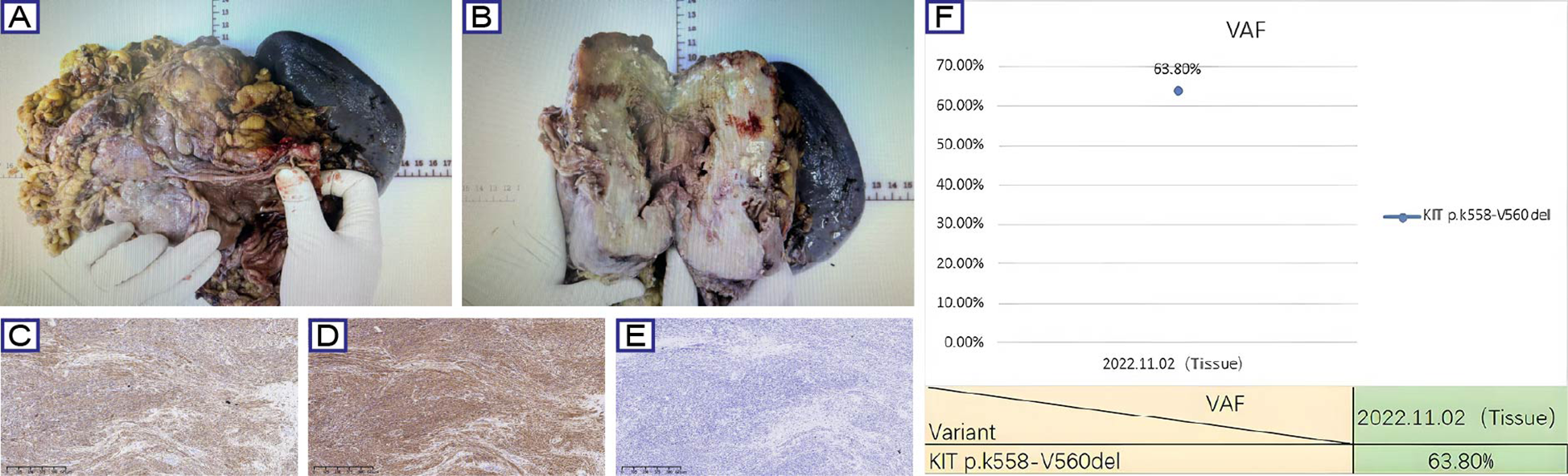
Surgical, histopathological, and molecular characteristics of GIST case. (A, B) Gross examination of the resected specimen demonstrates a lobulated mass (19 cm maximal diameter) with hemorrhagic necrosis (asterisk) and fibrous pseudocapsule (arrows). (C-E) Representative immunohistochemical staining for CD117, CD34 and Ki-67. (F) NGS identified a pathogenic KIT exon 11 deletion (p.K558_V560del) with 63.80% variant allele frequency.
In March 2024, the patient returned to our hospital with recurrent abdominal distension. Contrast-enhanced CT revealed progressive GIST features include anastomotic soft-tissue thickening, cystic-solid masses and compression changes in the inferior vena cava (Figure 3A). As the disease progressed, the patient refused to undergo a repeat biopsy, and Plasma ctDNA analysis was performed after full communication with the family,Plasma ctDNA analysis identified the residual KIT mutation (VAF 1.10%) and an recurrent ETV6:NTRK3 fusion (ETV6 exon4:NTRK3 exon14; VAF 35.29%). Entrectinib therapy (600 mg daily) was initiated on March 28, 2024. Follow-up CT at 4 weeks demonstrated partial response (RECIST 1.1) (Figure 3B). Repeat ctDNA profiling in June 2024 confirmed complete clearance of both mutations (Figure 3C). The patient remained asymptomatic with sustained radiographic response at final telephone follow-up (October 31, 2024). So far, the patient was continuing entrectinib without reported adverse events.
Figure 3
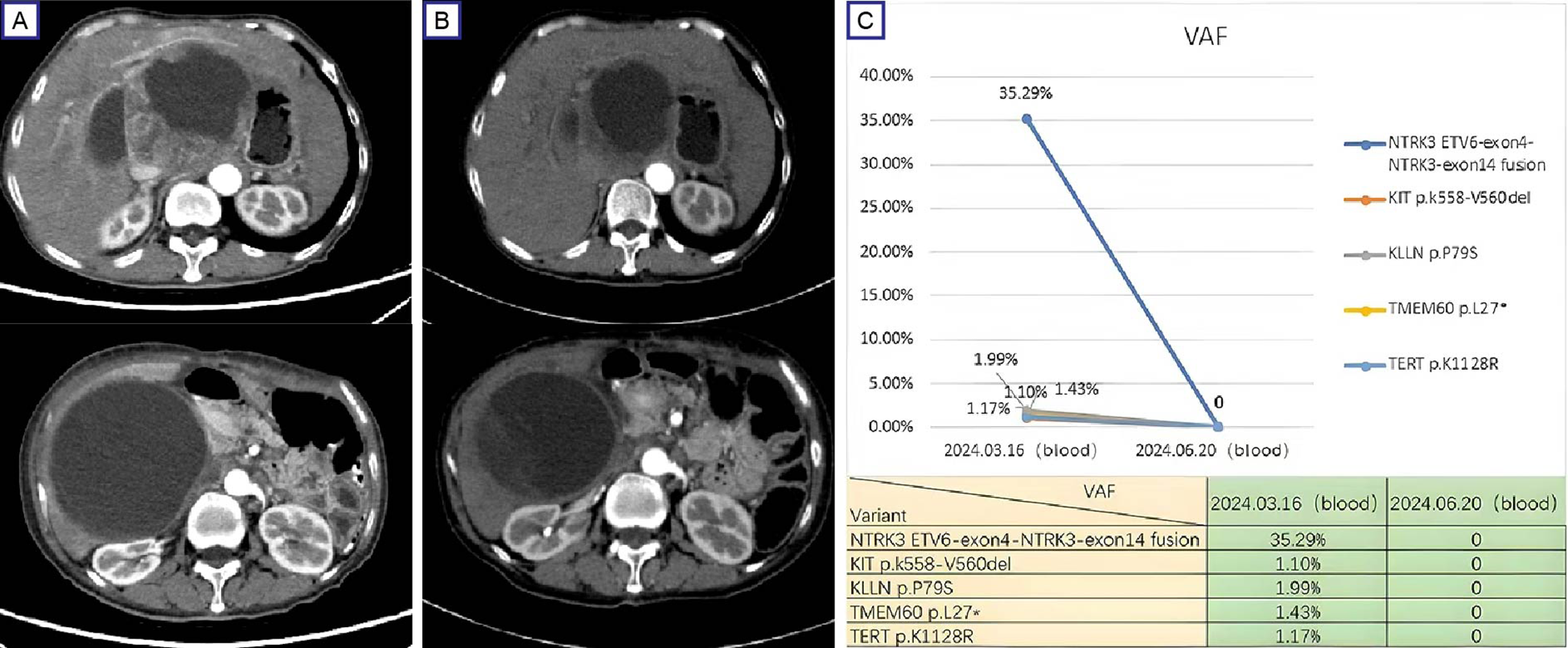
Therapeutic response and mutation dynamics in progressive GIST. (A) Pre-treatment contrast-enhanced CT (March 28, 2024) demonstrates a dominant cystic-solid mass (12.6 cm×10.8 cm, arrows) in the hepatogastric space with IVC compression; (B) Post-treatment (entrectinib) contrast-enhanced CT (April 25, 2024) shows partial response (RECIST 1.1) with 27% tumor reduction (9.6 cm × 8.4 cm) and improved IVC compression. (C) ctDNA profiling reveals clearance of KIT exon 11 deletion (p.K558_V560del) and ETV6:NTRK3 fusion (ETV6 exon4:NTRK3 exon14) following entrectinib therapy. IVC, inferior vena cava.
Discussion
Gastrointestinal stromal tumors (GISTs) is the most common mesenchymal neoplasms that originated from the interstitial cells of Cajal (ICCs) or their precursors within the gastrointestinal mesenchymal tissue (3). Epidemiological studies indicate a global incidence of 10 to 15 cases per million population, with gastric (60%) and small intestinal (30%) predominance. Less frequent sites include the duodenum (4 to 5%), rectum (2 to 4%), colon (1 to 2%), and esophagus (<1%) (4). Approximately 20 to 30% of GISTs demonstrate malignant behavior, with 5-year survival rates ranging from 35 to 65% for metastatic disease (4). More recent studies also suggest GISTs may also arise in extraintestinal sites such as the mesentery and omentum (5–7). Based on anatomical growth patterns, GISTs are classified into four subtypes: submucosal, intramural, subserosal, and extragastrointestinal (5–7). Most GISTs present as solitary lesions with nonspecific clinical manifestations, often incidentally detected during endoscopic or cross-sectional imaging evaluations for symptoms like upper abdominal pain or gastrointestinal bleeding (8). Immunohistochemical profiling remains pivotal for diagnosis, with 95% of cases expressing CD117 (KIT protein) and 70% showing CD34 positivity (9). Therefore, in practice, CD117 often considered to be best defining feature of GISTs, while CD34 provides complementary diagnostic value, particularly in CD117-negative cases. Genetics characterization plays a critical role in therapeutic decision-making and prognosis. Approximately 85% of GISTs harbor mutually exclusive activating mutations in KIT (exons 9, 11, 13, 17) or PDGFRA (exons 12, 18), driving constitutive receptor tyrosine kinase (RTK) activation and tumor progression (3, 10). The remaining 15% lack these canonical mutations and are classified as KIT/PDGFRA wild-type (WT) GISTs (11). Notably, recent studies identify NTRK gene rearrangement in approximately 16% of KIT WT GISTs, expanding the molecular landscape of this subset (12).Serological biomarker detection is also of great significance. Relevant studies have shown that preoperative and postoperative serum CA125 abnormalities can be regarded as independent risk factors for gastrointestinal stromal tumor progression, which is of great value to the comprehensive treatment of patients (13).
For localized and resectable GIST, surgical resection remains the only potentially curative treatment. However, postoperative recurrence occurs in 40 to 90% of patients (14). Neoadjuvant therapy is therefore recommended for patients with large GISTs to reduce tumor volume, minimize intraoperative rupture risk and improve resectability (15). Current National Comprehensive Cancer Network (NCCN) guidelines endorse imatinib for locally advanced or marginally resectable primary GISTs (16). While complete surgical excision combined with adjuvant imatinib constitutes the standard treatment for intermediate- and high-risk GISTs, bulky tumors often necessitate multivisceral resection or functional organ compromise. Preoperative imatinib administration facilitates R0 resection and organ preservation through tumor downsizing (17). Clinical studies demonstrate that neoadjuvant imatinib (400 mg daily) achieves significant tumor regression in massive GISTs (>10 cm), enabling radical resection in most cases (18–21). In the present case, the patient was found to harbor a KIT exon 11 deletion (p.K558_V560del) with a 63.80% VAF, achieving PR after imatinib therapy. Beyond tumor downsizing, three phase III clinical trials (ACOSOG Z9001, SSGXVIII/AIO, and EORTC 62024) have confirmed the long-term survival benefits of adjuvant imatinib, the 10-year overall survival rates reached 79% in high-risk cohorts (22–24). Based on this evidence and beyond, current clinical guidelines recommend 3-year adjuvant imatinib (400 mg daily) for high-risk GIST patients carrying imatinib-sensitive KIT or PDGFRA mutations (25).
Unfortunately, follow-up contrast-enhanced CT on March 28, 2024, revealed disease progression, which indicates an acquired resistance to imatinib. To our knowledge, approximately 50% of GIST patients develop imatinib resistance within 24 months, primarily due to acquired cis-mutations in the KIT and PDGFRA. Common resistance mutations include KIT V654A (exon 13), T670I (exon 14), D816V/D820G/N822K/Y823D (exon 17), and PDGFRA D842V (exon 18), which likely arise through Darwinian selection under TKI pressure (26, 27). Other drug resistance mechanisms include KIT overexpression, activation of downstream and/or alternative pathways, and BRAF mutations. Importantly, drug-resistant cells may undergo clonal proliferation, with different metastatic lesions potentially exhibiting distinct mutation profiles. Therefore, ctDNA profiling has emerged as a critical tool for comprehensive resistance mutation analysis, providing essential insights to guide therapeutic decision-making in resistant malignancies (28). In this case, KIT mutation and NTRK3 fusion were identified through plasma ctDNA analysis, and entrectinib 600 mg daily was initiated on March 28, 2024. Subsequent CT results (April 25, 2024) demonstrated partial response (RECIST 1.1), with complete molecular remission (both alterations undetectable, VAF 0%) confirmed at 3 months. While rare in adult malignancies, NTRK fusions are hallmark alterations in WT GIST lacking KIT/PDGFRA/RAS pathway mutations (29–31). The prototypical ETV6-NTRK3 fusion, first identified in infantile fibrosarcoma (32), has been reported in <2% of quadruple WT GISTs (31, 33). Brenca et al. proposed that ETV6-NTRK3 may drive tumorigenesis through IGF1R/IRS1 pathway activation (30). TRK inhibitors (entrectinib/larotrectinib) demonstrate remarkable efficacy across NTRK-fusion cancers, with reported response rates exceeding 75% in basket trials (31, 34–37). Notably, all three GIST patients in the larotrectinib NAVIGATE trial achieved >30% tumor shrinkage, including one pathologic complete response (37). The Belgian consensus guidelines now recommend larotrectinib as first-line therapy for NTRK-fusion GIST (38). Remarkably, follow-up CT at 1 month (April 25, 2024) showed PR, with complete clearance of both mutations at 3 months. This correlates with emerging evidence that ctDNA dynamics predict clinical outcomes in GIST. Studies demonstrate that ctDNA clearance associates with superior PFS (HR=0.28) and OS (HR=0.19), along with improved ORR (41.7% vs 12.1%) and DCR (97.5% vs 67.2%) (39–41). Our case highlights the clinical utility of serial liquid biopsies for monitoring molecular response and guiding precision therapy.
Conclusion
While NTRK fusions occur at extremely low frequencies (<1%) in GIST, this case highlights the clinical potential of TRK inhibitors (e.g., entrectinib) for overcoming imatinib resistance in molecularly selected patients.Based on the above,the integration of NGS and liquid biopsy has revolutionized the molecular profiling of GIST, enabling the detection of rare mutations and early identification of secondary resistance mechanisms. Although our findings are limited by the short follow-up duration and single-case nature, the observed rapid molecular clearance (VAF 0%) and radiographic response (PR) provide compelling preliminary evidence. These results align with recent basket trials demonstrating >75% response rates to TRK inhibitors in NTRK-fusion solid tumors (33–35).
Moving forward, systematic collection of real-world data through multicenter registries is critical to validate the long-term efficacy and safety of this targeted approach. Furthermore, prospective studies should explore optimal sequencing strategies combining TRK inhibitors with other targeted therapies to delay resistance. This paradigm-shifting case underscores the necessity of comprehensive molecular profiling in imatinib-resistant GIST and expands the therapeutic arsenal for precision oncology.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Shaanxi Provincial Cancer Hospital, No.309 West Road, Yanta District, Xi 'an City, Shaanxi Province. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GD: Writing – original draft. PH: Writing – review & editing. ZhiZ: Writing – review & editing. QG: Writing – review & editing. JJ: Writing – review & editing. SL: Writing – review & editing. JM: Writing – review & editing. JB: Writing – review & editing. HW: Writing – review & editing. ZheZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Project type: Xi'an Science and Technology Bureau, Applicant: Yili Zhang Project number: 2024JH-ZCLGG-0040, Project name: Technical development of automated tumor organoid and organ chip culture/determination/sensitivity detection system (living pathological engineering). Project type: Beijing Science and Technology Innovation Medical Development Foundation research project, Applicant: Zheng Zhao, Project number: KC2023-JX-0288-PM92, Project name: Application of circulating tumor DNA and peripheral blood T cell subsets in prognosis assessment of immunotherapy for advanced non-small cell lung cancer. Project type: “New Emerging Supportive Therapy for Cancer Research” public welfare project, Applicant: Zheng Zhao Project number: cphcf-2022-218, Project name: Study on the correlation between dynamic changes of Memory T cell subtypes and the efficacy of immune checkpoint inhibitors. Project type: Xi'an Science and Technology Bureau, Applicant: Zheng Zhao, Project number: 2024JH-YLYB-0176, Contract No.: 24YXYJ0133, Project name: Construction of an immunotherapy prediction model for advanced NSCLC based on ctDNA and peripheral blood T cell subsets. Project type: China Medical and Health Development Foundation, Applicant: Zheng Zhao Project code: chmdf2024-xrzx09-20, Project name: Discussion on the T cell related immune mechanism of clinically used zhengzheng Chinese patent medicines against lung cancer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Nishida T Naito Y Takahashi T Saito T Hisamori S Manaka D et al . Molecular and clinicopathological features of KIT/PDGFRA wild-type gastrointestinal stromal tumors. Cancer Sci. (2024) 115:894–904. doi: 10.1111/cas.16058
2
Cao Z Li J Sun L Xu Z Ke Y Shao B et al . GISTs with NTRK gene fusions: A clinicopathological, immunophenotypic, and molecular study. Cancers (Basel). (2022) 15:105. doi: 10.3390/cancers15010105
3
Serrano C Martín-Broto J Asencio-Pascual JM López-Guerrero JA Rubió-Casadevall J Bagué S et al . 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. (2023) 15:17588359231192388. doi: 10.1177/17588359231192388
4
Zhang B Zhu F Li P Zhu J . Artificial intelligence-assisted endoscopic ultrasound in the diagnosis of gastrointestinal stromal tumors: a meta-analysis. Surg Endosc. (2023) 37:1649–57. doi: 10.1007/s00464-022-09597-w
5
Yalcin Y Bozkurt KK Ciris IM Cerci SS Erdemoglu E . Primary extra-gastrointes- tinal stromal tumor of mesenteric root: a rare version of a soft tis-sue tumor located on a critical region. J Gastrointest Cancer. (2017) 49:513–6. doi: 10.1007/s12029-017-9944-7
6
Tarchouli M Bounaim A Essarghini M Aitidir B Lomdo M Benmoussa M et al . Extra-gastrointes- tinal stromal tumor of the greater omentum: unusual case report. J Gastrointest Cancer. (2016) 47:489–93. doi: 10.1007/s12029-015-9784-2
7
Fujimoto A Kobayashi T Uchida S Ichinose Y Sasaoki T Goto K et al . Laparoscopic total gastrectomy for multiple sporadic gastric gastrointestinal stromal tumors: report of a case. Surg Today. (2012) 42:84–8. doi: 10.1007/s00595-011-0011-x
8
Venkataraman V George S Cote GM . Molecular advances in the treatment of advanced gastrointestinal stromal tumor. Oncologist. (2023) 28:671–81. doi: 10.1093/oncolo/oyad167
9
Munteanu A Patrascu S Bordu S Laskou S Surlin V Radu P . Clinical and morphological characteristics of gastrointestinal stromal tumor. Chirurgia (Bucur). (2023) 118:618–23. doi: 10.21614/chirurgia.2023.v.118.i.6.p.618
10
Serrano C George S . Gastrointestinal stromal tumor: challenges and opportunities for a new decade. Clin Cancer Res. (2020) 26:5078–85. doi: 10.1158/1078-0432.CCR-20-1706
11
Ali RH Alsaber AR Mohanty AK Alnajjar A Mohammed EMA Alateeqi M et al . Molecular profiling of KIT/PDGFRA-mutant and wild-type gastrointestinal stromal tumors (GISTs) with clinicopathological correlation: an 18-year experience at a tertiary center in Kuwait. Cancers (Basel). (2024) 16:2907. doi: 10.3390/cancers16162907
12
Lee JH Shin SJ Choe EA Kim J Hyung WJ Kim HS et al . Tropomyosin-related kinase fusions in gastrointestinal stromal tumors. Cancers (Basel). (2022) 14:2659. doi: 10.3390/cancers14112659
13
Sui C Lin C Tao T Guan W Zhang H Tao L et al . Prognostic significance of serum CA125 in the overall management for patients with gastrointestinal stromal tumors. BMC Gastroenterol. (2023) 23(1):98. doi: 10.1186/s12876-023-02655-0
14
Alsaud JS Alruqayi S Alomair A . The role of neoadjuvant therapy in a giant gastric gastrointestinal stromal tumour: A case report and review of the literature. Cureus. (2024) 16:e55655. doi: 10.7759/cureus.55655
15
Alassani F Tchangai B Bagny A Adani-Ife AA Amavi KA Darre T et al . Excision of a large gastrointestinal stromal tumour following 16 months of neoadjuvant therapy with imatinib (case report). Oncol Ther. (2019) 7:159–64. doi: 10.1007/s40487-019-00101-4
16
von Mehren M Randall RL Benjamin RS Boles S Bui MM Ganjoo KN et al . Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2018) 16:536–63. doi: 10.6004/jnccn.2018.0025
17
Yang W Yu J Gao Y Shen Q Zhang Q Liu X et al . Preoperative imatinib facilitates complete resection of locally advanced primary GIST by a less invasive procedure. Med Oncol. (2014) 31:133. doi: 10.1007/s12032-014-0133-2
18
Luo H Peng L Wang N Zhang J Zheng X Sun Y et al . Early brain metastasis of advanced gastric cancer with a pathological complete response to neoadjuvant chemotherapy followed by surgery: a case report and literature review. Cancer Biol Ther. (2018) 19:875–8. doi: 10.1080/15384047.2018.1456600
19
Parab TM DeRogatis MJ Boaz AM Grasso SA Issack PS Duarte DA et al . Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. (2019) 10:144–54. doi: 10.21037/jgo.2018.08.20
20
Jumniensuk C Charoenpitakchai M . Gastrointestinal stromal tumor: clinicopathological characteristics and pathologic prognostic analysis. World J Surg Oncol. (2018) 16:231. doi: 10.1186/s12957-018-1532-1
21
Mohammadi M Roets E Bleckman RF Oosten AW Grunhagen D Desar IME et al . Impact of mutation profile on outcomes of neoadjuvant therapy in GIST. Cancers (Basel). (2025) 17:634. doi: 10.3390/cancers17040634
22
Dematteo RP Ballman KV Antonescu CR Maki RG Pisters PW Demetri GD et al . Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. (2009) 373:1097–104. doi: 10.1016/S0140-6736(09)60500-6
23
Joensuu H Eriksson M Sundby Hall K Hartmann JT Pink D Schütte J et al . One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. (2012) 307:1265–72. doi: 10.1001/jama.2012.347
24
Casali PG Le Cesne A Poveda Velasco A Kotasek D Rutkowski P Hohenberger P et al . Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European organisation for research and treatment of cancer soft tissue and bone sarcoma group intergroup randomized trial in collaboration with the Australasian gastro-intestinal trials group, UNICANCER, French sarcoma group, Italian sarcoma group, and Spanish group for research on Sarcomas. J Clin Oncol. (2015) 33:4276–83. doi: 10.1200/JCO.2015.62.4304
25
Joensuu H Eriksson M Sundby Hall K Reichardt A Hermes B Schütte J et al . Survival outcomes associated with 3 years vs 1 year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: an analysis of a randomized clinical trial after 10-Year follow-up. JAMA Oncol. (2020) 6:1241–6. doi: 10.1001/jamaoncol.2020.2091
26
Serrano C Fletcher JA . Overcoming heterogenity in imatinib-resistant gastrointestinal stromal tumor. Oncotarget. (2019) 10:6286–7. doi: 10.18632/oncotarget.27277
27
Shima T Taniguchi K Tokumaru Y Inomata Y Arima J Lee SW et al . Glucose transporter-1 inhibition overcomes imatinib resistance in gastrointestinal stromal tumor cells. Oncol Rep. (2022) 47:7. doi: 10.3892/or.2021.8218
28
SSerrano C Vivancos A López-Pousa A Matito J Mancuso FM Valverde C et al . Clinical value of next generation sequencing of plasma cell-free DNA in gastrointestinal stromal tumors. BMC Cancer. (2020) 20:99. doi: 10.1186/s12885-020-6597-x
29
Cocco E Scaltriti M Drilon A . NTRK fusion - positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. (2018) 15:731 –747. doi: 10.1038/s41571-018-0113-0
30
Brenca M Rossi S Polano M Gasparotto D Zanatta L Racanelli D et al . Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. (2016) 238:543–9. doi: 10.1002/path.4677
31
Shi E Chmielecki J Tang CM Wang K Heinrich MC Kang G et al . FGFR1 and NTRK3 actionable alterations in “Wild - Type” gastrointestinal stromal tumors. J Transl Med. (2016) 14:339. doi: 10.1186/s12967-016-1075-6
32
Knezevich SR McFaddn DE Tao W Lim JF Sorensen PH . A novel ETV6- NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. (1998) 18:184–7. doi: 10.1038/ng0298-184
33
Rrenca M Rossi S Polaino M Gasparotto D Zanatta L Racanelli D et al . Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. (2016) 238:543–9. doi: 10.1002/path.4677
34
MaChado I Claramunt-Alonso R Lavernia J Romero I Barrios M Safont MJ et al . ETV6::NTRK3 fusion-positive wild-type gastrointestinal stromal tumor (GIST) with abundant lymphoid infiltration (TILs and tertiary lymphoid structures): A report on a new case with therapeutic implications and a literature review. Int J Mol Sci. (2024) 25:3707. doi: 10.3390/ijms25073707
35
Drilon A Siena S Ou SI Patel M Ahn MJ Lee J et al . Safety and antitumor activity of the multitargeted pan - TRK, ROS 1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. (2017) 7:400–9. doi: 10.1158/2159-8290.CD-16-1237
36
Doebele RC Drilon A Paz-Ares L Siena S Shaw AT Farago AF et al . Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. (2020) 21:271–82. doi: 10.1016/S1470-2045(19)30691-6
37
Laetsch TW DuBois SG Mascarenhas L Turpin B Federman N Albert CM et al . Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. (2018) 19:705–14. doi: 10.1016/S1470-2045(18)30119-0
38
Awada A Berghmans T Clement PM Cuppens K De Wilde B Machiels JP et al . Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib. Crit Rev Oncol Hematol. (2022) 169:103564. doi: 10.1016/j.critrevonc.2021.103564
39
Pécuchet N Zonta E Didelot A Combe P Thibault C Gibault L et al . Base-position error rate analysis of next-generation sequencing applied to circulating tumor DNA in non-small cell lung cancer: A prospective study. PloS Med. (2016) 13:e1002199. doi: 10.1371/journal.pmed.1002199
40
Incorvaia L De Biase D Nannini M Fumagalli E Vincenzi B De Luca I et al . KIT/PDGFRA variant allele frequency as prognostic factor in gastrointestinal stromal tumors (GISTs): results from a multi-institutional cohort study. Oncologist. (2024) 29:e141–51. doi: 10.1093/oncolo/oyad206
41
Song Y Hu C Xie Z Wu L Zhu Z Rao C et al . Written on behalf of AME Lung Cancer Collaborative Group. Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort. Transl Lung Cancer Res. (2020) 9:269–79. doi: 10.21037/tlcr.2020.03.17
Summary
Keywords
gastrointestinal stromal tumor, acquired resistance, next-generation sequencing, NTRK3 fusion, ctDNA dynamics predict
Citation
Dong G, Han P, Zhang Z, Ge Q, Jiang J, Li S, Ma J, Bai J, Wei H and Zhao Z (2025) Case Report: Dramatic response to entritinib in a patient with gastrointestinal stromal tumor positive for NTRK3 fusion. Front. Oncol. 15:1588950. doi: 10.3389/fonc.2025.1588950
Received
06 March 2025
Accepted
24 July 2025
Published
18 August 2025
Volume
15 - 2025
Edited by
Matiullah Khan, AIMST University, Malaysia
Reviewed by
Lucia Guadalupe Taja Chayeb, National Institute of Cancerology (INCAN), Mexico
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, Germany
Updates
Copyright
© 2025 Dong, Han, Zhang, Ge, Jiang, Li, Ma, Bai, Wei and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wei, 276077154@qq.com; Zheng Zhao, 15964433993@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.