- 1Cancer Center, Department of Ultrasound Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China
- 2Department of Ultrasonography, Hangzhou Third People’s Hospital & Hangzhou Third Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, China
Great saphenous vein leiomyosarcoma is an extremely rare tumor clinically, which is often misdiagnosed as superficial venous thrombosis due to atypical clinical manifestations. This report describes a rare presentation of great saphenous vein leiomyosarcoma in a 37-year-old man who was referred to our hospital due to a gradually enlarging painless mass on his left shank. Ultrasound revealed that the mass in the left calf was a solid tumor originating from the left great saphenous vein. He underwent radical resection for the mass ultimately. Subsequent histopathological and immunohistochemical analyses resulted in a conclusive diagnosis of primary leiomyosarcoma originating from the great saphenous vein. There are only 56 cases of great saphenous vein leiomyosarcoma reported in medical databases worldwide at present, which are summarized and analyzed here.
Introduction
Leiomyosarcoma (LMS) is a rare malignant tumor, and LMS originating from the vessel is even rarer (1). Vascular LMS usually occurs in the inferior vena cava and is relatively rare in the great saphenous vein (GSV) (2). Herein, we report a case of leiomyosarcoma of the great saphenous vein (GSV-LMS) and review the literature. The patient provided written informed consent for the publication of this manuscript and any identifying images or data. The clinical manifestations of GSV-LMS are atypical (3), and only 56 cases have been publicly reported so far. There is a lack of experience in its diagnosis and treatment, which leads to a lack of understanding. As a consequence, this disease is prone to misdiagnosis and mistreatment. By means of the introduction of a rare case, we analyzed the diagnostic value of GSV-LMS based on ultrasound, combined with the contents of previous literature, and we tried to summarize the effective diagnosis and treatment information of GSV-LMS so as to improve the preoperative diagnostic accuracy and the prognosis of patients.
Case description
Case presentation
A previously healthy 37-year-old man was admitted to our hospital due to a 4-month history of a subcutaneous mass about the size of a walnut on his left calf. He did not take it seriously at first. The patient reported that the mass gradually increased and was currently about the size of an egg. In the past week, the skin of the lump was accidentally broken. Prior to hospitalization, he had been undergoing symptomatic treatment with no obvious improvement. On the first day of admission, the patient’s physical examination revealed that there was a 6 cm × 4 cm mass in the inner part of the lower left calf. It was hard, slightly compressible, with low mobility and no obvious tenderness. The skin tension on the surface was high, accompanied by pigmentation, local skin rupture, and a small amount of serous exudates.
Ultrasound findings
Ultrasonography showed a subcutaneous heterogeneous hypoechoic mass located in the inside of his lower left calf, the size of which was approximately 5.9 cm × 3.6 cm × 4.5 cm, the shape was lobed, the boundary was clear, and the edge was not smooth (Figures 1A–D). The interior of the mass was detected to have a number of honeycomb-like anechoic areas (Figures 1B, C). Ultrasound scan showed that the upper and lower poles of the mass connected to the GSV (Figures 1A, D), and the residual GSV of the left calf had a wide diameter and was filled with a heterogeneous hypoechoic material (Figures 1G, H). Color Doppler flow imaging (CDFI) showed abundant blood flow signals in the mass and punctate blood flow signals in the heterogeneous hypoechoic mass in the residual GSV of the left calf (Figures 1E–G). In addition, there was no obvious abnormality in the inferior vena cava, iliac vein, and other deep or superficial veins of the lower limbs, and the blood flow signal was normal. The ultrasound diagnosis was a solid tumor originating from the GSV. The patient has a history of diabetes for more than 1 year with regular medication and a satisfactory level of control. The patient’s chest radiograph, electrocardiogram, and other preoperative routine laboratory tests were normal.
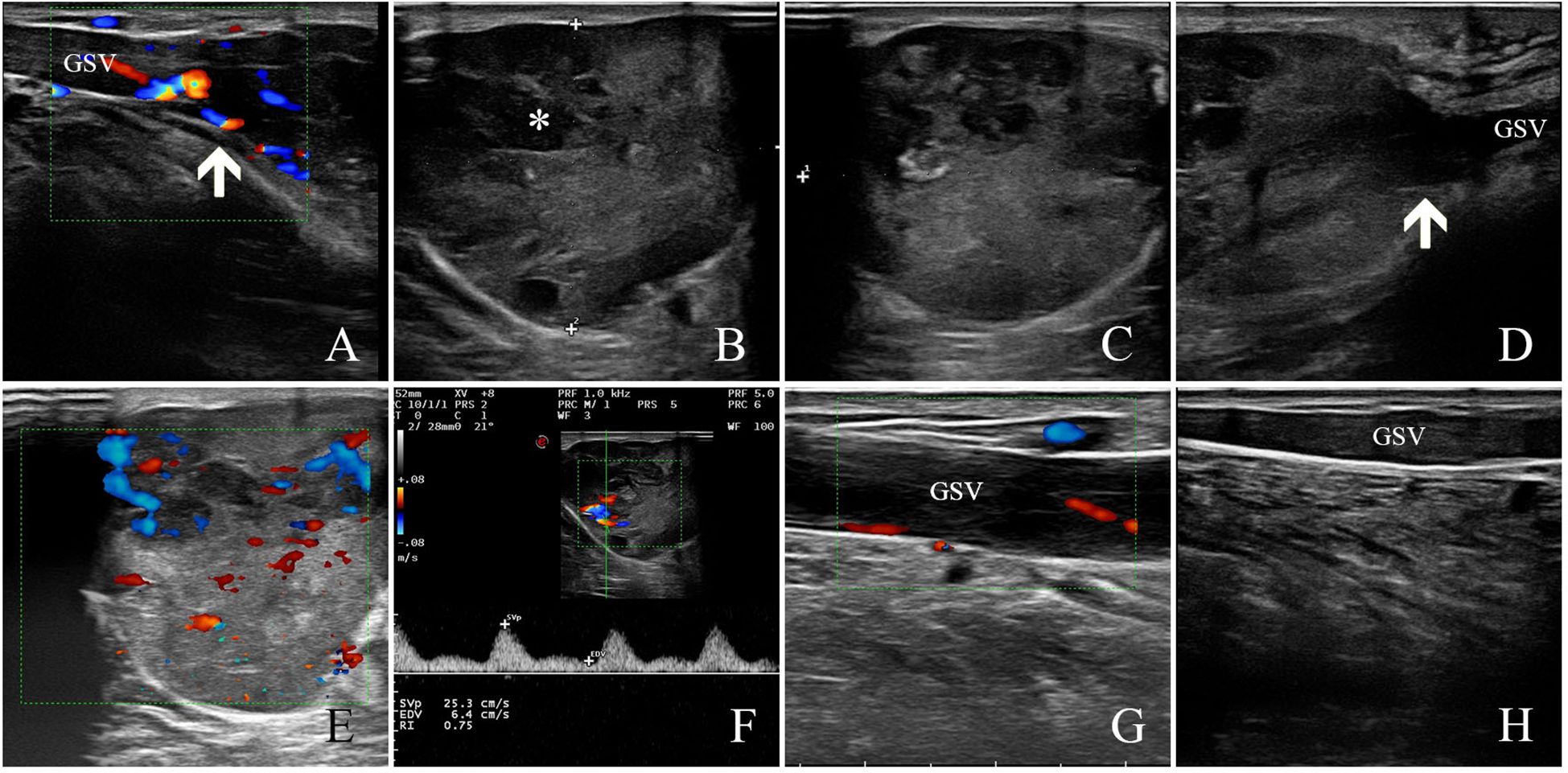
Figure 1. Ultrasound findings of GSV-LMS in a male patient. (A) The white arrow indicates the upper pole of the lesion. (B, C) The figure shows that the internal echo was heterogeneous hypoechoic, and a number of honeycomb-like anechoic areas were detected (as shown by white * in the figure). (D) The white arrow indicates the lower pole of the lesion. (E) Color Doppler flow imaging (CDFI) showed abundant blood flow signals in the mass. (F) The arterial spectrum was detected in the internal blood flow signal of the lesion with a resistance index of approximately 0.75. (G) The GSV at the proximal side of the lesion was full of heterogeneous hypoechoic mass, and a punctured blood flow signal could be detected. (H) The GSV at the distal side of the lesion was full of heterogeneous hypoechoic mass, and no blood flow signal could be detected. GSV, great saphenous vein; LMS, leiomyosarcoma.
Treatment
The patient underwent excision of soft tissue mass in his lower left calf under general anesthesia (Figure 2A). Intraoperative view showed a mass with rich blood supply approximately 6 cm × 7 cm × 5 cm in size found wrapped around the GSV (Figure 2B). The mass was isolated and confirmed to be of GSV origin (Figure 2C). Rich blood supply was seen on the surface and surrounding area of the mass (Figure 2B). The upper and lower blood vessels that connected with the tumor were separated and cut off at approximately 2 cm of the normal segment, not only to avoid embolization risk but also to prevent tumor invasion. The tumor and surrounding adipose tissue were completely removed. No other adjuvant therapy such as radiotherapy or chemotherapy was given after surgery, and the incision healed well.
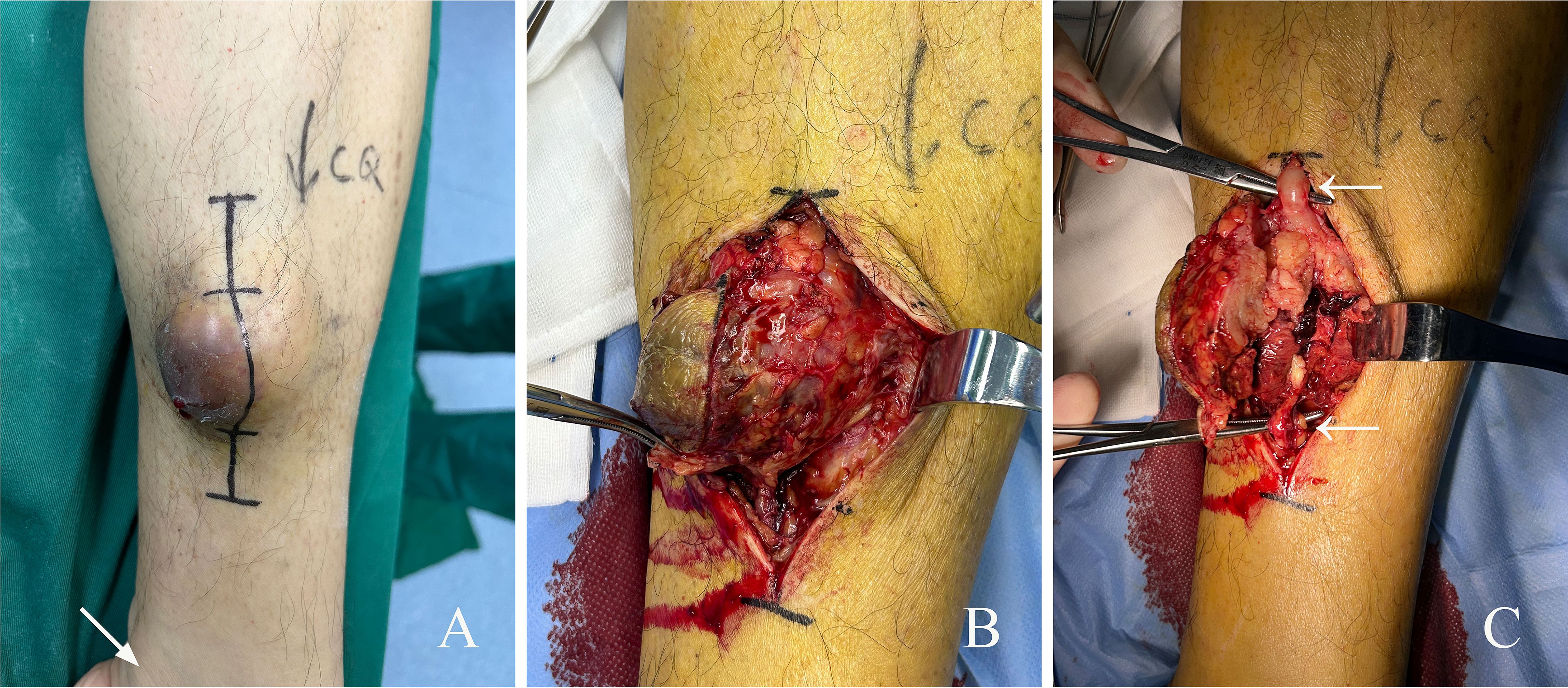
Figure 2. Surgical findings of GSV-LMS in a male patient. (A) Appearance of the subcutaneous mass on his left calf. The area indicated by the arrow is the medial malleolus. (B) Intraoperative view showing a mass with rich blood supply approximately 6 cm × 7 cm × 5 cm in size was found wrapped around the GSV. More proliferative vessels can be seen on the surface and around the mass. (C) The mass was isolated and confirmed to be of GSV origin. The white arrows mark the GSV. GSV, great saphenous vein; LMS, leiomyosarcoma.
Pathological examination
Gross pathology showed that the section of the tumor was grayish-white and grayish-red with medium texture (Figure 2C). Microscopic pathology showed fusiform tumor cells with different nuclear sizes, irregular shapes, and large atypia, suggesting that the soft tissue pleomorphic tumor was more likely to be sarcoma (Figures 3A, B). Immunohistochemical (IHC) results (Figures 3C–J) showed CK (−), SMA (focally +), Desmin (focally +), h-caldesmon (focally +), CD31 (vascular +), CD34 (vascular +), S-100 (−), and Ki-67 (+, 30%). CD31 (vascular +) and CD34 (vascular +) confirmed extensive vascular proliferation within and on the surface of LMS. Ki-67 (+, 30%) supported the diagnosis of high-grade angioleiomyosarcoma.
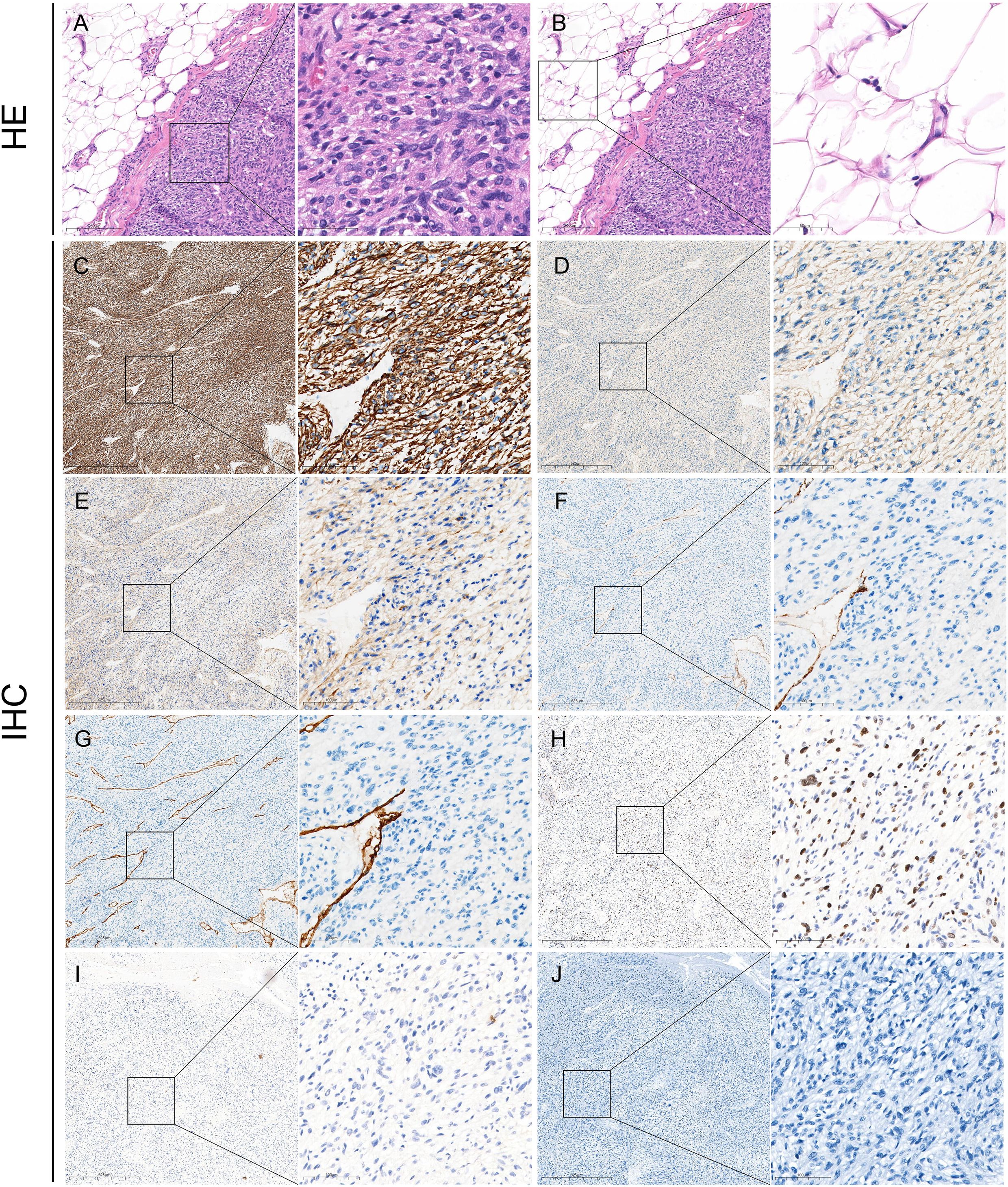
Figure 3. Pathological findings of GSV-LMS in a male patient. (A) Left panel: H&E-stained pathological section of this case (×100). Right panel: a locally magnified view (×400) of the area within the box in the left panel showing the venous leiomyosarcoma tissue. It showed fusiform tumor cells with different nuclear sizes, irregular shapes, and large atypia, suggesting that the soft tissue pleomorphic tumor was more likely to be sarcoma. (B) Left panel: H&E-stained pathological section of this case (×100), identical to the left panel in panel (A) Right panel: a locally magnified view (×400) of the area within the box in the left panel, showing the surrounding lesion tissue. (C) IHC staining for Desmin is partially positive. (D) IHC staining for SMA is partially positive. (E) IHC for h-caldesmon is partially positive. (F) IHC staining for CD31 shows positivity only in vascular structures. (G) IHC staining for CD34 shows positivity only in vascular structures. (H) IHC staining for Ki-67 is positive (30%). (I) IHC staining for CK is negative. (J) IHC staining for S-100 is negative. GSV, great saphenous vein; LMS, leiomyosarcoma; IHC, immunohistochemistry.
Follow-up
The patient has been followed up for approximately 13 months after surgery. Liver metastasis and lung metastasis were found 10 months after operation. The patient received subsequent systemic treatment, including chemotherapy and transarterial chemoembolization (TACE) interventional therapy for liver metastasis. Currently, the patient is alive.
Literature review
The PubMed, China National Knowledge Infrastructure, Wanfang Data, and Embase databases were searched for articles published from 1868 to 2024. A total of 56 cases in 53 reports were identified: 27 men (48.2%) and 29 women (51.8%). The details are shown in Table 1. The age ranged from 2 to 85 years (average 55.64 ± 16.43). Except for six patients whose reasons for visiting the hospital were not reported, 45 patients (90%) were treated for palpable masses, of which 30 masses were painless; two patients (4%) were treated for lower limb edema, and three patients (6%) were treated for superficial phlebitis. The thigh (28 cases, 54.9%) was the most common site of GSV-LMS, followed by the groin (10 cases, 19.6%), shank (nine cases, 17.6%), ankle (three cases, 5.9%), and knee (one case, 2.0%); and no specific site of disease was reported in five cases.
Except for one case with incomplete data, the remaining 55 patients underwent surgical treatment, 41 (74.5%) underwent complete resection, and 11 (20%) underwent wide resection of which three (5.5%) underwent intraoperative revascularization. Intraoperative measurements showed that the maximum diameter of the mass was 10–170 (48.69 ± 32.37) mm. A total of 43 cases (76.8%) were followed up for 1–216 (37.53 ± 52.847) months, only nine cases (20.9%) were followed up for more than 5 years, and seven cases’ follow-up (16.3%) was terminated due to death. Except for nine cases whose postoperative adjuvant therapy was not reported, 18 cases (38.3%) received postoperative adjuvant therapy, including 12 cases who received radiotherapy alone, four cases who received chemotherapy alone, and two cases who received radiotherapy combined with chemotherapy. In addition, except for 11 cases with unknown medical history, five cases (11.1%) had in situ recurrence, and 11 cases (24.4%) had distant metastasis. The most common site of metastasis was the lung (eight cases), followed by the liver (two cases), heart (one case), and bone (one case).
Discussion
Soft tissue sarcomas are rare, accounting for less than 1% of all malignancies, and LMS accounts for only 5%–10% of all soft tissue sarcomas (1, 4). LMS of vascular origin is extremely rare and accounts for less than 2% of all LMS cases. The number of venous origins is five times that of arterial origins (30), and less than 2% of patients had large and middle vein origins (18). The most common site of venous LMS is the inferior vena cava, followed by the renal vein and GSV (2). The GSV is the most commonly involved site in lower limb blood vessels (approximately 30%) (44). However, only 56 cases of GSV-LMS have been reported globally at present, and all of them are case reports and series. The etiology of this disease is unknown, with an insidious onset, difficult diagnosis, and a high susceptibility to misdiagnosis.
GSV-LMS is extremely rare, with only 56 cases reported in the literature (Table 1), and our patient is the 57th documented case and the youngest ever reported in China. Moreover, the average age of onset of GSV-LMS is 55.89 ± 17.67 years, and the prevalence is equal between men and women, which was consistent with those previously reported (7). The site of the disease is more common around the knee, while occurrence in the distal end of the knee is relatively rare (3). In this case, the site is the calf, which is an unusual position. The growth patterns of GSV-LMS include intracavitary growth, extracellular growth, and mixed growth (55). The clinical manifestations of GSV-LMS are atypical, and most patients are treated for palpable subcutaneous masses with possible symptoms such as pain and limb swelling when the lesion blocks the lumen or when secondary thrombosis occurs. It is difficult to distinguish the disease from venous thrombosis, superficial phlebitis, and other subcutaneous masses. However, the combination of ultrasonography or other auxiliary examinations can improve diagnostic accuracy (44).
On account of the advantages of its simple and non-invasive nature and low cost, ultrasound is the most frequently used auxiliary examination method for finding lower limb masses. With the development and wide application of diagnostic imaging technology, non-invasive ultrasound has been able to provide surgeons with more useful information before surgery. It can clearly show the extent of involvement and the internal blood supply of the lesions, which are helpful for differential diagnosis. The typical sonographic feature of GSV-LMS is a heterogeneous hypoechoic mass in the GSV with a regular or irregular shape that cannot be flattened after compression by a probe. Sometimes, it is difficult to distinguish and easy to be misdiagnosed. When venous thrombosis with an unknown cause or poor therapeutic effect is encountered in clinical work, it is necessary to be vigilant by expanding the scope of the ultrasound scan and carefully observing the relationship between the hypoechoic mass in the venous channel and the venous wall and the internal blood supply of the hypoechoic mass. Ultrasound-guided puncture biopsy can result in a definite diagnosis preoperatively, but due to the unclear origin of the lesion and the possibility of thrombosis, the results may be false negative (47). Contrast-enhanced ultrasound (CEUS) can clearly show the blood supply in a hypoechoic mass, and needle biopsy combined with CEUS can improve the accuracy of diagnosis. Regrettably, due to the patient’s strong willingness to undergo surgery, CEUS, puncture biopsy, and other preoperative examinations such as computed tomography (CT) or magnetic resonance imaging (MRI) were not completed. Therefore, we failed to obtain other images of the lesion.
GSV-LMS needs to be differentiated from other venous diseases. When a venous aneurysm is complicated by thrombosis (56), two-dimensional ultrasound also reveals focal aneurysmal dilation of the GSV with the lumen filled with a hypoechoic material. However, CDFI showed no blood flow signal within the hypoechoic area of the venous aneurysm, whereas abundant blood flow signals can be detected within the aneurysmal lumen of GSV-LMS. Other venous tumors (57–59), such as venous leiomyoma, may exhibit intraluminal growth leading to luminal occlusion or be complicated by venous thrombosis. Compared to GSV-LMS, venous tumors generally demonstrate a slower growth rate. Conventional ultrasound examination poses challenges in differential diagnosis due to overlapping features. Ultrasound-guided needle biopsy can be employed to determine the pathological characteristics, thereby aiding in the formulation of an appropriate treatment plan.
At present, radical resection is the most effective treatment for GSV-LMS. The scope of surgical resection is usually extended to the proximal and distal segments of the affected venous margin of approximately 2–3 cm so as to reduce local recurrence and minimize the negative margin. When there is obvious adhesion between the lesion and the surrounding tissues, the scope of resection can be further expanded according to the actual situation, and vascular reconstruction or autologous vein transplantation can be performed when necessary (1). Only three of the reported cases underwent intraoperative vascular reconstruction. Preoperative ultrasound evaluation can help to identify the nature of the lesion, evaluate the scope of lesion involvement, and guide the formulation of a surgical plan, which can reduce partly the recurrence rate of patients.
Currently, the indications and efficacy of adjuvant chemoradiotherapy for venous LMS are unclear. Postoperative adjuvant chemoradiation is often performed for high-grade tumors clinically, which can effectively prevent local recurrence and metastasis after surgery. A total of 17 cases in this study received adjuvant treatment after surgery, and none of these cases had local recurrence or metastasis during the reported postoperative follow-up period. Ultrasound is of great value in evaluating whether patients have recurrence or metastasis and can effectively assist in judging patients’ condition to aid in guiding chemoradiotherapy. Patients with GSV-LMS have a high probability of tumor recurrence within 2 to 3 years after surgery, so patients should have regular postoperative follow-ups. Evaluations such as ultrasound every 3 months and other imaging examinations such as CT every 6 months are usually recommended (48).
GSV-LMS is an extremely rare malignant tumor originating from veins, which is difficult to diagnose and prone to misdiagnosis and mistreatment. Preoperative ultrasound examination has a certain evaluation value. It can assist in guiding clinical diagnosis and treatment and in improving the diagnosis and treatment effect. GSV-LMS patients may have recurrence and metastasis, and ultrasound can be used as the most convenient follow-up tool after surgery to detect new lesions in time, which is of great value in improving the long-term survival rate of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. BD: Conceptualization, Methodology, Writing – original draft. CH: Investigation, Methodology, Writing – review & editing. LS: Investigation, Methodology, Writing – review & editing. GH: Data curation, Writing – review & editing. QH: Data curation, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cangiano R, Orlandino G, Patitucci G, Lembo F, and Fabrizio T. Primary leiomyosarcoma of the great saphenous vein: case report and review of the literature. Ann Vasc Surg. (2017) 38:315.e1–e7. doi: 10.1016/j.avsg.2016.04.021
2. Gage MJ, Patel AV, Koenig KL, and Newman E. Non-vena cava venous leiomyosarcomas: a review of the literature. Ann Surg Oncol. (2012) 19:3368–74. doi: 10.1245/s10434-012-2379-2
3. Zhang X, Wang YH, Chen YX, Liu B, and Zheng YH. Leiomyosarcoma of the great saphenous vein: report of one case and meta-analysis. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae. (2019) 41:435–42. doi: 10.3881/j.issn.1000-503X.10738
6. Deweese JA, Terry R, and Schwartz SI. Leiomyoma of the greater saphenous vein with preoperative localization by phlebography. Ann Surg. (1958) 148:859–61. doi: 10.1097/00000658-195811000-00027
8. Dorfman HD and Fishel ER. Report of a case and review of the literature. Am J Clin Pathol. (1963) 39:73–8. doi: 10.1093/ajcp/39.1.73
9. Christiansen J. Malignant tumors originating from the venous system. Review and a case of leiomyosarcoma from the long saphenous vein. Ugeskr Laeger. (1964) 126:483–5.
10. Allison MF. Leiomyosarcoma of the femoral vein. Report of a case in a child. Clin Pediatr (Phila). (1965) 4:28–31. doi: 10.1177/000992286500400110
11. Szasz IJ, Barr R, and Scobie TK. Leiomyosarcoma arising from veins: two cases and a review of the literature on venous neoplasms. Can J Surg. (1969) 12:415–9.
12. Hughes S. A case of leiomyosarcoma of the long saphenous vein. Vasc Surg. (1973) 7:71–3. doi: 10.1177/153857447300700202
13. Jernstrom P and Gowdy RA. Leiomyosarcoma of the long saphenous vein. Am J Clin Pathol. (1975) 63:25–31. doi: 10.1093/ajcp/63.3.25
14. Gross E and Horton MA. Leiomyosarcoma of the saphenous vein. J Pathol. (1975) 116:37–41. doi: 10.1002/path.1711160106
15. Stringer BD. Leiomyosarcoma of artery and vein. Am J Surg. (1977) 134:90–4. doi: 10.1016/0002-9610(77)90289-6
16. Orsi G, Camera S, and Zanlungo U. Primary peripheral venous tumors. Minerva Cardioangiol. (1979) 27:241–4.
17. Fischer MG, Gelb AM, Nussbaum M, Haveson S, and Ghali V. Primary smooth muscle tumors of venous origin. Ann Surg. (1982) 196:720. doi: 10.1097/00000658-198212001-00019
18. Berlin Ö, Stener B, Kindblom LG, and Angervall L. Leiomyosarcomas of venous origin in the extremities. A correlated clinical, roentgenologic, and morphologic study with diagnostic and surgical implications. Cancer. (1984) 54(10):2147e2159. doi: 10.1002/1097-0142(19841115)54:10<2147::aid-cncr2820541015>3.0.co;2-9
19. Leu HJ and Makek M. Intramural venous leiomyosarcomas. Cancer. (1986) 57:1395e1400. doi: 10.1002/1097-0142(19860401)57:7<1395::AID-CNCR2820570726>3.0.CO;2-O
20. Humphrey M, Neff J, Lin F, and Krishnan L. Leiomyosarcoma of the saphenous vein. A case report and review of the literature. J Bone Joint Surg Am. (1987) 69:282–6. doi: 10.2106/00004623-198769020-00019
21. Song KY, Jang YW, Kim MK, Lee GY, and Sung RH. Leiomyosarcoma arising in the great saphenous vein-a case report. J Korean Med Sci. (1991) 6:372–5. doi: 10.3346/jkms.1991.6.4.372
22. Welk E, Bindewald H, and Berndt R. Vascular leiomyosarcomaa surgical rarity. Chirurg. (1991) 62:223–5.
23. Dzsinich C, Gloviczki P, Heerden van JA, Nagorney DM, Pairolero PC, and Johnson CM. Primary venous leiomyosarcoma: a rare but lethal disease. J Vasc Surg. (1992) 15:595e603. doi: 10.1016/0741-5214(92)90003-Q
24. Saglik Y, Icli F, Uluoglu O, and Isiklar ZU. Leiomyosarcoma of the great saphenous vein. Int Orthop. (1992) 16:185e187. doi: 10.1007/BF00180214
25. Stallard D, Sundaram M, Johnson FE, and Janney C. Case report 747: leiomyosarcoma of great saphenous vein. Skeletal Radiol. (1992) 21(6):399e401. doi: 10.1007/BF00241821
26. Stambuk J, Oddo D, Perez R, and Bravo M. Leiomyosarcoma of the saphenous vein. Rev Med Chile. (1993) 121:673e676.40.
27. Byard RW, Bourne AJ, Phillips GE, Rice MS, and Davey RB. Leiomyosarcoma of the saphenous vein in a child with 12-year follow-up. J Pediatr Surg. (1993) 28:271–4. doi: 10.1016/s0022-3468(05)80294-5
28. Reix T, Sevestre H, Sevestri-Pietri MA, Szychta P, and Pietri J. Primary Malignant tumors of the venous system in the lower extremities. Ann Vasc Surg. (1998) 12:589–96. doi: 10.1007/s100169900205
29. Chang SP, Tsai TF, Chen YF, Chen HH, and Hung CM. Intravenous leiomyosarcoma. Chin J Dermatol. (2001) 19:52–7.
30. Le Minh T, Cazaban D, Michaud J, Ginestet-Auge MC, Laporte MH, Patelli A, et al. Great saphenous vein leiomyosarcoma: a rare Malignant tumor of the extremity-two case reports. Ann Vasc Surg. (2004) 18:234–6. doi: 10.1007/s10016-003-0090-2
31. Marle AG, Bronkhorst MW, and Brouwers MA. Leiomyosarcoma of the great saphenous vein: a case report and review of the literature. Sarcoma. (2004) 8:135–9. doi: 10.1155/S1357714X04000234
32. Yao QD, Yu HY, Wang M, and Qu MT. A clinicopathological observation of leiomyosarcoma in the large vein of the left lower limb: a case report. Prac J Med & Pharm. (2004) 21:1070–2. doi: 10.14172/j.cnki.issn1671-4008.2004.12.007
33. Zhang XS, Zou YH, Zhao CQ, Lv YX, Wang C, and Wang WL. Primary leiomyosarcoma of the great saphenous vein: a case report. Chin J Gen Surg. (2005) 05:323–4. doi: 10.3760/j.issn:1007-631X.2005.05.026
34. El Khoury M, Mesurolle B, Trassard M, Cherel P, Talma V, and Hagay C. Leiomyosarcoma of the great saphenous vein. Australas Radiol. (2006) 50:500e503. doi: 10.1111/j.1440-1673.2006.01636.x
35. Zhang XS and Wang ZG. Primary leiomyosarcoma of the great saphenous vein: case report. Eur J Vasc Endovasc Surg. (2006) 32:222–5. doi: 10.1016/j.ejvs.2005.12.018
36. Mammano E, Zanon A, Picchi G, Rossi CR, Rossi G, Cosci M, et al. Primary great saphenous vein leiomyosarcoma: report of a case. Surg Today. (2008) 38:161e162. doi: 10.1007/s00595-007-3588-3
37. Yanada M, Shimada J, Kohnosu H, Sawabe Y, Tomita H, and Tokui M. A case of primary great saphenous vein leiomyosarcoma showing high uptake on fdg-pet. Nihon Rinsho Geka Gakkai Zasshi (Journal Japan Surg Association). (2010) 71:1663–6. doi: 10.3919/jjsa.71.1663
38. Bibbo C and Schroeder M. Review of vascular leiomyosarcoma and report of a case localized to the greater saphenous vein of the ankle. J foot ankle Surg. (2011) 50:329e335. doi: 10.1053/j.jfas.2011.01.002
39. Røpcke DM, Lorenzen JA, and Jørgensen PH. Leiomyosarkom udgående fra vena saphena magna [Leiomyosarcoma of the great saphenous vein. Ugeskr Laeger. (2012) 174:1384–5.
40. Cüneyt ERIS, Gucu A, Toktas F, Yumun G, and Ozyazicioglu A. Primer büyük safen ven leiomyosarkomu: olgu sunumu [Primary great saphenous vein leiomyosarcoma: case report. Damar Cerrahi Dergisi. (2012) 21:294–6. doi: 10.9739/uvcd.2012-31702
41. Amato B, Compagna R, Gasbarro V, Serra R, and De Franciscis S. Great saphenous vein and leiomyosarcoma. EJVES Extra. (2013) 26:e15ee16. doi: 10.1016/j.ejvsextra.2013.03.001
42. Xu C, Liu C, Yang Z, and Cui GH. Ultrasonographic manifestations of leiomyosarcoma in the great saphenous vein: a case report. Chin J Ultrasonography. (2013) 22:382. doi: 10.3760/cma.j.issn.1004-4477.2013.05.006
43. Werbrouck C, Marrannes J, Gellens P, Van Holsbeeck B, and Laridon E. Leiomyosarcoma of the great saphenous vein. J Belg Radiol. (2013) 96:183. doi: 10.5334/jbr-btr.265
44. Fremed DI, Faries PL, Schanzer HR, Marin ML, and Windsor T. Primary leiomyosarcoma of saphenous vein presenting as deep venous thrombosis. Vascular. (2014) 22:450–3. doi: 10.1177/1708538113516446
45. Amato ACM, Silva AJdeD, Santos RV, Amato, and de Toledo Arruda SJ. Leiomyosarcoma of the great saphenous vein. Jornal Vasc Brasileiro. (2015) 14:186–8. doi: 10.1590/1677-5449.0077
46. Lin WW, Erkan S, Smith RC, and Kishore S. Leiomyosarcoma of great saphenous vein localised to the calf. BMJ Case Rep. (2016) 2016:bcr2016215829. doi: 10.1136/bcr-2016-215829
47. Macarenco RS, Filippi RZ, D’Almeida Costa F, and Jesus-Garcia R. Leiomyosarcoma of the great saphenous vein (vena saphena magna) with granular cell change: Report of a superficial neoplasm. J Cutan Pathol. (2018) 45:141–5. doi: 10.1111/cup.13062
48. Naouli H, Lathelize H, and Bouarhroum A. Leiomyosarcoma of the great saphenous vein: case report and literature review. Ann Vasc Surg. (2018) 56:353.e1–353.e6. doi: 10.1016/j.avsg.2018.08.111
49. Guner A, Güner EG, Sancar KM, Onan B, and Yldz M. Cardiac metastasis of great saphenous vein leiomyosarcoma. J Card Surg. (2020) 35:2029e2032. doi: 10.1111/jocs.14744
50. Tresgallo-Parés R, De Virgilio-Salgado L, Torres-Lugo NJ, AsenjoMolina NA, Ramirez N, and Bibiloni-Rodríguez J. Primary leiomyosarcoma of the great saphenous vein: a case report. Int J Surg Case Rep. (2021) 88:106565. doi: 10.1016/j.ijscr.2021.106565
51. Alkhaled AA, Alkazmi SS, Altheyab HI, Cheikhzen EA, and Abu Shakra RI. Great saphenous vein leiomyosarcoma: a case report. Ann Med Surg. (2022) 74:103307. doi: 10.1016/j.amsu.2022.103307
52. Fu X and Lu G. Leiomyosarcoma of the great saphenous vein with granulosa cell morphology. Chin J Diagn Pathol. (2022) 006:570–1. doi: 10.3969/j.issn.1007-8096.2022.06.026
53. Dziekiewicz M, Obara A, and Makowski K. Multifocal sarcoma mimicking superficial vein thrombosis of the Leg: a case report. Am J Case Rep. (2022) 23:e937317. doi: 10.12659/AJCR.937317
54. Atieh A, Allaw H, Ashouri M, and Zafarghandi M. Great saphenous vein leiomyosarcoma mimicking thrombosed aneurysm: A case report and review of the literature. J Vasc Surg cases Innov Tech. (2023) 10:101399. doi: 10.1016/j.jvscit.2023.101399
55. Bednarova I, Frellesen C, Roman A, and Vogl TJ. Leiomyosarcoma of the inferior vena cava. Radiology. (2018) 288:901–8. doi: 10.1148/radiol.2018160821
56. Irsara S, Sommariva A, Visonà A, and Ferretto L. The dark side of deep vein thrombosis: intraluminal femoral vein leiomyosarcoma. Acta Phlebologica. (2022) 23:31–6. doi: 10.23736/S1593-232X.21.00506-3
57. Matei SC, Matei M, Anghel FM, Olariu A, and Olariu S. Great saphenous vein giant aneurysm. Acta Phlebol. (2022) 23:87–92. doi: 10.23736/S1593-232X.22.00517-3
58. Erfurt-Berge C. Malignant tumours presenting as chronic leg or foot ulcers. J Clin Med. (2021) 10:2251. doi: 10.3390/jcm10112251
Keywords: leiomyosarcoma, great saphenous vein, ultrasound, hypoecho, case report
Citation: Liu Y, Dong B, Hou C, Sun L, He G and Hu Q (2025) Great saphenous vein leiomyosarcoma: a rare case report and literature review. Front. Oncol. 15:1589001. doi: 10.3389/fonc.2025.1589001
Received: 06 March 2025; Accepted: 06 June 2025;
Published: 02 July 2025; Corrected: 07 July 2025.
Edited by:
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, GermanyReviewed by:
Fuchun Yang, University of Cincinnati, United StatesMladen Anđić, University of Belgrade, Serbia
Sergiu-Ciprian Matei, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2025 Liu, Dong, Hou, Sun, He and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaohong Hu, Y2Fycm90aHFoQDE2My5jb20=
†These authors share first authorship
 Ying Liu
Ying Liu Bin Dong2†
Bin Dong2† Litao Sun
Litao Sun