- 1State Key Laboratory of Translational Oncology, Department of Clinical Oncology, Prince of Wales Hospital, Hong Kong Cancer Institute, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2State Key Laboratory of Translational Oncology, Department of Chemical Pathology, Prince of Wales Hospital, Li Ka Shing Institute of Health Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Surgery, Prince of Wales Hospital, Shatin, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4Department of Diagnostic and Interventional Radiology, Prince of Wales Hospital, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Background: An increasing number of immuno-therapeutic agents have proven efficacy in hepatocellular carcinoma (HCC). Inflammatory markers (c-reactive protein, interleukin-8 and inflammatory score) have been found to be prognostic factors in HCC patients. These inflammatory markers have demonstrated correlations with quality of life (QOL) disturbances in fatigue, appetite loss and nutritional concern. Type I interferon response triggered by HCC could be responsible for an inflammatory state and anorexia-cachexia syndrome leading to these specific QOL impairment. Peripheral blood Interferon Stimulated Gene 15 (ISG15) messenger ribonucleic acid (mRNA) transcript level, a biomarker for type I interferon response, was evaluated for its prognostic significance for overall survival (OS) in a prospective cohort of HCC patients. QOL measurement was employed to systemically capture and quantify patients’ clinical manifestations for correlations with ISG15 mRNA transcript level.
Methods: Clinical, QOL and laboratory data of 340 treatment naïve HCC patients were collected at study entry. ISG15 mRNA transcript levels in circulating leucocytes were quantified. Independent prognostic factors for OS were identified. Correlation analyses between ISG15 mRNA transcript level and scores of QOL factors were performed.
Results: High ISG15 mRNA transcript level in circulating leucocytes was an independent prognostic factor for poor OS (hazard ratio 1.62 [1.23-2.15]; p-value<0.01). The median OS of patients with high ISG15 gene expression was significantly shorter than those with low expression, 4.7 versus 14.3 months respectively (p-value<0.03). There were significant correlations between high ISG15 mRNA transcript level and worse scores in QLQ-C30 fatigue, appetite loss and QLQ-HCC18 nutritional disturbances (p-values <0.05).
Conclusions: Elevated ISG15 mRNA transcript level in peripheral blood leucocytes was an independent poor prognostic factor for OS in HCC patients. Patients with higher ISG15 gene expression, suggesting more intense type I interferon response, had significantly worse OS. High ISG15 gene expression demonstrated significant correlations with QOL disturbances in fatigue, appetite loss and nutritional concern. These QOL factors could be capturing the anorexia-cachexia manifestations from interferon response induced by HCC.
1 Introduction
Hepatocellular carcinoma (HCC) is the 3rd leading cause of cancer death worldwide (1). Over one-half of HCC occur in China (2). More than 70% of HCC patients present with advanced disease and could only receive palliative or supportive therapy (3, 4). Prognostication therefore plays an important role, whereas two groups of prognostic factors have been gaining recognition: inflammatory markers and quality of life (QOL).
Immune-response and inflammation are closely related to HCC pathogenesis and progression (5). Inflammatory markers such as interleukin-8 (IL-8), inflammatory score (6) and c-reactive protein (CRP) (7) have been reported to be prognostic for overall survival (OS) in HCC patients. QOL assessment in HCC patients using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-HCC18 (8) also carried prognostic value.
Traditionally QOL disturbances in cancer patients were thought to be mostly related to mass effect from tumors. However, for uncertain reasons, QOL factors measuring appetite loss, nutritional disturbances, fatigue and functional impairment have been demonstrated to correlate with inflammatory markers (6, 9) in HCC patients. These QOL disturbances might involve a complex interplay between HCC and inflammatory cascade. IL-8, among these inflammatory markers, is a chemokine induced by type I interferon response (10). Interferon-α administration to mice, triggering type I interferon response, can lead to reduction in oral intake and wasting (11). In human studies, therapeutic use of interferon-α frequently caused adverse effects including anorexia, nausea, fatigue and asthenia in cancer as well as non-cancer patients (12–14). In other words, the above mentioned specific QOL factors appeared to be measuring the symptoms from type I interferon response. Therefore we postulated that type I interferon response could be activated in HCC patients, have prognostic significance and have association with QOL disturbances.
Plasma level of interferon could reflect interferon response, however, its measurement has been technically challenging with limited accuracy due to its low concentration with short half-life (15). On the other hand, Interferon Stimulated Gene 15 (ISG15) expression, reflecting type I interferon response activation, could be measured with higher accuracy (10, 16–20). Both endogenous type I interferon response or exogenous administration of type I interferon can lead to ISG15 expression and enhanced IL8 production (17, 18). ISG15 encodes for interferon-stimulated protein of 15 kilodalton, which is responsible for ISGylation of various targets to regulate immunologic functions (21). Specifically, ISG15 expression was upregulated in viral infections including Coronarvirus Disease-2019 (COVID-19) (22–24), autoimmune diseases (25–27) and in some types of cancer (28–33). ISG15 has demonstrated tumor-suppressing and tumor-promoting effects in different tumor types (30). For instance, high ISG15 expression has been associated with progression of endometrial cancer (31) and earlier relapse in patients with resected HCC (32). On the contrary, ISG15 expression has been implicated to suppress adenocarcinoma of lung (33) and cervical squamous cell carcinoma (34). While reported studies focused on intra-tumoral ISG15 expression, there has been evidence to support that disease-associated interferon response gene expressions also manifested in circulating leucocytes (35). Hence we utilized ISG15 mRNA level in circulating leucocytes to gauge the degree of type I interferon response (10, 16, 35).
In this study, we investigated the prognostic significance of ISG15 mRNA transcript levels in peripheral blood leucocytes, clinical and QOL factors in HCC patients. QOL measurement was employed to systemically capture and quantify patients’ symptoms for statistical analyses and clinical correlation. The associations between ISG15 mRNA transcript levels and QOL factors were evaluated.
2 Materials and methods
2.1 Patients
This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Prince of Wales Hospital (PWH), Hong Kong. Patients presented to PWH with newly diagnosed HCC were recruited prospectively. HCC diagnosis was established by either (i) the finding of hyper-vascular hepatic tumor(s) in dynamic imaging with elevated alpha-fetoprotein (AFP) or (ii) tumor biopsy. Patients were excluded from the study if they received prior HCC treatment, had a history of another malignancy, evidence of hepatic encephalopathy, cognitive impairment or illiteracy. All patients provided written informed consent for the study.
2.2 Data collection
Demographic, clinical, QOL data and peripheral blood were collected at study entry. After consenting, all patients had venesection for blood collection for once before HCC treatment. Blood was tested for ISG15 mRNA transcript level, cell counts, renal and liver functions, clotting profile, AFP, hepatitis B surface-antigen and anti-hepatitis C antibody. Patients were followed-up until death.
2.3 Laboratory procedures for quantification of peripheral blood ISG15 mRNA transcript level
2.3.1 Peripheral blood leukocytes isolation and total RNA extraction
For ISG15 gene expression analysis, peripheral blood in ethylenediaminetetraacetic acid (EDTA) was used. Density gradient centrifugation, by Lymphoprep™ (Serumwerk Bernburg AG, Germany), was used to isolate peripheral blood mononuclear cells (PBMC) and granulocytes. Extraction of total RNA from whole blood and leukocytes was performed using a modified acid guanidinium thiocyanate-phenol-chloroform (AGPC) protocol (36), according to the manufacturer’s instructions (Molecular Research Center, Inc., Ohio, USA). This process effectively isolated nucleated cells, the resulting total RNA yield would overwhelmingly be derived from leukocytes, with negligible contribution from anucleated erythrocytes and platelets. 250 µL TRI Reagent® LS (TB126) was added to the PBMC and granulocytes and 300 µL TRI Reagent® BD (TB120) was added to the whole blood. Phase separation was performed using 1-bromo-3-chloropropane (B9673, Sigma-Aldrich). Subsequent washing and elution were by isopropanol (I9516, Sigma-Aldrich) and ethanol (4116-4104, Daejung).
2.3.2 Reverse transcription
Synthesis of first strand complementary deoxyribonucleic acid (cDNA) from the total RNA was performed using the PrimeScript RT Reagent Kit (#RR037A, Takara Bio, Shiga, Japan). 10 µL reverse transcription system was used, including 2 µL of 5X PrimeScript Buffer (for Real-Time), 0.5 µL PrimeScript RT Enzyme Mix I, 0.5 µL Oligo dT Primer (50 µM), 2 µL Random-6mers (100 µM), and 5 µL of RNA (Up to 1 µg/µL) from the samples. Reverse transcription was performed in the C1000 Touch Thermal Cycler (Bio-Rad, Hercules, California, USA), under these conditions: incubation at 37°C for 15 minutes, then enzyme denaturation at 85°C for 5 seconds, followed by storage at 4°C for short-term preservation before refrigeration storage.
2.3.3 Quantification of ISG15 gene expression
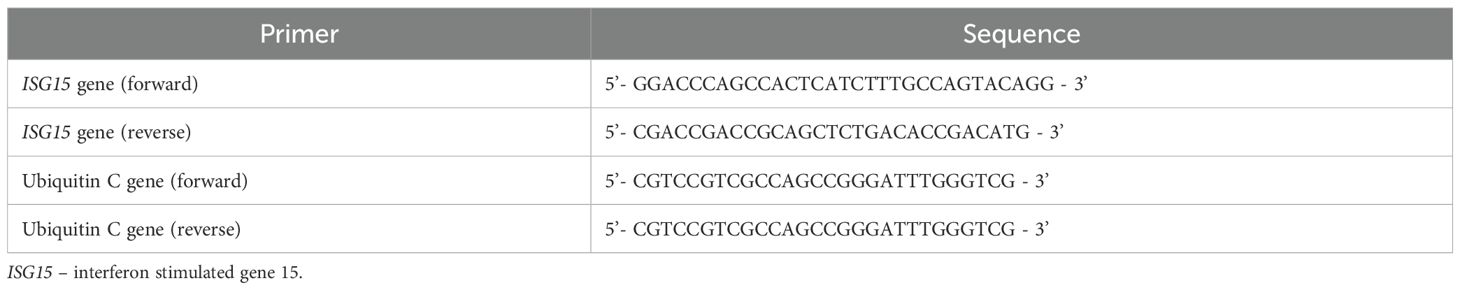
The cDNA was diluted and duplicated for optimization of the real time quantitative polymerase chain reaction (RT-qPCR) performance. In each RT-qPCR batch, the same reference sample was used and a serial 2-fold dilution was performed to establish PCR efficiency. The TB Green® Premix Ex Taq™ II (Tli RNase H Plus) kit (#RR820A, Takara Bio, Shiga, Japan) and ISG15 gene primers (see Table 1) were used in the reaction, carried out in LC480 thermal cycler (Roche, Basel, Switzerland). The qPCR conditions were: pre-incubation at 95°C for 30 seconds, then 45 cycles of amplification (95°C for 5 seconds, 55°C for 30 seconds, and 72°C for 20 seconds), melting (95°C for 1 minute, 40°C for 1 minute and 65°C for 20 seconds) and final cooling at 40°C for 30 seconds. Quantification of the expression level of the patients’ ISG15 gene was by the efficiency (eff) corrected delta delta cycle threshold (ddCT) method. Gene UBC was used as the internal housekeeping gene. A constant cDNA sample from one healthy donor was used as the calibrator for all the tests. The formula for fold change (FC) calculation is shown in Table 2. All qPCR were performed in duplicate and the duplicate cycle threshold (Ct) value difference must be less than 1 for quality control (36–38).
2.4 QOL measurement
The EORTC QLQ-C30 (39) and QLQ-HCC18 (40) questionnaires were completed by patients at study entrance. C30 and HCC18 index scores, summary scorings of QLQ-C30 and QLQ-HCC18 respectively, were calculated (see Supplementary Table 1) (8).
2.5 Estimation of sample size and statistical analyses
The median OS of local HCC patients was previously reported to be 6 months (41). We assumed a hazard ratio (HR) of 2.0 for high ISG15 gene expression, with two-sided alpha level at 0.05 and power at 80%, the estimated target sample size was 298 patients. Complete-case analysis was adopted since index scores calculation required all scores from QLQ-C30 and QLQ-HCC18 to be available and there has been no prior knowledge to allow logical imputation for missing data in a novel variable of peripheral blood ISG15 mRNA transcript level. Allowing for 10% of patients with missing data, we aimed to recruit 331 patients. Clinical and QOL data were assessed by standard descriptive tests. OS was defined as the duration between study-entry and death. Time-to-death analysis was performed using the Kaplan-Meier method, censoring patients alive or lost to follow-up. Survival distributions were compared using the log-rank test. Prognostic clinical and QOL factors for OS were identified using Cox regressions. Significant non-overlapping clinical factors and QOL factors were analyzed in multivariate Cox regression models to adjust for confounding variables in order to identify independent prognostic factors. Two-tailed p-values of less than 0.05 were considered statistically significant. Receiver operating characteristic (ROC) curve of ISG15 mRNA transcript level thresholds using leucocytosis as a surrogate outcome was generated to determine an optimal cut-off for high ISG15 gene expression for correlation tests. Correlations between ISG15 mRNA transcript level and QOL factors were analyzed using Student’s t-test, Mann-Whitney U test (since the novel variable of ISG15 mRNA level had unknown distribution, both parametric and non-parametric tests were performed as sensitivity analyses), univariate and multivariate logistic regressions. P-values of less than 0.05 were considered significant. Analyses were performed using SAS version 9.4 (SAS institute, Cary, NC, USA).
3 Results
3.1 Patient characteristics
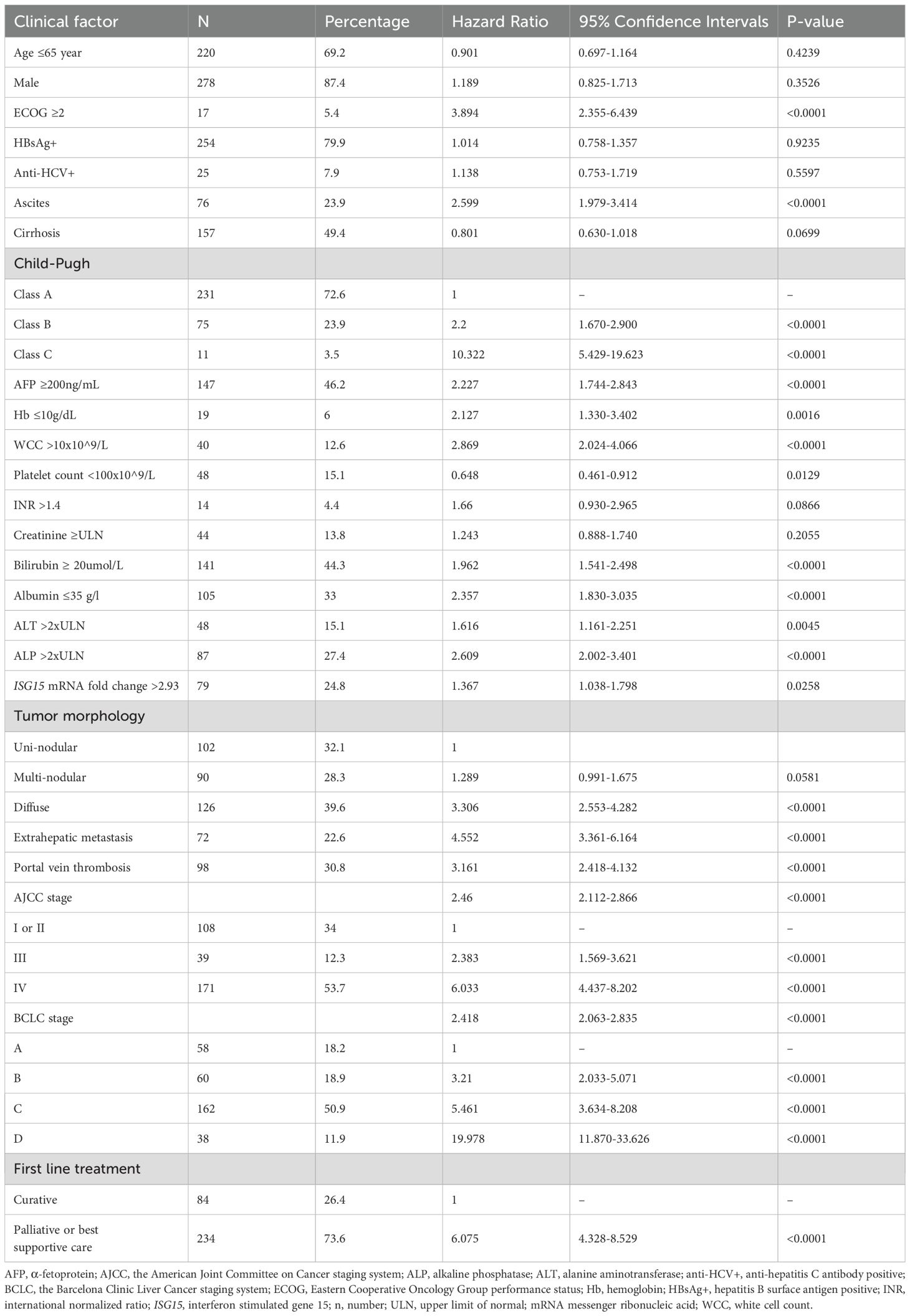
From 2009 to 2017, 340 HCC patients were recruited (318 patients with complete data for analyses, 20 with incomplete QOL data and 2 with unsuccessful ISG15 mRNA quantification). Patients’ baseline characteristics, clinical and laboratory data are presented in Table 3. The median age at presentation was 59 (range 27 to 86) years. Most patients (87%) were male. Concerning the pattern of liver tumors: 32% of patients had uni-nodular disease, 28% had multi-nodular disease and 40% had diffuse-infiltrative disease. Portal-vein thrombosis was found in 98 (31%) patients, while metastasis beyond liver in 72 (23%) patients. Evidence of hepatitis B was found in 80% of patients. Two-hundred and thirty-one (73%) patients were in Child-Pugh class A, 75 (24%) in class B and 11 (4%) in class C. The median follow-up duration was 116 (95% confidence interval [CI] 87-120) months. The median OS was 9.5 (95% CI 6.1-14.3) months.
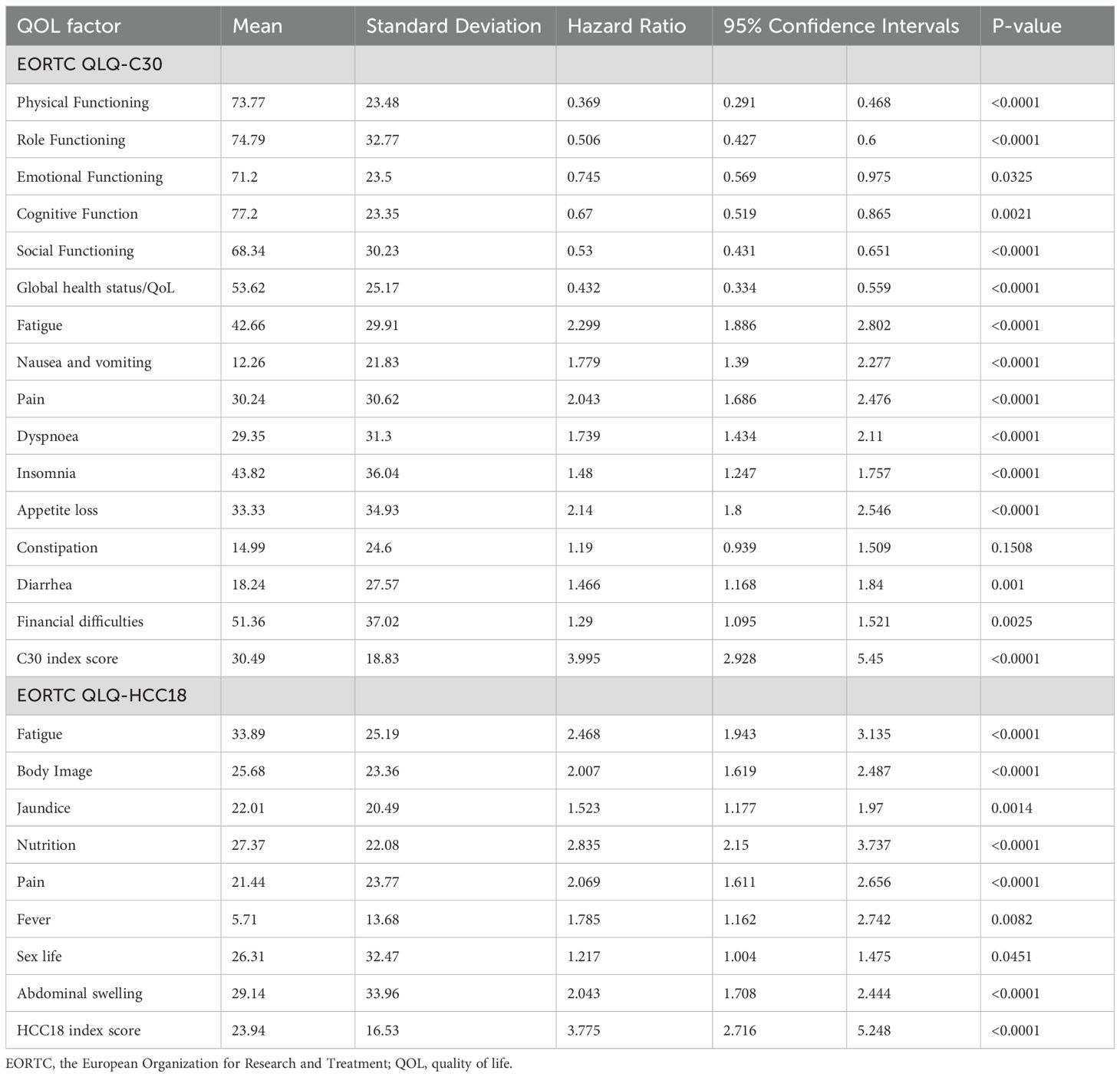
Table 4 presents the QOL data of the patients. The mean QLQ-C30 physical functioning score was 74 (standard deviation [SD] 23), fatigue score was 43 (SD 30), appetite loss score was 33 (SD 35), QLQ-HCC18 jaundice score was 22 (SD 20), nutritional concern score was 27 (SD 22) and pain score was 21 (SD 24).
3.2 ISG15 mRNA analyses
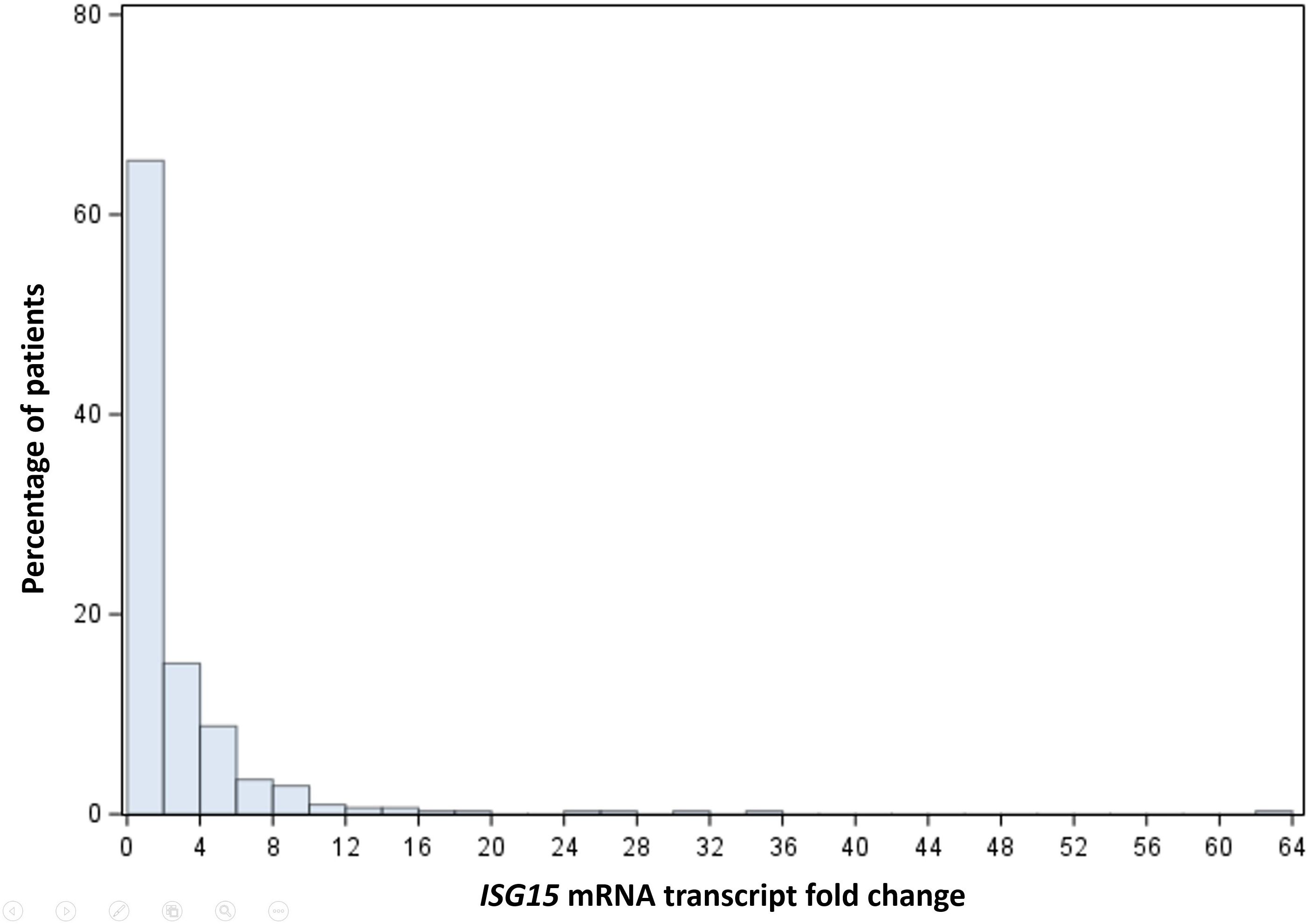
The median peripheral blood ISG15 mRNA transcript level was 1.24 (interquartile range 0.57-2.93) FC. The distribution of ISG15 mRNA transcript FC in HCC patients is shown in Figure 1. ISG15 mRNA level exhibited a skewed distribution, therefore levels of more than 75th percentile (2.93 FC) were grouped as high expression in Cox regressions for survival analyses. The median OS was significantly worse in patients with high ISG15 gene expression (signifying a higher degree of type I interferon response) when compared to those with low expression, 4.7 (95% CI 3.3-8.6) versus 14.3 (95% CI 8.9-20.4) months respectively (log-rank p-value <0.03). Figure 2 presents the survival curves of patients according to ISG15 gene expression levels.

Figure 2. Overall survival plots of patients with low versus high peripheral blood ISG15 gene expression.
3.3 Univariate Cox analyses of ISG15 mRNA level, clinical and QOL prognostic factors
Univariate Cox regressions of clinical and QOL factors are presented in Tables 3, 4 respectively. Patients with high ISG15 mRNA level had significantly higher risk of death than those with low level (p<0.03). Compared to patients in Child-Pugh class A, patients in Child-Pugh’s B or C classes had significantly worse OS (p-values <0.01). Compared to patients with American Joint Committee on Cancer stage I and II diseases, patients with stage III or IV diseases had significantly worse OS (p-values <0.01). Raised white cell count (WCC), lower hemoglobin level and extrahepatic metastasis were also significantly associated with worse OS (p-values <0.01).
Better (higher) scores in QLQ-C30 physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning and global health status/QoL were significantly associated with better OS (p-values <0.05). Worse (higher) scores in QLQ-C30 fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, diarrhea, financial difficulties, QLQ-HCC18 fatigue, body image, jaundice, nutrition, pain, fever, sex life and abdominal swelling were significantly associated with worse OS (p-values <0.05). Worse (higher) C30 and HCC18 index scores were also significantly associated with worse OS (p-values <0.01).
3.4 Multivariate Cox analyses of prognostic factors
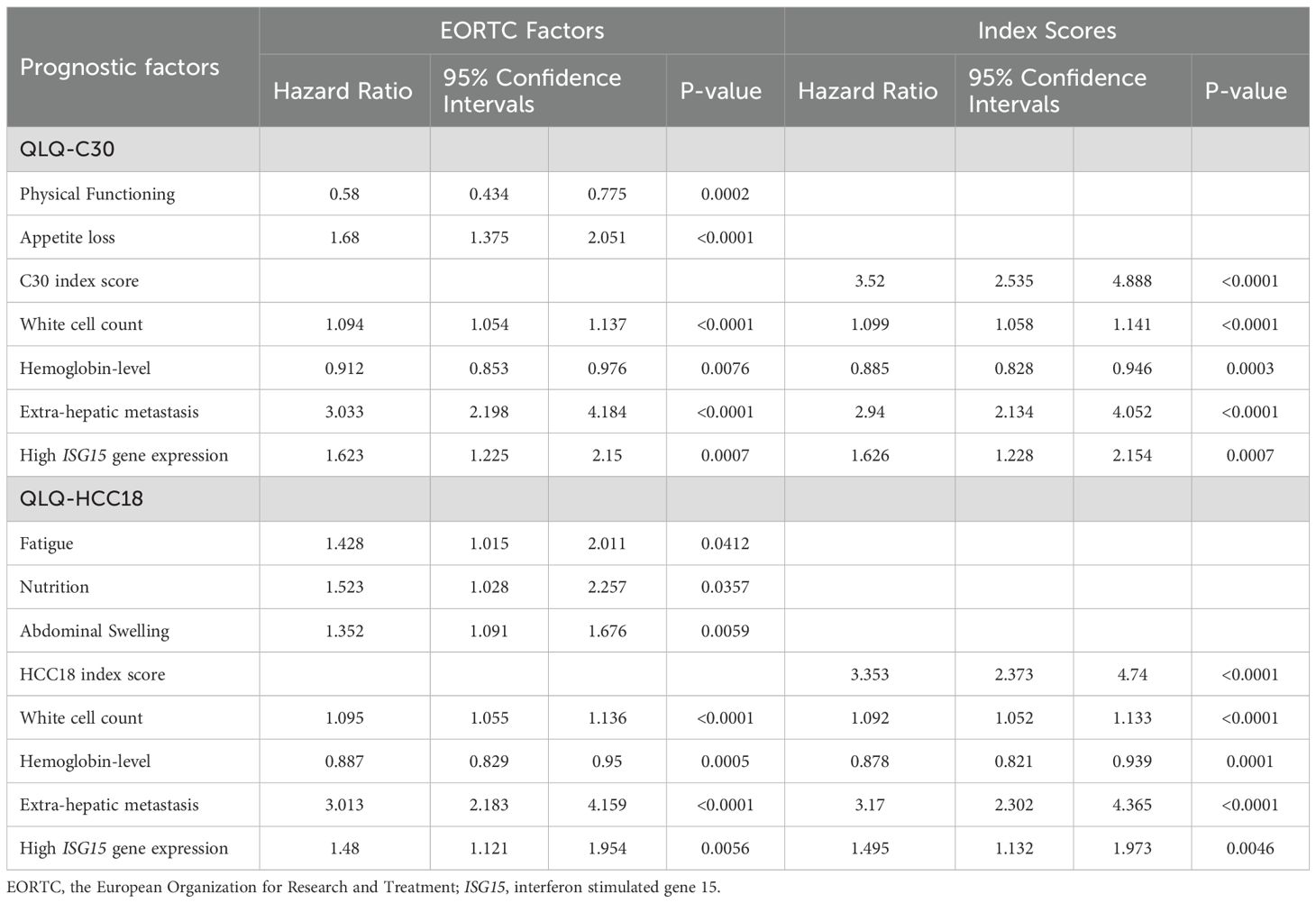
Multivariate Cox regressions using ISG15 mRNA transcript level, clinical and QOL factors are presented in Table 5. Using QLQ-C30 as QOL factors, higher ISG15 mRNA transcript level in circulating leucocytes (HR 1.62, 95% CI 1.23-2.15; p-value <0.01), higher white cell count (HR 1.09, 95% CI 1.05-1.14; p-value <0.01), the presence of extra-hepatic metastasis (HR 3.03, 95% CI 2.20-4.18; p-value <0.01), worse (higher) scores in appetite loss (HR 1.68, 95% CI 1.38-2.05; p-value <0.01) and C30 index score (HR 3.52, 95% CI 2.54-4.89; p-value <0.01) were independent prognostic factors for worse OS. Whereas higher hemoglobin level (HR 0.91, 95% CI 0.85-0.98; p-value <0.01) and better (higher) QLQ-C30 physical functioning score (HR 0.58, 95% CI 0.43-0.78; p-value <0.01) were independent prognostic factors for better OS.
Using QLQ-HCC18 as QOL factors, higher ISG15 mRNA transcript level (HR 1.48, 95% CI 1.12-1.95; p-value <0.01), presence of extra-hepatic metastasis (HR 3.01, 95% CI 2.18-4.16; p-value <0.01), higher white cell count (HR 1.10, 95% CI 1.06-1.14; p-value <0.01), worse (higher) scores in fatigue (HR 1.43, 95% CI 1.02-2.01; p-value <0.05), nutrition (HR 1.52, 95% CI 1.03-2.26; p-value <0.05), abdominal swelling (HR 1.35, 95% CI 1.09-1.68; p-value <0.01) and HCC18 index score (HR 3.35, 95% CI 2.37-4.74; p-value <0.01) were independent prognostic factors for worse OS. Whereas higher hemoglobin level (HR 0.89, 95% CI 0.83-0.95; p-value <0.01) was an independent prognostic factor for better OS.
3.5 Correlations between ISG15 mRNA transcript level and clinical symptomatology
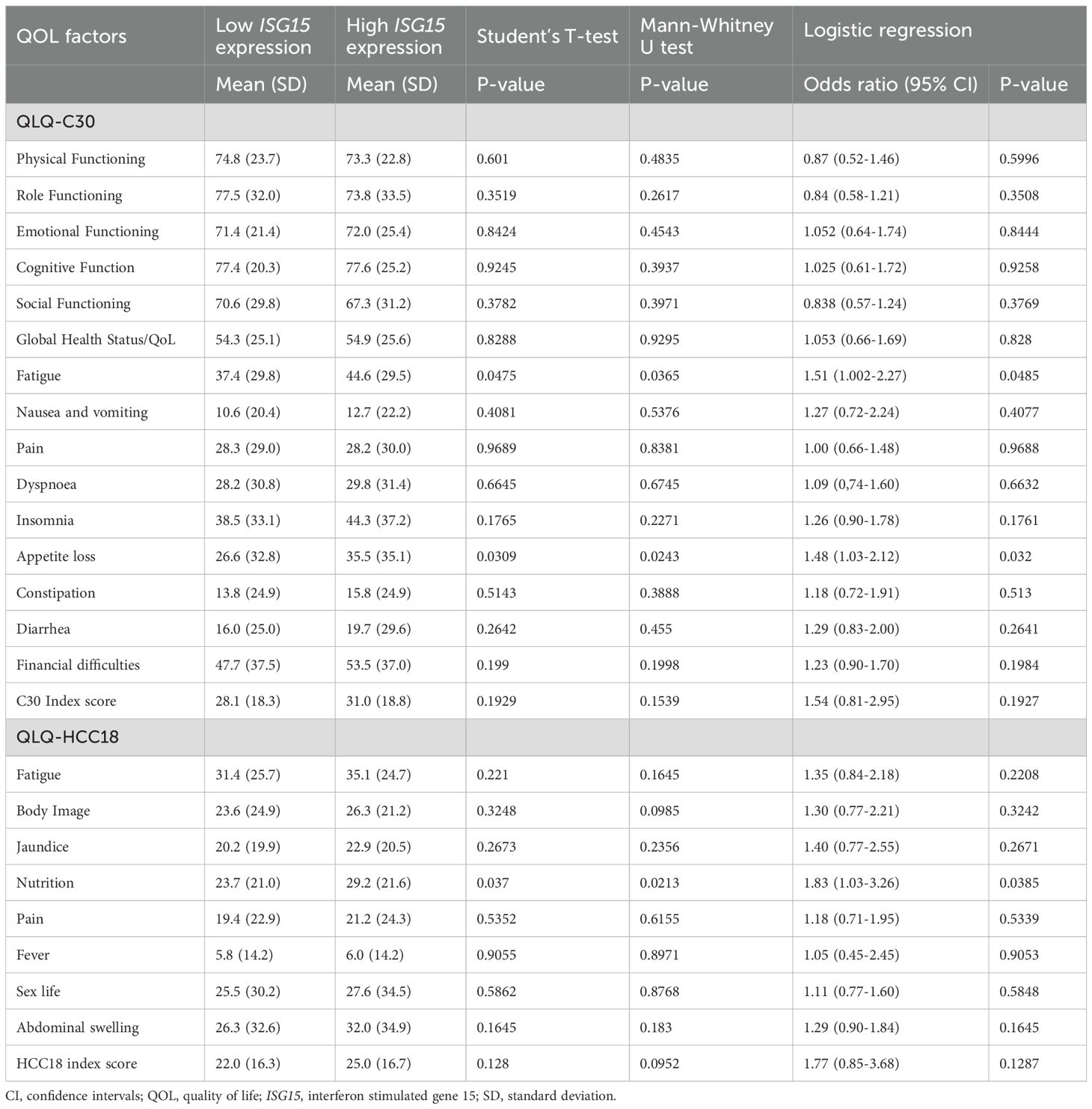
Correlation analyses between ISG15 mRNA transcript level and QOL factors are presented in Table 6. From ROC analysis, the optimal threshold for high ISG15 mRNA transcript level for correlation analyses was 1.3 FC or above. Worse (higher) scores in QLQ-C30 fatigue (T-test p-value <0.05; Mann-Whitney U p-value <0.05; logistic regression odds ratio [OR] 1.51, 95% CI 1.002-2.270; p-value <0.05), appetite loss (T-test p-value <0.05; Mann-Whitney U p-value <0.03; OR 1.48, 95% CI 1.03-2.12; p-value <0.05) and QLQ-HCC18 nutritional concern (T-test p-value <0.05; Mann-Whitney U p-value <0.03; OR 1.83, 95% CI 1.03-3.26; p-value <0.05) demonstrated significant correlations with higher ISG15 mRNA transcript level.
Results of multivariate logistic regressions between ISG15 mRNA transcript level and QOL factors adjusting for clinical factors are presented in Supplementary Table 2. Factors that best predict a higher peripheral blood ISG15 mRNA transcript level were worse (higher) scores in QLQ-C30 appetite loss (OR 1.48, 95% CI 1.03-2.12; p-value <0.05) and QLQ-HCC18 nutritional concern (OR 1.83, 95% CI 1.03-3.26; p-value <0.05).
4 Discussion
This is the first study to show peripheral blood ISG15 mRNA transcript level as an independent prognostic factor for OS in HCC patients. Patients with high level of ISG15 mRNA in circulating leucocytes compared to patients with low level had significantly worse survival, with median OS of 4.7 and 14.3 months respectively. In contrast to a previous report which has shown prognostic value of intra-tumoral ISG15 protein expression confining to early stage HCC patients who received surgical resection, the current study using peripheral blood ISG15 mRNA transcript level demonstrated prognostic significance applicable to all stages of HCC. Moreover, measuring ISG15 mRNA transcript level in peripheral blood (commonly referred to as liquid biopsy) has the advantage of being more practical than measuring ISG15 expression in tumor-tissue. Since majority of HCC are diagnosed by imaging findings with tumor markers and risk factors evaluation (42), tumor biopsy is usually unavailable for testing.
This is also the first study to report ISG15 mRNA level in circulating leucocytes as an independent prognostic factor in the cancer field. Since some other cancers have demonstrated ISG15 protein overexpression in tumor-tissue, further studies are warranted to investigate whether peripheral blood ISG15 mRNA level is also prognostic in other cancer types.
As part of the survival analyses, QOL has demonstrated significant prognostic value. Patients with worse baseline scores in physical functioning, fatigue, appetite loss, nutritional disturbance and abdominal swelling had worse survival compared to those with better scores. QLQ-C30 appetite loss, QLQ-HCC18 nutritional disturbances and abdominal swelling were independent prognostic factors for OS that were not previously reported.
Apart from viral infection, cancer has also been implicated to activate interferon response (43–45). Since ISG15 gene expression has been reported to be specific for type I interferon response pathway activation (16), the current results of elevated ISG15 expression in treatment-naïve HCC patients suggested that type I interferon response was involved in HCC. All recruited patients were treatment-naïve, therefore they did not have any confounding inflammatory response from HCC therapy.
QOL measurement systematically assesses common symptoms and functional disturbances in HCC patients and provides quantification of severity of symptoms in ratings. These data allow correlation analyses between intensity of interferon response pathway activation (as reflected by ISG15 mRNA transcript level) and clinical symptomatology.
ISG15 mRNA level was correlated with HCC patients’ symptomatology. In particular, QOL factors (fatigue. appetite loss and nutrition [which includes questioning for patient’s worries about poor nourishment and recent weight loss]) demonstrated significant correlations with ISG15 gene expression, a pattern consistent with results from analyses using IL-8, CRP and inflammatory score (6, 7). This is logical, since fatigue and anorexia are common clinical manifestations after interferon-α injection (triggering type I interferon response). The cytokines released from type I interferon response have been implicated in inducing anorexia in the central nervous system level, causing reduced oral intake and wasting in cancer patients (the cancer anorexia-cachexia syndrome) (46). The current data suggested that these QOL disturbances have strong underlying biological basis related to interferon response.
In other words, QOL disturbances involving fatigue, appetite loss and nutrition altogether could suggest type I interferon response triggered by HCC. Such grouping of QOL factors could be utilized as a biomarker itself. Future studies could evaluate whether transforming these QOL item scores into an aggregate-score could reflect the intensity of type I interferon response.
Since these QOL impairment could be due to interferon response activation, controlling interferon response might relieve HCC patients’ symptoms and thereby improve their QOL. HCC control with anti-cancer therapy might reduce the intensity of type I interferon response. Furthermore, clinical studies suggested that adverse effects from therapeutic interferon-α could be treated with various measures, e.g. methylphenidate and corticosteroid for fatigue; megestrol acetate and metoclopramide for anorexia; as well as dietary supplementation for nutritional disturbance (47). Further research is necessary to investigate the effectiveness of these measures in improving patients’ QOL.
In recent years, various immune checkpoint inhibitors including anti-programmed death-1 (anti-PD-1), anti-programmed death ligand-1 (anti-PD-L1) and anti-cytotoxic T-lymphocyte-associated antigen 4 (anti-CTLA-4) classes have proven efficacy in treatment for HCC (48–53). ISG15’s key involvement in immune defense system has gained research interest in developing it as a target for cancer immunotherapy. For instance, vaccine against ISG15 protein antigen, ISG15 gene knockdown, ISG15 targeting to potentiate anti-PD-1’s efficacy in pre-clinical cancer models showed some early promises (54–56). Since ISG15 has demonstrated both tumor-suppressing as well as tumor-promoting properties even within the same tumor type (57, 58), complex mechanistic interplays between ISG15 and other immunologic factors are likely involved in immune response against cancer cells. Further investigation is warranted to elucidate ISG15’s pivotal immune-regulatory function. Nevertheless, the current findings established the prognostic characteristics of ISG15 gene expression in HCC patients. Peripheral blood ISG15 mRNA transcript level is a candidate for evaluation as predictive biomarker in future HCC immunotherapy clinical trials. For instance, neutrophil-to-lymphocyte ratio (an inflammatory marker) has been suggested to be predictive of efficacy of immunotherapy in head and neck cancer (59).
We acknowledge there were several limitations in this study. First, our study focused on HCC patients, it is unknown whether the current findings related to ISG15 mRNA transcript level is generalizable to other malignant and non-malignant diseases. Second, in order to capture patient-reported QOL, patients with cognitive impairment were excluded. This prevented recruitment of some terminally-ill patients with encephalopathy due to hepatic failure. To minimize this potential selection-bias in future studies, we could include patients with cognitive impairment by accepting carer-proxy rated QOL data for analysis. Third, our study did not capture post-treatment ISG15 expression and QOL data, which could provide information regarding whether treatment response would alter patients’ ISG15 expression or QOL. However, the current findings pave the path for future research in this direction.
Our study possessed several strengths. First, the sample size was large enough to compensate for the number of missing cases (6.4%) while maintaining adequate power for statistical analyses. Second, this study had a median follow-up duration of 9 years which allow capturing of long survival data, thereby enhancing the precision of survival analyses.
5 Conclusion
This is the first study to demonstrate ISG15 mRNA transcript level in peripheral blood leucocytes as an independent prognostic factor for OS in HCC patients. Patients with higher ISG15 gene expression, reflecting more intense interferon response pathway activation, had significantly worse OS. High ISG15 mRNA transcript level in circulating leucocytes was significantly correlated with QOL disturbances in terms of fatigue, appetite loss and nutritional concern in HCC patients. These QOL factors could be registering the anorexia-cachexia manifestations from type I interferon response triggered by HCC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Prince of Wales Hospital, Hong Kong, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Data curation, Resources. NT: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Data curation. FM: Writing – review & editing, Data curation, Formal analysis, Investigation, Writing – original draft. JK: Writing – review & editing, Data curation, Investigation. EH: Writing – review & editing, Data curation, Investigation. BM: Writing – review & editing, Data curation, Investigation. SC: Writing – review & editing, Data curation, Investigation. KL: Writing – review & editing, Data curation, Investigation. SY: Writing – review & editing, Data curation, Investigation. WY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1589053/full#supplementary-material
Abbreviations
AFP, α-fetoprotein; AGPC, acid guanidinium thiocyanate-phenol-chloroform; AJCC, American Joint Committee on Cancer; cDNA, deoxyribonucleic acid; CI, confidence interval; CRP, C-reactive protein; Ct, cycle threshold; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; ddCT, delta delta cycle threshold; ECOG, Eastern Cooperative Oncology Group; EDTA, ethylenediaminetetraacetic acid; Eff, efficiency; EORTC, the European Organization for Research and Treatment of Cancer; FC, fold change; QOL, quality of life; IL, interleukin;
ISG15, interferon stimulated gene 15; OS, overall survival; PBMC, peripheral blood mononuclear cells; qPCR, quantitative polymerase chain reaction; PD-1, programmed death-1;
PD-L1, programmed death ligand-1; RNA, ribonucleic acid; ROC, receiver operating characteristic; SD, standard deviation; WCC, white cell count.
References
1. Forner A, Reig M, and Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
2. El-Serag HB and Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
3. Xiang X, Zhong JH, Wang YY, You XM, Ma L, Xiang BD, et al. Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin Trans oncology: Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico. (2017) 19:891–7. doi: 10.1007/s12094-017-1621-6
4. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Hernandez-Gea V, Toffanin S, Friedman SL, and Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. (2013) 144:512–27. doi: 10.1053/j.gastro.2013.01.002
6. Li L, Chan SL, Mo F, Hui EP, Koh J, Chan AKC, et al. Correlations of health-related quality of life with serum inflammatory indicators IL-8 and mIBI in patients with hepatocellular carcinoma. Cancer Manag Res. (2019) 11:2719–27. doi: 10.2147/CMAR.S178482
7. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. (2015) 22:803–10. doi: 10.1245/s10434-014-4048-0
8. Li L, Mo FK, Chan SL, Hui EP, Tang NS, Koh J, et al. Prognostic values of EORTC QLQ-C30 and QLQ-HCC18 index-scores in patients with hepatocellular carcinoma - clinical application of health-related quality-of-life data. BMC cancer. (2017) 17:8. doi: 10.1186/s12885-016-2995-5
9. Li L, Chan SL, Mo F, Hui EP, Koh J, Chan AK, et al. Status of inflammation in relation to health related quality of life in hepatocellular carcinoma patients. Qual Life Res. (2019) 28(9):2597–607. doi: 10.1007/s11136-019-02190-0
10. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. (2013) 14:e218–28. doi: 10.1016/S1470-2045(12)70582-X
11. Stutte S, Ruf J, Kugler I, Ishikawa-Ankerhold H, Parzefall A, Marconi P, et al. Type I interferon mediated induction of somatostatin leads to suppression of ghrelin and appetite thereby promoting viral immunity in mice. Brain Behav Immun. (2021) 95:429–43. doi: 10.1016/j.bbi.2021.04.018
12. Motzer RJ, Rakhit A, Ginsberg M, Rittweger K, Vuky J, Yu R, et al. Phase I trial of 40-kd branched pegylated interferon alfa-2a for patients with advanced renal cell carcinoma. J Clin Oncol. (2001) 19:1312–9. doi: 10.1200/JCO.2001.19.5.1312
13. Smith D, Wagstaff J, Thatcher N, and Scarffe H. A phase I study of rDNA alpha-2b interferon as a 6-week continuous intravenous infusion. Cancer chemotherapy Pharmacol. (1987) 20:327–31. doi: 10.1007/BF00262586
14. Woo MH and Burnakis TG. Interferon alfa in the treatment of chronic viral hepatitis B and C. Ann Pharmacother. (1997) 31:330–7. doi: 10.1177/106002809703100312
15. Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. (2017) 214:1547–55. doi: 10.1084/jem.20161451
16. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. (2005) 5:375–86. doi: 10.1038/nri1604
17. Oh SJ, Gim JA, Lee JK, Park H, and Shin OS. Coxsackievirus B3 infection of human neural progenitor cells results in distinct expression patterns of innate immune genes. Viruses. (2020) 12(3):325. doi: 10.3390/v12030325
18. Serrano-Fernández P, Möller S, Goertsches R, Fiedler H, Koczan D, Thiesen HJ, et al. Time course transcriptomics of IFNB1b drug therapy in multiple sclerosis. Autoimmunity. (2010) 43:172–8. doi: 10.3109/08916930903219040
19. Tang FH, Chang WA, Tsai EM, Tsai MJ, and Kuo PL. Investigating novel genes potentially involved in endometrial adenocarcinoma using next-generation sequencing and bioinformatic approaches. Int J Med Sci. (2019) 16:1338–48. doi: 10.7150/ijms.38219
20. Yuan Y, Ma H, Ye Z, Jing W, and Jiang Z. Interferon-stimulated gene 15 expression in systemic lupus erythematosus: Diagnostic value and association with lymphocytopenia. Z Rheumatol. (2018) 77:256–62. doi: 10.1007/s00393-017-0274-8
21. Wan XX, Chen HC, Khan MA, Xu AH, Yang FL, Zhang YY, et al. ISG15 inhibits IFN-α-resistant liver cancer cell growth. BioMed Res Int. (2013) 2013:570909. doi: 10.1155/2013/570909
22. Skaug B and Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. (2010) 143:187–90. doi: 10.1016/j.cell.2010.09.033
23. Teng TS, Foo SS, Simamarta D, Lum FM, Teo TH, Lulla A, et al. Viperin restricts chikungunya virus replication and pathology. J Clin Invest. (2012) 122:4447–60. doi: 10.1172/JCI63120
24. Fassan M, Collesei A, Angerilli V, Sbaraglia M, Fortarezza F, Pezzuto F, et al. Multi-design differential expression profiling of COVID-19 lung autopsy specimens reveals significantly deregulated inflammatory pathways and SFTPC impaired transcription. Cells. (2022) 11(6):1011. doi: 10.3390/cells11061011
25. Xiang M, Chen Q, Feng Y, Wang Y, Wang J, Liang J, et al. Bioinformatic analysis of key biomarkers and immune filtration of skin biopsy in discoid lupus erythematosus. Lupus. (2021) 30:807–17. doi: 10.1177/0961203321992434
26. Piera-Velazquez S, Mendoza FA, Addya S, Pomante D, and Jimenez SA. Increased expression of interferon regulated and antiviral response genes in CD31+/CD102+ lung microvascular endothelial cells from systemic sclerosis patients with end-stage interstitial lung disease. Clin Exp Rheumatol. (2021) 39:1298–306. doi: 10.55563/clinexprheumatol/ret1kg
27. Yang Y, Song J, Zhao H, Zhang H, and Guo M. Patients with dermatomyositis shared partially similar transcriptome signature with COVID-19 infection. Autoimmunity. (2023) 56:2220984. doi: 10.1080/08916934.2023.2220984
28. Jessen C, Kreß JKC, Baluapuri A, Hufnagel A, Schmitz W, Kneitz S, et al. The transcription factor NRF2 enhances melanoma Malignancy by blocking differentiation and inducing COX2 expression. Oncogene. (2020) 39:6841–55. doi: 10.1038/s41388-020-01477-8
29. Zuo D, Chen Y, Zhang X, Wang Z, Jiang W, Tang F, et al. Identification of hub genes and their novel diagnostic and prognostic significance in pancreatic adenocarcinoma. Cancer Biol Med. (2021) 19(7):1029–46. doi: 10.20892/j.issn.2095-3941.2020.0516
30. Álvarez E, Falqui M, Sin L, McGrail JP, Perdiguero B, Coloma R, et al. Unveiling the multifaceted roles of ISG15: from immunomodulation to therapeutic frontiers. Vaccines (Basel). (2024) 12(2):153. doi: 10.3390/vaccines12020153
31. Zhao X, Wang J, Wang Y, Zhang M, Zhao W, Zhang H, et al. Interferon−stimulated gene 15 promotes progression of endometrial carcinoma and weakens antitumor immune response. Oncol Rep. (2022) 47(6):110. doi: 10.3892/or.2022.8321
32. Qiu X, Hong Y, Yang D, Xia M, Zhu H, Li Q, et al. ISG15 as a novel prognostic biomarker for hepatitis B virus-related hepatocellular carcinoma. Int J Clin Exp Med. (2015) 8:17140–50.
33. Qu T, Zhang W, Yan C, Ren D, Wang Y, Guo Y, et al. ISG15 targets glycosylated PD-L1 and promotes its degradation to enhance antitumor immune effects in lung adenocarcinoma. J Transl Med. (2023) 21:341. doi: 10.1186/s12967-023-04135-1
34. Zhao W, Liu YK, Li DJ, Zhao XW, Deng HY, and Du NY. The role of interferon-stimulated gene 15 in the occurence and progression of cervical squamous cell carcinoma. J Physiol Pharmacol. (2023) 74(1):77–83. doi: 10.26402/jpp.2023.1.08
35. Hong G, Li H, Li M, Zheng W, Li J, Chi M, et al. A simple way to detect disease-associated cellular molecular alterations from mixed-cell blood samples. Brief Bioinform. (2018) 19:613–21. doi: 10.1093/bib/bbx009
36. Chomczynski P and Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. (1987) 162:156–9. doi: 10.1016/0003-2697(87)90021-2
37. Pfaffl MW. Quantification strategies in real-time PCR. In: Bustin SA, editor. A-Z of quantitative PCR International University Line (IUL) (2004) p: 87 –112.
38. Bustin SA. Improving the quality of quantitative polymerase chain reaction experiments: 15 years of MIQE. Mol Aspects Med. (2024) 96:101249. doi: 10.1016/j.mam.2024.101249
39. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
40. Blazeby JM, Currie E, Zee BC, Chie WC, Poon RT, and Garden OJ. Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer. (2004) 40:2439–44. doi: 10.1016/j.ejca.2004.06.033
41. Yeo W, Mo FK, Koh J, Chan AT, Leung T, Hui P, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol. (2006) 17:1083–9. doi: 10.1093/annonc/mdl065
42. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. Eur Assoc Study Liver. J Hepatol. (2001) 35:421–30. doi: 10.1016/S0168-8278(01)00130-1
43. Snell LM, McGaha TL, and Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. (2017) 38:542–57. doi: 10.1016/j.it.2017.05.005
44. Yu R, Zhu B, and Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci. (2022) 79:191. doi: 10.1007/s00018-022-04219-z
45. Fuertes MB, Woo SR, Burnett B, Fu YX, and Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. (2013) 34:67–73. doi: 10.1016/j.it.2012.10.004
46. Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, and Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. (2004) 7:427–34. doi: 10.1097/01.mco.0000134363.53782.cb
47. Kirkwood JM, Bender C, Agarwala S, Tarhini A, Shipe-Spotloe J, Smelko B, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. (2002) 20:3703–18. doi: 10.1200/JCO.2002.03.052
48. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. (2022) 1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070
49. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
50. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2022) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
51. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkMate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
52. Verset G, Borbath I, Karwal M, Verslype C, Van Vlierberghe H, Kardosh A, et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. (2022) 28:2547–54. doi: 10.1158/1078-0432.CCR-21-3807
53. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
54. Wood LM, Pan ZK, Seavey MM, Muthukumaran G, and Paterson Y. The ubiquitin-like protein, ISG15, is a novel tumor-associated antigen for cancer immunotherapy. Cancer Immunol Immunother. (2012) 61:689–700. doi: 10.1007/s00262-011-1129-9
55. Nguyen HM, Gaikwad S, Oladejo M, Paulishak W, and Wood LM. Targeting ubiquitin-like protein, ISG15, as a novel tumor associated antigen in colorectal cancer. Cancers (Basel). (2023) 15(4):1237. doi: 10.3390/cancers15041237
56. Burks J, Fleury A, Livingston S, and Smith JP. ISG15 pathway knockdown reverses pancreatic cancer cell transformation and decreases murine pancreatic tumor growth via downregulation of PDL-1 expression. Cancer Immunol Immunother. (2019) 68:2029–39. doi: 10.1007/s00262-019-02422-9
57. Burks J, Reed RE, and Desai SD. Free ISG15 triggers an antitumor immune response against breast cancer: a new perspective. Oncotarget. (2015) 6:7221–31. doi: 10.18632/oncotarget.3372
58. Bolado-Carrancio A, Lee M, Ewing A, Muir M, Macleod KG, Gallagher WM, et al. ISGylation drives basal breast tumour progression by promoting EGFR recycling and Akt signalling. Oncogene. (2021) 40:6235–47. doi: 10.1038/s41388-021-02017-8
59. Hirasawa Y, Kubota Y, Mura E, Suzuki R, Tsurui T, Iriguchi N, et al. Maximum efficacy of immune checkpoint inhibitors occurs in esophageal cancer patients with a low neutrophil-to-lymphocyte ratio and good performance status prior to treatment. Anticancer Res. (2024) 44:3397–407. doi: 10.21873/anticanres.17160
Keywords: type I interferon response, anorexia cachexia syndrome, liver cancer, interferon stimulated gene 15, qPCR, cDNA, EORTC QLQ-C30, HCC18 index score
Citation: Li L, Tang NLS, Mo F, Koh J, Hui EP, Ma B, Chan SL, Lee KF, Yu SCH and Yeo W (2025) ISG15 mRNA transcript level in circulating leucocytes prognostic of overall survival in hepatocellular carcinoma patients and correlated with quality of life disturbances involved in anorexia-cachexia. Front. Oncol. 15:1589053. doi: 10.3389/fonc.2025.1589053
Received: 06 March 2025; Accepted: 12 August 2025;
Published: 27 August 2025.
Edited by:
Lekshmi R. Nath, Amrita Vishwa Vidyapeetham, IndiaReviewed by:
Komal Ramani, Cedars Sinai Medical Center, United StatesManish Kumar Singh, Oklahoma Medical Research Foundation, United States
Copyright © 2025 Li, Tang, Mo, Koh, Hui, Ma, Chan, Lee, Yu and Yeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leung Li, bF9saUBjbG8uY3Voay5lZHUuaGs=; Nelson L. S. Tang, bmVsc29udGFuZ0BjdWhrLmVkdS5oaw==
†ORCID: Leung Li, orcid.org/0000-0002-0251-0253
Nelson L. S. Tang, orcid.org/0000-0002-3607-5819
Frankie Mo, orcid.org/0000-0002-0566-3212
Jane Koh, orcid.org/0000-0002-1034-2042
Edwin P. Hui, orcid.org/0000-0002-8134-7019
Brigette Ma, orcid.org/0000-0003-4802-1102
Stephen L. Chan, orcid.org/0000-0001-8998-5480
Simon C. H. Yu, orcid.org/0000-0002-8715-5026
Winnie Yeo, orcid.org/0000-0002-0863-8469
 Leung Li
Leung Li Nelson L. S. Tang
Nelson L. S. Tang Frankie Mo
Frankie Mo Jane Koh1†
Jane Koh1† Brigette Ma
Brigette Ma Stephen L. Chan
Stephen L. Chan Kit F. Lee
Kit F. Lee Simon C. H. Yu
Simon C. H. Yu