- 1Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
- 2Haematology Department, Hospices Civils de Lyon, Lyon, France
- 3Kite, A Gilead Company, Santa Monica, CA, United States
- 4Real World Evidence, Oracle Life Sciences, Austin, TX, United States
- 5Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Introduction: The objectives of this study were to identify key treatment attributes that drive physician and patient preferences for second line (2L) and third line (3L) treatments in relapsed/refractory (R/R) follicular lymphoma (FL).
Methods: A multi- country, internet-based survey was administered to patients(N=195) with R/R FL and treating physicians (N=300) from the United States, United Kingdom, France, Germany, Brazil, and Japan. The survey included two discrete choice experiments – one for 2L and one for 3L treatment options – that prompted respondents to select their preferred option between two hypothetical treatment profiles varying on seven attributes associated with treatment for R/RFL: progression-free survival (PFS), overall survival (OS), serious adverse events (AE), cytokine release syndrome (CRS) events, neurological events, fatigue, and administration. Mean preference weights and relative attribute importance were estimated in each sample, overall and by country, using hierarchical Bayesian models. Physician estimates were also stratified by practice setting.
Results: Treatment preferences for physicians and patients were most influenced by PFS. Beyond PFS, patients placed greater emphasis on the administration of medications, whereas physicians tended to focus more on five-year OS and toxicity profiles of agents. Preference for PFS above all other 2L and 3L treatment attributes was consistent for physicians, regardless of practice setting and country. However, patient treatment preferences varied by country.
Discussion: These results offer key perspectives on how physicians and patients evaluate treatment options in 2L and 3L treatment settings; this information is essential for facilitating shared decision-making in an expanding, complex treatment landscape.
1 Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma globally (1). In the United States (US), incident FL cases are projected to rise from approximately 11,800 in 2020 to 14,700 in 2030, with similar increases expected in other regions (1). FL burdens patients, with notable impairments in health-related quality of life (HRQoL), working, and daily living, especially during later lines of therapy (2–9).
Although the outcome of first-line (1L) treatment of FL patients has improved in the era of chemo-immunotherapy and immunotherapy maintenance (10, 11), many patients experience refractory disease or have a disease recurrence within five years of receiving 1L therapy. Once patients develop relapsed or refractory (R/R) disease, the success of therapies decreases with each subsequent treatment line (1, 12–14), and in the second-line (2L) setting, roughly 70-75% of patients will have a subsequent relapse within five years (1, 15).
Clinical trial data evaluating the efficacy and safety of novel therapies, including CD-19 direct chimeric antigen receptor T-cell (CAR T) therapies, CD3 X CD20 bispecific antibodies, and EZH2, PI3K, and BTK inhibitors, demonstrate increases in the complete treatment response rate, even among pre-treated individuals (16). While an expanding treatment landscape for R/R FL is generally positive, increases in available treatments can also create challenges. Indeed, treatment selection for R/R FL is a nuanced process that increases in complexity as more therapeutic options become available and requires careful consideration of various factors (i.e., treatment goals, patient treatment history, and performance status (17). Perceptions of these factors can differ between patients and physicians (18, 19). Therefore, understanding how patients and physicians perceive treatment options is essential for shared decision-making.
The past two decades have demonstrated a growing focus on patient autonomy and the involvement of patients in treatment planning and decision-making. This complex and important process requires an understanding of patient preferences, both in terms of disease management and their overall health and includes addressing psychosocial needs in the context of cancer treatment (20). Evidence suggests that patients are more likely to adhere to medications and experience more positive outcomes when physicians engage their patients in treatment selection and share decision-making (21–27). Research evaluating patient and physician preferences for R/R FL treatment may produce knowledge that facilitates shared decision-making amidst an evolving and complex treatment landscape. However, robust preference research on R/R FL treatment is limited (28, 29), and extending such research to study designs that focus on later lines of treatment across diverse geographical regions may advance our understanding of patients’ treatment needs and promote shared decision-making in treatment selection.
The objectives of this study were to identify key treatment attributes that drive physician and patient preferences for 2L and third-line (3L) treatments in R/R FL and describe the congruence between patient and physician preferences.
2 Materials and methods
2.1 Study design
A cross-sectional, multi-country, online survey was administered to physicians between May and August 2023 and administered to patients with FL between September 2023 and January 2024. Participating countries included the US, the United Kingdom (UK), France, Germany Brazil, and Japan. The survey included two discrete choice experiments (DCE) to evaluate preferences for attributes associated with 2L and 3L treatment for R/R FL. The study protocol (10589-AMartin01) received exemption status from the Sterling Institutional Review Board on December 8, 2022. This study was conducted in accordance with the Declaration of Helsinki. All participants provided electronic consent and received fair-market compensation for their participation.
2.2 Participants
Recruitment was led by Global Perspectives, which specializes in online healthcare and health outcomes research. Physicians were recruited from an existing panel of healthcare providers who agreed to participate in online surveys. Patients were recruited through multiple sources, including patient databases, patient panels, social media, patient associations, and physician referrals. Recruitment quotas were applied to ensure a minimum number of physicians from academic and community settings in each country and a minimum number of patients from each country were included in our samples.
Physicians currently treating patients with FL, who treated patients with cancer for 3–40 years, and who treated at least five patients with FL in the past six months were eligible to participate in the study. Patients were required to be ≥18 years old, diagnosed with FL, and self-report that their FL had relapsed or was refractory unless the patient was from Japan. Due to recruitment challenges, patients from Japan were required to have received at least first-line treatment.
2.3 Survey content
Two DCE exercises (30–32) were conducted to evaluate preferences for 2L and 3L treatments in R/R FL, respectively. Each exercise included a series of 12 choice tasks, where respondents were instructed to select their preferred option from two hypothetical treatment profiles (Figure 1). For the 2L DCE, patients and physicians were asked to assume they were choosing (in the case of patients) or prescribing (in the case of physicians) 2L (second round)/2L treatment; in the 3L DCE, they were asked to assume they were choosing or prescribing 3L (third round)/3L treatment. The DCEs included attributes and levels associated with CAR Ts, bispecific antibodies, chemotherapy, and stem-cell transplantation. Specific therapies, like CAR T, are not currently available for R/R FL treatment in the UK and are not yet approved for 2L or 3L therapy in Europe. However, the hypothetical nature of the DCE allowed us to assess CAR T characteristics in these regions to provide insights into potential future treatment landscapes. Each hypothetical treatment profile consisted of seven attributes with three to six levels each: PFS, five-year overall survival (OS), serious adverse events (AE), cytokine release syndrome (any grade; CRS), neurological events (any grade), fatigue (any grade), and administration (Table 1). Attributes were identified via a focused literature review and refined with input from clinical experts. The experimental design for the DCEs was a balanced design with minimal overlap (31). The design was generated to optimize overall design efficiency in terms of (a) level balance (each level is shown approximately an equal number of times); (b) minimal level overlap (levels repeat within the same task); and (c) orthogonality (levels may be evaluated independently of other levels).
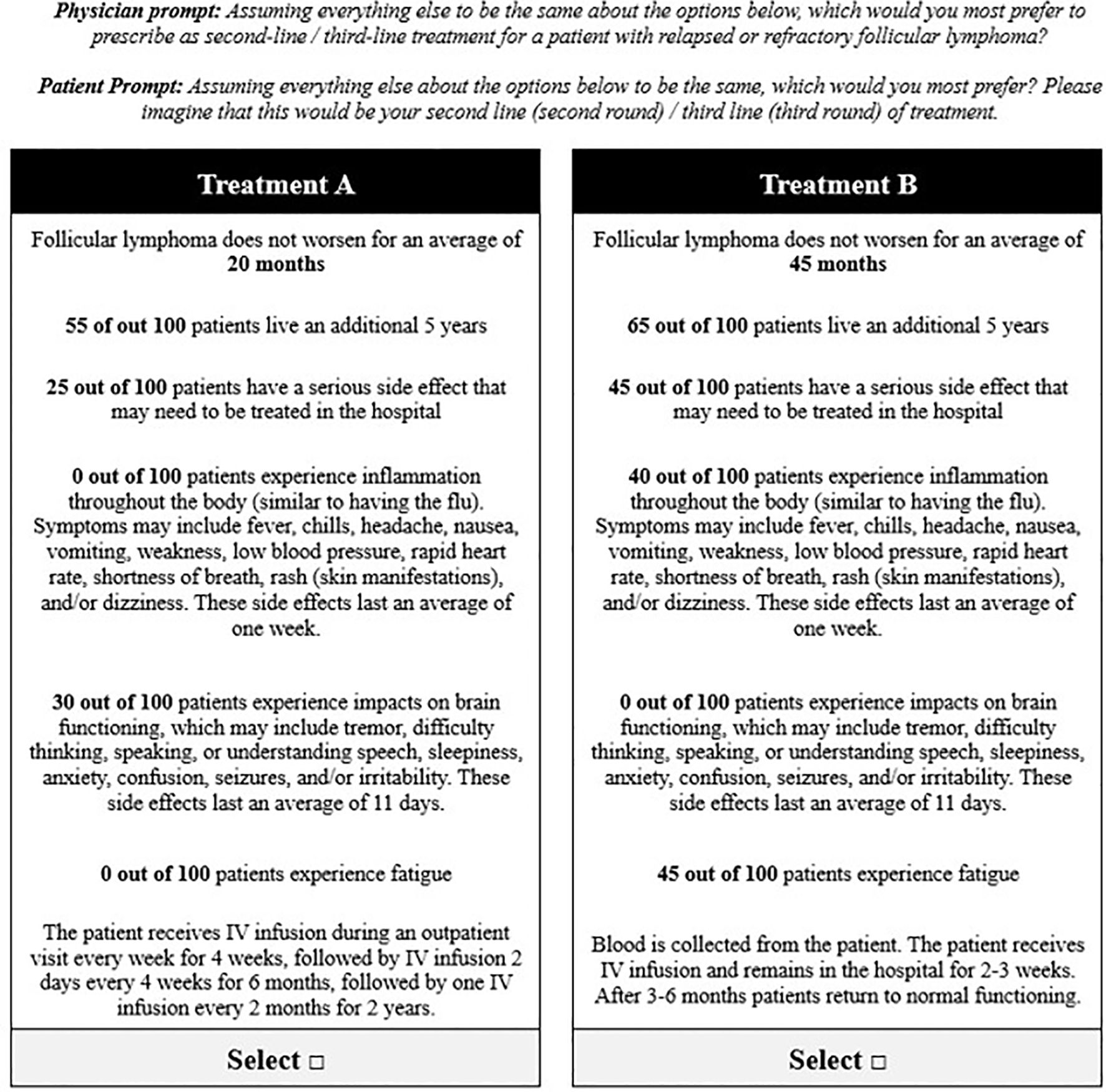
Figure 1. Example choice task from the discrete choice experiment to evaluate preferences for key treatment attributes.
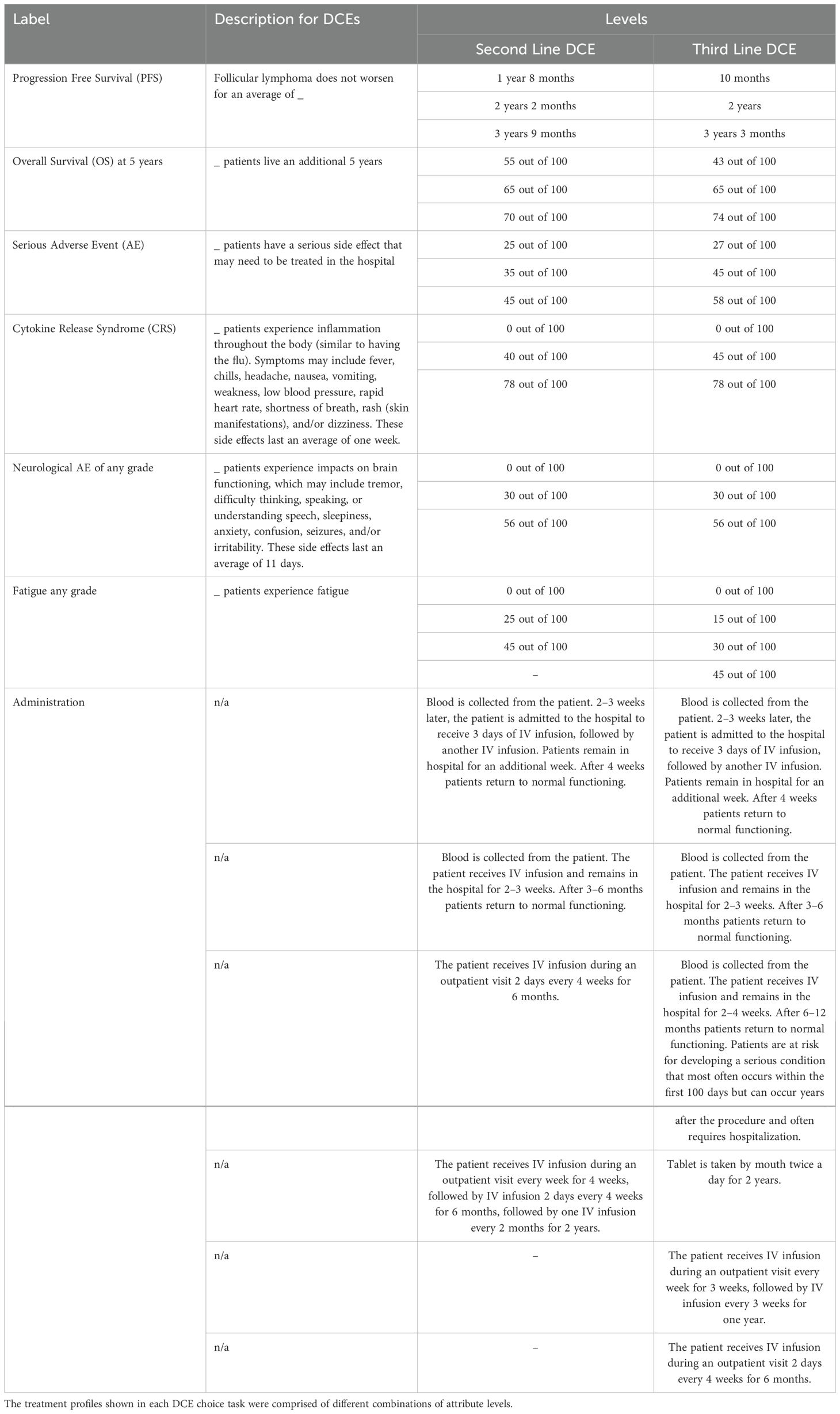
Table 1. Attributes and levels included in the second and third-line discrete choice experiments (DCE).
For the physician survey, sociodemographic characteristics, practice characteristics, and experiences treating patients with FL were collected. Patient sociodemographic, general health, clinical, and treatment characteristics were collected in the patient survey. The patient survey also included several patient-reported outcome measures (PROMs), including the Work Productivity and Activity Impairment (WPAI) – General Health Questionnaire (33), the EQ-5D utility index score, the EQ Visual Analogue Scale (VAS) (34), the Functional Assessment of Cancer Therapy – General (FACT-G) (35), and the Lymphoma Scale (36, 37) to supplement the FACT-G. A description of these instruments can be found in Supplementary Methods. Draft surveys were pilot tested via cognitive interviews (N=18 in each cohort) to ensure the survey content, including the DCEs, was clearly worded and performing as expected. The final physician and patient surveys took an average of 21.2 and 36.9 minutes to complete, respectively.
2.4 Statistical analysis
Descriptive statistics (mean and standard deviation [SD] for continuous variables; frequencies and percentages for categorical variables) were used to characterize the overall physician and patient samples. PROMs were also summarized descriptively.
To estimate preference weights for each attribute level in the DCEs, hierarchical Bayes (HB) multinomial logit models were utilized (30). These models generate utility estimates from individual-level choices and information from other respondents to estimate optimal coefficients. The prior distributions of the estimates are assumed to be normally distributed across the sample. HB models are considered hierarchical because they have two levels such that individual-level preference weights are described by a multivariate normal distribution at the higher level but governed by a multinomial logic model at the lower level. To ensure model estimates in the aggregate physician and patient samples were precise, a priori sample size calculations were completed using the formula (38), where n is the number of participants, t is the number of choice tasks, a is the number of profiles show in each task, and c represents the largest number of levels for any one attribute:
With N=195 patients, seven attributes with a maximum of six levels, two alternatives per task, and 12 choice tasks, the formula results in 780, which is above 500, indicating that there was sufficient sample size to obtain relatively precise utility estimates for each of our DCE exercises in the patient cohort. With N=300 physicians and the same levels and tasks, the result is 1,200, again indicating that there was sufficient sample size for the physicians.
Mean preference weights (MPW) were estimated for each attribute level in the DCEs to measure relative preference. These weights measure relative preferences, such that the magnitude of the difference between attribute levels reflects how influential the difference has on treatment choice. MPWs allow for the assessment of trade-offs between utility gains and losses across attributes. As such, the difference between the highest (most preferred) and lowest (least preferred) MPW for each treatment indicates the magnitude of attributes influence on treatment preferences.
Relative importance (RI) for each attribute was also estimated using the MPWs to show the total impact on the utility of treatment associated with each attribute. RI estimates were derived at the respondent level by calculating the range of each attribute (utility of the most favorable minus the least favorable), then divided by the sum of all attribute ranges and multiplying by 100. Bivariate comparisons using one-way analysis of variance (ANOVA; α=0.05) were performed on RI estimates to examine unadjusted differences in physician treatment preferences, relative to academic setting (academic vs. community) and country. Given the smaller sample size for the patient cohort, descriptive comparisons of attribute RI were performed by country. All data were analyzed using Statistical Package for Social Sciences (SPSS) version 28 (IBM SPSS Statistics for Windows, Version 28.0, Armonk, NY: IBM Corp) for descriptive statistics and Sawtooth Software Lighthouse Studio version 9.12.0 or higher for the DCE analyses.
3 Results
3.1 Demographics
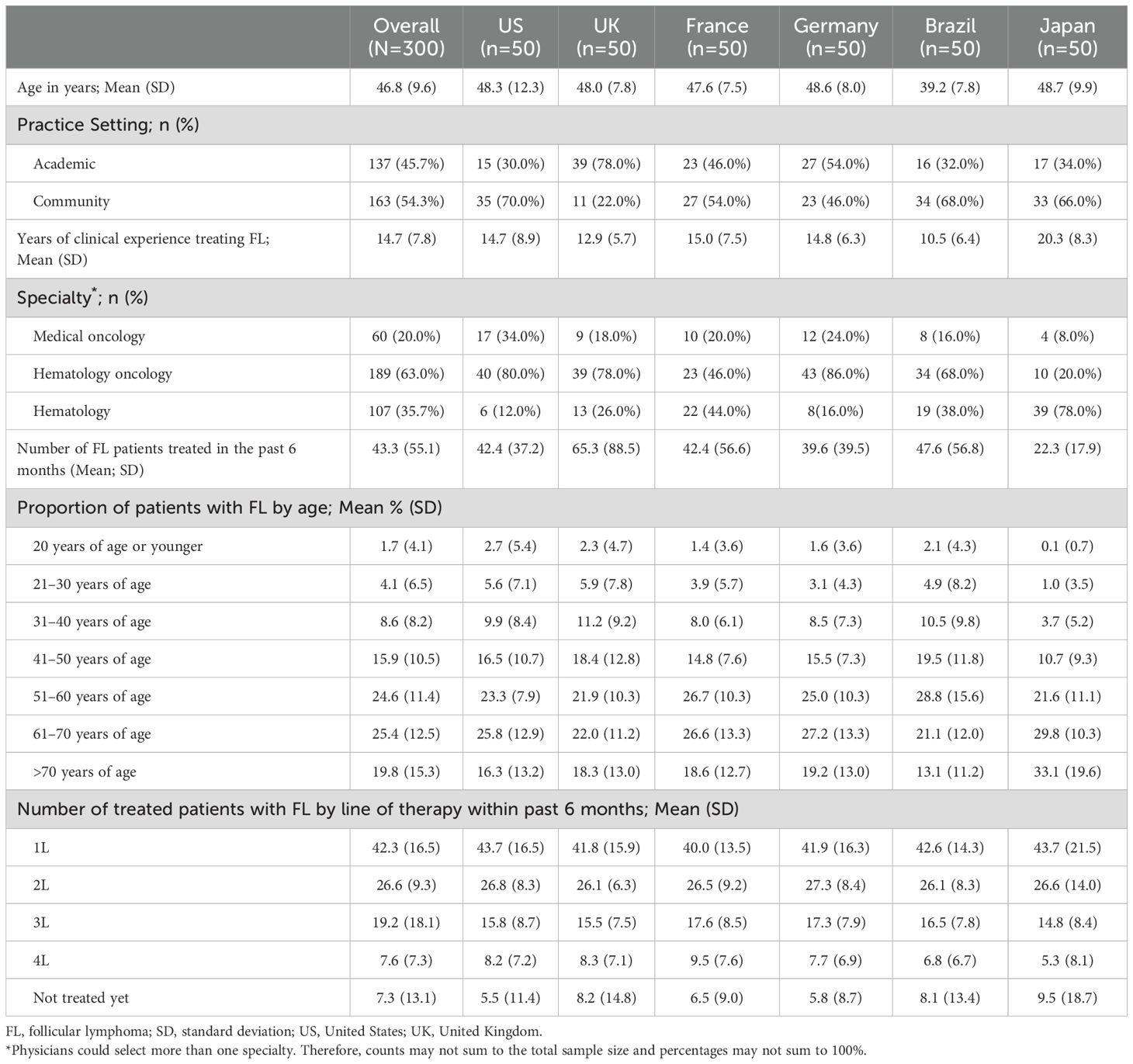
Participating physicians (N=300) were equally distributed across countries (Table 2). Mean (SD) age was 46.8 (9.6) years, with the oldest physicians in Japan (48.7 years) and the youngest in Brazil (39.2 years). More than half (54.3%) of physicians practiced in community settings, though this varied by country. Physicians had an average of 14.7 years of clinical experience treating FL (range: 10.5 years in Brazil to 20.3 years in Japan) and treated 44 patients, on average, with FL in the past six months.
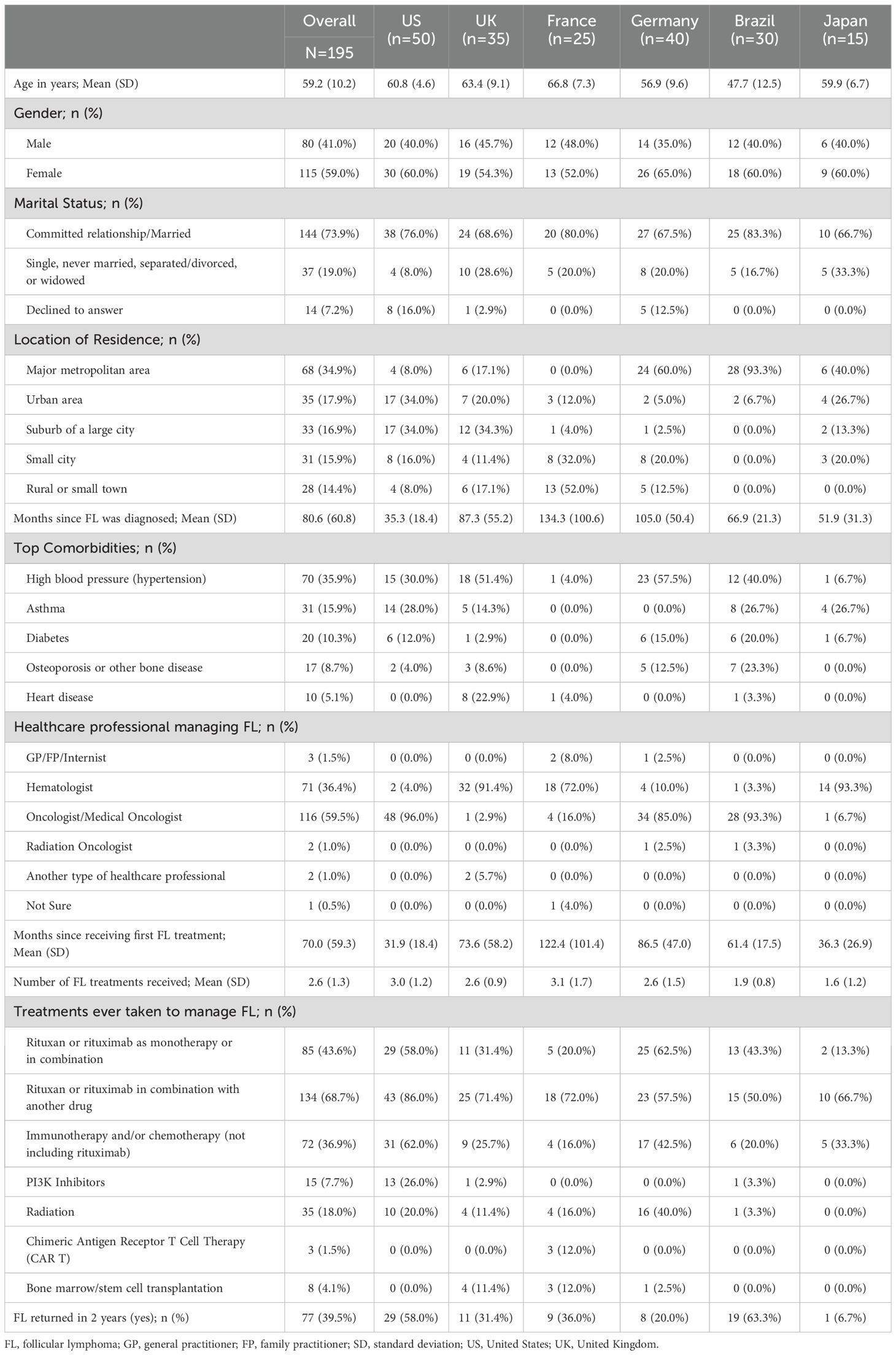
Participating patients (N=195) included those from the US (n=50, 25.6%), Germany (n=40, 20.5%), the UK (n=35, 17.9%), Brazil (n=30, 15.4%), France (n=25, 12.8%), and Japan (n=15, 7.7%) (Table 3). Women comprised a large proportion of patients (59.0%; range: 54.3% in the UK to 65.0% in Germany) and the mean (SD) age of patients was 59.2 (10.2) years. The mean (SD) number of months since patients were diagnosed with FL was 80.6 (60.8). The time since receiving the first FL treatment was the shortest in the US (31.9 [18.4] months) and the longest in France (122.4 [101.4]). The average number of FL treatments that patients received was 2.6 (SD=1.3) and 39.5% of patients said their FL returned within 2 years after starting their first FL treatment.
3.1.1 Patient-reported outcome measures
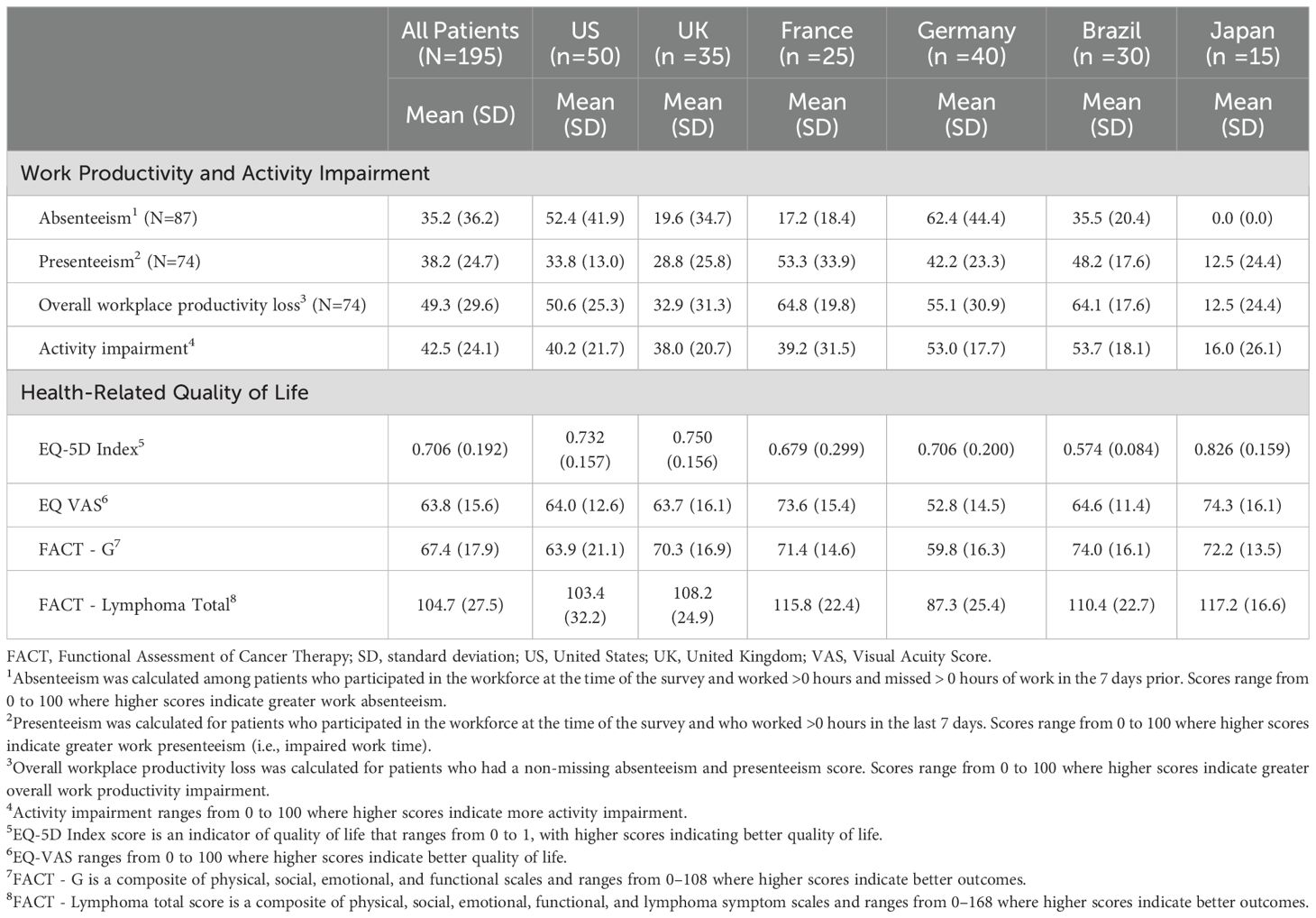
Among all patients, the mean (SD) percentage of absenteeism, presenteeism, overall work productivity impairment, and overall activity impairment over the past seven days was 35.2 (36.2), 38.2 (24.7), 49.3 (29.6), and 42.5 (24.1), respectively (Table 4). Patients from Japan reported the lowest levels of work productivity and activity impairment, across all metrics. Patients from France, on average, reported the highest levels of presenteeism (53.3%) and overall work productivity impairment (62.4%). Patients from Germany experienced the most absenteeism (62.4%), whereas patients from Brazil experienced the highest percentage of overall activity impairment (53.7%).
The mean (SD) EQ-5D index score ranged from 0.574 (0.084) in Brazil to 0.826 (0.159) in Japan, with an overall score of 0.706 (0.192) in the pooled patient sample (Table 4). The mean (SD) EQ VAS was 63.8 (15.6) in the pooled patient sample, with the lowest values noted among patients from Germany (52.8 [14.5]) and the highest from Japan (74.3 [16.1]). The mean (SD) FACT-G score was 67.4 (17.9) in the overall patient sample (range: 59.8 [16.3] in Germany to 72.2 [13.5] in Japan). When the Lymphoma Subscale was factored into HRQoL, the overall patient cohort had a mean (SD) score of 104.7 (27.5), with Germany patients once again having the lowest score on average (87.3 [25.4]) and Japan patients having the highest average score (117.2 [16.6]).
3.2 2L DCE findings
On average, both physician and patient treatment preferences were most influenced by increases in PFS, followed by decreases in the risk of neurological events, compared to other treatment attributes (Figure 2, Supplementary Table 1). Among physicians, increasing PFS from one year, eight months (MPW = -1.84) to three years, nine months (MPW = 2.48), reflects an absolute difference of 4.32, whereas decreasing risk of neurological events from 56% (MPW = -1.34) to 0% (MPW = 1.45) reflects an absolute difference of 2.79. Similarly, for patients, the absolute difference in increasing PFS from one year, eight months (MPW = -2.12) to three years, nine months (MPW = 2.37) was 4.49, and decreasing risk of neurological events from 56% (MPW = -1.58) to 0% (MPW = 1.55) was 3.13. As such, both physicians and patients were willing to accept an increased neurological event risk of 56% in exchange for an increase in PFS of 25 months.
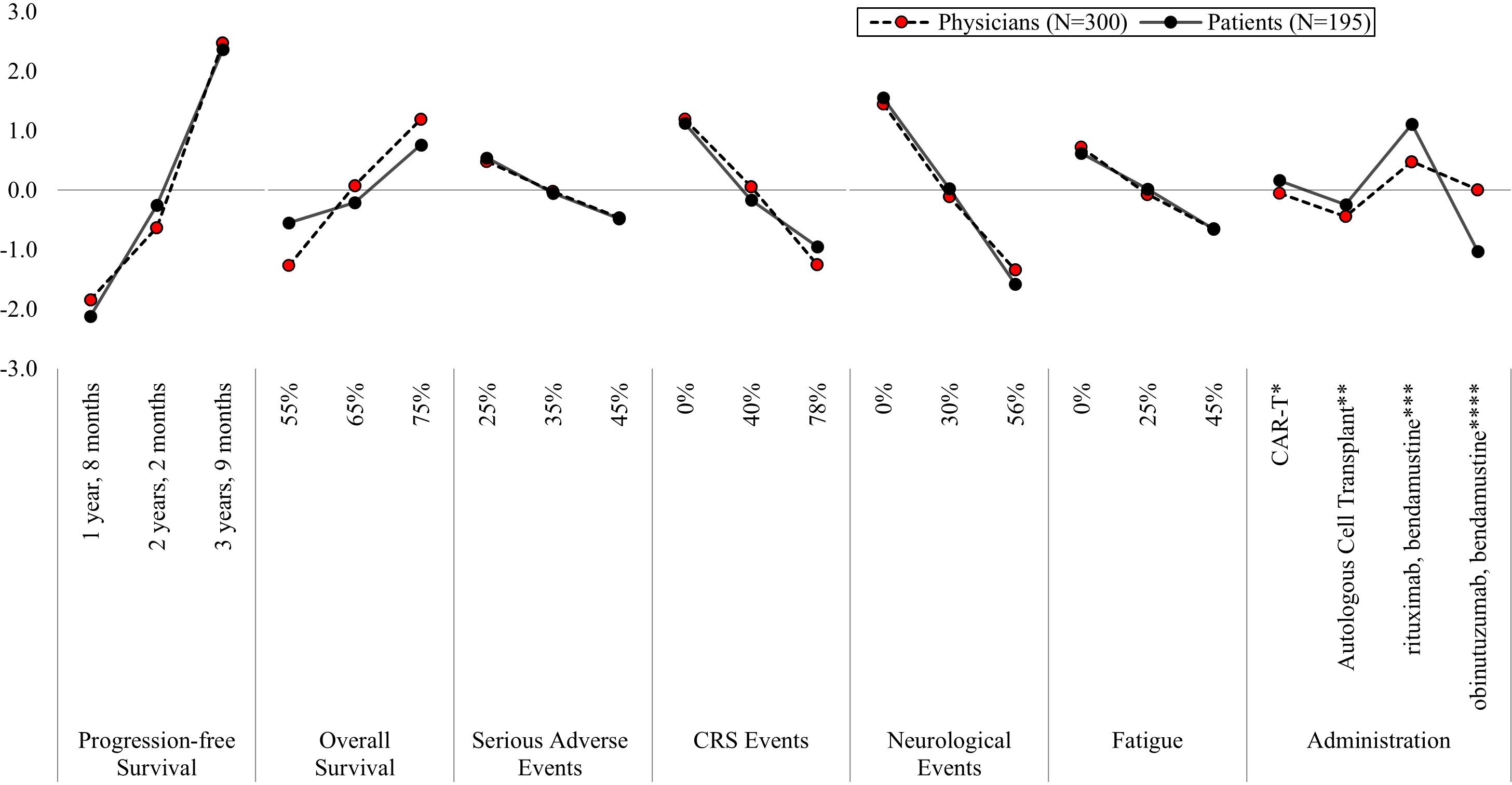
Figure 2. Mean preference weights for attributes associated with second-line treatment for relapsed/refractory follicular lymphoma. CRS, Cytokine Release Syndrome; IV, intravenous; PFS, progression free survival. Preference weights should not be interpreted by themselves. Instead, the magnitude of change within one attribute should be compared to change within another attribute. All preference weights of levels within an attribute sum to 0. *Blood is collected from the patient. 2–3 weeks later, the patient is admitted to the hospital to receive 3 days of IV infusion, followed by another IV infusion. Patients remain in the hospital for an additional week. After 4 weeks patients return to normal functioning. **Blood is collected from the patient. The patient receives IV infusion and remains in the hospital for 2–3 weeks. After 3–6 months patients return to normal functioning. ***The patient receives IV infusion during an outpatient visit 2 days every 4 weeks for 6 months. ****The patient receives IV infusion during an outpatient visit every week for 4 weeks, followed by IV infusion 2 days every 4 weeks for 6 months, followed by one IV infusion every 2 months for 2 years.
Following PFS and risk of neurological events, physician treatment preferences were most influenced by increasing five-year OS from 55% (MPW = -1.27) to 75% (MPW = 1.19) and decreases in the CRS risks from 78% (MPW = -1.25) to 0% (MPW = 1.19) (Figure 2, Supplementary Table 1). In contrast, patients prioritized treatment administration over reductions in CRS risks and five-year OS improvements. Patients preferred treatments administered in an outpatient setting on two days, every four weeks for six months (MPW = 1.11) over an outpatient infusion with extended follow-up treatments every two months for up to two years (MPW = -1.03). For physicians, administration had the least influence on 2L treatment preferences overall, compared to the other attributes.
3.3 3L DCE findings
For both physicians and patients in the 3L setting, increasing PFS from 10 months (MPW for physicians = -3.50 and patients = -3.34) to three years and three months (MPW for physicians = 3.14 and patients = 2.77) most influenced treatment preferences (Figure 3, Supplementary Table 2). While increasing five-year OS from 43% (MPW = -2.38) to 74% (MPW = 1.91) was the second most influential attribute for physicians’ treatment preferences, treatment administration was the next most influential treatment attribute for patients. Patients least preferred the administration method of allogeneic stem cell transplant (ASCT), which carries a risk of developing acute graft versus host disease, a serious condition that typically occurs within the first 100 days and requires hospitalization (MPW = -3.62). Instead, patients preferred the option of taking oral medication twice a day for two years (MPW = 2.14). Patient 3L treatment preferences were most influenced by increasing five-year OS from 43% (MPW = -1.07) to 74% (MPW = 1.21) and reducing the risk of neurological events from 56% (MPW = -1.05) to 0% (MPW = 1.17).
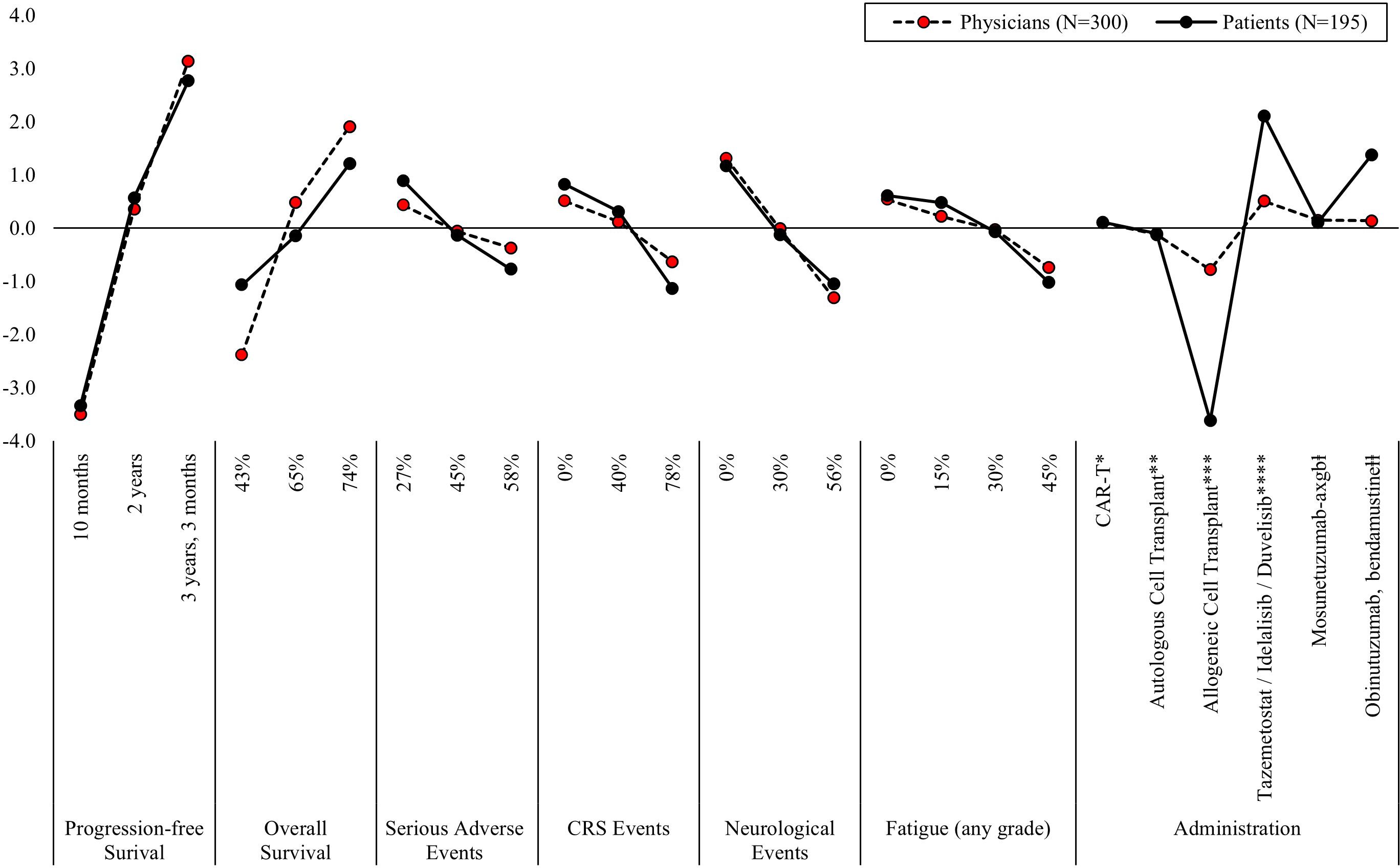
Figure 3. Mean preference weights for attributes associated with third-line treatment for relapsed/refractory follicular lymphoma. PFS, progression free survival; CRS, cytokine release syndrome. Note: Preference weights should not be interpreted by themselves. Instead, the magnitude of change within one attribute should be compared to change within another attribute. All preference weights of levels within an attribute sum to 0. *Blood is collected from the patient. 2-3 weeks later, the patient is admitted to the hospital to receive 3 days of IV infusion, followed by another IV infusion. Patients remain in the hospital for an additional week. After 4 weeks patients return to normal functioning. **Blood is collected from the patient. The patient receives IV infusion and remains in the hospital for 2-3 weeks. After 3-6 months patients return to normal functioning. ***Blood is collected from the patient. The patient receives IV infusion and remains in the hospital for 2-4 weeks. After 6-12 months patients return to normal functioning. Patients are at risk for developing a serious condition that most often occurs within the first 100 days but can occur years after the procedure and often requires hospitalization. ****Tablet is taken by mouth twice a day for 2 years. ⱡThe patient receives IV infusion during an outpatient visit every week for 3 weeks, followed by IV infusion every 3 weeks for one year. ⱡⱡThe patient receives IV infusion during an outpatient visit 2 days every 4 weeks for 6 months.
Beyond PFS and OS, the risk of neurological events and type of administration were among the most influential treatment attributes for physicians (Figure 3, Supplementary Table 2). Like patients, physicians least preferred ASCT (MPW = -0.78) and most preferred oral tablets twice a day for two years (MPW = 0.51), though the method of administration was not as important to physicians relative to patients.
Due to the influence of the ASCT on patient 3L treatment preferences, we conducted a post-hoc sensitivity analysis where the ASCT attribute level was removed from the model (Supplementary Table 3, Supplementary Figure 1). After removal, administration was the least influential 3L treatment attribute on physician preference, relative to the others assessed. For patients, the overall influence of administration on 3L treatment preferences decreased, such that the influence of administration was roughly equal to that of five-year OS, and the risk of neurological events (evidenced by the absolute difference between the most and least preferred attribute levels).
3.4 Subgroup comparisons of physician treatment preferences
Physician preferences for 2L and 3L treatment attributes were comparable between community and academic settings (i.e., all p values were >0.05) (Figure 4), with PFS being the most influential and equally valued by both groups. The influence of PFS was also consistent across countries, for both 2L and 3L settings (Figure 5). However, variation by country was noted in the 2L RI estimates for neurological events (p<0.001), OS (p<0.05), and serious AEs (p<0.05). Specifically, among physicians from France and Germany, the RI of neurological events was lower than other countries, such that both OS and CRS events were perceived as being relatively more important than neurological events. Consequently, the RI of OS in the 2L setting was highest among physicians in France and Germany. While serious AEs were consistently the least important 2L treatment attribute relative to others assessed, the magnitude of influence of serious AEs on treatment preferences was higher among physicians from France (RI = 9.1) and lowest among those from the US (RI = 6.3). In the 3L setting, the relative importance of the risk of neurological events varied by country (p<0.05), such that the relative importance was lowest among physicians from France (RI=10.9) and highest among those from the US (RI=16.6).
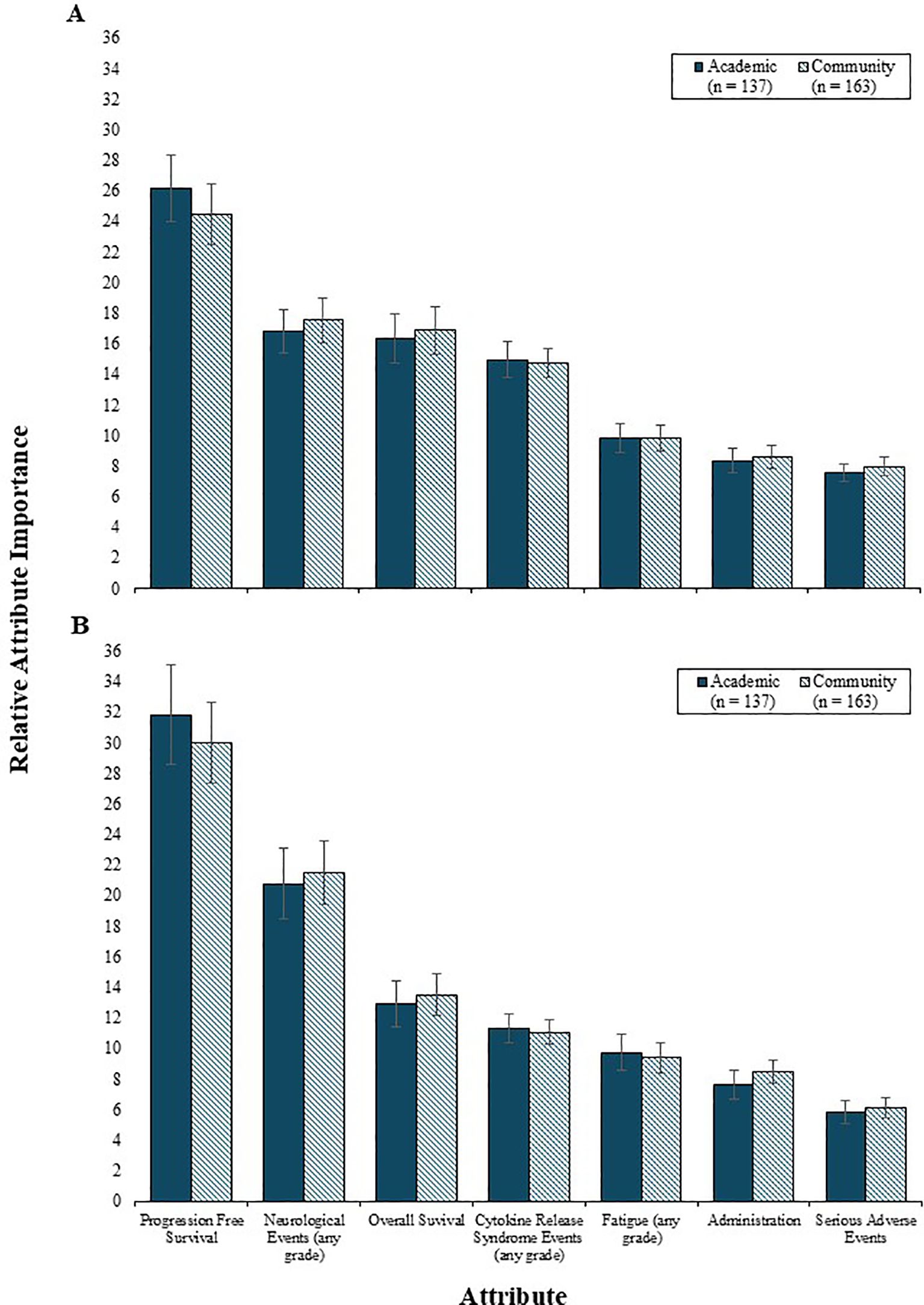
Figure 4. The relative importance of 2L (A) and 3L (B) treatment attributes by physician treatment setting. 2L, second line; 3L, third line.
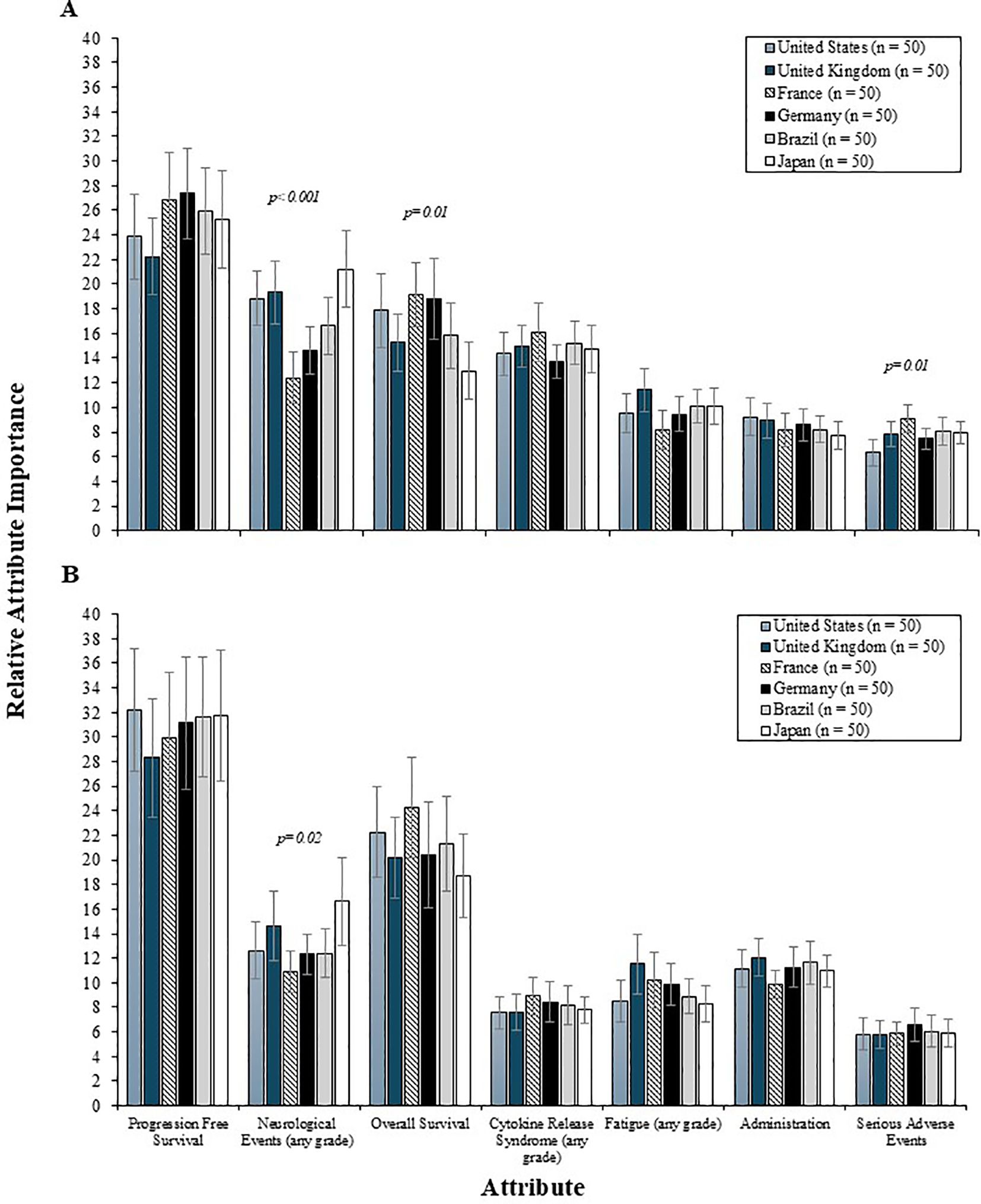
Figure 5. The relative importance of 2L (A) and 3L (B) treatment attributes among physicians by country. 2L, second line; 3L=third line.
3.5 Subgroup comparisons of patient treatment preferences
In the 2L setting, PFS was the most important attribute, relative to the others among patients from all countries except Brazil, who placed greater value on reducing the risk of neurological events (RI = 25.3) over increases in PFS (RI = 22.0) (Figure 6). For most countries, administration and reducing the risk of neurological events were among the most important 2L treatment attributes, whereas OS and serious AEs were consistently among the least important attributes.
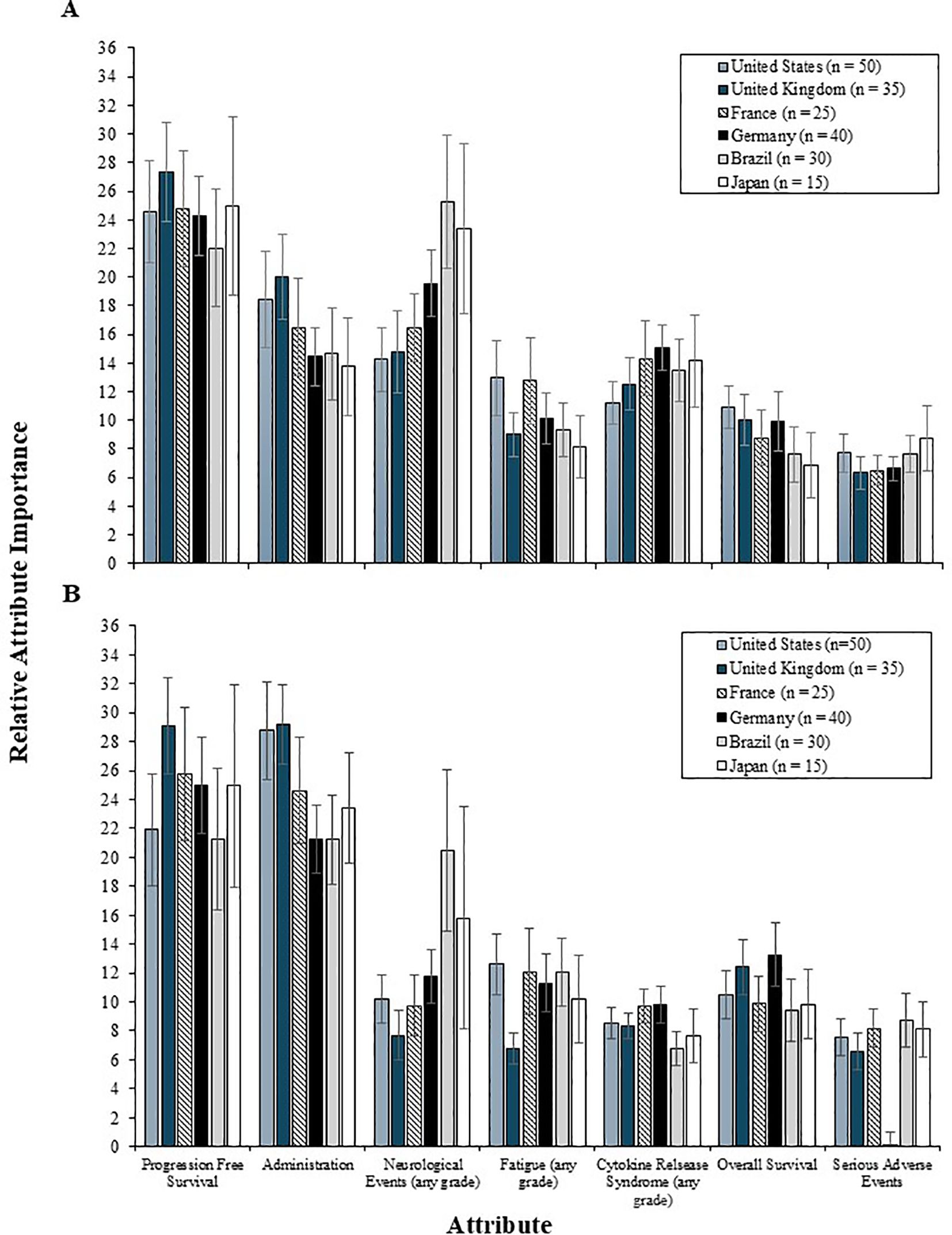
Figure 6. The relative importance of 2L (A) and 3L (B) treatment attributes among patients by country. 2L, second line; 3L, third line.
In the 3L setting, the relative importance of PFS and administration was similar to that of patients (Figure 6). Patients from the US found administration relatively more important (RI = 28.8) than PFS (RI = 21.9), whereas patients from France, Germany, and Japan found PFS most important (RI = 25.8, 25.0, and 24.9, respectively), followed by administration (RI = 24.6, 21.3, and 23.4, respectively). Patients from the UK and Brazil weighted PFS and administration similarly in importance (UK RI = 29.1 vs 29.2, respectively; Brazil RI = 21.3 vs. 21.2, respectively). Variation in the relative importance of other 3L treatment attributes was limited. However, patients from Brazil and Japan continued to value reductions in the risk of neurological events (RI = 20.5 and 15.8, respectively) more than OS and fewer side effects.
4 Discussion
We used DCE within geographically diverse samples of treating physicians and patients with R/R FL to evaluate preferences for 2L and 3L treatment attributes. Treatment preferences for physicians and patients were most influenced by PFS. Beyond PFS, patients in the aggregate placed greater emphasis on the route of administration of medications while physicians tended to focus more on five-year OS and safety profiles of agents. Preference for PFS above all other 2L and 3L treatment attributes was consistent among physicians, regardless of practice setting and country. However, variation in treatment preferences relative to country was observed among the patient cohort. Finally, this study further established that the burden of R/R FL is high among patients regarding HRQoL and WPAI. Combined, these results offer key perspectives on how physicians and patients evaluate treatment options in 2L and 3L treatment settings, understanding this information is essential to facilitate shared decision-making in an expanding and complex treatment landscape.
Our results showed physicians and patients preferred treatments with longer PFS time, regardless of the other treatment characteristics assessed. Our findings echo results from previous preference studies in R/R FL treatment. For example, Shafey and colleagues found that treatment preferences among Canadian patients and physicians were most influenced by survival free of relapse time, relative to other treatment characteristics like mode of administration, side effects, and health costs (28). Thomas et al. showed that treatment preferences among US-based patients with R/R FL and treating physicians were most influenced by increases in PFS from 6 months to 36 months (29). We extend this research by corroborating such findings with geographically diverse samples of patients and physicians. Moreover, our DCEs included five-year OS as a treatment attribute, which was also perceived as an influential treatment characteristic, particularly among physicians in the 3L setting. While both PFS and OS suggest survival benefits from treatment, longer periods of stability without disease progression, which are likely to contribute to HRQoL benefits where patients can engage in their daily lives with greater predictability (39), may explain why respondents in our study consistently identified PFS as more influential than OS. Indeed, contemporary research notes that patients prioritize holistic factors when considering their cancer care experiences, including emotional and psychological factors, like hope and opportunities to avoid suffering (40), which may speak to the nuance behind how patients view highly functional living and good health. Combined, our results suggest that PFS is a relevant endpoint for physicians and patients in the context of treatment selection and may be an important topic to discuss while engaging in shared decision-making.
Beyond PFS, physicians and patients differed in their preferences for other treatment attributes. For 2L treatments, patients prioritized safety and favored more convenient administration over five-year OS. Conversely, the influence of safety measures and five-year OS was more balanced among physicians. Five-year OS influenced 3L treatment preferences more for both patients and physicians. Yet, even in the context of 3L treatments, patients continued to place greater importance on administration. This result was largely driven by the inclusion of an administration attribute level in our 3L DCE that was designed to describe ASCT, which uniquely includes a risk of acute graft versus host disease, a condition that can require hospitalization (41). Notably, the attribute level describing ASCT was also least preferred among physicians, but the magnitude of the preference weight was significantly lower than that observed in the patient sample, suggesting that the aversion to ASCT is greater among patients than physicians. As seen in other disease settings, the attractiveness of ASCT likely decreases with increased availability of less toxic and effective. Therefore, we opted for sensitivity analysis with the ASCT level dropped from the administration attribute and found that the influence of administration on patient preferences for 3L treatment decreased, making it more like the influence of OS and risk of neurological events. As expected, administration remained the least influential 3L treatment attribute among physicians.
Compared to physicians, patients showed a stronger preference for 3L treatments with oral tablets over IV infusions. In prior research, Thomas and colleagues also noted that patients found administration/monitoring of R/R FL treatments significantly more important than physicians, and physicians prioritized administration/monitoring below safety outcomes, like CRS and laboratory abnormalities (29). Combined, these results suggest that treatment goals and expectations are likely to differ between patients with R/R FL and their physicians, particularly as they relate to the potential quality of life gains from more convenient modes of administration in later lines of treatment. Therefore, prioritizing effective communication and shared decision-making is essential when selecting appropriate treatment regimens.
Finally, this study builds upon previous literature that shows the burden of R/R FL is high (42–45). In our patient sample, the average EQ-5D index and EQ VAS were lower in each country assessed compared to previously published population norms (46, 47). Approximately one-third of patients experienced work productivity impairment in the form of absenteeism and presenteeism, suggesting that the burden of R/R FL may also have negative societal impacts. Importantly, we noted regional variations in patients’ HRQoL and WPAI measures. Patients from Japan consistently reported comparatively better outcomes across nearly all measures, possibly due to the smaller sample size of Japanese patients and the inclusion of patients who had yet to experience R/R disease due to recruitment challenges. These results underscore the complex interactions between the availability of supportive care services, healthcare systems, and cultural norms that can contribute to geographical variation in patient treatment experiences and outcomes.
4.1 Limitations
Participants were recruited via convenience sampling using an online panel that may not reflect the broader population of physicians treating R/R FL and patients with R/R FL. That said, recruitment quotas ensured key segments of the target populations were represented in study sampling. Further, whereas DCE methodology has been shown to provide rigorous and robust insight into treatment decision-making across a host of clinical settings, as with any study utilizing DCE methodology, the treatment profiles and scenarios are hypothetical and therefore may not capture the complexities in decision-making in real-world settings of R/R FL and may be further influenced by experiences and characteristics not evaluating in this study. Finally, our DCE did not include economic factors (e.g., average out-of-pocket costs) in our list of attributes, despite such factors being influential in both patient and physician treatment preferences (28). The decision to exclude such factors was due to the heterogeneity in the role of health systems and health insurance policies across countries assessed. Future studies focused on individual countries would benefit from the inclusion of economic factors in their evaluations of treatment preferences in R/R FL, as such research may further contextualize preferences for more traditional clinical treatment characteristics.
4.2 Conclusions
Selecting optimal treatment regimens that meet patients’ individual needs is essential. Our results show that treatments with superior PFS are consistently preferred by patients and physicians. However, physician and patient preferences may diverge when considering other treatment characteristics. For example, physicians may prioritize treatment efficacy in 3L settings while patients may prioritize treatment options that balance efficacy with safety and HRQoL considerations. Our findings support a shared decision-making model in the evolving treatment landscape for R/R FL to ensure individualized treatment planning and optimization. More research is needed to understand the risk/benefit ratio of novel treatment approaches, particularly with the introduction of bispecific antibodies and CAR T treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Sterling Institutional Review Board, (IRB ID: 10589-AMartin01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JG: Writing – review & editing. EB: Writing – review & editing. MR: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. KK: Methodology, Project administration, Supervision, Writing – original draft. KB: Conceptualization, Methodology, Supervision, Writing – review & editing. LK: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. SB: Writing – review & editing. TB: Writing – review & editing. GB: Writing – review & editing. OW: Formal analysis, Methodology, Writing – review & editing. MP: Project administration, Writing – review & editing. AP: Supervision, Writing – review & editing. PG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by Kite, a Gilead Company.
Acknowledgments
Thanks to Ashley Martin, Patrick Olsen, and Kacper Perkowski for valuable contributions to the conceptualization and execution of the study design and analysis plan. The study team would also like to thank the patients and physicians who participated in this study for the valuable time and perspectives. Medical writing support was provided by Errol J. Philip of Precision Health California and funded by Kite, a Gilead Company.
Conflict of interest
Authors MR, SB, TB, GB, MP, and AP were employed by the company Kite, a Gilead company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Kite, a Gilead company. The funder had the following involvement in the study: study design, interpretation of data, writing of this article, and decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1589722/full#supplementary-material
References
2. Johnson PC, Bailey A, Ma Q, Milloy N, Biondi E, Quek RGW, et al. Quality of life evaluation in patients with follicular cell lymphoma: A real-world study in europe and the United States. Adv Ther. (2024) 41:3342–61. doi: 10.1007/s12325-024-02882-1
3. Oerlemans S, Issa DE, Van Den Broek EC, Nijziel MR, Coebergh JW, Mols F, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol. (2014) 93:229–38. doi: 10.1111/ejh.2014.93.issue-3
4. Pettengell R, Donatti C, Hoskin P, Poynton C, Kettle PJ, Hancock B, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. (2008) 19:570–6. doi: 10.1093/annonc/mdm543
5. Wasse SK, Mounier M, Assogba E, Rossi C, Adnet J, Gauthier S, et al. Factors affecting health-related quality of life among survivors of non-hodgkin lymphoma: A population-based study. Cancers (Basel). (2023) 15(15):3885. doi: 10.3390/cancers15153885
6. Cheung MC, Imrie KR, Friedlich J, Buckstein R, Lathia N, and Mittmann N. The impact of follicular (FL) and other indolent non-Hodgkin’s lymphomas (NHL) on work productivity-a preliminary analysis. Psychooncology. (2009) 18:554–9. doi: 10.1002/pon.v18:5
7. Foster T, Miller JD, Boye ME, and Russell MW. Economic burden of follicular non-Hodgkin’s lymphoma. Pharmacoeconomics. (2009) 27:657–79. doi: 10.2165/11314820-000000000-00000
8. Morrison VA, Bell JA, Hamilton L, Ogbonnaya A, Shih HC, Hennenfent K, et al. Economic burden of patients with diffuse large B-cell and follicular lymphoma treated in the USA. Future Oncol. (2018) 14:2627–42. doi: 10.2217/fon-2018-0267
9. Ren J, Asche CV, Shou Y, and Galaznik A. Economic burden and treatment patterns for patients with diffuse large B-cell lymphoma and follicular lymphoma in the USA. J Comp Eff Res. (2019) 8:393–402. doi: 10.2217/cer-2018-0094
10. Press OW, Unger JM, Rimsza LM, Friedberg JW, Leblanc M, Czuczman MS, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. (2013) 31:314–20. doi: 10.1200/JCO.2012.42.4101
11. Shadman M, Li H, Rimsza L, Leonard JP, Kaminski MS, Braziel RM, et al. Continued excellent outcomes in previously untreated patients with follicular lymphoma after treatment with CHOP plus rituximab or CHOP plus (131)I-tositumomab: long-term follow-up of phase III randomized study SWOG-S0016. J Clin Oncol. (2018) 36:697–703. doi: 10.1200/JCO.2017.74.5083
12. Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. (2020) 10:74. doi: 10.1038/s41408-020-00340-z
13. Fuji S, Tada Y, Nozaki K, Saito H, Ozawa T, Kida T, et al. A multi-center retrospective analysis of patients with relapsed/refractory follicular lymphoma after third-line chemotherapy. Ann Hematol. (2020) 99:2133–9. doi: 10.1007/s00277-020-04126-y
14. Rivas-Delgado A, Magnano L, Moreno-Velázquez M, García O, Nadeu F, Mozas P, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. (2019) 184:753–9. doi: 10.1111/bjh.2019.184.issue-5
15. Townsend W, Hiddemann W, Buske C, Cartron G, Cunningham D, Dyer MJS, et al. Obinutuzumab versus rituximab immunochemotherapy in previously untreated iNHL: final results from the GALLIUM study. Hemasphere. (2023) 7:e919. doi: 10.1097/HS9.0000000000000919
16. Morabito F, Martino EA, Nizzoli ME, Talami A, Pozzi S, Martino M, et al. Comparative analysis of bispecific antibodies and CAR T-cell therapy in follicular lymphoma. Eur J Haematol. (2024) 114(1):4–16. doi: 10.1111/ejh.14335
17. Gupta G, Garg V, Mallick S, and Gogia A. Current trends in diagnosis and management of follicular lymphoma. Am J Blood Res. (2022) 12:105–24.
18. Mühlbacher AC and Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. (2013) 11:163–80. doi: 10.1007/s40258-013-0023-3
19. Mühlbacher AC, Juhnke C, Beyer AR, and Garner S. Patient-focused benefit-risk analysis to inform regulatory decisions: the european union perspective. Value Health. (2016) 19:734–40. doi: 10.1016/j.jval.2016.04.006
20. Shickh S, Leventakos K, Lewis MA, Bombard Y, and Montori VM. Shared decision making in the care of patients with cancer. Am Soc Clin Oncol Educ Book. (2023) 43:e389516. doi: 10.1200/EDBK_389516
21. Fifer S, Ordman R, Briggs L, and Cowley A. Patient and clinician preferences for genetic and genomic testing in non-small cell lung cancer: A discrete choice experiment. J Pers Med. (2022) 12(6):879. doi: 10.3390/jpm12060879
22. Fong J, Venables M, D’souza D, and Maskerine C. Patient communication preferences for prostate cancer screening discussions: A scoping review. Ann Fam Med. (2023) 21:448–55. doi: 10.1370/afm.3011
23. González JM, Ogale S, Morlock R, Posner J, Hauber B, Sommer N, et al. Patient and physician preferences for anticancer drugs for the treatment of metastatic colorectal cancer: a discrete-choice experiment. Cancer Manag Res. (2017) 9:149–58. doi: 10.2147/CMAR.S125245
24. Herrmann A, Sanson-Fisher R, Hall A, Wall L, Zdenkowski N, and Waller A. A discrete choice experiment to assess cancer patients’ preferences for when and how to make treatment decisions. Support Care Cancer. (2018) 26:1215–20. doi: 10.1007/s00520-017-3944-9
25. Stone RL, Cambron-Mellott MJ, Beusterien K, Maculaitis MC, Ritz S, Mulvihill E, et al. Patients’ and oncologists’ preferences for second-line maintenance PARP inhibitor therapy in epithelial ovarian cancer. Future Oncol. (2022) 18:491–503. doi: 10.2217/fon-2021-0567
26. Yeo HY, Liew AC, Chan SJ, Anwar M, Han CH, and Marra CA. Understanding patient preferences regarding the important determinants of breast cancer treatment: A narrative scoping review. Patient Prefer Adherence. (2023) 17:2679–706. doi: 10.2147/PPA.S432821
27. Zhang M, He X, Wu J, and Xie F. Differences between physician and patient preferences for cancer treatments: a systematic review. BMC Cancer. (2023) 23:1126. doi: 10.1186/s12885-023-11598-4
28. Shafey M, Lupichuk SM, Do T, Owen C, and Stewart DA. Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplant. (2011) 46:962–9. doi: 10.1038/bmt.2010.225
29. Thomas C, Marsh K, Trapali M, Krucien N, Worth G, Cockrum P, et al. Preferences of patients and physicians in the United States for relapsed/refractory follicular lymphoma treatments. Cancer Med. (2024) 13:e70177. doi: 10.1002/cam4.v13.19
30. Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR conjoint analysis good research practices task force. Value Health. (2016) 19:300–15. doi: 10.1016/j.jval.2016.04.004
31. Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. (2013) 16:3–13. doi: 10.1016/j.jval.2012.08.2223
32. Szinay D, Cameron R, Naughton F, Whitty JA, Brown J, and Jones A. Understanding uptake of digital health products: methodology tutorial for a discrete choice experiment using the bayesian efficient design. J Med Internet Res. (2021) 23:e32365. doi: 10.2196/32365
33. Reilly MC, Zbrozek AS, and Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. (1993) 4:353–65. doi: 10.2165/00019053-199304050-00006
34. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) 20:1727–36. doi: 10.1007/s11136-011-9903-x
35. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. (1993) 11:570–9. doi: 10.1200/JCO.1993.11.3.570
36. Hlubocky FJ, Webster K, Beaumont J, Cashy J, Paul D, Abernethy A, et al. A preliminary study of a health related quality of life assessment of priority symptoms in advanced lymphoma: the National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy - Lymphoma Symptom Index. Leuk Lymphoma. (2013) 54:1942–6. doi: 10.3109/10428194.2012.762977
37. Hlubocky FJ, Webster K, Cashy J, Beaumont J, and Cella D. The development and validation of a measure of health-related quality of life for non-hodgkin’s lymphoma: the functional assessment of cancer therapy—Lymphoma (FACT-lym). Lymphoma. (2013) 2013:147176. doi: 10.1155/2013/147176
38. Orme BK. Getting started with conjoint analysis: strategies for product design and pricing research. Madison (WI: Research Publishers LLC (2010).
39. Booth CM and Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. (2012) 10:1030–3. doi: 10.1200/JCO.2011.38.7571
40. Corn BW, Feldman DB, and Wexler I. The science of hope. Lancet Oncol. (2020) 9:e452–9. doi: 10.1016/S1470-2045(20)30210-2
41. Balassa K, Danby R, and Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med (Lond). (2019) 80:33–9. doi: 10.12968/hmed.2019.80.1.33
42. Ahlschlager L, Mccabe S, Deal AM, Guo A, Gessner KH, Lipman R, et al. The effect of treatment on work productivity in patients with bladder cancer. Urol Oncol. (2023) 41:293.e15–293.e21. doi: 10.1016/j.urolonc.2023.01.020
43. Cleeland CS, Mayer M, Dreyer NA, Yim YM, Yu E, Su Z, et al. Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: Results from a substudy of the VIRGO observational cohort study. Breast. (2014) 23:763–9. doi: 10.1016/j.breast.2014.08.004
44. Ohno S, Chen Y, Sakamaki H, Matsumaru N, and Tsukamoto K. A population-based study of the humanistic burden among cancer patients in Japan. J Med Econ. (2020) 23:429–41. doi: 10.1080/13696998.2019.1707213
45. Wood R, Taylor-Stokes G, Smith F, and Chaib C. The humanistic burden of advanced non-small cell lung cancer (NSCLC) in Europe: a real-world survey linking patient clinical factors to patient and caregiver burden. Qual Life Res. (2019) 28:1849–61. doi: 10.1007/s11136-019-02152-6
46. Janssen BEA. Population norms for the EQ-5D. In: Szende AJB and Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Springer, Dordrecht (NL (2014).
Keywords: treatment preferences, relapsed/refractory, follicular lymphoma, discrete choice experiment, progression-free survival
Citation: Gribben JG, Bachy E, Ray M, Krupsky K, Beusterien K, Kopenhafer L, Beygi S, Best T, Ball G, Will O, Palivela M, Patel A and Ghione P (2025) Patient and physician treatment preferences in relapsed/refractory follicular lymphoma: a discrete choice experiment in the United States, United Kingdom, France, Germany, Brazil, and Japan. Front. Oncol. 15:1589722. doi: 10.3389/fonc.2025.1589722
Received: 07 March 2025; Accepted: 02 June 2025;
Published: 10 July 2025.
Edited by:
Tadeusz Robak, Medical University of Lodz, PolandReviewed by:
Guido Gini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyNishma Patel, University College London, United Kingdom
Copyright © 2025 Gribben, Bachy, Ray, Krupsky, Beusterien, Kopenhafer, Beygi, Best, Ball, Will, Palivela, Patel and Ghione. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John G. Gribben, ai5ncmliYmVuQHFtdWwuYWMudWs=
 John G. Gribben
John G. Gribben Emmanuel Bachy
Emmanuel Bachy Markqayne Ray3
Markqayne Ray3 Kathryn Krupsky
Kathryn Krupsky Timothy Best
Timothy Best Madhu Palivela
Madhu Palivela