- 1Department of Pediatrics, Blood and Marrow Transplantation and Cellular Therapy, King Hussein Cancer Center, Amman, Jordan
- 2Adult Blood and Marrow Transplantation and Cellular Therapy Program, King Hussein Cancer Center, Amman, Jordan
- 3Faculty of Medicine, University of Jordan, Amman, Jordan
- 4Department of Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, Memphis, TN, United States
Introduction: Survival post-hematopoietic stem cell transplantation (HCT) is improving, with an increasing number of survivors. Subsequent neoplasms (SNs) following HCTs are of particular concern.
Methods: Between January 2003 and December 2022, HCT recipients’ records were retrospectively reviewed.
Results: At a median follow-up of 108 months (range, 0.13-215), 2659 patients received HCTs. Of those, 1131 (43%) were <18 years old. Allogeneic HCTs were conducted in 1476 (56%) patients. Myeloablative conditioning (MAC) was utilized in 2157 (81%), and 583(22%) received TBI. At a median of 9 years following transplant, forty-three patients developed SNs (1.6%) with a median age at time of HCT of 27.6 years (range, 2.8-64.8). Of those: 32 were males (74%), 20 received full HLA-matched allogeneic HCTs (46.5%), two (4.6%) had unrelated cord blood HCT (UCB), and one (2.3%) received haplo-HCT, whereas autologous HCTs accounted for 46.6% (n=20). Underlying diseases were: ALL(13.9%), AML(11.6%), Hodgkin Lymphoma(13.9%), Non-Hodgkin lymphoma(13.9%), Multiple Myeloma(18.6%), Fanconi Anemia(6.9%), CML(6.9%),Neuroblastoma(2.3%), and thalassemia (2.3%).); cGVHD occurred in (74%), and CMV infection/reactivation in (60.5%). Stem cell source included peripheral blood in (81.4%), BM in (3.9%), and UCB in (4.7%). Conditioning regimens were MAC (81.4%) vs RIC (18.6%). TBI-based regimen was utilized in 14 patients (32.5%). Subsequent hematologic malignancies accounted for 32.5% of SNs. While subsequent solid neoplasms occurred in 65.2%, and PTLD occurred in 2.3%. The probability of 5-year overall survival after a SN was 58.2%.
Conclusions: SNs adversely impact the overall survival and quality of life of HCT survivors. In our cohort, the rate of post-HCT SNs was lower than that in the literature; however, longer follow-up of our cohort is needed.
Introduction
Hematopoietic cell transplantation (HCT) is a curative modality for many malignancies and nonmalignant disorders. Significant advances in HCT have been attained. Consequently, more HCT recipients are becoming long-term survivors (1–3). As survivors age, their risk of experiencing post-HCT late effects increases.
HCT survivors face unique long-term challenges after their primary disease has been cured (3). In this setting, subsequent neoplasms (SNs) are among the biggest challenges that can develop after autologous or allogeneic HCT. Although rare, the impact of a SN is concerning due to its high mortality rates (4, 5). In fact, SNs account for 12%-27% of deaths among long-term HCT survivors (6, 7).
The relative risk associated with SNs in HCT recipients is influenced by patient- and treatment-related factors, with estimates ranging from a 2- to 10-fold increased risk (8). Cumulative incidence (CI) varies with follow-up duration: at 10 years, CIs range 2.2%-6.4% (9, 10); at 15 years, 10%-12% (11); and at 20 years, 6.9%-8.6% (12). The CI of a subsequent solid neoplasm (SSN) developing does not appear to plateau and was 3.8% (95% CI, 2.2-5.4) at 20 years post-HCT (12). Moreover, the CI of SNs at 5, 10, and 15 years post-HCT in survivors who received total body irradiation (TBI) conditioning was 0.7%, 2.2%, and 6.7%, respectively, compared to that of SNs at the same time points among those who did not receive TBI, which was 0.3%, 0.6%, and 0.8%, respectively (13). The risk of SNs becomes apparent 5 years post-HCT (4), and the incidence rises over time; several studies with 20-year follow-up have not shown any plateau in SN occurrence (3, 4, 14, 15).
SNs can be categorized into post-HCT Epstein-Barr virus–related B-cell lymphoproliferative diseases (PTLD), acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) after autologous HCT for lymphoma, and SSNs (3, 16–18). PTLD and AML/MDS develop early post-HCT, but SSNs have a longer latency (19). Complex interplay among host, tumor, and environmental characteristics causes SNs to develop (20, 21). These include younger age at HCT (22), higher TBI dose (22–24), genetics (19), graft T-cell depletion, anti–thymocyte globulin use, human leukocyte antigen (HLA)-mismatched donors, chronic graft-versus-host disease (cGVHD), primary disease, and high-intensity chemotherapy and radiotherapy in primary treatment and conditioning regimens (25–27). Furthermore, an immunocompromised state post-HCT and viral infection are associated with increased risk (16, 28). Similarly, cyclophosphamide use is a risk factor for SNs, independent of TBI (29).
Outcomes of patients with SNs have been described by several groups (3, 6, 12, 30). The 5-year overall survival (OS) is 42%-44% after aSN diagnosis (12, 30). In one report, the median OS was 15.7 months for patients with SNs that developed after reduced-intensity conditioning (RIC); OS was 66.4%, 53.3%, and 30.6% at 6, 12, and 60 months, respectively (9).
Here we reviewed the incidence, risk factors, and potential outcomes of SNs among pediatric and adult recipients of HCTs at King Hussein Cancer Center (KHCC) during an 18-year period.
Methods
Patient population and data source
Data from 2659 pediatric and adult HCT recipients at KHCC (January 2003–December 2022) were retrospectively reviewed and extracted from the KHCC-HCT Program database and patients’ electronic medical records. The study was conducted in compliance with the KHCC Institutional Review Board’s approval (approval number: [17 KHCC 63], dated [10-May-2017]).
Variables included age at HCT, stem cell source [i.e., bone marrow, peripheral blood stem cells (PBSCs), or umbilical cord blood (UCB)], donor type (related or unrelated), and conditioning regimen intensity. TBI use in conditioning regimens included no TBI, 200 cGy, or 1200 cGy. The timing of HCT was grouped into two eras: 2003–2010 vs 2010–2022. HCT indications were grouped into malignant disorders [i.e., AML, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), MDS, lymphomas, and solid tumors] and nonmalignant disorders. SN documentation included neoplasm type and treatment received (i.e., chemotherapy and/or radiotherapy and palliation). Age at SN diagnosis, time from HCT to SN diagnosis, and presence of active GVHD at the time of SN diagnosis were also captured.
Conditioning regimens and GVHD prophylaxis
Conditioning regimen intensity was classified as myeloablative conditioning (MAC) or RIC based on criteria from the Center for International Blood and Marrow Transplant Research (31). GVHD prophylaxis consisted of calcineurin inhibitors combined with methotrexate, mycophenolate mofetil, and/or alemtuzumab, or anti–thymocyte globulin. A combination of methotrexate and prednisone was also used in some patients.
Post-HCT follow-up, evaluations, and screening:
After their HCT, patients were monitored at KHCC and late effects clinics; they were followed indefinitely, per international guidelines (32, 33). All SNs were confirmed by biopsies, and biopsy specimens were examined by an experienced pathologist at KHCC. Patients whose primary disease was acute or chronic leukemia or AML/MDS had to have a different immunophenotype, FAB subtype, or conventional karyotype for the new tumor to be classified a SN.
Statistical analyses
Descriptive statistics were used to summarize patient and HCT characteristics at baseline. The main outcome variables of interest were the CI of SNs and OS. CIs of SNs were calculated using the Kaplan-Meier method at various time points post-HCT, with corresponding 95% CIs. The incidence of SNs was determined by dividing the total number of events by the total number of patient-years.
Chi-square statistics were used to assess significant differences in the distribution of parameters between patients who experienced SNs and those who did not. Time-to-event analysis was conducted to determine survival time from the date of HCT or SN diagnosis. OS probabilities and median survival times were estimated using the Kaplan-Meier estimator. The CI of death due to SNs, considering death by all causes, was also calculated.
Cox proportional-hazards models were used to evaluate the impact of various factors on OS. Univariate and multivariate Cox-regression analyses were performed to measure the association between potential risk factors and SN development, and the influence of SNs on OS. Hazard ratios with corresponding 95% CIs were calculated to assess the strength of these associations. Covariates included in multivariate analyses were selected based on their significance in univariate analyses and their clinical relevance. The following variables were examined as potential risk factors for SNs developing after HCT: age at HCT, sex, initial diagnosis, disease duration, latency between HCT and SN, prior radiotherapy, conditioning regimen intensity, use of TBI-based conditioning regimens compared to non-TBI regimens, stem cell source, and donor type. Gray’s test was used to compare the cumulative incidence of SNs between subgroups, including age and gender. A p-value of less than 0.05 was considered statistically significant.
Result
Patient characteristics
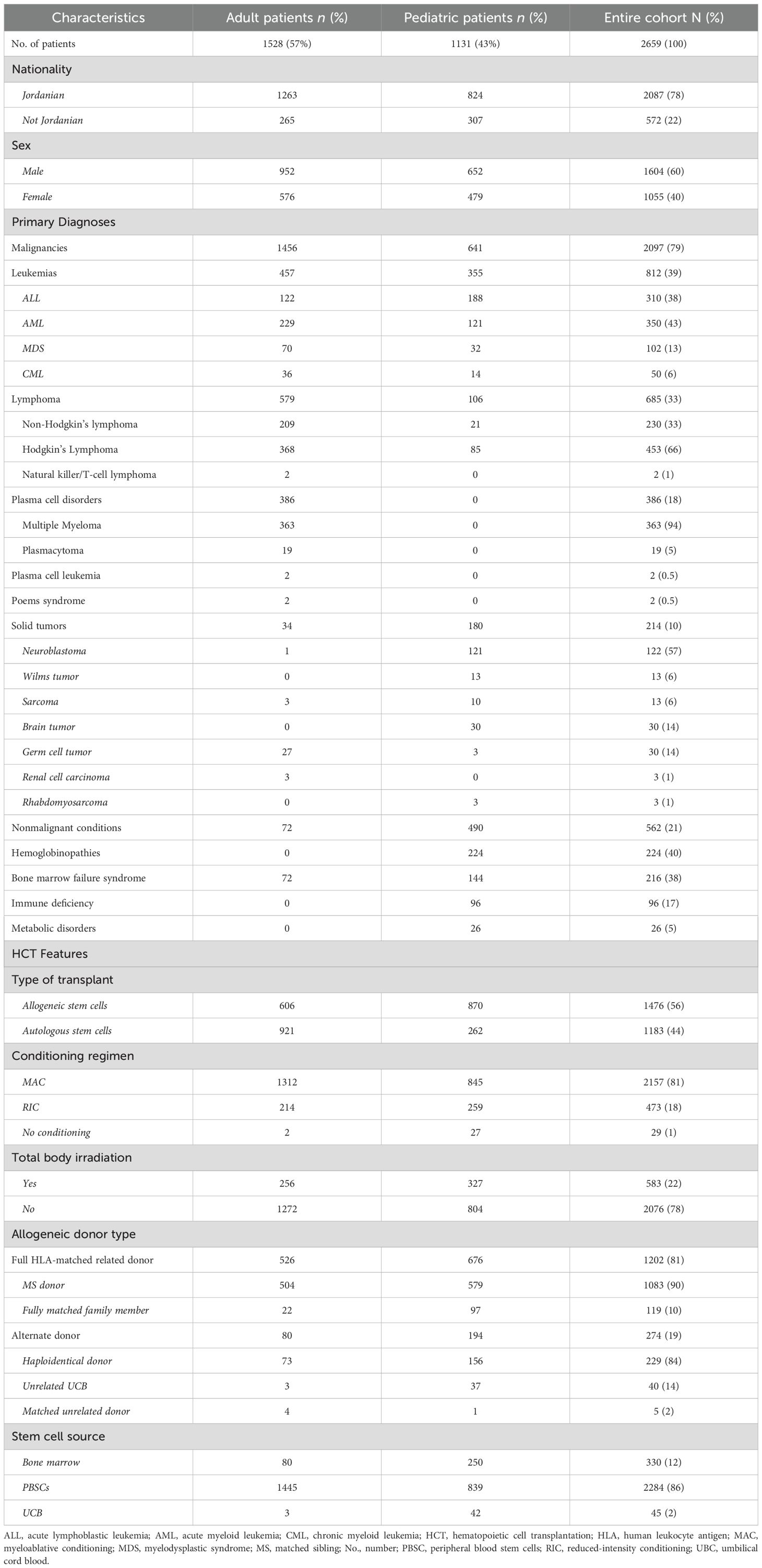
At median follow-up of 108 months (range, 0.13–215 months), 2659 patients were retrospectively reviewed: 1131 (43%) were younger than 18 years at the time of their HCT; median age at the time of HCT was 27.6 years (range, 2.8-64.8). In the entire cohort, 1604 (60%) were males, and 2087 (78%) were Jordanians. Malignant diseases accounted for 2097 (79%) indications for HCT (Table 1). Of the 2097 patients who had a primary malignant disease, 1883 (90%) received HCT for hematologic malignancies: 310 (14.8%), for ALL; 350 (16.7%) for AML; 50 (2.4%) for CML; 102 (4.9%) for MDS; 386 (18.3%) for plasma cell disorders of which the majority were due to multiple myeloma (363/386 (17.3%)); and 685 (32.7%) for lymphoma, the majority of which were for Hodgkin’s lymphoma (453/685 (21.6%)). Solid tumors accounted for 214 (10.2%) of the malignant indications for HCT, whereas the nonmalignant conditions accounted for 562 (21%) (Table 1).
Hematopoietic cell transplantation features and outcomes
Allogeneic HCTs were conducted in 1476 (56%) patients; median time from diagnosis to HCT was 1.3 months (range, 0.4–101 months). MAC conditioning was utilized in 2157 (81%) cases, and TBI-based conditioning was administered in 583 (22%) cases. Leukemia (812, 39% of malignant conditions) was the most common indication for allogeneic HCT. Full HLA-matched related donors were used in 1202 (81%) HCTs, and alternate donors were used in 274 (19%) HCTs: 229 (84%) involved a haploidentical donor, 40 (14%) used UCB and 5 (2%) matched unrelated donors. PBSCs were the predominant source (2284, 86%), followed by bone marrow (330, 12%) and UCB (45, 2%) (Table 1). CGVHD was reported in 243 (16%) patients who underwent Allogeneic HCTs.
Among the 2,659 patients included in the study, a total of 1,166 patients (43.8%) experienced either persistent or relapsed disease following HCT. Of the entire cohort, 222 patients (8.3%) underwent more than one transplant, either due to disease relapse or graft failure. Among the 43 patients who developed SNs, only 4 had undergone more than one transplant: 3 patients with multiple myeloma and 1 patient with Fanconi anemia. The remaining majority received a single transplant as part of their treatment course.
Median OS of the entire cohort was 115 months (95% CI, 13.7–216 months); the 5-year OS was 89.3% (95% CI, 87.9-90.8), and the 10-year OS was 75% (95% CI, 73-77). There was no difference in the risks of SN developing related to the type of HCT (allogeneic vs autologous), intensity of conditioning regimens (RIC vs MAC), or alkylators use.
Risk of subsequent neoplasms
At a median of 8.98 years (range, 0.13–72 years) post-HCT, SNs occurred in 43 (1.6%) patients (Table 2). Median age at SN diagnosis was 21 years (range, 0.2–66) for the entire cohort: 8 years (1.95–17.7) for pediatric patients and 44.8 years (21.7-63) for adult patients. Twenty-eight (65%) patients were older than 18 years, and 32 (74%) were male. Twenty-three (53%) patients received allogeneic and 20 (47%) autologous HCTs, respectively. Twenty (87%) allogeneic HCTs were from full HLA-matched donors; 2 (8.6%) were from UCB, and 1 (4.4%) was from an HLA-mismatched donor. Although the difference between adult and pediatric patients’ CI of SNs (Figure 1A) did not reach statistical significance (p= 0.133), adult patients had a progressively increased risk over time, rising from 0.1% at 3 years to 6.2% at 15 years post-transplant. Pediatric patients demonstrated a more stable pattern, with a CI of 0.3% at both 3 and 5 years, increasing to 2.6% at 15 years. No SNs were observed in either group within the first year post-transplant. Gender-based analysis also showed no statistically significant difference in the CI of SNs between males and females (p= 0.472). By 15 years post-HCT, the CI was 5.2% (95% CI: 1.2%–9.1%) in females and 4.2% (95% CI: 1.6%–6.8%) in males. The CI of SNs for the entire cohort was 1.3%. The probability of incidence of SNs at 5, 10, and 15 years after HCT was 0.3%, 1.3%, and 4.6%, respectively (Figure 1B). The underlying indication for the majority (79%) of the HCTs performed at KHCC during the study period was malignancy. Of the 43 patients in whom post-HCT SNs developed, 6 (13.9%) had ALL, 5 (11.6%) had AML, 3 (7%) had CML, 1 (2.3%) had idiopathic myelofibrosis, 12 (28%) had lymphoma, 8 (18.6%) multiple myeloma, 2 (4.7%) had aplastic anemia, and 2 (4.6%) had solid tumors. In 4 (9.3%) pediatric patients, the indication for HCT was a nonmalignant condition: 3 (7%) had Fanconi anemia, and 1 (2.3%) had thalassemia (Figure 2A). TBI-based regimens were utilized in 14 (32.5%) patients; cGVHD occurred in 17 of 23 Allogenic HCT (74%), and cytomegalovirus infection/reactivation occurred in 26 (60.5%) patients. Sources of stem cells included PBSCs in 35 (81.4%) patients, BM in 6 (13.9%), and UCB in 2 (4.7%). MAC was administered to 35 (81.4%) patients, and RIC to 8 (18.6%). Median follow-up from the time of SN diagnosis was 10.3 years (range, 0.41–15.9 years). There was no clear temporal pattern of SN development among diseases.
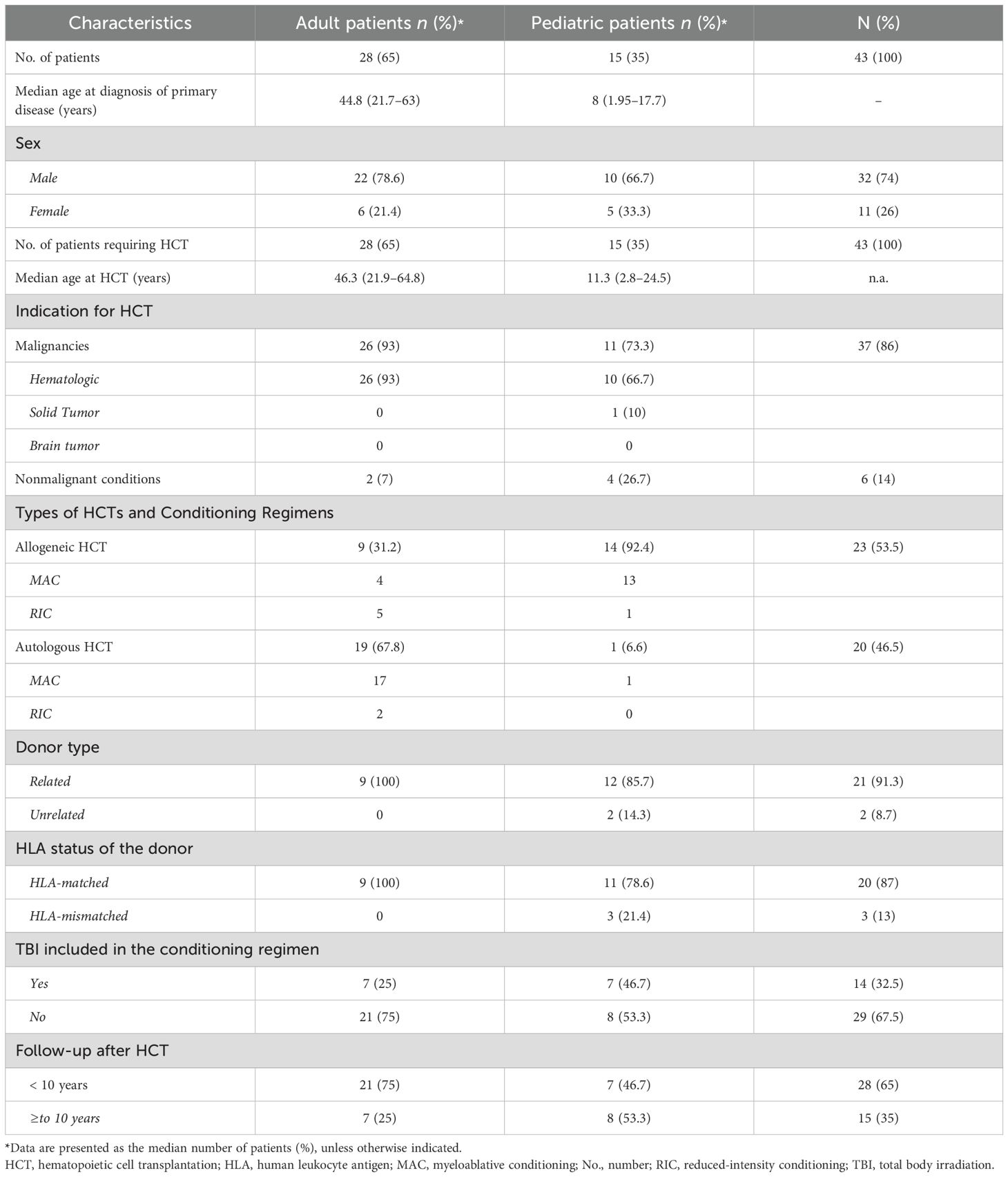
Table 2. Characteristics of patients and features of HCTs after which a subsequent neoplasm developed.
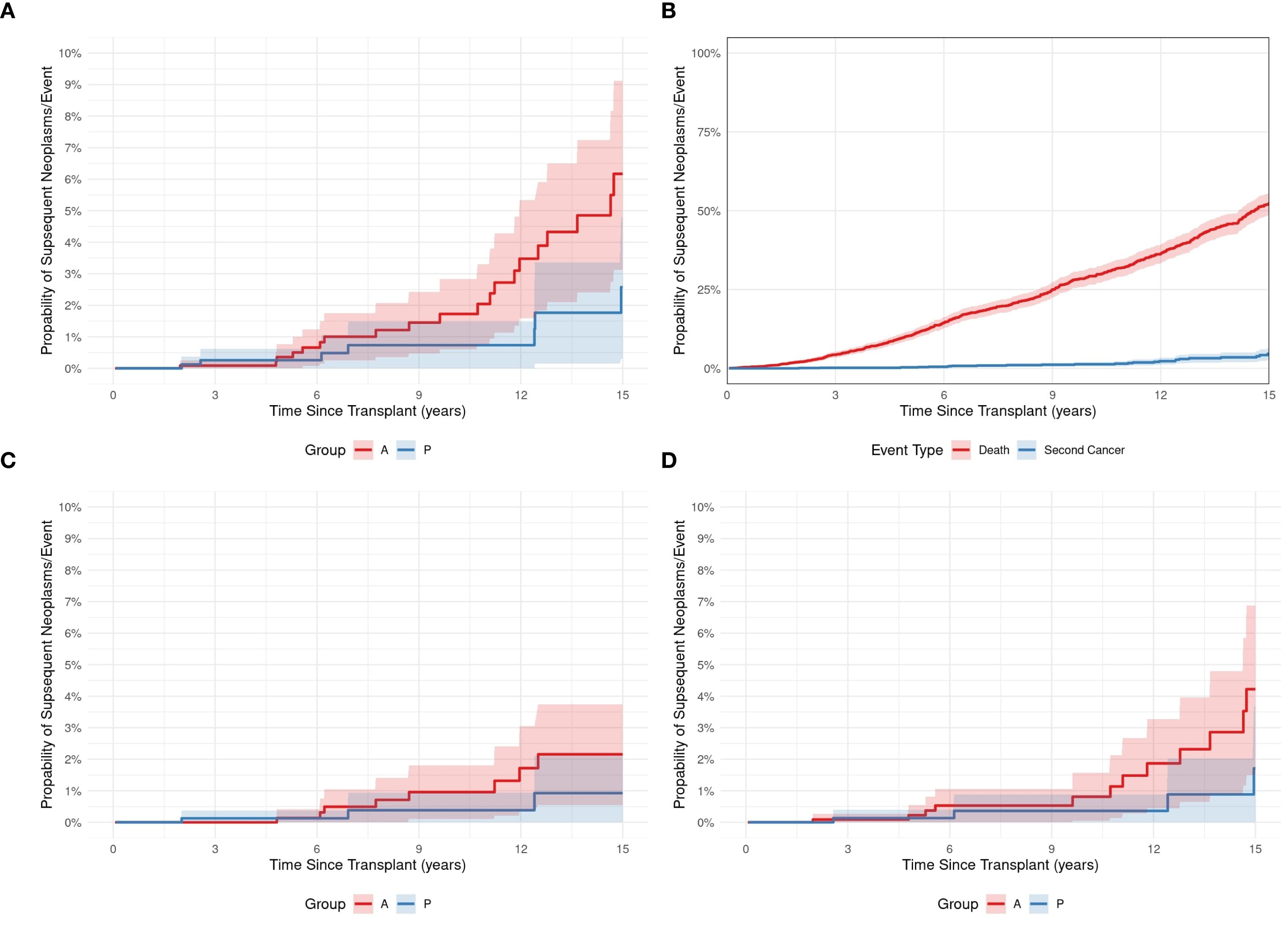
Figure 1. (A) The cumulative incidence of subsequent neoplasms in pediatric (P) and adult (A) HCT recipients. (B) The cumulative incidence of SN development (Blue plot) and death (red plot) after a subsequent neoplasm in 43 pediatric and adult HCT recipients. (C, D) The cumulative incidences of hematologic malignancy (C) or solid tumors (D) developing as a subsequent neoplasm after HCT in pediatric (P) and adult (A) patients.
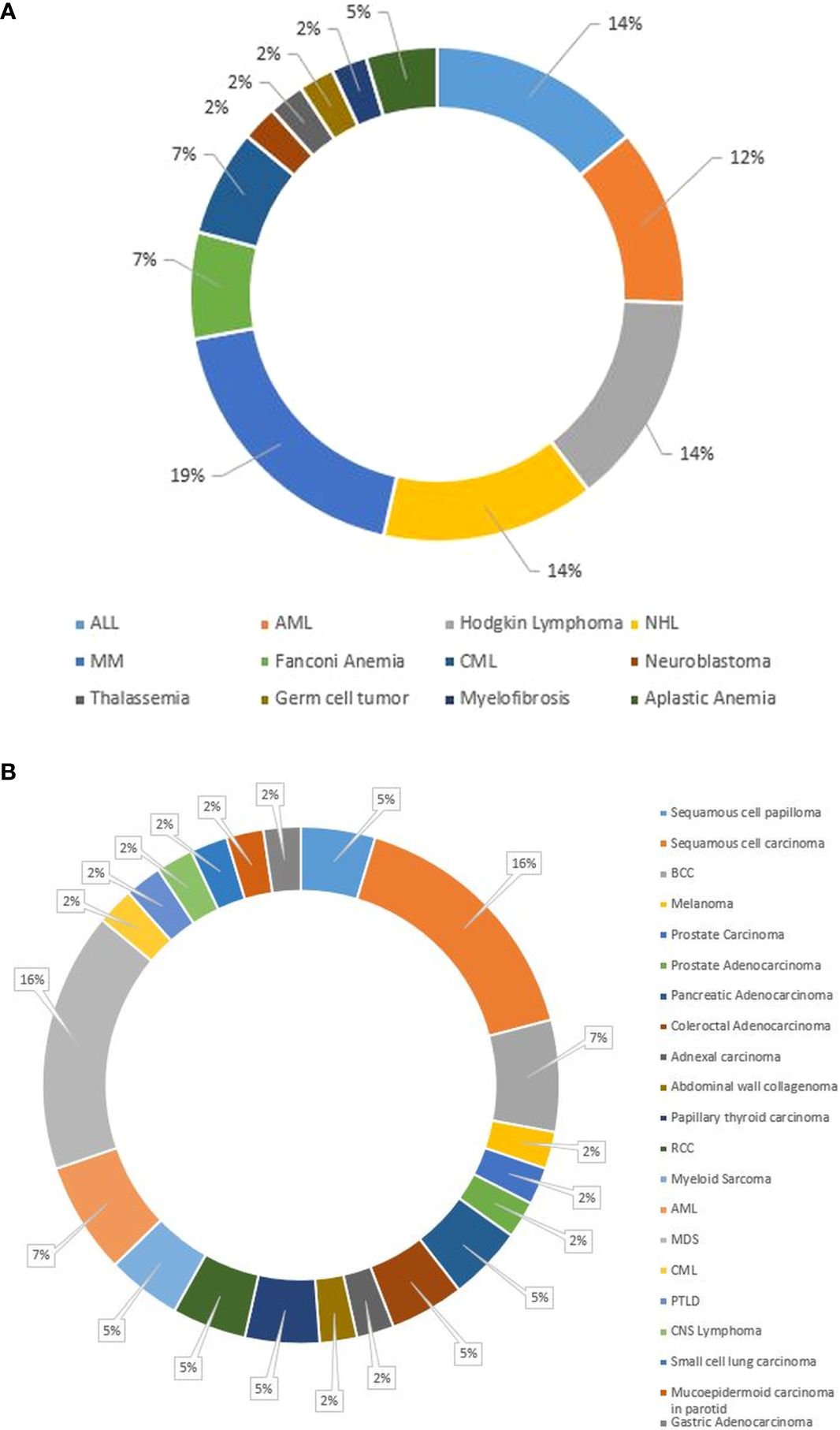
Figure 2. (A) Pie chart of primary diagnoses of the 43 pediatric and adult patients who experienced a subsequent neoplasm after HCT. (B) Pie chart showing the subsequent neoplasm diagnoses in our cohort of pediatric and adult HCT recipients. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCC, basal cell carcinoma; CML, chronic myeloid leukemia; CNS, central nervous system; HCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; PTLD, Post-transplant lymphoproliferative disease; RCC, renal cell carcinoma.
Subsequent hematologic malignancies
Subsequent hematologic malignancies (SHMs) developed in 14 (32.5%) patients (Figure 2B) at a median age of 40.7 years. SHMs were mostly of myeloid origin (3 AML, 1 CML and 7 MDS) (Figure 2B), with a median onset time from HCT of 2.84 years (Table 3). Eight (57%) patients received etoposide in their conditioning regimens, and 2 (14%) received TBI. Twelve (85.7%) patients received MAC before HCT. Ten (71.4%) received an autologous HCT, and 4 (28.6%) received an allogeneic HCT.
Table 3. Characteristics of subsequent neoplasms that developed in pediatric and adult recipients after HCT.
Subsequent solid neoplasms
Subsequent solid neoplasms (SSNs) developed in most 28 (65%) patients with SNs (Figure 2B), at median time to onset from HCT of 6.8 years (Table 3). SSNs were mainly carcinoma (85%, n=24): 7 (25%), squamous cell carcinoma (SCC); 6 (21.4%), adenocarcinoma; 3 (10.7%), Basal cell carcinoma; 4 (14.3%), carcinoma; 2 (7.1%) each renal cell carcinoma, Thyroid papillary carcinoma, and squamous papilloma; and 1 (3.6%) each, melanoma and collagenoma (Figure 2B).
SSNs arose in patients with a median age at the time of HCT of 23.9 years and mainly in those with leukemia [6 (14%), ALL; 5 (12%), AML; and 2 (5%), CML] or lymphoma [3 (11%) non-Hodgkin lymphoma, and 1 Hodgkin lymphoma (Figure 2A). Most transplants were allogeneic: 13 from matched sibling donors, 3 from matched related donors (MRDs), and 2 UCB. Twenty-two (79%) patients received MAC, 12 (43%) received TBI, and 4 (14%) received etoposide in their conditioning. Characteristics of patients with SSNs are summarized in Table 3.
Of the entire 43 patients who developed subsequent neoplasms, only one patient, whose primary diagnosis was aplastic anemia, developed PTLD at 0.13 years post HCT (Table 3).
Outcome of SNs
Among the 43 patients with SNs, 25 (58%) were alive at last follow-up, and 18 (42%) had died of SNs. The 5-year OS after SN development was 58%. There was no difference in OS between patients who had received TBI vs no-TBI (p = 0.258) (Figure 3A), nor between patients with SHM vs SSNs (p = 0.208) (data not shown). There was also no difference in those who received RIC vs MAC, (p = 0.113) (Figure 3C), or with vs. without etoposide (p = 0.251) (Figure 3D). While the cumulative incidence of second malignancies differed significantly between patients who underwent allogeneic (Allo) and autologous (Auto) HCT. Among Allo recipients, the cumulative incidence increased from 0.0% at 1 year to 0.2% (95% CI: 0.0%-0.5%) at 3 years, 0.3% (95% CI: 0.0%-0.7%) at 5 years, 0.7% (95% CI: 0.1%-1.3%) at 10 years, and 2.6% (95% CI: 1.2%-4.0%) at 15 years post-transplant. In contrast, Auto recipients showed a steeper increase in cumulative incidence, starting at 0.0% at 1 year, reaching 0.1% (95% CI: 0.0%-0.3%) at 3 years, 0.3% (95% CI: 0.0%-0.6%) at 5 years, 2.1% (95% CI: 1.0%-3.2%) at 10 years, and peaking at 7.4% (95% CI: 5.1%-9.7%) at 15 years. (P = 0.013), indicating that patients undergoing Auto transplants had a higher cumulative incidence of second malignancies compared to those receiving Allo transplants (Figure 3B).
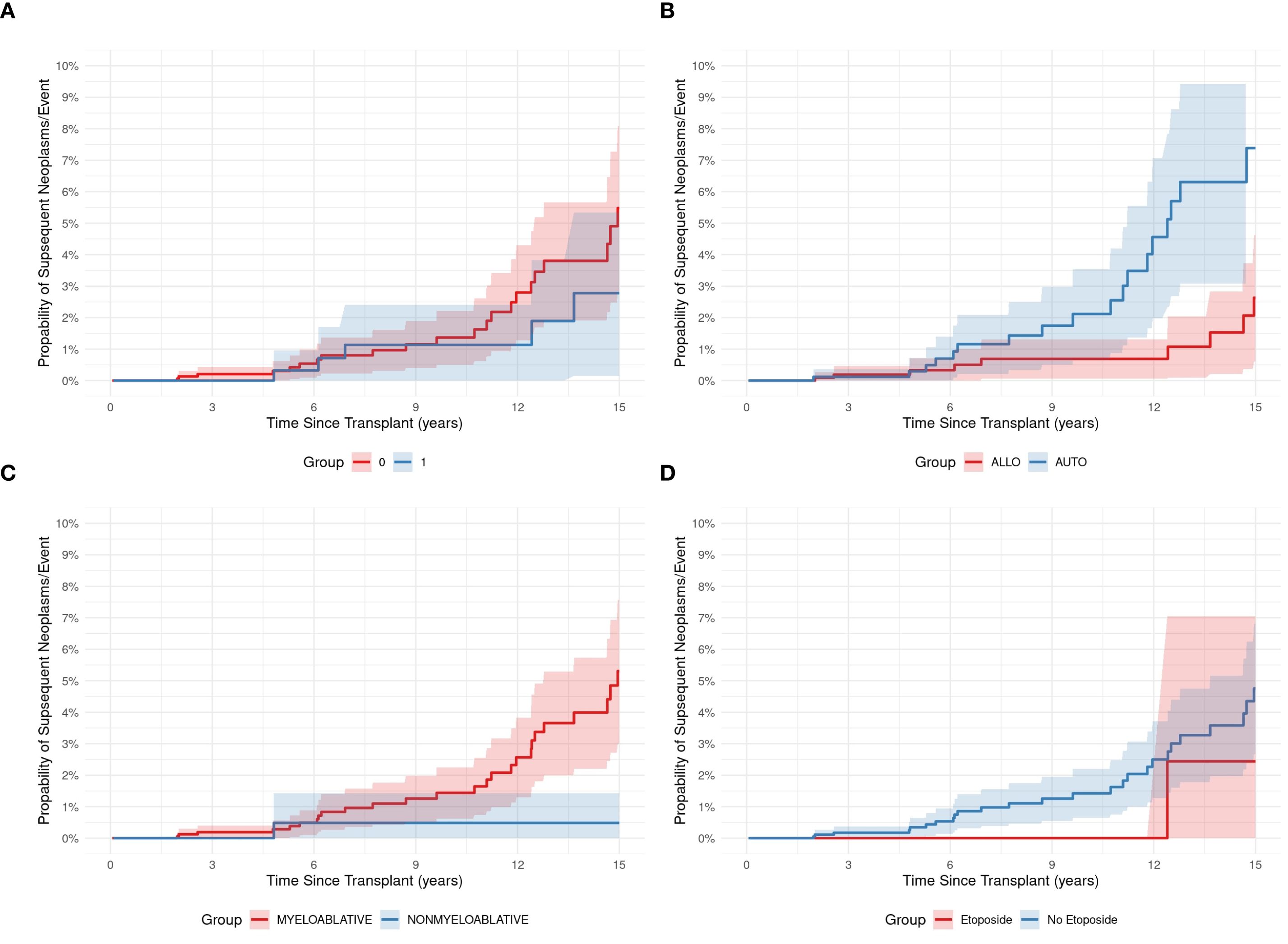
Figure 3. The development of subsequent neoplasms was not associated with HCT features, except for type of transplant. The use of total body irradiation (TBI; 1 vs. No TBI; 0) (A), the type of transplant, i.e., autologous (AUTO) vs allogeneic (ALLO) (B), the conditioning regimen (myeloablative vs reduced intensity (nonmyeloablative)) (C), and the use of etoposide (Etoposide vs No Etoposide) in the conditioning regimen (D).
Survival data are presented in Table 3. OS probabilities after SN development at Year 1 was 58.2% (95% CI: 40.9% to 82.9%). This survival estimate remained consistent through Year 5, with an OS probability of 58.2% (95% CI: 40.9% to 82.9%) (Figure 4). The 5- and 10-year CIs of death due to SNs were 0.3 and 1.3, respectively (Figure 1B). SHM was the most reported cause of death (57%) after SN development, followed by SSN (36%) (Figures 1C, D).
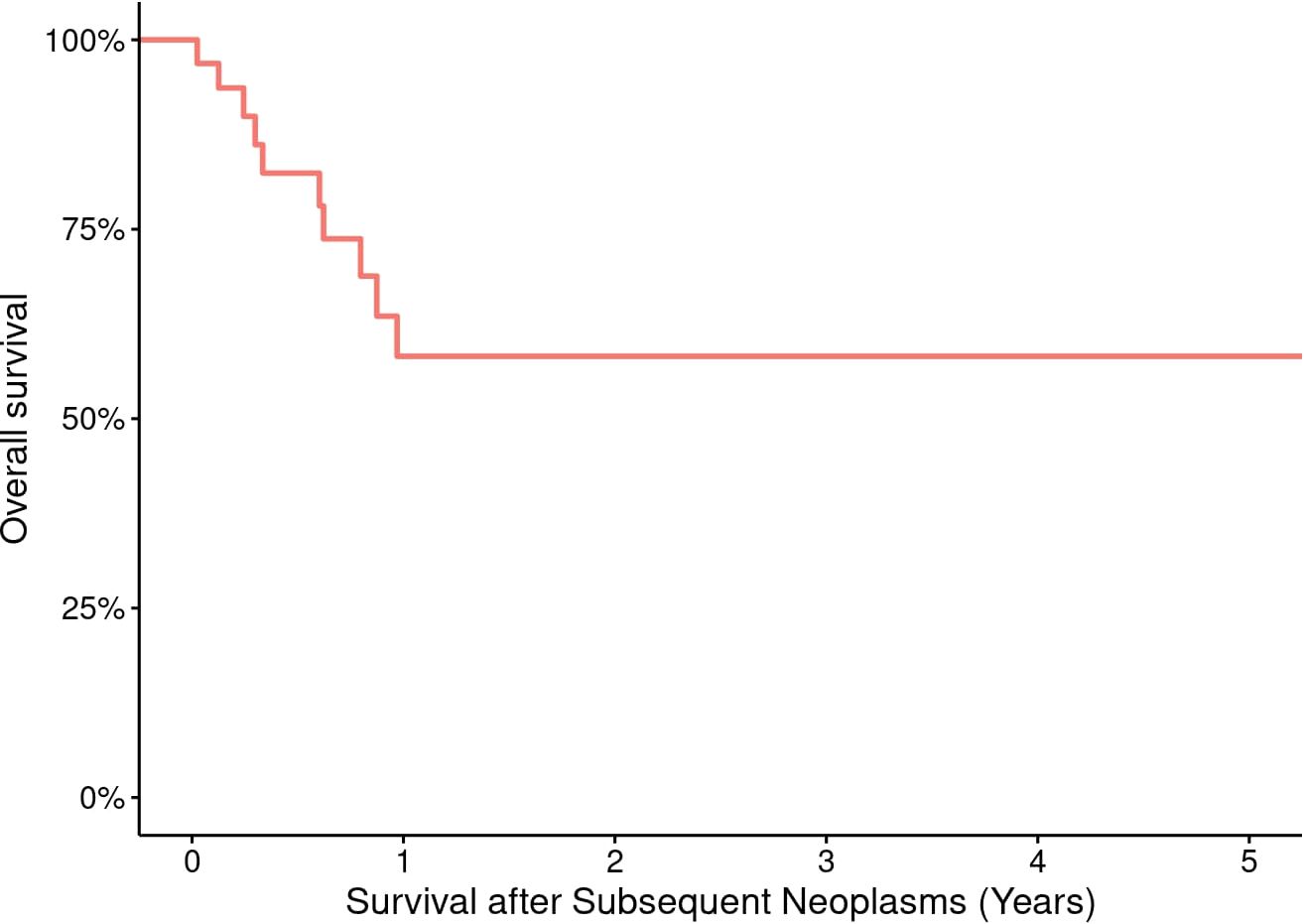
Figure 4. Overall survival of 43 pediatric and adult HCT recipients who experienced a subsequent neoplasm.
Discussion
Recent studies confirm that HCT recipients face a significantly higher risk of SN compared to the general population (34). In a Korean cohort of 5,177 allogeneic HCT recipients, the 10-year CI of subsequent non-hematologic malignancies was 4.23% versus 2.3% in matched controls (HR 1.73, 95% CI 1.32–2.25). In AML patients conditioned with chemotherapy-only regimens, the 10- and 15-year CI of SCC reached 4.2% and 8.1%, respectively, exceeding general population rates (35). Moreover, large registry data (n = 28,874) demonstrated cumulative SSN incidences of 1%, 2.2%, and 3.3% at 10, 15, and 20 years post-transplant (36), with cGVHD as a significant predictor. Pediatric ALL survivors who underwent HCT also exhibited a standardized incidence ratio of 2.6 for SN over 30 years as reported by Westerveld, A.S.R et al. (37) The incidence of SNs in our study was relatively lower than most values in the literature, but it was consistent with reports from Zamora-Ortiz et al. (10) Differences in methods and patient populations across these studies may have contributed to the variations.
Elevated SN risk after HCTs has been consistently observed, albeit with various incidences (18, 24, 30, 38). Hasegawa et al. (8) reported a CI of 4.2% of secondary malignancies at 10 years post-HCT, and Baker et al. (39) reported an estimated actuarial incidence of 9.9% for any post-HCT malignancy within 13 years and 22.0% by 30 years post-HCT (23), surpassing general population expectations. Whether a SN develops post-HCT is influenced by multiple factors, including chemotherapy, radiotherapy, immunologic environment post-HCT, immunosuppression, and reactivation of oncogenic viruses. In addition, recent evidence suggests that chronic inflammation and cytokine dysregulation play a central role in post-transplant tumorigenesis (40, 41). Conditioning regimens and GVHD trigger persistent activation of the NLRP3 inflammasome and increased levels of pro-inflammatory cytokines such as IL−1β, TNF−α, and IL−6, which can induce oxidative DNA damage, angiogenesis, and fibroblast activation (40, 42). These cytokines also activate key oncogenic pathways, including NF−κB and STAT3, supporting malignant transformation and immune evasion (43). Furthermore, elevated TGF−β levels in chronic GVHD promote extracellular matrix remodeling and stromal fibrosis, fostering a microenvironment conducive to neoplastic growth (44). Together, these processes, combined with impaired immune surveillance and the effects of viral reactivation, create a permissive environment for the development of SNs in HCT recipients. Young pediatric cancer survivors are particularly vulnerable. Among our patients who experienced SNs, 35% were pediatric. Furthermore, using certain chemotherapeutic agents (e.g., alkylators, topoisomerase II inhibitors) is a risk factor for SNs independent of TBI (29). Among our patients who experienced SNs, 12 had received etoposide as part of their conditioning regimen, 22 received high-dose cyclophosphamide, and 35 received MAC before HCT. Moreover, radiotherapy use and dose during conditioning are also risk factors for SNs (23). Eleven of 14 patients with SNs who had received 1200 cGy TBI for 3 days experienced carcinomas; the remaining 4 patients had AML, melanoma, collagenoma, and MDS, respectively. Rizzo et al. (33) demonstrated a 9-fold increased SN risk in younger patients who received TBI; this risk persists for decades after HCT and increases over time (13, 45). In contrast, some reports failed to identify TBI as a risk factor (10, 25, 46, 47,), indicating that the association between TBI and the development of post-HCT SNs is complex and not well understood (29).
In a study by Inamoto et al. (45), data from 31,867 patients who underwent a first HCT were analyzed for the development of SSN. Of these, 30% occurred after Auto HCT, while 70% developed following Allo HCT. Despite this difference, the OS probability of SSN did not show a statistically significant difference between the two transplant types. In contrast, within our cohort, the distribution of SN was more balanced, with 53.5% occurring post-Allo HCT and 46.5% post-Auto HCT. Notably, we observed a statistically significant higher cumulative incidence of SN following Auto HCT (p = 0.013). Several factors may contribute to this finding. First, AutoHCT recipients often receive intensive chemotherapy and/or radiotherapy prior to transplant, including alkylating agents and topoisomerase II inhibitors, both of which are established risk factors for therapy-related malignancies (14, 28, 48). Second, the absence of a graft-versus-tumor (GvT) effect in AutoHCT may reduce immune surveillance, potentially allowing pre-malignant or damaged cells to evade detection and progress to malignancy (49, 50). In contrast, the immune-mediated GvT effect in AlloHCT may play a role in reducing the risk of SNs by targeting abnormal clones. Third, AutoHCT is often used for relapsed or indolent malignancies such as lymphomas and multiple myeloma, where patients may live longer post-transplant, thus increasing the at-risk period for SN development (18). Finally, although chronic immunosuppression is more commonly associated with AlloHCT, underlying immune dysfunction in AutoHCT recipients, particularly in plasma cell disorders, may also promote carcinogenesis through impaired immune surveillance or persistent inflammatory signaling (51).
Cancer-predisposition disorders also contribute to SN development. Deeg et al. (52) described 18 SNs in 79 patients with FA at 6–11 years post-HCT. In our study, only 3 patients with FA experienced SNs, which could be attributed to eliminating radiotherapy and using RIC. Also, chronic mucosal inflammation associated with cGVHD and immunosuppression is associated with 2- to 3-fold higher rate of SNs than that in the general population of cancer survivors (14). Moreover, cGVHD appears to increase the risk of SCC (53); among our patients, SCC occurred in 7, and 5 of those also had cGVHD. Similarly, the risk of post-HCT SSN is more than twice that of the general cancer survivor population (54). The CI of SSN at 5, 10, and 15 years post-HCT is 2.2% (22), 6.55% (55), and 12.8%, respectively (3.8-fold higher than that in an age-matched control population) (22). Notably, SSNs tend to occur at younger ages than primary cancers (45) and exhibit longer latency post-HCT (16), with significant risks extending beyond 5 years post-HCT. Moreover, the incidence of SSN increases over time, without reaching a plateau (14, 29, 42, 55–57). Among SN subtypes, there is a high incidence of skin malignancies (54) and elevated risk of tumors of the oral cavity, esophagus, lung, soft tissue, and brain after busulfan-cyclophosphamide conditioning (58). In our study, SSNs comprised 65% of SNs; they manifested at a median of 6.8 years post-HCT and at a median age of 23.9 years. The most prevalent subtype was SSC. The incidence of these SSNs in our cohort exhibited a similar upward trend without plateauing. Leukemia and lymphoma were underlying indications for HCTs in these patients. Younger age at HCT is a major risk factor for SSNs; children younger than 10 years had a 33- to 36.6-fold higher risk of SSN than expected (22), which was 4.6 times higher than expected for those who were 10–29 years old at the time of HCT (12). In multivariate analysis, age older than 35 years at the time of autologous HCT and more than 36 months from diagnosis to autologous HCT were associated with greater SN risks (28). SHMs represent aggressive diseases characterized by a high incidence of adverse histologic and immune-biologic features, including therapy-related AML/MDS, a consequence of cumulative therapeutic exposure to pre-HCT alkylating agents, topoisomerase II inhibitors, radiation, and using PBSCs (48). Moreover, SHMs are more likely to be encountered after HCT (59). Median time to develop AML/MDS in our cohort was 29 months from the time of autologous HCT (range, 12–62 months). Among 14 patients with SHM, 10 had received autologous HCT and 4 had received allogeneic HCT. Two patients had received TBI, and 8 had received etoposide. Similar to results from a previous report (25), in our cohort, SHM accounted for 32.5% of SNs; all were of myeloid origin, mainly AML/MDS, which occurred at a median of 2.72 years post-HCT. This confirms shorter latency of AML/MDS post-HCT (59), occurring at a median of 1–2 years (16) and other leukemias developing relatively early after HCT (15, 57, 58).
The 5-year probability of OS after SN diagnosis depends on the SN type (53). Ehrhardt et al. (53) demonstrated that the highest risk of mortality after SNs occurred primarily within the first 5 years after SN diagnosis, as evidenced by 10- and 15-year OS estimates of 46% and 40%, respectively (53). Nevertheless, after 6 years from SN diagnosis, there was no significant increase in death risk linked to longer time between HCT and SN diagnosis, as per regression analysis. Among our HCT recipients who experienced SNs, 18 died; 13 died of their SN at a median of 7 months from SN diagnosis.
Similarly, outcomes of SSNs post-HCT show variability, with mortality rates approaching 100% within 5 years of SN diagnosis (53). Higher risks of mortality and poorer survival were noted in patients with SSNs at a younger age compared to the same type of primary cancer in the general population (45). Likewise, SHMs are linked to an unfavorable prognosis characterized by median survival of a few months (47). In our cohort, patients who experienced SHMs had high mortality rates, and those with AML/MDS died at a median of 6.8 months (range, 0–39.9 months) from the diagnosis of SHM.
Defining SNs can be challenging, and retrospective observational studies can have several limitations. Some events may be underreported due to survivors being lost to follow-up, leading to an underestimated incidence of SNs and, in turn, overestimated survival. The number of SNs in our cohort was limited and may reflect under-detection or benign tumors in patients and those lost to follow-up. Therefore, OS for individual tumor types should be cautiously interpreted. Moreover, the limited number of SNs and the heterogeneity of our cohort precluded our ability to investigate the impact of individual risk factors on OS or calculate carcinogenesis risk with reasonable accuracy. Additionally, subgroup analyses based on age and gender did not reveal any statistically significant differences in the incidence of SNs, likely due to the small event rate, further limiting the strength of comparative conclusions. Other limitations included a lack of comparison with age-matched controls to determine risk factors, the lack of detailed information on pre-transplant chemotherapy regimens, including the number of cycles and lines of prior treatment, as well as genetic testing, including cytogenetic and molecular data, such as TP53 mutation status. Given the large and heterogeneous nature of the cohort, spanning both pediatric and adult populations over 18 years, standardized data on pre-transplant therapies were not consistently available across all patients. Given these limitations, we could not estimate any associations between potential risk factors and SN development or prognosis. Despite these limitations, the outcomes of this historic cohort provide generalizable guidance for future HCT recipients with SNs. Future studies with more granular, prospective data collection may help clarify the influence of prior treatments on the risk of SNs post-HCT.
Conclusions
The development of post-HCT SNs poses significant risks to the survival and quality of life of HCT recipients. The incidence of SNs continues to increase over time. Although the SN rate observed in our cohort was lower than that reported in the literature, it is imperative to conduct longer follow-up studies with larger cohorts to more accurately determine SN risks. With longer follow-up, more SSNs will develop. Moreover, the rising incidence of SNs underscores the importance of comprehensive, lifelong surveillance, screening programs, and preventive measures in mitigating the impact of SNs and improving the outcomes of HCT survivors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by King Hussein institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective data, no intervention.
Author contributions
RR: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Supervision. SA: Writing – original draft, Writing – review & editing. KH: Writing – original draft, Writing – review & editing. HH: Writing – original draft, Writing – review & editing. ZA: Writing – original draft, Writing – review & editing. LB: Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing. IS: Writing – original draft, Writing – review & editing. AQ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Angela J. McArthur for editing the manuscript and Tahani Sarrawi and Mohannad Shathily for assisting with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Majhail NS and Douglas Rizzo J. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. (2013) 48:1145–51. doi: 10.1038/bmt.2012.258
2. Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematology. (2015) 2:e91–100. doi: 10.1016/S2352-3026(15)00028-9
3. Battiwalla M, Tichelli A, and Majhail NS. Long-term survivorship after hematopoietic cell transplantation: roadmap for research and care. Biol Blood Marrow Transplant. (2017) 23:184–92. doi: 10.1016/j.bbmt.2016.11.004
4. Tichelli A, Beohou E, Labopin M, Socié G, Rovó A, Badoglio M, et al. Evaluation of second solid cancers after hematopoietic stem cell transplantation in European patients. JAMA Oncol (2019) 5:229–35. doi: 10.1001/jamaoncol.2018.4934
5. Mohty B and Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J (2011) 1:e16–6. doi: 10.1038/bcj.2011.14
6. Atsuta Y, Hirakawa A, Nakasone H, Kurosawa S, Oshima K, Sakai R, et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2016) 22:1702–9. doi: 10.1016/j.bbmt.2016.05.019
7. Martin PJ, Counts GW, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. JCO (2010) 28:1011–6. doi: 10.1200/JCO.2009.25.6693
8. Hasegawa W, Pond GR, Rifkind JT, Messner HA, Lau A, Daly AS, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant. (2005) 35:51–5. doi: 10.1038/sj.bmt.1704706
9. Zamora-Ortiz G, Velázquez-Sánchez-de-Cima S, Ponce-de-León S, Gutiérrez-Aguirre CH, Ruiz- Delgado GJ, Gomez-Almaguer D, et al. Secondary malignancies after allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning and outpatient conduction. Hematology (2014) 19:435–40. doi: 10.1179/1607845414Y.0000000154
10. Shimoni A, Shem-Tov N, Chetrit A, Volchek Y, Tallis E, Avigdor A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia (2013) 27:829–35. doi: 10.1038/leu.2012.299
11. Heydari K, Shamshirian A, Lotfi-Foroushani P, Aref A, Hedayatizadeh-Omran A, Ahmadi M, et al. The risk of malignancies in patients receiving hematopoietic stem cell transplantation: a systematic review and meta-analysis. Clin Transl Oncol (2020) 22:1825–37. doi: 10.1007/s12094-020-02322-w
12. Liu HL, Chen YH, Chung CH, Wu GJ, Tsao CH, Sun CA, et al. Risk of secondary malignancies in hematopoietic stem cell transplantation recipients: a nationwide population-based study in Taiwan. Balkan Med J (2023) 40:131–8. doi: 10.4274/balkanmedj.galenos.2023.2022-10-56
13. Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socié G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med (1997) 336:897–904. doi: 10.1056/NEJM199703273361301
14. Majhail NS. Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Haematol (2011) 154:301–10. doi: 10.1111/j.1365-2141.2011.08756.x
15. Chaulagain CP, Sprague KA, Pilichowska M, Cowan J, Klein AK, Kaul E, et al. Clinicopathologic characteristics of secondary squamous cell carcinoma of head and neck in survivors of allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Bone Marrow Transplant. (2019) 54:560–6. doi: 10.1038/s41409-018-0299-x
16. Ortega JJ, Olivé T, De Heredia CD, and Llort A. Secondary malignancies and quality of life after stem cell transplantation. Bone Marrow Transplant. (2005) 35:S83–7. doi: 10.1038/sj.bmt.1704854
17. Ringdén O, Brazauskas R, Wang Z, Ahmed I, Atsuta Y, Buchbinder D, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant. (2014) 20:1777–84. doi: 10.1016/j.bbmt.2014.07.009
18. Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. JCO (2011) 29:814–24. doi: 10.1200/JCO.2010.28.9777
19. Kwag D, Park SS, Lee SE, Kim HJ, and Lee JW. Incidence and outcomes of subsequent malignancy after allogeneic hematopoietic stem cell transplantation in adult patients with severe aplastic anemia. Blood Res (2024) 59:44. doi: 10.1007/s44313-024-00046-2
20. Hudson MM. A model for care across the cancer continuum. Cancer (2005) 104:2638– 42. doi: 10.1002/(ISSN)1097-0142
21. Morton LM, Onel K, Curtis RE, Hungate EA, and Armstrong GT. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book. (2014) 34):e57–67. doi: 10.14694/EdBook_AM.2014.34.e57
22. Socié G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. JCO (2000) 18:348–8. doi: 10.1200/JCO.2000.18.2.348
23. Baker KS, Leisenring WM, Goodman PJ, Ermoian RP, Flowers ME, Schoch G, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood (2019) 133:2790–9. doi: 10.1182/blood.2018874115
24. Ladbury C, Armenian S, Bosworth A, He T, Wong FL, Dandapani S, et al. Risk of subsequent malignant neoplasms following hematopoietic stem cell transplantation with total body irradiation or total marrow irradiation: insights from early follow-up. Transplant Cell Ther (2022) 28:860. doi: 10.1016/j.jtct.2022.09.013
25. Michelis FV, Kotchetkov R, Grunwald RM, Azeem A, Atenafu EG, Lipton JH, et al. Long-term incidence of secondary malignancies after allogeneic hematopoietic cell transplantation: a single- center experience. Biol Blood Marrow Transplant. (2017) 23:945–51. doi: 10.1016/j.bbmt.2017.02.015
26. Danylesko I and Shimoni A. Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol (2018) 19:9. doi: 10.1007/s11864-018-0528-y
27. Atsuta Y, Suzuki R, Yamashita T, Fukuda T, Miyamura K, Taniguchi S, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol (2014) 25:435–41. doi: 10.1093/annonc/mdt558
28. Forrest DL, Nevill TJ, Naiman SC, Le A, Brockington DA, Barnett MJ, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. (2003) 32:915–23. doi: 10.1038/sj.bmt.1704243
29. Omori M, Yamashita H, Shinohara A, Kurokawa M, Takita J, Hiwatari M, et al. Eleven secondary cancers after hematopoietic stem cell transplantation using a total body irradiation-based regimen in 370 consecutive pediatric and adult patients. SpringerPlus (2013) 2:424. doi: 10.1186/2193-1801-2-424
30. Shimada K, Yokozawa T, Atsuta Y, Kohno A, Maruyama F, Yano K, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. (2005) 36:115–21. doi: 10.1038/sj.bmt.1705020
31. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplantation. (2009) 15:1628–33. doi: 10.1016/j.bbmt.2009.07.004
32. Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2012) 18:348–71. doi: 10.1016/j.bbmt.2011.12.519
33. Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European group for blood and marrow transplantation, the center for international blood and marrow transplant research, and the American society of blood and marrow transplantation. Biol Blood Marrow Transplant. (2006) 12:138–51. doi: 10.1016/j.bbmt.2005.09.012
34. Park S, Park S, and Han S. Risk of secondary nonhematologic malignancies after allogeneic stem cell transplantation: a nationwide case-control cohort study. Intl J Cancer (2022) 151:1024– 32. doi: 10.1002/ijc.v151.7
35. Isabella G, Katharina A, Matthias E, Oliver K, and Daniel W. Secondary solid malignancies and precancerous lesions after allogeneic hematopoietic stem cell transplantation using non-total body irradiation-based conditioning in acute myeloid leukemia. J Cancer Res Clin Oncol (2024) 150:152. doi: 10.1007/s00432-024-05679-5
36. Tan TD and Chiou LW. Second primary malignancy after hematopoietic stem cell transplantation: a single institute experience. J Cancer Res Practice. (2024) 11:67–72. doi: 10.4103/ejcrp.eJCRP-D-23-00054
37. Westerveld ASR, Roesthuis P, van der Pal HJH, Bresters D, Bierings M, Loonen J, et al. Increased risk of subsequent neoplasm after hematopoietic stem cell transplantation in 5-year survivors of childhood acute lymphoblastic leukemia. Blood Cancer J (2024) 14:150. doi: 10.1038/s41408-024-01122-7
38. Au WY, Chan EC, Pang A, Lie AKW, Liang R, Yuen APW, et al. Nonhematologic malignancies after allogeneic hematopoietic stem cell transplantation: incidence and molecular monitoring. Bone Marrow Transplant. (2004) 34:981–5. doi: 10.1038/sj.bmt.1704674
39. Baker KS, DeFor TE, Burns LJ, Ramsay NKC, Neglia JP, and Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. JCO (2003) 21:1352–8. doi: 10.1200/JCO.2003.05.108
40. Espinoza JL, Kamio K, Lam VQ, and Takami A. The impact of NLRP3 activation on hematopoietic stem cell transplantation. IJMS (2021) 22:11845. doi: 10.3390/ijms222111845
41. Singh V, Ubaid S, Kashif M, Singh T, Singh G, Pahwa R, et al. Role of inflammasomes in cancer immunity: mechanisms and therapeutic potential. J Exp Clin Cancer Res (2025) 44:109. doi: 10.1186/s13046-025-03366-y
42. Curtis RE. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood (2005) 105:3802–11. doi: 10.1182/blood-2004-09-3411
43. Akkız H, Şimşek H, Balcı D, Ülger Y, Onan E, Akçaer N, et al. Inflammation and cancer: molecular mechanisms and clinical consequences. Front Oncol (2025) 15:1564572. doi: 10.3389/fonc.2025.1564572
44. Chung JYF, Chan MKK, Li JSF, Chan ASW, Tang PCT, Leung KT, et al. TGF-β signaling: from tissue fibrosis to tumor microenvironment. IJMS (2021) 22:7575. doi: 10.3390/ijms22147575
45. Inamoto Y, Matsuda T, Tabuchi K, Kurosawa S, Nakasone H, Nishimori H, et al. Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation. Blood Advances. (2018) 2:1901–13. doi: 10.1182/bloodadvances.2018020966
46. Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol (2010) 11:331–8. doi: 10.1016/S1470-2045(09)70352-3
47. Howe R, Micallef INM, Inwards DJ, Ansell SM, Dewald GW, Dispenzieri A, et al. Secondary myelodysplastic syndrome and acute myelogenous leukemia are significant complications following autologous stem cell transplantation for lymphoma. Bone Marrow Transplant. (2003) 32:317–24. doi: 10.1038/sj.bmt.1704124
48. Sill H, Olipitz W, Zebisch A, Schulz E, and Wölfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol (2011) 162:792–805. doi: 10.1111/j.1476-5381.2010.01100.x
49. Lee SJ, Vogelsang G, and Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2003) 9:215–33. doi: 10.1053/bbmt.2003.50026
50. Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood (2008) 112:4371–83. doi: 10.1182/blood-2008-03-077974
51. Metzner B, Müller TH, Casper J, Kimmich C, Petershofen EK, Thole R, et al. Secondary malignancies after autologous stem cell transplantations in patients with malignant lymphoma and multiple myeloma. Eur J Haematology. (2025) 114:536–44. doi: 10.1111/ejh.v114.3
52. Deeg HJ and Witherspoon RP. Risk factors for the development of secondary malignancies after marrow transplantation. Hematol Oncol Clin North Am (1993) 7:417–29. doi: 10.1016/S0889-8588(18)30249-1
53. Ehrhardt MJ, Brazauskas R, He W, JD R, and Shaw BE. Survival of patients who develop solid tumors following hematopoietic stem cell transplantation. Bone Marrow Transplant (2016) 51:83–8. doi: 10.1038/bmt.2015.203
54. Omland SH, Gniadecki R, Hædersdal M, Helweg-Larsen J, and Omland LH. Skin cancer risk in hematopoietic stem-cell transplant recipients compared with background population and renal transplant recipients: a population-based cohort study. JAMA Dermatol (2016) 152:177. doi: 10.1001/jamadermatol.2015.3902
55. Bhatia S, Louie AD, Bhatia R, O’Donnell MR, Fung H, Kashyap A, et al. Solid cancers after bone marrow transplantation. JCO (2001) 19:464–71. doi: 10.1200/JCO.2001.19.2.464
56. Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant (2015) 50:1013– 23. doi: 10.1038/bmt.2015.63
57. Socié G, Baker KS, and Bhatia S. Subsequent malignant neoplasms after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2012) 18:S139–50. doi: 10.1016/j.bbmt.2011.10.005
58. Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood (2011) 117:316–22. doi: 10.1182/blood-2010-07-294629
Keywords: hematopoietic cell transplantation (HCT), second cancer, hematologic malignancies, cancer survivors, human leukocyte antigen (HLA), myeloablative conditioning (MAC), reduced-intensity conditioning (RIC), total body irradiation (TBI)
Citation: Rihani R, Amer S, Halahleh K, Hashem H, Abdel Rahman Z, Baqain L, Abu Shanap M, Sultan I and Qudeimat A (2025) Exploring the aftermath of hematopoietic cell transplantation: 18-year insights into post-transplant neoplasms. Front. Oncol. 15:1589755. doi: 10.3389/fonc.2025.1589755
Received: 07 March 2025; Accepted: 30 June 2025;
Published: 22 July 2025.
Edited by:
Ruxandra Irimia, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, RomaniaSonia Cismas, Deva Emergency County Hospital, Romania
Copyright © 2025 Rihani, Amer, Halahleh, Hashem, Abdel Rahman, Baqain, Abu Shanap, Sultan and Qudeimat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rawad Rihani, cnJpaGFuaUBraGNjLmpv
 Rawad Rihani
Rawad Rihani Shrouq Amer
Shrouq Amer Khalid Halahleh2
Khalid Halahleh2 Hasan Hashem
Hasan Hashem Zaid Abdel Rahman
Zaid Abdel Rahman Laith Baqain
Laith Baqain Mayada Abu Shanap
Mayada Abu Shanap Iyad Sultan
Iyad Sultan Amr Qudeimat
Amr Qudeimat