- 1Department of Pharmacy, Beijing Health Vocational College, Beijing, China
- 2Department of Medical Oncology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
Background: Perioperative immunotherapy has shown promising results in patients with resectable stage II-III non-small cell lung cancer (NSCLC). However, its benefits for the specific subgroup of elderly patients remain unclear. This study aims to evaluate the efficacy of perioperative immunotherapy in elderly NSCLC patients aged 65 and above, focusing on key metrics such as pathological complete response (pCR), event-free survival (EFS), and overall survival (OS).
Methods: We conducted a comprehensive meta-analysis of randomized clinical trials that reported subgroup data on elderly patients regarding pCR rates and hazard ratios (HRs) for EFS and OS. Data were retrieved from PubMed, EMBASE, and proceedings of oncology conferences from January 2020 to June 2025.A fixed effects model was used for the meta-analysis. Aggregated pooled HRs for time-to-event outcomes (EFS and OS), odds ratios (OR) and risk ratios (RRs) for dichotomous outcomes (pCR) were calculated specifically for patients aged ≥65 years who received perioperative immunotherapy or placebo.
Results: A total of 8 randomized controlled trials involving 1561 patients aged ≥65 years with resectable NSCLC were included. A significant benefit was observed in terms of pCR (risk ratio, 5.26; 95% CI, 3.54 – 7.82; I² = 0%) and EFS (HR, 0.64; 95% CI, 0.55 – 0.74; I² = 7%) for patients aged ≥65 years who received perioperative immunotherapy compared with placebo.
Conclusion: Our systematic review and meta-analysis demonstrated that perioperative immunotherapy was superior to placebo in terms of pathological and event-free survival for patients aged ≥65 years. These findings provide age-specific evidence to inform precision decision-making for treating the elderly patients.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420250654072.
Introduction
Lung cancer poses a major public health challenge globally, being the foremost reason for cancer-related deaths, with non-small-cell lung cancer (NSCLC) comprising more than 80% of all lung cancer cases (1). The incidence of NSCLC is more prevalent among the geriatric population, with those aged 65 and above accounting for approximately 46.9%, and those aged 70 and above making up around 20% (2). There is no chronological age threshold to define an older adult. However, age 65 and above is commonly accepted as the chronological definition of an older adult, primarily because it aligns with the eligibility age for Medicare benefits. This criterion has been adopted by the National Comprehensive Cancer Network and other authoritative bodies (3). Although ≥65 years account for 61% of new cancers and 70% of deaths, they constituted only 25% of oncology trial participants, which is substantial underrepresentation (4).
For patients diagnosed with stage I to III NSCLC, surgical resection continues to be the primary treatment option, irrespective of age. Despite undergoing surgical resection, patients have a high risk of postoperative recurrence, ranging from 20% to 60% based on the disease stage (5).
Immune checkpoint inhibitors enhance antitumor immunity by blocking inhibitory signaling through checkpoint receptors on T lymphocytes and their ligands in tumor cells, has revolutionized the treatment of NSCLC. Perioperative immunotherapy has been approved as standard of care for resectable stage II-III NSCLC without driver mutations. However, T lymphocytes functions are subject to age-related alterations (6), which may contribute to differences in treatment outcomes between elderly and younger patients. The efficacy of perioperative immunotherapy in elderly patients remains unclear.
In this study, we undertook a systematic review and meta-analysis of clinical trials that focused on perioperative immunotherapy in aged ≥ 65 years resectable NSCLC patients. The efficacy and safety outcomes were evaluated across different groups.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline (7). The protocol was prospectively registered with PROSPERO (CRD420250654072).
Data sources and search strategy
A comprehensive literature search was conducted across databases including PubMed, Embase, and proceedings of oncology conferences from January 1, 2020, to the present (search last updated June 30, 2025). Search parameters encompassed primary terms as PD - 1/PD-L1, neoadjuvant, adjuvant, perioperative, NSCLC.
Inclusion criteria
Included studies (1) focused on patients with resectable NSCLC; (2) involved PD - 1 or PD-L1 inhibitors in neoadjuvant and/or adjuvant therapy; (3) compared groups receiving immunotherapy with placebo or chemo-immunotherapy with chemotherapy; (4) reported pCR, EFS and/or OS data in ≥65 years subgroup; and (5) were designed to be randomized clinical prospective studies.
Data extraction and assessment of study quality
Two authors (Yue Cao and Yumeng Tian) independently extracted data and resolved discrepancies by consensus. Collected data pertained to outcomes including pCR, EFS, OS and its subgroup data. Ancillary details were recorded in the predefined information sheet. Methodological integrity was assessed using the Cochrane Risk of Bias Tool (8).
Main outcomes
The co-primary outcomes encompass pCR, which is defined as the absence of residual invasive cancer on hematoxylin and eosin stained slides of the resected lung specimen and lymph nodes after the completion of neoadjuvant therapy, and EFS, which is defined as the duration from randomization to either local progression that impedes planned surgery, the presence of an unresectable tumor, disease progression or recurrence, or death in ≥65 years patients. The secondary outcome was OS in ≥65 years patients.
Statistical analysis
Depending on the level of detail in the data disclosure for the elderly subgroup, the analysis encompasses three distinct strategies: (1) Neoadjuvant treatment regimen impacts the pathological response rate. Consequently, studies on neoadjuvant therapy were categorized and included in the analysis of pCR; (2) The studies that included neoadjuvant plus adjuvant therapy were considered for the EFS outcomes; (3) Due to the limitations of follow-up duration, the subgroup data regarding overall survival in the elderly population are likely constrained. Consequently, the data from the intention-to-treat (ITT) population were subjected to a meta-analysis, which was then compared with the disclosed data of the ≥65 years subgroup.
Initially, we performed direct meta-analysis comparing neoadjuvant chemoimmunotherapy with chemotherapy. The pooled relative risks (RRs) for pCR were derived via the Mantel-Haenszel method, whereas HRs for both EFS and OS were determined through the generic inverse-variance methods model. Intertrial heterogeneity was examined through the Cochran Q test, with P < .10 and I2 > 50% demarcating significant heterogeneity—in such instances, a randomized effects model was used; otherwise, a fixed-effects model was applied (9).
For the subgroup analysis, we extracted relevant data from each study`s subgroups, pooled them through direct meta-analysis. Other statistical analyses were conducted with Review Manager software, version 5.4 (The Cochrane Collaboration). All tests were 2-sided, and a P value < .05 was considered significant unless otherwise indicated.
Results
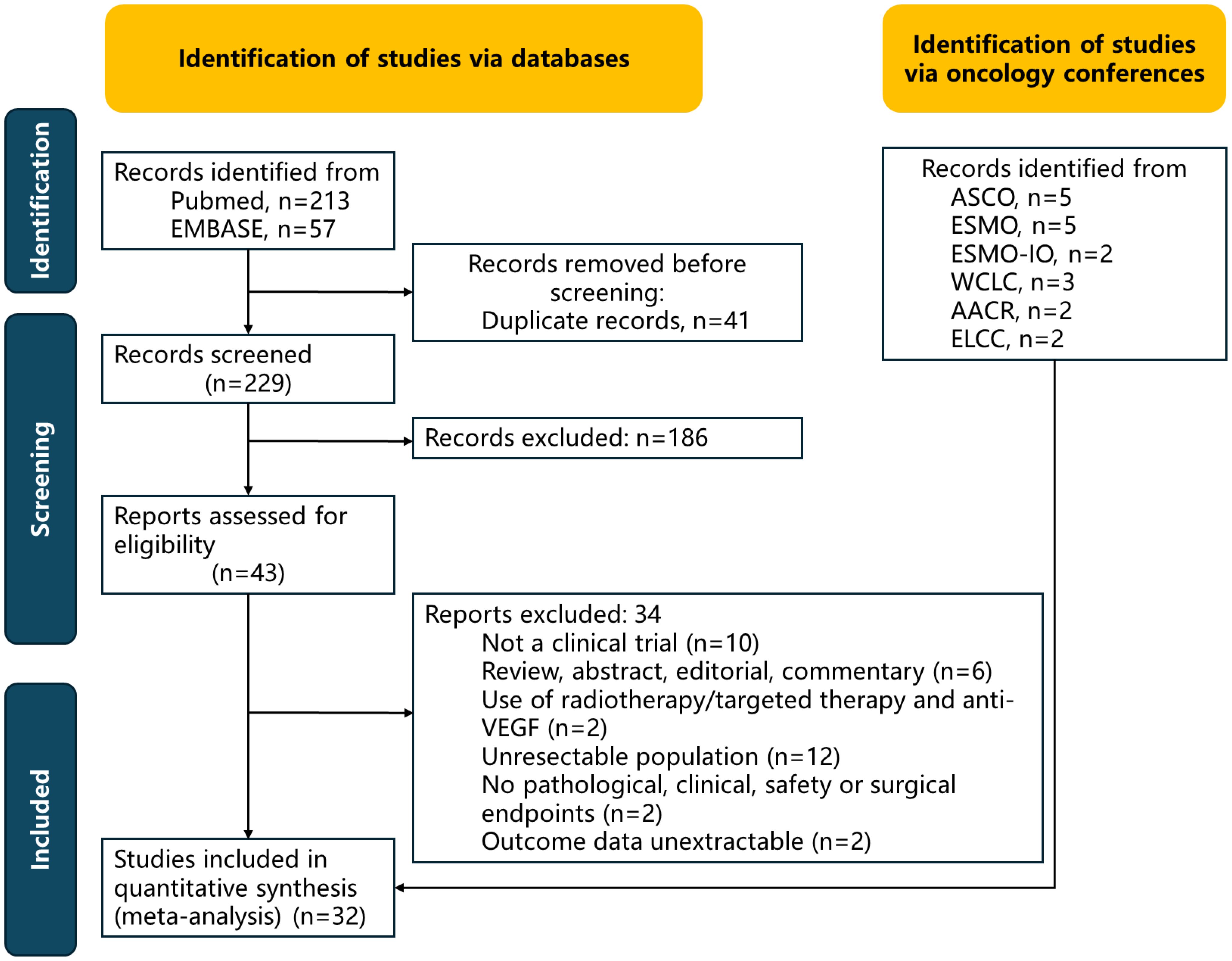
Eight randomized trials (3384 patients in total, including 1561 patients aged ≥65 years) were analyzed; the detailed selection flowchart is presented in Figure 1. Details of the bias assessment are provided in Supplementary Table S1 and Supplementary Figure S1. A primary source of bias arose from the Neotorch study because short follow-up time.
Trial characteristics
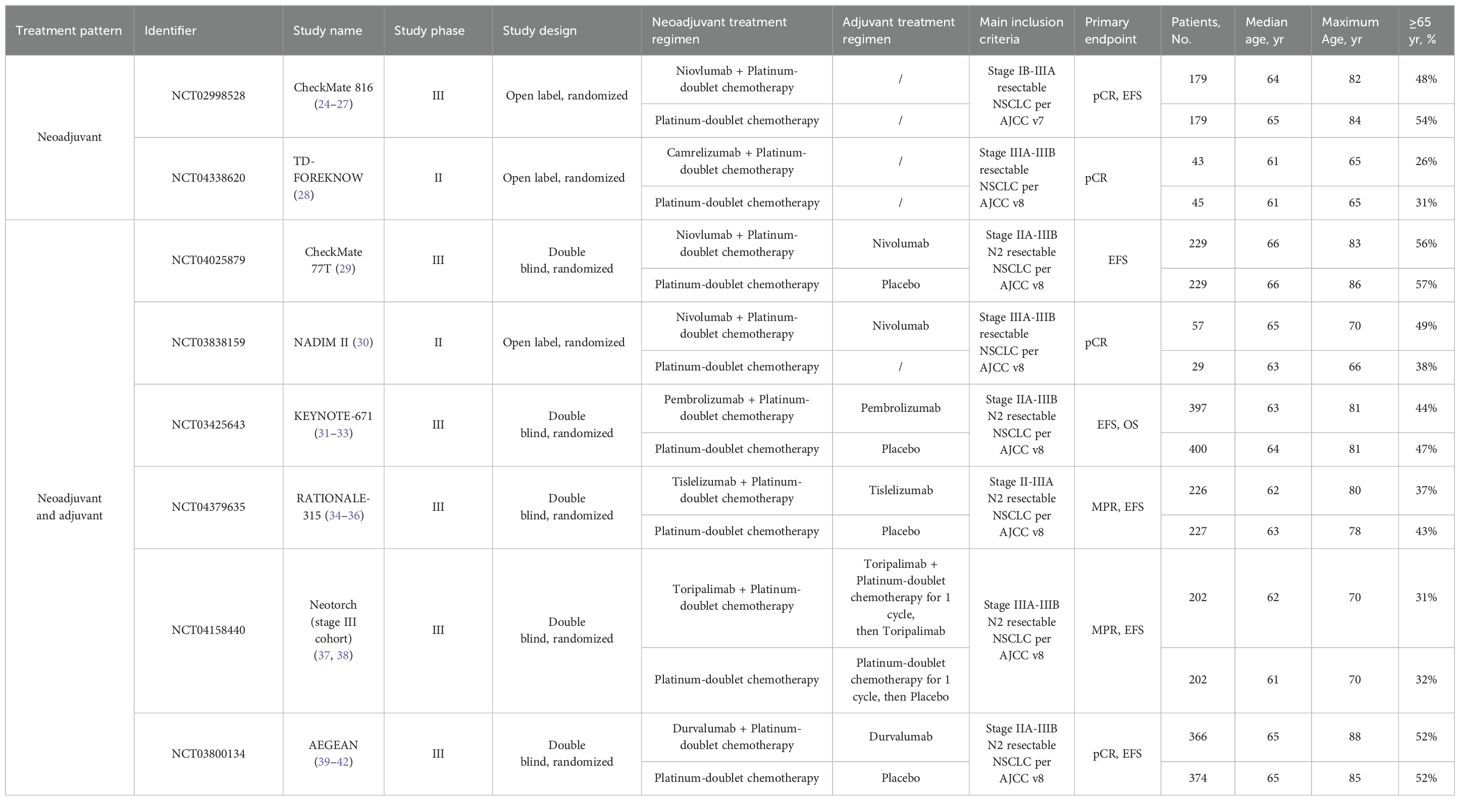
The characteristics and outcomes of the included trials are presented in Table 1. Six trials (AEGEAN, NADIM II, CheckMate 77T, KEYNOTE - 671, RATIONALE - 315 and Neotorch) explored perioperative immunotherapy vs placebo, while 2 trials (CheckMate 816 and TD-FOREKNOW) investigated neoadjuvant immunotherapy only.
Due to the different American Joint Committee on Cancer staging versions used during the study, Checkmate 816 enrolled patients with resectable NSCLC from stage IB to III according to the 7th edition, while other studies followed the 8th edition and enrolled patients with resectable NSCLC from stage II to III. Although the inclusion criteria of Neotorch included patients with stage II - III, only the data of the stage III cohort has been published so far.
In all experimental groups across these trials, 3 to 4 cycles of neoadjuvant PD - 1 or PD-L1 inhibitor plus platinum-based chemotherapy were administered. Predominantly, patients received a year of adjuvant immunotherapy, except in CheckMate 816 and TD-FOREKNOW. With a median follow-up time ranging from 18.3 to 57.6 months, the KEYNOTE - 671 and NADIM II study reported the OS data of the elderly patient subgroup.
Comparison of pCR
In the neoadjuvant immunotherapy group, the pCR rate across the included trials ranged from 17.2% to 40.7%. Through direct meta-analysis, compared with chemotherapy alone, the addition of neoadjuvant immunotherapy was associated with a higher pCR rate (risk ratio, 5.98; 95% CI, 4.67 – 7.66; I² = 30%) in the intention-to-treat (ITT) population (Figure 2). This benefit was observed across all age subgroups, including those aged <65 years (risk ratio, 5.87; 95% CI, 3.60 – 8.84; I² = 10%) and those aged ≥65 years (risk ratio, 5.26; 95% CI, 3.54 – 7.82; I² = 0%). The advantage in terms of the pathological response rate was further enhanced in the PD - 1 antibody drug group for ≥65 years patients, with a risk ratio of 6.16 (95% CI, 3.82 – 9.95; I² = 0%).
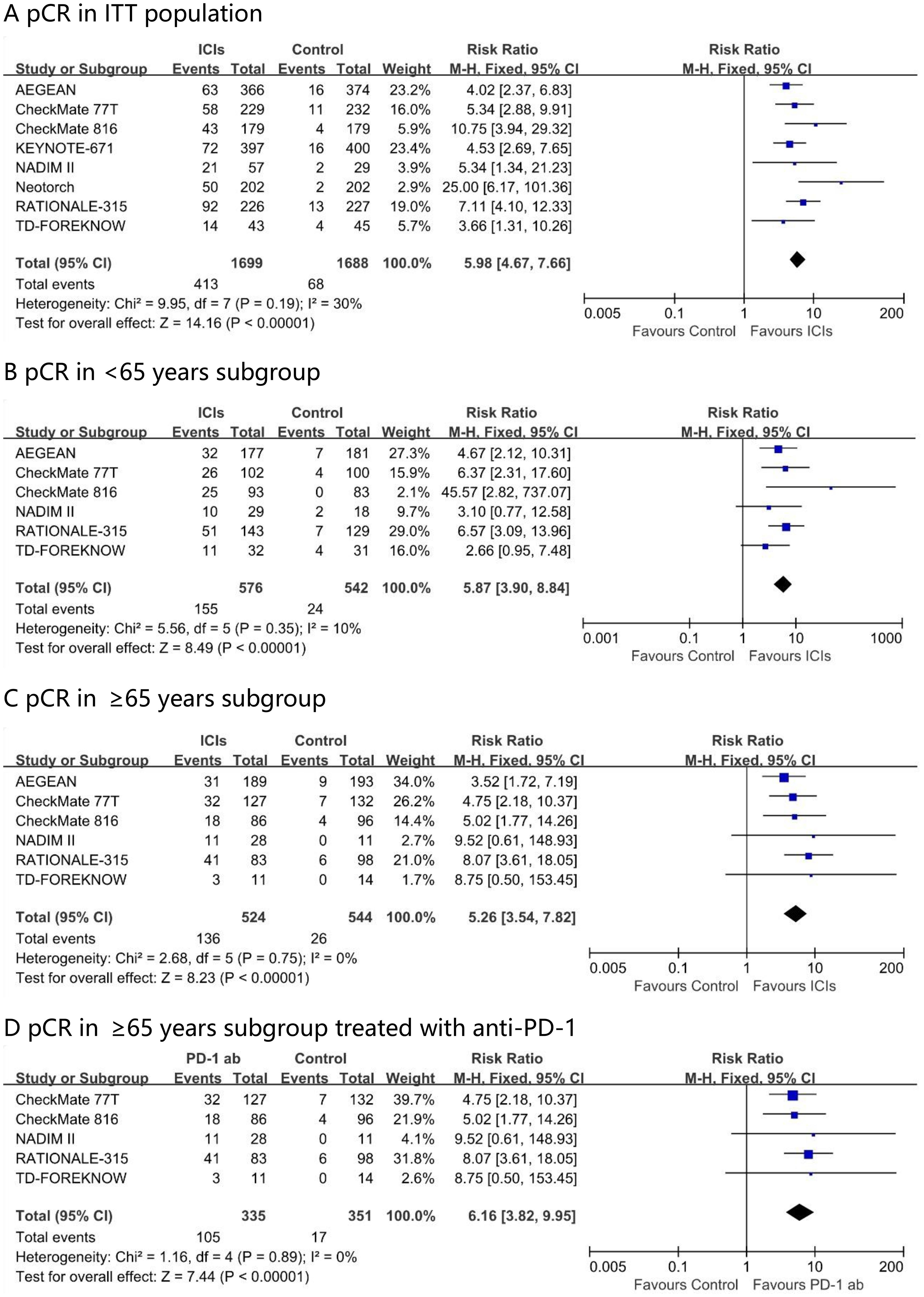
Figure 2. Pooled risk ratios of pCR in ITT population (A), <65 years subgroup (B), ≥65 years subgroup (C) and ≥65 years subgroup treated with anti-PD-1 (D) across randomized clinical trials.
Comparison of EFS
Fixed-effect meta-analysis estimated pooled EFS hazard ratios for perioperative immunotherapy versus placebo. A significant difference favoring perioperative immunotherapy over placebo was found in terms of EFS in the ITT population (HR, 0.59; 95% CI, 0.53 - 0.65; I² = 11%) (Figure 3). This benefit was observed across all age subgroups, including those aged <65 years (HR, 0.55; 95% CI, 0.47 – 0.63; I² = 0%) and those aged ≥65 years (HR, 0.64; 95% CI, 0.55 - 0.74; I² = 7%). The EFS advantage conferred by PD - 1 antibody in ≥65 years patients was comparable, with a hazard ratio of 0.62 (95% CI 0.52 – 0.73; I² = 15%).
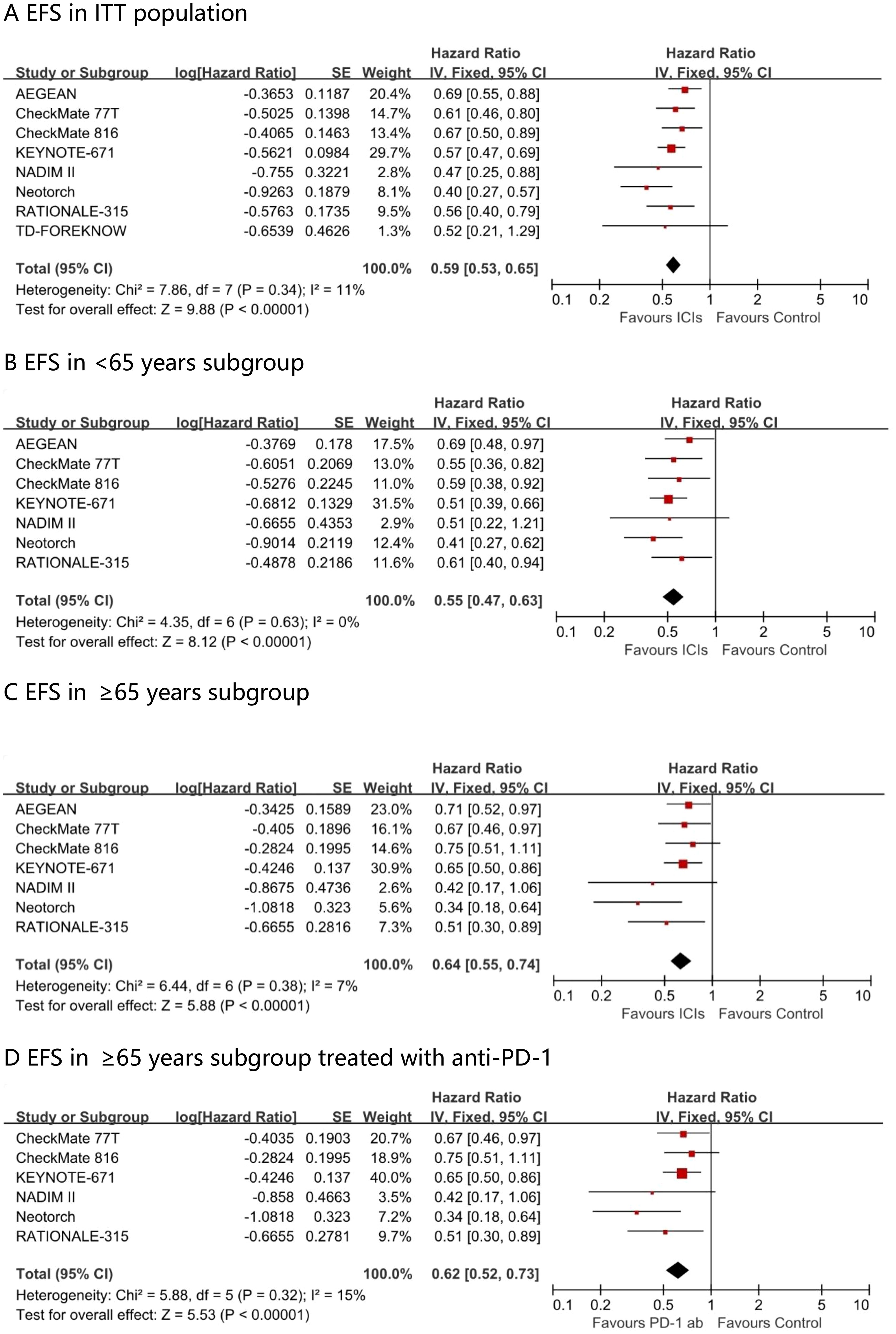
Figure 3. Pooled hazard ratios of event-free survival in ITT population (A), <65 years subgroup (B), ≥65 years subgroup (C) and ≥65 years subgroup treated with anti-PD-1 (D) across randomized clinical trials.
Comparison of OS
Among the perioperative immunotherapy studies, seven trails (AEGEAN, CheckMate 77T, CheckMate 816, KEYNOTE - 671, NADIM II, Neotorch, and RATIONALE - 315) reported the overall survival (OS) in the intention-to-treat (ITT) population, with KEYNOTE - 671 and NADIM II providing OS data for the ≥ 65 years subgroup. A significant difference favoring perioperative immunotherapy over placebo was found in terms of OS in the ITT population (HR, 0.75; 95% CI, 0.66 - 0.86; I² = 0%) (Figure 4). Patients younger than 65 years gained a significant survival advantage (OS HR 0.54; 95% CI 0.40–0.73; I² = 0%), whereas no such benefit was observed in patients aged ≥65 years at the current follow-up time (OS HR 0.99; 95% CI 0.72–1.36; I² = 0%).
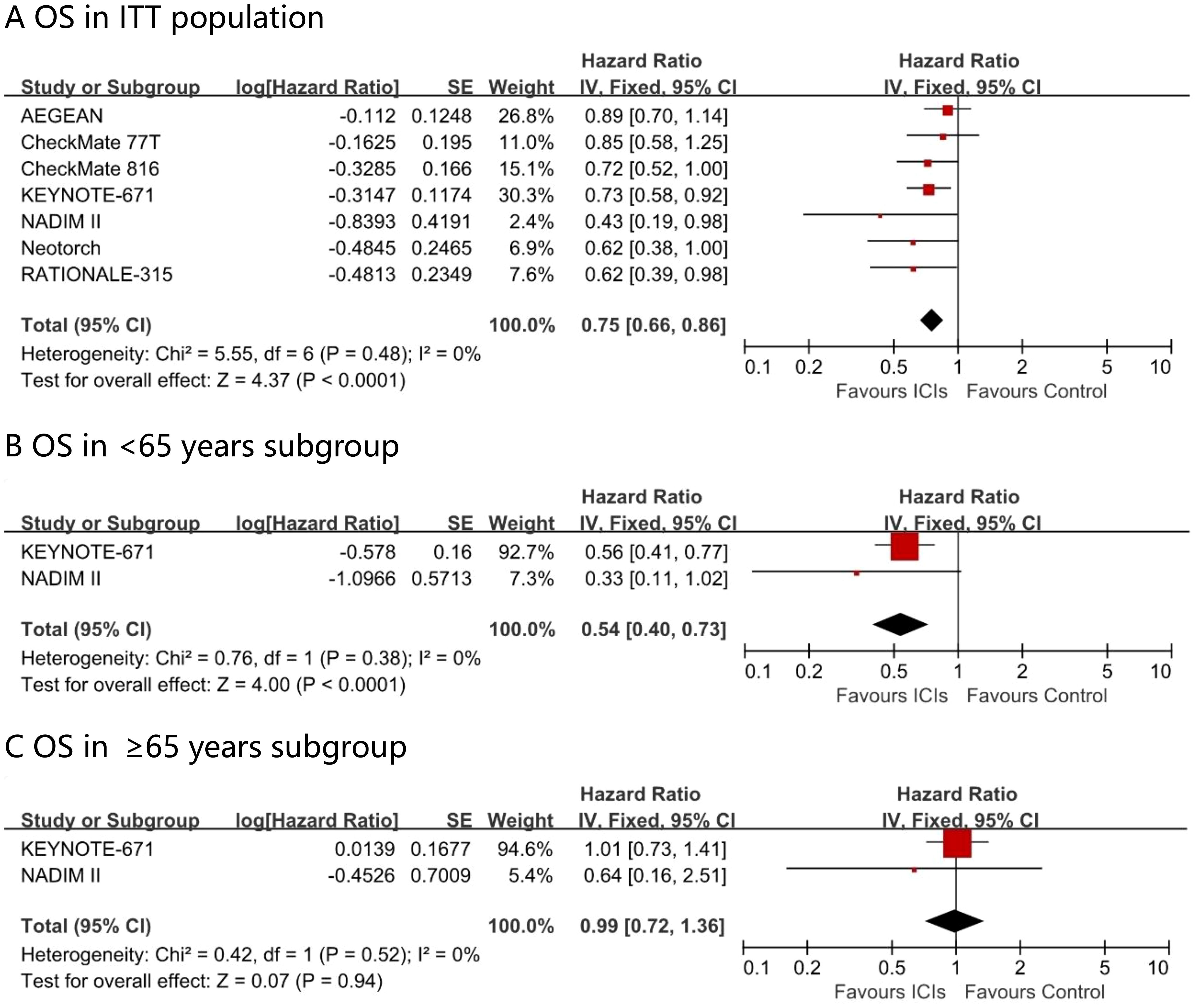
Figure 4. Pooled hazard ratios of overall survival in ITT population (A), <65 years subgroup (B), ≥65 years subgroup (C) and ≥65 years subgroup across randomized clinical trials.
Comparison of Surgical outcomes
Perioperative immunotherapy increased the rate of minimally invasive surgery (risk ratio, 1.13; 95% CI, 1.00 – 1.28; I² = 0%) and the proportion of lobectomy (risk ratio, 1.07; 95% CI, 1.01 – 1.14; I² = 58%) in the ITT population (Figure 5). None of the eight studies reported surgical outcome data specifically for the subgroup of patients aged 65 years or older.
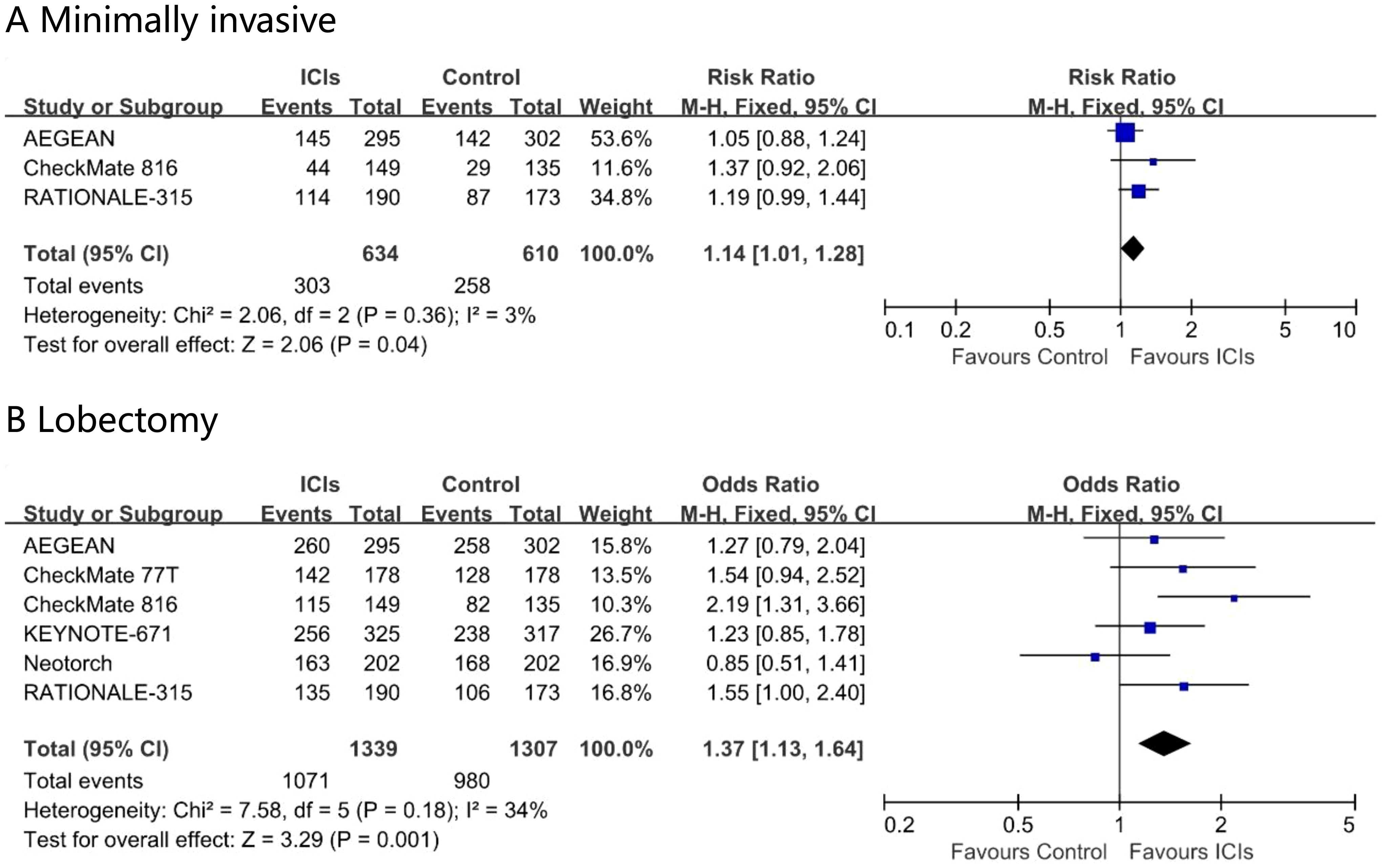
Figure 5. Pooled risk ratios of surgical outcomes, minimally invasive (A) and Lobectomy (B) in ITT population across randomized clinical trials.
Comparison of treatment related adverse events
In the ITT population, the incidence of TRAEs did not differ significantly between perioperative immunochemotherapy and chemotherapy alone (odds ratio 1.21; 95% CI 0.85 – 1.74; I² = 23%). Nevertheless, the addition of immunotherapy conferred a modest yet statistically significant increase in grade ≥3 TRAEs (odds ratio 1.24; 95% CI 1.08 – 1.43; I² = 21%) (Figure 6). Safety outcomes stratified by age were not reported.
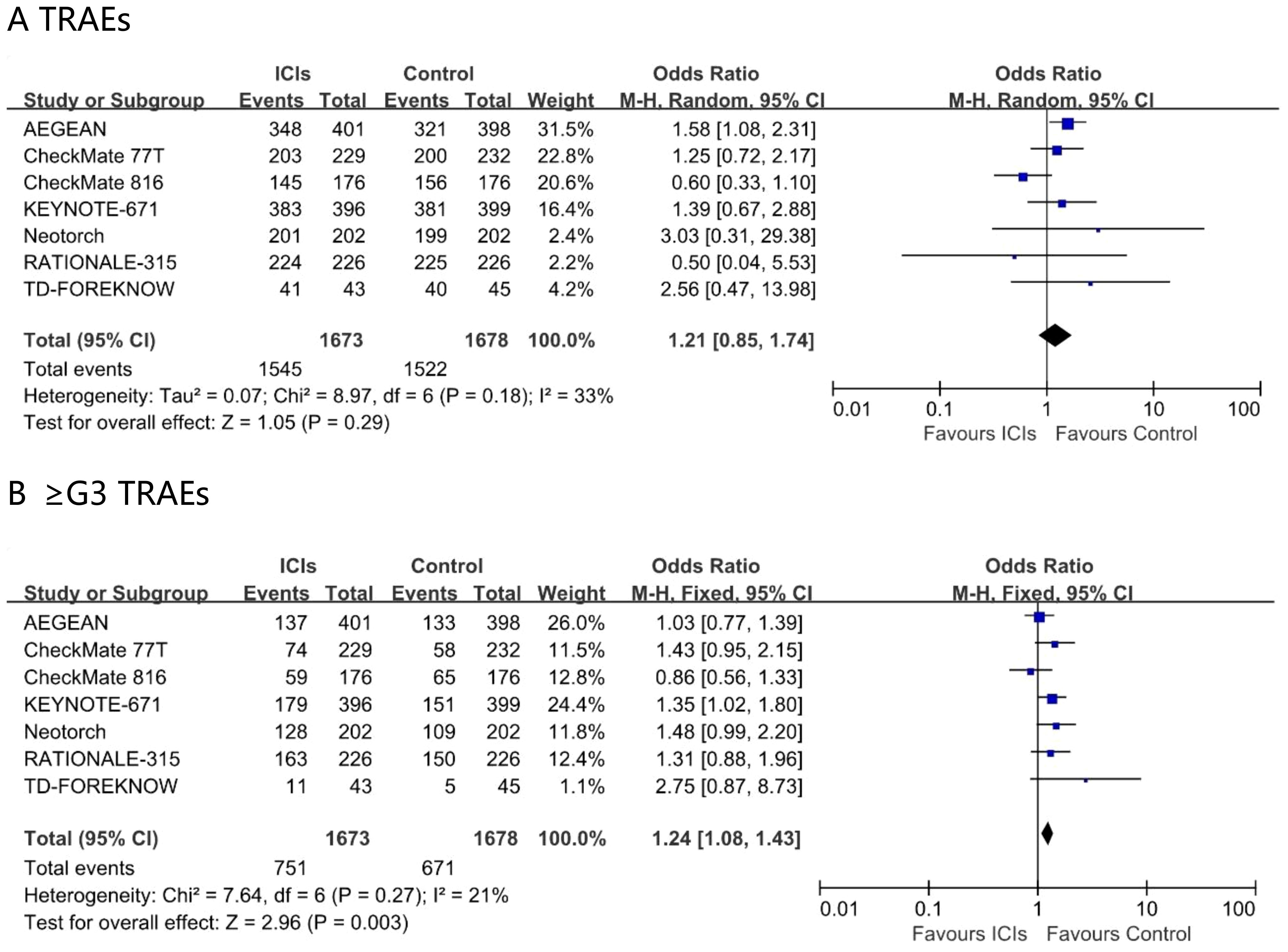
Figure 6. Pooled risk ratios of TRAEs (A), ≥G3 TRAEs (B) in ITT population across randomized clinical trials.
Discussion
To our knowledge, our meta-analysis is the first to show that adding perioperative PD - 1 or PD-L1 antibodies improves pCR and EFS in elderly patients with stage II-III resectable NSCLC. Moreover, the trend of benefit for elderly patients aligns with that of the ITT population and the <65 years subgroup, and increasing age does not diminish this advantage. Nevertheless, although an overall survival advantage was evident in the ITT population, this benefit was not replicated in the elderly subgroup.
Research by the British Thoracic Association indicates that advanced age is not a contraindication for surgery (10). In elderly early-stage NSCLC patients, the overall survival rate and lung cancer specific survival rate after surgery are higher than with radiotherapy or no treatment, and surgery may offer greater potential survival benefits for elderly NSCLC patients (11). Among the factors influencing surgery, age ranks relatively low. Age alone does not preclude patients from undergoing surgery. Comorbidities, surgical approach, and the extent of resection have a more significant impact on surgical decision-making in elderly patients. Minimally invasive surgery (12), and lobectomy (13) are more suitable for elderly patients. Perioperative immunotherapy increased the rate of minimally invasive surgery and the proportion of lobectomy, which is a positive sign for curative-intent therapy.
As elderly patients often exhibit diminished tolerance to conventional anticancer therapies, immune checkpoint inhibitors—characterized by a more favorable toxicity profile—offer renewed therapeutic option for this vulnerable population. In resectable NSCLC, CheckMate 159 (14) and CheckMate 816 (15) offer two neoadjuvant paradigm, mono-immunotherapy and chemo-immunotherapy, demonstrate that neoadjuvant single-agent immunotherapy still confers meaningful improvements in pCR EFS and OS. Nevertheless, when platinum-based chemotherapy is contraindicated by age-related frailty or comorbidity, peri-operative single-agent immunotherapy remains a clinically feasible and well-tolerated option.
Regarding treatment modalities, both neoadjuvant-only and perioperative immunotherapy have yielded positive results, but it remains to be determined which approach is superior. The individual patient level data analysis of CheckMate 77T and CheckMate 816 shows that perioperative nivolumab demonstrated an improvement in EFS compared to neoadjuvant nivolumab alone in patients with resectable NSCLC (16). In our study, the perioperative EFS (HR 0.62, Supplementary Figure S2) from the meta-analysis of the elderly subgroup was superior to that from Checkmate 816 (HR 0.75) (15), which is consistent with previous findings.
We also observe that anti-PD-1 demonstrates improved pCR rate compared with anti-PD-L1. In terms of molecular mechanisms, PD - 1 antibodies bind to PD - 1 and simultaneously block the binding of PD - 1 to its ligands (PD-L1 and PD-L2). However, while PD-L1 antibodies inhibit the binding of PD - 1 to PD-L1, the interaction between PD - 1 and PD-L2 remains intact, potentially inhibiting T cell activation. Thus, tumors may escape antitumor immune responses through the PD - 1/PD-L2 axis when treated with anti-PD-L1.
In the ITT population, the incidence of TRAEs was comparable between perioperative chemo-immunotherapy and chemotherapy alone; however, grade ≥3 TRAEs were more frequent with chemo-immunotherapy. Since the randomized controlled trial did not conduct an age-based subgroup analysis for adverse events, it was not possible to perform a meta-analysis. However, we have identified some data from real-world studies. Liu et al. retrospective compared the efficacy and safety of neoadjuvant immunochemotherapy in young and elderly patients with IIA-IIIB NSCLC in real world practice and found that the incidence of AEs was similar in young and elderly patients (17). Muhammad et al. retrospectively analyzed 19177 cancer patients and found that elderly recipients of immune checkpoint inhibitors are not at increased risk of irAE compared to younger patients (18). In parallel, three retrospective analyses reported that elderly patients experience higher incidences of dermatologic, pulmonary, and gastrointestinal immune-related adverse events during checkpoint inhibitor therapy (19–21).
Notably, immunotherapy yielded a favorable EFS yet unfavorable OS in elderly patients; several factors may explain this paradox. Firstly, elderly patients were not a predefined subgroup, which may lead to baseline imbalance. Secondly, death events in elderly patients are caused by multiple factors, with a higher incidence of non-tumor-related mortality. Using nationwide Japanese registry data, Yasufumi et al. found that non-cancer mortality rose to 31.6% by 4 years since diagnosis, driven mainly by heart (21.8%) and cerebrovascular disease (9.8%); both are markedly more common in the elderly cancer patients (22). Additionally, immune senescence impairs both innate and adaptive immunity: naive T/B cells drop while memory subsets rise, dendritic cell and natural killer cell functions decline, yet myeloid-derived suppressor cells and macrophages expand and hyper-function (23). Together, these changes may blunt the efficacy of immune-checkpoint inhibitors in elderly cancer patients. Therefore, lung cancer-specific survival may serve as a more precise endpoint for anti-tumor therapy research in elderly patients.
Limitations
This study had several limitations that make any recommendations preliminary. First, given the inclusion and exclusion criteria of the randomized controlled trial, the study involved elderly patients who were fit for surgical resection and chemoimmunotherapy, typically those with better physical fitness. As a result, the study’s findings are mainly applicable to this specific group of patients. Second, the follow-up duration in our study was relatively short, and only two studies reported the overall survival data for elderly patients aged 65 and above, so we could not clearly determine the long-term efficacy in elderly NSCLC patients. Third, the safety meta-analysis investigating the incidence of adverse events in elderly patients aged 65 and above was not conducted, due to insufficient original literature data.
Conclusions
In summary, this meta-analysis concluded that perioperative immunotherapy was superior to placebo in terms of pathological and efficacy outcomes for patients aged ≥65 years. Elderly patients derived benefits in terms of pathological complete response and event-free survival with perioperative immunotherapy. The study has spotted positive signs of perioperative immunotherapy for elderly patients with resectable stage II-III NSCLC. However, further prospective studies are needed to address this clinical question.
Author contributions
YC: Writing – original draft, Writing – review & editing. YT: Conceptualization, Formal analysis, Writing – review & editing. LL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1589846/full#supplementary-material
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Shi JF, Wang L, Wu N, Li JL, Hui ZG, Liu SM, et al. Clinical characteristics and medical service utilization of lung cancer in China, 2005-2014: Overall design and results from a multicenter retrospective epidemiologic survey. Lung Canc. (2019) 128:91–100. doi: 10.1016/j.lungcan.2018.11.031
3. Dotan E, Walter LC, Browner IS, Clifton K, Cohen HJ, Extermann M, et al. NCCN guidelines(R) insights: older adult oncology, version 1.2021. J Natl Compr Canc Netw. (2021) 19:1006–19. doi: 10.6004/jnccn.2021.0043
4. Shenoy P and Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res. (2015) 6:184–89. doi: 10.4103/2229-3485.167099
5. Akamine T, Takenaka T, Yano T, Okamoto T, Yamazaki K, Hamatake M, et al. Impact of timing and initial recurrence site on post-recurrence survival in resected non-small cell lung cancer. Eur J Surg Oncol. (2024) 50:108374. doi: 10.1016/j.ejso.2024.108374
6. Xu Y, Wang Z, Li S, Su J, Gao L, Ou J, et al. An in-depth understanding of the role and mechanisms of T cells in immune organ aging and age-related diseases. Sci China Life Sci. (2025) 68:328–53. doi: 10.1007/s11427-024-2695-x
7. Moher D, Liberati A, Tetzlaff J, Altman DG, and Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
8. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomzsed trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
9. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
10. British Thoracic S and Society of Cardiothoracic Surgeons of Great B, Ireland Working P. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. (2001) 56:89–108. doi: 10.1136/thorax.56.2.89
11. Guo Q, Hu S, Ye J, Su L, Wang S, Zhang D, et al. Surgery offers survival advantage over radiotherapy in patients who are 80 years and older with Stage I and II NSCLC: A retrospective cohort study of 7,045 patients. Front Surg. (2022) 9:1018320. doi: 10.3389/fsurg.2022.1018320
12. Mao Y, Gao Z, and Yin Y. Complete video-assisted thoracoscopic surgery and traditional open surgery for elderly patients with NSCLC. Front Surg. (2022) 9:863273. doi: 10.3389/fsurg.2022.863273
13. Zhang X, Lin G, and Li J. Comparative effectiveness of lobectomy, segmentectomy, and wedge resection for pathological stage I non-small cell lung cancer in elderly patients: A population-based study. Front Surg. (2021) 8:652770. doi: 10.3389/fsurg.2021.652770
14. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078
15. Forde PM, Spicer JD, Provencio M, Mitsudomi T, Awad MM, Wang C, et al. Overall survival with neoadjuvant nivolumab plus chemotherapy in lung cancer. N Engl J Med. (2025). 393(8):741–752. doi: 10.1056/NEJMoa2502931
16. Forde PM, Peters S, Donington J, Meadows-Shropshire S, Tran P, Lucherini S, et al. PL02.08 Perioperative vs Neoadjuvant Nivolumab for Resectable NSCLC: Patient-Level Data Analysis of CheckMate 77T vs CheckMate 816. J Thorac Oncol. (2024) 19:S2. doi: 10.1016/j.jtho.2024.09.014
17. Liu J, Huang X, Yang Y, Lv W, Wang Y, Xia P, et al. Comparison of efficacy and safety of neoadjuvant immunochemotherapy in young and elderly patients with IIA-IIIB non-small-cell lung cancer in real-world practice. BMC Pulm Med. (2024) 24:592. doi: 10.1186/s12890-024-03417-8
18. Muhammad A, Ahmed A-A, Mostafa A, and Muhammad Junaid T. Safety of Immune checkpoint inhibitors in elderly patients over 75 years. J Immunother Canc. (1235) 2023):11. doi: 10.1136/jitc-2023-SITC2023.1235
19. Muchnik E, Loh KP, Strawderman M, Magnuson A, Mohile SG, Estrah V, et al. Immune checkpoint inhibitors in real-world treatment of older adults with non-small cell lung cancer. J Am Geriatr Soc. (2019) 67:905–12. doi: 10.1111/jgs.15750
20. Tsukita Y, Tozuka T, Kushiro K, Hosokawa S, Sumi T, Uematsu M, et al. Immunotherapy or chemoimmunotherapy in older adults with advanced non-small cell lung cancer. JAMA Oncol. (2024) 10:439–47. doi: 10.1001/jamaoncol.2023.6277
21. Gao J, Zhang P, Tang M, Nie X, Yuan Y, Yang F, et al. Predictors of immune checkpoint inhibitor-related adverse events in older patients with lung cancer: a prospective real-world analysis. J Cancer Res Clin Oncol. (2023) 149:8993–9006. doi: 10.1007/s00432-023-04792-1
22. Gon Y, Zha L, Morishima T, Kimura Y, Asai K, Kudo H, et al. Non-cancer-related deaths in cancer survivors: A nationwide population-based study in Japan. J Epidemiol. (2025) 35:147–53. doi: 10.2188/jea.JE20240230
23. Lian J, Yue Y, Yu W, and Zhang Y. Immunosenescence: a key player in cancer development. J Hematol Oncol. (2020) 13:151. doi: 10.1186/s13045-020-00986-z
24. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
25. Girard N, Spicer J, Provencio M, Lu S, Broderick S, Awad MM, et al. Abstract CT012: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment for resectable (IB-IIIA) non-small cell lung cancer (NSCLC): Event-free survival (EFS) results from the phase 3 CheckMate 816 trial. Cancer Res. (2022) 82:CT012–CT12. doi: 10.1158/1538-7445.Am2022-ct012
26. Forde PM, Spicer J, Girard N, Provencio M, Lu S, Wang C, et al. 84O Neoadjuvant nivolumab (N) + platinum-doublet chemotherapy (C) for resectable NSCLC: 3-y update from CheckMate 816. J Thorac Oncol. (2023) 18:S89–90. doi: 10.1016/s1556-0864(23)00338-6
27. Spicer J, Girard N, Provencio M, Wang C, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab (NIVO) + chemotherapy (chemo) vs chemo in patients (pts) with resectable NSCLC: 4-year update from CheckMate 816. J Clin Oncol. (2024) 42:LBA8010–LBA10. doi: 10.1200/JCO.2024.42.17_suppl.LBA8010
28. Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian F, et al. Neoadjuvant camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for chinese patients with resectable stage IIIA or IIIB (T3N2) non-small cell lung cancer: the TD-FOREKNOW randomized clinical trial. JAMA Oncol. (2023) 9:1348–55. doi: 10.1001/jamaoncol.2023.2751
29. Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. (2024) 390:1756–69. doi: 10.1056/NEJMoa2311926
30. Provencio M, Nadal E, Gonzalez-Larriba JL, Martinez-Marti A, Bernabe R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2023) 389:504–13. doi: 10.1056/NEJMoa2215530
31. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983
32. Spicer JD, Gao S, Liberman M, Kato T, Tsuboi M, Lee SH, et al. LBA56 Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage non-small-cell lung cancer (NSCLC). Ann Oncol. (2023) 34:S1297–S98. doi: 10.1016/j.annonc.2023.10.052
33. Majem M, Garassino MC, Zhao G, Mennecier B, Grohe C, Ikeda N, et al. LBA3 Perioperative pembrolizumab (pembro) plus neoadjuvant chemotherapy (chemo) in early-stage non-small-cell lung cancer (NSCLC): A 4-year update of KEYNOTE-671. Immuno-Oncol Technol. (2024) 24. doi: 10.1016/j.iotech.2024.101027
34. Yue D, Wang W, Liu H, Chen Q, Chen C, Liu L, et al. Perioperative tislelizumab plus neoadjuvant chemotherapy for patients with resectable non-small-cell lung cancer (RATIONALE-315): an interim analysis of a randomized clinical trial. Lancet Respir Med. (2025) 13:119–29. doi: 10.1016/S2213-2600(24)00269-8
35. Yue D, Tan L, Xu S, Mao N, Hu J, Zhang L, et al. 108O Surgical outcomes from RATIONALE-315: Randomized, double-blind, phase III study of perioperative tislelizumab with neoadjuvant chemotherapy in resectable NSCLC. ESMO Open. (2024) 9. doi: 10.1016/j.esmoop.2024.102687
36. Yue D, Wang W, Liu H, Chen Q, Chen C, Liu L, et al. VP1-2024: RATIONALE-315: Event-free survival (EFS) and overall survival (OS) of neoadjuvant tislelizumab (TIS) plus chemotherapy (CT) with adjuvant TIS in resectable non-small cell lung cancer (NSCLC). Ann Oncol. (2024) 35:332–33. doi: 10.1016/j.annonc.2024.01.005
37. Lu S, Zhang W, Wu L, Wang W, Zhang P, Neotorch I, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. (2024) 331:201–11. doi: 10.1001/jama.2023.24735
38. Lu S, Wu L, Zhang W, Zhang P, Wang W, Fang W, et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): Interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol. (2023) 41:425126–26. doi: 10.1200/JCO.2023.41.36_suppl.425126
39. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Abstract CT005: AEGEAN: A phase 3 trial of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res. (2023) 83:CT005–CT05. doi: 10.1158/1538-7445.Am2023-ct005
40. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. (2023) 389:1672–84. doi: 10.1056/NEJMoa2304875
41. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Gao S, Urban L, et al. OA13.03 perioperative durvalumab for resectable NSCLC (R-NSCLC): updated outcomes from the phase 3 AEGEAN trial. J Thorac Oncol. (2024) 19:S38–9. doi: 10.1016/j.jtho.2024.09.069
Keywords: perioperative immunotherapy, resectable NSCLC, elderly patients, meta-analysis, pathologic complete response, event-free survival (EFS)
Citation: Cao Y, Tian Y and Li L (2025) Perioperative immune checkpoint inhibitors in elderly patients with resectable NSCLC: a systematic review and meta-analysis. Front. Oncol. 15:1589846. doi: 10.3389/fonc.2025.1589846
Received: 08 March 2025; Accepted: 21 August 2025;
Published: 17 September 2025.
Edited by:
Vera Rebmann, University of Duisburg-Essen, GermanyReviewed by:
Ran Wang, Anhui Medical University, ChinaMaria Anna Siciliano, AO Pugliese Ciaccio, Italy
Copyright © 2025 Cao, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Li, bGlsaW5fNTFAaG90bWFpbC5jb20=
 Yue Cao
Yue Cao Yumeng Tian2
Yumeng Tian2 Lin Li
Lin Li