- 1Department of Oncology, Shangrao People’s Hospital, Shangrao, China
- 2Department of Interventional Therapy, Ganzhou People’s Hospital, Ganzhou, China
- 3Department of Thoracic Surgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Background: Previous research has confirmed that integrating PD-1/PD-L1 inhibitors with chemotherapy (PC) represents a more effective strategy for treating advanced non-small-cell lung cancer (NSCLC). However, with the increasing number of phase 3 randomized controlled trials (RCTs) published in recent years, it is essential to re-evaluate the validity of this conclusion and to comprehensively assess the efficacy and safety across diverse patient subgroups.
Methods: We systematically reviewed phase 3 RCTs comparing PC with chemotherapy alone for stage IIIb-IV NSCLC. Data were extracted and analyzed for overall survival (OS), progression-free survival (PFS), response rates, and adverse events (AEs). Subgroup analyses were performed based on factors such as disease stage, pathological type, etc.
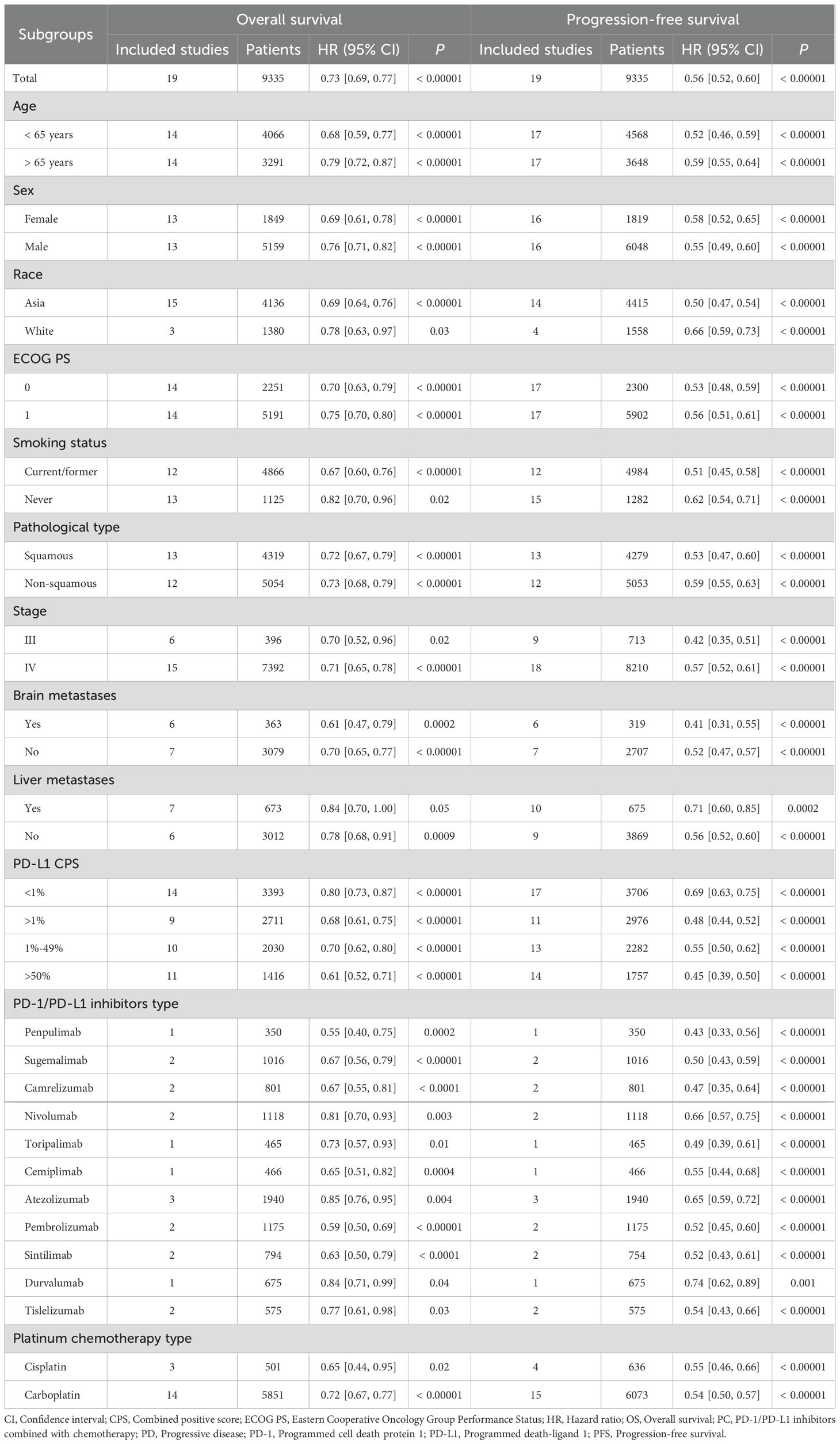
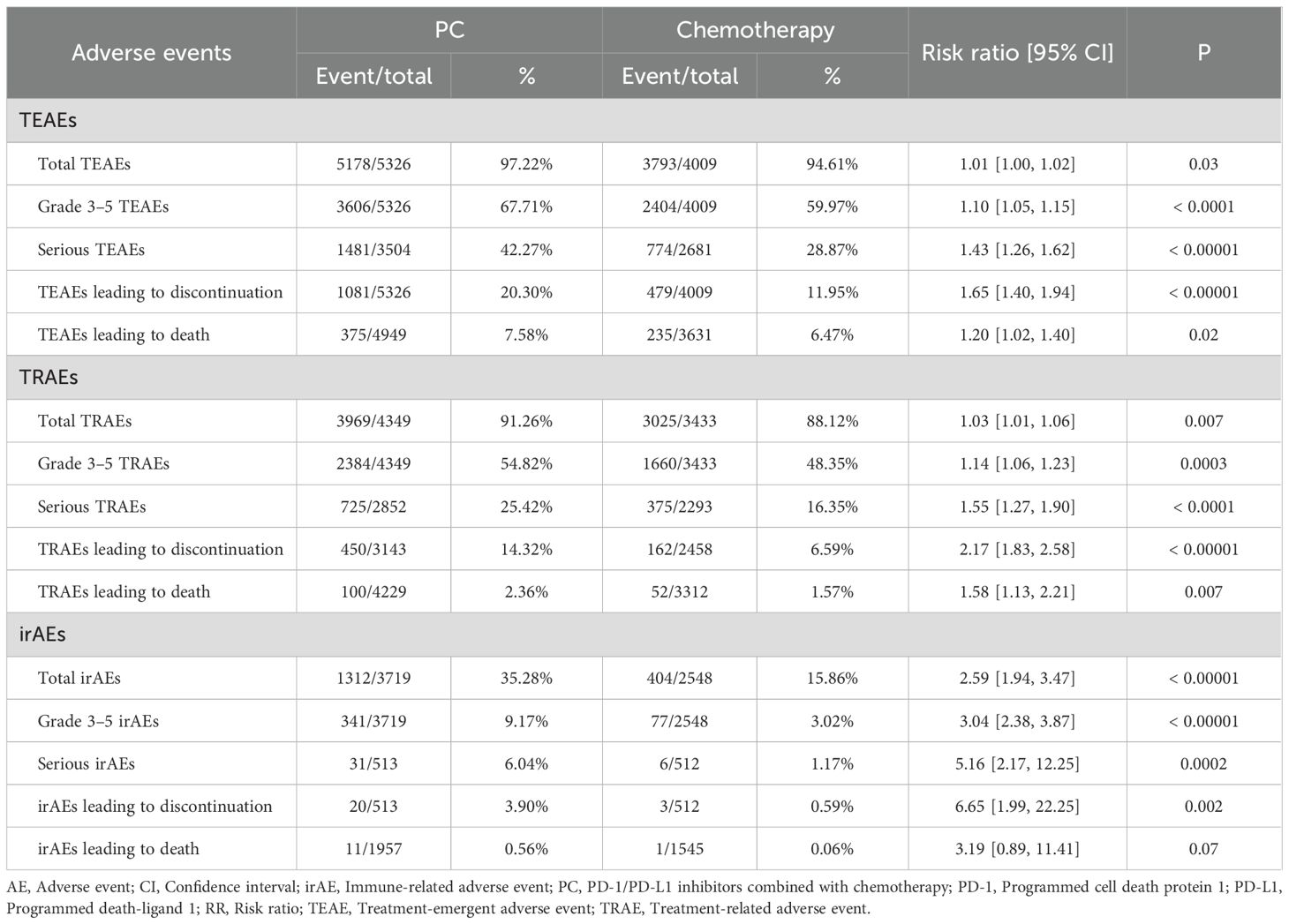
Results: After screening, 19 phase 3 RCTs involving 9335 patients were included. Our updated analysis confirmed at PC therapy significantly improves OS (hazard ratio [HR]: 0.73 [0.69, 0.77], P < 0.00001), PFS (HR: 0.56 [0.52, 0.60], P < 0.00001), duration of response (DOR, HR: 0.50 [0.45, 0.54], P < 0.00001) and objective response rate (ORR, risk ratio [RR]: 1.59 [1.51, 1.67], P < 0.00001) compared to chemotherapy alone. The survival benefits were consistent across all subgroups and increases with longer follow-up. Brain metastases and PD-L1 combined positive score (CPS) > 50% were the favorable factors for PC group. However, the combined treatment was associated with an increased incidence of total/grade 3–5 treatment emergent AEs (TEAEs), and immune-related AEs (irAEs), although the overall safety profile remained manageable. The most common AEs in the PC group were blood toxicity related AEs (anemia, neutrophil count decreased, etc).
Conclusion: The PC therapy continues to provide a substantial survival benefit for patients with stage IIIb-IV NSCLC. However, its higher incidence of AEs, especially irAEs, needs to be taken seriously.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251005925, identifier CRD420251005925.
Introduction
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related mortality globally, with advanced-stage disease often presenting limited treatment options and poor survival outcomes (1). One of the major challenges in current NSCLC treatment is the development of primary and acquired resistance to both chemotherapy and targeted therapies, which often leads to treatment failure. Moreover, conventional chemotherapy alone is limited by modest survival benefits and cumulative toxicity, while targeted therapies are only applicable to subsets of patients with specific driver mutations (2). The emergence of immune checkpoint inhibitors (ICIs), particularly those targeting the PD-1/PD-L1 pathway, has transformed the therapeutic landscape for advanced NSCLC. The rationale behind the PC regimen lies in its potential to overcome these limitations: chemotherapy not only reduces tumor burden but may also induce immunogenic cell death, thereby enhancing tumor antigen presentation, while PD-1/PD-L1 inhibitors restore T cell activity and help to counteract immune evasion and resistance mechanisms. By enhancing the immune system’s antitumor activity, these agents have demonstrated significant improvements in overall survival (OS) and progression-free survival (PFS) when administered in combination with chemotherapy (3).
However, the field is rapidly evolving, with numerous new phase 3 randomized controlled trials (RCTs) continually refining our understanding of these combinations (4–7). Recent studies have examined their efficacy across diverse patient subgroups, including those with varying PD-L1 expression levels, different histologic subtypes, and specific genetic mutations (4–7). While some trials reaffirm the superiority of PD-1/PD-L1 inhibitors combined with chemotherapy (PC), others report more nuanced outcomes, particularly in patients with low or negative PD-L1 expression, raising questions about universal applicability (8, 9). Meanwhile, safety remains a critical consideration. Immune-related adverse events (irAEs), such as pneumonitis and colitis, are well-documented risks of ICIs and may be exacerbated when combined with chemotherapy (10). Elderly patients and those with pre-existing autoimmune conditions are particularly susceptible, necessitating a careful evaluation of the risk-benefit profile in these populations (11).
Given the growing body of evidence, an updated meta-analysis is warranted to synthesize findings from recent phase 3 RCTs and provide a comprehensive evaluation of the benefits and risks of PC. This analysis aims to address key questions: (1) Does this combination continue to outperform chemotherapy alone in advanced NSCLC? (2) How do efficacy and safety profiles vary across patient subgroups? (3) What are the most frequent and severe adverse events (AEs) associated with these regimens? By integrating data from recent phase 3 RCTs, this meta-analysis seeks to offer evidence-based insights into optimizing treatment strategies for advanced NSCLC, ensuring that the benefits of these novel therapies are maximized while minimizing risks.
Materials and methods
Search strategy
Keywords including “PD-1/PD-L1 inhibitors”, “Lung cancer”, and “Randomized” were used in the search process. Six major databases-PubMed, ScienceDirect, Cochrane Library, Scopus, EMBASE, and Web of Science-were systematically searched. The investigation covered all available records from the inception of these databases up to February 13, 2025 (Supplementary Table S1).
Selection criteria
The inclusion criteria: (1) Participants: patients diagnosed with stage IIIb-IV NSCLC; (2) Intervention and control: PC compared to chemotherapy; (3) Outcomes: survival, response rates, and AEs; (4) Study design: phase 3 RCTs.
We excluded studies if they were retrospective studies, letters, review articles, editorials, and conference abstracts.
Data extraction
Two investigators independently collected data on study details (registration No., study name, etc), patient characteristics (age, pathological type, etc), survival outcomes (OS, PFS, etc), response rates (duration of response [DOR], objective response rate [ORR], etc), and AEs (treatment emergent AEs [TEAEs], irAEs, etc). In cases of missing data, corresponding authors were contacted for clarification, and discrepancies were resolved through re-evaluation by the investigators.
Outcome assessments
OS and PFS were subgroup analyzed based on age, sex, race, ECOG PS, smoking status, pathological type, stage, brain metastases, liver metastases, PD-L1 combined positive score (CPS), PD-1/PD-L1 inhibitors type, and platinum chemotherapy type. If specific subgroup data were missing in individual studies, those studies were excluded from the relevant subgroup analysis but remained in the overall analyses. The survival rates of OS (OSR) and PFS (PFSR) were evaluated at 6 to 60 months, and the duration of response rate (DORR) were assessed at 6 to48 months.
Quality assessment
Two instruments, the Cochrane Risk Assessment Tool and the Jadad scale, were used to assess the methodological quality of RCTs. The Jadad scale employs a 7-point scoring method, with scores ranging from 4 to 7 indicating high-quality studies (12, 13). Additionally, the outcomes were analyzed using the GRADE framework, which classifies evidence into four distinct levels: high, moderate, low, and very low (14).
Statistical analysis
STATA 12.0 and Review Manager 5.3 were used to perform data analysis. For survival outcomes, hazard ratios (HR) were used, whereas risk ratios (RR) were utilized for dichotomous data. Different articles from the same trial were considered only if they reported distinct outcomes, while for the same outcome we used the most recent or most complete dataset. A fixed-effects model was used for low heterogeneity (I² < 50% or P > 0.1), whereas a random-effects model was applied when heterogeneity was higher. Meanwhile, for outcomes exhibiting substantial heterogeneity, sensitivity analyses were also conducted by sequentially excluding individual studies to evaluate the robustness of the pooled estimates. A P-value below 0.05 was considered statistically significant. Publication bias was examined using funnel diagrams, along with Egger’s and Begg’s statistical tests (15, 16).
Results
Search results
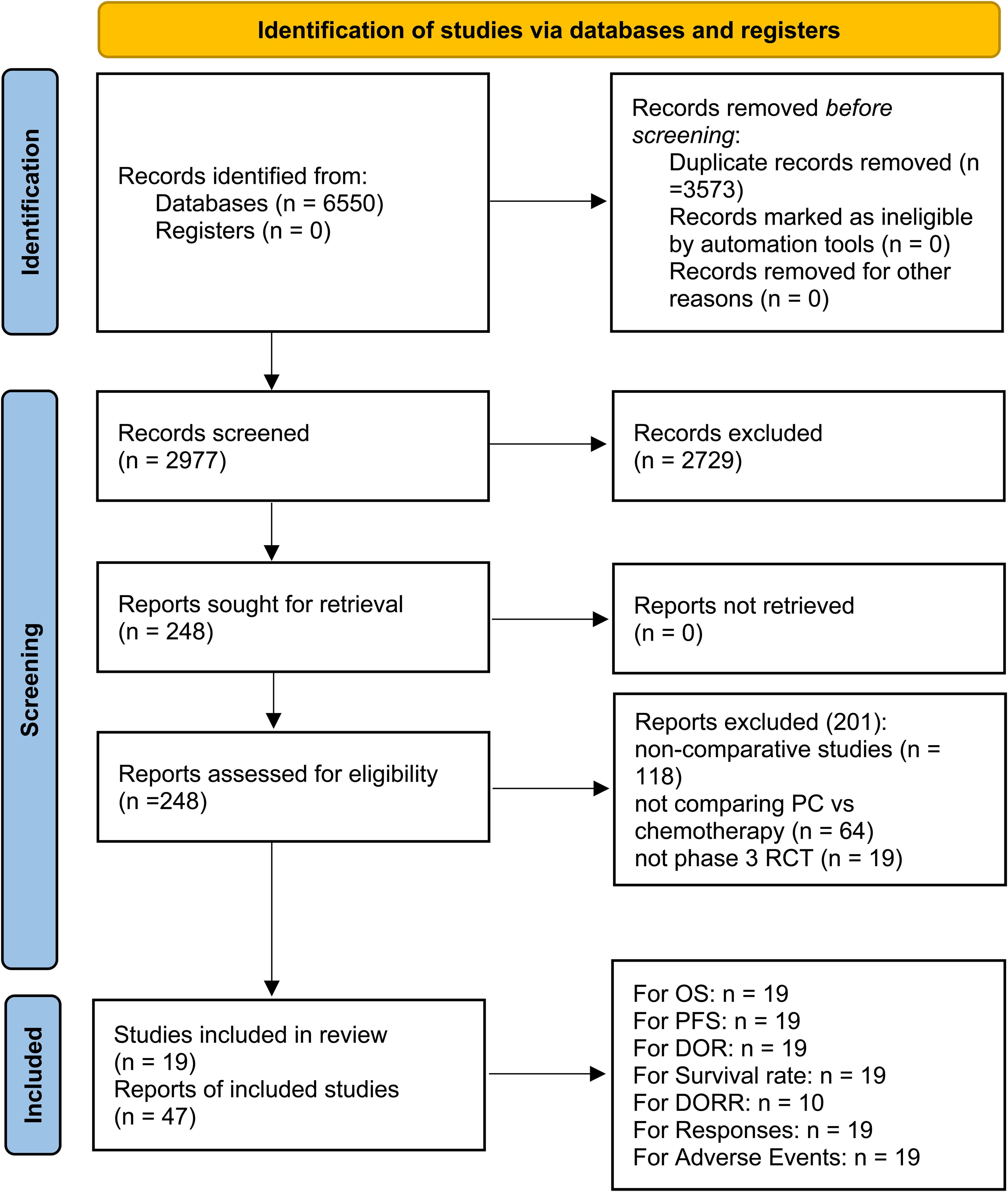
Among the 2977 screened studies, 47 reports from 19 phase 3 RCTs (AK105-302, ASTRUM-004, CameL, CameL-Sq, CheckMate 227 Part 1b, CheckMate 227 Part 2, CHOICE-01, EMPOWER-Lung 3, GEMSTONE-302, IMpower130, IMpower131, IMpower132, KEYNOTE-189, KEYNOTE-407, ORIENT-11, ORIENT-12, POSEIDON, RATIONALE-304, and RATIONALE-307), encompassing a total of 9335 patients, were selected (Figure 1) (4–9, 17–57). Table 1 summarizes the baseline characteristics of these studies. Of these, ten RCTs (5, 6, 8, 9, 21, 32–34, 37, 43) were international multicenter trials, while the remaining nine (4, 7, 17, 20, 30, 49, 52, 54, 56) were multicenter studies conducted in China. All included studies were considered high quality (Supplementary Figure S1, Supplementary Table S2). According to the GRADE framework, the evidence quality ranged from moderate to high (Supplementary Table S3).
Survival
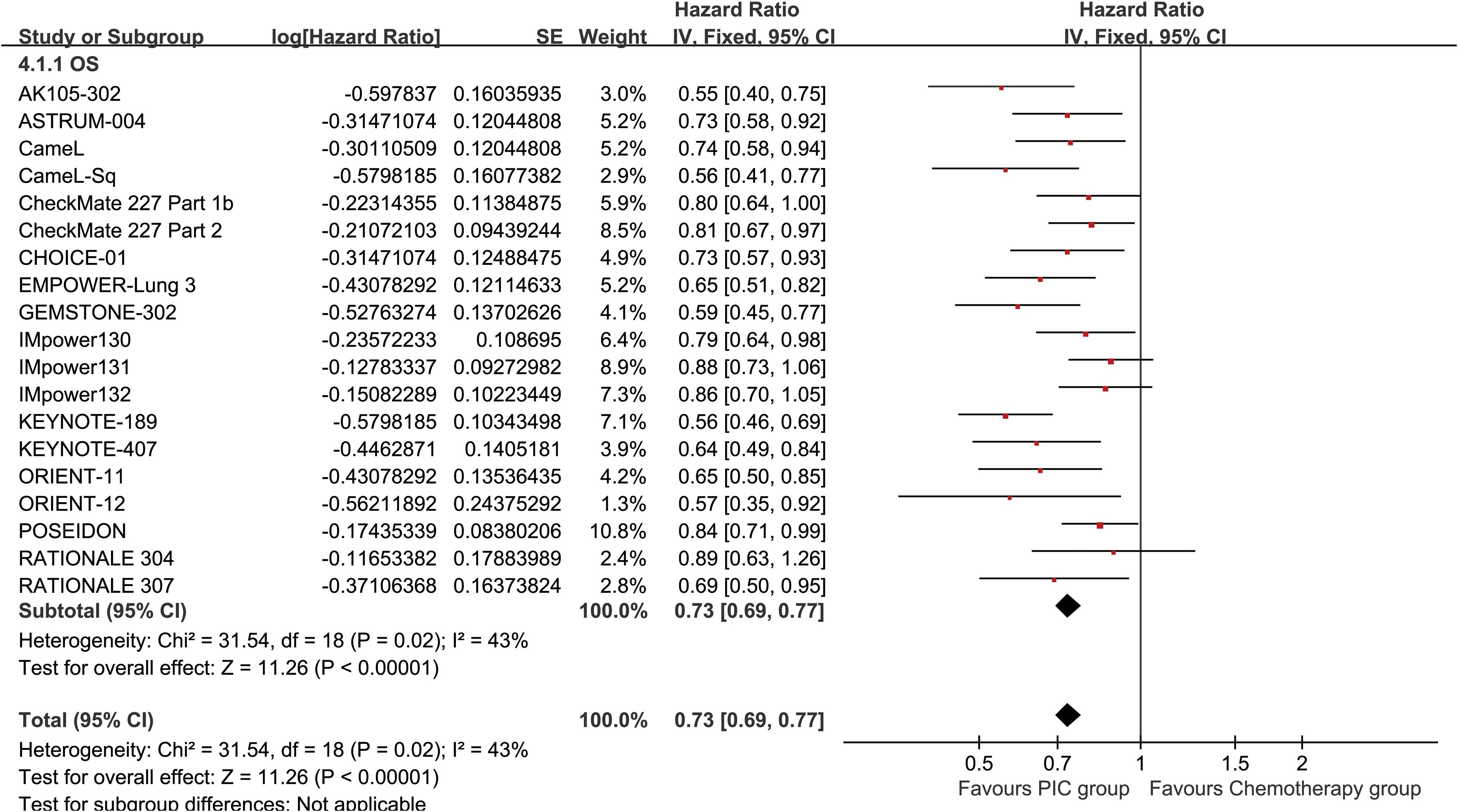
The PC group demonstrated superior OS (HR: 0.73 [0.69, 0.77], P < 0.00001) (Figure 2). OSR showed a significant advantage for the PC group over a period of 6 to 60 months. The OS benefit became more pronounced as survival time extended (Figure 3; Supplementary Figure S2).
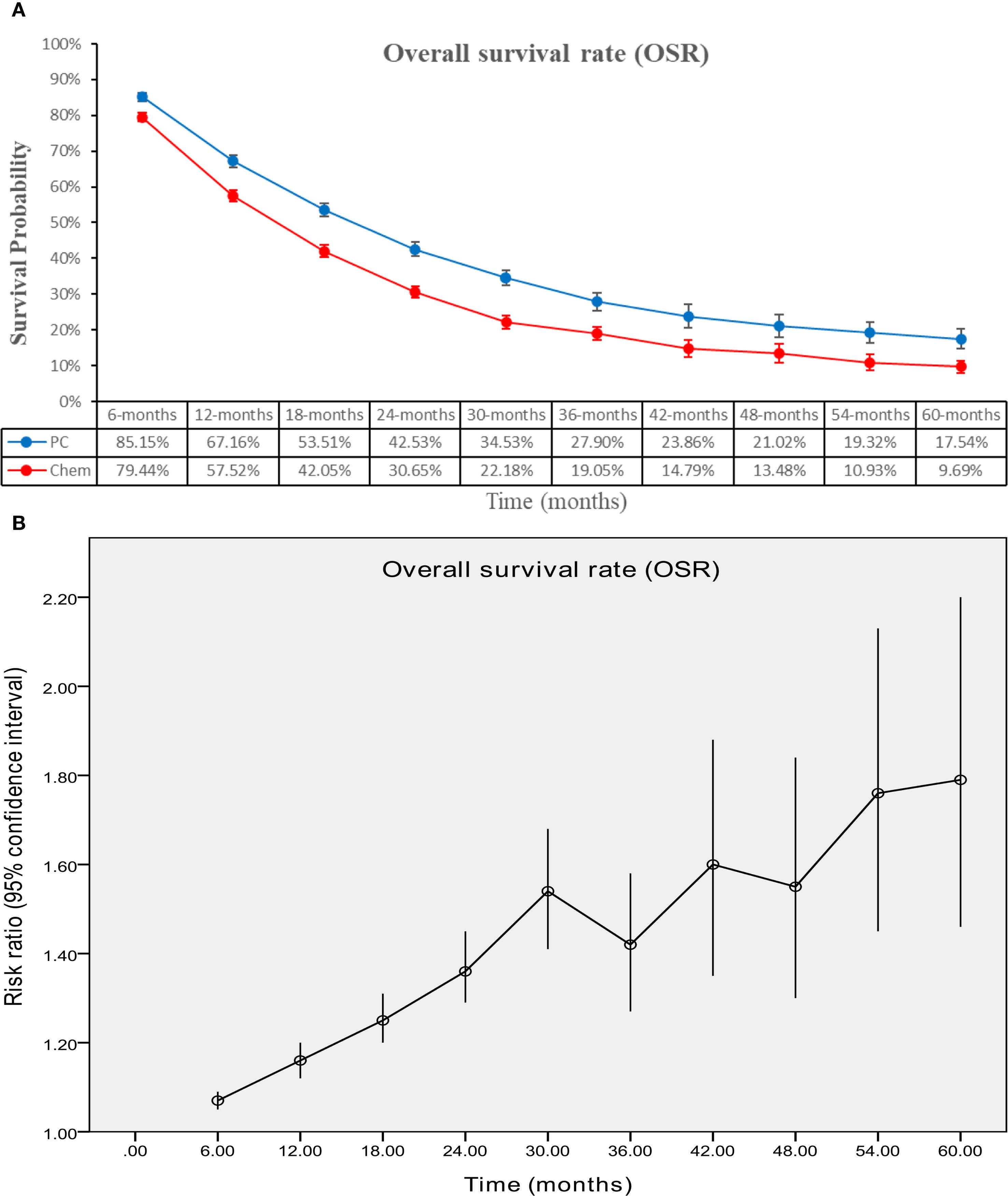
Figure 3. Comparisons of OSR associated with PC versus chemotherapy. (A) OSR at 6–60 months; (B) Trend of risk ratios in OSR.
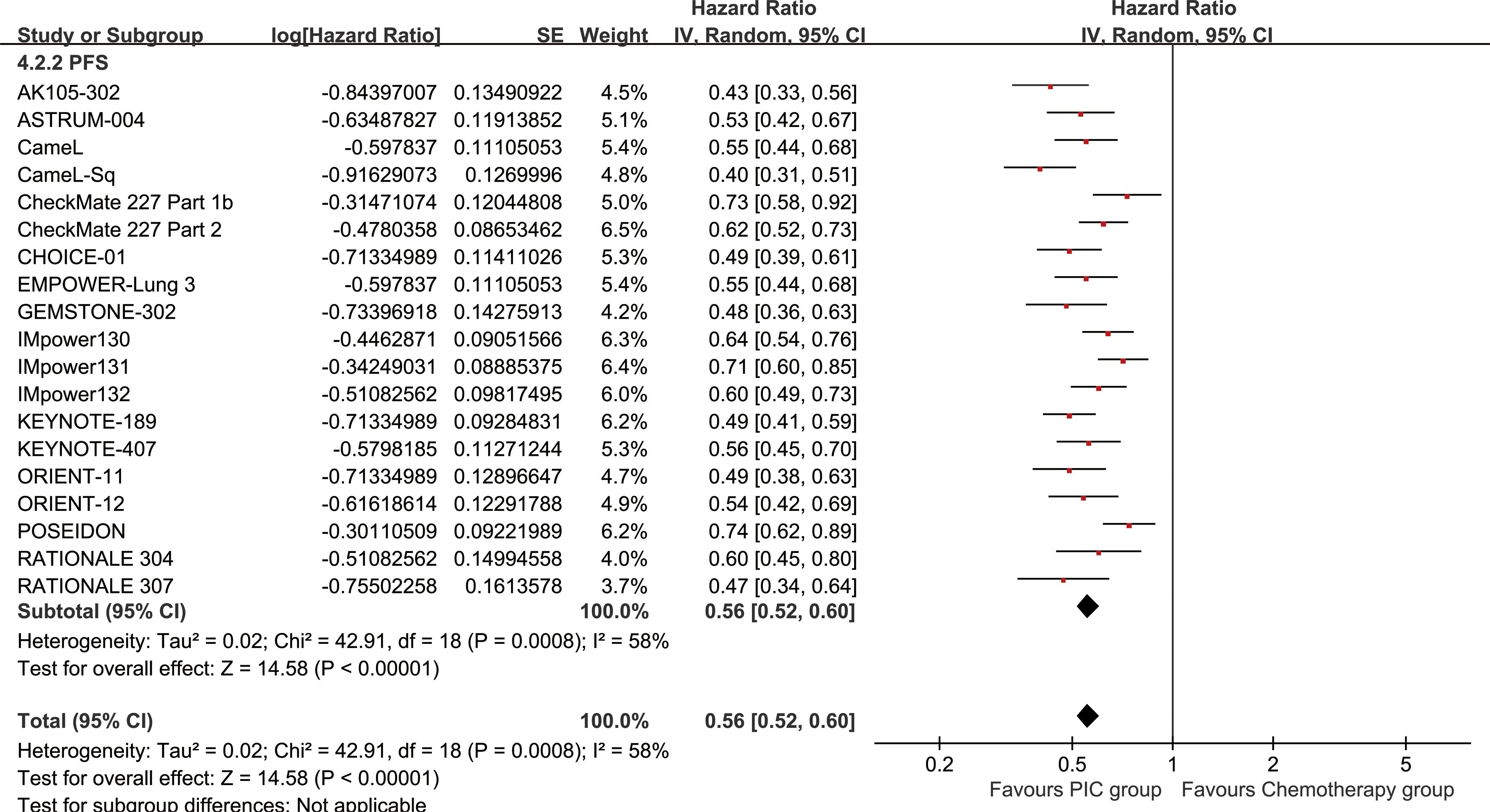
The PC group demonstrated enhanced PFS (HR: 0.56 [0.52, 0.60], P < 0.00001) (Figure 4). PFSR displayed a significant advantage for the PC group over a duration of 6 to 60 months. The PFS also benefit became more evident as survival time extended (Figure 5; Supplementary Figure S3).

Figure 5. Comparisons of PFSR associated with PC versus chemotherapy. (A) PFSR at 6–60 months; (B) Trend of risk ratios in PFSR.
Subgroup analysis of survival
OS and PFS consistently favored PC in all subgroups (as described in the outcome assessments). Brain metastases and PD-L1 CPS > 50% were the favorable factors for PC group in both OS and PFS (Table 2).
Responses
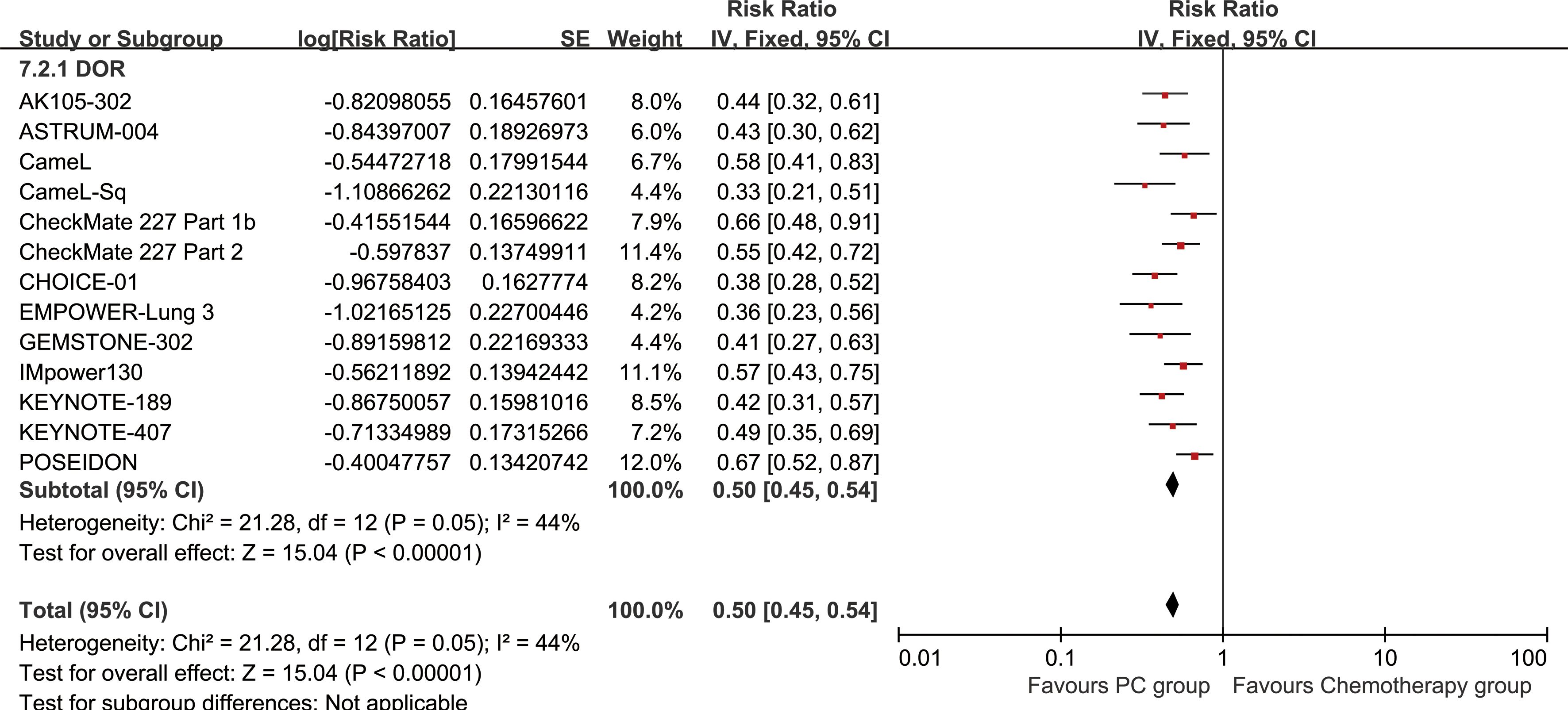
The PC group exhibited superior DOR (HR: 0.50 [0.45, 0.54], P < 0.00001) (Figure 6). DORR consistently favored the PC group over a period of 6 to 48 months (Figure 7; Supplementary Figure S4).
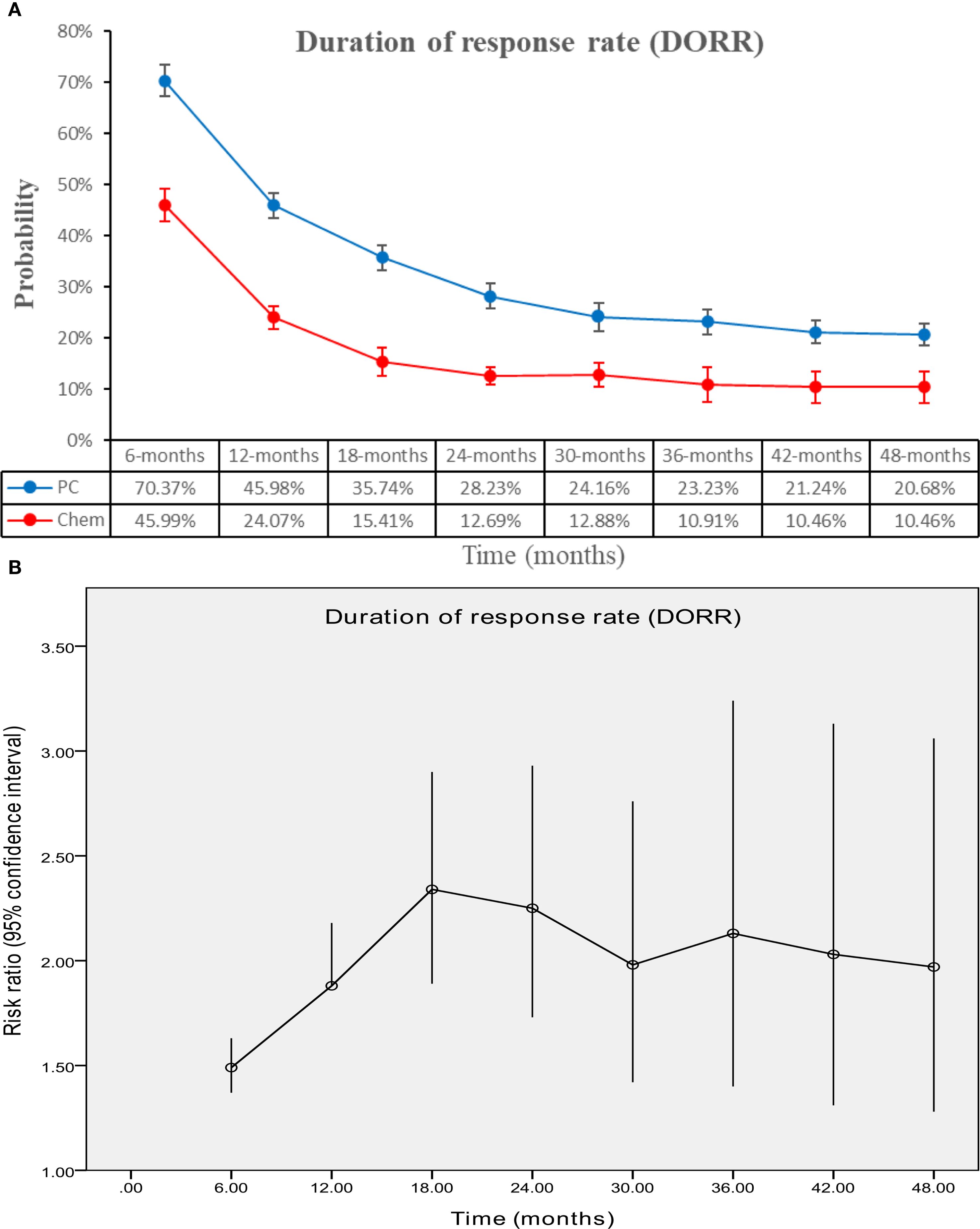
Figure 7. Comparisons of DORR associated with PC versus chemotherapy. (A) DORR at 6–48 months; (B) Trend of risk ratios in DORR.
The PC group achieved superior ORR (RR: 1.59 [1.51, 1.67], P < 0.00001), disease control rate (DCR) (RR: 1.12 [1.07, 1.18], P < 0.00001), complete response (CR) (RR: 2.30 [1.64, 3.23], P < 0.00001), and partial response (PR) (RR: 1.55 [1.47, 1.64], P < 0.00001). In contrast, the chemotherapy group had higher rates of stable disease (SD) (RR: 1.59 [1.51, 1.67], P < 0.00001) and progressive disease (PD) (RR: 1.55 [1.47, 1.64], P < 0.00001) (Table 3; Supplementary Figure S5).
Safety
Overall, the PC group experienced higher incidences of total TEAEs/TRAEs/irAEs, grade 3–5 TEAEs/TRAEs/irAEs, serious TEAEs/TRAEs/irAEs, TEAEs/TRAEs/irAEs leading to discontinuation, and TRAEs/irAEs leading to death (Table 4).
In TEAE analysis, the PC group showed higher occurrence of any grade nausea, alopecia, platelet count decreased, fatigue, alanine ALT increased, AST increased, decreased appetite, constipation, diarrhea, vomiting, pyrexia, hypoalbuminaemia, rash, arthralgia, edema peripheral, peripheral sensory neuropathy, pruritus, hyperglycemia, hypothyroidism, pneumonia, blood creatinine increased, hyperthyroidism, hypercholesteraemia, and interstitial lung disease (Supplementary Table S4). Meanwhile, the PC group also experienced higher rates of grade 3–5 platelet count decreased, fatigue, decreased appetite, diarrhea, arthralgia, and rash (Supplementary Table S5).
In irAEs analysis, the PC group showed higher occurrence of any grade hypothyroidism, pneumonitis, pneumonia, hepatitis, hyperthyroidism, severe skin reactions, infusion reactions, colitis, nephritis, adrenal insufficiency, and pancreatitis (Supplementary Table S6). Meanwhile, the PC group also experienced higher rates of grade 3–5 hepatitis, pneumonitis, severe skin reactions, colitis, hypothyroidism, and nephritis (Supplementary Table S7).
Sensitivity analysis
The findings for PFS, DCR, and total TEAEs remained robust after excluding individual studies in the sensitivity analysis (Supplementary Figure S6).
Publication bias
Funnel plots for OS, PFS, ORR, and grade 3–5 TEAEs appeared symmetrical, suggesting an acceptable level of publication bias (Supplementary Figure S7). Furthermore, no significant publication bias was found by Egger’s and Begg’s tests for these outcomes (all p > 0.05) (Supplementary Figure S8).
Discussion
The PC therapy has revolutionized the treatment landscape for advanced NSCLC, particularly for stage IIIb-IV disease. However, the rapid evolution of immunotherapy and the publication of numerous phase 3 RCTs in recent years have introduced new complexities and controversies (4–7). Given the persistent challenges of drug resistance and the limitations of current therapies, the PC regimen offers a rational approach by combining cytotoxic and immune-mediated mechanisms to achieve more durable responses. While earlier studies established the superiority of PC over chemotherapy alone, emerging evidence indicates that the benefits may vary across patient subgroups, particularly those with low or negative PD-L1 expression (17, 20). Additionally, the safety profile of PC, especially the incidence of irAEs, remains a critical concern, particularly for vulnerable populations such as elderly patients or those with pre-existing autoimmune conditions (21, 27). These uncertainties underscore the need for an updated meta-analysis to synthesize the latest evidence and conduct a thorough assessment of PC’s effectiveness and safety in advanced NSCLC. This updated meta-analysis, encompassing 19 phase 3 RCTs and 9,335 patients, confirms the significant survival benefits of PC over chemotherapy alone. The pooled results demonstrate substantial improvements in OS, PFS, DOR, and ORR. Subgroup analyses further reveal that patients with brain metastases and those with a PD-L1 CPS > 50% derive the greatest benefit from PC. Nevertheless, the combined treatment showed increased occurrences of AEs, including TEAEs, TRAEs, and irAEs, necessitating careful risk-benefit assessment in clinical practice.
The survival benefits of PC in advanced NSCLC are robust, as evidenced by marked improvements in OS (HR: 0.73) and PFS (HR: 0.56) in our meta-analysis. These findings align with recent studies, such as the KEYNOTE-189 and IMpower130 trials, which reported similar hazard ratios for OS and PFS in favor of PC (35, 37). Notably, the survival benefits of PC appear to increase over time, with OS and PFS rates showing greater divergence between the PC and chemotherapy groups at longer follow-up intervals. This suggests that the immunomodulatory effects of PD-1/PD-L1 inhibitors may provide durable clinical benefits, a phenomenon also observed in other malignancies treated with immune checkpoint inhibitors (4, 20). Subgroup analyses further illuminate the differential efficacy of PC across patient populations. Patients with brain metastases, a historically poor prognostic group, exhibited particularly pronounced survival benefits from PC. This finding is consistent with recent studies highlighting the potential of immunotherapy to penetrate the blood-brain barrier and exert antitumor effects in the central nervous system (37, 49). Meanwhile, all evaluated PD-1/PD-L1 inhibitors confer OS and PFS benefits versus chemotherapy, supporting a class effect in advanced NSCLC. While numerical differences in pooled HRs are apparent across agents, these arise from indirect, across-trial contrasts with heterogeneous populations, backbones, PD-L1 assays, and follow-up durations. Notably, patients with PD-L1 CPS >50% have consistently demonstrated a greater magnitude of benefit from immunotherapy-based regimens, highlighting the potential of CPS as a predictive biomarker in clinical decision-making. However, variability in testing methods and thresholds remains a challenge for universal application (58). In our subgroup analysis, patients with elevated PD-L1 expression (CPS > 50%) showed greater survival advantages, underscoring PD-L1 as a key predictor of immunotherapy effectiveness. In contrast, the survival benefits in patients with low or negative PD-L1 expression, though statistically significant, were less pronounced, raising considerations regarding the cost-effectiveness of PC in this subgroup (37, 39). Tumor mutational burden (TMB) has emerged as another promising biomarker, as tumors with high TMB tend to harbor more neoantigens, which can enhance immune recognition and response to immune checkpoint inhibitors. Recent evidence suggests that TMB may serve as an independent predictor of treatment efficacy across multiple cancer types. Incorporating both PD-L1 CPS and TMB into predictive models may improve the precision of patient stratification in future clinical trials (59). These findings highlight the need for further research to identify additional biomarkers that can refine patient selection for PC. Enhanced DOR further reinforces PC’s survival advantage, with a significantly prolonged duration in the PC group (HR: 0.50). This suggests that PC not only delays disease progression but also sustains tumor control over a more extended period, a key factor in improving long-term outcomes. The durability of response is particularly important in the context of immunotherapy, where the immune system’s memory effect can lead to prolonged antitumor activity even after treatment discontinuation (17, 21). Although our meta-analysis focuses on clinical outcomes, emerging preclinical and translational studies provide insight into the potential mechanisms underlying the superior efficacy of PD-1/PD-L1 inhibitors combined with chemotherapy. Chemotherapy can enhance tumor immunogenicity by increasing neoantigen presentation and promoting immunogenic cell death, thereby synergizing with PD-1/PD-L1 blockade to enhance cytotoxic T-cell responses. Furthermore, PC therapy has been shown to modulate the tumor microenvironment by reducing immunosuppressive cells such as regulatory T cells and myeloid-derived suppressor cells, and by increasing the infiltration and activation of effector CD8+ T cells. Cytokine profiling studies have also suggested that combined therapy may augment pro-inflammatory cytokine production, contributing to durable antitumor responses (60). Recent studies have further broadened the landscape of NSCLC research in ways that may intersect with immunotherapy. For example, analysis of bronchoalveolar lavage fluid microbiota has revealed significant associations with prognosis and immune modulation in NSCLC, while novel agents such as cycloastragenol have shown antitumor efficacy through apoptosis and autophagy pathways, potentially enhancing immunotherapeutic responses. These findings highlight the need to integrate clinical, microbiological, and molecular perspectives to optimize future immunotherapy-based strategies (61, 62).
In addition to survival outcomes, this meta-analysis highlights the superior response rates associated with PC. The PC group exhibited an ORR approximately 60% higher than the chemotherapy group (RR: 1.59), with similar improvements observed in DCR, CR, and PR rates. These findings are consistent with recent trials, such as the ORIENT-11 and RATIONALE-307 studies, which reported ORRs exceeding 60% in the PC arms (49, 56). The improved response rates likely contribute to the observed survival benefits, as deeper and more durable responses are associated with prolonged disease control and delayed progression. The DOR, a key measure of treatment response durability, was notably extended in the PC group (HR: 0.50). This finding aligns with the hypothesis that immunotherapy enhances the immune system’s ability to maintain long-term tumor control, even after the cessation of treatment (9, 43). However, the higher rates of PD in the chemotherapy group suggest that PC may be particularly effective in preventing disease progression, a key determinant of survival in advanced NSCLC. The improved response rates and DOR observed in the PC group may also have implications for patient quality of life. Patients who achieve a complete or partial response are more likely to experience symptom relief and improved functional status, which are critical considerations in the management of advanced NSCLC (4, 17). Furthermore, the higher rates of disease control in the PC group may reduce the need for subsequent lines of therapy, thereby minimizing the cumulative toxicity associated with multiple treatment regimens.
While the efficacy of PC is well-established, its safety profile remains a critical consideration. Our meta-analysis verifies that PC leads to increased incidences of TEAEs, TRAEs, and irAEs relative to chemotherapy alone. The most common AEs in the PC group were hematologic toxicities, such as anemia and decreased neutrophil count, which are likely attributable to the chemotherapy component of the regimen. However, the higher incidence of irAEs, including pneumonitis, hepatitis, and colitis, underscores the unique toxicity profile of immunotherapy (54, 56). The higher occurrence of grade 3–5 AEs, especially irAEs, in the PC group underscores the importance of close monitoring and proactive toxicity management. Recent studies have emphasized the importance of multidisciplinary care teams and standardized protocols for managing irAEs, which can significantly reduce morbidity and mortality associated with these events (63). Additionally, the higher rates of treatment discontinuation and death due to TRAEs/irAEs in the PC group underscore the importance of patient selection and risk stratification, particularly for vulnerable populations such as elderly patients or those with pre-existing autoimmune conditions (49, 52). The safety profile of PC also has implications for treatment sequencing and combination strategies. For example, a greater occurrence of irAEs in the PC group might hinder the viability of future immunotherapy for patients with severe toxic effects. Although rare, severe irAEs such as myocarditis and Guillain–Barré syndrome were observed in the PC group. These findings underscore the importance of vigilant monitoring and long-term follow-up, as some rare toxicities may emerge late or be underreported in clinical trials (64). This underscores the need for personalized treatment approaches that balance the potential benefits of PC with the risks of toxicity, particularly in patients with comorbidities or poor performance status (58).
Compared with the study by Meng et al. (2022), which analyzed a broader NSCLC population and demonstrated consistent OS and PFS benefits across PD-L1 subgroups, our findings align in confirming the robust efficacy of ICI plus chemotherapy but also expand upon their work by including additional trials and updated evidence (65). In contrast, Chen et al. (2022) focused specifically on squamous NSCLC, reporting stronger relative benefits in OS and PFS, likely reflecting histology-specific sensitivity to chemo-immunotherapy, but with a higher incidence of hematologic and hepatic toxicities (66). These differences can be largely attributed to variations in patient populations (all NSCLC vs. squamous only), the scope of included trials, and the weighting of safety endpoints (67). Taken together, our meta-analysis complements prior work by providing a more comprehensive overview across different PD-1/PD-L1 inhibitors and NSCLC subtypes, while also highlighting that efficacy and safety profiles may vary depending on histology and study design.
Despite its comprehensive scope, this meta-analysis has several limitations. First, heterogeneity among the included studies in patient populations, treatment regimens, and follow-up durations might have impacted the pooled outcomes. Second, the subgroup analyses, while informative, were limited by the availability of data in the original studies. For example, the impact of specific genetic mutations, such as EGFR or ALK alterations, on the efficacy of PC could not be fully explored due to insufficient data. Third, the long-term safety profile of PC remains incompletely characterized, as many of the included studies had relatively short follow-up periods. Fourth, the meta-analysis was unable to assess the cost-effectiveness of PC, a important consideration for healthcare systems worldwide. Fifth, our study is limited by the lack of data on the optimal sequencing of PD-1/PD-L1 inhibitors and chemotherapy, as most trials used induction chemoimmunotherapy followed by maintenance immunotherapy. Sixth, a further limitation is the absence of direct RCTs comparing different PD-1/PD-L1 inhibitors; our agent-level subgroup results versus chemotherapy cannot be interpreted as between-agent comparisons. Seventh, another limitation is the potential bias in AE reporting across trials, as differences in grading systems, monitoring intensity, and reporting standards may affect the comparability of safety outcomes. Finally, the long-term incidence of rare irAEs could not be fully characterized due to limited follow-up in the included trials.
Conclusion
This updated meta-analysis confirms the significant survival and response benefits of PC therapy in advanced NSCLC. The survival benefits were consistent across all subgroups (particularly effective in patients with brain metastases and high PD-L1 expression) and increases with the prolongation of survival time. Nevertheless, the increased occurrence of AEs, especially irAEs, requires careful patient selection and proactive toxicity control. Future studies should aim to discover novel biomarkers for better patient stratification and develop strategies to mitigate PC-related risks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. CY: Conceptualization, Data curation, Formal Analysis, Writing – original draft. QH: Conceptualization, Data curation, Formal Analysis, Writing – original draft. WC: Conceptualization, Data curation, Formal Analysis, Writing – original draft. WZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. WXZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. TZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors appreciate all the public health workers who participated in the databases (PubMed, ScienceDirect, the Cochrane Library, Scopus, EMBASE, and Web of Science).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1590017/full#supplementary-material
Supplementary Figure 1 | Cochrane Risk Assessment.
Supplementary Figure 2 | Forest plots of OSR at 6–60 months associated with PC versus chemotherapy.
Supplementary Figure 3 | Forest plots of PFSR at 6–60 months associated with PC versus chemotherapy.
Supplementary Figure 4 | Forest plots of DORR at 6–48 months associated with PC versus chemotherapy.
Supplementary Figure 5 | Forest plots of responses associated with PC versus chemotherapy.
Supplementary Figure 6 | Sensitivity analysis of PFS (A), DCR (B), and total TEAEs (C).
Supplementary Figure 7 | Funnel plots of OS (A), PFS (B), ORR (C), and grade 3–5 TEAEs (D).
Supplementary Figure 8 | Egger’s and Begg’s tests of OS (A), PFS (B), and grade TEAEs (C).
Abbreviations
AE, Adverse event; ALK, Anaplastic lymphoma kinase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CI, Confidence interval; CPS, Combined positive score; CR, Complete response; DCR, Disease control rate; DOR, Duration of response; DORR, Duration of response rate; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, Epidermal growth factor receptor; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HR, Hazard ratio; ICI, Immune checkpoint inhibitor; irAE, Immune-related adverse event; M/F, Male/Female; Non-sq, Non-squamous non-small-cell lung cancer; NSCLC, Non-small-cell lung cancer; ORR, Objective response rate; OS, Overall survival; OSR, Overall survival rate; PC, PD-1/PD-L1 inhibitors combined with chemotherapy; PD, Progressive disease; PD-1, Programmed cell death protein 1; PD-L1, Programmed death-ligand 1; PFS, Progression-free survival; PFSR, Progression-free survival rate; PR, Partial response; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International prospective register of systematic reviews; Sq, Squamous cell carcinoma; RCT, Randomized controlled trial; RET, Rearranged during transfection; ROS1, ROS Proto-Oncogene 1, receptor tyrosine kinase; RR, Risk ratio; SD, Stable disease; TEAE, Treatment-emergent adverse event; TRAE, Treatment-related adverse event.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Leighl NB, Ismaila N, Durm G, Florez N, Freeman-Daily J, Pellini B, et al. Therapy for stage IV non-small cell lung cancer without driver alterations: ASCO living guideline, version 2024.3. J Clin Oncol. (2025) 43(10):e17–30. doi: 10.1200/JCO-24-02786
3. Wang L, Yang Y, Yu J, Zhang S, Li X, Wu X, et al. Efficacy and safety of anti-PD-1/PD-L1 in combination with chemotherapy or not as first-line treatment for advanced non-small cell lung cancer: A systematic review and network meta-analysis. Thorac Cancer. (2022) 13:322–37. doi: 10.1111/1759-7714.14244
4. Zhong H, Sun S, Chen J, Wang Z, Zhao Y, Zhang G, et al. First-line penpulimab combined with paclitaxel and carboplatin for metastatic squamous non-small-cell lung cancer in China (AK105-302): a multicentre, randomised, double-blind, placebo-controlled phase 3 clinical trial. Lancet Respir Med. (2024) 12:355–65. doi: 10.1016/S2213-2600(23)00431-9
5. Zhou C, Hu Y, Arkania E, Kilickap S, Ying K, Xu F, et al. A global phase 3 study of serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer (ASTRUM-004). Cancer Cell. (2024) 42:198–208.e3. doi: 10.1016/j.ccell.2023.12.004
6. Borghaei H, O’Byrne KJ, Paz-Ares L, Ciuleanu TE, Yu X, Pluzanski A, et al. Nivolumab plus chemotherapy in first-line metastatic non-small-cell lung cancer: results of the phase III CheckMate 227 Part 2 trial. ESMO Open. (2023) 8:102065. doi: 10.1016/j.esmoop.2023.102065
7. Zhong J, Fei K, Wu L, Li B, Wang Z, Cheng Y, et al. Toripalimab plus chemotherapy for first line treatment of advanced non-small cell lung cancer (CHOICE-01): final OS and biomarker exploration of a randomized, double-blind, phase 3 trial. Signal Transduct Target Ther. (2024) 9:369. doi: 10.1038/s41392-024-02087-6
8. Baramidze A, Makharadze T, Gogishvili M, Melkadze T, Giorgadze D, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer with PD-L1 ≥ 1%: A subgroup analysis from the EMPOWER-Lung 3 part 2 trial. Lung Cancer. (2024) 193:107821. doi: 10.1016/j.lungcan.2024.107821
9. Peters S, Cho BC, Luft AV, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy in first-line metastatic NSCLC: five-year overall survival outcomes from the phase 3 POSEIDON trial. J Thorac Oncol. (2025) 20:76–93. doi: 10.1016/j.jtho.2024.09.1381
10. Wang H, Xia J, Yu A, Cao M, Zhao Y, Qin X, et al. Observation on the therapeutic efficacy of camrelizumab combined with chemotherapy in non-small cell lung cancer and the cutaneous immune-related adverse events: A retrospective study. Anticancer Agents Med Chem. (2025) 25(8):574–87. doi: 10.2174/0118715206350978241105080452
11. Bertaglia V, Petrelli F, Dottorini L, Carnio S, Morelli AM, Nepote A, et al. Chemotherapy plus immunotherapy as first line combination in older patients with extensive stage small cell lung cancer: A systematic review and meta-analysis. Semin Oncol. (2024) 52:14–8. doi: 10.1053/j.seminoncol.2024.11.001
12. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
13. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, and Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
15. Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ. (1998) 316:471.
16. Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
17. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed as first-line therapy for advanced non-squamous non-small-cell lung cancer: 5-year outcomes of the CameL randomized phase 3 study. J Immunother Cancer. (2024) 12:e009240. doi: 10.1136/jitc-2024-009240
18. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed as first-line treatment for advanced nonsquamous NSCLC: extended follow-up of cameL phase 3 trial. J Thorac Oncol. (2023) 18:628–39. doi: 10.1016/j.jtho.2022.12.017
19. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
20. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
21. Reck M, Ciuleanu TE, Lee JS, Schenker M, Zurawski B, Kim SW, et al. Systemic and intracranial outcomes with first-line nivolumab plus ipilimumab in patients with metastatic NSCLC and baseline brain metastases from checkMate 227 part 1. J Thorac Oncol. (2023) 18:1055–69. doi: 10.1016/j.jtho.2023.04.021
22. Brahmer JR, Lee JS, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in checkMate 227. J Clin Oncol. (2023) 41:1200–12. doi: 10.1200/JCO.22.01503
23. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 checkMate 227 part 1 trial. J Thorac Oncol. (2022) 17:289–308. doi: 10.1016/j.jtho.2021.09.010
24. Reck M, Schenker M, Lee KH, Provencio M, Nishio M, Lesniewski-Kmak K, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. (2019) 116:137–47. doi: 10.1016/j.ejca.2019.05.008
25. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
26. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
27. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. (2023) 41:651–63. doi: 10.1200/JCO.22.00727
28. Makharadze T, Gogishvili M, Melkadze T, Baramidze A, Giorgadze D, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in advanced NSCLC: 2-year follow-up from the phase 3 EMPOWER-lung 3 part 2 trial. J Thorac Oncol. (2023) 18:755–68. doi: 10.1016/j.jtho.2023.03.008
29. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. (2022) 28:2374–80. doi: 10.1038/s41591-022-01977-y
30. Zhou C, Wang Z, Sun M, Cao L, Ma Z, Wu R, et al. Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC. Nat Cancer. (2023) 4:860–71. doi: 10.1038/s43018-023-00578-z
31. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. (2022) 23:220–33. doi: 10.1016/S1470-2045(21)00650-1
32. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
33. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. (2020) 15:1351–60. doi: 10.1016/j.jtho.2020.03.028
34. Lu S, Fang J, Wang Z, Fan Y, Liu Y, He J, et al. Results from the IMpower132 China cohort: Atezolizumab plus platinum-based chemotherapy in advanced non-small cell lung cancer. Cancer Med. (2023) 12:2666–76. doi: 10.1002/cam4.5144
35. Nishio M, Saito H, Goto K, Watanabe S, Sueoka-Aragane N, Okuma Y, et al. IMpower132: Atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci. (2021) 112:1534–44. doi: 10.1111/cas.14817
36. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. (2021) 16:653–64. doi: 10.1016/j.jtho.2020.11.025
37. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
38. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. (2021) 32:881–95. doi: 10.1016/j.annonc.2021.04.008
39. Horinouchi H, Nogami N, Saka H, Nishio M, Tokito T, Takahashi T, et al. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study. Cancer Sci. (2021) 112:3255–65. doi: 10.1111/cas.14980
40. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) 21:387–97. doi: 10.1016/S1470-2045(19)30801-0
41. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
42. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
43. Sugawara S, Tanaka K, Imamura F, Yamamoto N, Nishio M, Okishio K, et al. Pembrolizumab plus chemotherapy in Japanese patients with metastatic squamous non-small-cell lung cancer in KEYNOTE-407. Cancer Sci. (2023) 114:3330–41. doi: 10.1111/cas.15816
44. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
45. Cheng Y, Zhang L, Hu J, Wang D, Hu C, Zhou J, et al. Pembrolizumab plus chemotherapy for chinese patients with metastatic squamous NSCLC in KEYNOTE-407. JTO Clin Res Rep. (2021) 2:100225. doi: 10.1016/j.jtocrr.2021.100225
46. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
47. Mazieres J, Kowalski D, Luft A, Vicente D, Tafreshi A, Gümüş M, et al. Health-related quality of life with carboplatin-paclitaxel or nab-paclitaxel with or without pembrolizumab in patients with metastatic squamous non-small-cell lung cancer. J Clin Oncol. (2020) 38:271–80. doi: 10.1200/JCO.19.01348
48. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
49. Zhang L, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer. (2022) 171:56–60. doi: 10.1016/j.lungcan.2022.07.013
50. Yang Y, Sun J, Wang Z, Fang J, Yu Q, Han B, et al. Updated overall survival data and predictive biomarkers of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC in the phase 3 ORIENT-11 study. J Thorac Oncol. (2021) 16:2109–20. doi: 10.1016/j.jtho.2021.07.015
51. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
52. Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol. (2021) 16:1501–11. doi: 10.1016/j.jtho.2021.04.011
53. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. (2023) 41:1213–27. doi: 10.1200/JCO.22.00975
54. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ma Z, et al. Tislelizumab plus chemotherapy as first-line treatment of locally advanced or metastatic nonsquamous non-small-cell lung cancer (final analysis of RATIONALE-304: a randomized phase III trial). ESMO Open. (2024) 9:103728. doi: 10.1016/j.esmoop.2024.103728
55. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol. (2021) 16:1512–22. doi: 10.1016/j.jtho.2021.05.005
56. Wang J, Lu S, Yu X, Hu Y, Zhao J, Sun M, et al. Tislelizumab plus chemotherapy versus chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: final analysis of the randomized, phase III RATIONALE-307 trial. ESMO Open. (2024) 9:103727. doi: 10.1016/j.esmoop.2024.103727
57. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
58. Deng Y, Li Y, Xu R, Li J, and Cui W. The critical role of PD-L1 expression in immunotherapy for advanced non-small cell lung cancer. Quant Imaging Med Surg. (2025) 15:4894–5. doi: 10.21037/qims-2025-209
59. Vryza P, Fischer T, Mistakidi E, and Zaravinos A. Tumor mutation burden in the prognosis and response of lung cancer patients to immune-checkpoint inhibition therapies. Transl Oncol. (2023) 38:101788. doi: 10.1016/j.tranon.2023.101788
60. Yang Q, Na J, Qin S, Su J, Huang H, and Zhong L. Anticancer mechanisms and applications based on γδ T cells in cancer immunotherapy. Crit Rev Oncol Hematol. (2025) 214:104814. doi: 10.1016/j.critrevonc.2025.104814
61. Cheng C, Wang Z, Ding C, Liu P, Xu X, Li Y, et al. Bronchoalveolar lavage fluid microbiota is associated with the diagnosis and prognosis evaluation of lung cancer. Phenomics. (2024) 4:125–37. doi: 10.1007/s43657-023-00135-9
62. Zhu LH, Liang YP, Yang L, Zhu F, Jia LJ, and Li HG. Cycloastragenol induces apoptosis and protective autophagy through AMPK/ULK1/mTOR axis in human non-small cell lung cancer cell lines. J Integr Med. (2024) 22:503–14. doi: 10.1016/j.joim.2024.05.004
63. Yu Y, Chen N, Yu S, Shen W, Zhai W, Li H, et al. Association of immune-related adverse events and the efficacy of anti-PD-(L)1 monotherapy in non-small cell lung cancer: adjusting for immortal-time bias. Cancer Res Treat. (2024) 56:751–64. doi: 10.4143/crt.2023.1118
64. Ogunniyi KE, Djunadi TA, Adewara O, Babawale I, Akinmoju OD, Olaiya VO, et al. Immunotherapy-induced cardiotoxicity: A narrative review of real-world case reports, recent information and clinical evidence. Cardiovasc Toxicol. (2025) 25:1381–410. doi: 10.1007/s12012-025-10038-y
65. Meng LF, Huang JF, Luo PH, Huang SX, and Wang HL. The efficacy and safety of immune checkpoint inhibitor plus chemotherapy in patients with advanced non-small-cell lung cancer: a meta-analysis. Invest New Drugs. (2022) 40:810–7. doi: 10.1007/s10637-022-01232-8
66. Chen Q, Zhang Z, Li X, and Bu L. Chemotherapy combined with immunotherapy as a first-line treatment brings benefits to patients with lung squamous cell carcinoma but different risks of adverse reactions: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:940567. doi: 10.3389/fphar.2022.940567
Keywords: PD-1/PD-L1 inhibitors, chemotherapy, non-small-cell lung cancer, meta-analysis, phase 3 randomized controlled trials
Citation: Xu Y, Zhong B, Yu C, Hou Q, Chen W, Zheng W, Zhang W and Zhou T (2025) The benefits and risks of adding PD-1/PD-L1 inhibitors to chemotherapy for stage IIIb-IV non-small-cell lung cancer: an updated meta-analysis based on phase 3 randomized controlled trials. Front. Oncol. 15:1590017. doi: 10.3389/fonc.2025.1590017
Received: 08 March 2025; Accepted: 28 August 2025;
Published: 11 September 2025.
Edited by:
Julie Tabiasco, INSERM UMR1291 Institut Toulousain des Maladies Infectieuses et Inflammatoires, FranceReviewed by:
Chen Ling, Fudan University, ChinaLing Yang, Xinyu People’s Hospital, China
Xia Hu, Jiangxi Provincial People’s Hospital, China
Copyright © 2025 Xu, Zhong, Yu, Hou, Chen, Zheng, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonggang Zhou, emhvdXRvbmdnYW5nYUAxNjMuY29t
 Yun Xu1
Yun Xu1 Wen Zheng
Wen Zheng Tonggang Zhou
Tonggang Zhou