- Department of Hepatobiliary Surgery, Yueyang Affiliated Hospital of Hunan Normal University, Yueyang, China
Background: Intrahepatic cholangiocarcinoma (iCCA) is a malignant tumor second only to hepatocellular carcinoma in terms of incidence among primary liver cancers. Surgical resection is currently the preferred treatment for iCCA. However, the prognostic significance, complications, and clinical benefits of lymph node dissection (LND) in iCCA patients remain a topic of debate within the academic community.
Methods: To evaluate the impact of LND on overall survival (OS) and prognosis in patients with resectable iCCA, studies published from various databases, including PubMed, Embase, Web of Science, and the Cochrane Library. A meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The impact of LND on prognosis was analyzed.
Results: A total of 5,787 patients from twenty-one retrospective cohorts were included in the final analysis. The results indicated that clinically node-negative patients who underwent LND had significantly better survival outcomes compared to those who did not undergo LND (P<0.01). In the R0 resection subgroup, LND was associated with improved survival compared to non-LND (P<0.01), while in the non-R0 resection subgroup, the LND group exhibited significantly fewer survival benefits than the non-LND group (P<0.01). When Compared to patients in the non-LND group, those in the LND N- group demonstrated significantly greater survival (P<0.05), while patients in the LND N+ group experienced significantly shorter OS (P<0.01).
Conclusion: Patients with resectable iCCA who underwent LND had better survival outcomes compared to those who did not undergo LND. Therefore, routine LND should be performed for clinically lymph node-negative (cLNM-) iCCA patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024564741.
1 Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver cancer after hepatocellular carcinoma and accounts for approximately 20% of all malignant liver tumors (1). Over the past two decades, the global incidence of iCCA has gradually increased, which may reflect both an increase in disease prevalence and an increasing trend toward early diagnosis (2). Surgical resection is considered the only treatment option that may extend patient survival, with approximately 20%-30% of patients being suitable candidates for this procedure (3). Lymph node metastasis (LNM) has been identified as a significant prognostic factor that affects iCCA outcomes (4), and lymph node dissection (LND) is crucial for managing this aspect of the disease. Despite controversial findings regarding its efficacy, routine LND for iCCA is recommended by the National Comprehensive Cancer Network (NCCN) guidelines (5, 6). Among the studies supporting LND, Umeda (4) et al. suggested that LND may significantly improve tumor prognosis, particularly in patients with hilar iCCA. However, LND does not appear to have a significant impact on survival rates among peripheral iCCAs compared with hilar carcinomas due to the greater potential of iCCA for intrahepatic or extrahepatic metastasis. Additionally, the study by Ke (7) et al. indicated that LND benefits clinical practice by guiding postoperative management specifically for iCCA patients with negative LNM results. Furthermore, research by Sposito (8) et al. demonstrated that adequate LND leads to improved survival outcomes among cN0 (clinically negative) iCCA patients with positive pathological lymph node negativity, thereby supporting the routine use of adequate LND in the treatment of patients with cN0 iCCA. In contrast, studies opposing LND, such as those by Kim (9) and Chang (10) et al. have shown that routine LND does not improve survival of iCCA patients. However, lymph node sampling remains valuable for accurate staging and plays a pivotal role in predicting outcomes and determining whether adjuvant therapy should be administered.
Due to some controversy remains regarding the beneficial effect and role of LND in post-iCCA patients, we performed this meta-analysis of published literature to assess the impact of LND on overall survival (OS) and prognosis among patients with resectable iCCA.
2 Materials and methods
2.1 Eligibility criteria
This study was reported in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Assessing the Methodological Quality of Systematic Reviews guidelines (11, 12). The inclusion criteria were as follows: (1) Study type: Cohort studies including retrospective or prospective designs were considered. Publications from January 1, 2013, to September 31, 2024, were included regardless of country of origin or language (Chinese or English). (2) Subjects: All cases included in the literature had a confirmed diagnosis of iCCA through pathological analysis. (3) Interventions and Terminology Definitions: Patients were categorized into two groups: those who underwent LND and those who did not undergo lymph node dissection (nLND). Patients with LND are categorized based on pathological results into two groups: LND N+ (those undergoing LND with pathological confirmation of positive lymph nodes) and LND N- (those undergoing LND with pathological confirmation of negative lymph nodes). The classification of lymph node status is as follows: cLNM- indicates preoperative clinical lymph node negative status (cLNM- patients are defined as individuals who show no positive evidence of lymph node involvement through imaging examinations, including computed tomography (CT), ultrasound, positron emission tomography-computed tomography (PET-CT), and magnetic resonance imaging (MRI), as well as biomarkers such as CA 19-9); cLNM+ indicates preoperative clinical lymph node positive status; pLNM- indicates pathological lymph node negative status; and pLNM+ indicates pathological lymph node positive status. Finally, based on intraoperative surgical margins, the classifications are as follows: R0 indicates microscopically negative resection margins (complete tumor resection), and R1 indicates incomplete resection. (4) Outcome measures: OS (OS was defined as the time from randomization to death from any cause), DFS (DFS was defined as the time from randomization to disease recurrence or death from any cause), Kaplan–Meier survival curve, postoperative complications, and postoperative recurrence rate. The exclusion criteria were as follows: (1) duplicate publications; (2) sample size <60 patients; (3) nonprimary iCCA combined with other primary malignant tumors; (4) lack of relevant outcome indicators. And (5) The grouping method was inconsistent, as it was not clearly delineated into the LND group and the non-LND group.
2.2 Information sources and search strategy
By searching computer databases such as PubMed, Embase, Web of Science, the Cochrane Library, Wanfang, China Knowledge Network, and other relevant literature published in the past 10 years, the most recent literature was included, with a search time limit from January 1, 2013, to September 31, 2024. The search strategy was developed according to the Cochrane Collaboration’s handbook: the search terms included “Bile Duct Neoplasms,” “Cholangiocarcinoma,” “Intrahepatic cholangiocarcinoma,” “Biliary tract cancers,” “Intrahepatic bile duct carcinoma,” “iCCA,” “Lymph Node Excision,” “Lymphatic clearance,” and “Lymphadenectomy.”
2.3 Data collection process and data items
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses standard was used to screen the literature. Two researchers independently read the literature, excluding obviously unqualified studies, such as duplicate literature, and then independently read the titles and abstracts of the literature obtained. They ensured that the literature met the inclusion criteria, and finally, they read the full texts carefully to determine final inclusion of each study. If any discrepancies were found during the screening process and if it was difficult to determine whether or not to include a study, its inclusion was discussed, sometimes by consultation with a third researcher. The extracted data included authors’ names, publication years, countries, sample sizes, patient sex, LND and nLND sample sizes, study types, matching methods, median follow-up times, multicenter study status, surgical margin status, OS, DFS, postoperative complications, and postoperative recurrence rate. Postoperative complications were evaluated according to the Clavien-Dindo classification of surgical complications (13).
2.4 Quality assessment of the literature
The Newcastle-Ottawa Scale was used to assess the quality of the literature. The quality assessment criteria included the selection of study subjects, comparability between groups, and measurement of exposure factors. Each study was scored from 0 to 9 points, and two researchers independently assessed the scores. Discrepancies were resolved through discussion and consultation with a third researcher. Cohort studies with scores ≥6 were considered to be of high quality, and studies with scores ≥4 were included in this meta-analysis.
2.5 Statistical analysis
RevMan 5.3 software was used for the data analysis. Patients were divided into the LND and nLND groups based on whether LND was performed. The LND group was further divided into a lymph node-positive group (N1 group) and a lymph node-negative group (N0 group). OS and DFS were used as effect sizes and are represented by hazard ratio (HR). For the included studies, the HR values for the risk rate were obtained through two approaches: 1. The risk rate values were explicitly given in the included literature. 2. If the risk rate values were not given in the original literature, Engauge-Digitizer data extraction software was used. The HR obtained from the preliminary study underwent multivariable adjustment. This software is used to extract data from target curves in the literature, and in this study, the target curve was the Kaplan–Meier survival curve. After extracting the target data, the risk rate was calculated using the risk rate calculation method summarized by Tierney, J.F (14). The postoperative recurrence rate and postoperative complication rate were analyzed using odds ratios. The chi-square test and I2 test were used to assess heterogeneity between study results. A P>0.1 and I2<50% indicated low heterogeneity between studies, and a fixed-effects model was used to combine effect sizes. A P<0.1 or I2>50% indicated heterogeneity between studies, and a random-effects model was used to combine effect sizes. Subgroup analysis was performed if necessary to explore the source of heterogeneity and to evaluate the reliability of the results. To control the family-wise error rate in subgroup analyses, P-values were adjusted using the Holm-Bonferroni method. All reported P-values in subgroup comparisons reflect this adjustment unless otherwise specified. A funnel plot was drawn to test for publication bias.
3 Results
3.1 Study selection and study characteristics
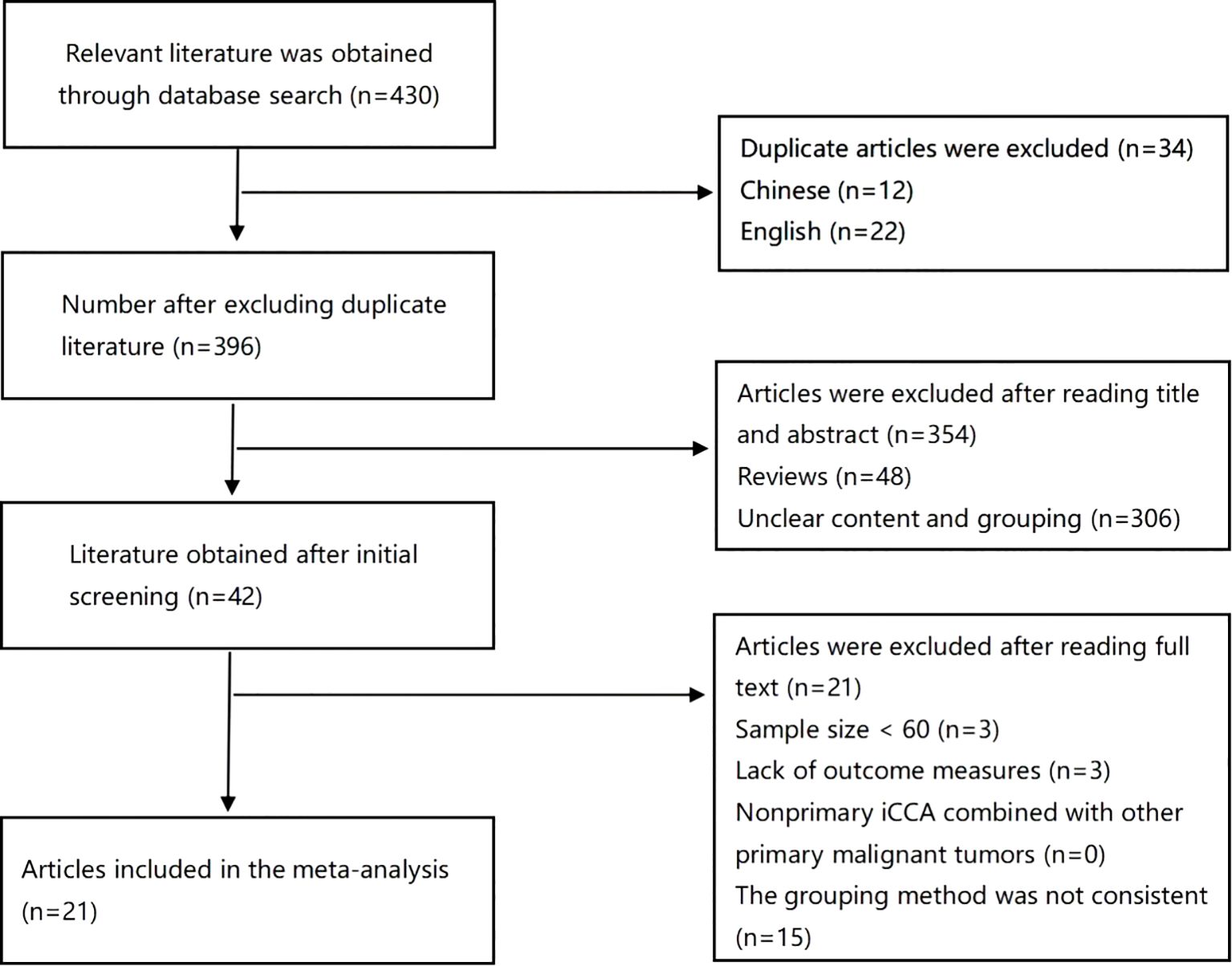
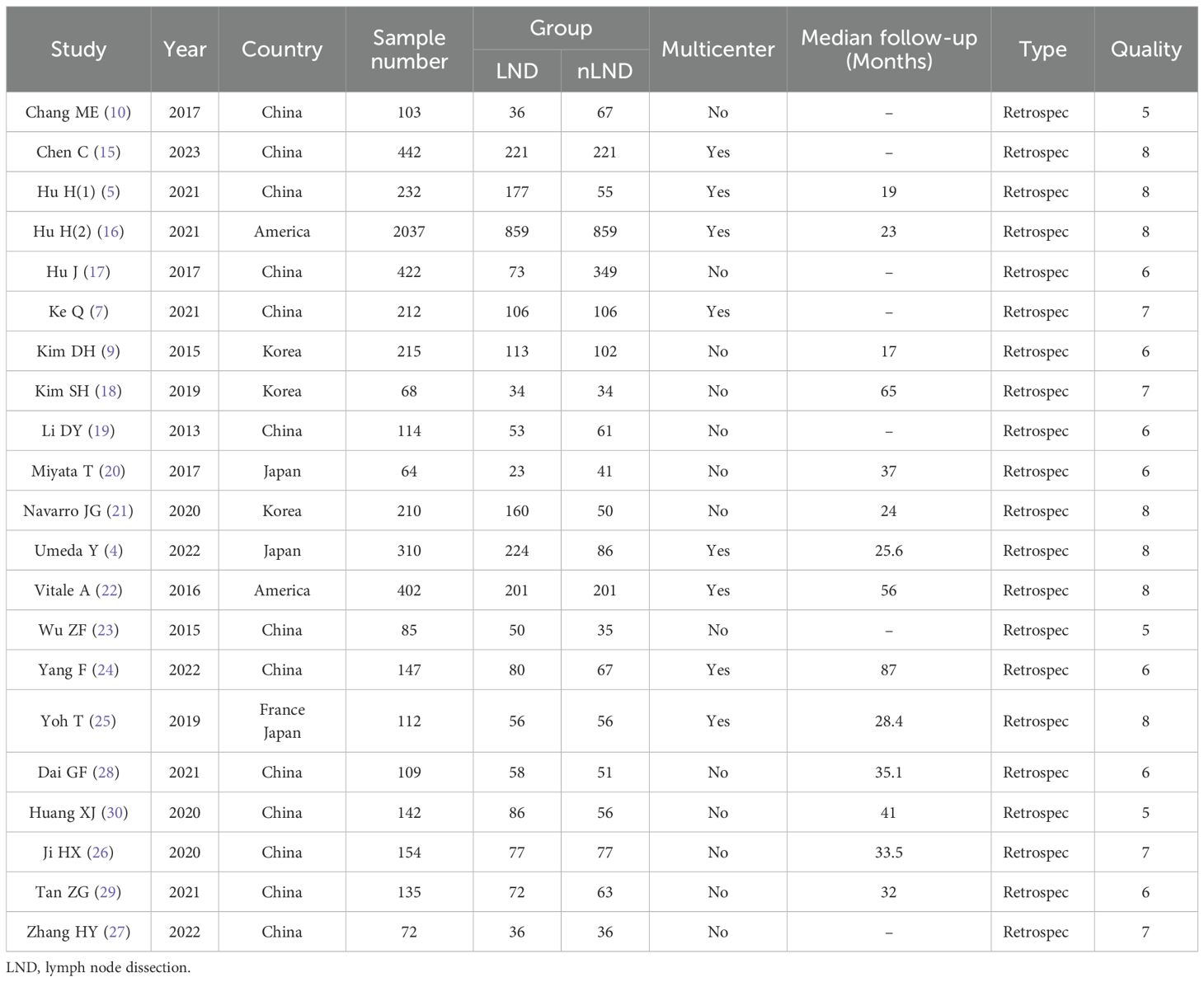
The search results are summarized in Figure 1. In all, 430 articles were retrieved through database searches, 34 duplicate articles were excluded, 354 articles were excluded after reading the abstracts, and 21 articles were excluded after reading the full texts; finally, 21 eligible studies were included in the analysis (4, 5, 7, 9, 10, 15–30) with a total of 5787 patients. No discrepancies occurred between the researchers regarding inclusion and exclusion criteria. The characteristics of the 21 included studies are shown in Table 1. All included studies were retrospective, with 18 studies from Asia, including 13 from China (5, 7, 10, 15, 17, 19, 23, 24, 26–30), 2 from Japan (4, 20), 3 from South Korea (9, 18, 21), 2 from the United States (16, 22), and one joint study from Japan and France (25).
3.2 Survival outcomes and meta-analysis results
3.2.1 Meta-analysis of LND and nLND patients
Eighteen studies (4, 5, 7, 9, 15–18, 20–22, 24–30) (18 studies,91.11%,5273/5787)were included in the analysis of OS (Figure 2a). Three studies (10, 19, 23) did not conduct a comprehensive survival analysis comparing LND and nLND, so they were excluded.High heterogeneity was observed between the studies (I2 = 73%, P<0.1), and a random-effects model was used for the analysis. The results indicated no significant difference in OS between the LND and nLND groups (HR=0.84, 95% CI: 0.70-1.01; P=0.06, Padj=0.0166).Twelve studies (5, 9, 15, 17, 18, 20, 21, 25–29) (12 studies,38.62%,2235/5787) were included in the analysis of DFS (Figure 2b). High heterogeneity was observed between the studies (I2 = 78%, P<0.1), and a random-effects model was used for the analysis. The results indicated no significant difference in DFS between the LND and nLND groups (HR=0.81, 95% CI: 0.63-1.04; P=0.10, Padj=0.025). The subgroup analyses for patients with cLNM- and R0 resection were pre-specified in our protocol based on clinical relevance. Subgroup analyses were conducted as follows.
![Forest plots displaying meta-analysis results of hazard ratios for two groups: (a) involves 18 studies with a total effect estimate of 0.84 [0.70, 1.01] and significant heterogeneity (I² = 73%); (b) involves 12 studies with a total effect estimate of 0.81 [0.63, 1.04] and significant heterogeneity (I² = 78%). The plots illustrate individual study estimates and confidence intervals, with overall effect sizes represented by diamond shapes. The x-axis shows a logarithmic scale of hazard ratios favoring experimental or control interventions.](https://www.frontiersin.org/files/Articles/1590019/fonc-15-1590019-HTML/image_m/fonc-15-1590019-g002.jpg)
Figure 2. Forest plot comparing OS (a) and DFS (b) in the LND and nLNDgroups. LND, lymph node dissection; OS, Overall Survival; DFS, Disease-Free Survival; IV, Inverse variance method; CI, confidence interval; SE, standard error.
Among them, 7 studies (7, 15, 17, 25, 27, 28, 30) included only cLNM- patients (Figure 3). High heterogeneity was observed between the studies, and analysis revealed that 4 studies (7, 15, 25, 27) used matching methods (4 studies,14.48%,838/5787), such as propensity score matching, while the remaining 3 studies (17, 28, 30) did not use any matching methods. After excluding these studies, the heterogeneity significantly decreased (I2 = 0%, P=0.94), and a fixed-effects model was used for the analysis. The results indicated that the LND group had a survival benefit compared with the nLND group for patients in the cLNM- subgroup, and the difference was statistically significant (HR=0.66, 95% CI: 0.56-0.79, P<0.001, Padj=0.005).
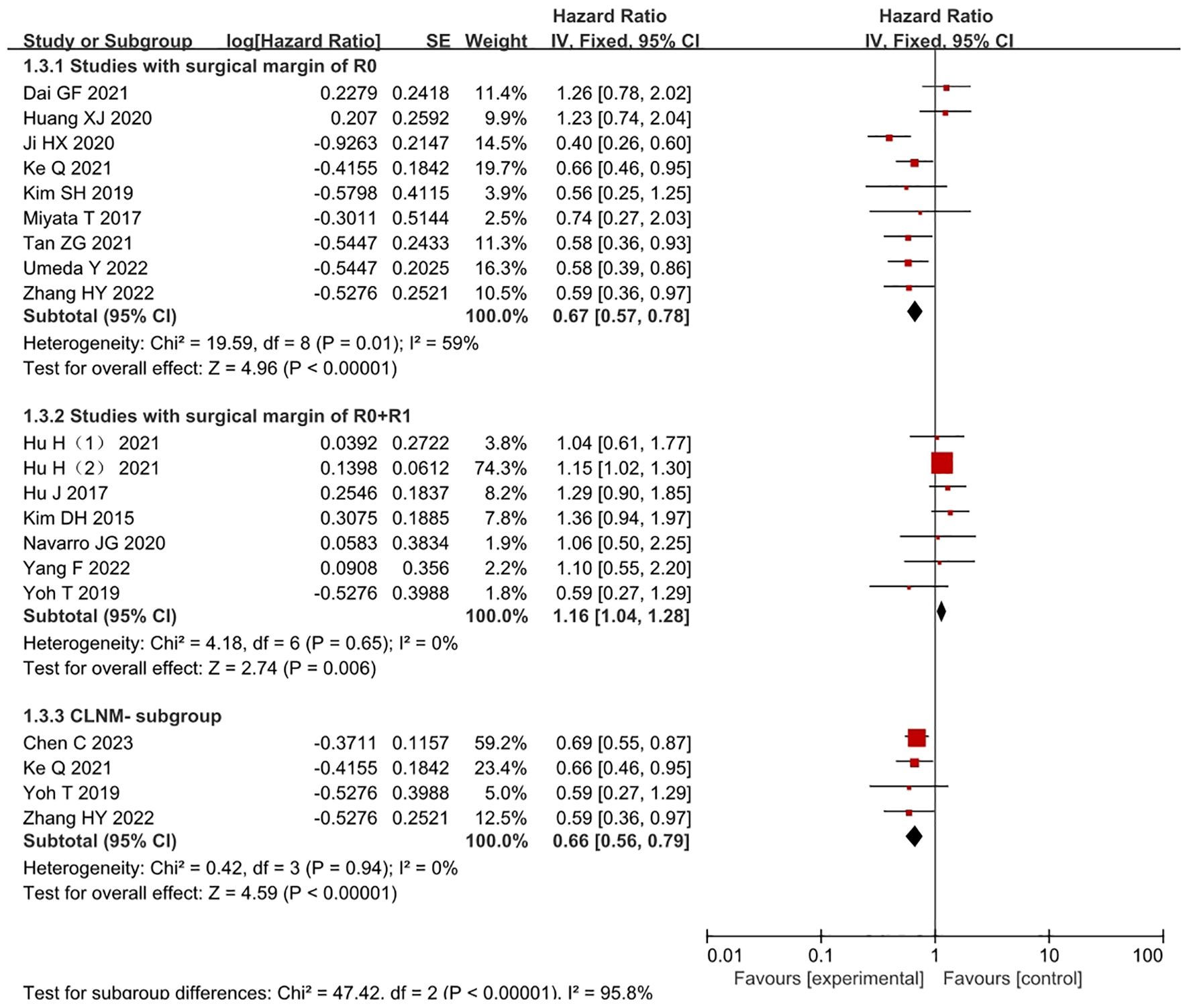
Figure 3. Forest plot comparing the OS in studies where the surgical margins were R0 or R0+R1 within the LND and nLND groups. And comparing OS in cLNM- patients. R0: negative surgical margin. R1: positive surgical margin. cLNM-, clinical lymph node metastasis-negative.
Complete R0 surgical resection was performed in 9 studies (4, 7, 18, 20, 26–30) (Figure 3) (9 studies,21.88%,1266/5787). The results indicated that the LND group had significantly longer OS than the R0 resection subgroup within the nLND group (HR=0.68, 95% CI: 0.52-0.88; P<0.001, Padj=0.0056). R0+R1 surgical resection was performed in 9 studies (5, 9, 15–17, 21, 22, 24, 25). Compared with the same subgroup, the study by Chen C (15) included only lymph node-negative samples, while the study by Vitale A (22) did not provide detailed descriptions of surgical margins. After excluding these two studies (7 studies,58.32%,3375/5787), the heterogeneity decreased (I2 = 0%, P<0.01), and a fixed-effects model was used for the analysis. The results indicated that the LND group had significantly shorter OS than the non-R0 resection subgroup within the nLND group (HR=1.16, 95% CI: 1.04-1.28; P=0.006, Padj=0.0125).
3.2.2 Meta-analysis of LND N- and nLND patients
Ten studies (4, 7, 10, 17, 19, 20, 23, 24, 26, 30) (10 studies,29.98%,1735/5787) compared the OS of LND N- and nLND patients (Figure 4a). Low heterogeneity was observed between the studies (I2 = 31%, P=0.16), and a fixed-effects model was used for the analysis. The results indicated that the LND N- group had a greater survival benefit than the nLND group, and the difference was statistically significant (HR=0.81, 95% CI: 0.68-0.98, P=0.003, Padj=0.01).
![Forest plot with three panels (a, b, c), showing hazard ratios and confidence intervals from various studies. Each panel presents subgroups with data including log[Hazard Ratio], standard error (SE), weight, and hazard ratios with fixed interval confidence intervals. Diamonds represent overall effect estimates. Panel a shows a total hazard ratio of 0.81; b shows 1.97; c shows 0.35. Heterogeneity statistics are provided for each panel.](https://www.frontiersin.org/files/Articles/1590019/fonc-15-1590019-HTML/image_m/fonc-15-1590019-g004.jpg)
Figure 4. Forest plot comparing OS in the LND N- and nLNDgroups (a). Comparing OS in the LND N+ and nLNDgroups (b) and comparing OS in the LND N- and LND N+ groups (c). LND, lymph node dissection; N-, Negative lymph nodes; N+, Positive lymph nodes.
3.2.3 Meta-analysis of LND N+ and nLND patients
Seven studies (4, 7, 10, 20, 23, 24, 30) compared the OS of LND N+ and nLND patients (Figure 4b), and the interstudy heterogeneity was high (I2 = 54%, P < 0.1). The analysis revealed that the LND rate in the study by Chang (10) et al. (34.9%) was lower than that in other studies in the same group,heterogeneity decreased after exclusion(I2 = 39%, P < 0.01).The results of the fixed effects model (6 studies,16.58%,960/5787)showed that the OS of patients in the LND N+ group was lower than that in the nLND group, and the difference was statistically significant (HR=1.97, 95% CI: 1.57-2.48, P < 0.001, Padj=0.0063).
3.2.4 Meta-analysis of LND N+ and LND N- patients
Fourteen studies (4, 5, 7, 9, 10, 16, 17, 20, 23, 24, 27–30) (14 studies,74.05%,4285/5787) compared the OS of LND N- and LND N+ patients (Figure 4c). Low heterogeneity was observed between studies (I2 = 0%, P=0.48), and a fixed-effects model was used for analysis. The results suggested that patients in the LND N- group had significantly longer OS than those in the LND N+ group (HR=0.35, 95% CI: 0.31-0.40; P<0.001, Padj=0.0071).
3.2.5 Meta-analysis of postoperative complications and recurrence rate
Five studies (9, 17, 19, 25, 28) (5 studies,16.79%,972/5787) reported the postoperative recurrence rate (Figure 5a), with low heterogeneity between studies (I2 = 2%, P=0.39). A fixed-effects model was used for analysis, and the results indicated no statistically significant difference in the postoperative recurrence rate between the LND group and the nLND group (HR=1.16, 95% CI=0.85-1.59, P=0.34, Padj=0.05).
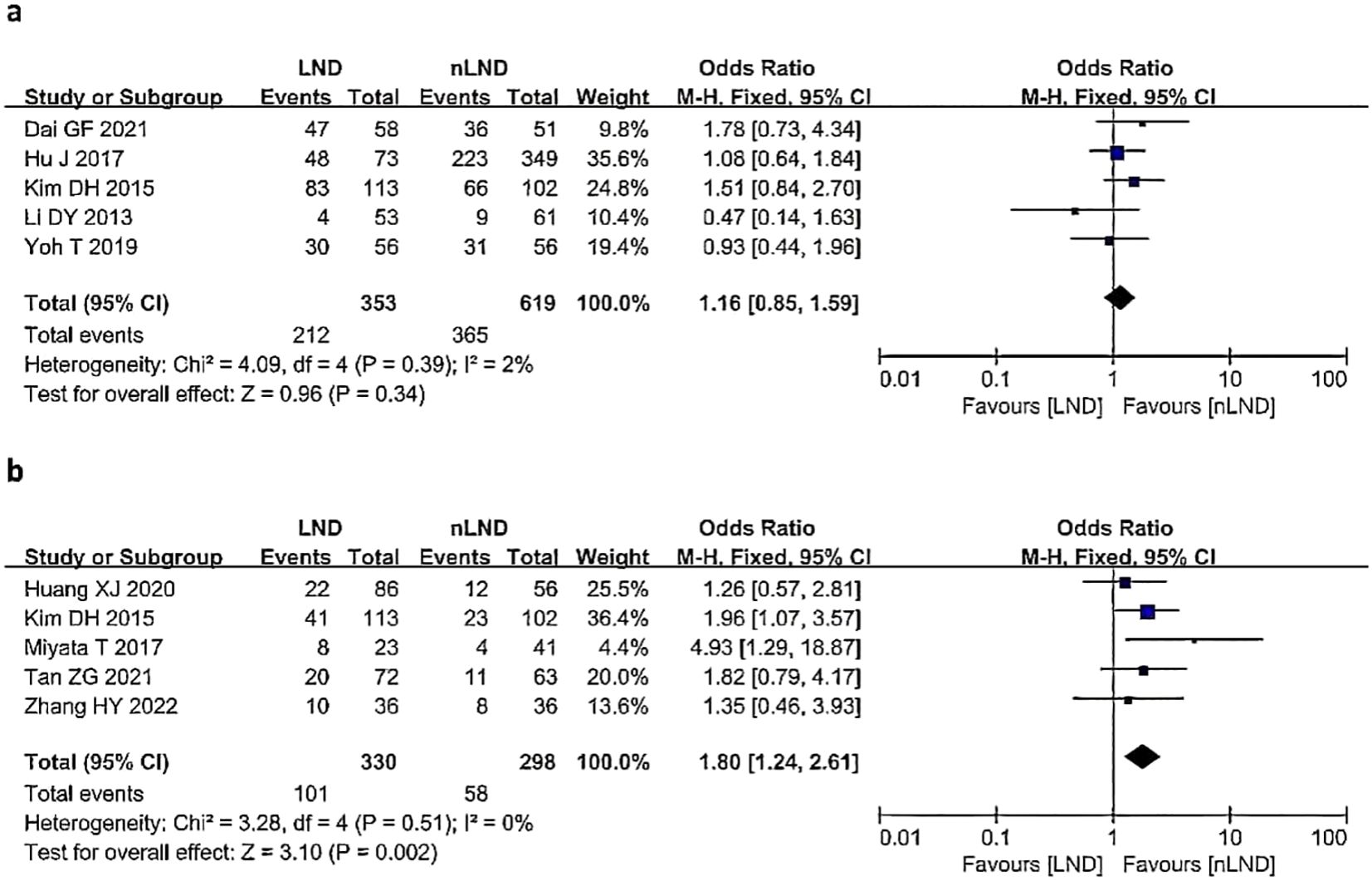
Figure 5. Postoperative outcomes following LND versus nLND in terms of recurrence (a) and postoperative morbidity (b). LND, lymph node dissection; M−H, Mantel–Haenszel method; CI, confidence interval.
Five studies (9, 20, 27, 29, 30)(5 studies,10.85%,628/5787) reported the incidence of postoperative complications (Figure 5b), with no heterogeneity among the studies (I2 = 0%, P=0.51). A fixed-effects model was used for analysis, and the results suggested that the LND group had a significantly greater incidence of postoperative complications than the nLND group (HR=1.80, 95% CI: 1.24-2.61;P=0.002,Padj=0.0083).
3.3 Publication bias
The funnel plot of the studies included in this meta-analysis was relatively concentrated and symmetrical, which indicates no significant publication bias (Figure 6). To quantitatively assess publication bias, we performed Egger’s linear regression test for the primary outcome (OS). The results were as follows: OS analysis (Egger’s intercept =1.32, 95%CI: -0.87-3.51, P=0.184). These findings confirm no significant asymmetry in the funnel plots, aligning with our initial conclusion that publication bias is unlikely to substantially affect the results.
4 Discussion
iCCA is the second most common malignant primary liver cancer, as it accounts for approximately 20% of all malignant liver tumors (1). iCCA is relatively rare in clinical practice and has an overall 5-year survival rate of less than 10%. In recent years, the incidence and mortality rates of iCCA have continued to increase (31). Surgical resection is currently the preferred treatment for iCCA, but early diagnosis is difficult, and only 20-40% of patients have the opportunity for surgical resection. The postoperative recurrence rate is high, with a 5-year recurrence rate up to 80% and a 5-year survival rate less than 20% (32–34). LNM, with a rate of 45-60%, is one of the most important adverse prognostic factors for curative surgery in iCCA patients (31). LND has important value in the diagnosis of LNM, tumor staging, prognostic guidance, and adjuvant treatment for iCCA. The prognostic value, complications, and clinical benefits of LND in iCCA patients are currently a focus of debate in the academic community.
This study presents a quantitative analysis of the effectiveness and safety of LND in iCCA patients. In all, 21 articles and 5781 patients were included in the analysis. These studies included both single-center and multicenter studies, all of which were retrospective. Most of these studies described the range of LND but did not specify the number of lymph nodes dissected. These patients received curative R0 surgical treatment regardless of their cLNM- status, as assessed by imaging and discussion among clinical doctors.
The results of the analysis suggested that compared with patients who underwent nLND, patients who underwent LND did not experience a survival benefit. Due to its significant heterogeneity, subgroup analyses were conducted to investigate the sources of this variability (for example, R0 versus non-R0 resection, cLNM status, etc.). These analyses revealed that factors such as the status of surgical margins and the accuracy of preoperative lymph node staging contributed to the observed heterogeneity. Subgroup analysis suggested that patients diagnosed with cLNM- before surgery had better survival outcomes with LND than nLND. Hu J et al. (17) reported that routine LND may not improve the survival rate of patients with resectable iCCA and cLNM-. The analysis of various studies revealed that the cLNM- group also included some N+ patients. Chen C et al. (15) included all patients with pLNM- and found that patients in the LND group had better OS and DFS. The sensitivity, specificity, and accuracy of PET/CT vs. MRI for the diagnosis of regional LNM were 70.0% vs. 50%, 91.7% vs. 83.3%, and 81.8% vs. 68.2%, respectively (35). With improvements in imaging technology and the increase in the specificity of experimental indicators, accompanied by a decrease in the false-negative rate of LNM, cLNM- patients may benefit from LND. In addition, subgrouping based on surgical margin status, R0 and R0+R1, suggested that the survival benefit of LND was significantly greater than that of nLND in the R0 group but was smaller in the R0+R1 group than in the nLND group. This suggests that the surgical margin status affects the prognosis of patients undergoing LND. Consequently, for patients with iCCA who have undergone non-R0 resection, it is our position that lymphadenectomy may be deemed unnecessary, given that these tumors are predominantly at an advanced stage and exhibit vascular invasion.
The analysis of 10 studies revealed that patients with pLNM- had better survival outcomes if they underwent LND(i.e., LND N-) than those who underwent nLND, and the analysis of 14 studies showed that patients with pLNM- who underwent LND also had significantly better survival prognosis than those with pLNM+. These data further suggests that routine LND should be considered for patients with cLNM-negative iCCA. This study also revealed that the OS of patients with LNM who underwent LND was shorter than that of patients with lymph node negativity and that of patients who underwent nLND. Ke et al. (7) reported that patients who underwent LND were more likely to receive postoperative adjuvant treatment, and compared with pLNM- and nLND patients, pLNM+ patients had a worse prognosis (P<0.05), but only LNM+ patients benefited from postoperative adjuvant treatment (P<0.05). This indicates that LND may also play an important role in the postoperative management of iCCA patients. For such high-risk patients, postoperative adjuvant radiotherapy may further decrease the likelihood of local recurrence. For instance, the Mayo regimen, which combines external radiation with brachytherapy, has demonstrated an increase in the 5-year survival rate for hilar cholangiocarcinoma, indicating its potential applicability to iCCA (36). In addition, Chafoori et al. (37) assert that for advanced and unresectable tumors, radiotherapy and chemotherapy can achieve local control of extrahepatic cholangiocarcinoma for up to two years. Similarly, Esmail et al. (38) proposed that integrating various treatment modalities, such as combining immunotherapy with chemotherapy, may result in improved survival outcomes. Statistical analysis indicates that patients diagnosed with pLNM+ who undergo lymphadenectomy experience less benefit from the procedure compared to those who do not. It is important to note, however, that the majority of patients in the pLNM+ cohort often present with compromised physical conditions, advanced tumor stages characterized by vascular and serosal invasion, and a higher incidence of complications. Despite these challenges, it has been observed that patients with pLNM+ can achieve improved outcomes through adjuvant therapy. Consequently, it is imperative to engage in a discussion regarding the appropriateness of lymphadenectomy for patients with positive LNM. A thorough preoperative assessment should be conducted, taking into account variables such as age, nutritional status, tumor size and quantity, the extent of LNM, CA19-9 levels, and any existing comorbidities. If the advantages of postoperative adjuvant therapy surpass the potential detriments associated with lymphadenectomy, the procedure should be considered; conversely, if the disadvantages of lymphadenectomy outweigh the benefits of postoperative adjuvant therapy, the procedure should be avoided. Achieving a balance between these two considerations necessitates further experimental research, which will be a focus of our future investigations.
This study revealed no significant difference in the postoperative recurrence rate between LND and nLND patients (I2 = 2%, P=0.34, Padj=0.05). However, the analysis revealed that LND significantly increased the incidence of postoperative complications compared with nLND. According to various studies, patients who undergo LND may have longer surgery times, greater blood loss, and longer drainage tube removal times. The occurrence of postoperative complications is one factor that contributes to a poor prognosis in cancer treatment (39). Therefore, iCCA patients should pay attention to the indications for LND, ensure safe surgery, and reduce postoperative complications to improve patient prognosis.This inquiry highlights a critical clinical consideration regarding the equilibrium between the advantages of survival and the potential risks associated with postoperative complications. Among the five studies examined in relation to postoperative complications, three (27, 29, 30) addressed the prognostic significance of LND in patients with cLNM-. The findings suggested that although LND may prolong the duration of surgery and the length of postoperative hospitalization, there was no notable difference in intraoperative blood loss or the incidence of postoperative complications. Moreover, for patients with iCCA and cLNM-, prophylactic LND appears to offer greater benefits than risks and can be integrated as a standard surgical procedure. It is posited that the implementation of standardized protocols and the application of proficient surgical techniques are vital for the successful execution of the surgery. Additionally, the patient’s capacity to endure the surgical procedure is a fundamental prerequisite, and conducting a thorough preoperative assessment may mitigate the likelihood of postoperative complications in iCCA patients, thereby enhancing their prognosis.
Currently, the practices for LND during the treatment of iCCA are not standardized. This lack of standardization reflects a lack of guidelines, which can be attributed to conflicting evidence in the literature. Insufficient evaluation of lymph node status may lead to inaccurate staging and insufficient application of adjuvant chemotherapy. Zhang et al. (40) demonstrated that at least 6 LNs should be routinely dissected and that examination beyond the 12 stations should be performed to obtain the maximum opportunity for accurate staging. Based on their recommendation, they proposed a new N stage model: N0 (LNM 0), N1 (LNM 1-2), and N2 (LNM > 2). Similarly, Sposito et al. (8) also suggested that adequate LND, which involves the resection of at least 6 lymph nodes during liver resection, provides significant benefits for patients with less advanced tumors (i.e., isolated tumors, maximum tumor size <5 cm, CA 199 <200 U/ml, no evidence of major vessel invasion). In patients with these characteristics, the possibility of systemic disease dissemination is low, and the tumor may be localized to the liver and regional lymph nodes. Therefore, adequate LND can eliminate all micrometastatic disease, thereby improving recurrence-free survival and OS. Kim et al. (41) attempted to determine the minimum number of lymph nodes required for iCCA using a Bayesian Weibull model. Their results suggested that at least 5 LNs should be dissected in patients undergoing curative iCCA surgery to promote accurate staging. Chen et al. (15) reported that clearing more than 8 lymph nodes was associated with a better patient prognosis, and not only accurate staging, without increasing the incidence of postoperative complications or the length of hospital stay. The range of LND is still controversial, and further research studies may be needed to standardize the scope of LND. The liver has multiple lymphatic drainage pathways, which may also require further research, including the concept of sentinel lymph nodes.
This meta-analysis does have some limitations. First, all the studies included are retrospective, highlighting the scarcity of prospective research in this field. This limitation makes it challenging to control for external factors. Second, while frameworks such as the 8th edition AJCC staging system (42) and NCCN guidelines (6) recommend lymph node evaluation for iCCA, they do not provide granular specifications for the extent of LND (e.g., minimum lymph node yield or anatomical stations to dissect). Consequently, the indications and scope of LND remain surgeon-dependent, guided by institutional protocols, clinical experience, and preoperative imaging. This variability complicates cross-study comparisons of LND outcomes. Moreover, factors such as the experience of surgeons, institutional protocols for adjuvant therapy, and the heterogeneity in patient selection may inevitably confound the observed survival benefits.Third, most studies have focused on the prognosis of iCCA patients after LND and have neglected the role of surgical margins, tumor size and location, CA19-9, CEA, number of LNM, and number of lymph nodes dissected in the prognosis of iCCA patients with LND.
Our research findings advocate for routine LND in patients with cLNM - iCCA who undergo R0 resection, while cautioning against the use of LND in non-R0 cases. This stratified approach optimizes survival benefits while minimizing unnecessary morbidity. Compared to previous meta-analyses, our study innovatively addresses a long-standing controversy by: (1) integrating large-scale data; (2) isolating the cLNM cohort to quantify the true benefits of LND; (3) introducing resection margin status as a key stratification variable; and (4) employing p-value adjusted subgroup analyses to enhance reliability. These advancements provide a roadmap for updating clinical guidelines and standardizing surgical practices.
5 Conclusion
In summary, current evidence suggests that iCCA patients who undergo curative surgery benefit more from LND than from nLND, a procedure that is accompanied by improvements in imaging technology and experimental indicator specificity. cLNM- patients will also benefit from LND, and routine LND should be performed on cLNM- iCCA patients. This study revealed that more postoperative complications are associated with LND than with nLND; however, with limited sample data, more prospective studies are needed to standardize the indications and scope of LND, reduce postoperative complications, and improve prognosis.
Author contributions
JY: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. FZ: Funding acquisition, Methodology, Writing – review & editing. XT: Methodology, Supervision, Writing – review & editing. JG: Investigation, Writing – review & editing. WF: Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Province, China(2024JJ7596).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hewitt DB, Brown ZJ, and Pawlik TM. Surgical management of intrahepatic cholangiocarcinoma. Expert Rev Anticancer Ther. (2022) 22:27–38. doi: 10.1080/14737140.2022.1999809
2. Zhang H, Yang T, Wu M, and Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. (2016) 379:198–205. doi: 10.1016/j.canlet.2015.09.008
3. Moris D, Palta M, Kim C, Allen PJ, Morse MA, and Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. (2023) 73:198–222. doi: 10.3322/caac.21759
4. Umeda Y, Mitsuhashi T, Kojima T, Satoh D, Sui K, Endo Y, et al. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J Hepatobiliary Pancreat Sci. (2022) 29:217–29. doi: 10.1002/jhbp.1038
5. Hu H, Xu G, Du S, Luo Z, Zhao H, and Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: a multicenter retrospective study. BMC Surg. (2021) 21:359. doi: 10.1186/s12893-021-01363-4
6. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
7. Ke Q, Wang L, Lin Z, Lou J, Zheng S, Bi X, et al. Prognostic value of lymph node dissection for intrahepatic cholangiocarcinoma patients with clinically negative lymph node metastasis: A multi-center study from China. Front Oncol. (2021) 11:585808. doi: 10.3389/fonc.2021.585808
8. Sposito C, Ratti F, Cucchetti A, Ardito F, Ruzzenente A, Di Sandro S, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol. (2023) 78:356–63. doi: 10.1016/j.jhep.2022.10.021
9. Kim DH, Choi DW, Choi SH, Heo JS, and Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. (2015) 157:666–75. doi: 10.1016/j.surg.2014.11.006
10. Chang ME, Lei HJ, Chen MH, Yeh YC, Li CP, Hung YP, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc. (2017) 80:140–6. doi: 10.1016/j.jcma.2016.09.010
11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
13. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
14. Tierney JF, Stewart LA, Ghersi D, Burdett S, and Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
15. Chen C, Su J, Wu H, Qiu Y, Song T, Mao X, et al. Prognostic value of lymphadenectomy in node-negative intrahepatic cholangiocarcinoma: A multicenter, retrospectively study. Eur J Surg Oncol. (2023) 49:780–7. doi: 10.1016/j.ejso.2022.11.008
16. Hu H, Zhao H, and Cai J. The role of lymph node dissection and a new N-staging system for intrahepatic cholangiocarcinoma: a study from the SEER database. J Int Med Res. (2021) 49:3000605211012209. doi: 10.1177/03000605211012209
17. Hu J, Chen FY, Zhou KQ, Zhou C, Cao Y, Sun HC, et al. Intrahepatic cholangiocarcinoma patients without indications of lymph node metastasis not benefit from lymph node dissection. Oncotarget. (2017) 8:113817–27. doi: 10.18632/oncotarget.22852
18. Kim SH, Han DH, Choi GH, Choi JS, and Kim KS. Oncologic impact of lymph node dissection for intrahepatic cholangiocarcinoma: a propensity score-matched study. J Gastrointest Surg. (2019) 23:538–44. doi: 10.1007/s11605-018-3899-2
19. Li DY, Zhang HB, Yang N, Quan Y, and Yang GS. Routine lymph node dissection may be not suita ble for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol. (2013) 19:9084–91. doi: 10.3748/wjg.v19.i47.9084
20. Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, et al. Clinical benefits of lymph node dissection in intrahepatic cholangiocarcinoma: A retrospective single-institution study. Anticancer Res. (2017) 37:2673–7. doi: 10.21873/anticanres.11615
21. Navarro JG, Lee JH, Kang I, Rho SY, Choi GH, Han DH, et al. Prognostic significance of and risk prediction model for lymph node metastasis in resect able intrahepatic cholangiocarcinoma: do all require lymph node dissection. HPB (Oxford). (2020) 22:1411–9. doi: 10.1016/j.hpb.2020.01.009
22. Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, and Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol. (2016) 113:685–91. doi: 10.1002/jso.24213
23. Wu ZF, Wu XY, Zhu N, Xu Z, Li WS, Zhang HB, et al. Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. (2015) 21:935–43. doi: 10.3748/wjg.v21.i3.935
24. Yang F, Wu C, Bo Z, Xu J, Yi B, Li J, et al. The clinical value of regional lymphadenectomy for intrahepatic cholangiocarcinoma. Asian J Surg. (2022) 45:376–80. doi: 10.1016/j.asjsur.2021.06.031
25. Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery. (2019) 166:975–82. doi: 10.1016/j.surg.2019.06.025
26. Hong-xiang J, Ma-Hui S, Yue K, Tian-geng Y, Long-jiu C, et al. Significance of lymphadenectomy in intrahepatic cholangiocarcinoma with radical resection(in Chinese with English abstract). Chin J Pract Surgery. (2020) 40:703–9.
27. Zhang H, Feng X, Tianqiang J, Junqi W, Jinming F, and Chaoliu D. Effect of lymph node dissection upon on imaging examination for intrahepatic cholangiocarcinoma with negative lymph nodes. J Abdominal Surg. (2022) 35:252–7. doi:
28. Gao-fan D. The prognostic factors of radical resection for intrahepatic cholangiocarcinoma and the clinical value of intraoperative lymph node dissection (in Chinese with English abstract). Fujian Medical University. (2021). doi: 10.27020/d.cnki.gfjyu.2021.000737
29. Zhi-guo T. Prognostic factors and significance of lymphadenectomy in intrahepatic cholangiocarcinoma (in Chinese with English abstract). Hunan Normal University. (2021). doi: 10.27137/d.cnki.ghusu.2021.002120
30. Xu-jian H, Fa-cai Y, Meng L, Xiao-peng L, Tai-an C, Yi H, et al. Prognostic value of hepatectomy combined with lymph node dissection in patients with intrahepatic cholangiocarcinoma and clinical negative lymph node metastasis(in Chinese with English abstract). Chin J Hepat Surg(Electronic Edition). (2020) 9:533–7.
31. Sposito C, Droz Dit Busset M, Virdis M, Citterio D, Flores M, Bongini M, et al. The role of lymphadenectomy in the surgical treatment of intrahepatic cholangiocarcinoma: A review. Eur J Surg Oncol. (2022) 48:150–9. doi: 10.1016/j.ejso.2021.08.009
32. El-Diwany R, Pawlik TM, and Ejaz A. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. (2019) 28:587–99. doi: 10.1016/j.soc.2019.06.002
33. Zhou SN, Lu SS, Ju DW, et al. A new prognostic model covering all stages of intrahepatic cholangiocarcinoma. J Clin Transl Hepatol. (2022) 10:254–62. doi: 10.14218/jcth.2021.00099
34. Li Q, Chen C, Su J, Qiu Y, Wu H, Song T, et al. Recurrence and prognosis in intrahepatic cholangiocarcinoma patients with different etiology after radical resection: a multi-institutional study. BMC Cancer. (2022) 22:329. doi: 10.1186/s12885-022-09448-w
35. Jiang L, Tan H, Panje CM, Yu H, Xiu Y, and Shi H. Role of 18F-FDG PET/CT imaging in intrahepatic cholangiocarcinoma. Clin Nucl Med. (2016) 41:1–7. doi: 10.1097/rlu.0000000000000998
36. Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. (2012) 143:88–98.e3. doi: 10.1053/j.gastro.2012.04.008
37. Ghafoori AP, Nelson JW, Willett CG, Chino J, Tyler DS, Hurwitz HI, et al. Radiotherapy in the treatment of patients with unresect able extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. (2011) 81:654–9. doi: 10.1016/j.ijrobp.2010.06.018
38. Zhang Y, Esmail A, Mazzaferro V, and Abdelrahim M. Newest therapies for cholangiocarcinoma: an updated overview of approved treatments with transplant oncology vision. Cancers (Basel). (2022) 14:5074. doi: 10.3390/cancers14205074
39. Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, et al. Prognostic impacts of postoperative complications in patients with intrahepatic cholangiocarcinoma after curative operations. Int J Clin Oncol. (2017) 22:526–32. doi: 10.1007/s10147-017-1099-9
40. Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg. (2021) 274:e1187–95. doi: 10.1097/sla.0000000000003788
41. Kim SH, Han DH, Choi GH, Choi JS, and Kim KS. Recommended minimal number of harvested lymph nodes for intrahepatic cholangiocarcinoma. J Gastrointest Surg. (2021) 25:1164–71. doi: 10.1007/s11605-020-04622-6
Keywords: intrahepatic cholangiocarcinoma, lymph node dissection, lymphadenectomy, prognosis, ICC
Citation: Yu J, Zhou F, Tan X-G, Guo J and Feng W (2025) Impact of lymph node dissection on the prognosis of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Front. Oncol. 15:1590019. doi: 10.3389/fonc.2025.1590019
Received: 08 March 2025; Accepted: 11 July 2025;
Published: 30 July 2025.
Edited by:
Abdullah Esmail, Houston Methodist Hospital, United StatesReviewed by:
Qi Li, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaRuoyu Zhang, Chinese Academy of Medical Sciences, China
Copyright © 2025 Yu, Zhou, Tan, Guo and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Zhou, MTU1ODAxMTUwMDFAMTYzLmNvbQ==
 Jun Yu
Jun Yu Fan Zhou
Fan Zhou Xing-Guo Tan
Xing-Guo Tan