- 1The Postgraduate Training Base of Jinzhou Medical University (The 960th Hospital of PLA), Jinan, China
- 2The 960th Hospital of People's Liberation Army (PLA), Jinan, Shandong, China
Low-grade myxofibrosarcoma (LGMFS) is a rare subtype of soft tissue sarcoma, with an estimated incidence of approximately 0.18 cases per million population, accounting for about 0.6% of all soft tissue sarcomas. It is characterized by a high local recurrence rate and malignant potential. Orbital involvement secondary to maxillary sinus LGMFS represents an exceptionally rare occurrence. The management of such cases is complicated by anatomical complexity (e.g., proximity to optic nerve, lacrimal apparatus, and orbital vasculature) and limitations of conventional therapies, including radical resection combined with chemoradiation. In this anatomically complex case of recurrent Low-Grade Myxofibrosarcoma, iodine-125 brachytherapy achieved durable local control (12-month progression-free survival), demonstrating superior precision targeting and reduced systemic toxicity compared to conventional therapies. No acute radiation toxicity was observed, while facial swelling and eye pain were relieved with concurrent improvement in visual function.
1 Introduction
Low-Grade Fibromyxoid Sarcoma (LGFMS) is a rare soft tissue sarcoma, with an incidence of approximately 0.18 per million population, accounting for 0.6% of all soft tissue sarcomas. It predominantly affects young adults, primarily involving the deep soft tissues of the extremities (especially the thighs and shoulders) and trunk (1). Rare cases may occur in unusual sites such as the head and neck, paranasal sinuses, or breast (2). Characterized by aggressive local invasiveness and high recurrence rates (40%–60%), LGFMS exhibits a distant metastasis risk of less than 10% at initial diagnosis. Despite its histological classification as “low-grade,” the tumor demonstrates infiltrative growth patterns and occult satellite lesions, leading to frequent recurrences (3). These features significantly increase the risk of patient disability and transformation into high-grade sarcoma, posing a critical challenge in clinical management. Standard treatment primarily involves surgical resection, with adjuvant radiotherapy or chemotherapy recommended postoperatively to reduce recurrence risk (4, 5). The management of maxillary sinus LGFMS presents particular challenges due to its unique anatomical constraints and exceptional rarity (6, 7). Although surgical intervention remains the primary approach for recurrent cases (5), managing recurrences in the maxillary sinus with orbital involvement is particularly challenging due to: 1) Surgical limitations due to adjacent critical structures (surgical manipulation may risk damage to the optic nerve and retina); 2)Risk of cumulative radiation toxicity from radiotherapy equipment (radiation-induced optic neuropathy; radiation-induced cerebral edema); and 3) reduced tolerance for systemic therapies.Iodine-125 (I-125I) brachytherapy offers precise, minimally invasive continuous low-dose-rate radiation delivery (8), particularly suitable for inoperable, post-radiation recurrent, or metastatic solid tumors (9). To address these clinical dilemmas, we propose I-125 brachytherapy as a novel therapeutic option, precise radiotherapy control for recurrent cases unsuitable for surgical intervention or conventional chemoradiotherapy.
2 Case report
2.1 Relevant past interventions
A 28-year-old female presented with nasal swelling in September 2022. MRI revealed a space-occupying lesion in the left maxillary sinus with maxillary bone invasion. She underwent initial surgical resection in October 2022. Tumor margins and lymph nodes (zone 2, 0/4) were negative, with a total tumor size of 4.5×4.5×3 cm. She received adjuvant radiotherapy (33 fractions) and showed good recovery during follow-up. In August 2023 (11 months post-surgery), she developed recurrent swelling at the primary site unresponsive to anti-inflammatory therapy. Mucosal biopsy confirmed tumor recurrence, and a second R0 resection of the left sinonasal tumor was performed in September 2023. Adjuvant chemotherapy was initiated 4 weeks postoperatively: Doxorubicin 60 mg/m²Cisplatin 75 mg/m² (Day 1); Ifosfamide 1.2 g/m² (Days 1–5); Repeated every 21 days. The patient completed 5 cycles of treatment but discontinued it due to severe chemotherapy - related toxicities, including nausea, vomiting, neutropenia, thrombocytopenia, and alopecia.
2.2 MRI and CT examination
In February 2024, the patient presented with recurrent symptoms including left facial swelling, left orbital displacement, diplopia, progressive visual acuity deterioration, and ocular distension. MRI revealed local tumor recurrence, demonstrating superior growth through the superior wall of the maxillary sinus into the orbital cavity, with optic nerve compression and orbital bone destruction (Figure 1). CT findings suggest tumor recurrence. (Figure 2).
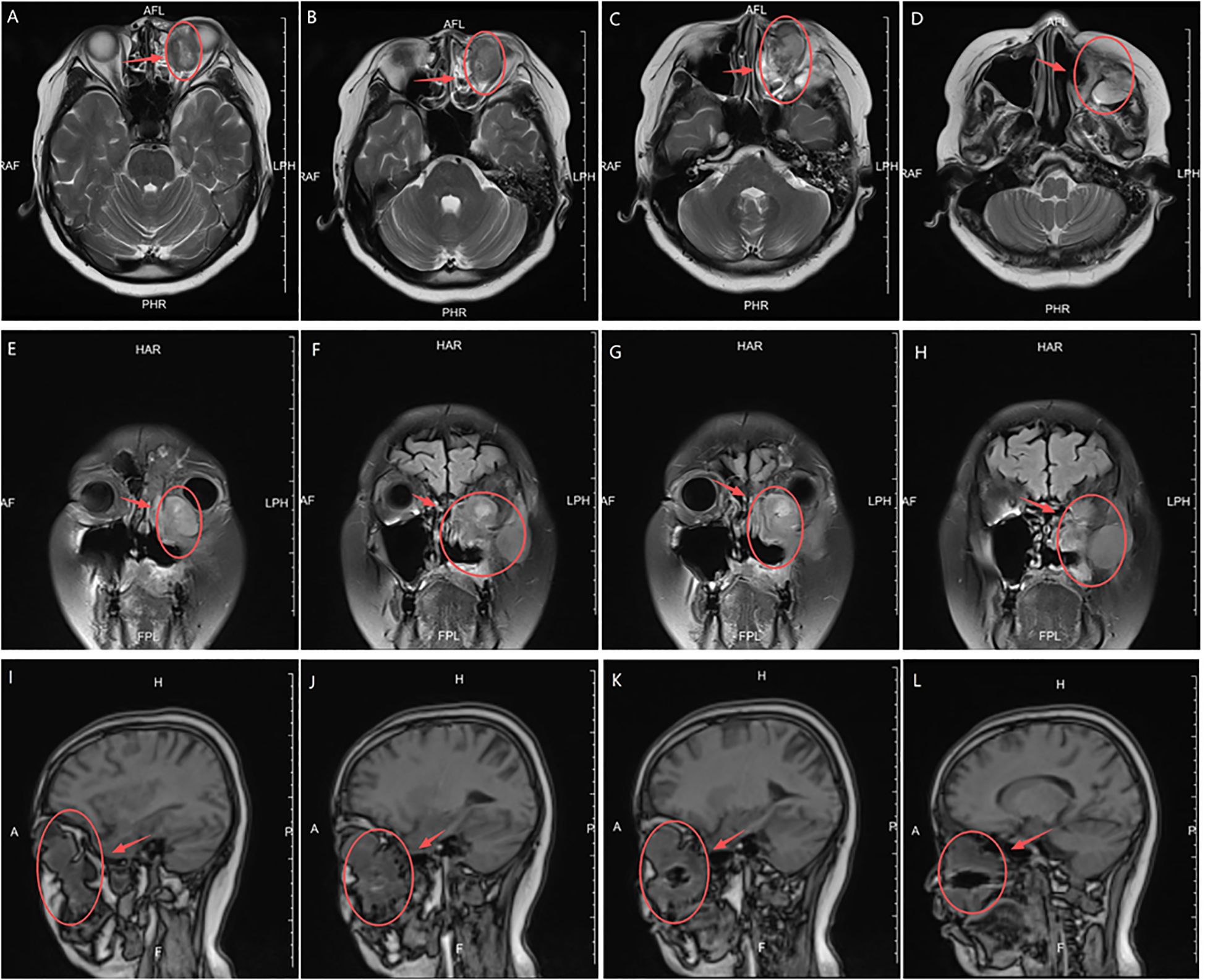
Figure 1. MRI Anatomical imaging: Findings consistent with (LGMFS) tumor recurrence involving the orbit, (as indicated by the arrows). (A–D) Axial T2-weighted image. (E–H) Coronal Short Tau Inversion Recovery (STIR) sequence image. (I–L) Sagittal T1-weighted image.
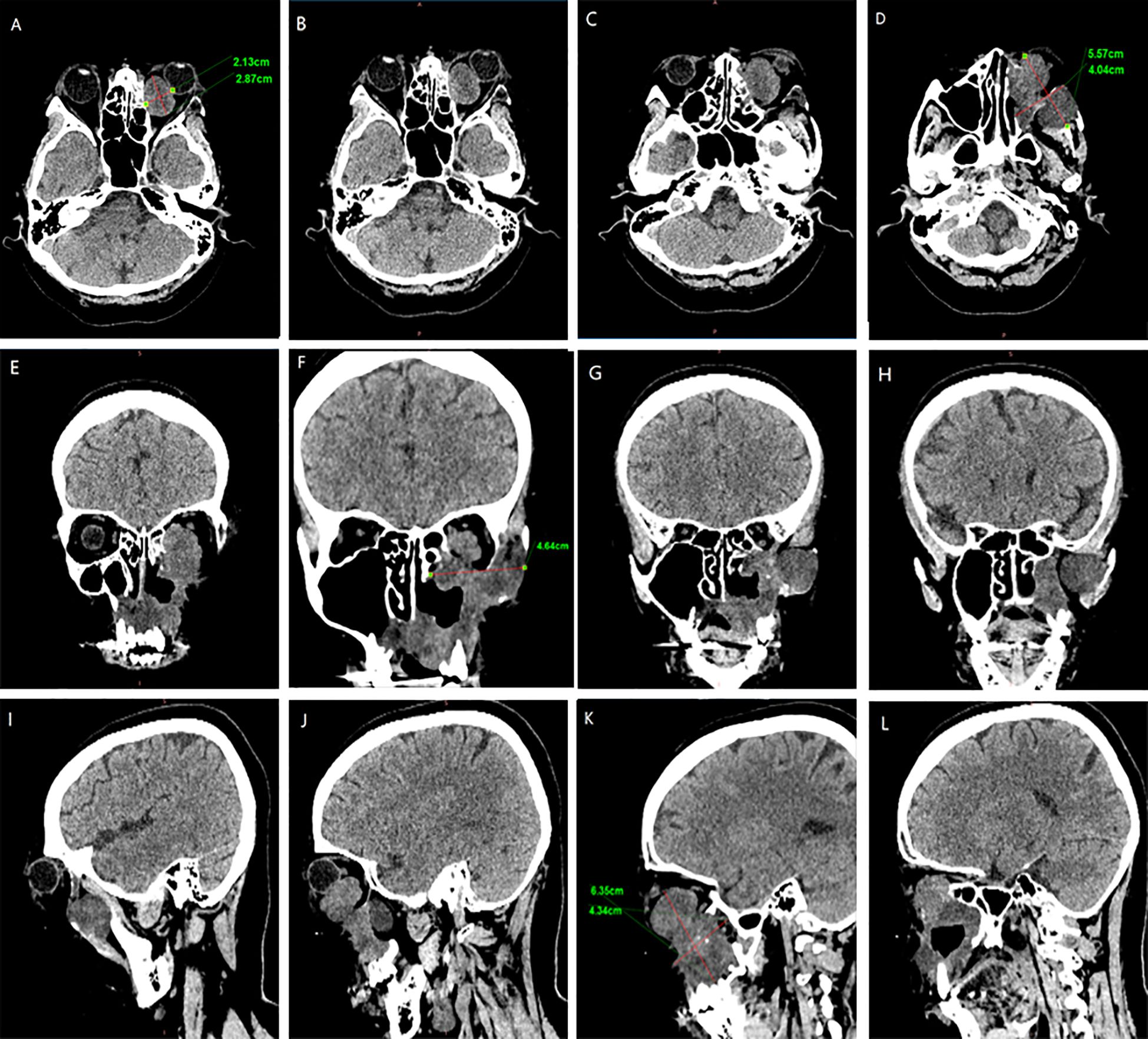
Figure 2. CT Anatomical imaging: Findings consistent with (LGMFS) tumor recurrence involving the orbit. (A–D) Axial CT image. (E–H) Coronal CT image. (I–L) Sagittal CT image.
2.3 Treatment
Preoperative Pathological Diagnosis confirmed Low-Grade Myxofibrosarcoma (left maxillary sinus) with spindle cell morphology, fibrous histiocytoma-like architecture (Figure 3), and myxoid degeneration. Immunohistochemistry showed Vimentin (+), SATB2 (+), MDM-2 (+), and Ki-67 (+30%). Considering the patient’s treatment history and the limitations of the anatomical structure, we proposed the use of Iodine-125 particle implantation therapy. Given the unique location of the tumor (which directly invaded the orbital region and compressed the optic nerve), this form of brachytherapy inherently carries the risk of optic nerve and retinal damage, presenting a significant clinical challenge. Prior to the surgery, we extensively reviewed a large number of relevant studies and planned the surgical procedure using the Treatment Planning System (TPS)to ensure the scientific basis of the surgical plan and to minimize the safety risks to adjacent organs (such as the optic nerve) (10, 11). Based on the patient’s tolerable surgical duration, the treatment was divided into two stages. Preoperative TPS planning guided precise implantation of radioactive seeds before each procedure (Supplementary Figures 1, 2). A total of 197 I-125 seeds (0.3 mCi/seed) were implanted to deliver a total prescription dose of 12,000 cGy (Stage 1: 71 seeds, Stage 2: 126 seeds). The initial surgery was performed in March 2024 (Figure 4). Postoperative CT-based TPS dosimetric evaluation confirmed high concordance between the spatial distribution/dosimetry of implanted I-125 seeds and preplanned configurations (Supplementary Figures 3, 4). The preceding description summarizes the patient’s core clinical profile (Table 1). All treatment plans were generated using the domestic Fei Tian Program TPS, with its specialized Fei Tian Brachy v3.00.00 module for brachytherapy planning.
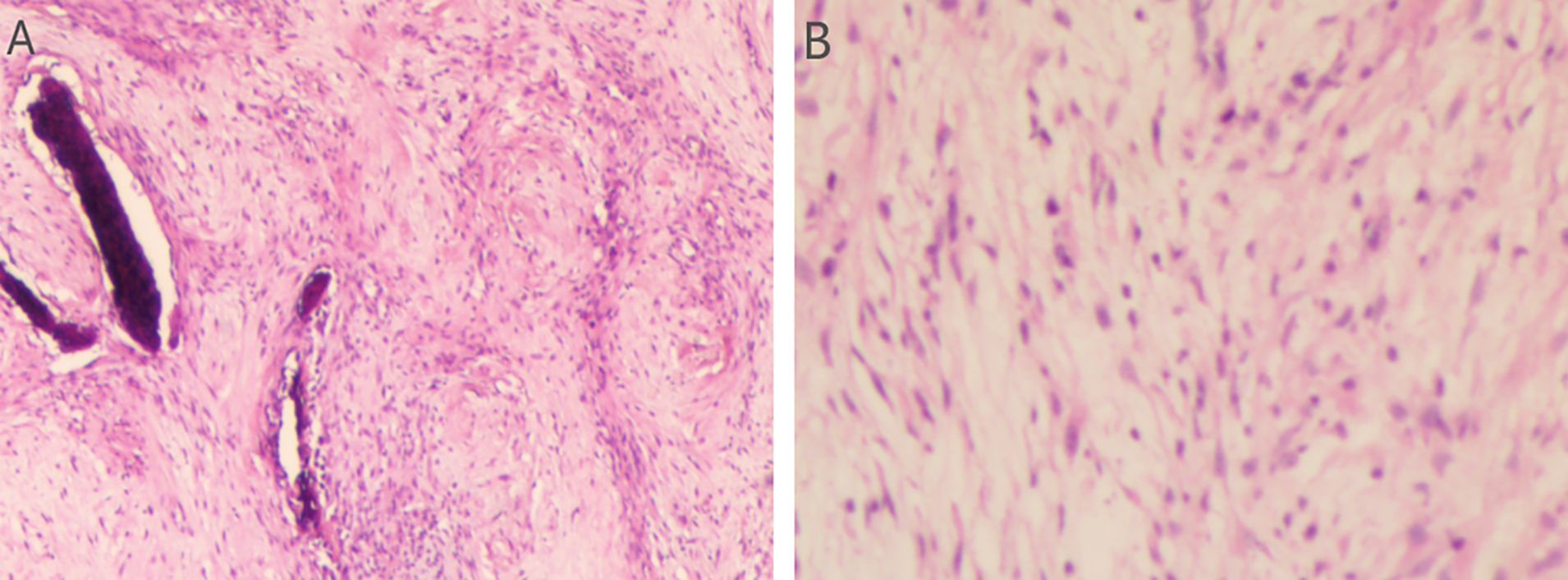
Figure 3. Assessment of the H&E stained tumor tissue. (A) The histopathological evaluation in lower magnification, seen in the left panel, the tissue exhibits a fascicular growth pattern. The cells possess elongated nuclei and include a significant number of spindle-shaped cells. Evident myxoid change is noted within the stroma. (B) In higher magnification, in the right panel, the section demonstrates a relatively uniform population of spindle-shaped cells. The nuclei are slender with evenly distributed chromatin. Mitotic figures are inconspicuous, suggesting low proliferative activity.
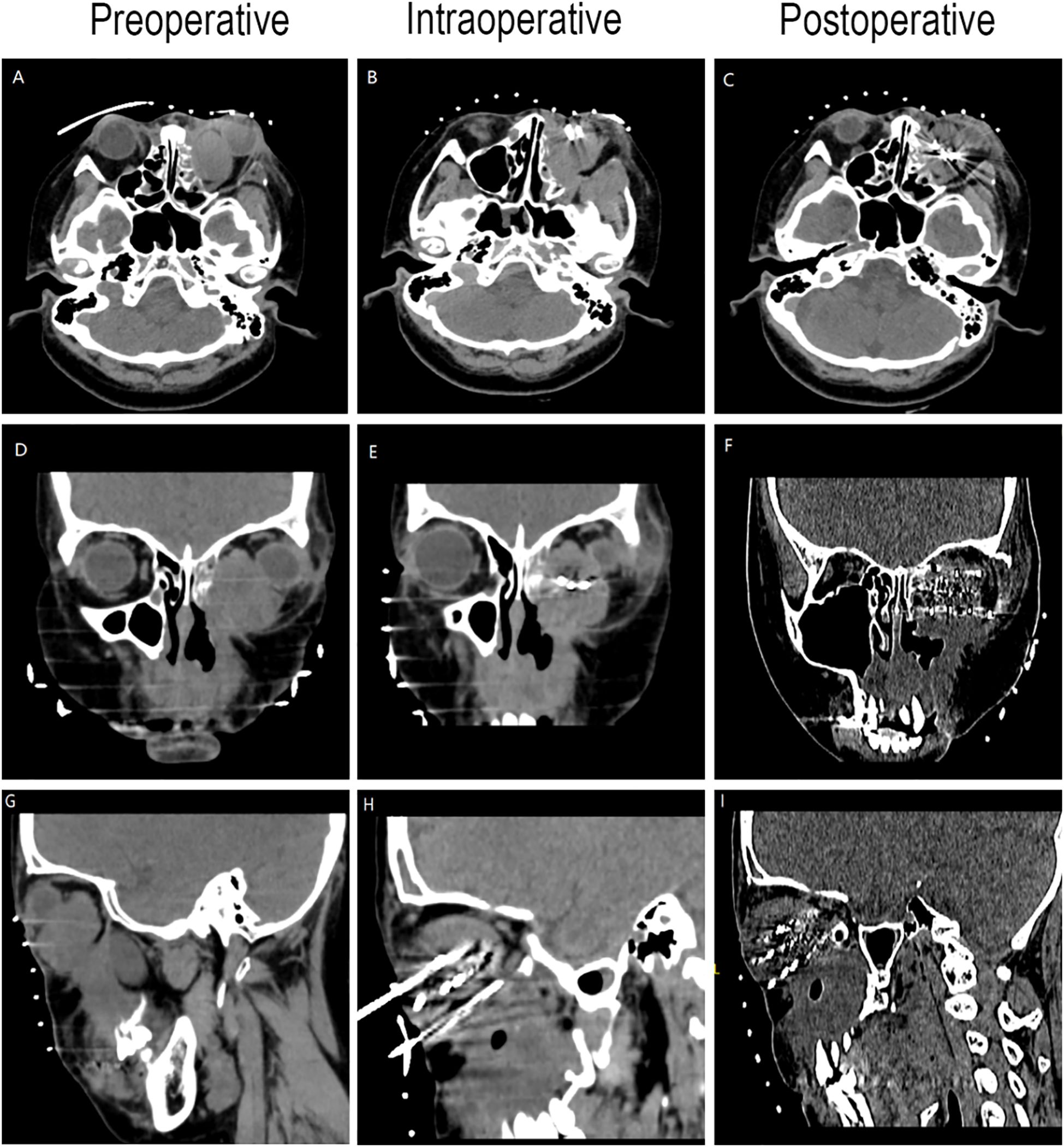
Figure 4. CT-guided iodine-125 seed implantation. (A–C) Axial CT image. (D–F) Coronal CT image. (G–I) Sagittal CT image.
2.4 Follow up
We followed up with the patient at 1 month, 3 months, 6 months, and 1 year postoperatively. After one year of follow-up, the volume of the primary tumor significantly decreased (the tumor size was approximately 5.57×4.04×6.35 cm before treatment, and 2.32×0.96×3.15 cm after treatment) (Figure 5). The intraorbital tumor decreased in size from 2.87×2.12×3.52 cm to 1.94×0.99×2.78 cm.The compression of the optic nerve by the tumor was alleviated, and both visual field and vision showed signs of recovery. The proptosis of the eyeball was significantly improved. The swelling and deformity of the eye were markedly improved, resulting in a more natural facial appearance (Figure 6). The patient’s visual acuity improved significantly from 20/200 (LogMAR 1.0) preoperatively to 20/30 (LogMAR 0.2) at 1 year postoperatively. The patient’s quality of life was significantly enhanced. No complications such as radiation-induced cataracts, optic neuropathy, or cerebral edema were observed during the last follow-up in March 2025.
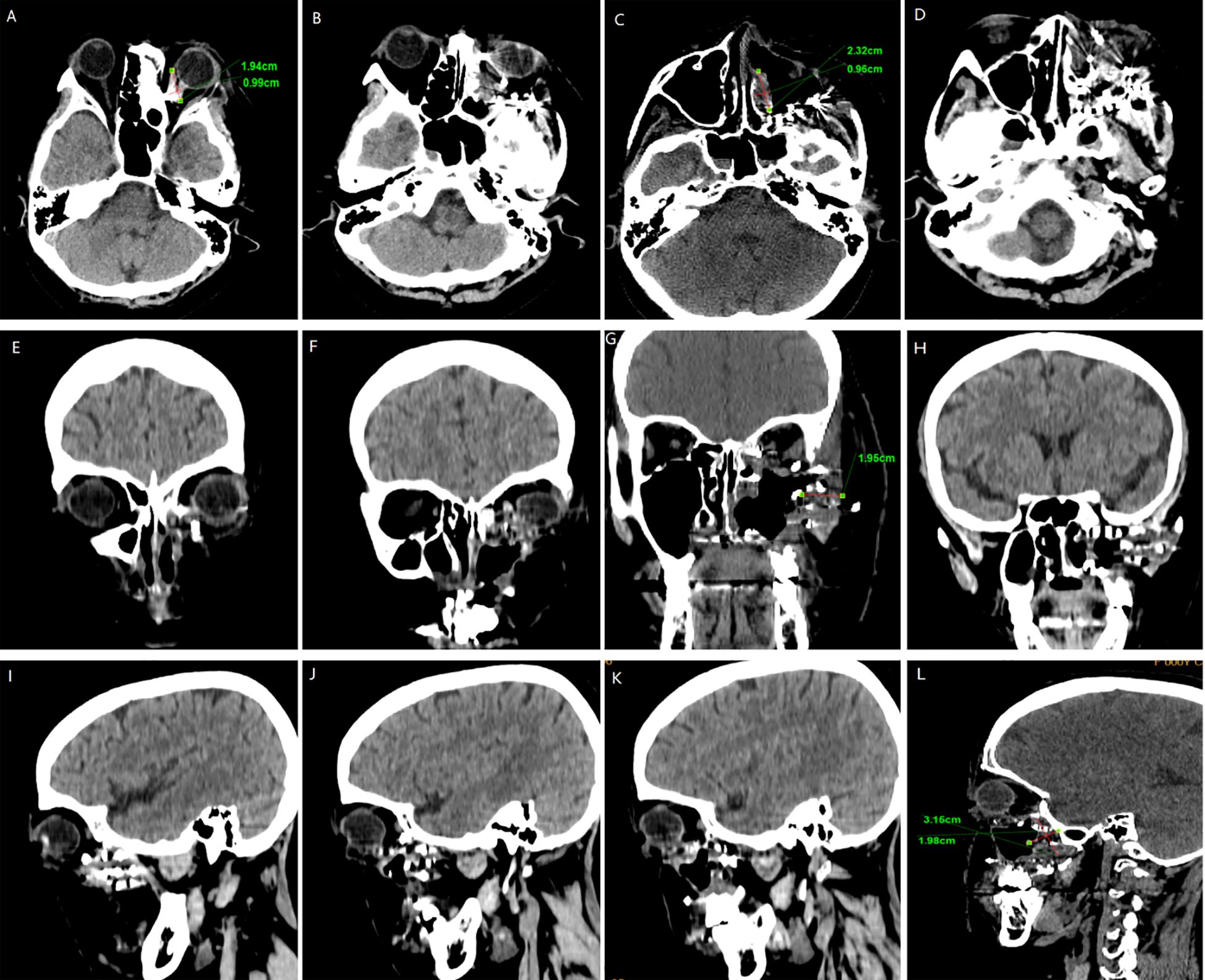
Figure 5. CT surveillance findings: (A–D) Axial CT image. (E–H) Coronal CT image. (I–L) Sagittal CT image (12-month follow-up).
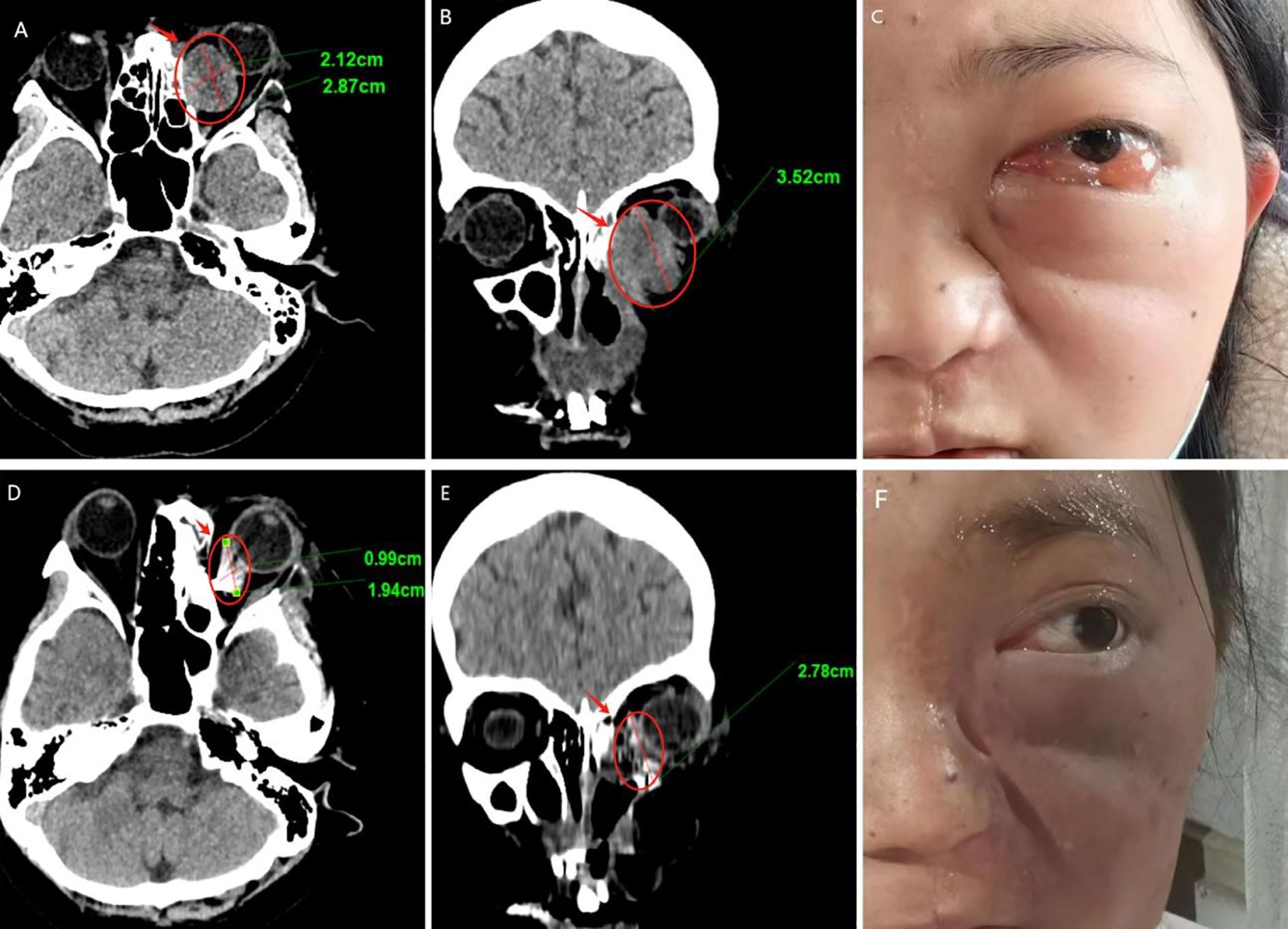
Figure 6. Follow-up results (A–C) Pre-therapeutic imaging series, (D–F) Post-therapeutic imaging series (12-month follow-up) (A, D) CT axial views, (B, E) CT coronal views.
3 Discussion
Low-Grade Myxofibrosarcoma (LGMFS) is a rare, aggressive soft tissue sarcoma with a high local recurrence rat (7). LGMFS occurring in the maxillary sinus and invading the orbit is even more uncommon. Current evidence demonstrates that adjuvant therapy (e.g., chemoradiation) following negative surgical margins (R0 resection) significantly reduces local recurrence risk (12). However, in this case, the tumor was located in the maxillary region, adjacent to critical structures such as the orbit, skull base, and cavernous sinus, making it difficult to achieve negative surgical margins. Research by Professor Li Wengang’s team notes (13): “Fibrosarcoma presents challenges in determining precise surgical resection margins and carries a risk of hematogenous metastasis, leading to a high postoperative recurrence rate.” Despite undergoing two prior surgical resections, conventional external beam radiotherapy, and chemotherapy, this patient experienced recurrence with orbital invasion due to the tumor’s intrinsic or acquired resistance to traditional radiotherapy and chemotherapy, demonstrating the limitations of surgery and conventional chemoradiotherapy in treating advanced low-grade myxofibrosarcoma of the maxilla. Conventional radiotherapy also faces significant challenges in such cases. A Mayo Clinic research team studying radiotherapy for soft tissue sarcomas states (14): “Radiation therapy in anatomically constrained regions often fails to deliver tumoricidal doses, resulting in local control failure.” In this patient, tumor invasion of the orbit with optic nerve compression made conventional external beam radiotherapy extremely difficult, as the radiation tolerance of the optic nerve (a radiosensitive structure) is far below the dose required for effective fibrosarcoma control (60–70 Gy). Furthermore, the radiosensitivity of the skull base and brain tissue further limited the delivery of therapeutic radiation doses (15).
Iodine-125 (I-125) seed implantation therapy, as a precise radiotherapy technique, demonstrated significant advantages in treating this case of recurrent maxillary fibrosarcoma. Its core mechanism lies in the continuous release of low-energy gamma rays (27.4-35.5 keV), enabling sustained irradiation of tumor tissue while maximizing protection of adjacent critical structures. This makes it particularly suitable for tumors abutting radiosensitive organs. Compared to conventional external beam radiotherapy, I-125 seed implantation achieves spatiotemporal redistribution of the radiation dose distribution. In the spatial dimension, it achieves high conformity of the high-dose region to the tumor target volume. In the temporal dimension, continuous low-dose-rate irradiation induces irreversible double-strand DNA breaks in tumor cells. These spatiotemporal characteristics significantly enhance the therapeutic ratio, delivering a higher biologically effective dose to the tumor while sparing critical structures such as the optic nerve and brain tissue.
Prior research indicates that I-125 brachytherapy demonstrates efficacy and safety in treating skull base and orbital tumors (16, 17). This safety profile is particularly crucial for optic nerve preservation, as seed implantation enables rapid dose fall-off, maintaining the optic nerve dose within safe tolerance limits. In this specific case, the patient’s tumor invaded the orbit and compressed the optic nerve, making it difficult for conventional radiotherapy to achieve tumor control while preserving visual function. Through meticulous planning of seed placement and quantity, we delivered a lethal radiation dose to the tumor while maintaining the optic nerve dose within its tolerance limit (18). Follow-up at one year showed no evidence of visual impairment or radiation injury to brain tissue, confirming the safety advantage of this technique in critical anatomic locations. To our knowledge, it is the first report of I-125 brachytherapy to offer a safe, effective, and repeatable therapeutic option for patients with recurrent LGMFS of the maxillary sinus who are unable to tolerate repeat surgery or chemoradiotherapy.
This case report demonstrates the potential feasibility of I-125 seed implantation for recurrent maxillary sinus LGMFS with orbital involvement, showing encouraging initial results at 12-month follow-up. However, several important limitations must be emphasized: (1) the short follow-up period precludes conclusions about long-term efficacy and late toxicity; (2) as a single case, generalizability remains uncertain; and (3) optimal patient selection criteria are yet to be established. While our preliminary results suggest potential benefits in anatomically challenging cases where conventional approaches are limited, larger prospective studies with extended follow-up periods are essential to establish the role of I-125 brachytherapy in managing recurrent LGMFS.
4 Conclusion
Iodine-125 seed implantation represents a promising treatment option for recurrent maxillary sinus LGMFS, particularly in cases where anatomical constraints or limitations of conventional approaches exist. Multicenter studies with longer follow-up periods are needed to establish optimal patient selection criteria and confirm long-term efficacy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Research Ethics Committee of the 960th Hospital of the Chinese People’s Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study is a retrospective case - report. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QS: Conceptualization, Data curation, Writing – original draft, Investigation, Software, Validation, Writing – review & editing. SL: Investigation, Writing – review & editing. QQ: Formal Analysis, Writing – review & editing. YH: Conceptualization, Writing – original draft. RH: Conceptualization, Writing – original draft. YC: Investigation, Writing – original draft. ZC: Investigation, Writing – original draft. JY: Software, Writing – original draft. XM: Writing – review & editing. ML: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the The 960th Hospital of PLA.
Acknowledgments
We thank all the participants of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1590061/full#supplementary-material
References
1. Norval EJ and Raubenheimer EJ. Myxofibrosarcoma arising in the maxillary sinus: a case report with a review of the ultrastructural findings and differential diagnoses. J Maxillofac Oral Surg. (2011) 10(4):334–339. doi: 10.1007/s12663-011-0259-0
2. Tajudeen BA, Fuller J, Lai C, Grogan T, Elashoff D, Abemayor E, et al. Head and neck sarcomas: the UCLA experience. Am J Otolaryngol. (2014) 35(4):476–81. doi: 10.1016/j.amjoto.2014.02.003
3. Oh AJ, Singh P, Pirakitikulr N, Roelofs K, Glasgow BJ, and Rootman DB. Low-grade fibromyxoid sarcoma of the orbit. Orbit. (2024) 43(3):375–9. doi: 10.1080/01676830.2022.2149820
4. Afacan MY, Ayoglu N, Ozsahin MK, and Botanlioglu H. A case of Malignant myxofibrosarcoma with hypoglycemia attacks. Cureus. (2023) 15(5):e38387. doi: 10.7759/cureus.38387
5. Indap S, Dasgupta M, Chakrabarti N, and Agarwal A. Low grade fibromyxoid sarcoma (Evans tumour) of the arm. Indian J Plast Surg. (2014) 47(2):259–62. doi: 10.4103/0970-0358.138973
6. Koucky V, Kalfert D, Kodetova Novakova D, and Plzak J. Low-grade fibromyxoid sarcoma of the maxillary sinus. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2021) 165(3):342–5. doi: 10.5507/bp.2020.032
7. Zhao D, Dai J, Hu Y, and Wang T. A case report on the short-term recurrence of low-grade fibromyxoid sarcoma in the maxillary sinus. Ear Nose Throat J. (2025) 104(6):346–50. doi: 10.1177/01455613241276673
8. Gao FL, Wang Y, Huang XZ, Pan TF, and Guo JH. I-125 seeds brachytherapy with transcatheter arterial chemoembolization for subcapsular hepatocellular carcinoma. BMC Gastroenterol. (2022) 22(1):273. doi: 10.1186/s12876-022-02356-0
9. Mao G and Theodore N. Spinal brachytherapy. Neuro Oncol. (2022) 24(Suppl 6):S62–S68. doi: 10.1093/neuonc/noac094
10. Marcu LG and Lawson JM. Technical and dosimetric aspects of iodine-125 seed reimplantation in suboptimal prostate implants. Br J Radiol. (2013) 86(1026):20130058. doi: 10.1259/bjr.20130058
11. Zhang M, Liu G, He X, and Chu C. Dosimetric evaluation of iodine-125 brachytherapy for brain tumors using MR guidance combined with a three-dimensional non co-planar template. Brachytherapy. (2023) 22(2):242–9. doi: 10.1016/j.brachy.2022.11.008
12. Li Z, Liu X, Zhang Q, Zhang J, Huang M, and Liu S. Myxofibrosarcoma of the mandible: a case report and review of the literature. BMC Oral Health. (2020) 20(1):113. doi: 10.1186/s12903-020-01094-7
13. Wang Z, Miao F, Gu L, Zhang R, Ma Y, Li Y, et al. Stimulator of interferon genes-activated biomimetic dendritic cell nanovaccine as a chemotherapeutic booster to enhance systemic fibrosarcoma treatment. ACS Nano. (2024) 18(35):24219–35. doi: 10.1021/acsnano.4c05657
14. Tibbo ME, Landau R, Markowitz MI, Abuodeh Y, Thomas Temple H, and Crawford B. The effect of radiation or chemotherapy on the local recurrence, overall survival, and distant metastasis in patients with myxofibrosarcoma: A systematic review. J Surg Oncol. (2024) 130(3):586–93. doi: 10.1002/jso.27782
15. Carey AR, Page BR, and Miller N. Radiation-induced optic neuropathy: a review. Br J Ophthalmol. (2023) 107(6):743–9. doi: 10.1136/bjo-2022-322854
16. Ma N, Wang P, Zhang S, Ning X, Guo C, Zhang Q, et al. Surgical resection and orbital iodine-125 brachytherapy for orbital Malignancy: a novel treatment for orbital lymphoma. Int Ophthalmol. (2023) 43(6):1945–55. doi: 10.1007/s10792-022-02594-x
17. Abrishami M, Beiki-Ardakani A, and Krema H. Iodine-125 plaque brachytherapy for the treatment of iris cavernous hemangioma. Can J Ophthalmol. (2025) 60(2):e320–e323. doi: 10.1016/j.jcjo.2024.09.007
Keywords: low-grade myxofibrosarcoma, iodine-125 brachytherapy, orbital-invasive, case report, CT-guided
Citation: Sun Q, Li S, Qiu Q, Hu Y, Huang R, Chen Y, Cui Z, Yang J, Ma X and Li M (2025) Iodine-125 brachytherapy for orbital-invasive low-grade myxofibrosarcoma of the maxillary sinus: a case report challenging conventional therapeutic paradigms. Front. Oncol. 15:1590061. doi: 10.3389/fonc.2025.1590061
Received: 11 March 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Yujing Li, Emory University, United StatesReviewed by:
Hong Ma, OriCell Therapeutics, ChinaJoko Wibowo Sentoso, Sebelas Maret University, Indonesia
Copyright © 2025 Sun, Li, Qiu, Hu, Huang, Chen, Cui, Yang, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowen Ma, eXVldGlhbnpoaXhpdUBzaW5hLmNvbQ==; Min Li, bGltaW55aW5neGlhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qiyu Sun
Qiyu Sun Shuai Li2†
Shuai Li2† Jiaxin Yang
Jiaxin Yang Min Li
Min Li