- 1Lankenau Institute for Medical Research, Wynnewood, PA, United States
- 2Lankenau Medical Center, Main Line Health, Wynnewood, PA, United States
- 3Breastcancer.org, Ardmore, PA, United States
- 4Center for Population Health Research, Main Line Health, Wynnewood, PA, United States
- 5College of Population Health, Thomas Jefferson University, Philadelphia, PA, United States
- 6Department of Psychiatry, Columbia University Irving Medical Center, New York, NY, United States
- 7Department of Neural Sciences, Center for Substance Abuse Research, Temple University Lewis Katz School of Medicine, Philadelphia, PA, United States
- 8Department of Neurology, Baylor University Medical Center, Houston, TX, United States
- 9New York State Psychiatric Institute, New York, NY, United States
Introduction: Chemotherapy-induced peripheral neuropathy (CIPN) can greatly impair function, leading to disability or truncated treatment in cancer patients. Previous animal studies show that cannabidiol (CBD) and delta-9- tetrahydrocannabinol (THC) can ameliorate CIPN. This study assessed the effect of combined CBD and THC on CIPN symptoms amongst cancer patients treated with taxane- or platinum-based agents.
Materials and methods: This 12-week randomized, double-blind, placebo-controlled trial included participants with nonmetastatic breast, colorectal, endometrial, or ovarian cancer experiencing grade 2–3 CIPN. The active group received CBD (125.3-135.9 mg) combined with THC (6.0-10.8 mg) in gelcaps. The Quality-of-Life Questionnaire-CIPN twenty-item scale (QLQ-CIPN20) sensory subscale was used as the primary outcome. Additional outcomes assessed pain, sleep, and function. Neurologic exams evaluated touch, pressure, and vibration sense. Following the randomized controlled trial, participants were invited to enroll in a 12-week open-label observational study.
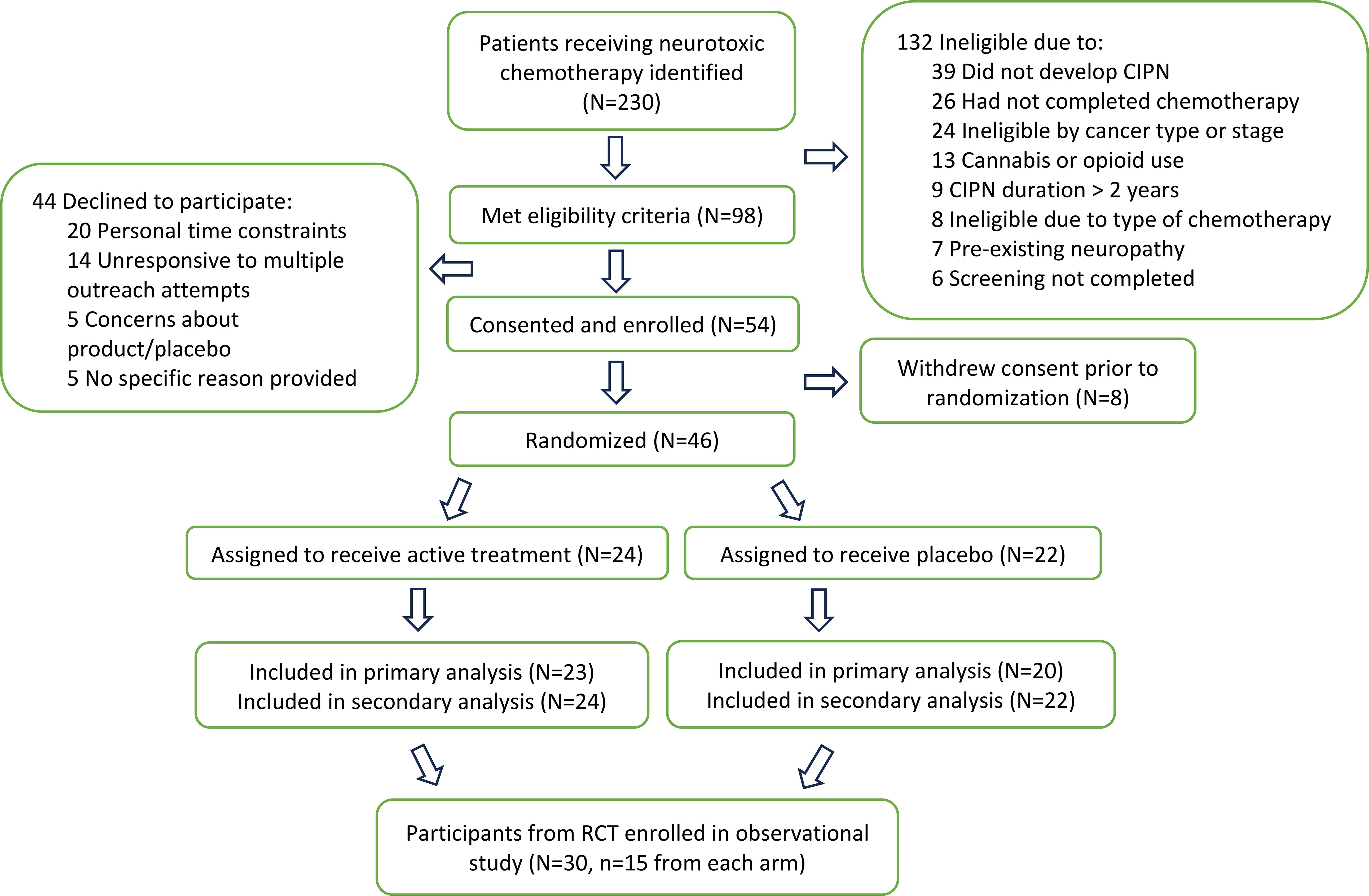
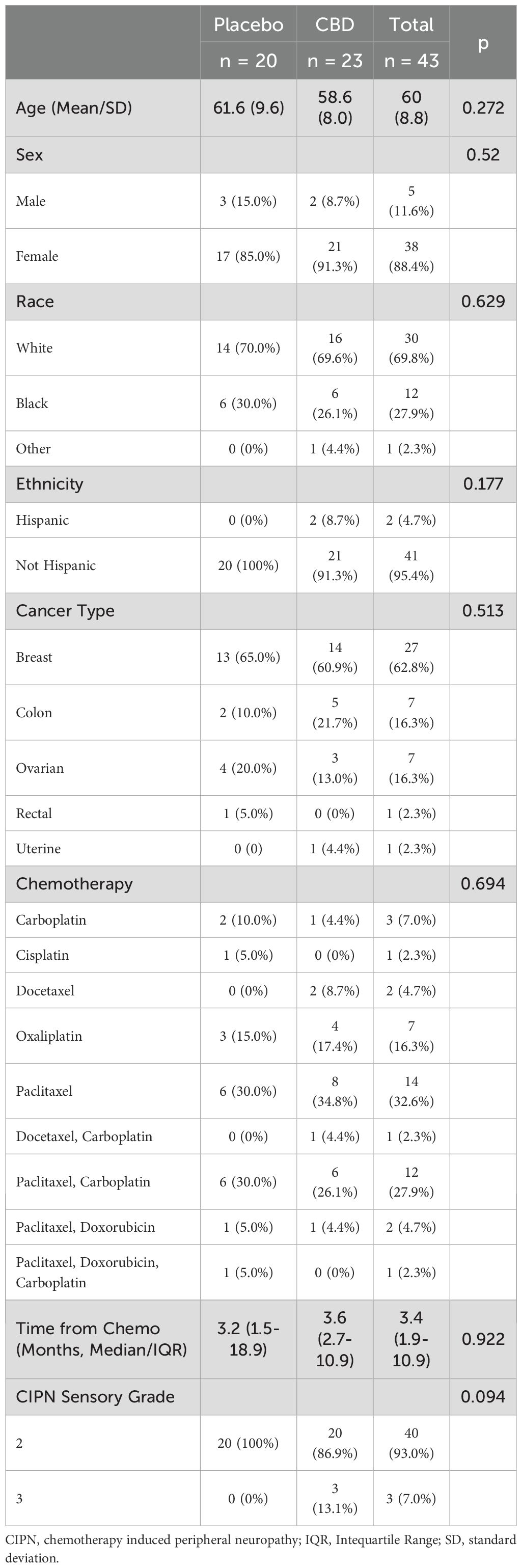
Results: Of 230 participants identified, 124 met eligibility, 54 were enrolled, 46 were randomized, and 43 completed 12 weeks of treatment. This was lower than our goal of 100 randomized participants. The mean age was 60 +/- 9 years, 88% were female, 63% had breast cancer. All participants had completed chemotherapy. The primary analysis showed no differences in outcome measures between active and placebo groups, likely due to sample size. Although an increase in bilirubin (one participant in active group, and one in placebo) and alkaline phosphatase (one participant in active group) was seen, this did not exceed the exit criteria. A secondary analysis showed that the active group experienced greater improvement in the QLQ-CIPN20 measures of sensory impairment relative to placebo (-10.4 (95% -20.5, -0.3), p = 0.044). There was also improvement in light touch and vibration sensation of the feet on neurological exam that approached significance. There was no effect on other measures, including pain, and no between-group differences in side effects. The uncontrolled observational study showed similar results.
Discussion: The primary analysis showed no between-group difference in CIPN symptoms. The secondary analysis indicated that CBD with THC could improve sensory impairment and might increase touch and vibration sense, although these findings require confirmation in a future, more fully powered study. Nonetheless, our results show that combination CBD/THC can be safely delivered to participants with CIPN and suggest that these cannabinoids should be further investigated for this indication.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a disabling and dose-limiting complication of cytotoxic agents. The symptoms include distal extremity numbness, tingling, pain, and loss of function. CIPN can impair quality of life and impede patients’ ability to complete curative therapy (1, 2). Research shows that 40-70% of patients treated with taxanes (e.g., paclitaxel, docetaxel) or platinum-based agents (e.g., oxaliplatin, cisplatin, carboplatin) develop CIPN (3, 4). Additionally, the rate of CIPN has remained unchanged for decades, globally, without a decrease in prevalence (4).
Previous studies have investigated a range of therapies for this disorder. These include topical agents, gabapentinoids, antidepressants, physical therapy, acupuncture, and more (5). However, to date, only duloxetine has shown level I evidence of efficacy for CIPN (1, 6). Thus, there is a need to develop additional treatments for this common, refractory disorder.
In mice, cannabidiol (CBD) and delta 9- tetrahydrocannabinol (THC) can ameliorate CIPN caused by paclitaxel and oxaliplatin (7–12). Previous studies show that CBD administration prevented the development of mechanical and cold sensitivity in mice treated with paclitaxel through a potential serotonergic mechanism (7), without psychoactive effects or interference with chemotherapeutic efficacy (8). Follow-up studies showed that CBD was also effective against oxaliplatin-associated mechanical sensitivity and synergized when co-administered with THC (9). These preventative effects of cannabinoids on paclitaxel-associated mechanical sensitivity in mice have also been shown by Kalvala et al. (10, 11) Additionally, Ortiz et al. (12) reported that CBD treatment can reverse paclitaxel-induced mechanical sensitivity.
Previous studies in cancer patients have also investigated cannabinoids for CIPN. In 2014, Lynch et al. (13) compared nabiximols (an oral spray of THC and CBD) to placebo in 18 participants with CIPN and found no significant change in CIPN symptoms (13). In contrast, a retrospective study showed that the use of medicinal cannabis in patients treated with oxaliplatin or 5-fluorouracil-based combinations was associated with a decrease in CIPN severity compared to patients not using cannabis (14). An open label trial administered CBD to two groups of patients receiving chemotherapy: oxaliplatin combined with capecitabine or paclitaxel with carboplatin (15). The results showed decreased cold sensitivity to touch and less throat discomfort in participants who received CBD with capecitabine and oxaliplatin, with no effect on pain for either group (15). Meanwhile, a pilot randomized, placebo-controlled trial of oral cannabinoids (300 mg CBD/15mg THC) showed no improvement (16), while a study of topical CBD cream also showed a lack of an effect on pain (17).
Our goal was to investigate hemp-derived cannabinoids for CIPN using a randomized, double-blind, placebo-controlled design of 12 weeks duration. We enrolled participants with cancer who developed CIPN following taxane- or platin-based chemotherapy. The active medication consisted of gelcaps with cannabinoids that contained CBD combined with THC. Each gelcap contained between CBD (13.9 and 15.1 mg) and THC (between 0.67 and 1.20 mg) and the dose was titrated to 3 gelcaps three times a day. Change in CIPN symptoms was measured with questionnaires while neurologic examination evaluated touch, pressure, and vibration in the extremities. Participants completing the randomized controlled trial were invited to enroll in an open-label, 12-week observational study where the dose was reduced to 1 gelcap twice daily. The outcomes included questionnaires but not neurologic examination.
Materials and methods
Randomized controlled trial
This randomized, double-blind, placebo-controlled clinical trial was conducted from 6/1/2020 to 8/8/2022. Approval was obtained from Main Line Hospitals Institutional Review Board and registered with clinicaltrials.gov (NCT04398446). The inclusion criteria were: 1) diagnosed nonmetastatic breast, colorectal, or endometrial cancer or a diagnosis of ovarian cancer (any stage with no evidence of active disease at the time of enrollment); 2) completed treatment with taxane- or platin-based chemotherapy; 3) CIPN of sensory grade 2 or 3 and motor grade < 2 (severity determined using the Common Terminology Criteria for Adverse Events, CTCAE) (18). Exclusion criteria included: 1) current cannabis use (negative urine drug screen required); 2) unstable medical illness and serious psychiatric disorders; 3) history of neuropathy prior to chemotherapy; 4) CIPN of > 2 years; and 5) pregnancy or breastfeeding. Additional details on eligibility criteria are provided in the Supplementary Material.
Participants were assigned to active or placebo groups using random blocks of size 2 and 4, with cancer type (breast, colorectal, ovarian/uterine) as the stratification variable. The active medication, delivered in gelcaps, contained CBD (between 13.9 and 15.1 mg per gelcap) and THC (between 0.67 and 1.20 mg per gelcap) while the placebo consisted of hemp-derived product in gelcaps devoid of CBD and THC. The combination of CBD with THC was chosen based on animal data showing synergy when used for CIPN (8). The doses were derived from allometric scaling based on previous animal studies. Because plant-derived cannabinoids were used, there was some variability in the dosages of CBD and THC contained within gelcaps. Details on the dosages selected and the gelcap contents, including the use of third-party testing, are provided in the Supplementary Material.
For both groups, the following titration schedule was used: days 1-3, 1 gelcap 3 times daily; days 4-6, 2 gelcaps 3 times daily; day 7 and beyond, 3 gelcaps 3 times daily. Thus, after week 1, participants in the active group took between 125.3 to 135.9 mg CBD daily and between 6.0 to 10.8 mg THC daily. Both active and placebo gelcaps were provided by Ecofibre/Ananda Health and dispensed through an independent pharmacy using codes to maintain blinding. Ecofibre/Ananda Health was not involved in the study design, regulatory approval, research procedures, analysis of data, interpretation of results, or publication of findings.
The following outcomes were measured every 2 weeks for 12 weeks:
1) The primary outcome was the sensory subscale of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-CIPN twenty-item scale (QLQ-CIPN20) (19). This questionnaire assesses sensory, motor, and autonomic symptoms: the sensory subscale includes 9 items that measure numbness, tingling, distinguishing temperature, hearing, and pain (19). We also separated the scores for numbness and tingling from pain from the sensory questions so that these items could be investigated independently (questions 31-34, 39, 40 were averaged for numbness/tingling; questions 35 and 36 were averaged for pain). This approach has precedent in CIPN research (20), though it is not formally validated as a primary endpoint.
Additional outcomes included:
2) Neurological examinations were performed using the following assessments (see Supplementary Material for additional details):
a. The Semmes-Weinstein monofilament examination, which evaluates touch and pressure sensation (21), was performed five times on the palm using five sizes of monofilament, and five times on the big toe using one size of monofilament (see Supplementary Material for monofilament sizes). Delivery of the monofilament was alternated in a non-regular pattern with sham delivery. The outcome measure was the number of times the monofilament was accurately detected.
b. A 128Hz tuning fork to measure vibratory sensation of the big toe, performed five times (with alternating vibration and no vibration) measured the number of times participants accurately detected vibration or no vibration.
c. A 40g semi-sharp sterile tip to measure pinprick pain sensation of the big toe was performed five times. Actual pinprick was alternated (in a non-regular pattern) with sham pinprick. The outcome was the number of times the pinprick was accurately detected by the participant.
3) The Brief Pain Inventory short form (BPI-SF) questionnaire was used to gauge pain (22). The outcomes included the worst and average pain in the past 24 hours (questions 3 and 5) while pain severity was reported as the average of questions 3-6. The pain interference score was obtained using the total score of items 9a-9g.
4) The EORTC QLQ-C30 Quality of Life Survey (QLQ-C30) was used to assess global health status, including physical, psychological and social function (23). The outcomes included a summary score and global health QOL score (23, 24).
5) The PROMIS Sleep Disturbance Scale was used to rate sleep-related impairment (sleep quality, sleep depth, and restoration) (25). The score provides a standardized T-score with a mean of 50 and a standard deviation of 10.
In addition, side effects were assessed at each participant visit and liver function tests were obtained at baseline followed by every 4 weeks for 12 weeks total.
Statistical analysis (randomized controlled trial)
Baseline patient characteristics, demographics, CIPN grade, cancer type and stage, chemotherapy type, time since completion of chemotherapy and primary outcome results were compared between the two arms at baseline. Continuous variables were summarized using means (standard deviation) if normally distributed, or medians (interquartile range) if non-normally distributed and compared between the two arms using two-sample t-tests (for normal distributions) or Wilcoxon rank-sum tests (non-normal distributions). Categorical variables were summarized using frequencies and percentages and compared between the two arms using Chi-square tests of independence.
The primary data analysis investigated the change from baseline to week 12 and included only participants with complete data (n=20 placebo, n=23 active). The primary outcome was change in the QLQ-CIPN20 sensory subscore and secondary outcomes were change in numbness and tingling (from the QLQ-CIPN20), pain (from the QLQ-CIPN20), other QLQ-CIPN20 sub-scores (motor, autonomic), neurological exams, BPI, QLQ-30, and PROMIS from baseline to week 12. The mean differences from baseline and week 12 were compared between each arm with a two-sample t-test and Cohen’s d for effect size. Multiple imputation was not performed for the primary analysis, as applying it in a small sample could increase the risk of bias.
To address missing data in the primary analysis, we conducted additional secondary analyses using a modified intention-to-treat approach that included all participants who completed at least 8 weeks of study visits. Multivariable linear mixed effects models were built for each scale, with random intercepts to account for repeated measures. With these models, we compared the outcomes between study arms while adjusting for baseline CIPN grade and number of visits. In addition, we further adjusted for baseline scale values for BPI, QLQ-C30, and PROMIS sleep because of differences at baseline. The interaction between arm and time was also added to the model and the predicted probabilities graphed. The interactions were tested for significance with a Wald test. For the linear mixed models, no adjustments were made for missing assessments and all patients who had at least 8 weeks of follow-up were included. Finally, a sensitivity analysis of the CIPN20 sensory and motor scales was conducted for all patients who had completed chemotherapy 18 months or less at baseline.
All statistical analyses were performed in Stata/MP 17.0 (StataCorp LP., Texas, USA). Significance was assessed at the 0.05 level, 95% confidence intervals (CI) were presented, and all tests are two-sided unless noted otherwise. A sample size calculation was performed (see Supplementary Material for details).
Observational study
Participants who completed the RCT were invited to enroll in an optional 12-week open-label observational study. This study was separated by the RCT by a 4-week washout period. Gelcaps from Ecofibre/Ananda Health were sent directly to participants who took one gelcap twice daily containing CBD (between 13.9 and 15.1 mg per gelcap) and THC (between 0.67 and 1.20 mg per gelcap), as above. The same primary outcomes (sans neurological exam) were measured by digital survey at baseline and weeks 4, 8, and 12.
Statistical analysis (observational study)
Descriptive statistics were performed. Scores were compared between baseline and week 12 of the observational study, as well as between baseline and week 12 of the RCT (for those enrolled in both studies). Because we saw an effect in the RCT for sensory CIPN20 scores, for this scale only we combined the results of the RCT and the observational study. We used two-sample t-tests and Cohen’s D to compare the RCT placebo and RCT active groups for each of these three timescales. We built linear adjusted main effects regression models, using the RCT placebo group as reference, to account for the correlation between participants, time from baseline, baseline CIPN grade, and other CIPN drugs as covariates. Marginal graphs were created by adding time and active vs. placebo group as an interaction in the mixed effects model.
Results
Main study: randomized controlled trial
Of 230 potential participants, 124 met eligibility criteria and 54 were consented and enrolled. Of these, 46 were randomized and 43 completed 12 weeks (23 assigned to the active group, 20 to placebo) as shown in Figure 1. Demographic information and participant characteristics are shown in Table 1 for those who completed 12 weeks. The median time from chemotherapy (in months) was 3.2 (IQR 1.5-18.9) for the placebo group and 3.6 (IQR 2.7-10.9) for the CBD group. No between-group differences were seen.
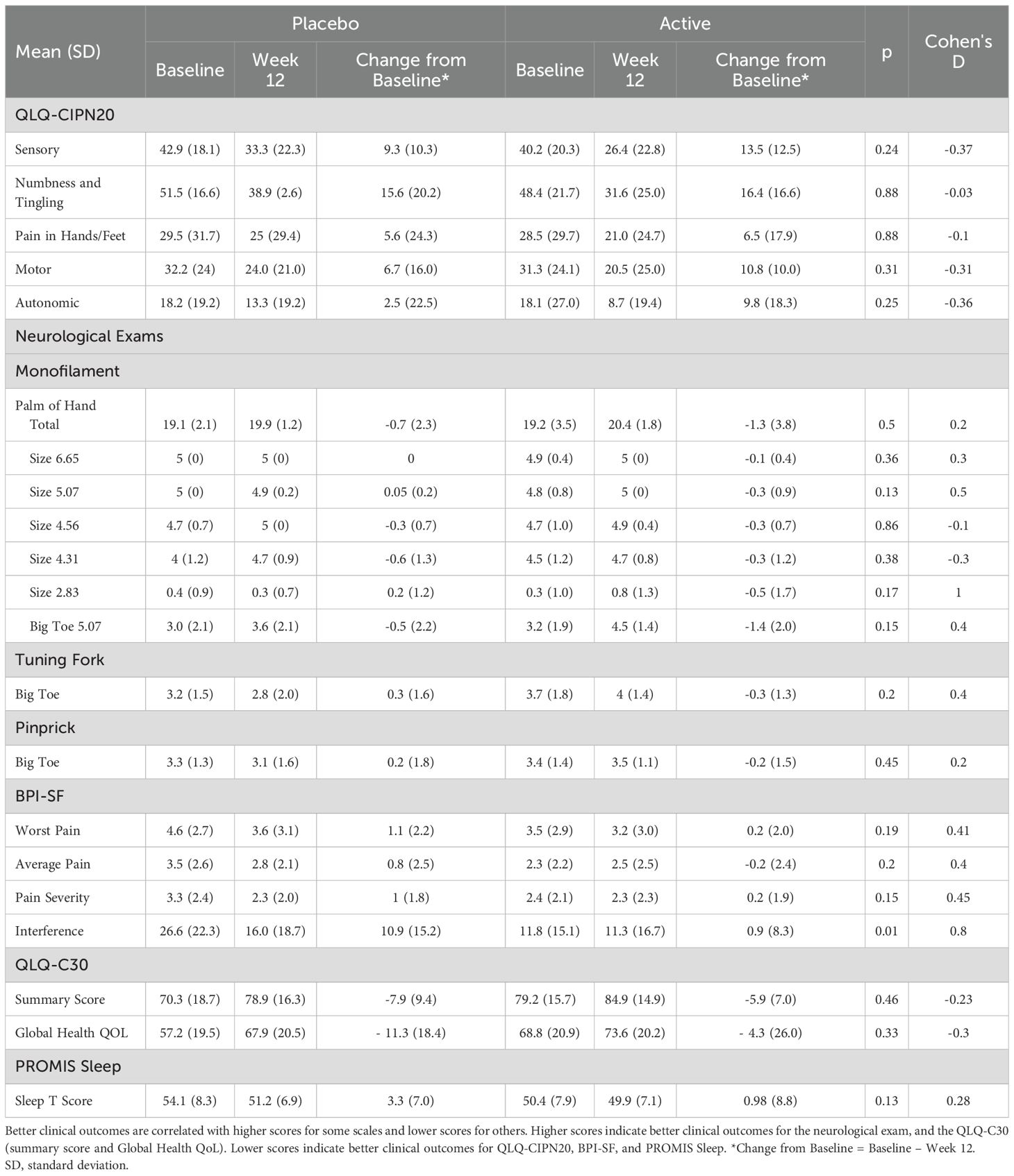
The result of the primary analysis is shown in Table 2 which compared outcomes at baseline, week 12, and change from baseline to week 12. No group differences were observed except the BPI score of pain interference, where the placebo group reported greater improvement compared to the active group (10.9 vs. 0.9, p=0.01). However, there was a difference in the baseline pain interference score between the two groups (26.6 (SD 22.3) vs. 11.8 (SD 15.1), p = 0.011). After adjustment for baseline score, no difference was observed.
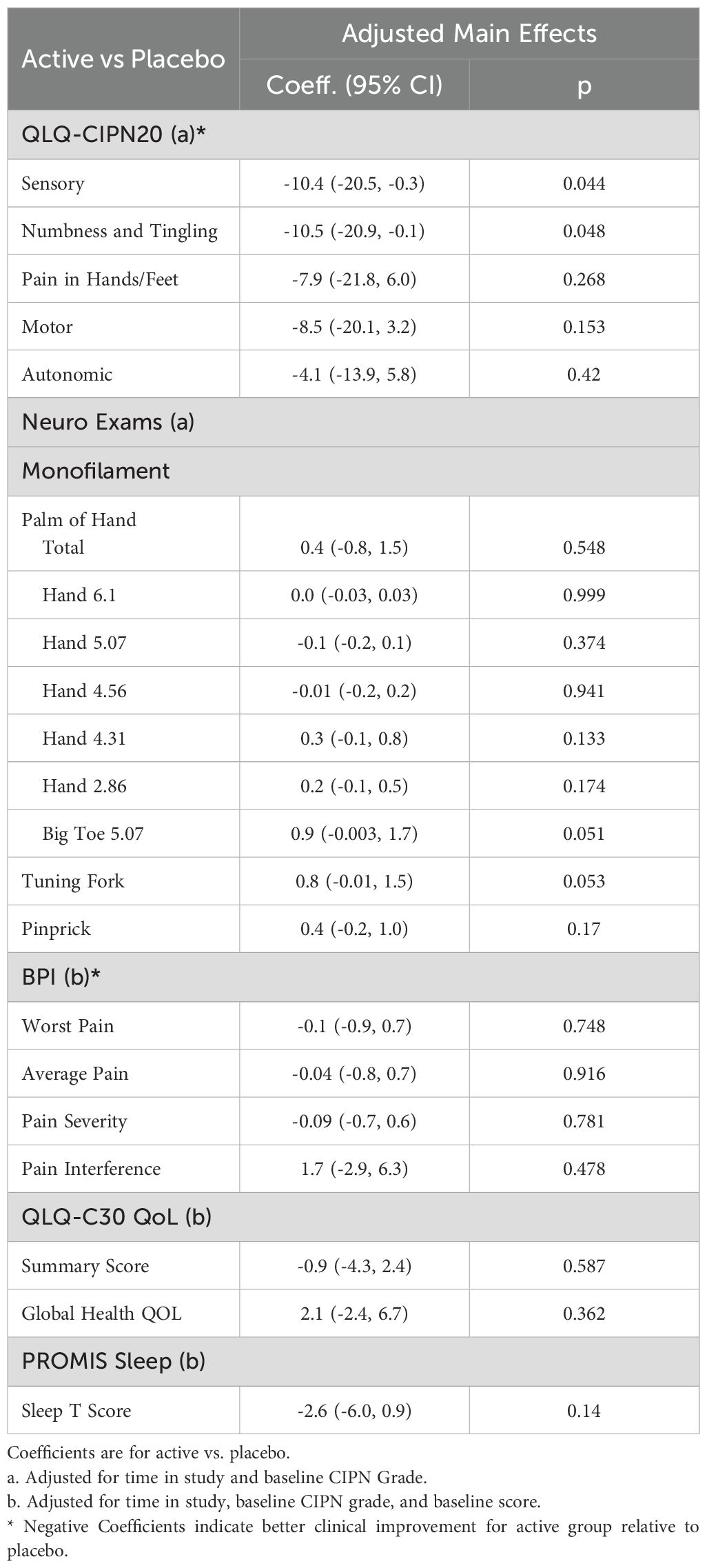
The secondary analysis included 46 participants who completed at least 8 weeks (see Supplementary Material for demographic information). The results are shown in Table 3. Here, greater improvement was seen in the active group in the sensory score of the QLQ-CIPN20 relative to the placebo group (-10.4 (95% CI -20.5, -0.3), p = 0.044). Additionally, the active group reported greater improvement in numbness and tingling compared to the placebo group (-10.5 (95% CI -20.9, -0.1), p = 0.048) with no difference in pain. No differences were seen in motor or autonomic symptoms. There were no differences in BPI-SF, QLQ-C30, or PROMIS scores. No adjustments were made for multiple comparisons in secondary analyses; as such, these findings should be considered hypothesis-generating and interpreted with caution.
The results of the neurologic exam are also shown in Table 3. Monofilament testing of the big toe (0.9 (95% CI -0.003, 1.7), p=0.051) and tuning fork testing (0.8 (95% CI -0.01, 1.5), p=0.053) approached significance for improvement in the active group relative to placebo. No differences were found in the pinprick exam of the big toe or monofilament testing of the hand.
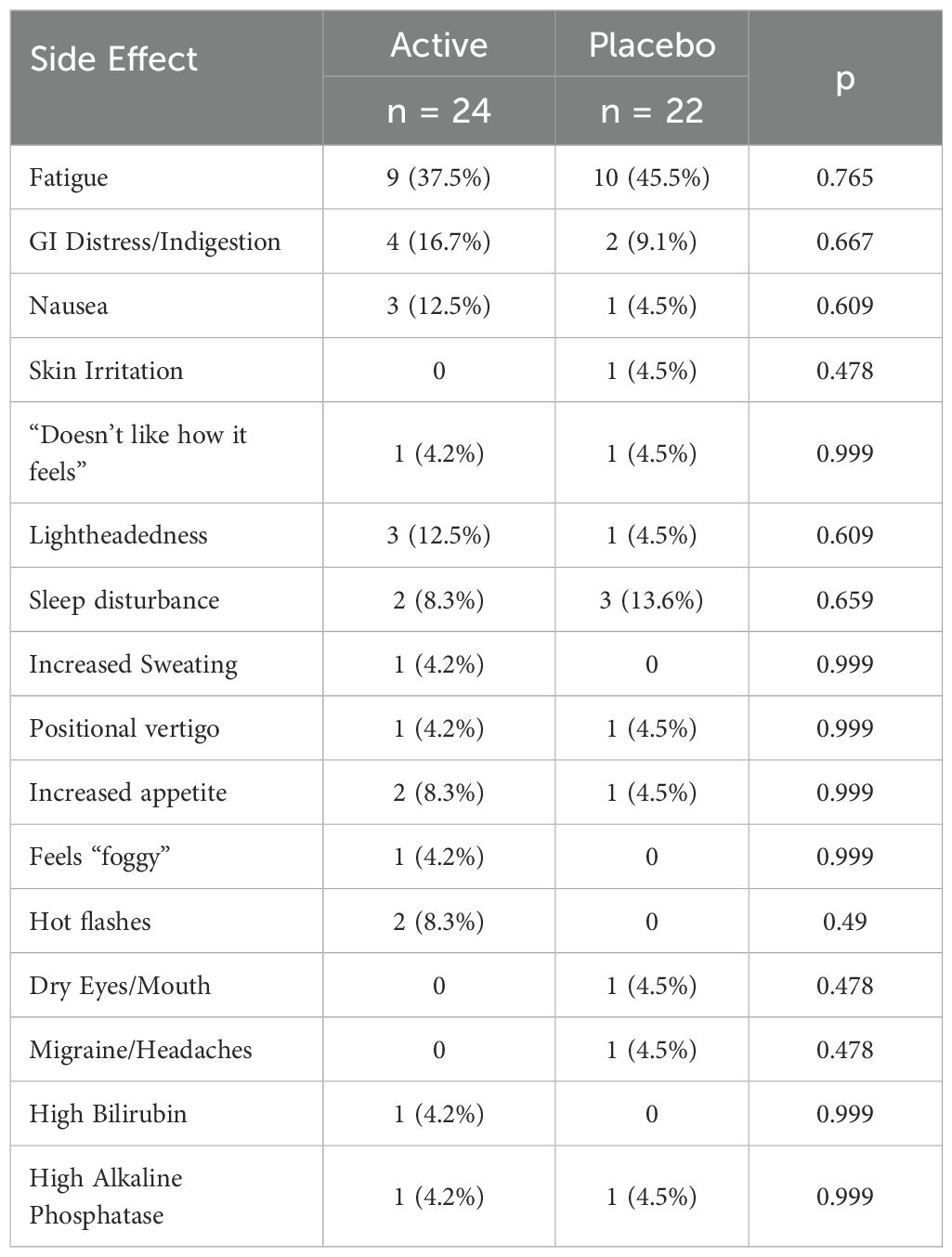
The side effects reported by participants are shown in Table 4. No between-group differences were seen. There were no serious adverse events, and no participant was removed from the study due to an adverse event. Please see Supplementary Material for side effects categorized by severity using CTCAE criteria (Supplementary Tables S2, S3). As shown in these tables, fatigue, gastrointestinal distress, and sleep disturbance were the most common side effects. The majority of side effects were rated as grade 2 (moderate symptoms).
Of note, two participants in the active group experienced an increase in lab values (one bilirubin and one alkaline phosphatase) while one in the placebo group had an increase in bilirubin. No participant had an increase in lab values that exceeded two times normal and met criteria for early discontinuation.
The sensitivity analysis including only patients whose time from chemotherapy at baseline was 18 months or less included 17 placebo and 21 CBD. The results were similar to the primary analysis (see Supplementary Tables S4, S5 in the Supplementary Material).
Observational study (following the RCT)
A total of 30 participants were enrolled in the observational study. Of these, 15 had previously been assigned to the active group and 15 were in the placebo group. The time between the RCT and observational study was 28–92 days, which could have impacted the outcomes. Participant demographic information and characteristics are shown in Table 5, with the participants separated by the group assigned to in the RCT. No differences were seen between the two groups.
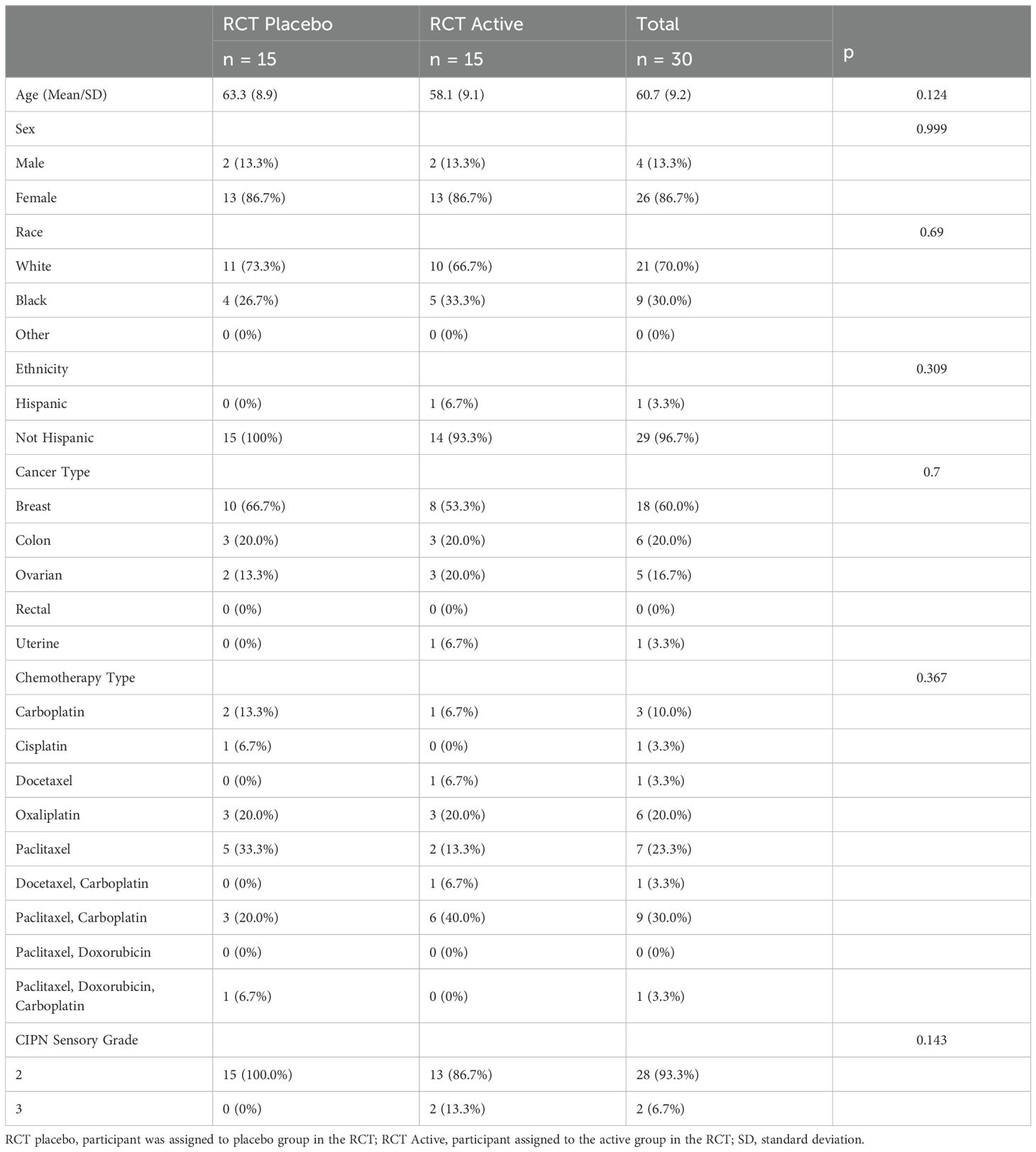
Table 5. Observational study: Participant characteristics and demographics by group in the observational study (separated by the group assigned to in the RCT).
The analysis of the observational study compared participants by their previously assigned group in the RCT (referred to as RCT placebo or RCT active). The results (Table 6) showed that the RCT active group reported an improvement in the QLQ-CIPN20 sensory score, and numbness/tingling compared to the RCT placebo group, with no change in pain. Within each group, there was no significant change for other outcome measures in the observational study only. No participants required removal from the study due to adverse events.
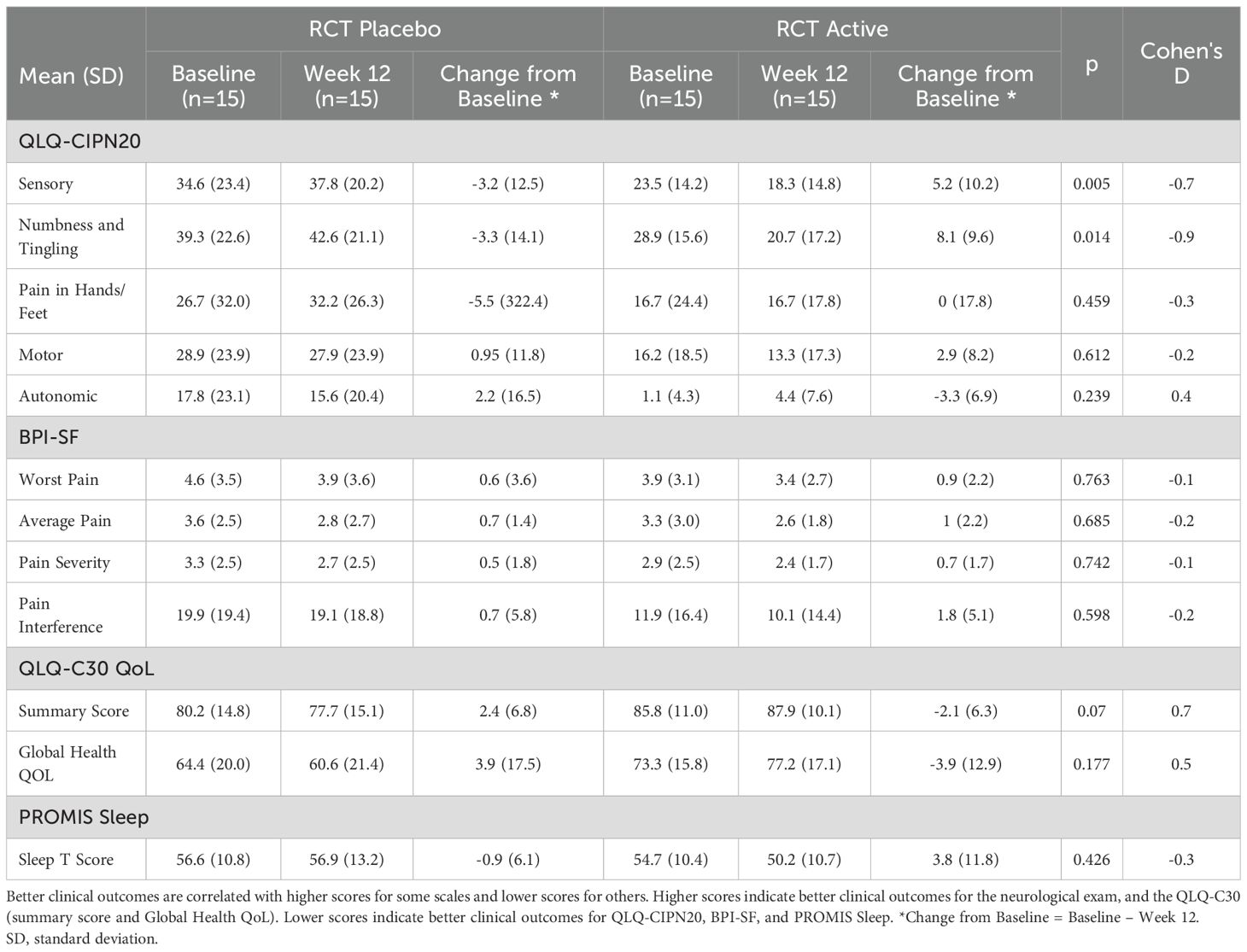
Table 6. Observational study: comparison of outcomes at baseline, week 12, and change (baseline - week 12).
Discussion
In this randomized, placebo-controlled clinical trial of plant-based CBD with THC for CIPN induced by platinum-based or taxane chemotherapy we saw no difference between the active and placebo groups in our primary analysis. This was likely due to the number of participants which did not reach our goal of 100, as indicated by an a priori sample size calculation. This may have limited statistical power and increased the risk of Type II error. As such, additional studies with larger samples are needed to validate these findings.
Our secondary, exploratory analysis included all randomized participants (adjusted for time in the study and baseline CIPN grade). It showed that the combination of CBD/THC could improve sensory impairment in CIPN. We saw a reduction in the sensory score of the QLQ-CIPN20 and a decrease in self-reported numbness and tingling in the active condition compared to the placebo. This finding was accompanied by a trend in the results of the neurologic exam, which suggested that the active treatment might increase touch and vibration sense in the feet compared to placebo. However, because of the sample size and the potential for type II errors, however, these findings need to be considered preliminary and in of validation in larger studies.
If validated in a larger study, the finding of improved sensory function has the potential to help patients with CIPN. Numbness and tingling are among the most common symptoms of sensory impairment in cancer patients (20, 26, 27) and lead to significant disability and fall-related injuries (28). However, there remains a lack of treatment options: only duloxetine shows clear evidence of efficacy for CIPN (1, 6), but has a modest impact on numbness and tingling (29).
On neurologic exam, we saw a trend towards improved protective sensation and vibration sense of the feet in the active group over the placebo group. Protective sensation is crucial for normal function and vibration sense is closely tied to the ability to sense position and maintain balance (30). Impaired postural control and loss of balance in CIPN is associated with functional impairment and a risk of physical harm (31).
We saw no serious adverse events and no difference in side effects between the active and placebo groups, indicating that CBD (125.1 to 135.9 mg daily) combined with THC (between 6.0 to 11.5 mg daily) was well tolerated. We did see a small increase in bilirubin and alkaline phosphatase although this occurred in both groups. However, this increase did not surpass the exit criteria of twice normal values for these laboratory measures.
In the open-label observational study, we compared participants who had previously been assigned to the active group in the RCT to those who received placebo in the RCT. These results showed that participants in the active group experienced greater improvement in the QLQ-CIPN20 sensory score and numbness/tingling compared to the placebo group. We also saw no adverse events leading to removal from the study for safety purposes. However, the observational portion of the study included no control group or neurological exams which limits the interpretation of these results.
Our findings add to the literature on cannabinoids for CIPN. An early pilot study investigated nabiximols for CIPN and did not find a significant effect on pain or sensory function, but showed that the medication was well tolerated by cancer patients (13). A more recent placebo-controlled pilot study (n=12) of oral cannabinoids (300 mg CBD/15 mg THC) showed improved CIPN in the placebo group (16). However, a retrospective analysis of patients (700+) treated with oxaliplatin and 5-fluorouracil-based combinations showed that cannabis use was associated with lower CIPN severity and a decrease in the development of neuropathy caused by oxaliplatin (14). A randomized, placebo-controlled trial of topical CBD cream for CIPN showed no effect on pain (17), but a case series reported that topical creams with THC and/or CBD might improve painful CIPN (32).
Recently, Nielsen et al. prospectively investigated the effect of CBD in participants (n=54) scheduled to receive carboplatin and paclitaxel or capecitabine and oxaliplatin (15). The active group received CBD (300 mg daily) administered for 8 days, starting the day before chemotherapy. A non-randomized control group was included who did not receive placebo. The results showed less cold sensitivity to touch, throat discomfort, and discomfort swallowing cold liquids in participants who received CBD (along with capecitabine with oxaliplatin) with no effect on pain or numbness/tingling compared to the control group.
Taken together, the results of these studies, including ours, indicate that CBD and THC are tolerated well in this patient population and could have a clinical use in addressing CIPN. However, the findings are mixed, including the effect on pain. Although previous placebo-controlled studies have shown that THC can reduce painful neuropathy caused by diabetes or HIV (33–36), studies using cannabinoids for CIPN are more mixed. This might result from the CIPN studies using lower doses of THC, which has been shown to reduce pain caused by a range of medical disorders (37). Nonetheless, given that CIPN generally includes numbness and tingling more commonly than pain (20, 26, 27) these studies suggest that cannabinoids may have a future therapeutic role.
The mechanism behind the ability of cannabinoids to improve the sensory symptoms of CIPN is not fully understood but likely includes a range of cellular processes. CBD has a low affinity for cannabinoid receptors, but regulates the endocannabinoid system as an allosteric modulator, and has up to 56 neurological molecular targets (38, 39). We and others have investigated three potential mechanisms of action using in vivo and in vitro preclinical assays: 5-HT1A receptor antagonism, GPR55 (G protein-coupled receptor 55) receptor antagonism, and the Na+-Ca2+ exchanger in mitochondria (mNCX).
In vivo, antagonism of the 5-HT1A receptor attenuates the neuroprotective effects of CBD in preclinical models of neuropathic pain (40), including CIPN (8). However, direct clinical studies testing 5-HT1A activation specifically for relief of sensory symptoms associated with neuropathies humans have not been performed. An analogue of CBD that is selective for GPR55 receptor antagonism, KLS-13019, effectively attenuates pain and inflammation associated with CIPN in vivo as well as in vitro (41, 42). We and others have demonstrated that CBD can engage mNCX to regulate intracellular calcium levels (43, 44), including in response to paclitaxel (39). While there is emerging evidence that the mNCX plays a role in pain and sensory function, direct human data is needed.
CBD also interacts with a wide range of other receptors, cellular signaling cascades, and proinflammatory cytokines, and has been shown to reduce oxidative stress and to inhibit proinflammatory pathways (38, 39, 45, 46). While these mechanisms contribute to neuronal damage in CIPN, the exact etiology underlying the sensory disturbances of parasthesias and dysesthesias are not known. As mentioned, Δ9-THC prevents symptoms in CIPN in rodent models and potentiates the effects of CBD; these effects are likely attributed to direct actions on canonical CB1 and CB2 receptors (47). Unfortunately, while biomarkers are being developed to predict and monitor CIPN, there is less evidence for their use in measuring response to specific CIPN treatments.
Overall, this study suggests that combination CBD/THC could help with the sensory impairment seen in CIPN. Since the disorder is prevalent and incurs significant hardship, even a modest sensory improvement could enhance patients’ quality of life, given the lack of alternatives. Further, this study included participants who had completed chemotherapy, suggesting that improvement may occur following the onset of CIPN, which might help some patients. Future studies should include dose-ranging trials, biomarker endpoints, and male-inclusive cohorts to better define therapeutic windows.
Limitations
A significant limitation of this study was the small sample size. However, we had expected an attrition rate of 30% when the actual rate was 15%, which may reflect the medication being well tolerated. In addition to sample size, other factors could have influenced the results such as suboptimal dosing or delayed timing of the intervention post-chemotherapy. This study included 88% women (12% men), which likely results from the types of cancer diagnoses (63% breast and 16% ovarian). This prevented an assessment of sex-related differences, including metabolism differences (48), and limits the generalizability to males. Baseline time from treatment did not differ between the groups, however, since this study occurred after chemotherapy, the treatment effect may have been attenuated. Future studies may include participants CIPN limited to a shorter duration.
The gelcaps contained cannabis product, which meant that there was variation in the dosages. Although this would be expected when using any plant-derived cannabis product, it could have an impact on reproducibility. Participants were asked to keep a logbook to record compliance with medication, which was close to 100%. However, pill counts were not performed which would have provided a more direct measure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Lankenau Institute for Medical Research. Our Human Research Protection Program, administered through the Office of Research Protections and the Main Line Hospitals Institutional Review Board, has received full accreditation by the Association for Accreditation of Human Subject Research Protection Programs (AAHRPP). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. MG: Data curation, Investigation, Project administration, Writing – review & editing. SK: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JG: Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. JB: Project administration, Resources, Supervision, Writing – review & editing. KD: Data curation, Methodology, Writing – review & editing. GB: Writing – review & editing. EE: Data curation, Investigation, Project administration, Writing – review & editing, Supervision. SM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. LS: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. SW: Project administration, Supervision, Writing – review & editing. KA: Data curation, Methodology, Project administration, Supervision, Writing – review & editing. AG: Investigation, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. JH: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. JM: Investigation, Visualization, Writing – review & editing. DH: Investigation, Writing – review & editing. ZA: Investigation, Visualization, Writing – review & editing. AS: Investigation, Visualization, Writing – review & editing. JS: Investigation, Visualization, Writing – review & editing. RC: Investigation, Visualization, Writing – review & editing. EZ: Investigation, Writing – review & editing. PG: Conceptualization, Investigation, Methodology, Project administration, Resources, Visualization, Writing – review & editing. SL: Conceptualization, Investigation, Supervision, Writing – review & editing. SS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was generously supported by the following sources: Ecofibre/Ananda Health, the Lawrence and Barbara Cohen Living Legacy Fund, the Dr. Philip Reeves Living Legacy Fund, and the Gordon Charter Foundation. Additional support was provided by the National Institute on Drug Abuse (NIDA) award K24DA050087 and the National Center for Complimentary and Integrative Health (NCCIH) award R01AT010778.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1590168/full#supplementary-material
References
1. Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. (2020) 38:3325–48. doi: 10.1200/JCO.20.01399
2. Gewandter JS, Freeman R, Kitt RA, Cavaletti G, Gauthier LR, McDermott MP, et al. Chemotherapy-induced peripheral neuropathy clinical trials: Review and recommendations. Neurology. (2017) 89:859–69. doi: 10.1212/WNL.0000000000004272
3. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. (2014) 155:2461–70. doi: 10.1016/j.pain.2014.09.020
4. D’Souza RS, Saini C, Hussain N, Javed S, Prokop L, and Her YF. Global estimates of prevalence of chronic painful neuropathy among patients with chemotherapy-induced peripheral neuropathy: systematic review and meta-analysis of data from 28 countries, 2000-24. Reg Anesth Pain Med. (2025), rapm-2024-106229. doi: 10.1136/rapm-2024-106229
5. D’Souza RS, Alvarez GAM, Dombovy-Johnson M, Eller J, and Abd-Elsayed A. Evidence-based treatment of pain in chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. (2023) 27:99–116. doi: 10.1007/s11916-023-01107-4
6. Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA. (2013) 309:1359. doi: 10.1001/jama.2013.2813
7. Ward SJ, Ramirez MD, Neelakantan H, and Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg. (2011) 113:947–50. doi: 10.1213/ANE.0b013e3182283486
8. Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, and Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. (2014) 171:636–45. doi: 10.1111/bph.12439
9. King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, et al. Single and combined effects of Δ9 -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol. (2017) 174:2832–41. doi: 10.1111/bph.13887
10. Kalvala AK, Bagde A, Arthur P, Kulkarni T, Bhattacharya S, Surapaneni S, et al. Cannabidiol-loaded extracellular vesicles from human umbilical cord mesenchymal stem cells alleviate paclitaxel-induced peripheral neuropathy. Pharmaceutics. (2023) 15:554. doi: 10.3390/pharmaceutics15020554
11. Kumar Kalvala A, Bagde A, Arthur P, Kumar Surapaneni S, Ramesh N, Nathani A, et al. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-induced neuropathic pain in rodents. Int Immunopharmacol. (2022) 107:108693. doi: 10.1016/j.intimp.2022.108693
12. Ortiz YT, Bilbrey JA, Felix JS, Kienegger EA, Mottinelli M, Mukhopadhyay S, et al. Cannabidiol and mitragynine exhibit differential interactive effects in the attenuation of paclitaxel-induced mechanical allodynia, acute antinociception, and schedule-controlled responding in mice. Pharmacol Rep PR. (2023) 75:937–50. doi: 10.1007/s43440-023-00498-w
13. Lynch ME, Cesar-Rittenberg P, and Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. (2014) 47:166–73. doi: 10.1016/j.jpainsymman.2013.02.018
14. Waissengrin B, Mirelman D, Pelles S, Bukstein F, Blumenthal DT, Wolf I, et al. Effect of cannabis on oxaliplatin-induced peripheral neuropathy among oncology patients: a retrospective analysis. Ther Adv Med Oncol. (2021) 13:1758835921990203. doi: 10.1177/1758835921990203
15. Nielsen SW, Hasselsteen SD, Dominiak HSH, Labudovic D, Reiter L, Dalton SO, et al. Oral cannabidiol for prevention of acute and transient chemotherapy-induced peripheral neuropathy. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. (2022) 30:9441–51. doi: 10.1007/s00520-022-07312-y
16. Haney M, Choo T-H, Tiersten A, Levin FR, Grasse1 A, DeSilva N, et al. Oral cannabis for taxane-induced neuropathy: A pilot randomized placebo-controlled study. Cannabis Cannabinoid Res. (2025). doi: 10.1089/can.2025.0028
17. D’Andre S, Novotny P, Walters C, Lewis-Peters S, Thomé S, Tonhagen CS, et al. Topical cannabidiol for established chemotherapy-induced neuropathy: A pilot randomized placebo-controlled trial. Cannabis Cannabinoid Res. (2024) 9(6):e1556-e1564. doi: 10.1089/can.2023.0253
18. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Bethesda, Maryland, USA: National Cancer Institute. (2017).
19. Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer Oxf Engl 1990. (2005) 41:1135–9. doi: 10.1016/j.ejca.2005.02.012
20. Wolf SL, Barton DL, Qin R, Wos EJ, Sloan JA, Liu H, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. (2012) 20:625–32. doi: 10.1007/s00520-011-1141-9
21. Bell-Krotoski JA, Fess EE, Figarola JH, and Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. J Handb Ther Off J Am Soc Handb Ther. (1995) 8:155–62. doi: 10.1016/s0894-1130(12)80314-0
22. Poquet N and Lin C. The brief pain inventory (BPI). J Physiother. (2016) 62:52. doi: 10.1016/j.jphys.2015.07.001
23. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
24. Husson O, de Rooij BH, Kieffer J, Oerlemans S, Mols F, Aaronson NK, et al. The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “Real-world”: results from the population-based PROFILES registry. Oncologist. (2020) 25:e722–32. doi: 10.1634/theoncologist.2019-0348
25. Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. (2010) 33:781–92. doi: 10.1093/sleep/33.6.781
26. Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol Off J Am Soc Clin Oncol. (2011) 29:1472–8. doi: 10.1200/JCO.2010.33.0308
27. Bonhof CS, van de Poll-Franse LV, Wasowicz DK, Beerepoot LV, Vreugdenhil G, and Mols F. The course of peripheral neuropathy and its association with health-related quality of life among colorectal cancer patients. J Cancer Surviv Res Pract. (2021) 15:190–200. doi: 10.1007/s11764-020-00923-6
28. Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. (2016) 73:860–6. doi: 10.1001/jamaneurol.2016.0383
29. Desforges AD, Hebert CM, Spence AL, Reid B, Dhaibar HA, Cruz-Topete D, et al. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. BioMed Pharmacother Biomedecine Pharmacother. (2022) 147:112671. doi: 10.1016/j.biopha.2022.112671
30. Gilman S. Joint position sense and vibration sense: anatomical organisation and assessment. J Neurol Neurosurg Psychiatry. (2002) 73:473–7. doi: 10.1136/jnnp.73.5.473
31. Monfort SM, Pan X, Loprinzi CL, Lustberg MB, and Chaudhari AMW. Impaired postural control and altered sensory organization during quiet stance following neurotoxic chemotherapy: A preliminary study. Integr Cancer Ther. (2019) 18:153473541982882. doi: 10.1177/1534735419828823
32. D’Andre S, McAllister S, Nagi J, Giridhar KV, Ruiz-Macias E, and Loprinzi C. Topical cannabinoids for treating chemotherapy-induced neuropathy: A case series. Integr Cancer Ther. (2021) 20:15347354211061739. doi: 10.1177/15347354211061739
33. Wallace MS, Marcotte TD, Umlauf A, Gouaux B, and Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain. (2015) 16:616–27. doi: 10.1016/j.jpain.2015.03.008
34. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. (2007) 68:515–21. doi: 10.1212/01.wnl.0000253187.66183.9c
35. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, and Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. (2013) 14:136–48. doi: 10.1016/j.jpain.2012.10.009
36. Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. (2012) 153:2073–82. doi: 10.1016/j.pain.2012.06.024
37. Jeddi HM, Busse JW, Sadeghirad B, Levine M, Zora1 MJ, Wang L, et al. Cannabis for medical use versus opioids for chronic non-cancer pain: a systematic review and network meta-analysis of randomised clinical trials. BMJ Open. (2024) 14:e068182. doi: 10.1136/bmjopen-2022-068182
38. Castillo-Arellano J, Canseco-Alba A, Cutler SJ, et al. The polypharmacological effects of cannabidiol. Mol Basel Switz. (2023) 28:3271. doi: 10.3390/molecules28073271
39. Luz-Veiga M, Azevedo-Silva J, and Fernandes JC. Beyond pain relief: A review on cannabidiol potential in medical therapies. Pharm Basel Switz. (2023) 16:155. doi: 10.3390/ph16020155
40. Aguiar DD, da Costa Oliveira C, Fonseca FCS, de Almeida DL, Campos Pereira WV, Guimarães FS, et al. Peripherally injected canabidiol reduces neuropathic pain in mice: Role of the 5-HT1A and TRPV1 receptors. Biochem Biophys Res Commun. (2023) 660:58–64. doi: 10.1016/j.bbrc.2023.04.022
41. Sunda F and Arowolo A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J Off Publ Fed Am Soc Exp Biol. (2020) 34:14083–92. doi: 10.1096/fj.202000975R
42. Brenneman DE, Kinney WA, McDonnell ME, Zhao P, Abood ME, and Ward SJ. Anti-inflammatory properties of KLS-13019: a novel GPR55 antagonist for dorsal root ganglion and hippocampal cultures. J Mol Neurosci MN. (2022) 72:1859–74. doi: 10.1007/s12031-022-02038-2
43. Ryan D, Drysdale AJ, Lafourcade C, et al. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci Off J Soc Neurosci. (2009) 29:2053–63. doi: 10.1523/JNEUROSCI.4212-08.2009
44. Brenneman DE, Kinney WA, and Ward SJ. Knockdown siRNA targeting the mitochondrial sodium-calcium exchanger-1 inhibits the protective effects of two cannabinoids against acute paclitaxel toxicity. J Mol Neurosci MN. (2019) 68:603–19. doi: 10.1007/s12031-019-01321-z
45. Martinez Naya N, Kelly J, Corna G, Golino M, Abbate A, and Toldo S. Molecular and cellular mechanisms of action of cannabidiol. Mol Basel Switz. (2023) 28:5980. doi: 10.3390/molecules28165980
46. Santos WBDR, Guimarães JO, Pina LTS, Serafini MR, and Guimarães AG. Antinociceptive effect of plant-based natural products in chemotherapy-induced peripheral neuropathies: A systematic review. Front Pharmacol. (2022) 13:1001276. doi: 10.3389/fphar.2022.1001276
47. Rahn EJ, Deng L, Thakur GA, Vemuri K, Zvonok AM, Lai YY, et al. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain. (2014) 10:27. doi: 10.1186/1744-8069-10-27
48. MacNair L, Kulpa J, Hill ML, Eglit GML, Mosesova I, Bonn-Miller MO, et al. Sex differences in the pharmacokinetics of cannabidiol and metabolites following oral administration of a cannabidiol-dominant cannabis oil in healthy adults. Cannabis Cannabinoid Res. (2024) 9:e1170–8. doi: 10.1089/can.2022.0345
Keywords: chemotherapy-induced peripheral neuropathy, cannabinoids, cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), sensory impairment, cancer
Citation: Weiss M, Giaddui M, Kjelstrom S, Gary J, Ward SJ, Burrell J, Diguilio K, Bidas G, Erebor E, Meske S, Saeed L, Windawi S, Aliano Ruiz K, Ghaneie A, Hibbs J, Marks J, Holtz D, Ali Z, Shevade A, Sabol J, Ciocca R, Zeger E, Gilman P, Larson S, Shimamoto S and Martinez D (2025) Combination CBD/THC in the management of chemotherapy-induced peripheral neuropathy: a randomized double blind controlled trial. Front. Oncol. 15:1590168. doi: 10.3389/fonc.2025.1590168
Received: 08 March 2025; Accepted: 29 August 2025;
Published: 23 October 2025.
Edited by:
Ryan S. D’Souza, Mayo Clinic, United StatesCopyright © 2025 Weiss, Giaddui, Kjelstrom, Gary, Ward, Burrell, Diguilio, Bidas, Erebor, Meske, Saeed, Windawi, Aliano Ruiz, Ghaneie, Hibbs, Marks, Holtz, Ali, Shevade, Sabol, Ciocca, Zeger, Gilman, Larson, Shimamoto and Martinez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marisa Weiss, bXdlaXNzQGJyZWFzdGNhbmNlci5vcmc=
 Marisa Weiss
Marisa Weiss Muath Giaddui1
Muath Giaddui1 Stephanie Kjelstrom
Stephanie Kjelstrom Sara Jane Ward
Sara Jane Ward Sam Meske
Sam Meske John Marks
John Marks Paul Gilman
Paul Gilman Diana Martinez
Diana Martinez