- Department of Gastroenterology, the First Affiliated Hospital of Medical College, Shihezi University, Shihezi, Xinjiang, China
We report a rare case of intestinal-type gastric cancer combined with submucosal ectopic gastric glands in a patient without Helicobacter pylori (HP) infection. A 55-year-old female presented for a routine health check-up. Gastroscopy revealed a lesion approximately 2.0 cm in size, classified as type O-IIa+IIc, located on the posterior wall of the upper gastric body. Endoscopic biopsy indicated high-grade intraepithelial neoplasia, which promoted endoscopic submucosal dissection (ESD). Pathological examination confirmed mucosal adenocarcinoma with submucosal ectopic gastric glands. Given the association of such lesions with gastric cancer, careful diagnosis and treatment are essential. The patient remained disease-free without recurrence during a 2-year follow-up period.
Submucosal ectopic gastric glands (SHGG) refer to the abnormal proliferation of gastric glandular tissue from the lamina propria into the submucosa. SHGG is often considered a benign condition (1), typically resulting from repeated mucosal injury. However, rare cases of malignant transformation have been reported (2–23). Here, we present a case of gastric dysplasia caused by SHGG, successfully diagnosed and treated with ESD.
Case presentation
A 55-year-old female presented for a routine health check-up. Physical examination and laboratory tests were unremarkable, with no history of HP infection. No family history of malignancies or HP infection; stable family dynamics; no history of psychiatric disorders.Gastroscopy revealed atrophic gastritis and a 2.0 cm O-IIa+IIc lesion on the posterior wall of the upper gastric body. The Paris classification ‘O-IIa+IIc’ describes a raised lesion with a central depression, a pattern often seen in early-stage gastric cancers. The lesion showed a central umbilicated depression with adherent mucus, surrounded by normal-appearing mucosa (Figure 1a). Narrow-band imaging (NBI) showed villous structures within the central depression (Figure 1b). Biopsy indicated a villous-tubular adenoma with focal high-grade intraepithelial neoplasia. The gastric mucosa showed no significant atrophy, with regular arrangement of collecting venules (RAC) observed from the gastric body to the angulus. Magnified imaging revealed villous structures around and within the central pit, with focal epithelial neoplasia-like irregular structures and disordered, dilated microvessels (Figure 1c, d). Endoscopic ultrasound (EUS) (TGF-UC180J)confirmed intact submucosal layers, and CT scans showed no distant metastasis. The patient underwent ESD for diagnostic and therapeutic purposes.
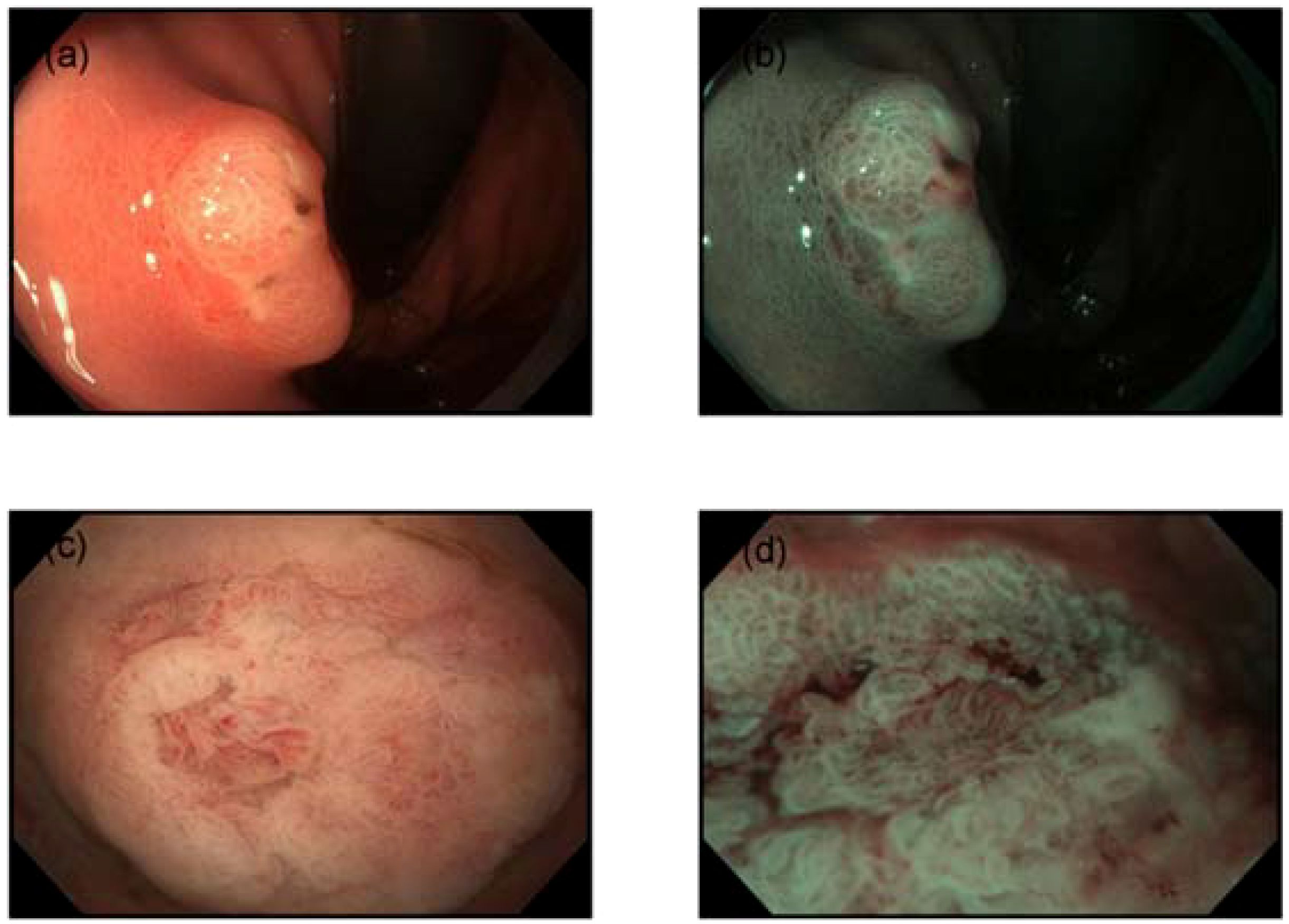
Figure 1. Endoscopic features. (a) Gastroscopy revealed a 2.0 cm subepithelial lesion on the posterior wall of the upper gastric body, with a central opening containing clear viscous fluid. (b) Narrow-band imaging (NBI) demonstrated villous structures within the central opening. (c) The mucosa surrounding and within the central pit exhibited villous structures. (d) Disordered and dilated microvessels were observed.
Pathological examination of the non-neoplastic mucosa (Figure 2a, b) and resected specimen (Figure 2c, d) revealed non-neoplastic mucosa extending into the submucosa, surrounded by the muscularis mucosae, consistent with SHGG. The boundary between cancerous and non-cancerous areas was clear. Final histopathological diagnosis confirmed well-differentiated tubular adenocarcinoma (SM1, <500 µm), with negative horizontal and vertical margins and no lymphovascular invasion. Immunohistochemistry showed MUC2+ (Figure 2e), CD10+ (Figure 2f), MUC5AC-, MUC6-, Pepsinogen-, H/K-ATPase-, CgA (focal+), P53 (wild-type), and Ki-67 (40%+). Whole-genome sequencing identified a KRAS mutation (exon 2, p.G12C) with a mutation frequency of 30.83%. Given the endoscopic and histological findings, the following diseases were considered and excluded, for instance, gastric inverted hyperplastic polyp (IHP), gastritis cystica profunda (GCP), primary submucosal adenocarcinoma and submucosal tumors, etc. The patient was advised to undergo a regulatory follow-up gastroscopy every 6 months for the first 2 years to monitor the potential recurrence. Given the KRAS mutation and background atrophic gastritis, long-term endoscopic follow-up was recommended, with further evaluation if new symptoms occurred. This study was approved by the ethics committee of The First Affiliated Hospital of Shihezi University, and written informed consent was obtained from the patient for publication and accompanying images.
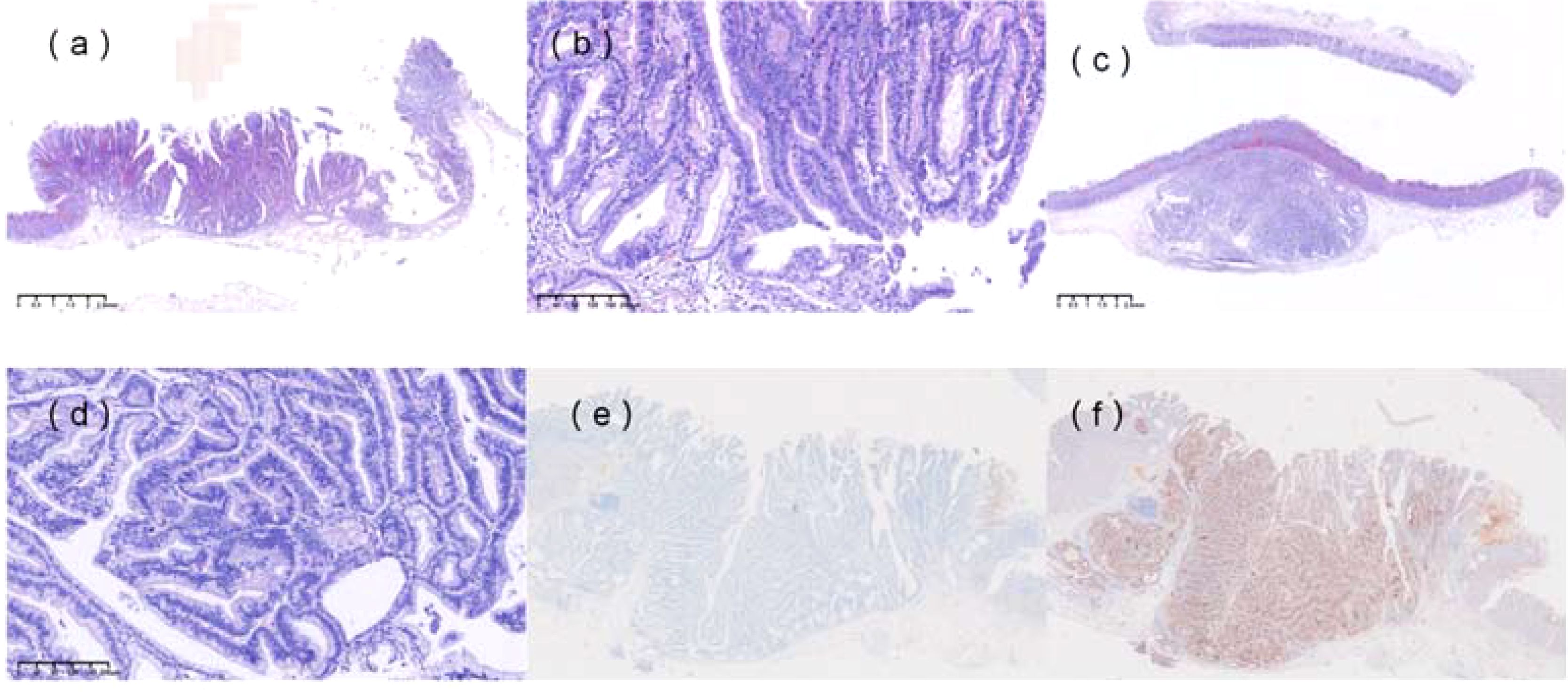
Figure 2. Histopathological features. (a) Non-neoplastic mucosa, including foveolar epithelium and pyloric glands, was observed within the submucosa, surrounded by the muscularis mucosae. (b) Hematoxylin and eosin (H&E) staining of the specimen (×100). (c) H&E staining of the resected specimen (×20) showed complete resection of the heterotopic gastric gland (HGG) component, with focal carcinomatous changes. The boundary between cancerous and non-cancerous areas was indistinct. (d) H&E staining of the specimen (×100). (e) Immunohistochemical staining revealed MUC2 positivity. (f) Immunohistochemical staining revealed CD10 positivity.
Discussion
This case represents a rare instance of intestinal-type gastric cancer in an HP-negative patient. Although HP-negative intestinal-type gastric cancers have been reported, the underlying carcinogenic mechanisms remain unclear, with potential factors including NSAID use, steroid therapy, superficial gastritis, and bile reflux. This case highlights the role of SHGG in carcinogenesis, supported by genetic analysis.
SHGG is characterized by cystic dilation of glandular structures within the submucosa, often accompanied by smooth muscle tissue continuous with the muscularis mucosae. Similar lesions include IHP and GCP, both of which have been associated with gastric cancer. A literature review identified 29 cases of SHGG combined with gastric cancer [Table 1: Basic Clinical Data of Patients (2–23)]. SHGG-related early gastric cancer predominantly occurs in middle-aged and elderly males, with 60% of cases located in the gastric body (Table 2), often infiltrating the submucosa. Immunohistochemical findings (Table 3) suggest that well-differentiated tumors are more common. These studies underlined the rarity of SHGG-associated gastric cancer, particularly in HP-negative patients. Consistently, the predominance of SM1 invasion and intestinal-type differentiation in line with our case, besides, the prevalence of KRAS mutations in SHGG lesions further supports their proliferative origin rather than inflammatory pathogenesis. There is distinct clinical and molecular heterogeneity for diagnostic and therapeutic approaches, with ESD being an effective approach for localized lesions. EUS is increasingly recognized as a valuable tool for preoperative diagnosis, with multilocular hypoechoic areas in the third layer being a characteristic feature. Diffuse SHGG with gastric cancer can complicate the assessment of tumor depth and extent. If SHGG-related cancer is confined to the mucosa or submucosa, ESD may be a viable treatment option.
The relationship between gastric cancer and SHGG may involve two pathways: (1) gastric cancer originating from SHGG and progressing into the mucosa, or (2) gastric cancer originating in the mucosa and extending into SHGG. In this case, the O-IIa+IIc lesion exhibited dilated vessels on the elevated surface, typically absent in submucosal invasive cancers but seen in carcinoids, fundic gland cancers, and mucosal cancers with submucosal invasion. NBI magnification revealed an irregular white opaque substance (WOS), characteristic of intestinal-type well-differentiated adenocarcinoma. The absence of stromal reaction or vascular invasion in the submucosal lesion, along with preserved mucosal architecture, suggests that the cancer may have originated in the mucosa and spread to SHGG. The presence of an activating KRAS mutation in SHGG supports the notion that SHGG is a proliferative lesion driven by oncogenic mutations.
KRAS encodes a small GTPase that acts as a molecular switch within the RAS/MAPK signaling pathway, thus involving cell growth, proliferation, and differentiation. KRAS is confirmed to be the most frequently mutated oncogene in various tumors, notably in colorectal cancer, pancreatic ductal adenocarcinoma, etc. Oncogenic KRAS mutations contribute to tumor progression not only by driving proliferation but also by modulating the tumor microenvironment. For instance, KRAS mutations upregulate PD-L1 expression, aiding immune escape, and activate inflammasomes like NLRP3, which further promote a pro-tumor inflammatory milieu (24). KRAS mutations often co-occur with other driver mutations such as TP53, PIK3CA, and APC, which can synergistically influence oncogenic signaling pathways and impact prognosis and therapeutic responses (25). KRAS mutations serve as key oncogenic drivers in many cancers by activating proliferative and immune-modulatory pathways, but are uncommon in gastric cancer, particularly in HP-negative cases (26). HP-negative gastric cancers follow distinct carcinogenic pathways involving genetic and epigenetic alterations such as CDH1 mutations and MSI, which contribute to their unique clinical and pathological features (27). KRAS p.G12C inhibitors, such as sotorasib and adagrasib, have demonstrated significant clinical efficacy, especially in KRAS G12C-mutant non-small cell lung cancer (28). However, monotherapy shows limited efficacy in colorectal cancer due to other resistance mechanisms (25). This suggests that combination therapies pairing KRAS G12C inhibitors with other approaches, such as chemoradiotherapy and immunotherapies, may help improve outcomes in gastric cancer, but high-quality clinical trials are further required.
The pathogenesis of SHGG is thought to involve chronic inflammation, such as erosion and regeneration. In this case, the background mucosa exhibited continuous atrophic gastritis extending to the antrum. Immunohistochemistry showed CD10 positivity, indicating intestinal differentiation. Previous reports have linked SHGG to HP-related chronic inflammation, with most cases involving well-differentiated adenocarcinoma. Chronic inflammation may lead to epigenetic abnormalities, such as DNA methylation, contributing to carcinogenesis. Gastric or duodenal reflux has also been implicated in SHGG development. However, this patient had no HP infection, and recent studies suggest that SHGG is not an inflammatory lesion but rather a proliferative disorder driven by frequent oncogenic mutations, particularly KRAS. Some other evidence suggests the possible pathogenesis in HP-negative intestinal-type gastric cancer, including genetic and epigenetic alterations, alternative environmental and host factors, epigenetic dysregulation, etc (29, 30). The absence of HP infection may delay preneoplastic lesion formation, but once genetic and epigenetic alterations accumulate can develop with aggressive features, understanding these underlying mechanisms is crucial for identifying therapeutic targets.
ESD has been successfully used for en bloc resection of SHGG-related lesions, demonstrating its feasibility as a treatment option. While SHGG may be a precursor to adenocarcinoma, the overall risk of malignant transformation is low, and resection of small SHGG lesions may not be necessary. However, larger SHGG lesions have a higher likelihood of containing dysplastic or cancerous components, warranting careful histological evaluation. Given the risk of metachronous lesions, long-term follow-up is recommended for patients with SHGG-related gastric cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Shihezi University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SP: Data curation, Methodology, Writing – original draft, Writing – review & editing. ST: Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by the National Natural Science Foundation of China (No. 82460539), Bintuan Science and Technology Program (Nos. 2023AB056 and 2022ZD023, 2022ZD038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kosugi S, Kanda T, and Hatakeyama K. Adenocarcinoma arising from heterotopic gastric mucosa in the stomach. J Gastroenterol Hepatol. (2006) 21:483–4. doi: 10.1111/j.1440-1746.2006.04108.x
2. Kamata Y, Kurotaki H, Onodera T, and Nishida N. An unusual heterotopia of pyloric glands of the stomach with inverted downgrowth. Acta Pathol Jpn. (1993) 43:192–7. doi: 10.1111/j.1440-1827.1993.tb01131.x
3. Yamashita M, Hirokawa M, Nakasono M, Kiyoku H, Sano N, Fujii M, et al. Gastric inverted hyperplastic polyp. Report of four cases and relation to gastritis cystica profunda. APMIS. (2002) 110:717–23. doi: 10.1034/j.1600-0463.2002.1101005.x
4. Kono T, Imai Y, Ichihara T, Miyagawa K, Kanemitsu K, Ajiki T, et al. Adenocarcinoma arising in gastric inverted hyperplastic polyp: a case report and review of the literature. Pathol Res Pract. (2007) 203:53–6. doi: 10.1016/j.prp.2006.08.010
5. Kim DH, Kim KM, Oh SJ, Oh JA, Choi MG, Noh JH, et al. Early gastric cancer arising from heterotopic gastric mucosa in the gastric submucosa. J Korean Surg Soc. (2011) 80 Suppl 1:S6–S11. doi: 10.4174/jkss.2011.80.Suppl1.S6
6. Kim HS, Hwang EJ, Jang JY, Lee J, and Kim YW. Multifocal adenocarcinomas arising within a gastric inverted hyperplastic polyp. Korean J Pathol. (2012) 46:387–91. doi: 10.4132/KoreanJPathol.2012.46.4.387
7. Nakamatsu D, Nishida T, Inoue T, Shigekawa M, Shinzaki S, Yamada T, et al. Advanced gastric cancer deriving from submucosal heterotopic gastric glands based on pathological diagnosis. Nihon Shokakibyo Gakkai Zasshi. (2013) 110:290–3.
8. Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, et al. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. (2013) 46:568–71. doi: 10.5946/ce.2013.46.5.568
9. Imamura T, Komatsu S, Ichikawa D, Kobayashi H, Miyamae M, Hirajima S, et al. Gastric carcinoma originating from the heterotopic submucosal gastric gland treated by laparoscopy and endoscopy cooperative surgery. World J Gastrointest Oncol. (2015) 7:118–22. doi: 10.4251/wjgo.v7.i8.118
10. Tanaka H, Baba Y, Isono Y, Matsusaki S, Sase T, Okano H, et al. Gastric intramucosal cancer spreading to submucosal heterotopic gastric glands. Nihon Shokakibyo Gakkai Zasshi. (2015) 112:1657–63. doi: 10.11405/nisshoshi.112.1657
11. Watanabe N, Mitsuda M, Yamashita E, Ito H, Toma A, Nakamura K, et al. A case report of early gastric cancer with submucosal heterotopic gastric glands. Gan To Kagaku Ryoho. (2016) 43:1881–3.
12. Manabe S, Mukaisho KI, Yasuoka T, Usui F, Matsuyama T, Hirata I, et al. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol. (2017) 23:7047–53. doi: 10.3748/wjg.v23.i38.7047
13. Takahashi K, Fujiya M, Ichihara S, Moriichi K, and Okumura T. Inverted gastric adenocarcinoma of fundic gland mucosa type colliding with well differentiated adenocarcinoma: A case report. Med (Baltimore). (2017) 96:e7080. doi: 10.1097/MD.0000000000007080
14. Watanabe Y, Watanabe M, Suehara N, Ishikawa N, Shinkawa T, Hosokawa T, et al. Early gastric cancer with diffuse heterotopic gastric glands and granular cell tumors mimicking advanced gastric cancer. Int J Surg Case Rep. (2018) 46:41–6. doi: 10.1016/j.ijscr.2018.04.009
15. Uchida A, Ozawa M, Ueda Y, Murai Y, Nishimura Y, Ishimatsu H, et al. Gastric adenocarcinoma of fundic gland mucosa type localized in the submucosa: A case report. Med (Baltimore). (2018) 97:e12341. doi: 10.1097/MD.0000000000012341
16. Uozumi T, Seki H, Matsuzono E, Sogabe S, Sugai N, Fujita J, et al. Gastric adenocarcinoma of fundic gland type arising from heterotopic gastric glands during a 19-year follow-up period. Clin J Gastroenterol. (2019) 12:556–61. doi: 10.1007/s12328-019-00989-5
17. Namikawa T, Ishida N, Yokota K, Munekage E, Iwabu J, Munekage M, et al. Early gastric cancer with multiple submucosal heterotopic gastric gland: A case report. Mol Clin Oncol. (2019) 10:583–6. doi: 10.3892/mco.2019.1846
18. Wada K, Tazawa H, Komo T, Hadano N, Onoe T, Sudo T, et al. Gastric adenocarcinoma appearance in leiomyoma: A case report. Int J Surg Case Rep. (2020) 71:327–30. doi: 10.1016/j.ijscr.2020.05.050
19. Tanaka Y, Tokubayashi Y, Fujii S, Kusaka T, Shibuya S, and Kokuryu H. Gastric miNEN arising from the heterotopic gastric glands. Intern Med. (2020) 59:3165–9. doi: 10.2169/internalmedicine.5333-20
20. Kim JY, Ahn S, Kim KM, Chang SH, Kim HS, Lee JH, et al. Gastric inverted polyps-distinctive subepithelial lesions of the stomach: clinicopathologic analysis of 12 cases with an emphasis on neoplastic potential. Am J Surg Pathol. (2021) 45:680–9. doi: 10.1097/PAS.0000000000001651
21. Inokuchi Y, Washimi K, Watanabe M, Hayashi K, Kaneta Y, Furuta M, et al. Successful resection of gastric cancer arising from a heterotopic gastric gland in the submucosa by endoscopic submucosal dissection. Clin Case Rep. (2022) 10:e5981. doi: 10.1002/ccr3.5981
22. Kobayashi M, Hamada Y, Usui M, and Nakagawa H. Gastric dysplasia arising from a submucosal heterotopic gastric gland. JGH Open. (2024) 8:e70047. doi: 10.1002/jgh3.70047
23. Cho H, Hashimoto T, Naka T, Yatabe Y, Oda I, Saito Y, et al. Activating KRAS and GNAS mutations in heterotopic submucosal glands of the stomach. J Gastroenterol. (2022) 57:333–43. doi: 10.1007/s00535-022-01863-x
24. Hamarsheh S, Groß O, Brummer T, and Zeiser R. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun. (2020) 11:5439. doi: 10.1038/s41467-020-19288-6
25. Huang L, Guo Z, Wang F, and Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. (2021) 6:386. doi: 10.1038/s41392-021-00780-4
26. Mondal K, Posa MK, Shenoy RP, and Roychoudhury S. KRAS mutation subtypes and their association with other driver mutations in oncogenic pathways. Cells. (2024) 13:1221. doi: 10.3390/cells13141221
27. Tsai KF, Liou JM, Chen MJ, Chen CC, Kuo SH, Lai IR, et al. Distinct clinicopathological features and prognosis of helicobacter pylori negative gastric cancer. PloS One. (2017) 12:e0170942. doi: 10.1371/journal.pone.0170942
28. Qunaj L, May MS, Neugut AI, and Herzberg BO. Prognostic and therapeutic impact of the KRAS G12C mutation in colorectal cancer. Front Oncol. (2023) :1252516. doi: 10.3389/fonc.2023.1252516
29. Shi J, Qu YP, and Hou P. Pathogenetic mechanisms in gastric cancer. World J Gastroenterol. (2014) 20:13804–19. doi: 10.3748/wjg.v20.i38.13804
Keywords: submucosal ectopic gastric glands, early gastric cancer, case report, a 55-year-old female, pathological examination
Citation: Peng S and Tian S (2025) Case report and literature analysis of ectopic gastric glands combined with intestinal-type gastric cancer in an HP-negative background. Front. Oncol. 15:1590544. doi: 10.3389/fonc.2025.1590544
Received: 09 March 2025; Accepted: 19 May 2025;
Published: 05 June 2025.
Edited by:
Babak Pakbin, Texas A and M University, United StatesReviewed by:
Malihe Rezaee, Shahid Beheshti University of Medical Sciences, IranRadman Mazloomnejad, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2025 Peng and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuxin Tian, ZW5kbzA3MjZAc2h6dS5lZHUuY24=
 Shiyu Peng
Shiyu Peng Shuxin Tian*
Shuxin Tian*