- 1Department of Pulmonary and Critical Care Medicine State Key Laboratory of Respiratory Health and Multimorbidity West China Hospital of Sichuan University, Precision Medicine Key Laboratory of Sichuan Province, Chengdu, Sichuan, China
- 2Institute of Respiratory Health, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Lung Cancer Center/Lung Cancer Institute, West China Hospital, Sichuan University, Chengdu, China
Background: The association between smoking status, cumulative smoking dose, and immunotherapy efficacy in non-small cell lung cancer (NSCLC) remains controversial. We sought to integrate the lifetime pack-years with smoking cessation status to identify optimal immunotherapy beneficiaries.
Methods: A total of 1,192 immunotherapy-treated NSCLC patients treated between November 2015 and April 2024 were enrolled. Data on demographics, clinical characteristics, pathologic characteristics, treatments, and clinical outcomes were collected. The objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS) were compared across different smoking statuses (never, current, and former smokers) and cumulative smoking doses (never smokers, non-heavy smokers: <20 pack-years, and heavy smokers: ≥20 pack-years). Multivariate logistic regression and Cox proportional hazards models were used to analyze ORR and PFS, respectively.
Results: Among the 1,192 patients, 377 were never smokers, 499 were current smokers, and 316 were former smokers. In terms of smoking status, former smokers exhibited the longest median PFS (17.0 months, P < 0.001), with the highest ORR (46.8%, P < 0.001) and DCR (86.7%, P = 0.008). Regarding cumulative smoking dose, the heavy smoker group demonstrated the longest median PFS (15.9 months, P = 0.001), with the highest ORR (46.6%, P < 0.001) and DCR (85.2%, P = 0.012). Notably, further multivariate analysis identified former heavy smokers as independent favorable predictors of ORR (OR = 1.93, 95% CI = 1.25–2.99, P = 0.003) and PFS (HR = 0.75, 95% CI = 0.57–0.99, P = 0.04) in advanced NSCLC patients receiving immunotherapy.
Conclusions: This real-world cohort analysis establishes a clinical stratification combining smoking cessation status with cumulative smoking dose, identifying former heavy smokers as optimal immunotherapy beneficiaries. These findings advocate integrated smoking history documentation and emphasize clinical prioritization of cessation interventions to enhance treatment efficacy in NSCLC.
1 Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1), with non-small cell lung cancer (NSCLC) representing approximately 85% of lung cancer cases (2). Due to the lack of prominent symptoms in early-stage NSCLC, most patients are diagnosed at advanced stages when symptoms become apparent, with a 5-year survival rate of only 27% (1). In recent years, the introduction of immune checkpoint inhibitors (ICIs), particularly targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death-ligand 1 (PD-L1), has led to significant breakthroughs in the treatment of advanced NSCLC (3). The immunotherapy has notably improved the prognosis of NSCLC patients with advanced disease and has become one of the standard treatment strategies in international lung cancer guidelines.
Despite these advancements, the clinical response to immunotherapy varies among individuals, with fewer than 20% of unselected NSCLC patients benefiting from anti-PD-1/PD-L1 therapies (4, 5). Therefore, identifying biomarkers that can assist in selecting patients who would benefit the most from immunotherapy is of critical importance. The PD-L1 expression level is one of the earliest indicators used to predict the effect of immunotherapy and select the target population. Compared to those with low or negative PD-L1 expression, patients with high PD-L1 expression in advanced NSCLC are more likely to benefit from immunotherapy, exhibiting higher objective response rates (ORRs) (6–8). However, its clinical utility remains limited, and not all patients with a high tumor proportion score (TPS) for PD-L1 expression experience clinical benefit (9). Other biomarkers related to the prediction of immunotherapy efficacy include tumor mutation burden (TMB), tumor-infiltrating lymphocytes (TILs), microsatellite instability (MSI), tumor genomic characteristics, and so on (9–11).
Smoking is a well-established risk factor for the development of NSCLC and is also thought to increase the overall risk of patients dying from cancer (12). With the development of tumor immunotherapy, several studies have suggested that current and former smokers may experience improved prognosis with immunotherapy (13), while a recent study argues that never smokers may exhibit increased progression-free survival (PFS) and overall survival (OS) (14). Nevertheless, the meta-analysis involving 17 phase III trials with 10,283 patients suggested that smoking status does not significantly affect the efficacy of immunotherapy (15).
In this study, we aimed to investigate the effect of smoking status and cumulative smoking dose on clinical outcomes in patients with advanced NSCLC undergoing immunotherapy.
2 Materials and methods
2.1 Study design and participants
This retrospective study included adult patients (≥18 years) who were histologically diagnosed with primary advanced NSCLC, including pathological stages III and IV, at West China Hospital of Sichuan University between November 2015 and April 2024. All included patients were confirmed via pathological diagnosis and received either ICI monotherapy or a combination of ICI and chemotherapy. Patients were excluded if they had 1) a history of other malignancies or 2) incomplete data on smoking history. The study was approved by the Medical Ethics Committee of West China Hospital of Sichuan University (approval number: 2024-1410), and the requirement for informed consent was waived because the data were deidentified.
2.2 Data collection
We collected the following data from the patients: gender, age at diagnosis, smoking history, body mass index (BMI), Eastern Cooperative Oncology Group Performance Status (ECOG PS), histological type of the tumor, the tumor–node–metastasis (TNM) stage, programmed cell death ligand 1 (PD-L1) expression levels, the combination therapy (including chemotherapy and radiotherapy), and lines of treatment. The categories of BMI were defined as follows: underweight, BMI less than 18.5; normal, BMI 18.5 to less than 24.9; and overweight or obese, BMI 25 or higher (16). The TNM stage was classified according to the 8th edition International Association for the Study of Lung Cancer (IASLC) staging system for lung cancer (17). PD-L1 expression was assessed using 22C3 pharmDx immunohistochemistry antibody and classified into three categories: tumor proportion score (TPS) <1% (negative), TPS 1%–49% (low expression), and TPS ≥50% (high expression) (18).
Never smokers were defined as individuals who had smoked fewer than 100 cigarettes in their lifetime (19). Current smokers were individuals who smoked regularly or occasionally, and former smokers were those who had quit smoking. Current and former smokers were categorized as ever smokers. The number of pack-years of cigarette smoking was calculated by multiplying the average number of cigarettes smoked per day by the total number of years the individual has smoked and then dividing this product by 20 (average number of cigarettes in a pack). Individuals were classified as non-heavy smokers if they had accumulated fewer than 20 pack-years of smoking, while those with 20 or more pack-years were categorized as heavy smokers (20).
2.3 Outcomes
The primary endpoint was PFS, defined as the time from the initiation of immunotherapy to disease progression or death from any cause (21). Secondary endpoints included ORR, defined as the proportion of patients achieving a complete response (CR) or partial response (PR) to treatment, and disease control rate (DCR), defined as the proportion of patients with CR, PR, or stable disease (SD). The efficacy of immunotherapy was evaluated by experienced clinicians according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (22). The follow-up deadline was on 30 December 2024.
2.4 Statistical analysis
Categorical variables were summarized as frequencies and percentages, with group differences assessed using chi-square tests. The Fisher’s exact test was used when expected frequencies were low. PFS was estimated using the Kaplan–Meier survival analysis, with the subgroup differences compared using log-rank tests. Logistic regression and Cox proportional hazards regression were employed to identify potential factors influencing ORR and PFS in advanced NSCLC, respectively. Variables with a P-value <0.05 in the univariate analysis were included in the multivariate model. All statistical analyses were performed using R version 4.3.3 (R Foundation, Vienna, Austria), with statistical significance defined as a two-tailed P-value <0.05.
3 Results
3.1 Baseline characteristics
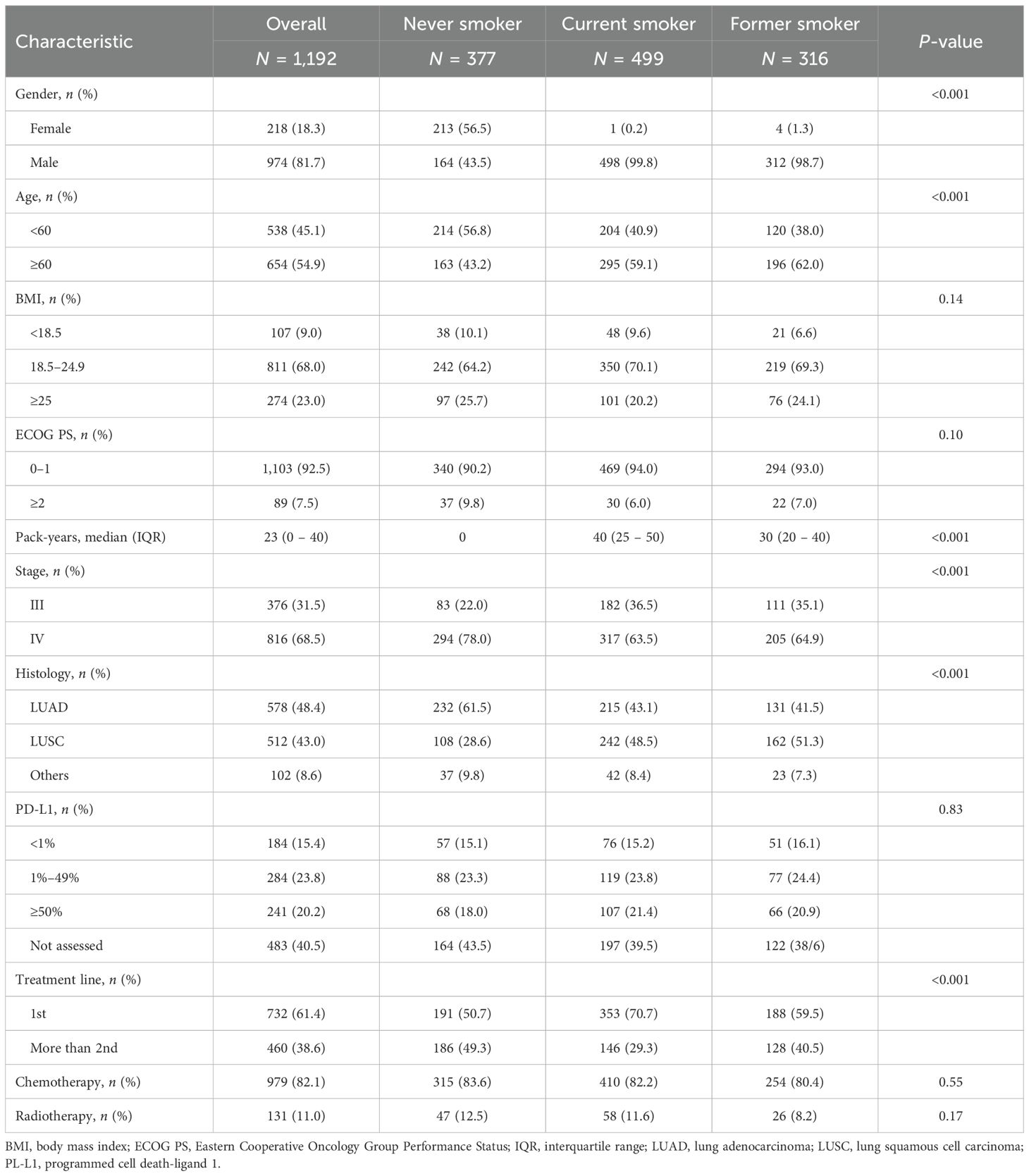
A total of 1,192 patients with advanced NSCLC who received immunotherapy between November 2015 and April 2024 were included in this study. Of these, 377 were never smokers, 499 were current smokers (median pack-years, 40), and 316 were former smokers (median pack-years, 30). The baseline characteristics of the patients are summarized in Table 1. The vast majority of the patients were men (974, 81.7%), and approximately 54.9% were aged 60 years or older. Of the total number of patients, 9.0% had a BMI less than 18.5 kg/m2, 23.0% had a BMI greater than 25 kg/m2, and 92.5% had an ECOG PS of 0 or 1. Stage IV disease was diagnosed in 68.5% of patients. In terms of histological subtypes, 48.4% had adenocarcinoma, 43.0% had squamous cell carcinoma (SCC), and 8.6% had other subtypes. Regarding PD-L1 expression levels, 15.4% were negative, 23.8% had low expression, and 20.2% had high expression. Of the patients, 61.4% received first-line immunotherapy, 82.1% received chemotherapy, and 11.0% received radiotherapy. Significant differences in age, sex, TNM stage, histological subtypes, and treatment line were observed among the three groups.
3.2 Immunotherapy efficacy stratified by smoking status
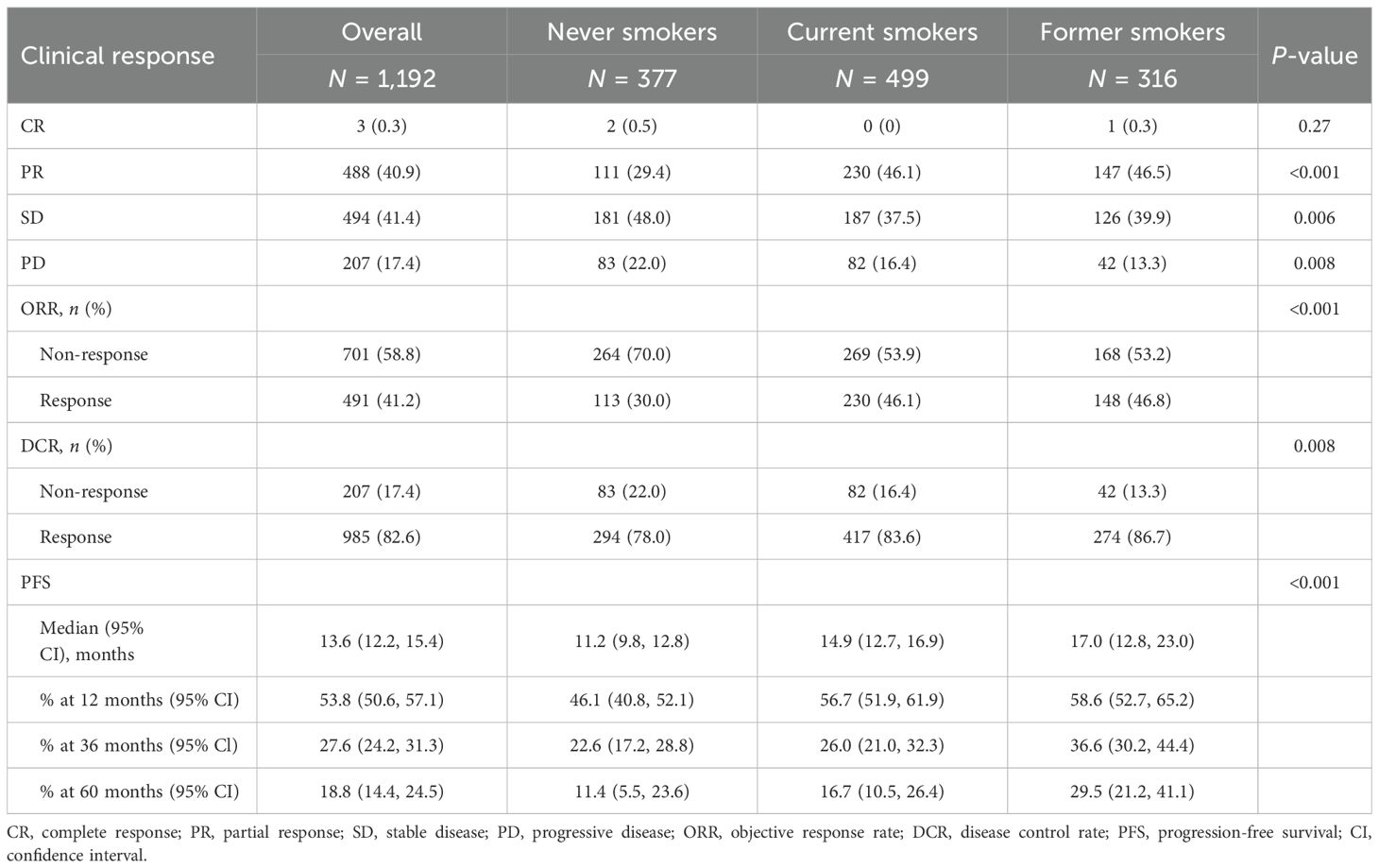
We first examined the relationship between ORR and smoking status among patients. The overall ORRs were 30.0% for never smokers, 46.1% for current smokers, and 46.8% for former smokers, with a statistically significant difference (P < 0.001). DCR was also the highest in former smokers at 86.7%, compared to 78.0% in never smokers and 83.6% in current smokers (P = 0.008) (Table 2).
In never smokers, the median PFS was 11.2 months (95% CI: 9.8–12.8), with 1-, 3-, and 5-year PFS rates of 46.1% (95% CI: 40.8–52.1), 22.6% (95% CI: 17.2–28.8), and 11.4% (95% CI: 5.5–23.6), respectively. Compared with never smokers, ever smokers (defined as individuals who currently or formerly smoked) exhibited significantly longer PFS (P < 0.001, Figure 1A). Further stratifying the ever-smoker group, current smokers had a median PFS of 14.9 months (95% CI: 12.7–16.9), with 1-, 3-, and 5-year PFS rates of 56.7% (95% CI: 51.9–61.9), 26.0% (95% CI: 21.0–32.3), and 16.7% (95% CI: 10.5–26.4), respectively. Former smokers had the longest median PFS at 17.0 months (95% CI: 12.8–23.0, P < 0.001), with 1-, 3-, and 5-year PFS rates of 58.6% (95% CI: 52.7–65.2), 36.6% (95% CI: 30.2–44.4), and 29.5% (95% CI: 21.2–41.1), respectively (Table 2, Figure 1B).
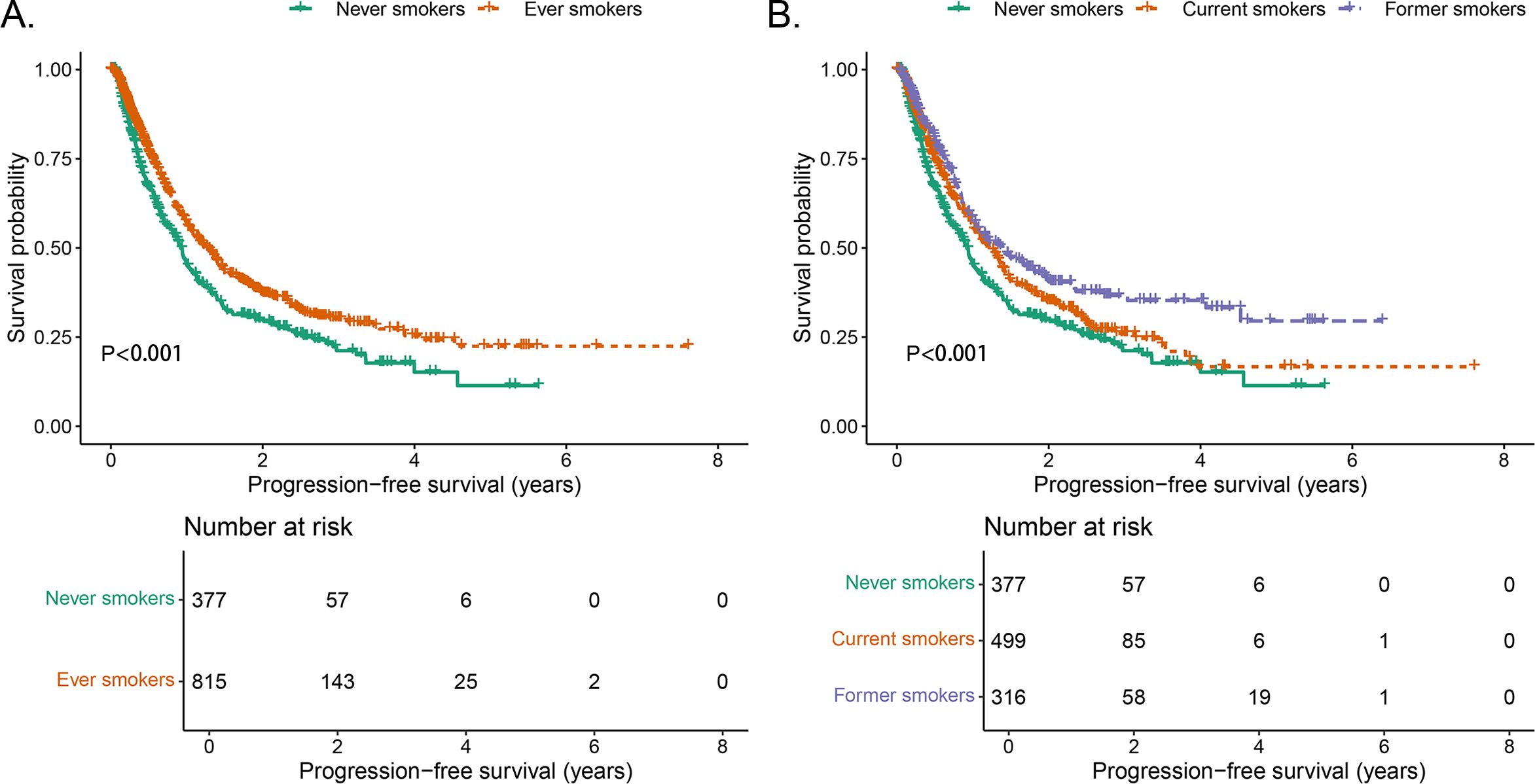
Figure 1. Comparison of PFS stratified by smoking status. (A) Comparison of PFS between never smokers and ever smokers. (B) Comparison of PFS between never smokers, current smokers, and former smokers.
3.3 Immunotherapy efficacy stratified by both smoking status and cumulative smoking dose
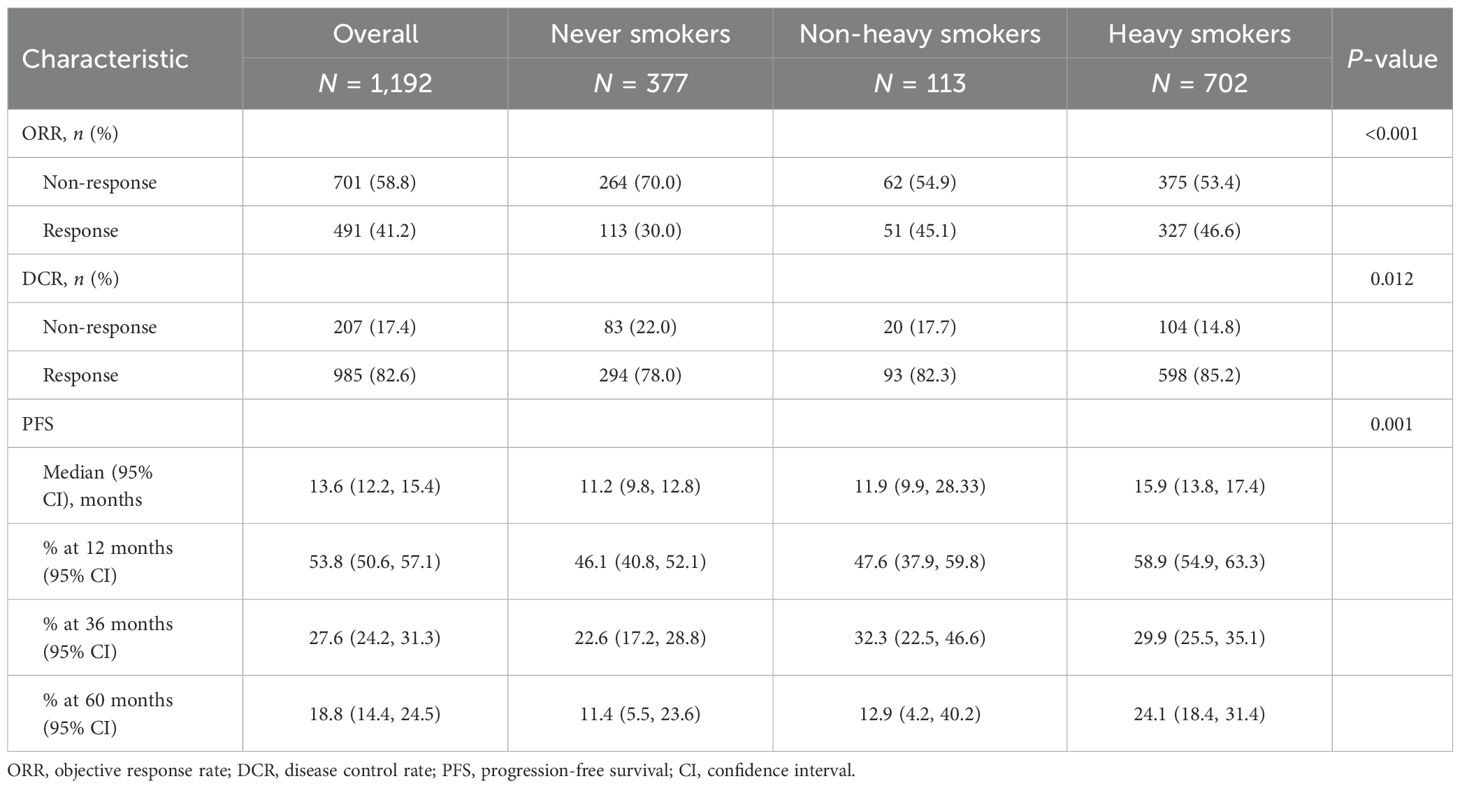
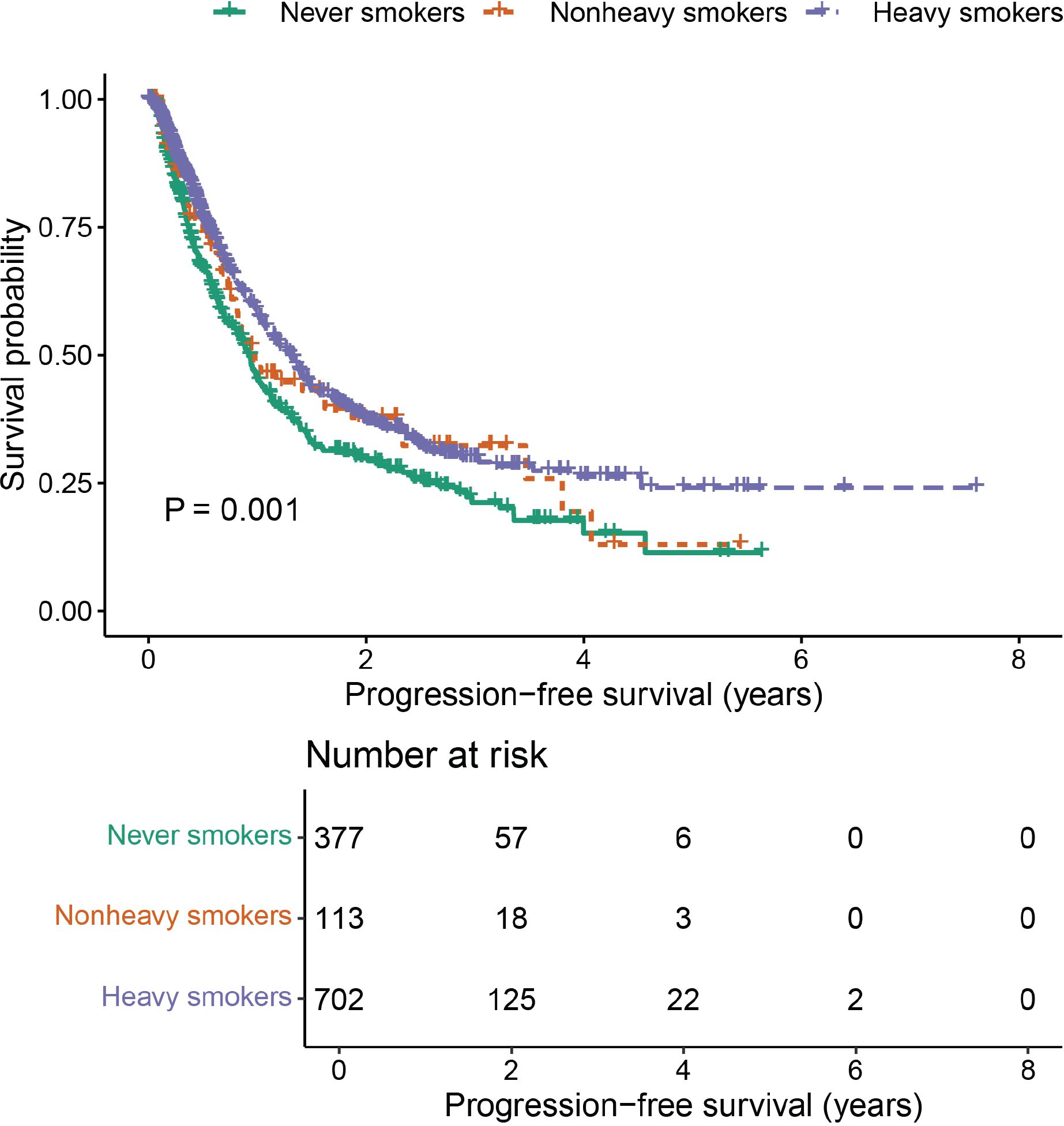
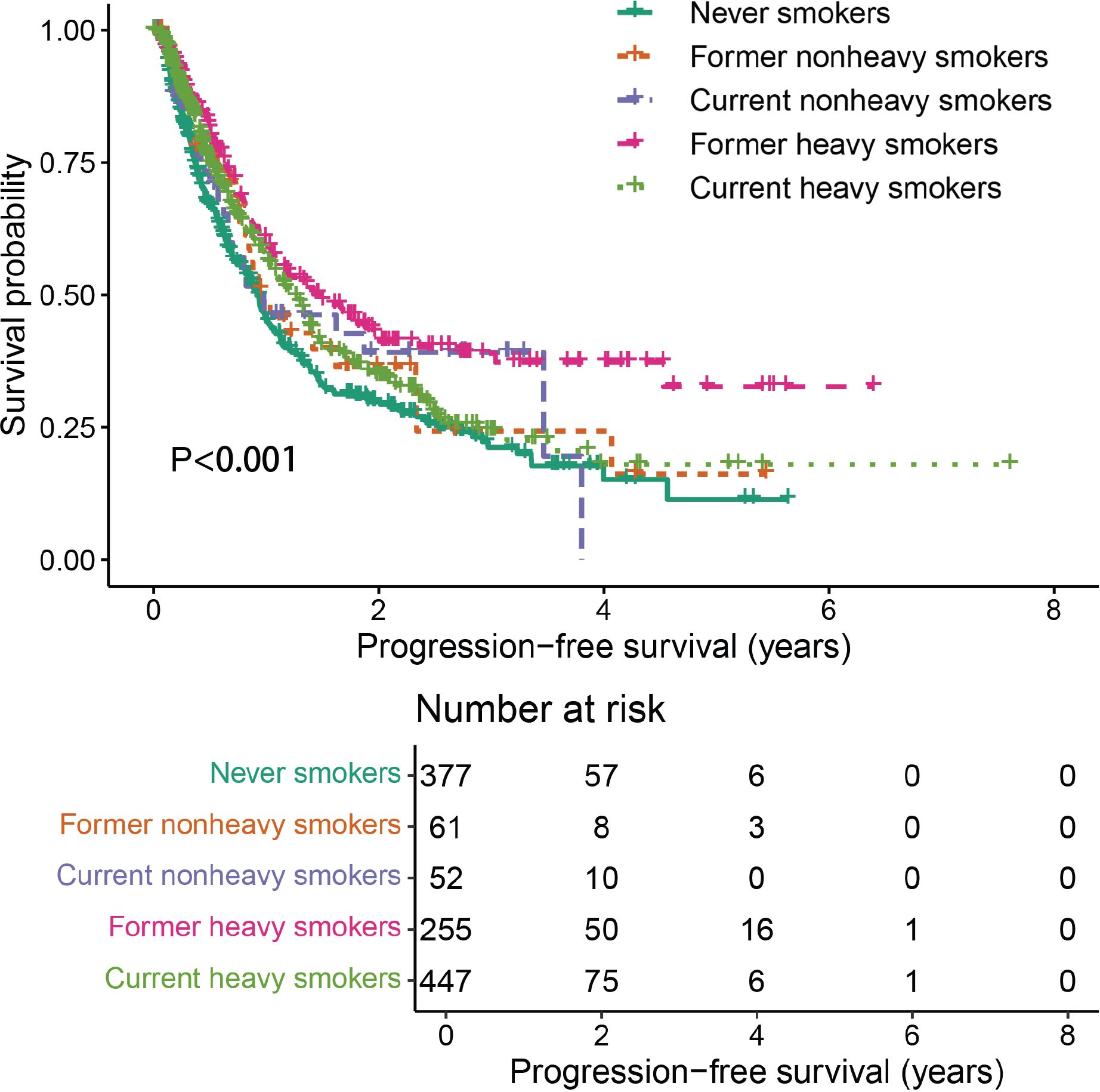
A positive correlation was observed between efficacy outcomes and cumulative smoking dose. The ORR in never smokers was 30.0%. As the cumulative smoking dose increased, ORR showed a significant improvement (P < 0.001, Table 2), rising to 45.1% in non-heavy smokers and 46.6% in heavy smokers. A similar trend was seen in DCR, with values of 78.0%, 82.3%, and 85.2% in never smokers, non-heavy smokers, and heavy smokers, respectively (P = 0.012, Table 2). Additionally, cumulative smoking dose exhibited a dose-dependent positive correlation with median PFS, which was statistically significant (P = 0.001, Table 3, Figure 2).
Further analysis was performed to evaluate the combined effects of smoking status and cumulative smoking dose. Compared with never smokers, former heavy smokers demonstrated significantly enhanced PFS (P < 0.001, Figure 3).
3.4 Analysis of prognostic factors for ORR and PFS
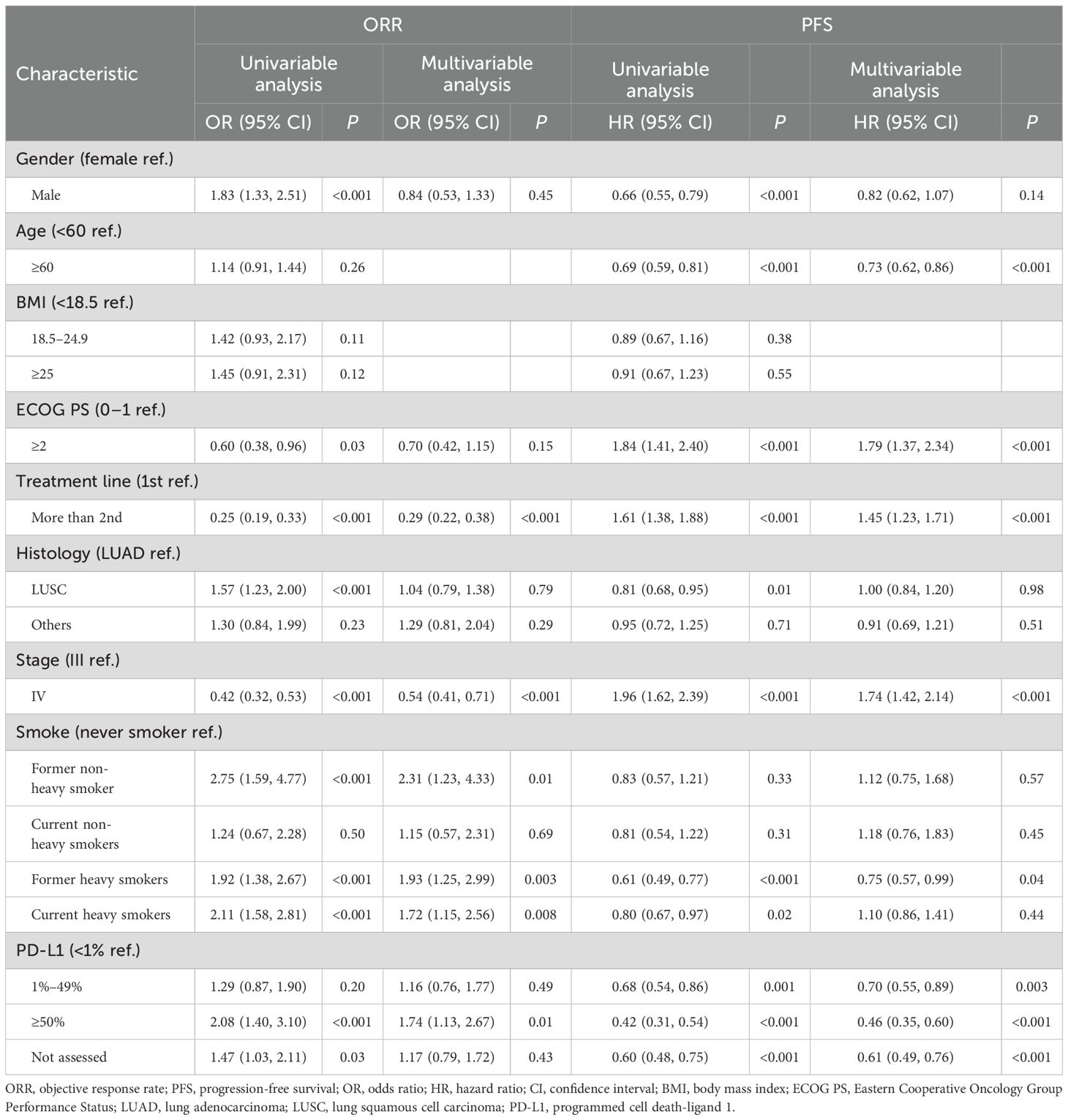
For smoking status, multivariate analysis after adjusting for baseline covariates revealed that it was significantly associated with increased ORR. Current smokers exhibited an OR of 1.65 (95% CI: 1.12–2.45, P = 0.01), while former smokers had an OR of 2.01 (95% CI: 1.32–3.05, P = 0.001) (Supplementary Table 1). However, compared with never smokers, no significant differences in PFS were observed in current smokers (HR = 1.11, 95% CI: 0.87–1.42, P = 0.38) and former smokers (HR = 0.82, 95% CI: 0.63–1.06, P = 0.13) (Supplementary Table 1).
With respect to cumulative smoking dose, both non-heavy smokers (OR = 1.70, 95% CI: 1.01–2.85, P = 0.04) and heavy smokers (OR = 1.80, 95% CI: 1.23–2.64, P = 0.002) demonstrated significantly improved ORR compared with never smokers. However, cumulative smoking dose was not independently associated with PFS in patients with advanced NSCLC receiving immunotherapy (non-heavy smokers: HR = 1.14, 95% CI: 0.82–1.58, P = 0.44; heavy smokers: HR = 0.95, 95% CI: 0.75–1.20, P = 0.67) (Supplementary Table 2).
Further analysis, incorporating both smoking status and cumulative smoking dose, revealed that former heavy smokers were significantly associated with improved ORR (OR = 1.93, 95% CI: 1.25–2.99, P = 0.003) and prolonged PFS (HR = 0.75, 95% CI: 0.57–0.99, P = 0.04). These findings suggest that former heavy smoking is an independent protective factor for better ORR and PFS in patients receiving immunotherapy (Table 4).
4 Discussion
This investigation represents the first real-world analysis to incorporate both smoking status and cumulative smoking dose in the evaluation of immunotherapy efficacy in patients with advanced NSCLC. Our findings suggest that immunotherapy efficacy is significantly greater in ever smokers compared to never smokers, with former heavy smokers emerging as an independent favorable predictor for both improved ORR and PFS in advanced NSCLC patients receiving immunotherapy.
Our study aligns with existing literature that underscores the relevance of smoking status in influencing immunotherapy outcomes (23, 24). This could be explained by the following reasons. Firstly, a significant dose–response relationship between smoking history and TMB has been established in advanced lung adenocarcinoma (25). Increased TMB correlates with enhanced CD8-positive T-cell infiltration, higher neoantigen load, and distinct immune response signatures, all of which contribute to improved outcomes with immunotherapy (26). Secondly, tobacco smoke contains a range of carcinogens, such as polycyclic aromatic hydrocarbons (PAHs) like benzo[a]pyrene (BaP) and aromatic amines, including 4-aminobiphenyl. Exposure to BaP has been shown to upregulate the mRNA and surface expression of PD-L1, which is mediated by aryl hydrocarbon receptor (AhR) (27, 28). A recent pooled analysis of 11 clinical trials has indicated that PD-L1 expression has an appreciable impact on clinical outcomes for patients with NSCLC treated with immunotherapy in the first- or second-line treatment setting (29). Furthermore, compared with smoking-related lung cancers, lung cancer in individuals who have never smoked (LCINS) presents a distinct genomic landscape (19, 30). In the Asian NSCLC population, EGFR mutations occur in 40%–60% of cases, especially among never smokers (31). However, EGFR-mutant tumors are often represented as exhibiting an immune-cold phenotype, characterized by an immunosuppressive microenvironment and reduced immunogenicity, leading to poor response to PD-1/PD-L1 monotherapy (32, 33).
Notably, our data demonstrated that former heavy smokers represent a distinct subgroup with particularly favorable outcomes, highlighting the importance of smoking cessation, even following a history of heavy smoking. Our findings complement the existing literature by adding to the understanding of the impact of smoking cessation on improving the efficacy of immunotherapy. These findings are similar to those of the KEYNOTE-024 trial (6, 34), which demonstrated that pembrolizumab significantly prolonged OS compared to chemotherapy in former smokers (HR = 0.59, 95% CI: 0.41–0.85). In contrast, the efficacy of immunotherapy was superior but insignificant in current smokers (HR = 0.81, 95% CI: 0.41–1.60) and never smokers (HR = 0.90, 95% CI: 0.11–7.59) (34). A possible explanation for the observed differences may involve multiple mechanisms. First, smoking-associated oxidative stress activates the inflammatory response pathway, leading to further generation of reactive oxygen species (ROS) (35). Oxidative metabolism and hypoxia influence the immune TME through multiple mechanisms, thereby weakening the beneficial effects of immune checkpoint blockade therapy (36). Second, nicotine in current smokers may enhance tumor proliferation and angiogenesis and compromise the therapeutic effects of treatment via the nicotinic acetylcholine receptor (nAChR) signaling pathway (37). Additionally, exposure to tobacco smoke constituents may upregulate the expression of immunosuppressive proteins, such as YAP1 (38, 39). These findings may indicate the importance of early smoking cessation to optimize immunotherapy strategies.
Nevertheless, our study has several inherent limitations. First, as a retrospective analysis, it is susceptible to selection bias. Additionally, because it depends on the accuracy and completeness of existing data, there may be issues with missing or inaccurate information. Second, OS data are not yet available due to the relatively early stage of follow-up at the time of analysis. We plan to continue monitoring OS in subsequent follow-up assessments, with results to be reported once the data reach sufficient maturity. Third, given that the data collection period spanned the COVID-19 pandemic, the lack of systematic recording of patients’ COVID-19 infection status limited our ability to evaluate its potential impact on treatment efficacy. Finally, while the strengths of the current study include a large sample size with high follow-up rates, further larger-scale, multicenter prospective studies are needed to validate our findings and explore the underlying molecular mechanisms, including the impact of different genetically driven mutations.
5 Conclusions
Our study demonstrates former heavy smokers (≥20 pack-years) as an independent favorable prognostic factor for advanced NSCLC patients receiving immunotherapy. These findings underscore the importance of early cessation in clinical settings. In addition, clinical assessments should prioritize the standardized documentation of detailed smoking history, including smoking status and cumulative smoking dose. Further prospective studies are needed to validate its clinical utility and explore the underlying biological mechanisms, with the aim of optimizing immunotherapy outcomes in patients with advanced NSCLC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin.
Author contributions
LY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Visualization. HW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Investigation, Resources, Writing – original draft. PT: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing. WL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0506105/2023ZD0506100 to Weimin Li), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0522806/2024ZD0522800 to Panwen Tian), Sichuan Science and Technology Program (2024NSFSC1528 to Lan Yang), and National Natural Science Foundation of China (No. 92159302 to Weimin Li).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1590825/full#supplementary-material
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA: Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Duma N, Santana-Davila R, and Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proc. (2019) 94:1623–40. doi: 10.1016/j.mayocp.2019.01.013
3. Li MSC, Chan ALS, Mok KKS, Chan LL, and Mok TSK. Next-generation immunotherapy: igniting new hope for lung cancer. Ther Adv Med Oncol. (2024) 16:17588359241302021. doi: 10.1177/17588359241302021
4. Huang Q, Zhang H, Hai J, Socinski MA, Lim E, Chen HQ, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology. (2018) 7:e1396403. doi: 10.1080/2162402X.2017.1396403
5. Thummalapalli R, Ricciuti B, Bandlamudi C, Muldoon D, Rizvi H, Elkrief A, et al. Clinical and molecular features of long-term response to immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Clin Cancer research: an Off J Am Assoc Cancer Res. (2023) 29:4408–18. doi: 10.1158/1078-0432.CCR-23-1207
6. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
7. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. New Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
8. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. New Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
9. Holder AM, Dedeilia A, Sierra-Davidson K, Cohen S, Liu D, Parikh A, et al. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat Rev Cancer. (2024) 24:498–512. doi: 10.1038/s41568-024-00705-7
10. Memmott RM, Wolfe AR, Carbone DP, and Williams TM. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. J Thorac Oncol. (2021) 16:1086–98. doi: 10.1016/j.jtho.2021.03.017
11. Wilbur HC, Le DT, and Agarwal P. Immunotherapy of MSI cancer: facts and hopes. Clin Cancer Res. (2024) 30:1438–47. doi: 10.1158/1078-0432.CCR-21-1935
12. Ordóñez-Mena JM, Schöttker B, Mons U, Jenab M, Freisling H, Bueno-de-Mesquita B, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. (2016) 14:62. doi: 10.1186/s12916-016-0607-5
13. Di Federico A, De Giglio A, Gelsomino F, Sperandi F, Melotti B, and Ardizzoni A. Predictors of survival to immunotherapy and chemoimmunotherapy in non-small cell lung cancer: A meta-analysis. JNCI: J Natl Cancer Institute. (2022) 115:29–42. doi: 10.1093/jnci/djac205
14. Liguori L, Giorgio G, Polcaro G, Pagliara V, Malandrino D, Perri F, et al. Checkpoint based immunotherapy in non-small cell lung cancer: a real-world retrospective study. Front Immunol. (2024) 15:1419544. doi: 10.3389/fimmu.2024.1419544
15. Luo D, Yang D, Cao D, Gong Z, He F, Hou Y, et al. Effect of smoking status on immunotherapy for lung cancer: a systematic review and meta-analysis. Front Oncol. (2024) 14:1422160. doi: 10.3389/fonc.2024.1422160
16. Ellison-Barnes A, Johnson S, and Gudzune K. Trends in obesity prevalence among adults aged 18 through 25 years, 1976-2018. Jama. (2021) 326:2073–4. doi: 10.1001/jama.2021.16685
17. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
18. Leapman MS, Presley CJ, Zhu W, Soulos PR, Adelson KB, Miksad RA, et al. Association of programmed cell death ligand 1 expression status with receipt of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. JAMA network Open. (2020) 3:e207205. doi: 10.1001/jamanetworkopen.2020.7205
19. Luo W, Zeng Z, Jin Y, Yang L, Fan T, Wang Z, et al. Distinct immune microenvironment of lung adenocarcinoma in never-smokers from smokers. Cell Rep Med. (2023) 4:101078. doi: 10.1016/j.xcrm.2023.101078
20. Faselis C, Nations JA, Morgan CJ, Antevil J, Roseman JM, Zhang S, et al. Assessment of lung cancer risk among smokers for whom annual screening is not recommended. JAMA Oncol. (2022) 8:1428–37. doi: 10.1001/jamaoncol.2022.2952
21. Azuma K, Xiang H, Tagami T, Kasajima R, Kato Y, Karakawa S, et al. Clinical significance of plasma-free amino acids and tryptophan metabolites in patients with non-small cell lung cancer receiving PD-1 inhibitor: a pilot cohort study for developing a prognostic multivariate model. J immunotherapy Cancer. (2022) 10:e004420. doi: 10.1136/jitc-2021-004420
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
23. Dai L, Jin B, Liu T, Chen J, Li G, and Dang J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: A systematic review and meta-analysis. EClinicalMedicine. (2021) 38:100990. doi: 10.1016/j.eclinm.2021.100990
24. Zhao W, Jiang W, Wang H, He J, Su C, and Yu Q. Impact of smoking history on response to immunotherapy in non-small-cell lung cancer: A systematic review and meta-analysis. Front Oncol. (2021) 11:703143. doi: 10.3389/fonc.2021.703143
25. Wang X, Ricciuti B, Nguyen T, Li X, Rabin MS, Awad MM, et al. Association between smoking history and tumor mutation burden in advanced non–small cell lung cancer. Cancer Res. (2021) 81:2566–73. doi: 10.1158/0008-5472.CAN-20-3991
26. Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non–small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. (2022) 8:1160–8. doi: 10.1001/jamaoncol.2022.1981
27. Taş İ, Varlı M, Pulat S, Sim HB, Kim JJ, and Kim H. TDO2 inhibition counters Benzo[a]pyrene-induced immune evasion and suppresses tumorigenesis in lung adenocarcinoma. Cancer Metab. (2024) 12:36. doi: 10.1186/s40170-024-00365-z
28. Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. (2019) 10:1125. doi: 10.1038/s41467-019-08887-7
29. Vallejo J, Singh H, Larkins E, Drezner N, Ricciuti B, Mishra-Kalyani P, et al. Impact of increasing PD-L1 levels on outcomes to PD-1/PD-L1 inhibition in patients with NSCLC: A pooled analysis of 11 prospective clinical trials. oncologist. (2024) 29:422–30. doi: 10.1093/oncolo/oyae006
30. LoPiccolo J, Gusev A, Christiani DC, and Jänne PA. Lung cancer in patients who have never smoked-an emerging disease. Nat Rev Clin Oncol. (2024) 21:121–46. doi: 10.1038/s41571-023-00844-0
31. Blechter B, Hsiung CA, Wang X, Zhang HY, Seow WJ, Shi JX, et al. Polygenic risk score and lung adenocarcinoma risk among never-smokers by EGFR mutation status: A brief report. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2024) 20:521–30. doi: 10.1158/1538-7445.AM2024-6149
32. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2017) 12:403–7. doi: 10.1016/j.jtho.2016.10.007
33. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:210–6. doi: 10.1001/jamaoncol.2017.4427
34. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin oncology: Off J Am Soc Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
35. Caliri AW, Tommasi S, and Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
36. Konen JM, Wu H, and Gibbons DL. Immune checkpoint blockade resistance in lung cancer: emerging mechanisms and therapeutic opportunities. Trends Pharmacol Sci. (2024) 45:520–36. doi: 10.1016/j.tips.2024.04.006
37. Chellappan S. Smoking cessation after cancer diagnosis and enhanced therapy response: mechanisms and significance. Curr Oncol (Toronto Ont.). (2022) 29:9956–69. doi: 10.3390/curroncol29120782
38. Liu T, Guo W, Luo K, Li L, Dong J, Liu M, et al. Smoke-induced SAV1 gene promoter hypermethylation disrupts YAP negative feedback and promotes Malignant progression of non-small cell lung cancer. Int J Biol Sci. (2022) 18:4497–512. doi: 10.7150/ijbs.73428
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, smoking hygiene, cumulative smoking dose, real-world evidence
Citation: Yang L, Wang H, Tian P and Li W (2025) Prognostic stratification through smoking status and cumulative smoking dose in advanced non-small cell lung cancer immunotherapy: a dose-dependent real-world analysis. Front. Oncol. 15:1590825. doi: 10.3389/fonc.2025.1590825
Received: 10 March 2025; Accepted: 15 July 2025;
Published: 06 August 2025.
Edited by:
Hongbing Zhang, Tianjin Medical University General Hospital, ChinaReviewed by:
Guoqing Zhang, First Affiliated Hospital of Zhengzhou University, ChinaYixing Wang, City of Hope National Medical Center, United States
Wei Yan, Peking University Third Hospital, China
Copyright © 2025 Yang, Wang, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panwen Tian, bXJhc2NlbmRAMTYzLmNvbQ==; Weimin Li, d2VpbWluMDAzQDE2My5jb20=
†These authors share first authorship
‡These authors share last authorship
 Lan Yang
Lan Yang Haoyu Wang
Haoyu Wang Panwen Tian
Panwen Tian Weimin Li
Weimin Li