Abstract
Introduction:
Colorectal cancer (CRC) is a leading cause of global cancer mortality, increasingly linked to circadian rhythm disruption—a critical yet underexplored driver of tumorigenesis.
Methods:
This bibliometric analysis evaluates 374 publications from the Web of Science Core Collection (1999–2024) using VOSviewer, CiteSpace, and Bibliometrix to map global research trends.
Results:
Annual publications surged post-2016, peaking in 2021, reflecting intensified focus on circadian-CRC interactions. The United States led in output (122 publications, H-index 46), followed by France (76 publications) and China (49 publications), with the Netherlands achieving the highest citation impact (88.06 citations per publication). French institutions, notably Assistance Publique–Hôpitaux de Paris (APHP), dominated translational research, while foundational studies by Levi et al. on chronomodulated chemotherapy remained pivotal. Keyword analysis identified “circadian rhythm” and “colorectal cancer” as core themes, with “inflammation” and “inflammatory bowel disease” showing significant citation bursts post-2014. Co-citation networks bridged molecular chronobiology (Science, PNAS) and clinical oncology (Cancer Research), though mechanistic studies prioritized clock genes (e.g., BMAL1, PER2) over environmental disruptors. Clinically, aligning chemotherapy with circadian rhythms reduced severe toxicity by 40% in metastatic CRC, yet gaps persist in biomarker validation and monitoring tools. Epidemiologically, shift workers faced a 20–30% elevated CRC risk, correlating with PER2 silencing in 45% of tumors and NF-κB/STAT3 pathway activation.
Discussion:
Future research should integrate AI-driven circadian profiling, global collaboration, and trials targeting circadian-immune-metabolic axes to advance precision chronotherapy. This study underscores circadian biology as a cornerstone of CRC management, advocating strategies that harmonize molecular insights with ecological relevance to improve outcomes.
1 Introduction
Circadian rhythms represent an evolutionarily optimized timekeeping machinery that orchestrates physiological and molecular processes within 24-hour cycles, synchronizing organisms with environmental zeitgebers like light-dark cycles and feeding patterns (1). Beyond regulating sleep-wake cycles, these biological oscillators regulate critical cellular functions including DNA repair, metabolism, and immune surveillance—processes now recognized as intricately linked to cancer pathogenesis (2, 3). The therapeutic rationale for chronomodulated chemotherapy stems from this circadian regulation of drug metabolism. Key enzymes like cytochrome P450 (CYP3A4) and dihydropyrimidine dehydrogenase (DPD) exhibit striking diurnal activity variations, with CYP3A4 peaking at dawn (ZT0-4) and DPD activity surging 2.3-fold during daylight phases (ZT8-12) (4, 5). This temporal enzymatic landscape directly dictates the efficacy of chemotherapeutics such as oxaliplatin (CYP3A4-dependent) and 5-fluorouracil (DPD-metabolized), where circadian-aligned administration during enzyme trough phases has demonstrated 40-60% improvement in therapeutic index across clinical trials (4). Recent advances in single-cell resolution reveal colorectal tumors maintain circadian oscillations in drug-metabolizing enzymes within distinct cellular subpopulations, creating spatiotemporal heterogeneity in chemosensitivity (6).
Mounting epidemiological evidence positions circadian disruption as an emerging oncogenic driver in colorectal cancer CRC (7, 8), a malignancy undergoing dramatic global transformation. CRC now accounts for 10% of global cancer burden, with 19.3 million new cases and 935,000 deaths annually (9). Strikingly, early-onset CRC (<50 years) has increased 22% globally since 2000, projected to constitute 11% of colon and 23% of rectal cancers by 2030 (10). Geographical disparities persist exhibiting 6-fold variations in age-standardized incidence rates from 23.5/100,000 in India to 158.3/100,000 in Denmark with age-standardized mortality rates ranging from 3.2/100,000 (Western Africa) to 15.6/100,000 (Eastern Europe) (11), while paradoxically rising 1.3% annually in high-income countries despite screening programs (12). This epidemiological shift coincides with molecular profile alterations, including emerging clock gene mutations.
At the molecular level, core clock genes (BMAL1, PER1/2, CRY1/2) are increasingly implicated in CRC aggressiveness, metastatic potential, and therapeutic resistance (13, 14). The convergence of artificial intelligence (AI) with single-cell omics is catalyzing a paradigm shift in circadian oncology. Advanced algorithms like scPrisma now enable circadian signal extraction from single-cell RNA-seq data through periodic expression analysis of 327 core clock-controlled genes (CCGs) (6). Applied to CRC liver metastases, this approach uncovered 23% of tumor-infiltrating immune cells maintaining functional circadian programs that govern temporal PD-1/CTLA-4 checkpoint expression (15).Deep learning breakthroughs using temporal single-cell proteomics datasets (24 timepoints/3h intervals) achieve 89% accuracy in predicting optimal chemotherapy timing by modeling cell cycle synchronization and drug efflux pump oscillations (12, 16). These technologies collectively map tumor microenvironment (TME) dynamics, with AI-driven analysis of 58,431 single cells identifying STAT1-driven myeloid subpopulations acquiring circadian immunosuppressive functions post-chemotherapy.
Clinical translation of these insights shows promise. Chronomodulated chemotherapy synchronized with drug metabolism rhythms improves survival and reduces toxicity in metastatic CRC patients (17). Mechanistically, colorectal tumors frequently harbor mutations in circadian regulators (NPAS2, PER3) (18, 19) that disrupt cell cycle checkpoints and DNA repair timing.
Despite these advancements, critical gaps persist in mapping research evolution, identifying collaborative networks, and highlighting emerging themes at the circadian-CRC interface. This is where bibliometric analysis becomes pivotal—by systematically evaluating scientific publications, it reveals research patterns, collaboration dynamics, and thematic evolution, providing a macroscopic field view to guide future directions (20).
In this study, we conduct a comprehensive bibliometric analysis to: (1) Elucidate how circadian disruptions at molecular, cellular, and systemic levels drive CRC initiation and progression. (2) Identify barriers impeding the clinical translation of chronotherapeutic strategies. (3) Explore how global collaboration and emerging technologies—such as AI-driven circadian monitoring and single-cell omics—can advance precision chrono-oncology.
We aim to explore the application of circadian biology in colorectal cancer treatment by integrating bibliometric trends, mechanistic insights, and clinical evidence. We hope for multidisciplinary approaches that balance precise timing with ecological relevance in order to improve patient outcomes.
2 Methods
2.1 Data source and search strategy
Data for this study were sourced from the Web of Science Core Collection (WoSCC), a comprehensive database frequently utilized in bibliometric research. To mitigate temporal biases associated with periodic database updates, a single retrieval session was conducted on March 1, 2025, encompassing publications from January 1, 1999, to December 31, 2024. The search strategy employed Boolean operators to combine two thematic domains: (1) Circadian rhythm terms:
TS=(“Circadian Rhythm” OR “Circadian Rhythms” OR “Rhythm, Circadian” OR “Rhythms, Circadian” OR “Twenty-Four Hour Rhythm” OR “Rhythm, Twenty-Four Hour” OR “Nyctohemeral Rhythm” OR “Diurnal Rhythm”) (2) Colorectal cancer terms: TS=(“Colorectal Neoplasm” OR “Colorectal Cancer” OR “Rectal Cancer” OR “Colon Cancer” OR “Colorectal Carcinoma” OR “Rectal Neoplasm” OR “Cancer of the Colon” OR “Colonic Cancer”).
The search was refined to include only peer-reviewed articles and reviews published in English, excluding non-research items such as editorials and meeting abstracts (Figure 1). The resulting data were exported in plain text format for subsequent analysis.
Figure 1
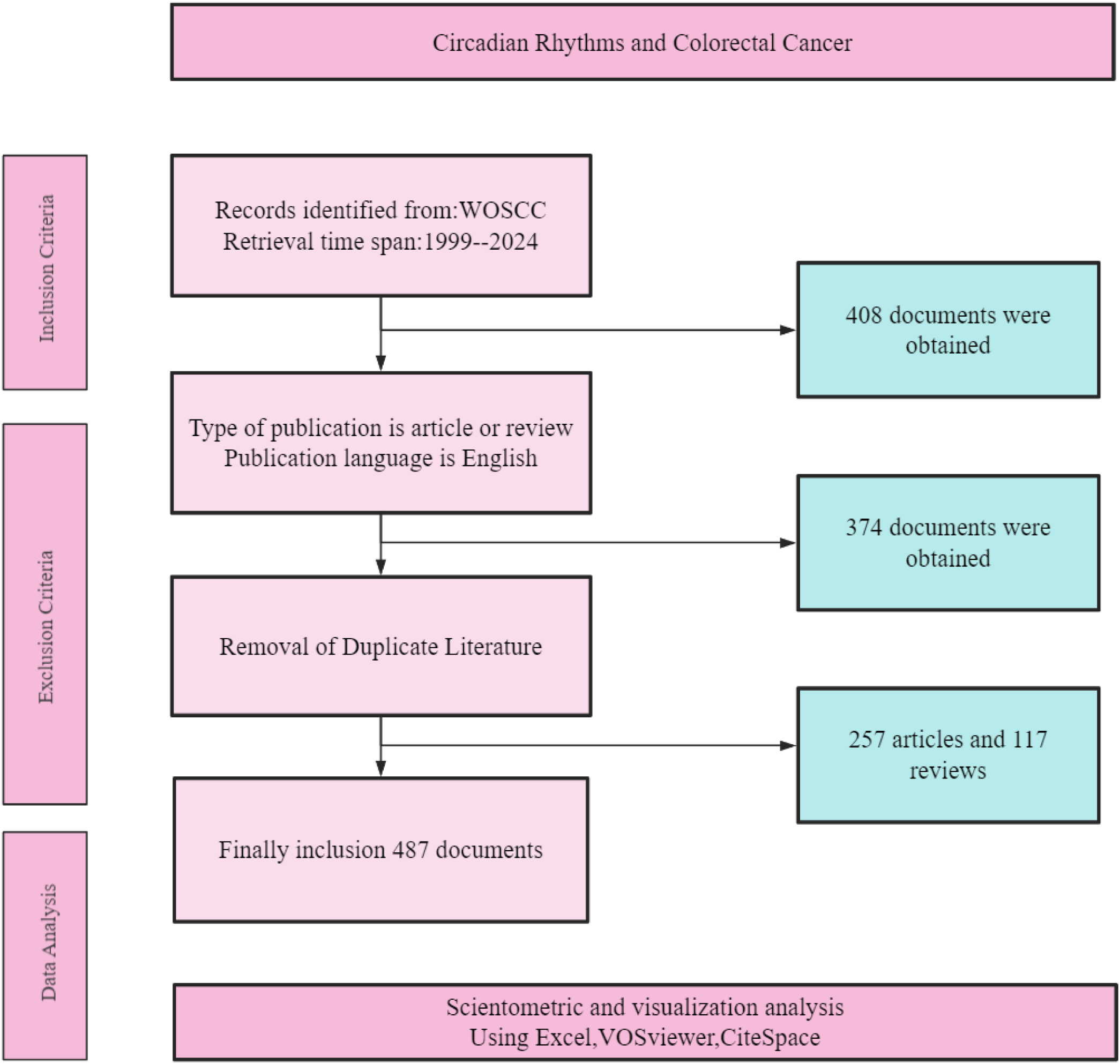
Flow chart for search strategy of publications.
2.2 Data analysis
This study employed bibliometric analysis to evaluate global research trends in circadian rhythm and CRC. Data were analyzed using established tools: VOSviewer (version 1.6.18) for constructing and visualizing bibliometric networks such as co-occurrence networks of keywords, and CiteSpace (version 6.3) for citation burst detection and visualizing collaborative networks among institutions, authors, keywords, and co-cited references. Additionally, R (version 4.1) was utilized for visual analysis of publication countries, further elucidating patterns and trends in global research collaborations.
To present the analysis results intuitively, valid data extracted from the WoSCC database were imported into CiteSpace [version 6.3] and VOSviewer [version 1.6.18]. CiteSpace was employed to visualize the collaborative networks among institutions, authors, keywords, and co-cited references, revealing citation bursts for references and keywords, as well as exploring the current status, focus, and trends of research. Cluster plots and temporal distribution plots were generated to identify field trends. VOSviewer facilitated visual analysis of the collaborative networks between countries and journals. Concurrently, R (version 4.1) was utilized for visual analysis of publication countries, further elucidating patterns and trends in global research collaborations. Publications were screened for relevance to circadian-CRC interactions, with non-research articles excluded. Analytical outputs included network visualizations of author collaborations, institutional partnerships, and thematic clusters. Results were cross-validated through independent coding by two researchers to ensure consistency.
2.3 Quantitative analysis
Research productivity and impact were assessed through a dual analytical framework, integrating quantitative metrics and network visualization. Productivity metrics focused on four dimensions (1): publication volume by leading authors (2), journal-level contributions (3), institutional output, and (4) national research activity, identifying key contributors through frequency rankings. Quality assessment employed co-citation dynamics, analyzing authors, journals, references, and keywords to map intellectual influence and thematic convergence.
Visual analytics were implemented using three complementary platforms:
CiteSpace (v6.3): Generated temporal burst detection plots for references/keywords and time-zone networks to trace thematic evolution. Institutional collaboration networks were pruned using pathfinder scaling (g-index = 25) to highlight dominant partnerships.
VOSviewer (v1.6.18): Constructed journal co-citation maps with full-counting normalization and Linlog/Modularity clustering (resolution = 1.0), revealing interdisciplinary bridges between chronobiology and oncology.
R (v4.1): Produced geospatial heatmaps via the bibliometrix package (threshold = 5-country minimum), visualizing geographic disparities in circadian-CRC research output.
3 Results
3.1 Annual trends in publications
Based on the specified search criteria and time span from January 1, 1999, to December 31, 2024, we conducted two separate searches in the WOSCC database. The first search, using keywords related to circadian rhythms, yielded 36,291 results. The second search, using keywords related to CRC, resulted in 235,092 records. By combining these two sets of keywords, we obtained an initial set of 408 records related to both circadian rhythms and CRC. After applying the inclusion criteria—filtering for articles and reviews published in English—the number of documents was reduced to 374. Subsequently, duplicate literature was meticulously removed, resulting in a final collection of 257 articles and 117 reviews. These 487 documents were then subjected to scientometric and visualization analysis using tools such as Excel, VOSviewer, and CiteSpace, as detailed in Figure 1.
The annual publication trends are depicted in Figure 2. From 1999 to 2009, there was a gradual increase in publications, indicating growing interest in this research area. The period between 2010 and 2015 saw steady growth, reflecting sustained exploration of the interplay between circadian regulation and CRC pathogenesis. A notable surge occurred from 2016 to 2021, with the peak annual output in 2021, underscoring intensified scientific efforts during this timeframe. Post-2021, there was a slight decline in publications; however, the numbers remained elevated compared to earlier decades, highlighting the enduring relevance of circadian biology in CRC research. Over the past 25 years, the total amount of data shows that researchers have been paying more and more attention to this field. This is because circadian mechanisms are becoming more and more recognized as important in controlling the progression of CRC. This makes the field a key area for future oncological studies.
Figure 2
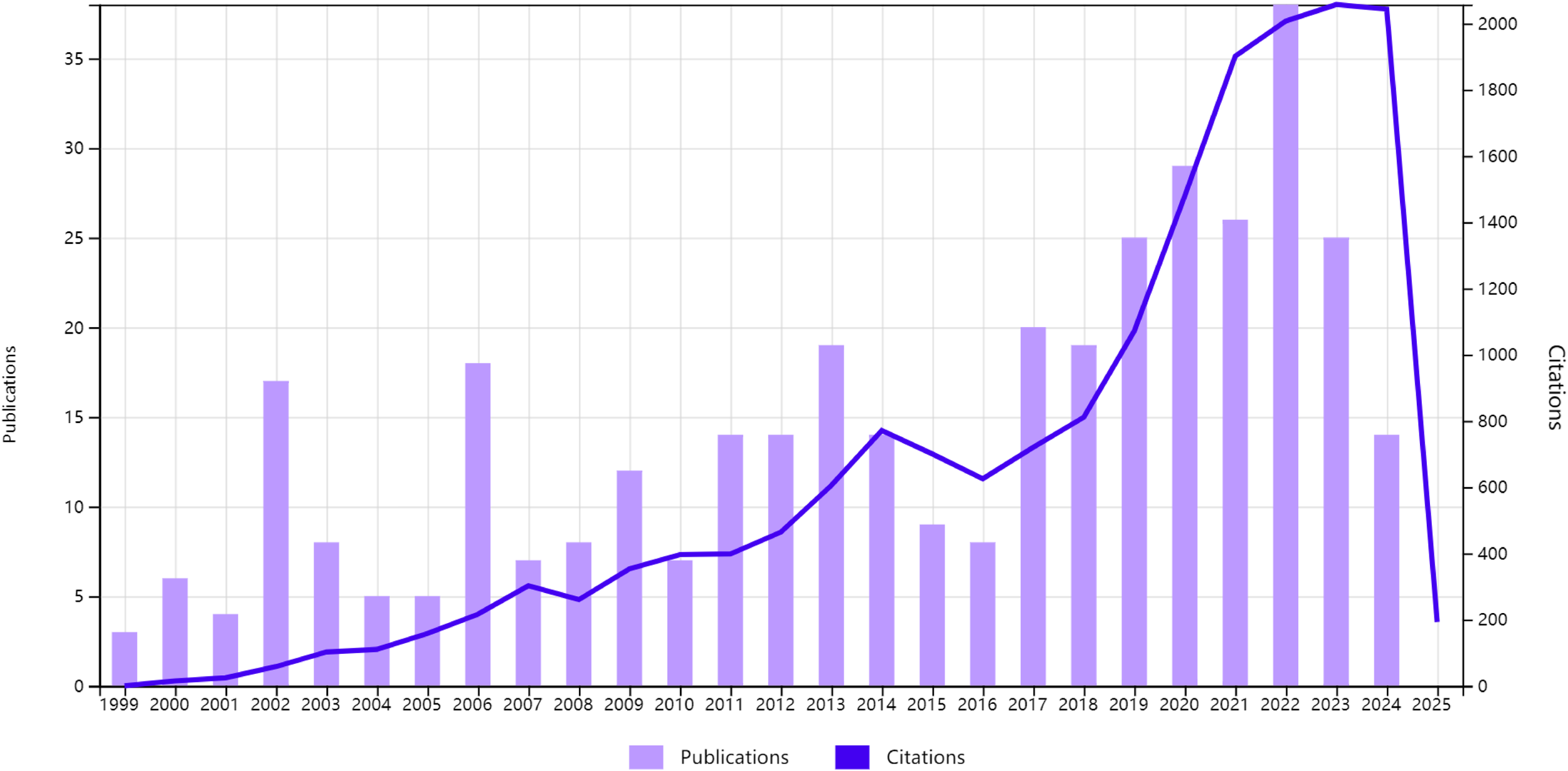
The annual growth trends of publications from 1999 to 2024.
3.2 Analysis of publishing countries/regions and institutions
The bibliometric analysis encompassed publications from 49 countries and 674 institutions. (Figure 3a) illustrates the global distribution of research output by country. (Figure 3b) also depicts the global distribution of scientific production in circadian rhythms and colorectal cancer research (1999–2024). The color gradient reflects the number of publications per country, with darker blue shades indicating higher scientific output. The color scale ranges from light blue (low production) to dark blue (high production), and countries with no publications are shown in gray. As detailed in Table 1, the United States emerged as the leading contributor to circadian rhythm and CRC research, with 122 publications and a total of 7,097 citations, averaging 58.17 citations per publication. Its H-index of 46, the highest among all countries, further reflects this prominence, indicating both prolific output and sustained scholarly impact. France ranked second with 76 publications and an H-index of 37, while China followed with 49 publications; however, its average citation rate (30.22) and H-index (23) were comparatively lower. Notably, the Netherlands demonstrated exceptional citation impact, achieving the highest average citations per publication (88.06) despite a smaller publication volume (16).
Figure 3
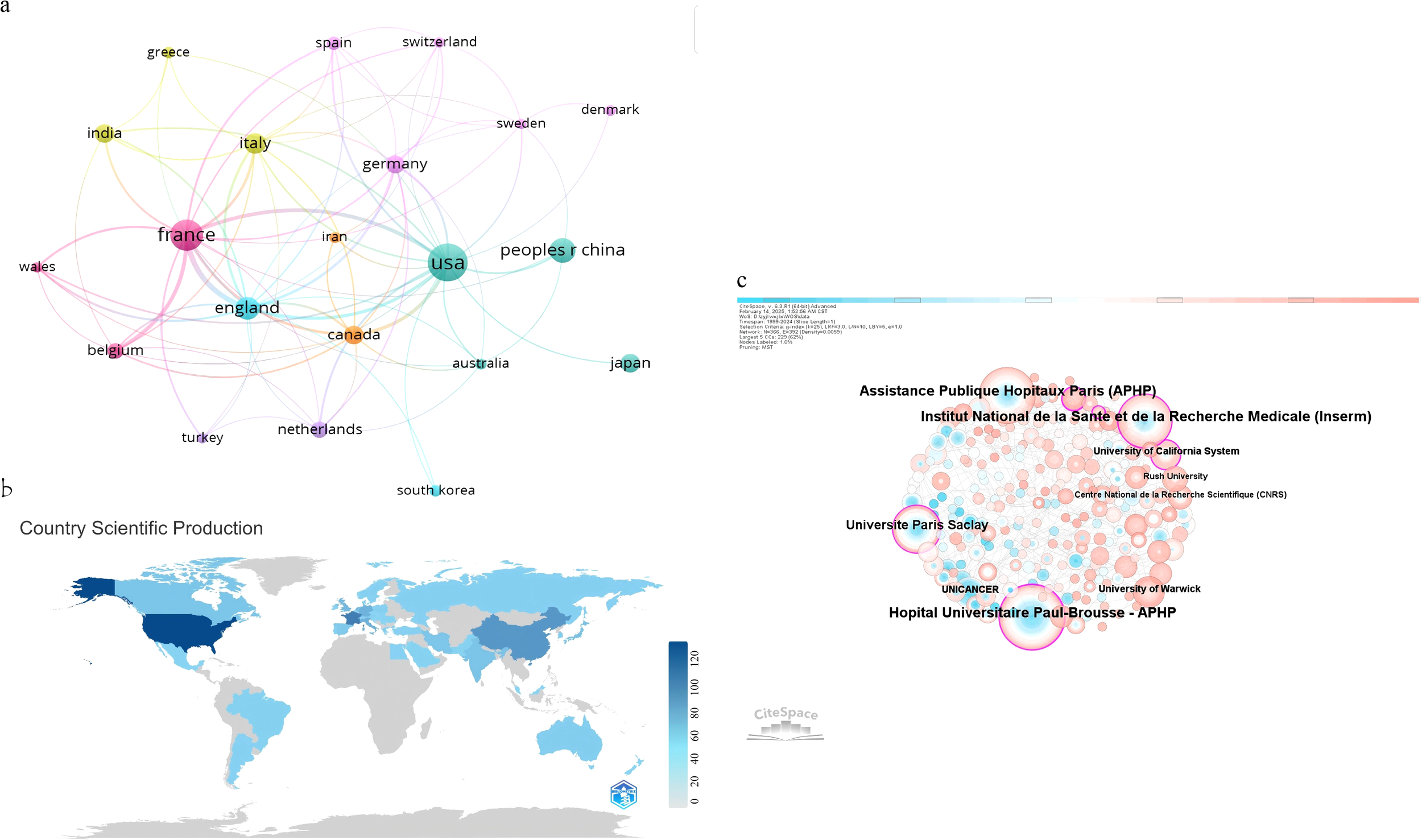
(a) Visualization analysis of countries collaborations generated by VOSviewer. (b) The network of institutions collaborations generated by Citespace. (c) Country/Region number of publications.
Table 1
| Rank | Country | Publications | Citations | Average Citation/Publication | H-index |
|---|---|---|---|---|---|
| 1 | USA | 122 | 7,097 | 58.17 | 46 |
| 2 | France | 76 | 5,145 | 67.7 | 37 |
| 3 | The People's Republic of China | 49 | 1,841 | 30.22 | 23 |
| 4 | England | 37 | 3,043 | 82.24 | 24 |
| 5 | Italy | 28 | 795 | 28.39 | 15 |
| 6 | India | 23 | 405 | 17.61 | 9 |
| 7 | Japan | 23 | 526 | 22.87 | 13 |
| 8 | Canada | 22 | 1,267 | 57.59 | 16 |
| 9 | Germany | 20 | 523 | 26.15 | 14 |
| 10 | Netherlands | 16 | 1,409 | 88.06 | 11 |
Top 10 active countries/regions.
Collaborative networks among countries are visualized in (Figure 3a). Node sizes correspond to publication counts, with thicker connecting lines indicating stronger research partnerships. The United States and China serve as central hubs within this network, maintaining robust bilateral collaborations. The U.S. also exhibits extensive ties to numerous nations, including France, England, Canada, and Japan. Countries such as India, Italy, and Germany display moderate engagement, while South Korea, Australia, and Switzerland occupy peripheral positions with fewer connections. This interconnected framework highlights the global integration of circadian rhythm-CRC research, driven by leading nations with high academic influence.
Table 2 showcases the leading research institutions based on publication volume in circadian rhythm and CRC studies. Assistance Publique–Hôpitaux de Paris (APHP) emerged as the most prolific contributor, producing 55 publications (14.2% of the total analyzed studies), accompanied by 4,070 citations and an H-index of 31. This underscores APHP’s dual role in driving high-volume research and generating impactful findings, particularly in clinical and translational studies linking circadian disruption to CRC progression. The Institut National de la Santé et de la Recherche Médicale (Inserm) came in second with 52 publications, or 13.4% of the total. These papers had 3,847 citations and an H-index of 30, which shows that they have made long-term contributions to understanding how circadian regulation affects tumorigenesis at the molecular level. Hôpital Universitaire Paul-Brousse APHP followed closely with 51 publications (13.2%), emphasizing France’s dominance in this field.
Table 2
| Rank | Organization | Publications | Citations | Average Citation/Publication | H-index |
|---|---|---|---|---|---|
| 1 | Assistance Publique – Hôpitaux de Paris (APHP) | 55 | 4,070 | 74 | 31 |
| 2 | Institut National de la Santé et de la Recherche Médicale (Inserm) | 52 | 3,847 | 73.98 | 30 |
| 3 | Hôpital Universitaire Paul-Brousse APHP | 51 | 3,711 | 72.76 | 30 |
| 4 | Université Paris-Saclay | 36 | 2,629 | 73.03 | 26 |
| 5 | Unicancer | 17 | 822 | 48.35 | 13 |
| 6 | University Of Warwick | 16 | 688 | 43 | 11 |
| 7 | University Of California System | 14 | 1,028 | 73.43 | 11 |
| 8 | Centre National de la Recherche Scientifique (CNRS) | 11 | 765 | 69.55 | 9 |
| 9 | Stanford University | 11 | 831 | 75.55 | 9 |
| 10 | Pt. Ravishankar Shukla University | 10 | 261 | 26.1 | 8 |
Top 10 active institutions.
Notably, Université Paris-Saclay ranked fourth with 36 publications, while institutions such as Stanford University and the University of California System demonstrated exceptional citation quality despite smaller publication volumes (11 and 14 publications, respectively). French institutions collectively accounted for over 40% of the top 10 entries, highlighting their centralized role in advancing circadian-CRC research.
Visualization via CiteSpace (Figure 3c) illustrates institutional collaboration networks. Node size corresponds to publication output—larger circles represent institutions like APHP and Inserm with higher productivity. Connecting lines denote collaborative ties, with thicker lines indicating stronger partnerships (e.g., between APHP and Inserm). This network reveals a tightly interconnected European core, complemented by transatlantic links to U.S. institutions such as Stanford University, facilitating cross-regional knowledge exchange. The spatial layout underscores how high-output institutions serve as hubs for innovation, disseminating critical insights into circadian biology’s role in CRC.
3.3 Authors and co-cited authors
A total of 1914 researchers contributed to publications investigating circadian rhythm and CRC. Among these, LEVI, Francis ranked first with 34 publications (Table 3), followed by Innominate, Pasquale F. (16 publications) and Giacchetti, S. (11 publications), reflecting their active engagement in advancing this field. The collaborative network, visualized in Figure 4a, represents authors as nodes where larger circles indicate higher publication volumes, and connecting lines denote collaborative relationships, with thicker lines reflecting stronger partnerships. For instance, dense clusters around LEVI, Francis highlight his central role in fostering research connections. Author co-citation analysis (Figure 4b, Table 4) identifies foundational contributors whose works are frequently cited together. Mormont, Marie Christine and LEVI, Francis were the most co-cited authors (231 citations each), followed by Iunominato, Pasquale F. (205 citations) and Levi F (191 citations). Centrality—specifically intermediary centrality—quantifies a node’s role as a bridge between disconnected parts of the network. Higher centrality values (e.g., Giacchetti, S. at 0.18) indicate authors whose work serves as a critical “pivot point,” linking distinct research clusters. Removing such high-centrality nodes would disrupt the shortest paths between other nodes, impairing information flow efficiency. For example, Filipski, Elisabeth (centrality 0.06) also acts as a connector but with less influence. These patterns underscore the dual importance of productivity and network position: top authors drive both publication output and interdisciplinary integration, while high-centrality researchers ensure cohesive knowledge exchange across the circadian-CRC research landscape.
Table 3
| Rank | Author | Publications | Citations | Average Citation/Publication | H-index |
|---|---|---|---|---|---|
| 1 | LEVI, Francis | 34 | 2,779 | 81.74 | 26 |
| 2 | Innominate, Pasquale F. | 16 | 934 | 58.38 | 15 |
| 3 | Giacchetti, S. | 11 | 1,034 | 94 | 11 |
| 4 | Parganiha, Arti | 10 | 261 | 26.1 | 8 |
| 5 | Filipski, Elisabeth | 10 | 1,154 | 115.4 | 10 |
| 6 | Focan, C. | 9 | 590 | 65.56 | 9 |
| 7 | Mormont, Marie-Christine | 8 | 1,185 | 148.13 | 8 |
| 8 | Ballesta, Annabelle | 8 | 418 | 52.25 | 7 |
| 9 | Relógio, Angela | 7 | 151 | 21.57 | 7 |
| 10 | Bishehsari, Faraz | 7 | 271 | 38.71 | 5 |
Top 10 authors in terms of total publications.
Figure 4
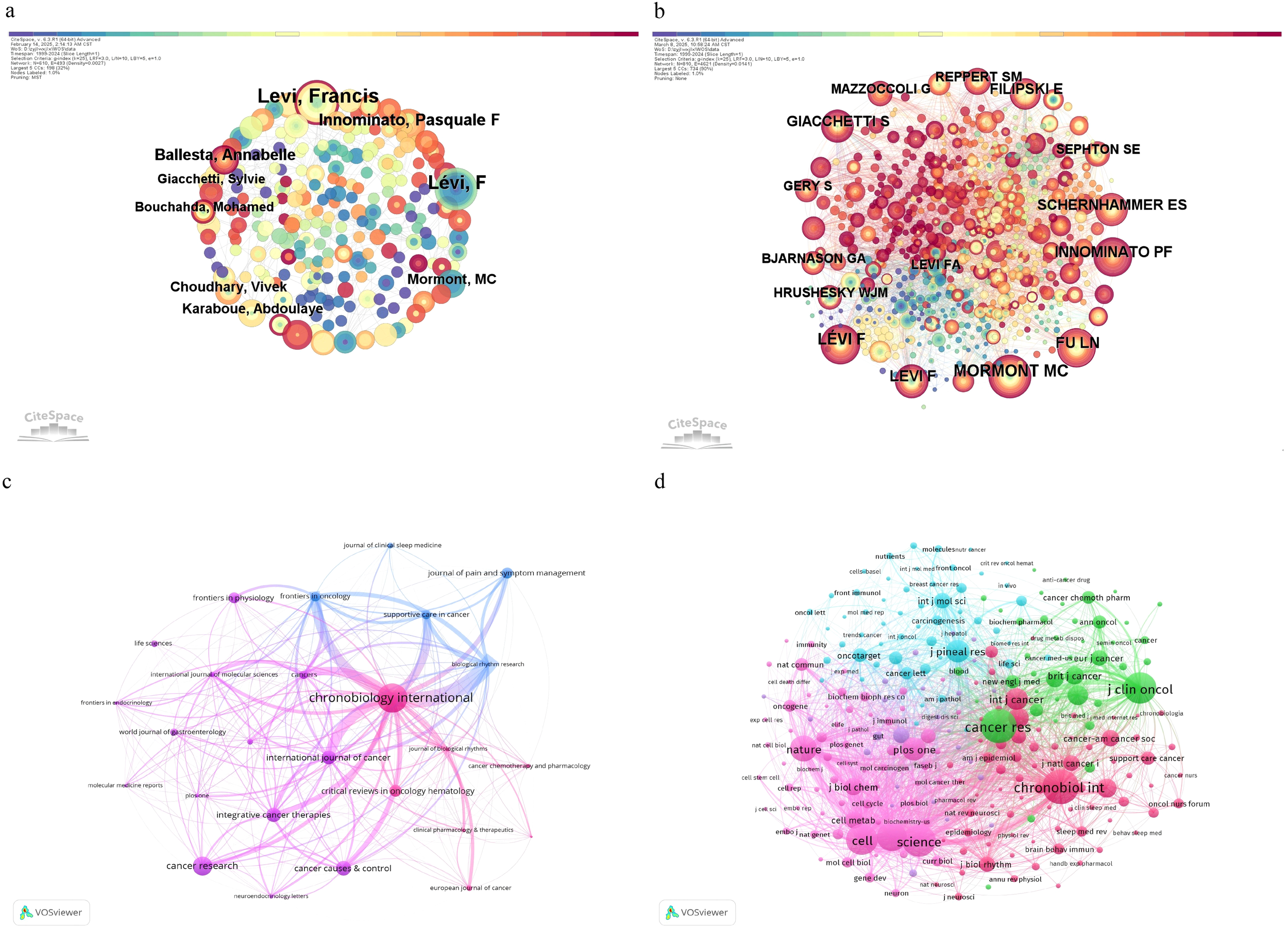
(a) Illustrate the network of author collaborations; (b) the co-occurrence network generated using CiteSpace; (c) The visualization of journals using VOSviewer; (d) the co-citation network visualization of journals using VOSviewer.
Table 4
| Rank | Cited Authors | Count | Centrality |
|---|---|---|---|
| 1 | Mormont, Marie Christine | 231 | 0.02 |
| 2 | LEVI,Francis | 231 | 0.01 |
| 3 | Innominato, Pasquale F. | 205 | 0.03 |
| 4 | Levi F | 191 | 0.1 |
| 5 | Fu, L. | 163 | 0.02 |
| 6 | Giacchetti, S. | 159 | 0.18 |
| 7 | Filipski, Elisabeth | 155 | 0.06 |
| 8 | Schernhammer, E.S. | 149 | 0.06 |
| 9 | Reppert, S.M. | 148 | 0.03 |
| 10 | Sephton, S.E. | 144 | 0.03 |
Top 10 co-cited authors in terms of total citations.
3.4 Journals and co-cited journals
Over the past two decades, research on circadian rhythm and colorectal cancer (CRC) has expanded significantly, as evidenced by the contributions of key academic journals. Table 5 summarizes the productivity and impact of leading journals, with Chronobiology International (Q2) ranking first in publication volume (32 articles), followed by Biological Rhythm Research (Q3, 10 articles) and Cancers (Q1, 10 articles). Notably, Cancer Research (Q1), despite a lower publication count (5 articles), achieved the highest citation impact (120.8 citations per article) and journal impact factor (IF 12.5), underscoring its pivotal role in disseminating high-impact findings. Journals such as Integrative Cancer Therapies (Q2) demonstrated exceptional citation efficiency (71.83 citations per article), while International Journal of Cancer (Q1) and European Journal of Cancer (Q1) maintained strong interdisciplinary relevance with moderate publication outputs. Figure 4c visualizes journal relationships via VOSviewer. Chronobiology International stands out as a central node, reflecting both its substantial publication volume and significant influence in circadian rhythm and colorectal cancer research.
Table 5
| Rank | Journals Rank | Publications | Citations | Average Citation/Publication | H-index | Journal IF (2023) |
|---|---|---|---|---|---|---|
| 1 | Chronobiology International(Q2) | 32 | 1,053 | 32.91 | 21 | 2.2 |
| 2 | Biological Rhythm Research(Q3) | 10 | 49 | 4.9 | 5 | 1.0 |
| 3 | Cancers(Q1) | 10 | 215 | 21.5 | 8 | 4.5 |
| 4 | International Journal of Cancer(Q1) | 9 | 415 | 46.11 | 9 | 5.7 |
| 5 | International Journal of Molecular Sciences(Q1) | 9 | 415 | 46.11 | 9 | 5.6 |
| 6 | Frontiers in Oncology(Q2) | 8 | 122 | 13.56 | 6 | 3.5 |
| 7 | Integrative Cancer Therapies(Q2) | 6 | 431 | 71.83 | 6 | 2.9 |
| 8 | Cancer Research(Q1) | 5 | 604 | 120.8 | 5 | 12.5 |
| 9 | European Journal of Cancer(Q1) | 5 | 136 | 27.2 | 5 | 7.7 |
| 10 | Supportive Care in Cancer(Q2) | 5 | 222 | 44.4 | 5 | 2.8 |
Top 10 journals in terms of total publications.
The co-citation network (Figure 4d, Table 6) highlights foundational journals that shape circadian-CRC research. Cancer Research emerged as the most co-cited journal (275 citations), followed by Chronobiology International (238 citations) and Journal of Clinical Oncology (197 citations). High-impact journals such as Science and Proceedings of the National Academy of Sciences exhibited elevated centrality values (0.05 each), indicating their role as critical bridges connecting diverse research domains. Centrality, a measure of a journal’s ability to integrate interdisciplinary knowledge, emphasizes how these journals facilitate cross-field dialogue—for instance, linking molecular biology with clinical oncology. Journals with higher centrality values, when removed, would disrupt knowledge pathways, underscoring their structural importance in the academic network.
Table 6
| Rank | Cited References | Count | Centrality |
|---|---|---|---|
| 1 | Cancer Research | 275 | 0.02 |
| 2 | Chronobiology International | 238 | 0.02 |
| 3 | Journal of Clinical Oncology | 197 | 0.03 |
| 4 | Clinical Cancer Research | 197 | 0.02 |
| 5 | Science | 195 | 0.05 |
| 6 | Journal of the National Cancer Institute | 187 | 0.02 |
| 7 | International Journal of Cancer | 182 | 0.03 |
| 8 | Proceedings of the National Academy of Sciences of the United States of America | 176 | 0.05 |
| 9 | Cell | 173 | 0.03 |
| 10 | PLOS ONE | 162 | 0.02 |
Top 10 co-cited journals in terms of total citations.
Collectively, these findings illustrate the dual dynamics of productivity and influence: while Chronobiology International dominates publication volume, Cancer Research and high-centrality journals like Science drive conceptual integration and knowledge dissemination across the circadian-CRC research landscape.
(Figure 5d) presents a double-graph overlay analysis depicting citation connections between journals in the context of circadian rhythm and CRC research. Citing journals are on the left, with cited journals on the right, each grouped by subject. Colored lines show citation pathways. Two main paths stand out: the orange path signifies that journals in molecular, biology, immunology are often cited by those in molecular biogenetics. In contrast, the green path indicates that articles in medicine, medical, clinical journals are frequently cited within health, nursing, and medicine. This visual analysis improves the understanding of interactions and knowledge flow between disciplines.
Figure 5
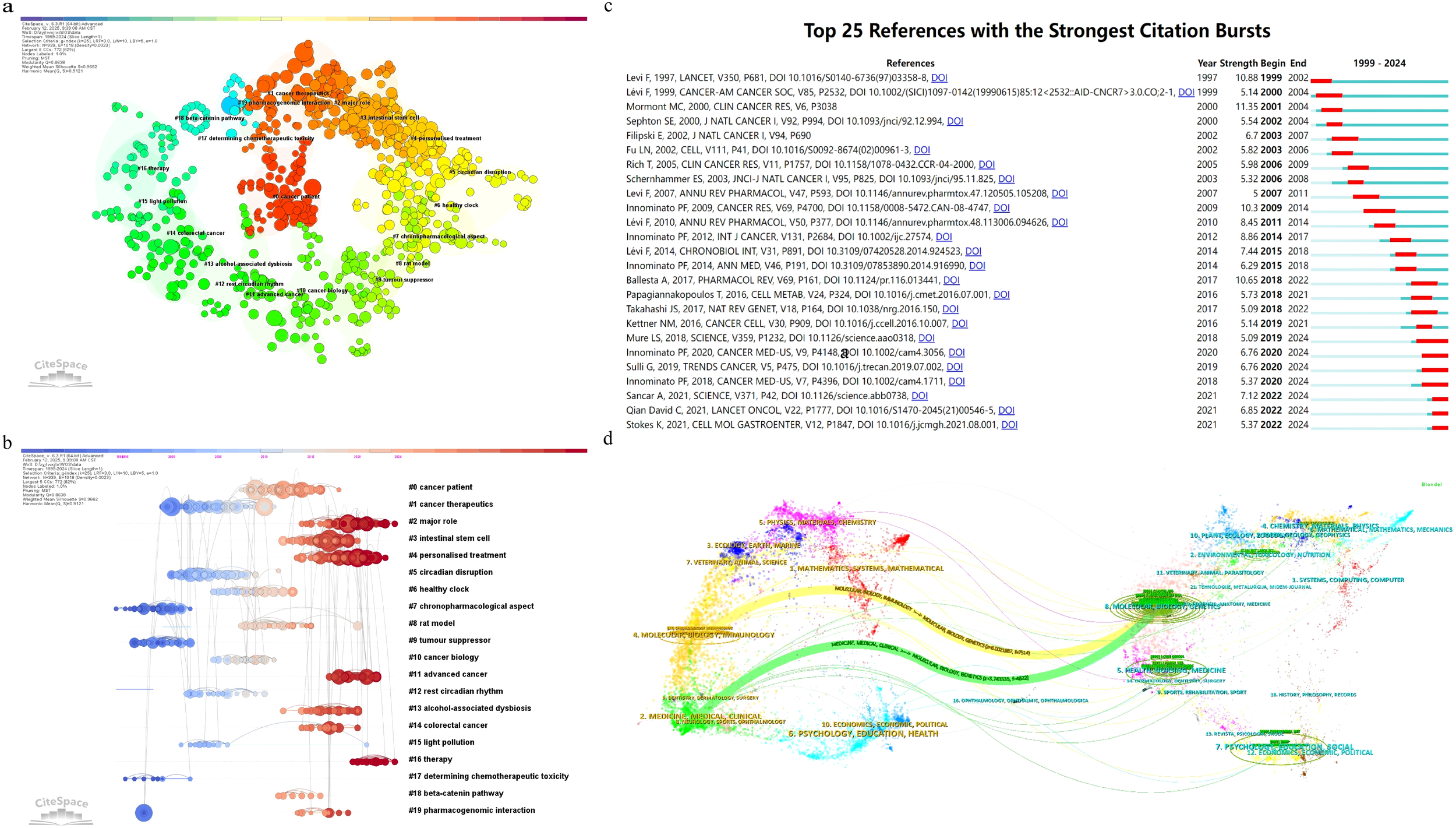
(a) The network of co-cited references clusters; (b) the labels clustering of co-cited literature based on LLR algorithm; (c) Top 25 references with strongest citation bursts; (d) Sankey diagram of journal analysis.
3.5 Co-cited references and highlights
The analysis of references in the field of CRC and circadian rhythm research uncovers the interplay and development of various sub-themes. This approach pinpoints references cited together across multiple papers, signaling thematic or content-related connections. Among the 487 co-cited references identified, Table 7 lists the top 10 most frequently co-cited works.
Table 7
| Rank | Title | First author | Cited Journals | Cited Journals | Count | Centrality |
|---|---|---|---|---|---|---|
| 1 | Systems Chronotherapeutics | Annabelle Ballesta | Pharmacological Review | Ballesta A, 2017, PHARMACOL REV, V69, P161, DOI 10.1124/pr.116.013441 | 27 | 0 |
| 2 | Circadian Rhythm in Rest and Activity: A Biological Correlate of Quality of Life and a Predictor of Survival in Patients with Metastatic Colorectal Cancer | Pasquale F. Innominato | Cancer Research | Innominato PF, 2009, CANCER RES, V69, P4700, DOI 10.1158/0008-5472.CAN-08-4747 | 22 | 0 |
| 3 | Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment | Gabriele Sulli | Trends in Cancer | Sulli G, 2019, TRENDS CANCER, V5, P475, DOI 10.1016/j.trecan.2019.07.002 | 19 | 0 |
| 4 | Sex-dependent least toxic timing of irinotecan combined with chronomodulated chemotherapy for metastatic colorectal cancer: Randomized multicenter EORTC 05011 trial | Pasquale F. Innominato | Cancer Medicine | Innominato PF, 2020, CANCER MED-US, V9, P4148, DOI 10.1002/cam4.3056 | 19 | 0 |
| 5 | Marked 24-h rest/activity rhythms are associated with better quality of life, better response and longer survival in patients with metastatic colorectal cancer and good performance status | Mormont MC, Lévi F | Clinical Cancer Research | Mormont MC, 2000, CLIN CANCER RES, V6, P3038 | 19 | 0 |
| 6 | Randomized multicenter trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer | Francis Lévi | The Lancet | Levi F, 1997, LANCET, V350, P681, DOI 10.1016/S0140-6736(97)03358-8 | 18 | 0 |
| 7 | Circadian Timing in Cancer Treatments | Francis Lévi | Annual Review of Pharmacology | Lévi F, 2010, ANNU REV PHARMACOL, V50, P377, DOI 10.1146/annurev.pharmtox.48.113006.094626 | 16 | 0 |
| 8 | Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer | Pasquale F. Innominato | International Journal of Cancer | Innominato PF, 2012, INT J CANCER, V131, P2684, DOI 10.1002/ijc.27574 | 16 | 0 |
| 9 | Diurnal transcriptome atlas of a primate across major neural and peripheral tissues | Ludovic S. Mure | Science | Mure LS, 2018, SCIENCE, V359, P1232, DOI 10.1126/science.aao0318 | 14 | 0 |
| 10 | Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-center, longitudinal study | David C Qian | The Lancet Oncology | Qian David C, 2021, LANCET ONCOL, V22, P1777, DOI 10.1016/S1470-2045(21)00546-5 | 14 | 0 |
Top 10 co-cited documents.
As shown in Figures 5a, b, the cluster analysis visually represents these 20 clusters, illustrating the interconnectedness of various research themes. Figure 5a presents the network of co-cited references clusters, while Figure 5b shows the labels clustering of co-cited literature based on LLR algorithm. Cluster analysis delves into research trends, thematic evolution, and knowledge frameworks within the field. The references are grouped into 20 clusters, such as #0 cancer patient, #1 cancer therapeutics, #2 major role, #3 intestinal stem cell, #4 personalized treatment, #5 circadian disruption, #6 healthy clock, #7 chronopharmacological aspect, #8 rat model, #9 tumor suppressor, #10 cancer biology, #11 advanced cancer, #12 rest circadian rhythm, #13 alcohol-associated dysbiosis, #14 colorectal cancer, #15 light pollution, #16 therapy, #17 determining chemotherapeutic toxicity, #18 beta-catenin pathway, and #19 pharmacogenomic interaction. These clusters capture the multifaceted nature of CRC research, underscoring its complexity and depth.
(Figure 5c) presents the top 25 most cited references, reflecting emerging trends and growing interest in the field. Notably, the study by Levi F et al., titled “Randomized multicenter trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer,” published in The Lancet in 1997, exhibits the strongest citation burst. This research highlights the efficacy of chronotherapy in enhancing treatment outcomes for metastatic CRC patients. The study employed a randomized multicenter trial to assess how circadian rhythm-based chemotherapy timing affects overall survival and quality of life. Results showed significant improvements in both overall survival and quality of life when chemotherapy was aligned with the patient’s circadian rhythm. These findings emphasize the importance of considering circadian rhythms in CRC treatment and reveal key connections between research topics, helping to identify core references and emerging research areas within the field.
Additionally, the most recent citation burst is observed in the study by Stokes K et al., titled “The Circadian Clock Gene, Bmal1, Regulates Intestinal Stem Cell Signaling and Represses Tumor Initiation,” published in 2021. This research explores the role of the Bmal1 gene in intestinal stem cell signaling and its impact on tumor initiation in colorectal cancer. The study uses a combination of genetic models, organoid assays, and transcriptomic profiling to demonstrate that the loss of Bmal1 disrupts circadian rhythms and increases tumor initiation. The findings highlight the importance of circadian clock genes in maintaining intestinal homeostasis and preventing tumorigenesis, providing new insights into the molecular mechanisms underlying the relationship between circadian rhythms and CRC development.
3.6 Keyword co-occurrence and keyword prominence
Keyword analysis of 374 publications, performed using CiteSpace, identified central themes and trends in circadian rhythm and colorectal cancer (CRC) research. Table 8 lists the 20 most cited keywords, with colorectal cancer (173 occurrences, centrality 0.12) and circadian rhythm (143 occurrences, centrality 0.07) dominating the field. These terms reflect the dual focus on CRC pathology and circadian biology. The evolution of these keywords reflects the interplay between scientific advancements and clinical priorities. For instance, the emergence of “circadian rhythms” as a prominent keyword since 1999 coincided with foundational discoveries in chronobiology, including the characterization of core clock genes (e.g., CLOCK, BMAL1). High-centrality keywords such as circadian rhythms (centrality 0.38) and chemotherapy (centrality 0.28) emerged as critical connectors between research clusters, emphasizing their integrative roles. The early prominence of “fluorouracil” (5-FU) aligns with its establishment as a chemotherapeutic cornerstone, while the recent emergence of “inflammatory bowel disease” (since 2019) reflects growing recognition of inflammation-driven CRC pathogenesis. Other prominent terms, including metastatic colorectal cancer (90 counts) and quality of life (77 counts), underscore clinical priorities, while night shift work (27 counts) highlights environmental influences on circadian disruption. Notably, the sustained citation burst of “gene expression” (1999–2007) correlates with the rise of high-throughput sequencing technologies, enabling mechanistic exploration of CRC-circadian interactions. Co-occurrence networks (Figures 6a, b) identified 20 thematic clusters, with gene expression (#0), clock genes (#1), and circadian rhythms (#2) representing core areas of focus. Figure 6b specifically illustrates the clustering timeline graph generated by CiteSpace. Recent keyword evolution (2020 onward) has been driven by discoveries linking circadian disruption to tumor microenvironment remodeling and immune evasion. A pivotal study in Science Advances demonstrated that BMAL1 deficiency promotes Apc loss of heterozygosity, activating Wnt/c-Myc signaling and accelerating colorectal tumorigenesis in both murine models and patient-derived organoids (21). These findings have reinvigorated research into circadian-metabolic crosstalk in CRC. Furthermore, (Figure 6c) illustrates that “folinic acid” is the keyword with the longest duration of prominence in the analysis, emerging in 1999 and persisting until 2007, indicating its sustained relevance. Inflammation-related keywords and clusters are also noteworthy in this analysis. Keywords such as “inflammation” and “inflammatory bowel disease” have shown significant citation bursts, indicating a growing interest in the role of inflammation in CRC research. The keyword “inflammation” has a citation burst strength of 4.46, beginning in 2014 and ending in 2021, reflecting its increasing importance in the field. Additionally, “inflammatory bowel disease” has a citation burst strength of 3, starting in 2019 and continuing through 2022. These trends suggest that inflammation-related research is a critical and evolving area within CRC studies, highlighting the potential therapeutic targets and mechanisms associated with inflammatory processes in cancer development and progression. The keywords in this study serve as indicators of the main themes and research focuses within the field of circadian rhythms and CRC. Their temporal evolution reveals two key drivers: 1) technological innovations (e.g., sequencing platforms enabling circadian transcriptome analysis), and 2) paradigm shifts in mechanistic understanding (e.g., circadian control of DNA repair fidelity). The frequent occurrence of keywords such as “colorectal cancer” and “circadian rhythm” underscores the central role these concepts play in the research. The co-occurrence of keywords provides insight into the complex relationships and interactions between different research areas, highlighting the interdisciplinary nature of the field. The identification of prominent keywords and their duration of prominence helps to trace the evolution of research interests and emerging trends, guiding future research directions.
Table 8
| Rank | Keywords | Count | Centrality |
|---|---|---|---|
| 1 | colorectal cancer | 173 | 0.12 |
| 2 | circadian rhythm | 143 | 0.07 |
| 3 | breast cancer | 108 | 0.04 |
| 4 | metastatic colorectal cancer | 90 | 0.05 |
| 5 | circadian rhythms | 80 | 0.38 |
| 6 | quality of life | 77 | 0.05 |
| 7 | chemotherapy | 43 | 0.28 |
| 8 | circadian clock | 39 | 0.1 |
| 9 | folinic acid | 37 | 0.16 |
| 10 | colon cancer | 34 | 0.23 |
| 11 | clock | 30 | 0.17 |
| 12 | risk | 29 | 0.03 |
| 13 | survival | 28 | 0.04 |
| 14 | gene expression | 28 | 0.12 |
| 15 | night shift work | 27 | 0.16 |
| 16 | expression | 25 | 0.03 |
| 17 | 5 fluorouracil | 25 | 0.08 |
| 18 | oxaliplatin | 23 | 0.01 |
| 19 | fluorouracil | 23 | 0.06 |
| 20 | shift work | 21 | 0.04 |
Top 20 active keywords.
Figure 6
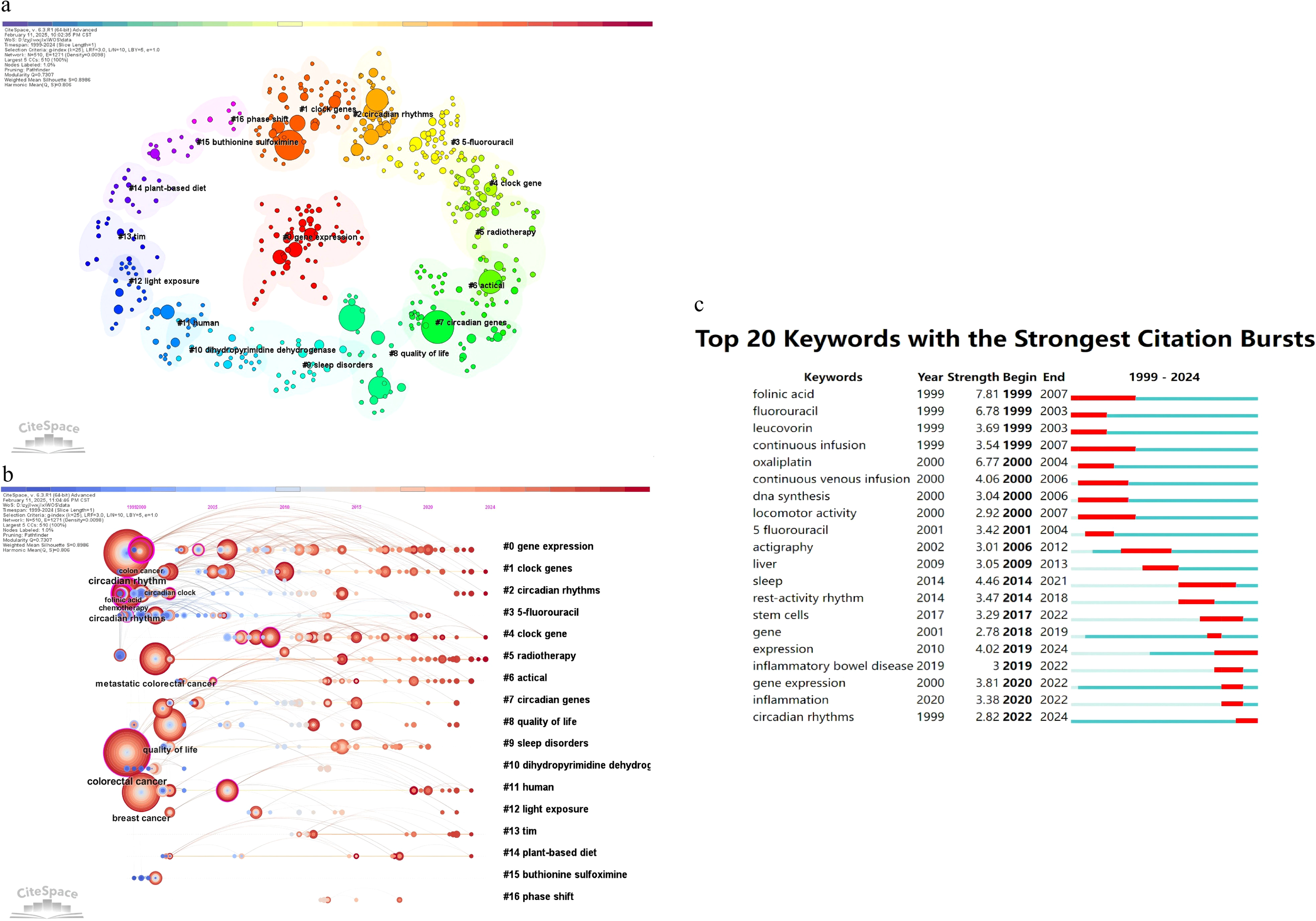
(a) Illustrate the keywords clustering map; (b) the clustering timeline graph; (c) Displays the top 25 keywords with strongest citation bursts.
4 Discussion
4.1 General information
The number of publications from 1999 to 2024 shows how the body of research connecting problems with circadian rhythms to the development of colorectal cancer (CRC) has changed over time. The gradual rise in publications from 1999 to 2009 coincided with seminal discoveries in circadian biology, notably the identification of core clock genes such as CLOCK, BMAL1, and PER, and their roles in cellular timekeeping. From 2010 to 2015, the field saw steady growth and important scientific discoveries. The marked escalation from 2016 to 2021 reflected two pivotal developments: First, large-scale genomic analyses found that circadian genes like PER1, PER3, and NPAS2 were frequently changed and out of whack in CRC (22, 23). Second, discoveries of molecular circadian mechanisms galvanized interdisciplinary research into clock-cancer crosstalk. While publications declined slightly post-2021, sustained elevation compared to earlier decades underscores persistent interest in circadian-targeted therapies (24, 25). Recent studies have shown that gut microbiota and circadian rhythms work together to affect CRC metastasis, mainly by changing myeloid-derived suppressor cells (26, 27). These cells play a pivotal role in shaping an immunosuppressive tumor microenvironment that facilitates cancer progression in a time-dependent manner.
In parallel, single-cell sequencing techniques have revealed that the circadian context within CRC tumors varies across different cell types (28). This difference between cell types indicates that problems with the circadian rhythm might affect the epithelial, stromal, and immune parts of the tumor in different ways. These findings indicate a change in the field, moving from just observing connections to exploring the detailed mechanisms and practical applications of how circadian rhythms interact with tumors.
The global research landscape of circadian rhythm and CRC demonstrates a complex interplay of productivity, collaboration, and scholarly influence. Bibliometric data from 49 countries and 674 institutions reveal distinct geopolitical and institutional hierarchies. The United States dominates in both output (122 publications) and academic impact, evidenced by its highest H-index (46) and average citations per publication (58.17), reflecting its entrenched role as a global scientific leader supported by interdisciplinary ecosystems and sustained funding. France comes in second with 76 publications and an H-index of 37. This is thanks to centralized institutions like Assistance Publique–Hôpitaux de Paris (APHP) and Inserm, which are responsible for over 40% of the top-ranked institutional outputs. This supports a national strategy that combines clinical and molecular research to speed up the translational outcomes. China, ranking third in volume (49 publications), faces a notable disparity in citation metrics (average 30.22 citations), potentially linked to linguistic isolation, methodological conservatism, or regional publication preferences.
To better understand how working together internationally and between institutions helps improve research on circadian rhythm and CRC, we need to look beyond just counting co-authors and examine the different types, motivations, and challenges of these collaborations. Several key factors have driven successful collaborations in this field. First, centralized funding mechanisms—such as Horizon 2020 in Europe and U54 consortia in the United States—have provided long-term, interdisciplinary financial support. Second, open-access platforms and genomic databases, including TCGA and the European Genome-phenome Archive (EGA), have fostered data transparency and reuse across institutions and borders.
Nevertheless, significant barriers remain. These include differences in regulatory frameworks (e.g., General Data Protection Regulation in the EU versus more lenient models elsewhere), linguistic and cultural gaps, and disparities in research capacity. Notably, collaboration with low- and middle-income countries (LMICs) remains sparse, despite the rising burden of CRC in regions such as South Asia and sub-Saharan Africa. These asymmetries hinder global equity and limit the generalizability of circadian-based interventions across populations. To optimize future collaboration models, three strategies are proposed. First, establishing regional chronomedicine hubs could enhance local-global synergy and build sustainable infrastructures in underrepresented areas. Second, adopting co-ownership models for data and outcomes may reduce epistemic dependency and promote balanced participation. Third, integrating implementation science within research consortia can bridge the gap between mechanistic findings and clinical application, ensuring that discoveries are embedded within real-world healthcare systems. In sum, collaboration in circadian rhythm and CRC research has evolved from opportunistic alliances into structured, impact-oriented networks. Continued investment in equitable, interdisciplinary, and implementation-focused collaborations will be essential for translating circadian science into global cancer control strategies.
Journals show this duality even more: Cancer Research (Impact Factor 12.5) has the most citations (120.8 per article) because it publishes mechanistic breakthroughs, while Chronobiology International has the most output (32 articles) because it publishes niche epidemiological studies. However, relying too much on impact factors could make the “Matthew Effect” worse. This is when bigger journals get more attention than smaller ones, like Integrative Cancer Therapies, which gets a lot of citations (71.83 per article) through patient-centered chronotherapy trials. Author productivity highlights Francis Lévi as a linchpin, with 34 publications and 231 co-citations cementing his role in advancing chronomodulated therapies.
Keyword analysis and co-citation clustering reveal an interconnected framework spanning molecular mechanisms, novel therapeutics, and environmental influences. Twenty thematic clusters were identified, covering topics such as “intestinal stem cells,” “β-catenin pathway,” “light pollution,” and “personalized treatment.” These clusters underscore the interdisciplinary nature of CRC-circadian research, integrating oncology, chronobiology, pharmacology, and environmental health.
Researchers are becoming more aware that inflammation can cause and worsen circadian disruption in CRC (29). Keyword co-occurrence and citation burst analyses support this idea. The prominence of “inflammation” (burst strength 4.46, 2014–2021) and “inflammatory bowel disease” (burst strength 3.0, 2019–2022) highlights a paradigm shift linking chronic inflammation to CRC pathogenesis. Notably, shift workers’ problems with their circadian rhythms have been shown to make their intestinal barrier function worse (30, 31). This lets microbiota-driven inflammation turn on oncogenic pathways like NF-κB and STAT3, which are controlled by BMAL1 and PER2 (32, 33). This creates a feedback loop in which inflammation disrupts circadian homeostasis, fueling tumorigenic microenvironments.
The fact that “circadian rhythms” (0.38) are more important than “inflammation” (0.12) suggests that clock gene manipulation is still the main focus of circadian research. Also, the lack of terms like “circadian metabolomics” or “immunochronotherapy” shows missed chances to study how the tryptophan-kynurenine axis and other immunometabolic pathways are controlled by time. For more research, we need to use a systems chronobiology approach that combines real-time measurements of lifestyle-related inflammation with single-cell sequencing of circadian-immune interactions in CRC biopsies.
These studies indicate that, despite significant advancements in research depth and breadth, notable gaps persist. Preclinical models, such as rat models and organoid assays, emphasize the regulation of genetic and environmental variables. On the other hand, clinical studies use basic circadian rhythm monitoring methods, like self-reported sleep logs, instead of real-time biomarker tracking and have a wide range of patients. As a result, the translation of these studies into beneficial outcomes for CRC patients is inconsistent.
To fill these gaps, we need to combine multi-omics approaches (e.g., spatial transcriptomics and metabolomics) with ecological transient assessment. To better untangle the “gene-environment-time” relationships that generate colorectal cancer heterogeneity (34). Meanwhile, modifying the way clinical trials are set up to include circadian biomarkers and adaptive temporal therapy algorithms could hasten the change from population-based treatment plans to tailored programs that take rhythms into consideration.
4.2 Future perspectives of chronotherapy in colorectal cancer: harnessing circadian biology for precision oncology
The integration of chronotherapy—timed interventions aligned with circadian rhythms—into CRC management represents a transformative shift in oncology, with the potential to optimize therapeutic efficacy while minimizing adverse effects. Accumulating evidence highlights the critical role of circadian oscillations in regulating tumorigenesis, drug metabolism, and immune surveillance, providing actionable targets for precision oncology (26, 35). This circadian-targeted paradigm is crystallizing along three axes of innovation: molecular chrono-reprogramming, immune timing precision, and AI-driven temporal engineering.
Building on this foundation, metabolic chrono-interventions are emerging as a first frontier. Circadian regulation of cellular metabolism and hormone signaling offers novel avenues for metabolic interventions in CRC. Timed administration of glycolytic inhibitors or mitochondrial modulators can exploit tumor metabolic vulnerabilities that fluctuate across circadian cycles (36–38). This metabolic chrono-vulnerability directly informs molecular targeting strategies for clock gene networks.
Parallel advances are redefining immunotherapy through circadian synchronization. The circadian regulation of immune checkpoint molecules (e.g., PD-L1) and T-cell trafficking necessitates a re-evaluation of immunotherapy schedules. Preclinical studies suggest that administering anti-PD-1/PD-L1 antibodies during peak T-cell receptor signaling enhances antitumor responses (39, 40). Circadian-coordinated cytokine therapy, such as timed IL-12 or IFN-γ administration, could generate synergistic “immune waves” that dismantle immunosuppressive niches in metastatic CRC (41). These findings establish immune timing as the second pillar of modern chronotherapy.
Meanwhile, restoring circadian homeostasis in CRC Targeting core clock genes (e.g., CRY, PER, BMAL1) via CRISPR-based epigenetic modifiers or small-molecule clock modulators (e.g., KL001 analogs) presents a promising approach to restoring circadian transcription in CRC (42, 43). Phase I trials of REV-ERB agonists, which regulate lipid metabolism and cell cycle progression, have demonstrated potential in CRC models with disrupted clock gene function (44). Additionally, synthetic biology approaches—such as engineered circadian circuits to drive tumor-suppressive gene expression in a phase-dependent manner—are advancing toward clinical translation (45). These molecular tools now converge with intelligent chronosystem design.
The final dimension unfolds through AI-powered temporal architecture. Emerging platforms integrating real-time circadian monitoring through wearable biosensors and single-cell transcriptomics, combined with AI-driven tumor clock modeling, could personalize infusion schedules (46, 47). Machine learning models trained on multi-omics circadian datasets may soon enable dynamic adjustments to FOLFOX regimens, maximizing efficacy while minimizing toxicity, particularly in microsatellite-stable CRC subtypes (48). This triad of innovation—molecular reprogramming, immune timing, and algorithmic optimization—collectively redefines therapeutic precision.
The future of CRC chronotherapy lies in transcending empirical timing strategies and embracing systems-level circadian engineering. Three transformative vectors now anchor this evolution (1): CRISPR-clock gene editing and synthetic circadian circuits (42–45) (2); Checkpoint inhibitor/cytokine synchronization with immune circadian dynamics (39–41) (3); AI-chronosystems integrating wearable biosensors and single-cell omics (46–48). By dissecting the intricate interplay between clock networks, tumor ecosystems, and therapeutic agents, researchers can unlock precision chrono-combinations that transform CRC into a chronobiologically manageable disease. Clinically, this necessitates biomarker-driven trials, the integration of circadian-informed algorithms into treatment planning, and the development of smart chronotechnology platforms. Advancing these strategies will position chronotherapy at the forefront of 21st-century oncology, redefining how CRC is treated in an era of precision medicine.
5 Conclusions
The integration of circadian biology into colorectal cancer (CRC) research has provided profound insights into the temporal regulation of oncogenesis, therapy resistance, and immune evasion. Over the past 25 years, bibliometric analysis of 374 publications has revealed an exponential growth in this field post-2016, driven by seminal discoveries linking circadian disruption to CRC risk and therapeutic outcomes. Key findings highlight the pivotal role of core clock genes (BMAL1, PER2) in mechanistic studies, with PER2 silencing observed in 45% of tumors, correlating with advanced disease stages and poor prognosis. Conversely, environmental disruptors such as night-shift work—an established proxy for chronic circadian misalignment—are associated with a 20–30% increase in CRC incidence, underscoring the necessity of contextualizing molecular findings within real-world behavioral and ecological frameworks.
Clinically, chronomodulated chemotherapy has demonstrated significant benefits, reducing severe toxicity by 40% in metastatic CRC. However, the slow integration of circadian principles into routine oncology practice reflects persistent systemic barriers, including inconsistent biomarker validation, the absence of scalable circadian monitoring tools, and regional disparities in research priorities. While Western nations continue to lead in molecular chronobiology, Asia’s emphasis on clinical epidemiology and Europe’s translational expertise—exemplified by Assistance Publique–Hôpitaux de Paris (APHP) trials—highlight the untapped potential for global collaboration to harmonize circadian research and its applications in cancer treatment.
The growing prominence of “inflammation” in keyword analyses signals a paradigm shift toward understanding circadian-immune crosstalk. However, the relatively weak centrality of inflammation-related terms compared to core clock genes exposes a critical imbalance—mechanistic studies frequently overlook how socioeconomic factors (e.g., urban light pollution, irregular meal timing) interact with genetic susceptibilities to drive CRC pathogenesis.
Future research must prioritize three strategic directions (1): AI-driven circadian profiling to decode patient-specific rhythms for personalized intervention (2), multi-omics integration (e.g., spatial transcriptomics, metabolomics) to unravel the intricate circadian-immune-metabolic interplay, and (3) equitable global clinical trials that address circadian disparities in underrepresented populations. By bridging these gaps, circadian biology can transition from a specialized research focus to a foundational pillar of precision oncology, offering spatiotemporally optimized strategies to mitigate the global CRC burden and improve patient outcomes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LC: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. NX: Investigation, Methodology, Writing – original draft, Writing – review & editing. JL: Investigation, Software, Writing – review & editing. YT: Investigation, Writing – review & editing. SZ: Supervision, Project administration, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Research projects of traditional Chinese medicine of Hunan 644 Province (No. A2023043); Hunan Provincial Health and Family Planning Commission Research 645 Project (No. 20200424); Hunan Provincial Clinical Medical Research Center Project (No. 646 2023SK4048); Hunan Provincial Chinese Medicine Discipline Leaders Funding Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SIRT5, Sirtuin 5, an NAD^+-dependent protein deacetylase; GLUD1, Glutamate Dehydrogenase 1; TCA, Tricarboxylic Acid Cycle; MTA1, Metastasis-Associated Protein 1; OXPHOS, Oxidative Phosphorylation; Skp2, S-phase kinase-associated protein 2; IDH1, Isocitrate Dehydrogenase 1; ETC, Electron Transport Chain; VEGF, Vascular Endothelial Growth Factor; NOX4, NADPH oxidase 4; 8-OH-dG, 8-hydroxy-2’-deoxyguanosine; CoQ10, Coenzyme Q10; SIRT1, Silent mating type information regulation 2 homolog-1; PGC-1α, Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha; NRF2, Nuclear Factor Erythroid 2-Related Factor 2; LPA3, Lysophosphatidic Acid Receptor 3; NIX, Nip-like protein X; BNIP3, BCL2/adenovirus E1B 19kDa interacting protein 3; FUNDC1, FUN14 domain containing 1; LC3, Microtubule-associated protein 1 light chain 3; CRISPR/Cas9, Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9;
References
1
Gerhart-Hines Z Lazar MA . Circadian metabolism in the light of evolution. Endocrine Rev. (2015) 36:289–304. doi: 10.1210/er.2015-1007
2
Fagiani F Di Marino D Romagnoli A Travelli C Voltan D Di Cesare Mannelli L et al . Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal transduction targeted Ther. (2022) 7:41. doi: 10.1038/s41392-022-00899-y
3
Quist M van Os M van Laake LW Bovenschen N Crnko S . Integration of circadian rhythms and immunotherapy for enhanced precision in brain cancer treatment. EBioMedicine. (2024) 109:1–14. doi: 10.1016/j.ebiom.2024.105395
4
Levi FA Okyar A Hadadi E Innominato PF Ballesta A . Circadian regulation of drug responses: toward sex-specific and personalized chronotherapy. Annu Rev Pharmacol Toxicol. (2024) 64:89–114. doi: 10.1146/annurev-pharmtox-051920-095416
5
Ector C Schmal C Didier J De Landtsheer S Finger AM Muller-Marquardt F et al . Time-of-day effects of cancer drugs revealed by high-throughput deep phenotyping. Nat Commun. (2024) 15:7205. doi: 10.1038/s41467-024-51611-3
6
Karin J Bornfeld Y Nitzan M . scPrisma infers, filters and enhances topological signals in single-cell data using spectral template matching. Nat Biotechnol. (2023) 41:1645–54. doi: 10.1038/s41587-023-01663-5
7
Gil-Martín E Egea J Reiter RJ Romero A . The emergence of melatonin in oncology: Focus on colorectal cancer. Medicinal Res Rev. (2019) 39:2239–85. doi: 10.1002/med.21582
8
Zhou L Zhang Z Nice E Huang C Zhang W Tang Y . Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Hematol oncology. (2022) 15:21. doi: 10.1186/s13045-022-01238-y
9
Murphy CC Zaki TA . Changing epidemiology of colorectal cancer - birth cohort effects and emerging risk factors. Nat Rev Gastroenterol Hepatol. (2024) 21:25–34. doi: 10.1038/s41575-023-00841-9
10
Aggarwal S Lavingiya V Krishna V Chitalkar P Ostwal V Parikh PM . Young onset colorectal cancer. South Asian J Cancer. (2024) 13(2):225–8. doi: 10.4103/SAJC-2024-001
11
Santucci C Mignozzi S Malvezzi M Boffetta P Collatuzzo G Levi F et al . European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann Oncol. (2024) 35:308–16. doi: 10.1016/j.annonc.2023.12.003
12
Gross SM Mohammadi F Sanchez-Aguila C Zhan PJ Liby TA Dane MA et al . Analysis and modeling of cancer drug responses using cell cycle phase-specific rate effects. Nat Commun. (2023) 14:3450. doi: 10.1038/s41467-023-39122-z
13
Cash E Sephton S Woolley C Elbehi AM Ri A Ekine-Afolabi B et al . The role of the circadian clock in cancer hallmark acquisition and immune-based cancer therapeutics. J Exp Clin Cancer Res. (2021) 40:1–14. doi: 10.1186/s13046-021-01919-5
14
Ortega-Campos SM Verdugo-Sivianes EM Amiama-Roig A Blanco JR Carnero A . Interactions of circadian clock genes with the hallmarks of cancer. Biochim Biophys Acta (BBA)-Reviews Cancer. (2023) 1878:188900. doi: 10.1016/j.bbcan.2023.188900
15
Palakurthi B Fross SR Guldner IH Aleksandrovic E Liu X Martino AK et al . Targeting CXCL16 and STAT1 augments immune checkpoint blockade therapy in triple-negative breast cancer. Nat Commun. (2023) 14:2109. doi: 10.1038/s41467-023-37727-y
16
Fraunhoffer N Hammel P Conroy T Nicolle R Bachet JB Harle A et al . Development and validation of AI-assisted transcriptomic signatures to personalize adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann Oncol. (2024) 35:780–91. doi: 10.1016/j.annonc.2024.06.010
17
Lévi F Focan C Karaboué A de la Valette V Focan-Henrard D Baron B et al . Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Advanced Drug delivery Rev. (2007) 59:1015–35. doi: 10.1016/j.addr.2006.11.001
18
Zhang L Chen Y Chong CS Ma X Tong S He X et al . The genomic and transcriptomic landscapes of clock genes reveal the significance of circadian rhythm in the progression and immune microenvironment of metastatic colorectal cancer. Clin Trans medicine. (2022) 12:e755. doi: 10.1002/ctm2.v12.3
19
Feng D Xiong Q Zhang F Shi X Xu H Wei W et al . Identification of a novel nomogram to predict progression based on the circadian clock and insights into the tumor immune microenvironment in prostate cancer. Front Immunol. (2022) 13:777724. doi: 10.3389/fimmu.2022.777724
20
Xiao N Huang X Zang W Kiselev S Bolkov MA Tuzankina IA et al . Health-related quality of life in patients with inborn errors of immunity: a bibliometric analysis. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1371124
21
Chun SK Fortin BM Fellows RC Habowski AN Verlande A Song WA et al . Disruption of the circadian clock drives Apc loss of heterozygosity to accelerate colorectal cancer. Sci Adv. (2022) 8:eabo2389. doi: 10.1126/sciadv.abo2389
22
Yu C-C Chen L-C Chiou C-Y Chang Y-J Lin VC Huang C-Y et al . Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. (2019) 19:1–9. doi: 10.1186/s12935-019-0811-4
23
Deng F Yang K . Current status of research on the period family of clock genes in the occurrence and development of cancer. J Cancer. (2019) 10:1117. doi: 10.7150/jca.29212
24
Fishbein AB Knutson KL Zee PC . Circadian disruption and human health. J Clin Invest. (2021) 131(19):1–12. doi: 10.1172/JCI148286
25
Benkli B Kim SY Koike N Han C Tran CK Silva E et al . Circadian features of cluster headache and migraine: a systematic review, meta-analysis, and genetic analysis. Neurology. (2023) 100:e2224–e36. doi: 10.1212/WNL.0000000000207240
26
Liu J-L Xu X Rixiati Y Wang C-Y Ni H-L Chen W-S et al . Dysfunctional circadian clock accelerates cancer metastasis by intestinal microbiota triggering accumulation of myeloid-derived suppressor cells. Cell Metab. (2024) 36:1320–34. e9. doi: 10.1016/j.cmet.2024.04.019
27
Liu J Shao N Qiu H Zhao J Chen C Wan J et al . Intestinal microbiota: A bridge between intermittent fasting and tumors. Biomedicine Pharmacotherapy. (2023) 167:115484. doi: 10.1016/j.biopha.2023.115484
28
Fortin BM Pfeiffer SM Insua-Rodríguez J Alshetaiwi H Moshensky A Song WA et al . Circadian control of tumor immunosuppression affects efficacy of immune checkpoint blockade. Nat Immunol. (2024) 25:1257–69. doi: 10.1038/s41590-024-01859-0
29
Pagel R Bär F Schröder T Sünderhauf A Künstner A Ibrahim SM et al . Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. (2017) 31:4707. doi: 10.1096/fj.201700141RR
30
Reynolds AC Paterson JL Ferguson SA Stanley D Wright KP Jr. Dawson D . The shift work and health research agenda: considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev. (2017) 34:3–9. doi: 10.1016/j.smrv.2016.06.009
31
Voigt RM Forsyth CB Keshavarzian A . Circadian rhythms: a regulator of gastrointestinal health and dysfunction. Expert Rev gastroenterology hepatology. (2019) 13:411–24. doi: 10.1080/17474124.2019.1595588
32
Pang X Tang Y-J Ren X-H Chen Q-M Tang Y-L Liang X-H . Microbiota, epithelium, inflammation, and TGF-β signaling: an intricate interaction in oncogenesis. Front Microbiology. (2018) 9:1353. doi: 10.3389/fmicb.2018.01353
33
Li C Cai C Wang C Chen X Zhang B Huang Z . Gut microbiota-mediated gut-liver axis: a breakthrough point for understanding and treating liver cancer. Clin Mol Hepatology. (2024) 31(2):350–81. doi: 10.3350/cmh.2024.0857
34
Gonsard A Genet M Drummond D . Digital twins for chronic lung diseases. Eur Respiratory Review. (2024) 33:240159. doi: 10.1183/16000617.0159-2024
35
Wang Y Narasimamurthy R Qu M Shi N Guo H Xue Y et al . Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis. Nat Cancer. (2024) 5:546–56. doi: 10.1038/s43018-024-00759-4
36
Fendt S-M Frezza C Erez A . Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer discovery. (2020) 10:1797–807. doi: 10.1158/2159-8290.CD-20-0844
37
Fuhr L El-Athman R Scrima R Cela O Carbone A Knoop H et al . The circadian clock regulates metabolic phenotype rewiring via HKDC1 and modulates tumor progression and drug response in colorectal cancer. EBioMedicine. (2018) 33:105–21. doi: 10.1016/j.ebiom.2018.07.002
38
Wolpaw AJ Dang CV . Exploiting metabolic vulnerabilities of cancer with precision and accuracy. Trends Cell Biol. (2018) 28:201–12. doi: 10.1016/j.tcb.2017.11.006
39
Wang Y Han J Wang D Cai M Xu Y Hu Y et al . Anti-PD-1 antibody armored γδ T cells enhance anti-tumor efficacy in ovarian cancer. Signal Transduction Targeted Ther. (2023) 8:399. doi: 10.1038/s41392-023-01646-7
40
Li T Niu M Zhou J Wu K Yi M . The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling. Cell Communication Signaling. (2024) 22:179. doi: 10.1186/s12964-024-01562-5
41
Aristin Revilla S Kranenburg O Coffer PJ . Colorectal cancer-infiltrating regulatory T cells: functional heterogeneity, metabolic adaptation, and therapeutic targeting. Front Immunol. (2022) 13:903564. doi: 10.3389/fimmu.2022.903564
42
Zhou Z Zhang R Zhang Y Xu Y Wang R Chen S et al . Circadian disruption in cancer hallmarks: novel insight into the molecular mechanisms of tumorigenesis and cancer treatment. Cancer Lett. (2024) 604:217273. doi: 10.1016/j.canlet.2024.217273
43
Kinouchi K Sassone-Corsi P . Metabolic rivalry: circadian homeostasis and tumorigenesis. Nat Rev Cancer. (2020) 20:645–61. doi: 10.1038/s41568-020-0291-9
44
Wang S Li F Lin Y Wu B . Targeting REV-ERBα for therapeutic purposes: promises and challenges. Theranostics. (2020) 10:4168. doi: 10.7150/thno.43834
45
Jiang W Zhao S Jiang X Zhang E Hu G Hu B et al . The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer letters. (2016) 371:314–25. doi: 10.1016/j.canlet.2015.12.002
46
Harkos C Hadjigeorgiou AG Voutouri C Kumar AS Stylianopoulos T Jain RK . Using mathematical modelling and AI to improve delivery and efficacy of therapies in cancer. Nat Rev Cancer. (2025) 25(5):324–40. doi: 10.1038/s41568-025-00796-w
47
Zhong N-N Wang H-Q Huang X-Y Li Z-Z Cao L-M Huo F-Y et al . Enhancing head and neck tumor management with artificial intelligence: Integration and perspectives. Semin Cancer Biol. (2023) 95:52–74. doi: 10.1016/j.semcancer.2023.07.002
48
Amniouel S Jafri MS . High-accuracy prediction of colorectal cancer chemotherapy efficacy using machine learning applied to gene expression data. Front Physiol. (2024) 14:1272206. doi: 10.3389/fphys.2023.1272206
Summary
Keywords
colorectal cancer, circadian rhythm disruption, tumor microenvironment, colorectal cancer intervention, bibliometric
Citation
Chen L, Wang Z, Xiao N, Liu J, Tao Y and Zhang S (2025) Deciphering the circadian rhythm in colorectal cancer: a bibliometric analysis of research landscape and trends. Front. Oncol. 15:1591257. doi: 10.3389/fonc.2025.1591257
Received
10 March 2025
Accepted
09 May 2025
Published
16 June 2025
Volume
15 - 2025
Edited by
Hongbing Zhang, Tianjin Medical University General Hospital, China
Reviewed by
Yuhao Xie, St. John’s University, United States
Piao Zhao, Sichuan Agricultural University, China
Yanjun Gao, George Washington University, United States
Updates
Copyright
© 2025 Chen, Wang, Xiao, Liu, Tao and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sifang Zhang, sifangzhang2005@csu.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.