- 1College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- 2Research Center, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia
- 3Comparative Medicine Department, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: Extracellular vesicles (EVs) play an integral role in cancer biology, influencing tumor progression, metastasis, and tumor microenvironment. Due to their distinctive molecular composition, including proteins, nucleic acids, and lipids, EVs present a promising candidate for cancer diagnostics and precision therapeutics.
Methods: This review was conducted by looking up recent studies obtained through PubMed, Scopus, and Web of Science databases using targeted keywords such as “Extracellular Vesicles,” “Cancer Therapy,” “Biomarkers,” “Exosomes,” “Tumor Microenvironment,” and “Precision Medicine.” From an initial 4,320 articles identified, 427 were screened after applying publication filters, resulting in the inclusion of 298 articles relevant to EV isolation, characterization, diagnostic sensitivity, specificity, and therapeutic efficacy.
Results: Biomarkers derived from EVs derived across various cancers showed high diagnostic performance. For example, four miRNA EVs showing sensitivity and specificity of 98% and 96% respectively was found in breast cancer. EV-RNA and surface antigen analyses for hepatocellular carcinoma with 93.8% sensitivity and 74.5% specificity. Additionally, EV biomarker cancers of the colorectal microRNA miR-23a and miR-301a had 89% sensitivity and >70% specificity. EVs in a therapeutic context were an effective drug delivery system for enhancing precision of chemotherapy and immunotherapy with reduced systemic toxicity.
Conclusion: The theranostics of EVs provide great capacity for early cancer diagnosis and personalized treatment based on their high diagnostic sensitivity and specificity. Future standardization protocols are essential to translate EV technologies into clinical oncology.
1 Introduction
Cancer is currently the fifth leading cause of mortality (1). This onus is predicted to increase to 35 million cases by 2050, due to reasons such as aging, population growth, and increasing prevalence of risk factors like obesity, tobacco use, and air pollution (1, 2). Despite ameliorations in cancer therapy, critical challenges prevail in financing its management, with only 39% of countries including basic cancer treatment in healthcare coverage and even fewer providing palliative care. This disparity is echoed further in low-income countries, where late diagnoses and limited access to treatment lead to higher mortality rates (1, 3). Extracellular vesicles (EVs), lipid-bilayer structures secreted by most cells, have recently gained attention for their potential in cancer diagnosis and therapy. These vesicles, ranging from 50 nm to 10 µm in size, are categorized into exosomes, microvesicles (MVs), and apoptotic bodies (ApoBDs) based on their size, biogenesis, and contents (4–6). Their ability to carry diverse cargo, including genetic, protein, and lipid material, makes them versatile tools for intercellular communication and disease spread (7, 8). Exosomes, the smallest subtype, are notable for their ability to cross the blood-brain barrier, enabling targeted drug delivery for neurological diseases and inflammation (5, 6). Likewise, MVs and ApoBDs play important roles in cellular communication and tumor progression, though further research is needed to fully explicate their roles (9–11). In the context of cancer, EVs have been shown to have a significant function in tumor progression and communication within the tumor microenvironment (TME). Cancer-derived EVs carry a diverse number of nucleic acids, proteins, and lipids, such as microRNAs, mutated epidermal growth factor receptors (EGFR), and vascular endothelial growth factors (VEGF), that facilitate intercellular signaling, immune modulation, and the advancement of aggressive phenotypes (12–14). Furthermore, EVs contribute to the horizontal transfer of oncogenic traits and mitigate processes like epithelial-mesenchymal transition (EMT), exacerbating cancer cell invasiveness and metastasis (15). These special characteristics make EVs central components of both cancer pathogenesis and targets for therapeutic intervention. The aim of this article is to provide a comprehensive review of the most recent data regarding the use of EVs in the diagnostic and therapeutic aspects of cancer as well as their clinical applications.
2 Methodology
This literature review of current trends in the theranostic applications of EVs in cancer, using articles from PubMed, Scopus, and Web of Science databases. Using keywords including “Extracellular Vesicles,” “Cancer Therapy,” “Biomarkers,” “Exosomes,” “Tumor Microenvironment,” and “Precision Medicine,” yielded approximately 4,320 articles. After applying filters to retain only English-language, systematic reviews, meta-analyses, reviews, and randomized clinical trials published in the last ten years, the number of articles was narrowed down to 427. Following an independent review of the titles, abstracts, and full texts for relevance and alignment with the review’s objective, 298 articles were ultimately included as references in this paper. Primary areas examined included methods of EV isolation and characterization, diagnostic potential (with emphasis on sensitivity and specificity), therapeutic utility of EV-based approaches, and advancements in digital imaging and AI-supported techniques for EV analysis. The table highlighted in future perspectives was aimed at analyzing clinical trials on the clinical applications of extracellular vesicles in cancer. The table was done based on active trials found on clinicaltrials.gov as of January 14th, 2025. The search included keywords like “exosomes,” extracellular vesicles,” and “cancer.” The search excluded studies not related to cancer, and non-diagnostic or therapeutic applications, yielding a total of 58 clinical trials. The table was made to identify the cancer type and subtype, the clinical use including a description of the use, and trial status with NCT identifiers.
3 Biogenesis of extracellular vesicles
EVs are classified into a variety of subtypes based on their size, origin, and function. These subtypes include microvesicles (MVs) (0.1–0.35 µm), apoptotic bodies (0.8–5 µm), and small EVs (50–120 nm) (16, 17). Whereas MVs are the result of the plasma membrane’s outward budding (18), apoptotic bodies are formed during programmed cell death (19). In contrast, exosomes are nanoscale vesicles that are produced from multivesicular bodies (MVBs) within late endosomes as shown in Figure 1 (21).
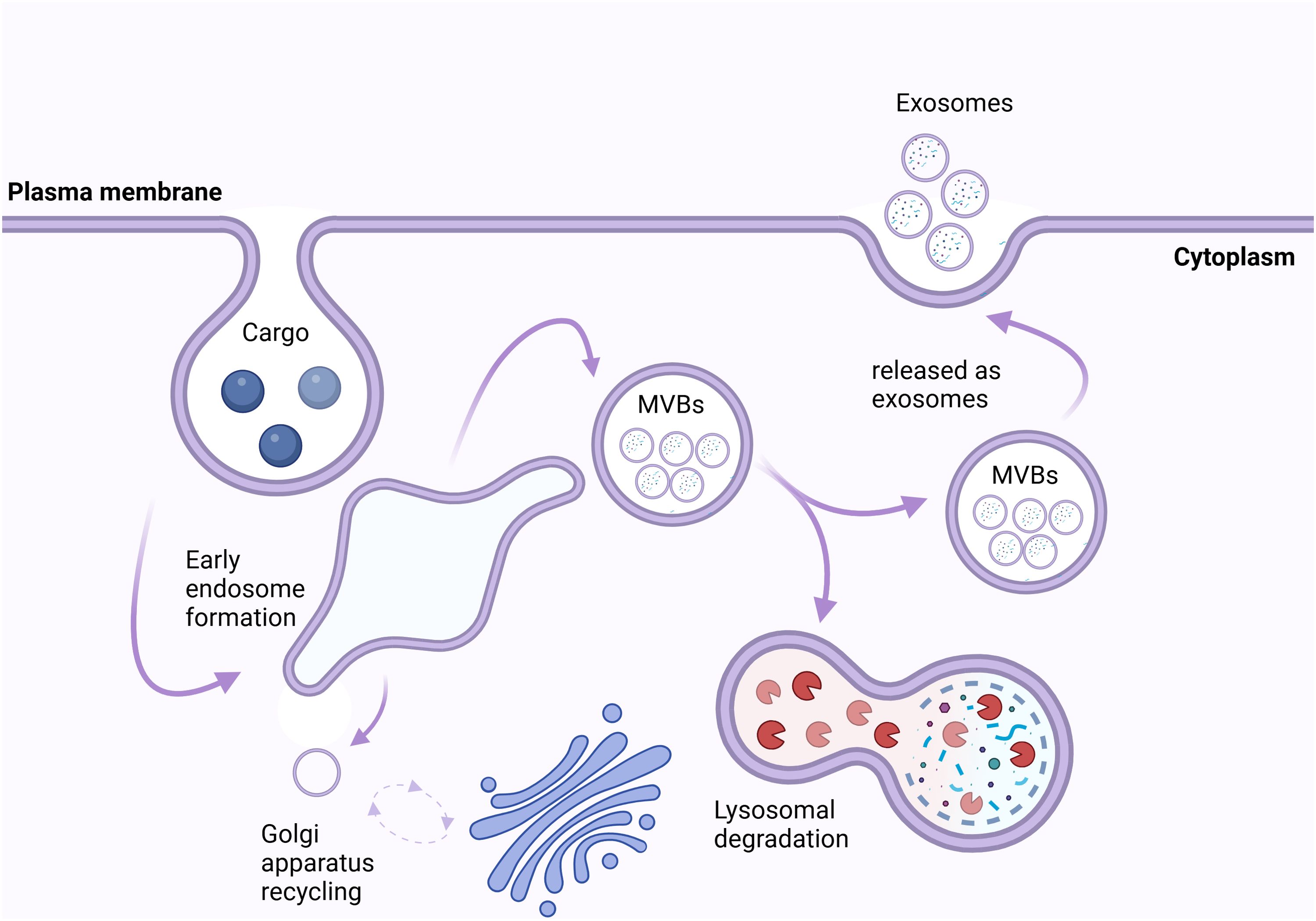
Figure 1. Biogenesis and Fate of Exosomes. The intracellular trafficking and fate of exosomes begins at the plasma membrane, where cargo (e.g., proteins, lipids, nucleic acids) is internalized via endocytosis, forming early endosomes. These early endosomes mature into multivesicular bodies (MVBs) containing intraluminal vesicles. MVBs have two potential fates: they can fuse with lysosomes, leading to lysosomal degradation, or they can fuse with the plasma membrane, resulting in the release of exosomes into the extracellular space. Additionally, vesicular trafficking is regulated by the Golgi apparatus, which recycles membrane components to maintain cellular homeostasis. Exosomes serve as critical mediators of cell-cell communication by transferring their cargo to recipient cells (20).
3.1 Exosomes biogenesis
When endocytosis occurs, early endosomes are created that can recycle cargo, break it down via lysosomes, or grow into MVBs with intraluminal vesicles (ILVs) that release exosomes when they fuse with the plasma membrane (Figure 1) (22, 23). Endosomal trafficking involves changes in endosomal composition, such as replacing sphingomyelin with ceramide and Rab5 with Rab11, promoting downstream transport and arrangement (22).
3.1.1 ESCRT-dependent pathway
MVB maturation, ILV formation, and cargo recognition are all regulated by the endosomal sorting complex required for transport (ESCRT). ESCRT-0 (Vps27/Hrs.) clusters ubiquitinated cargo, ESCRT-I (Vps23/TSG101) and ESCRT-II (Vps36/EAP45) encourage vesicle budding, and ESCRT-III (SNF7/CHMP4) causes ILV scission, which is completed by ATPase Vps4, whereas (22, 24). Exosome production is enhanced by other regulators, such as the syndecan-syntenin-ALIX pathway, especially in viral pathogenesis and cancer. Epstein-Barr Virus (EBV) uses this mechanism to load latent membrane protein 1 (LMP1) into exosomes, facilitating immune evasion, while oncogenic SRC kinase activates it in breast cancer cell lines like MCF7 cells (25–28). Additionally, Charcot-Marie-Tooth disease type 1C is caused by mutations in the SIMPLE protein that interfere with MVB formation (22, 29).
3.1.2 ESCRT-independent pathway
Other pathways include Rab31, which improves the exosomal packaging of the epidermal growth factor receptor (EGFR) in cancer cells, and Ceramide, which promotes ILV budding (26, 30, 31). Moreover, tetraspanins (CD63, CD81, and CD9) aid in exosome release and cargo sorting, and CD63 controls PMEL loading in melanocytes (26, 32).
3.2 Cargo sorting and release
Proteins, lipids, and nucleic acids must all be drawn in via different endosomal sorting processes prior to the production of inward vesicles. For instance, Monoubiquitination is used in cargo sorting to direct proteins into ILVs via ESCRT-0, and deubiquitination takes place prior to exosomal release (22). In melanoma, phosphorylated Vps27/Hrs. promotes the loading of PD-L1 into exosomes, preventing T-cell migration and causing resistance to anti-PD-1 therapy (22, 33). Although the exact mechanisms are still unknown, RNA-binding proteins (RBPs) also mediate RNA inclusion in exosomes (34).
The release of exosomes is dependent on Rab GTPases and SNARE proteins. V-SNAREs (VAMP7, VAMP8) mediate fusion by interacting with t-SNAREs (syntaxins, SNAPs) (22, 34). Hepatitis C transmission via exosomes, for instance, is influenced by syntaxin 4, whereas vesicle docking is controlled by Rab27a/b and Rab35. In cancer and neurodegeneration, exosome secretion is modulated by changes such phosphorylation and O-GlcNAcylation (23). The therapeutic potential of microRNAs (like miR-134 and miR-135b) and long non-coding RNAs (such PVT-1 and HOTAIR) is highlighted by their influence on exosome dynamics (22). These mechanisms highlight the intricate control of exosome release and its relevance in disease contexts.
3.3 Exosome cargo uptake
Through membrane fusion or endocytosis, EV cargo directly affects recipient cells, in contrast to receptor-mediated endocytosis. Tetraspanins, proteoglycans, and lectins are examples of surface proteins that help with targeting (Figure 2). The therapeutic importance of exosomal ligands PD-L1, TNF, FasL, and TRAIL in cancer comes from their interactions with tumor receptors (22, 26). These findings about the synthesis, sorting, and uptake of exosome emphasize their importance in both health and illness, offering prospects for therapeutic intervention.
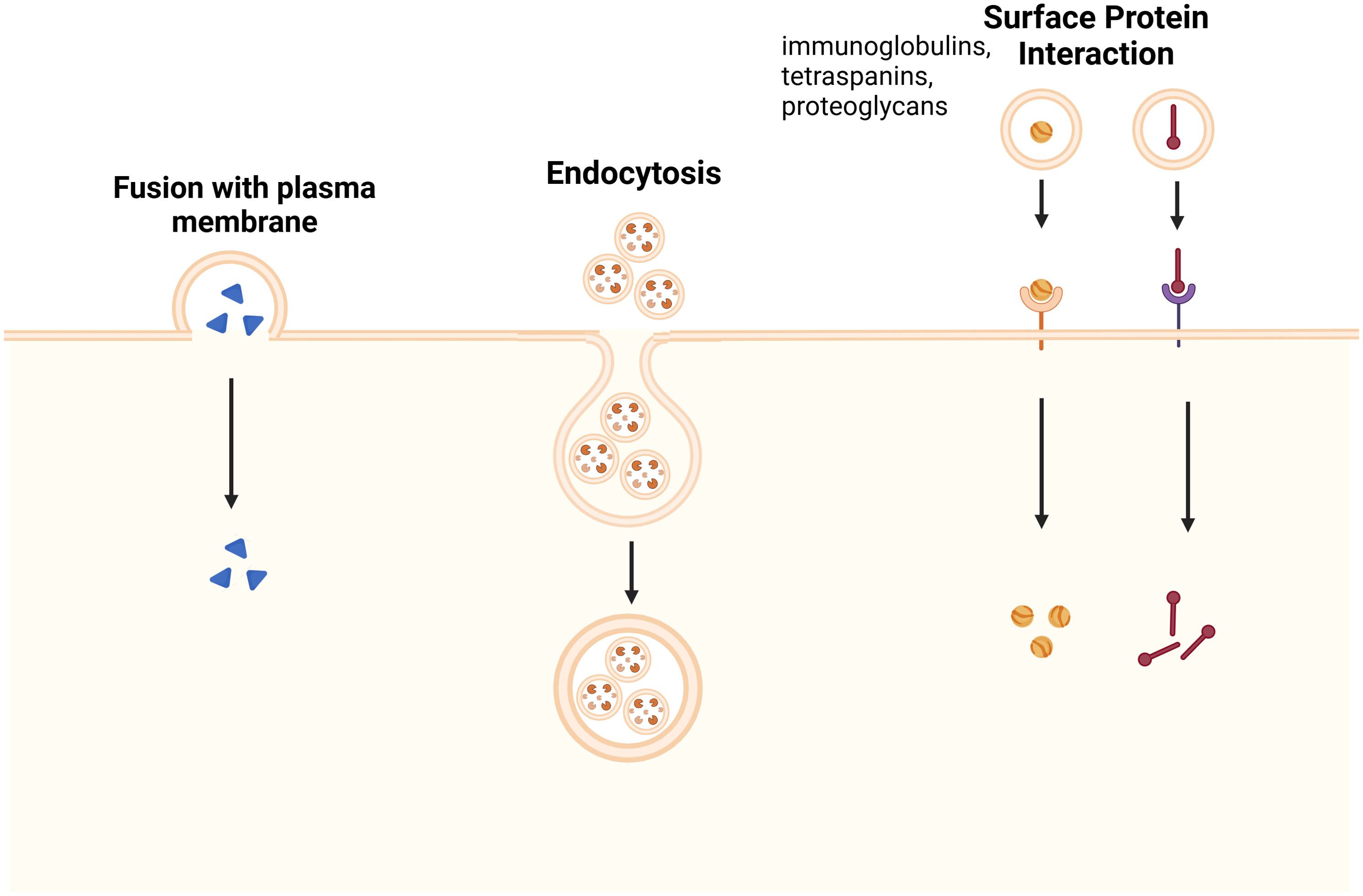
Figure 2. Mechanisms of Exosome Uptake by Recipient Cells. Exosome uptake occurs through three main pathways: fusion with the plasma membrane, where exosomes directly merge with the recipient cell’s membrane, releasing their cargo into the cytoplasm; endocytosis, in which exosomes are internalized via clathrin-mediated endocytosis, macropinocytosis, or caveolin-dependent uptake, leading to intracellular signaling; and surface protein interaction, where exosomal surface proteins, such as immunoglobulins, tetraspanins, and proteoglycans, engage with specific receptors on the recipient cell membrane, triggering signaling cascades or receptor-mediated endocytosis. These uptake mechanisms play essential roles in various physiological and pathological processes, including immune modulation, tissue regeneration, and disease progression (35).
4 Role of extracellular vesicles in tumor progression and metastasis
Because exosomes are vital for the crosstalk between cells either in the healthy or diseased state, it is crucial to examine their contribution in cancer progression (36). The released exosomes into the extracellular space by exocytosis carry host cell-specific cargo from the host cell that are internalized by recipient cells through endocytosis (37). Within the TME, this promotes interactions between TME cells and cancer stem cells (CSCs) (38). CSCs are subpopulations of cancer cells that have the capacity to self-renew and differentiate into many lineages promoting tumor development and heterogeneity (39). While conventional treatments focus on the tumor’s mass, the resilience of CSCs causes spread and recurrence (36).
Exosomes derived from CSCs can increase tumor aggressiveness by altering the TME, transporting bioactive chemicals to surrounding cells, and promoting tumor development and metastasis (40, 41). Within the TME, HCC-derived exosomes, for example, have been shown to transport tumor-promoting miRNAs like miR-21 and long non-coding RNAs (lncRNAs) like TUC339 that promote cell proliferation while inhibiting tumor-suppressive signaling pathways (36). Additionally, CSC-derived exosomes promote epithelial-mesenchymal transition (EMT) by upregulating TGFβ1, a key regulator of metastasis, and proteases such as matrix metalloproteinase-9 (MMP9) enhancing ECM degradation (36). These mechanisms collectively facilitate tumor cell invasion, dissemination, and the formation of pre-metastatic niches.
EVs released from cancer cells contain heat shock proteins (HSPs) such as HSP70 and HSP90 that maintain protein stability and prevent apoptosis under radiation or chemotherapy-induced damage (41). Therefore, EVs containing HSPs can enhance tumor cell motility, invasiveness, and metastasis, making EV-mediated communication a potential therapeutic strategy to overcome treatment resistance and improve patient outcomes (41).
EVs in the tumor microenvironment are crucial for cancer cell metabolic reprogramming, influencing angiogenesis and immune modulation (40). They also contribute to the remodeling of the extracellular matrix, facilitating cancer cell invasion and dissemination (42). Understanding these interactions can lead to targeted therapies to inhibit tumor progression and metastasis, thereby offering novel strategies for cancer treatment (43).
5 Methods of extracellular vesicles concentration and isolation
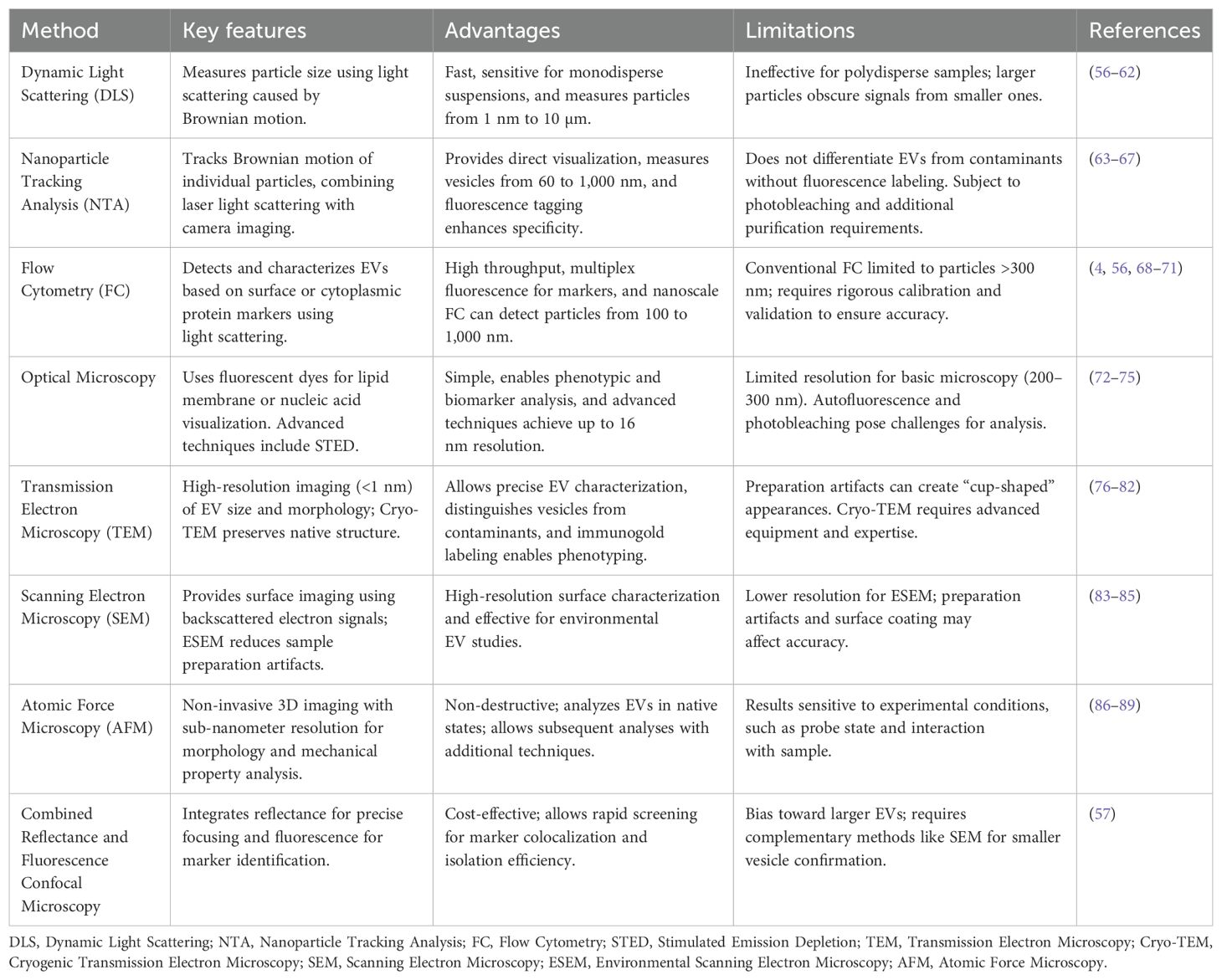
Several approaches have been developed to efficiently concentrate and isolate EVs from biological fluids by utilizing an EV feature that separates them from surrounding particles (Figure 3) (45). The yield, purity, and size distribution of isolated EVs vary depending on the procedure (46). As a result, high-quality EVs must be isolated using a method that is acceptable for the study’s objectives and consistent with future analyses (47).
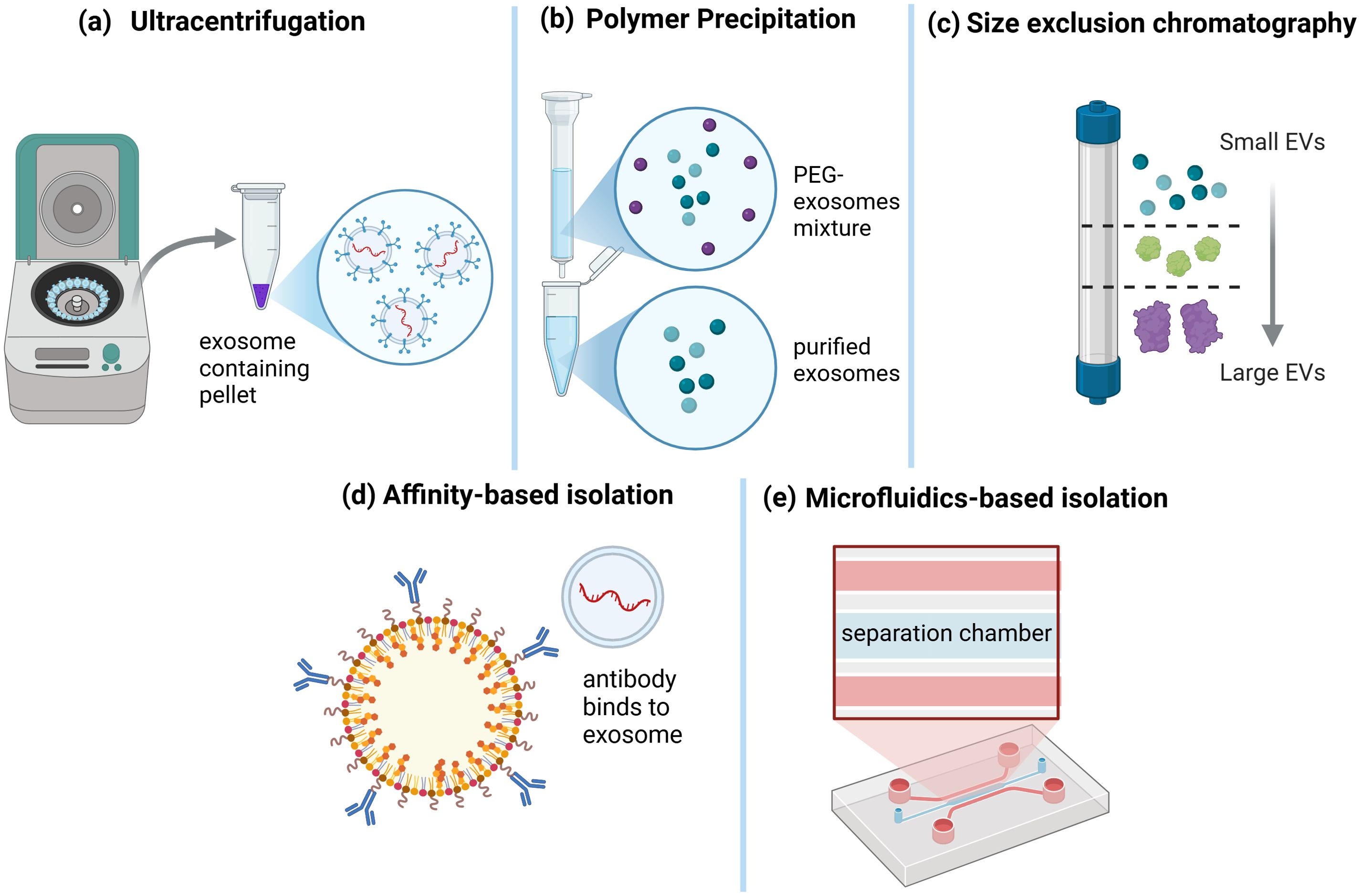
Figure 3. Exosome Isolation Techniques. Various methods have been developed for exosome isolation. (a) Ultracentrifugation is a widely used technique that involves high-speed centrifugation to pellet exosomes from biological fluids. (b) Polymer precipitation, using agents such as polyethylene glycol (PEG), facilitates exosome aggregation and subsequent precipitation. (c) Size exclusion chromatography separates exosomes based on their size, allowing for the enrichment of small extracellular vesicles (EVs) while removing larger particles. (d) Affinity-based isolation employs antibodies or ligands that specifically bind to exosomal surface proteins, enabling targeted capture. (e) Microfluidics-based isolation utilizes specialized lab-on-a-chip devices with separation chambers designed for high-throughput and precise exosome isolation. These diverse techniques cater to different research and clinical applications, depending on the required purity, yield, and specificity (44).
Separation/concentration can be carried out based on the EV’s biophysical parameters of size, density, charge, and surface composition (48). The optimum strategy is to use numerous approaches to optimize purity and yield (49). Table 1 presents a comparison of EV isolation methods. The table includes essential features of various approaches, such as purity (ability to eliminate impurities), yield (number of EVs produced), scalability (capacity for large-scale applications), cost (total reagent and equipment expenses), and time (process length). Each criterion is categorized as Low, Medium, or High based on current research and practical concerns (50). The differences between EV isolation methods can be summarized by comparing their performance in terms of purity, yield, scalability, cost, and time.
In terms of purity, size exclusion chromatography (SEC) and affinity-based approaches provide the highest levels of EV purity by effectively eliminating impurities such as proteins and lipoprotein (51). Ultracentrifugation (UC) achieves moderate purity but may co-isolate non-EV particles, whereas precipitation procedures produce the lowest purity due to the non-specific nature of polymer-induced precipitation, which can collect undesired proteins and other detritus (52). When it comes to yield, precipitation techniques and ultracentrifugation excel since they can process larger sample volumes and produce more EV amounts, albeit at the sacrifice of purity (53). SEC and micro-fluidics achieve moderate yields by selectively isolating EVs, whereas affinity-based approaches isolate specific EV subpopulations, resulting in lower total yields. Precipitation methods are the most scalable since they are simple and require little equipment, making them ideal for high-throughput applications (54). Ultracentrifugation and SEC are fairly scalable methods, but their time constraints and reliance on specialist equipment limit them (45). Affinity-based approaches and microfluidics, on the other hand, have low scalability due to sample volume limitations and the requirement for specialized reagents or devices (52). Precipitation methods are the most cost-effective since they require only basic reagents and equipment (49). Ultracentrifugation and SEC are somewhat expensive, including expenses incurred for equipment and consumables. The most expensive choices include microfluidics and affinity-based approaches, which require specialized reagents like antibodies and complex isolation devices.
The time necessary for isolation varies significantly (47). The fastest methods of isolation are precipitation and microfluidics, which take only a few hours to complete. SEC takes intermediate time for processing and elution; however, ultracentrifugation is the slowest procedure, requiring several hours to days due to the consecutive centrifugation processes (49). Overall, precipitation technologies are best suited for applications that require high throughput at cheap cost, where purity is less important. Ultracentrifugation strikes a balance between yield and purity, although it is labor-intensive and time-consuming (45). SEC provides excellent purity while maintaining EV integrity, making it ideal for delicate downstream applications. Affinity-based approaches are very specific to certain EV subpopulations, but they are expensive and difficult to scale. Microfluidics offers precision and speed but is restricted by its cost and sample volume capacity (55). The individual research goals, available resources, and expected downstream applications should all be considered when selecting an isolation strategy (50).
This comparative analysis demonstrates that no single isolation strategy is universally perfect; instead, the best methodology is strongly influenced by the study’s specific goals. For researchers who prioritize purity and EV integrity, particularly for downstream applications like proteomics or RNA analysis, SEC or affinity-based procedures may be the best option, but their yield or scalability might be limited. When large-scale EV synthesis is a top priority, particularly for diagnostic or therapeutic research, precipitation or ultracentrifugation may provide a more feasible cost-to-output ratio. Finally, a hybrid technique that incorporates complimentary methods may provide a personalized solution by optimizing both quality and efficiency based on the experimental situation.
6 Methods of extracellular vesicles characterization
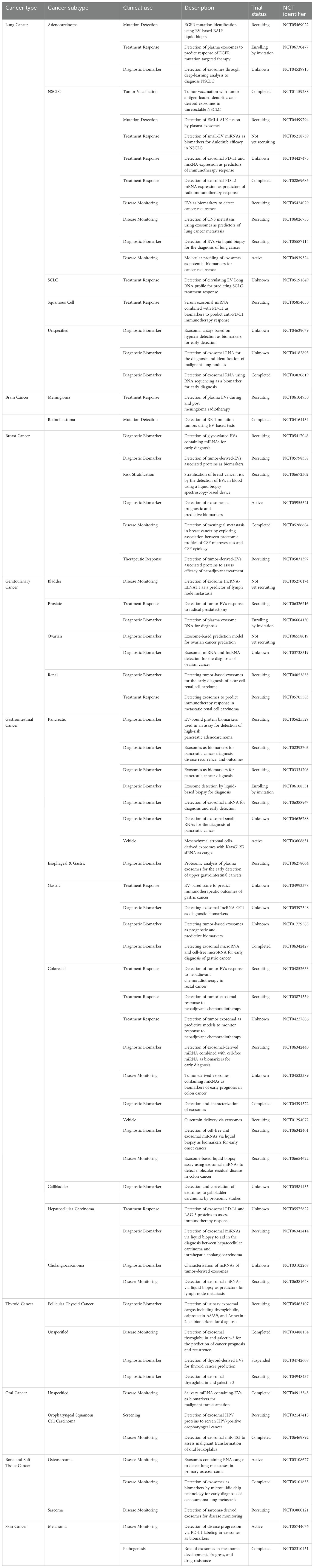
To enhance comprehension of EVs’ functions and evaluate the related diagnostic and therapeutic tools, correct classification is essential. Each approach harbors different advantages and disadvantages regarding the interpretation of results (Table 2).
6.1 Dynamic light scattering
Dynamic light scattering (DLS) is also known as photon correlation spectroscopy (PCS) which determines the size of the particle by measuring fluctuations of the scattered light caused by the Brownian movement of the particles in the solution (56). DLS is fast and highly sensitive for uniform suspensions because unlike imaging-based methods, DLS uses photon detectors to analyze the entire sample (60). This enables it to analyze particles from 1 nm to 10 µm without extensive preprocessing (90).
6.2 Nanoparticle tracking analysis
EV research has increasingly relied on nanoparticle tracking analysis (NTA) to quantify particle size and concentration. NTA tracks the Brownian motion of individual particles suspended in a solvent and uses the Stokes Einstein equation to calculate their hydrodynamic diameter (63–66). Further, EV subpopulations have been studied using fluorescent NTA. Thus, labeled surface markers or RNA-targeting molecular beacons have been used to stain particular vesicle subtypes and RNA cargo. These advancements have allowed insight into the diversity and function of EVs (64, 91, 92).
6.3 Flow cytometry
Flow cytometry (FC) is a common method used to detect and characterize EVs by their surface or cytoplasmic protein markers (4, 68, 69). Typically, traditional flow cytometers can measure relatively large EVs, with diameters almost always greater than 300 nm (69). This method involves passing a focused stream of the fluid carrying particles through a laser beam of a specific wavelength and strategically placed visible and fluorescent light detectors measuring scattered light (56, 93, 94). The forward scattered light (FSC), detected in the path of the laser, gives information on particle size, while side scattered light (SSC), perpendicular to the beam, is a measure of the internal complexity of the particles, such as granularity (70). Nevertheless, conventional FC suffers from low sensitivity and resolution, which limits its ability to detect smaller EVs, e.g. those below 300 nm (56, 69, 71, 95).
This challenge has recently been addressed through several advancements. This technology linearly detects particles from 100 to 1,000 nm (small to large EV) and allows for multiplexed fluorescent detection (96, 97). Nano-flow cytometry (nFC) labels EVs with disease specific markers and gives insights into cellular origin and pathological state (52, 96, 97).
6.4 Optical microscopy
Optical microscopy provides a straightforward method for observing EVs due to their relatively large size, despite the diffraction-limited resolution of approximately 200 to 300 nm. However, unless combined with sophisticated methods like stimulated emission depletion (STED) microscopy, confocal microscopy, or total internal reflection fluorescence microscopy (TIRFM), optical microscopy is insufficient for revealing finer structural detail of smaller EVs, i.e., exosomes (72, 73). Resolutions of up to 16 nm are obtained with STED microscopy, which is suitable for EV characterization and morphological studies (74, 75, 98).
6.5 Transmission electron microscopy
Transmission electron microscopy (TEM) is the gold standard for EV imaging because it allows for imaging of single EVs with resolutions <1 nm (76). Cryo-EM is used to overcome these artifacts in order to render native EV structures with more accuracy without chemical fixation or dehydration (77, 78). This method is especially useful for characterizing the three-dimensional architecture of EVs, differentiating them from other contaminants (99–101). TEM can be further enhanced by immunogold labeling to identify specific EV proteins using gold conjugated antibodies for EV phenotyping in complex media (79, 102, 103). Another electron microscopy technique is scanning electron microscopy (SEM). High resolution images of EV surfaces are obtained using SEM by utilizing backscattered electron signals to distinguish between sample components (83). Samples may be sputter coated with metallic or carbon layers to provide contrast enhancement for surface characteristic study (84, 104, 105). The study of environmental surface degradation of EVs and their interaction with other materials has been instrumental using SEM (106).
6.6 Atomic force microscopy
Atomic force microscopy (AFM) is a non-destructive way to characterize EVs with three dimensional topographical images, providing details of their morphology and sub-nanometer resolution (86, 107, 108). AFM obtains detailed insight into their morphology, size, mechanical properties and surface charge by scanning a sharp tip over the EV surface (86, 87, 109). Moreover, since its non-destructive approach it allows for further characterization by subsequent techniques (110). AFM’s utility in its ability to analyze EVs under physiological state without staining or fixation (86, 88). Specific antibody coated surfaces are then applied to further enable the identification of EV subpopulations (89).
6.7 Combined reflectance and fluorescence confocal microscopy
Reflectance and fluorescence confocal microscopy integration is a practical and cost-effective way to characterize EVs. Using this approach, EV fluorescence signals are differentiated from nonspecific artifacts, leaving phenotypic characterization possible using standard confocal laser scanning microscopes. Using high intensity reflection planes from the coverslip and glass slide as references, reflectance microscopy allows for sharp focusing on EVs. Using this setup, lateral and axial resolutions of 198 nm and 492 nm, respectively, are achieved and allow visualization of EVs and their aggregates (57).
When put together, these characterization techniques provide wide tools for examining EVs at a variety of resolution, sensitivity, and molecular detail levels. While TEM and AFM provide excellent structural insights, tools like NTA and flow cytometry allow for quantitative and phenotypic research. Each method has unique strengths and limits, and when used together, they frequently give the most thorough insight. As the field progresses, integrated techniques that combine high-resolution imaging and molecular profiling are anticipated to play an important role in improving EV-based diagnoses and therapies. The biological inquiry and the study’s technical constraints ultimately determine the best method or combination to use.
7 Usage of extracellular vesicles in cancer diagnostics
EVs have emerged as promising biomarkers for cancer diagnostics. These EVs can be isolated from various biofluids, such as plasma, bronchoalveolar lavage fluid (BALF), urine, and cerebrospinal fluid (CSF) (52), allowing a selective and non-invasive diagnostic approach for cancer detection. Among these, plasma-derived EVs are the most commonly used biofluid due to their accessibility and the presence of a diverse range of EVs containing tumor-specific markers (111, 112).
EVs possess the ability to carry a variety of biomarkers with significant diagnostic and prognostic potential. Tumor-associated miRNAs are frequently upregulated in cancer patients and have been studied for their role in early cancer detection and monitoring therapeutic responses. The integration of multiple biomarker types—genetic, protein, and lipid—into EV analysis holds promise for providing a more comprehensive approach to cancer diagnostics (113). However, challenges remain, such as variability in EV concentrations across individuals and different biofluids, which must be addressed to enhance diagnostic accuracy (114).
7.1 Breast cancer
In breast cancer, specific EV-associated miRNAs have demonstrated strong diagnostic potential. Elevated levels of miR-21 and miR-155 in EVs are linked to tumor aggressiveness, immune interactions, and metastasis. Conversely, reduced levels of miR-126 in BRCA mutation-positive patients suggests potential for personalized diagnosis (115). Similarly, EVs carrying HER2 and EGFR provide valuable non-invasive tools for monitoring tumor status and tracking treatment response, making them essential for improving breast cancer detection and treatment personalization (116). Furthermore, multi-miRNA diagnostic panels have been demonstrating improved accuracy over single biomarker approaches. For example, a four-miRNA panel (miR-1246, miR-206, miR-24, and miR-373) achieved 98% sensitivity and 96% specificity, significantly outperforming individual markers and offering an enhanced breast cancer diagnostic tool (117). Additionally, miR-10b and miR-639 have been found to promote tumor invasiveness, migration, and epithelial-mesenchymal transition in breast cancer stem cells. These traits make them potential biomarkers for early diagnosis, with sensitivity and specificity ranging from 64.8% to 83.3% (118, 119) (Table 3).
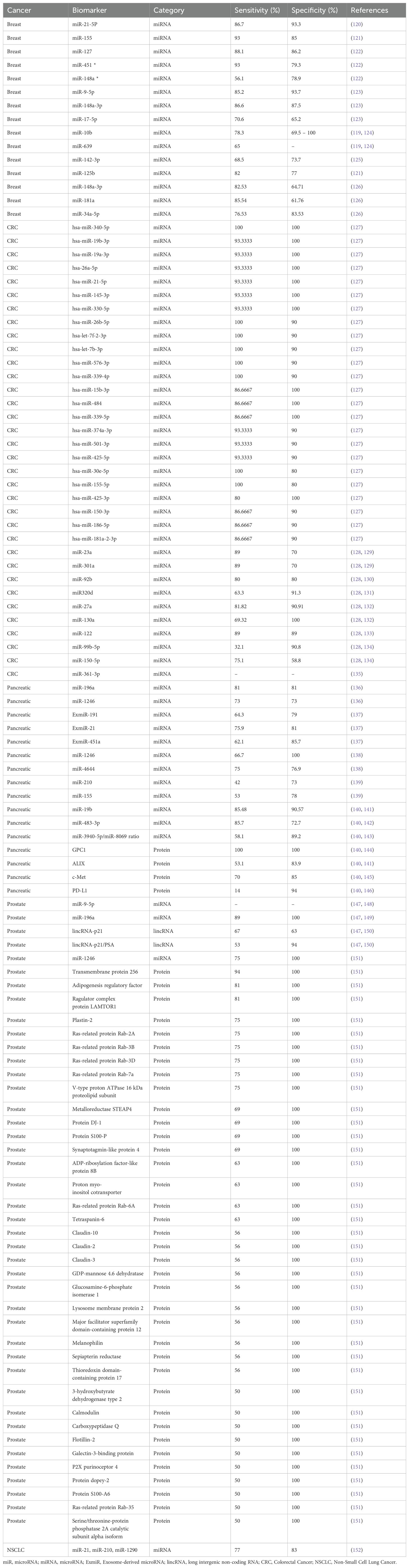
Table 3. EVs diagnostic biomarkers in different types of cancer EVs diagnostic biomarkers in different types of cancer.
7.2 Lung cancer
Studies identified differentially expressed miRNAs (DEMs) in plasma-derived small EVs (sEVs), such as miRNA-483-3p, upregulated in small cell lung cancer (SCLC), and miRNA-152-3p and miRNA-1277-5p, upregulated in non-SCLC (153). These DEMs regulate key biological processes, such as cAMP signaling and leukocyte transendothelial migration, aiding in early detection of SCLC and NSCLC. Additionally, miR-1246b found in BALF-derived EVs distinguishes malignant pulmonary nodules, while miR-505-5p in LA patients promotes tumor growth and inhibits apoptosis by targeting TP53AIP1 (118, 154). Furthermore, EGFR T790M/L858R-mutant non-SCLC cells produce EVs that accelerate the tumor growth by enhancing invasion, migration, and proliferation. Additionally, they complement EV-based diagnostic techniques by improving EGFRvIII mutation detection in circulating EV-RNA (152, 155). However, further research is required to refine and standardize these methodologies for clinical application.
7.3 Cervical cancer
EV-based biomarkers like squamous cell carcinoma antigen (SCCA) and miRNAs, such as miR-21, have been shown to be crucial for cervical cancer pathogenesis and progression. Notably, miR-486-5p has been shown to be overexpressed in cervical cancer and targets PTEN, activating the PI3K/Akt signaling pathway. This leads to enhanced cancer cell proliferation and growth, making miR-486-5p a potential biomarker for both cancer diagnosis and targeted therapy (156). Furthermore, proteomic analyses of serum-derived EVs from cervical cancer patients’ and healthy controls’ revealed 17 expressed proteins involved in metabolic processes and angiogenesis via the VEGF signaling pathway, including COX5A, IPO5, and ERI3 (157). Similarly, 19 upregulated proteins were associated with chemokine signaling pathways and increased cellular and metabolic regulation, highlighting their potential as therapeutic targets (157).
7.4 Ovarian cancer
EV-based biomarkers have also demonstrated promise in ovarian cancer detection. The miR-200 family, particularly miR-200a and miR-200c, is overexpressed in various ovarian cancer subtypes and associated with advanced disease stages and worse tumor-grades and survival outcomes. Furthermore, miR-200a has shown to have a strong correlation with tumor stage, grade, and lymph node metastasis, indicating its potential for early detection and prognosis (117). In addition to miR-200, recent studies show the enhanced diagnostic potential of exosomal miR-223. Where in combination with CA-125, demonstrated an increased sensitivity and specificity compared to CA-125 alone in the diagnosis of epithelial ovarian cancer patients, enhancing early-stage detection accuracy (158).
7.5 Hepatocellular carcinoma
Studies have demonstrated high diagnostic potential of combined EV-RNA and EV-surface antigen models for hepatocellular carcinoma (HCC), with sensitivity and specificity reaching 93.8% and 74.5% respectively. Similarly, combinations of serum-based EVs containing miR-21-5p, miR-92a-3p, and AFP show sensitivity of 95% and 86%, respectively. EV-lncRNA biomarkers like LINC00853 and lnc85 show superior diagnostic performance, surpassing serum AFP in sensitivity and specificity, making them effective for early HCC detection in high-risk groups. However, their ability to differentiate HCC from CCA remains limited, highlighting the need for EV-derived biomarkers (159). Furthermore, exosomal miRNAs play a crucial role in HCC diagnosis, metastasis, and disease progression. Where upregulated levels of miR-21, miR-221, and miR-222, and downregulated levels of miR-122, miR-145, and miR-199-a, shown to influence tumor growth, treatment resistance, and early cancer detection (160). EVs carrying IncRNA biomarkers have also shown the potential in enhancing HCC diagnosis, however, their low abundance requires highly sensitive detection methods (161). Moreover, a more recent study demonstrated the diagnostic potential of EV-based surface proteins for HCC, with the study showing significant differences in expressions observed between HCC patients and individuals with non-malignant liver disease (NMLD) (162). Furthermore, urinary EV glycoproteins, including 756 N-glycopeptides and 107 N-glycoproteins have been identified as potential non-invasive biomarkers for diagnosing HCC (163). Additionally, another study reveals six potential biomarkers for HCC, including EV-lncRNAs such as, DLEU2, HOTTIP, MALAT1, NEAT1, SNHG1, and TUG1. Among these biomarkers, TEV-MALAT1 showed good diagnostic ability for early-stage HCC, even in AFP-negative cases (164).
7.6 Colorectal cancer
Ren et al. discovered that exosomes secreted by colorectal cancer (CRC) cells, such as SW480 and HCT116, promote cell growth by activating STAT3 signaling under hypoxia conditions (128, 165). Similarly, Li et al. found that hypoxic EVs containing miR-361-3p increase tumor growth and block apoptosis by targeting TRAF3, activating the noncanonical NF-κB pathway (135). Accordingly, elevated levels of this miRNA in circulating exosomes are connected to poor prognosis and may serve as a potential marker and therapeutic target in CRC, as it is induced by hypoxia-inducible factor 1-alpha (HIF-1α) (135). Moreover, Liu et al. found that exosomes produced from CRC cells, such as mi-R106b-3p, stimulate cell invasion, migration, and the epithelial-to-mesenchymal transition (EMT) (166). Karimi et al. collected blood samples from CRC patients and showed higher expressions of exosomal miR-23a and miR-301a than normal controls with 89% and 70% sensitivity and specificity, respectively (129). Min et al. studies on individuals with CRC retain plasma EVs with distinct miRNA profiles, including Let-7b-3p, miR-1339-3p, miR-150-3p, and miR-145-3p, suggesting a new biomarker category for early diagnosis (127).
7.7 Pancreatic cancer
CA 19-9, a widely used diagnostic biomarker for pancreatic cancer, has limitations like low sensitivity, false negatives in Lewis negative phenotype, and higher false positive rates with obstructive jaundice, making early detection challenging (167). Recent studies have focused on the function of exosomes in relation to its potential diagnostic abilities in pancreatic cancer (168–170). For example, Li et al. revealed that exosomal miR-222 in pancreatic ductal adenocarcinoma (PDAC) promote invasion and proliferation of tumor cells through regulating p27 (168). Goto et al.’s study found three ExmiRs, ExmiR-191, -21, and -451a, outperformed CA 19–9 in early PC diagnosis, with sensitivity and specificity values exceeding 80% (Table 3) (167). Moreover, exosomes from pancreatic cancer patients overexpressed Glypican-1 (GPC1), suggesting its potential as a biomarker for pancreatic cancer diagnosis and stratification (140, 144).
7.8 Prostate cancer
Although prostate specific antigen (PSA) is the most used biomarker for the detection of prostate cancer, it lacks in its ability to stratify patients with high risk for early detection or with those with indolent prostate cancer (150, 171). Exosomes cargo that is distinct to prostate cancer can serve as a noninvasive tool for diagnosis (147, 172). For example, Logozzi et al. used PSA carried by exosomes (Exo-PSA) and showed superior performance than conventional PSA with almost 100% specificity and sensitivity (173). In such context of diagnostics, ExosomeDx, a pioneering company in oncology and precision medicine, uses urine-derived exosomal RNA in their flagship product, the ExoDx™ Prostate Test, to aid clinical decision-making (174, 175). Such actions encourage industries that bench to clinical shift can be possible for a therapeutic change.
Looking ahead, one could conclude from these findings that individualized therapeutic approaches in breast cancer, where miRNA panels like miR-1246, miR-206, and others demonstrate exceptional diagnostic accuracy, are among the most promising treatments associated with EV-based biomarkers (117). This opens the door to more specialized and efficient treatments. It is also a crucial area for clinical development because miR-200a-targeting ovarian cancer treatments, when combined with CA-125, offer improved early-stage diagnosis accuracy (176). Using a combination of exosomal miR-21 and miR-92a-3p biomarkers, hepatocellular carcinoma (HCC) exhibits encouraging findings with high sensitivity and specificity for early detection and distinction from non-malignant liver disorders (159). These advancements demonstrate how using EV-based biomarkers might enhance diagnostic skills and result in more individualized and successful treatment plans for a variety of malignancies.
8 Therapeutic uses of extracellular vesicles in cancer
8.1 EVs role in cancer immunotherapy
Immunotherapy, including cancer vaccines and immune checkpoint inhibitors, has emerged as a promising therapeutic regimen for cancer patients. Emerging as a promising adjunct to this domain in cancer management are the role of EVs, offering promising advantages such as precise targeting and improved immune responses (177, 178). Several studies have explored the role of EVs in enhancing drug-mediated apoptosis in cancers. For example, Cho et al., devised genetically engineered EVs derived from human CD8+ T cells incorporated with interleukin-2 and cetuximab against A549 lung cancer cells. The engineered EVs demonstrated greater cytotoxicity and enhanced susceptibility to immune-mediated cell death in the lung cancer cells. Additionally, the EVs exhibited EGFR- dependent targeting, which suggests their potential as precise and effective immunotherapeutic strategy for lung cancer (179). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer treatment that preferentially binds to DR5 to cause apoptosis in cancer cells with no systemic effects. However, tumor resistance poses numerous therapeutic hurdles for TRAIL-based medications (180). To counter the challenges with the clinical use of TRAIL-based agents, many studies explored the use of EVs to improve outcomes. For example, exosomes engineered with TRAIL and loaded with triptolide (TRAIL-Exo/TPL) have demonstrated significant therapeutic potential in malignant melanoma. TRAIL-Exo/TPL enhanced tumor targeting, cellular uptake, apoptosis induction with reduced drug toxicity in vivo (181). Similarly, Qiu et al., developed mesenchymal stem-cell derived exosomes (MSCT-EXO) loaded with cabazitaxel (CTX) and TRAIL targeted towards oral squamous cell carcinoma (OSCC). By preventing the PI3K/Akt/mTOR pathway from being activated, the exosomes containing CTX and TRAIL showed synergistic antitumor effects and triggered apoptosis, which resulted in a considerable tumor suppression and tumor volume decrease (182). EVs also modulate radiation resistance, especially in oral squamous cell cancer. For instance, Chen et al. revealed that hypoxic cancer cell exosomal miR-340-5p targets KLF10/UVRAG, driving radioresistance in OSCC, making it a promising biomarker for theranostics (183). Furthermore, metformin reversed this impact by restoring KLF10 expression, making it a promising OSCC radiosensitivity therapy (183). In lung cancer, EVs loaded with TRAIL and dinaciclib (EV-T-Dina) were seen to downregulate anti-apoptotic factors such as cFLIP, MCL-1, and Survivin. Moreover, this combination when nebulized, demonstrated lower drug resistance and higher stability and efficacy in the treatment of lung cancer (184). These studies demonstrate the potential of EVs to modulate apoptosis in cancers by improving the efficacy of drug-induced apoptosis and reducing drug resistance.
Additionally, some research has demonstrated that EVs can be used to boost the effectiveness of different immune activation drugs, especially those that trigger the Stimulator of Interferon Genes (STING) pathway. To increase CDN efficacy, Jang et al. designed an EV that contained the cyclic dinucleotide (CDN) STING agonist ExoSTING. By specifically targeting antigen-presenting cells inside the tumor microenvironment, ExoSTING was able to boost immune activation by encouraging local Th1 responses, CD8+ T cell recruitment, and systemic anti-tumor immunity against the tumor. This effect minimized systemic inflammation and improved CDN efficacy (185). Similarly, McAndrews et al., devised an engineered exosome, iExoSTINGa, that delivered the STING agonist cyclic GMP-AMP, that demonstrated enhanced targeting efficiency and pharmacokinetics compared to free STING agonists (186).
Exosomal PD-L1 has also emerged as an important factor in tumor immune evasion. Tumor-derived small EVs (TDSEVs) high in PD-L1 block T cell activation in draining lymph nodes, contributing to resistance to immune checkpoint inhibitors (187, 188). Interestingly, CRISPR/Cas9-mediated deletion of Rab27a or neutral sphingomyelinase-2 (nSMase2) decreased exosomal PD-L1 levels where nSMase2 deletion further reduced total PD-L1 without changing surface expression (187). Therefore, blocking exosomal PD-L1 dramatically decreased tumor growth, even in resistant mice, and worked in tandem with anti-PD-L1 antibodies, highlighting its potential as a new immunotherapeutic target (188).
A promising substitute for other immunotherapies is the therapeutic cancer vaccination. By exposing dendritic cells (DC) to tumor-specific antigens, cancer vaccines improve adaptive immunity and encourage long-lasting T cell responses (189). EVs are promising candidates for cancer vaccines against a variety of malignancies, including malignant melanoma, according to numerous research. For instance, by promoting DC maturation and elevating CD8+ T cells and serum interferon α (INF-ƛ), melanoma cell-derived EVs suppressed tumor growth and metastasis and produced anti-tumor immunity (190). Ma et al. demonstrated a similar effect using Melanoma tumor cell-derived microparticles (T-MPs). The T-MPs worked to activate a lysosomal pathway in DCs and thus facilitating tumor antigen presentation to CD8+ T cells (191). These studies show the potential of EVs in enhancing immune functions and their potential for clinical use.
8.2 EVs as chemotherapy-delivery vehicles in cancer management
The use of EVs as drug delivery vehicles has emerged due to their natural biocompatibility, ability to traverse biological barriers, and inherent targeting capacity. Unlike traditional synthetic delivery systems, EVs have been shown to provide a safer and more efficient alternative, capable of reducing systemic toxicity and enhancing therapeutic efficacy of agents in the management of various cancers (192, 193). EV-based drug delivery vehicles showed promising potential in treating triple-negative breast cancer (TNBC). Macrophage-derived EVs loaded with paclitaxel and doxorubicin (Dox) showed effective accumulation in TNBC cells with significant anti-proliferative effects, offering a novel strategy in addressing the challenges of treating cancers such as TNBC (194). Furthermore, various studies explore exosomes as potential drug delivery agents. Exosomes show promising natural nanoparticles for drug delivery, particularly in multidrug- resistant cancers. Kim et al. assessed the effect of macrophage-derived exosomes loaded with paclitaxel (exoPTX) in murine lung carcinoma model. EVs have the potential to be effective drug delivery vehicles for treatment-resistant malignancies, as evidenced by the study’s 50-fold enhanced cytotoxicity and notable anticancer effects (195). Additionally, exoPTX demonstrated completely accumulated in pulmonary metastatic cells without affecting normal tissue. These findings highlight the potential clinical benefits, as this delivery model could reduce systemic toxicity seen with conventional chemotherapy by selectively targets cancer cell and bypassing drug resistance mechanisms (195). In addition, compared to free Dox, another study created Exo-Dox, a mixture of Dox and exosomes produced from mesenchymal stem cells, to target osteosarcoma in vitro. Exo-Dox demonstrated less cardiotoxicity, improved anti-tumor effectiveness, and increased cellular absorption (196). Moreover, exosomes loaded with gemcitabine (ExoGEM) demonstrated similar findings against pancreatic cancer cells. ExoGEM demonstrated enhanced targeting and cytotoxicity compared to systemic gemcitabine, selectively increasing drug concentration at tumor sites with minimal systemic effects. ExoGEM was also seen to suppress tumor growth, prolong survival, and completely eradicate tumors in tumor-bearing mice (197). Interestingly, exosomes have been shown to demonstrate the ability to deliver chemotherapy to target brain cancer. In a zebrafish brain cancer model, brain endothelial cell-derived exosomes enhanced drug uptake via receptor-mediated endocytosis and effectively crossed the blood-brain barrier to target the brain. This method was shown to significantly reduce tumor growth supported by a reduction in the fluorescent markers of cancer cells, highlighting the utility of exosome-based delivery systems in targeting brain cancers (198). These findings highlights the potential clinical utility of exosome-based drug delivery systems, which could offer treatment options for brain cancers that were previously considered untreatable with conventional chemotherapeutics (198).
Furthermore, multiple studies explored the prospect use of functionalized exosomes with aptamers for targeted therapy. For example, Bagheri et al. devised an engineered exosome-based delivery system for Dox in a murine colon adenocarcinoma model (21, 21). Mesenchymal stem cell-derived exosomes encapsulated Dox and were functionalized with MUC1 aptamers for targeted delivery to MUC1-positive cancer cells. The exosomes demonstrated superior Dox transportation, enhanced accumulation in tumor cells, and enhanced clearance compared to free Dox. Additionally, the engineered exosomes significantly suppressed tumor growth in the adenocarcinoma model (199). These findings further support the important clinical implications of exosome-based delivery systems, as they can potentially reduce side effects and improve treatment outcomes in managing colorectal cancer (21). Another study also demonstrated similar potential clinical implications by exploring aptamer-functionalized exosomes loaded with Dox for colorectal cancer treatment. Another study explored aptamer-functionalized exosomes loaded with Dox for colorectal cancer treatment. The study functionalized Dox-loaded exosomes with AS1411 aptamers. The functionalized exosomes demonstrated enhanced cytotoxicity and significant tumor growth suppression through target accumulation and retention (200). These studies highlight the potential of functionalized exosomes as a safe and effective therapeutic strategy in cancer treatment. Moreover, other EVs such as nanovesicles have been shown to have promising drug delivery abilities. Jang et al. demonstrated that bioengineered exosome-mimetic nanovesicles loaded with chemotherapeutics outperformed traditional liposomal delivery systems. The nanovesicles demonstrated efficient tumor targeting and enhanced tumor cell death and reduced tumor growth with minimal adverse systemic effects (201).
Studies have been exploring the role of synthetic or engineered EVs in cancer treatment. Engineered EVs are bioengineered versions of naturally occurring EVs modified to enhance their therapeutic potential. These EVs are modified by surface modifications, cargo loading, or genetic alterations, offering greater biocompatibility to natural EVs (202). For example, engineered exosomes have been studied to reverse drug resistance in drug-resistant cancers. Liang et al. demonstrated that exosomes co-delivering 5-fluorouracil (5-FU) and a miR-21 inhibitor effectively reversed 5-FU-resistance in resistant colorectal cancer cells. As a possible biomarker for early identification and treatment response monitoring, EVs from plasma and bronchoalveolar lavage fluid are enriched with miR-21, a microRNA linked to non-small cell lung cancer (NSCLC) (203). Furthermore, Wang et al., showed that engineered mimic vesicles derived from erythrocyte membranes had the ability to overcome multidrug resistant tumors. The mimic vesicles were designed to co-deliver Dox and P-glycoprotein siRNA and achieved high drug loading rates able to effectively silence P-glycoprotein and enhancing Dox-induced tumor inhibition (204). Moreover, engineered macrophage-derived exosome-coated nanoparticles were shown to increase chemotherapy response in TNBC. This was done by modifying exosome surfaces with a c-Met-targeting peptide, which significantly increased tumor cellular uptake, targeting efficacy, and Dox-induced tumor inhibition, offering a promising strategy for TNBC treatment (205). These engineered EVs can hold extreme clinical potential by offering precise treatment while reducing off-target effects seen in conventional chemotherapy.
Proteolysis-targeting chimeras (PROTACs) serve as a new therapeutic approach to target select pathogenic proteins through hijacking the ubiquitin-proteasome system for protein breakdown (206, 207). Tumor-targeting PROTACs represent recent innovations that utilize receptors which specifically bind to overexpressed receptors found on cancer cells (208). Additionally, the development of “pro-PROTACs” represents a new approach for minimizing off-tumor toxicity by requiring tumor-associated enzymes to activate them (208). Nanoparticle-assisted delivery and PEGylation have also improved the stability, solubility, and pharmacokinetics of PROTACs, allowing for higher tumor accumulation and fewer systemic effects (209, 210). These engineered approaches have enabled scientists to generate specific potent and safe PROTAC candidates for treating breast and prostate cancers (211, 212).
These studies underscore the growing clinical potential of EVs as efficient drug delivery vehicles in cancer therapy. By enhancing drug targeting and availability, improving efficacy, and minimizing systemic toxicity, EV-based delivery systems present a promising alternative to conventional chemotherapeutic approaches. Additional studies exploring the use of EVs as drug delivery vehicles in clinical settings are needed to provide valuable clinical data.
9 Engineering therapeutic extracellular vesicles: preparation and storage challenges
9.1 Preparation strategies for therapeutic extracellular vesicles
EVs can inherently facilitate the transfer of macromolecules across cells, rendering them advantageous vehicles for drug delivery owing to their biocompatibility, and targeting efficacy. Drug incorporation into EVs can be accomplished via endogenous or exogenous techniques.
Endogenous loading is the process of incorporating therapeutic substances into parental cells, such as through gene transfection or co-incubation. For example, Chen et al. employed lentiviral transfection to overexpress miR-375 in adipose-derived MSCs, resulting in exosomes that stimulated bone repair (213). Co-incubation has also been used to successfully load hydrophobic medicines such as doxorubicin (214), paclitaxel (215) and curcumin (216), with Zhu et al. showing that paclitaxel-loaded MSC exosomes had increased antiproliferative properties (217).
On the other hand, exogenous loading introduces drugs directly into EVs post-isolation, typically achieving higher efficiency. Zhou et al. encompassed electroporation, utilized for siRNA delivery into EVs that was effective yet potentially leading to EV aggregation, which can be alleviated by the addition of EDTA as demonstrated by Kooijmans et al. (189, 218). Yerneni et al. used ultrasonication enhances drug loading by temporarily disrupting EV membranes, demonstrating the ability to reverse inflammation in vivo with curcumin-loaded EVs (219). Mechanical extrusion and freeze-thaw cycles have been also employed; however, they may compromise membrane integrity or exhibit reduced efficiency (220, 221).
Targeting can be improved by membrane alteration. For instance, Liu et al. used genetic engineering to alter EVs with RGD peptide for retinal treatment (222). Lee et al. developed exosomes that incorporated sodium azide-containing lipids and linked them to targeting peptides through copper-free click chemistry to improve the targeting of cancer cells (223) and Du et al. utilized ultrasonic drug loading and CD47 alteration to create EVs that could evade immune clearance and target tumors (224).
EV engineering methods should be chosen based on cargo type, therapeutic purpose, and targeting. Endogenous methods are gentler and retain EV integrity, making them suited for sensitive payloads, while exogenous methods are more efficient but may compromise EV stability (220, 225). Using gene transfection and ultrasonication together can improve multifunctionality and therapeutic performance. Membrane modification is useful for targeted administration but requires cytotoxicity and long-term biocompatibility testing (220).
9.2 Storage conditions and stability considerations
Efficient storage of EVs is essential for maintaining their structural integrity, molecular composition, and bioactivity, hence ensuring consistent particle count, cargo (protein/RNA), and morphology across diverse EV sources (226).
Proper storage of EVs is essential to maintain their structure, cargo integrity, and biological function (227). A systematic review by Ahmadian et al. analyzed 50 studies and found that −80 °C is the most reliable temperature for long-term EV preservation, effectively maintaining particle concentration, RNA/protein content, and morphology across various sources (227). However, repeated freeze-thaw cycles were shown to significantly damage EVs, reducing particle count, promoting aggregation, and degrading miRNA—up to 70% loss after a single cycle in one study (228). Short-term storage at 4 °C is acceptable for a few days, especially in native biofluids, but longer periods lead to protein degradation and membrane damage (229). Elevated temperatures (>25 °C) accelerate deterioration and are unsuitable for storage (39, 42, 56). The use of cryoprotectants, particularly trehalose, was shown to preserve EV morphology and function, including during freeze-drying (lyophilization), making it a promising strategy for maintaining stability without ultra-cold conditions (230, 231). Moreover, storage buffers like PBS alone are insufficient, often leading to aggregation, whereas formulations like PBS with human albumin and trehalose (PBS-HAT) significantly improve EV preservation (232). Storing EVs within their native biofluids, such as plasma or saliva, also enhances stability compared to purified suspensions (233). Interestingly, some findings suggest −80 °C may outperform liquid nitrogen (−196 °C) in preserving RNA content and membrane integrity (234).
Despite progress, standardized storage techniques for long-term EV preservation are still immature and require adjustment based on EV type and planned application (235). Key problems, such as heterogeneity, low yield, scalability, and stability, remain barriers to successful clinical translation (236, 237). Maintaining EV integrity during storage requires enhanced stabilization measures, such as freezing at -80°C (236, 237). Progress in EV characterization, imaging, and synthetic alternatives, together with enhanced technology and quality control, will be critical to the advancement of EV-based targeted therapeutics (236, 237).
9.3 In Vivo biodistribution and safety considerations of extracellular vesicles
9.3.1 Circulation, biodistribution, and metabolic fate
EVs are natural endogenous cargo delivery vehicles (238). They have inherent targeting properties that allow for interaction with target cells. Many factors contribute to the homing of EVs in vivo including components of the EVs like surface markers and phospholipids, the cell source of the EVs and the administration route all of which discussed in this section.
9.3.2 Role of surface markers and phospholipids in EV behavior
Surface markers and receptors on EVs serve multiple functions, including facilitating the targeting of certain cells and organs (239). For example, tetrasponins can form a complex with integrin α4 and CD49D to target CD54 endothelial cells and pancreatic cells (240, 241). This binding is pivotal in EV-mediated tumor development. Moreover, ligands such as transferrin (Tf), which are abundantly expressed on cancer cells, can bind to transferrin receptors (TfR) inherently present on EV surfaces, so enabling targeted delivery (241, 242). Interestingly, EVs generated from milk possess surface proteins that facilitate targeted absorption. For instance, Näslund et al. demonstrated that these connections facilitate the targeting of monocyte-derived dendritic cells (DCs) by EVs through the MUC1–DC-SIGN pathway (241, 243).
Beyond surface markers, the lipid composition of the EV membrane, especially phospholipids, plays a central role in directing EVs to specific cells. Phosphatidylserine (PS) can interact with PS receptors on immune cells, frequently resulting in the clearance of EVs by macrophages. For example, TIM4 binds PS to promote engulfment, while cloaking PS with annexin V reduced macrophage uptake by 66% (244). Furthermore, EV surface glycans can interact with cancer cells through the binding of CCR8 and CCL18 (245). These findings underscore the substantial impact of membrane composition on the targeting and uptake of EVs, which, if considered, will improve therapeutic applications.
9.3.3 Influence of cell source on EV homing
The targeting of EVs is significantly determined by their cell of origin, as EVs typically exhibit a preference for homing to tissues or cells that are closely associated with their parent cells (236). For instance, endothelial EVs from the brain can accumulate in cerebral tissue (198), while melanoma EVs target melanoma metastases (246). EVs from mesenchymal stem cells (MSC-EVs) have increased accumulation in mice kidney with acute kidney injury, suggesting disease-specific biodistribution (247). Tumor-derived EVs exhibit a natural affinity for tumor sites, facilitated by specific surface molecules such as E-cadherin present on prostate cancer-derived EVs, which enable targeting of homologous tumors (248). Immune cell-derived EVs, particularly from DCs, exhibit MHC molecules that facilitate the targeting of immune cells, thereby augmenting immune responses (249). MSC-derived EVs demonstrate efficacy in targeting conditions such as type 2 diabetes and neurodegenerative disorders (250). Endothelial cell-derived EVs demonstrate a significant affinity for bone, indicating potential for bone-targeted therapies (251). This emphasizes the potential of utilizing the inherent homing abilities of EVs, influenced by their cellular origin, for targeted therapeutic applications in diverse conditions.
EVs can also passively target tumor sites due to enhanced permeability and retention (EPR) effect in the tumor microenvironment (TME) that is characterized by permeable vasculature facilitating the passive accumulation of EVs. A study has shown that EVs originating from melanoma cells possess a natural propensity for lung tissues, resulting in high accumulation in lung metastases (236). Multiple myeloma and breast cancer cells get miR-15a or miR-16 from bone marrow-derived MSC (BM-MSC) EVs, limiting proliferation and angiogenesis (252). In contrast, the activation of cancer cells generally directs stromal cell-derived EVs towards pro-tumor phenotypes. For instance, fibroblasts activated by hepatoma cells demonstrate a notable elevation of SPOCK1/testican-1 pathways, facilitating the advancement of hepatoma cells (253, 254). When fibroblasts are stimulated by cancer cells like hepatoma, they release EVs with tumor-promoting cargo (e.g., SPOCK1/testican-1), which tend to home back to tumor sites and support cancer progression (252, 254). MSC-derived EVs also promote tumor migration via affecting integrin expression and MET. MiR-374a-5p-loaded EVs of gastric cancer-derived MSCs target HAPLN1 to boost gastric tumor integrin expression and cell migration (255).
Because the functions of the EVs imitate their originating cells, EVs can also possess antitumor activity if released from immune cells like DCs, natural killer (NK) cells and macrophages. NK cells, intrinsic tumor eradicators inside the TME, have been investigated in many immunotherapeutic approaches including adoptive NK cell transfer, CAR-NK treatment, and checkpoint inhibition (254, 256). EVs derived from activated primary natural killer (NK) cells or NK-92 cells stimulated by IL-12, IL-15, and IL-18 exhibit superior capacity to infiltrate and target solid tumors in comparison to those from dormant NK cell lines (257). DCs derived EVs show increased accumulation in spleen (236), giving antigens to T lymphocytes to elicit their antitumor response (258). Consequently, DC-EVs are inherently connected to T cell functionality and can directly and indirectly boost T cell immunity (254, 259). M1 macrophage-derived EVs similarly induce tumor death by decreasing the expression levels of CCR4, Foxp3, and CTLA-4 in canine peripheral mononuclear cells cocultured with tumor cells (254, 260). While specific targeting of EVs is not fully understood, integrins are key molecules involved in organ-specific metastasis of tumor cells (236).
These findings show how the source and activation state of EV-producing cells affect their targeting (homing) and biological consequences, providing a solid foundation for future EV-based targeted therapeutics.
9.3.4 Influence of administration routes on EV distribution and targeting
The route of administration of a drug influences its arrival rate to the target organ, its metabolism, and consequently the ultimate concentration achieved (261). Similarly, as EVs serve as vehicle for drug delivery, their mode of administration also affects the homing sites of these EVs (241).
Intravenous injection (IV) is the predominant route as it circumvents metabolism, hence preserving medication concentration (262). Wiklander et al. interestingly found that IV administered EVs accumulate in the liver more commonly than other routes by 60% (263). This was also emphasized by Zhou et al. who demonstrated that EVs tend to accumulate more in the liver and spleen through IV route compared to intraperitoneal (IP) injection where EVs seemed to accumulate more in adipose tissue broadly (264). Brossa et al. found out that administered human liver stem cells-derived EVs through the IV route inhibited subcutaneous tumors (265). However, IV injection poses problems such as off-target accumulation and rapid clearance rate (241, 266).
IP injections offer another viable approach. For example, Heidari et al. used MSC-derived EVs delivered intraperitoneally to treat acute colitis (267), while Nojehdehi et al. demonstrated immune-modulatory and glucose-stabilizing effects using adipose MSC-EVs via this route (268). This makes the IP route more suitable for metabolic diseases as EVs tend to accumulate in the pancreas (236, 263, 269).
Subcutaneous injections tend to accumulate in the digestive system (236), but face obstacles like adipose tissue and fibrovascular networks, affecting drug absorption. Research shows limited exosomes enter systemic circulation, with IP injections lasting longer due to reduced lymphatic flow (270).
The oral route is the favored approach because it is simple and does not require skilled staff for administration (271). Orally administered human breast milk-derived EVs have been demonstrated to mitigate gut inflammation by modulating immune responses (241, 272), specifically by promoting Treg and Th2 differentiation while inhibiting Th1 and Th17 cells (273). This indicates both direct and indirect effects on T cell-mediated immunity (272, 273). Therefore, this data suggests that oral route administration accumulates more in the small intestine which makes it suitable for digestive system diseases (241).
Local delivery, particularly intratumoral delivery, emphasizes targeting damaged tissue to enhance effectiveness of the injected therapy (274). In a study utilizing a prostate cancer mouse model, Rivoltini developed TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes for intratumoral injection. The results indicated an effective binding to tumor tissue, leading to a 58% reduction in tumor size (275). Although this method ensures efficient delivery and demonstrates a decrease in tumors, it is not desired by many patients due to its invasive nature and the possibility of an abrupt drug release that might be lethal (241, 276).
Betzer et al. investigated the impact of intranasal exosome injection. Interestingly, the study found that this approach efficiently accumulated in the brain, indicating their promise as a non-invasive treatment for neurological diseases (277). This finding demonstrate the possibility and effectiveness of intranasal delivery for targeted brain therapy.
As stated in the abovementioned routes, it is essential to correlate the targeted disease with the route of administration, as this relationship directly influences therapeutic potential. For example, IV administration is favored in systemic disorders, although there is a risk of off-targets (241), IP is more suitable for metabolic diseases (241, 269), and oral route targets more gastrointestinal diseases (241, 273). Among all, the local delivery is more likely the ideal choice of therapy as it offers fewer side effects, lower off-targets and augmented therapeutic effects (241).
Technical challenges such as rapid uptake and dye transfer hinder the understanding of EV clearance (278); however, recent studies employ that EVs have a short half-life, with blood levels influenced by secretion and rapid clearance (279).
9.4 Toxicity profiles of extracellular vesicles: the importance of standardized evaluation methods
EVs are involved in many physiological processes and diseases where they are generally considered safe, but not all have beneficial effects (280). For example, cancer-derived EVs can promote malignancy and may cause unexpected adverse reactions (280). Toxicity assessments conducted in rodents with EVs derived from MSCs, fibroblasts, HEK293T, and Expi293F cells indicated no significant adverse effects on hematological parameters, organ histopathology, or immune activation (280). However, mild inflammation in the liver and kidney was occasionally observed, though it did not result in significant immune toxicity (281). Furthermore, immunotoxicity studies indicated minimal immune responses, with certain EVs (e.g., derived from bovine milk) causing slight leukocyte proliferation and complement activation, whereas MSC-EVs exhibited largely inert characteristics (282). No instances of systemic anaphylaxis or significant cytokine responses were observed (283). Evaluations of gene toxicity through comet and micronucleus assays indicated no genotoxic effects from MSC- or milk-derived EVs (284). However, exosomes from chemo-resistant glioblastoma models demonstrated potential gene interaction (285). More importantly, concerns persist regarding tumorigenicity; MSC-exosomes facilitated cancer cell migration and colony formation, while exosomes derived from cancer sources enhanced tumor progression and established immunosuppressive microenvironments in various in vivo models (285–287). The findings highlight the necessity for comprehensive and standard guidelines for toxicity assessment to facilitate the safe clinical translation of EV therapies.
10 Future perspectives and current limitations
A number of cutting-edge technologies have been established to change cancer diagnostics to offer more accurate and non-invasive ways than those used for early detection (288). Phototherapy refers to the use of specific light wavelengths to activate therapeutic agents, such as photosensitizers, for the treatment of cancer (289). Current research in photodynamic therapy (PDT) has produced responsive photosensitizers which detect tumor environmental signals for precise targeting and decreased side effects in other tissues (290). Moreover, the use of nanotechnology in PDT enables better delivery and higher accumulation of photosensitizers in tumors, which improves treatment outcomes (291). These advancing technological innovations bring substantial progress to cancer phototherapy while creating paths for more accurate treatment methods with reduced invasiveness (292).
Another advances include the integration of nanotechnology to create biosensors and platforms for sensitive biomarkers detection that are exosome-based. For example, Patolsky et al. used Magnetically Amplified DNA Assays (MADA) to improve DNA detection by incorporating nucleic acid-modified magnetic nanoparticles. The method achieved high sensitivity and specificity, identifying single-base mismatches and achieving femtomolar detection limits, demonstrating its potential for advanced diagnostics (293).
Surface of exosomes can also be identified for active targeting, increasing the circulation time and producing the specific drug target vehicle (294) For instance, Stickney Z et al. developed a surface display technology using exosomes and tetraspanins to address the lack of a suitable mammalian display system. They created fluorescent reporters for both inner and outer display, demonstrated successful display, and validated the system in vivo. Their work has potential applications in exosome tracking, targeted drug delivery, and vaccines (295). Additionally, the use of aptamer-drug-exosome has been shown to have higher affinity to tumor environment other than drug-exosome alone (294). Zuo et al. created an aptamer-equipped exosomes (Exos) platform for effective chemotherauetic delivery to cancer targets using diacyllipid–aptamer conjugates. The aptamer-modified Exos (Apt-Exos) can specifically deliver drugs to cancer cells, providing an efficient delivery platform for targeted cancer therapy and diagnosis (296).
Because exosome profiling will contain massive data, machine learning and bioinformatics play an essential role in improving the analytical tools (288). The study developed a prognostic model using disulfidptosis-related gene expression patterns for breast cancer. It identified key DRGs and used unsupervised clustering to define immune subtypes. The model validated four DRGs, indicating potential for personalized treatment strategies (297).
Several ongoing clinical trials are exploring the diagnostic and therapeutic potential of EVs in various cancers (Table 4). These trials focus on utilizing EVs as non-invasive tools for early cancer detection, detection of various cancer-related mutations, and disease monitoring, offering a promising alternative to traditional invasive methods. Trials are furthermore assessing the role of EVs in predicting treatment responses, particularly in immunotherapy and targeted therapies by analyzing several EV-based biomarkers (298). Additionally, EVs are being explored as potential therapeutic agents, including their use in cancer vaccine development and drug delivery systems (298). These advancements highlight the growing interest in EV-based applications for diagnosing and treating cancer, however further research is necessary to fully understand their clinical benefits and practical applications.
The clinical translation of EVs as diagnostic and therapeutic modalities in cancer treatment is currently limited due to several significant challenges. Key challenges include the large-scale isolation of EVs, which remains a complex and costly process, and the heterogeneity of EV populations, which complexes standardization (288). Additionally, scalability and reproducibility in biomarker detection are concerns, as consistent isolation and characterization techniques are crucial for reliable results. The incremental validation of EV-based therapies presents another hurdle, requiring extensive preclinical and clinical testing to establish their safety and efficacy (288). Furthermore, navigating the regulatory landscape for EV-based therapeutics poses substantial challenges, given the need for rigorous validation and approval processes. Overcoming these limitations is essential for the successful clinical application of EVs in cancer therapy.
11 Conclusion
The field of cancer diagnostics and therapeutics is transforming through extra-cellular vesicles, as they present a minimally invasive precise platform for disease management. Through their biomarker role EVs enable important understanding of tumor evolution as well as therapy-related adjustments thereby enabling medical staff to detect tumors early and deliver personalized care. The carriers transport drugs effectively with decreased side effects in the body. Several obstacles stand in the way of clinical deployment because standard isolation methods need improvement alongside scale-up methods and the development of better detection accuracy. The enhancement of clinical diagnostics through EV-based methods can be achieved by implementing advanced EV-based techniques. Future innovations and translational work in the field must continue because ongoing research studies show EVs have a major role in changing cancer treatment through their clinical significance.
Author contributions
YA: Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. FM: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. HM: Writing – original draft, Writing – review & editing. NG: Methodology, Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. IS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AY: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. World Health Organization. Global cancer burden growing, amidst mounting need for services. (2024).
4. Doyle LM and Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. (2019) 8(7):727. doi: 10.3390/cells8070727
5. Tkach M and Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. (2016) 164(6):1226–32. doi: 10.1016/j.cell.2016.01.043
6. Hessvik NP and Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. (2018) 75(2):193–208. doi: 10.1007/s00018-017-2595-9
7. Lv YM, Tan J, Miao Y, and Zhang Q. The role of microvesicles and its active molecules in regulating cellular biology. J Cell Mol Med. (2019) 23(12):7894–904. doi: 10.1111/jcmm.v23.12
8. Cavallari C, Camussi G, and Brizzi MF. Extracellular vesicles in the tumour microenvironment: Eclectic supervisors. Int J Mol Sci. (2020) 21(18):6768. doi: 10.3390/ijms21186768
9. Battistelli M and Falcieri E. Apoptotic bodies: Particular extracellular vesicles involved in intercellular communication. Biology. (2020) 9(1):21. doi: 10.3390/biology9010021
10. Holmgren L, Szeles A, Rajnavölgyi E, Folkman J, Klein G, Ernberg I, et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood (1999) 93(11):3956–63. doi: 10.1182/blood.V93.11.3956
11. Shetty AK and Upadhya R. Extracellular vesicles in health and disease. Aging Dis. (2021) 12(6):1358–62. doi: 10.14336/AD.2021.0827
12. Kozomara A and Griffiths-Jones S. MiRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. (2014) 42. doi: 10.1093/nar/gkt1181
13. Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. (2008) 10(5):619–24. doi: 10.1038/ncb1725
14. Dalla PV, Santos J, Milthorpe BK, and Padula MP. Selectively-packaged proteins in breast cancer extracellular vesicles involved in metastasis. Int J Mol Sci. (2020) 21(14):4990. doi: 10.3390/ijms21144990
15. Dominiak A, Chełstowska B, Olejarz W, and Nowicka G. Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers. (2020) 12(5):1232. doi: 10.3390/cancers12051232
16. Gholizadeh S, Shehata Draz M, Zarghooni M, Sanati-Nezhad A, Ghavami S, Shafiee H, et al. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosensors Bioelectronics. (2017) 91:588–605. doi: 10.1016/j.bios.2016.12.062
17. Théry C, Zitvogel L, and Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. (2002) 2(8):569–79. doi: 10.1038/nri855
18. Raposo G and Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. (2013) 200(4):373–83. doi: 10.1083/jcb.201211138
19. Yáñez-Mó M, Siljander PRM, Andreu Z, Borrás FE, Buzas EI, Buzas K, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracellular Vesicles. (2015) 4:27066. doi: 10.3402/jev.v4.27066
20. Mustafa F. Path of exosomes formation. (2025). Available online at: https://app.biorender.com/profile/template/details/t-677e2b65b541a21652819186-path-of-exosomes-formation
21. Hadizadeh N, Bagheri D, Shamsara M, Hamblin MR, Farmany A, Xu M, et al. Extracellular vesicles biogenesis, isolation, manipulation and genetic engineering for potential in vitro and in vivo therapeutics: An overview. Front Bioengineering Biotechnol. (2022) 10. doi: 10.3389/fbioe.2022.1019821
22. Krylova SV and Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. (2023) 24(2):1337. doi: 10.3390/ijms24021337
23. Van Niel G, D’Angelo G, and Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
24. Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT, et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. (2020) 219(10):e201912074. doi: 10.1083/jcb.201912074
25. Imjeti NS, Menck K, Egea-Jimenez AL, Lecointre C, Lembo F, Bouguenina H, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc Natl Acad Sci U.S.A. (2017) 114:12495–500. doi: 10.1073/pnas.1713433114
26. Lee YJ, Shin KJ, and Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp Mol Med. (2024) 56:877–89. doi: 10.1038/s12276-024-01209-y
27. Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. (2012) 14:677–85. doi: 10.1038/ncb2502
28. Nkosi D, Sun L, Duke LC, et al. Epstein-barr virus lmp1 promotes syntenin-1-and hrsinduced extracellular vesicle formation for its own secretion to increase cell proliferation and migration. mBio. (2020) 11:1–23. doi: 10.1128/mBio.00589-20
29. Zhu H, Guariglia S, Yu RYL, et al. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol Biol Cell. (2013) 24(11):1619–S3. doi: 10.1091/mbc.e12-07-0544
30. Wei D, Zhan W, Gao Y, Wu Z, Yu M, and Wei X. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. (2021) 31(2):157–77. doi: 10.1038/s41422-020-00409-1
31. Leidal AM, Huang HH, Marsh T, Solvik T, Zhang D, Ye J, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. (2020) 22(2):187–99. doi: 10.1038/s41556-019-0450-y
32. van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. (2011) 21(4):708–21. doi: 10.1016/j.devcel.2011.08.019
33. Guan L, Wu B, Li T, Wang L, and Zhang Y. HRS phosphorylation drives immunosuppressive exosome secretion and restricts CD8+ T-cell infiltration into tumors. Nat Commun. (2022) 13(1):4078. doi: 10.1038/s41467-022-31713-6
34. Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, and D’Agostino VG. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J Extracellular Vesicles. (2020) 10(2):e12043. doi: 10.1002/jev2.12043
35. Mustafa F. Exosomal uptake mechanisms. (2025). Available online at: https://app.biorender.com/profile/template/details/t-677e3ae90fa9d82c6152d28f-exosomal-uptake-mechanisms
36. Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Al-Hazmi A, Taha RZ, and Abdel-Mageed MI. Potential effect of exosomes derived from cancer stem cells and MSCs on progression of DEN-Induced HCC in Rats. Stem Cells Int. (2018) 2018:8058979. doi: 10.1155/2018/8058979
37. He G, Peng X, Wei S, Zhang H, and Wu Q. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. (2022) 21(1):19. doi: 10.1186/s12943-021-01440-5
38. Li Q, He G, Yu Y, Li X, Peng X, and Yang L. Exosome crosstalk between cancer stem cells and tumor microenvironment: cancer progression and therapeutic strategies. Stem Cell Res Ther. (2024) 15:449. doi: 10.1186/s13287-024-04061-z
39. Ayob AZ and Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. (2018) 25(1):20. doi: 10.1186/s12929-018-0426-4
40. Tayanloo-Beik A, Eslami A, and Sarvari M. Extracellular vesicles and cancer stem cells: a deadly duo in tumor progression. Oncol Rev. (2024) 18:1411736. doi: 10.3389/or.2024.1411736
41. Alharbi MG, Lee SH, Abdelazim AM, Saadeldin IM, and Abomughaid MM. Role of extracellular vesicles in compromising cellular resilience to environmental stressors. BioMed Res Int. (2021) 2021:9912281. doi: 10.1155/2021/9912281
42. Encarnação CC, Faria GM, Franco VA, Botelho LGX, Moraes JA, and Renovato-Martins M. Interconnections within the tumor microenvironment: extracellular vesicles as critical players of metabolic reprogramming in tumor cells. J Cancer Metastasis Treat. (2024) 10:28. doi: 10.20517/2394-4722.2024.78
43. Bertolini G, Fortunato O, and Lucotti S. Editorial: Role of extracellular vesicles in promoting cancer stem cell properties, tumor aggressiveness and metastasis formation in solid tumors. Front Immunol. (2024) 15:1504676. doi: 10.3389/fimmu.2024.1504676
44. Mustafa F. Methods of exosome isolation. (2025). Available online at: https://app.biorender.com/profile/template/details/t-6799fe6cc1fa0c816deb7094-methods-of-exosome-isolation
45. Brennan K, Martin K, FitzGerald SP, O’Sullivan J, and Blanco A. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. (2020) 10:1–13. doi: 10.1038/s41598-020-57497-7
46. Zhao Z, Wijerathne H, Godwin AK, and Soper SA. Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucl Acids. (2021) 2:80–103. doi: 10.20517/evcna.2021.07
47. Kang H, Kim J, and Park J. Methods to isolate extracellular vesicles for diagnosis. Micro Nano Syst Lett. (2017) 5. doi: 10.1186/s40486-017-0049-7
48. Clos-Sansalvador M, Monguió-Tortajada M, Roura S, Franquesa M, and Borràs FE. Commonly used methods for extracellular vesicles’ enrichment: Implications in downstream analyses and use. Eur J Cell Biol. (2022) 101(3):151227. doi: 10.1016/j.ejcb.2022.151227
49. Shami-Shah A, Travis BG, and Walt DR. Advances in extracellular vesicle isolation methods: a path towards cell-type specific EV isolation. Extracell Vesicles Circ Nucl Acids. (2023) 4:447–60. doi: 10.20517/evcna.2023.14
50. Welsh JA, Goberdhan DCI, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. (2024) 13(2):e12404. doi: 10.1002/jev2.12404
51. Ter-Ovanesyan D, Gilboa T, Budnik B, et al. Improved isolation of extracellular vesicles by removal of both free proteins and lipoproteins. Elife. (2023) 12:1–13. doi: 10.7554/eLife.86394
52. Carnino JM, Lee H, and Jin Y. Isolation and characterization of extracellular vesicles from Broncho-Alveolar lavage fluid: A review and comparison of different methods. Respir Res. (2019) 20:1–11. doi: 10.1186/s12931-019-1210-z
53. Konoshenko MY, Lekchnov EA, Vlassov AV, and Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res Int. (2018) 2018:8545347. doi: 10.1155/2018/8545347
54. Liangsupree T, Multia E, and Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. (2021) 1636:461773. doi: 10.1016/j.chroma.2020.461773
55. Ter-Ovanesyan D, Norman M, Lazarovits R, et al. Framework for rapid comparison of extracellular vesicle isolation methods. Elife. (2021) 10:1–17. doi: 10.7554/eLife.70725
56. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, and Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. (2017) 18(6):1153. doi: 10.3390/ijms18061153
57. Bağcı C, Sever-Bahcekapili M, Belder N, Bennett APS, Erdener ŞE, and Dalkara T. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics. (2022) 9(2):021903. doi: 10.1117/1.NPh.9.2.021903
58. Palmieri V, Lucchetti D, Gatto I, et al. Dynamic light scattering for the characterization and counting of extracellular vesicles: a powerful noninvasive tool. J Nanoparticle Res. (2014) 16:2583. doi: 10.1007/s11051-014-2583-z
59. Kesimer M and Gupta R. Physical characterization and profiling of airway epithelial derived exosomes using light scattering. Methods. (2015) 87:59–63. doi: 10.1016/j.ymeth.2015.03.013
60. Stetefeld J, McKenna SA, and Patel TR. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev. (2016) 8(4):409–27. doi: 10.1007/s12551-016-0218-6
61. Bryant G, Abeynayake C, and Thomas JC. Improved particle size distribution measurements using multiangle dynamic light scattering. 2. Refinements and applications. Langmuir. (1996) 12:6224–8. doi: 10.1021/la00007a028
62. Hoo CM, Starostin N, West P, and Mecartney ML. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J Nanoparticle Res. (2008) 10(Suppl 1):89–96. doi: 10.1007/s11051-008-9435-7
63. Sokolova V, Ludwig AK, Hornung S, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. (2011) 87(1):146–50. doi: 10.1016/j.colsurfb.2011.05.013
64. Thane KE, Davis AM, and Hoffman AM. Improved methods for fluorescent labeling and detection of single extracellular vesicles using nanoparticle tracking analysis. Sci Rep. (2019) 9:12295. doi: 10.1038/s41598-019-48181-6
65. Oosthuyzen W, Sime NEL, Ivy JR, et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol. (2013) 591(23):5833–42. doi: 10.1113/tjp.2013.591.issue-23
66. Comfort N, Cai K, Bloomquist TR, Strait MD, Ferrante AW, and Baccarelli AA. Nanoparticle tracking analysis for the quantification and size determination of extracellular vesicles. J Visualized Experiments. (2021) 2021. doi: 10.3791/62447
67. Fortunato D, Mladenović D, Criscuoli M, et al. Opportunities and pitfalls of fluorescent labeling methodologies for extracellular vesicle profiling on high-resolution single-particle platforms. Int J Mol Sci. (2021) 22(19):10510. doi: 10.3390/ijms221910510
68. Nolan JP. Flow cytometry of extracellular vesicles: Potential, pitfalls, and prospects. Curr Protoc Cytom. (2015) 73:13.14.1–16. doi: 10.1002/0471142956.2015.73.issue-1
69. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, and Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. (2019) 8(4):307. doi: 10.3390/cells8040307
70. Chiang CY and Chen C. Toward characterizing extracellular vesicles at a single-particle level Tse-Hua Tan. J Biomed Sci. (2019) 26(1):9. doi: 10.1186/s12929-019-0502-4
71. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, and Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. (2018) 118(4):1917–50. doi: 10.1021/acs.chemrev.7b00534
72. Ter-Ovanesyan D, Kowal EJK, Regev A, Church GM, and Cocucci E. Imaging of isolated extracellular vesicles using fluorescence microscopy. Methods Mol Biol. (2017) 1660:233–41. doi: 10.1007/978-1-4939-7253-1_19
73. Panagopoulou MS, Wark AW, Birch DJS, and Gregory CD. Phenotypic analysis of extracellular vesicles: a review on the applications of fluorescence. J Extracellular Vesicles. (2020) 9(1):1710020. doi: 10.1080/20013078.2019.1710020
74. Westphal V and Hell SW. Nanoscale resolution in the focal plane of an optical microscope. Phys Rev Lett. (2005) 94(14):143903. doi: 10.1103/PhysRevLett.94.143903
75. Willig KI, Rizzoli SO, Westphal V, Jahn R, and Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. (2006) 440(7086):935–9. doi: 10.1038/nature04592
76. Pisitkun T, Shen RF, and Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U.S.A. (2004) 101(36):13368–73. doi: 10.1073/pnas.0403453101
77. Höög JL and Lötvall J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J Extracell Vesicles. (2015) 4:28680. doi: 10.3402/jev.v4.28680
78. Kadiu I, Narayanasamy P, Dash PK, Zhang W, and Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. (2012) 189(2):744–54. doi: 10.4049/jimmunol.1102244
79. Arraud N, Linares R, Tan S, et al. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J Thromb Haemostasis. (2014) 12(5):614–27. doi: 10.1111/jth.12554
80. Bachurski D, Schuldner M, Nguyen PH, et al. Extracellular vesicle measurements with nanoparticle tracking analysis–An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. (2019) 8(1):1596016. doi: 10.1080/20013078.2019.1596016
81. Malenica M, Vukomanović M, Kurtjak M, et al. Perspectives of microscopy methods for morphology characterisation of extracellular vesicles from human biofluids. Biomedicines. (2021) 9(6):603. doi: 10.3390/biomedicines9060603
82. Dong G, Douanne N, Fernandez-Prada C, and Olivier M. Unique Leishmania mexicana clones secrete populations of extracellular vesicles with unique protein profile and variable infectious capability. Front Cell Infect Microbiol. (2024) 14:1443262. doi: 10.3389/fcimb.2024.1443262
83. Gniadek M and Dąbrowska A. The marine nano- and microplastics characterisation by SEM-EDX: The potential of the method in comparison with various physical and chemical approaches. Mar pollut Bull. (2019) 148:210–6. doi: 10.1016/j.marpolbul.2019.07.067
84. Corcoran PL, Biesinger MC, and Grifi M. Plastics and beaches: A degrading relationship. Mar pollut Bull. (2009) 58(1):80–4. doi: 10.1016/j.marpolbul.2008.08.022
85. Collins SP, Pope RK, Scheetz RW, Ray RI, Wagner PA, and Little BJ. Advantages of environmental scanning electron microscopy in studies of microorganisms. Microsc Res Tech. (1993) 25(5-6):398–405. doi: 10.1002/jemt.1070250508
86. Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia J, van der Vegt B, et al. Atomic force microscopy: A novel approach to the detection of nanosized blood microparticles. J Thromb Haemostasis. (2010) 8(2):315–23. doi: 10.1111/j.1538-7836.2009.03654.x
87. Tiede K, Boxall ABA, Tear SP, Lewis J, David H, and Hassellöv M. Detection and characterization of engineered nanoparticles in food and the environment. In: Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment (York, UK: Taylor & Francis), vol. 25. (2008).
88. Siedlecki CA, Wang IW, Higashi JM, Kottke-Marchant K, and Marchant RE. Platelet-derived microparticles on synthetic surfaces observed by atomic force microscopy and fluorescence microscopy. Biomaterials. (1999) 20(16):1521–9. doi: 10.1016/S0142-9612(99)00065-4
89. Parisse P, Rago I, Ulloa Severino L, Candeloro P, Di Fabrizio E, Borghi F, et al. Atomic force microscopy analysis of extracellular vesicles. Eur Biophysics J. (2017) 46(8):813–20. doi: 10.1007/s00249-017-1252-4
90. Maguire CM, Rösslein M, Wick P, and Prina-Mello A. Characterisation of particles in solution–a perspective on light scattering and comparative technologies. Sci Technol Advanced Materials. (2018) 19(1):732–45. doi: 10.1080/14686996.2018.1517587
91. Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. (2011) 7(6):780–8. doi: 10.1016/j.nano.2011.04.003
92. Desgeorges A, Hollerweger J, Lassacher T, Rohde E, Helmbrecht C, and Gimona M. Differential fluorescence nanoparticle tracking analysis for enumeration of the extracellular vesicle content in mixed particulate solutions. Methods. (2020) 177:67–73. doi: 10.1016/j.ymeth.2020.02.006
93. Koritzinsky EH, Street JM, Star RA, and Yuen PST. Quantification of exosomes. J Cell Physiol. (2017) 232:1587–90. doi: 10.1002/jcp.v232.7
94. Coumans FAW, Gool EL, and Nieuwland R. Bulk immunoassays for analysis of extracellular vesicles. Platelets. (2017) 28(3):242–8. doi: 10.1080/09537104.2016.1265926
95. Kurian TK, Banik S, Gopal D, Chakrabarti S, and Mazumder N. Elucidating methods for isolation and quantification of exosomes: A review. Mol Biotechnol. (2021) 63:249–66. doi: 10.1007/s12033-021-00300-3
96. Gomes J, Lucien F, Cooper TT, Van Goethem E, D'Eer A, de Witte P, et al. Analytical considerations in nanoscale flow cytometry of extracellular vesicles to achieve data linearity. Thromb Haemost. (2018) 118(9):1612–24. doi: 10.1055/s-0038-1668544
97. Padda RS, Deng FK, Brett SI, Ashby JM, Wilson KM, Green JE, et al. Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma. Prostate. (2019) 79(6):592–603. doi: 10.1002/pros.23764
98. Mondal A, Ashiq KA, Phulpagar P, Singh DK, and Shiras A. Effective visualization and easy tracking of extracellular vesicles in glioma cells. Biol Proced Online. (2019) 21:4. doi: 10.1186/s12575-019-0092-2
99. Yuana Y, Koning RI, Kuil ME, Rensen PCN, and Koster AJ. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. (2013) 2. doi: 10.3402/jev.v2i0.21494
100. Yang JE, Rossignol ED, Chang D, Wang L, Gharibyan V, Cansizoglu AE, et al. Complexity and ultrastructure of infectious extracellular vesicles from cells infected by non-enveloped virus. Sci Rep. (2020) 10:7939. doi: 10.1038/s41598-020-64531-1
101. Votteler J, Ogohara C, Yi S, Hsia Y, Nattermann U, Belnap DM, et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature. (2016) 540(7632):292–5. doi: 10.1038/nature20607
102. Brisson AR, Tan S, Linares R, Gounou C, and Arraud N. Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets. (2017) 28(3):263–71. doi: 10.1080/09537104.2016.1268255
103. Goughnour PC, Park MC, Kim SB, Snyder AP, Meehan BM, Stöckl S, et al. Extracellular vesicles derived from macrophages display glycyl-tRNA synthetase 1 and exhibit anti-cancer activity. J Extracell Vesicles. (2020) 10(1):e12029. doi: 10.1002/jev2.12029
104. Stokroos I, Kalicharan D, van der Want JJL, and Jongebloed WL. A comparative study of thin coatings of Au/Pd, Pt and Cr produced by magnetron sputtering for FE-SEM. J Microsc. (1998) 189(Pt 1):79–89. doi: 10.1046/j.1365-2818.1998.00282.x
105. Caldwell J, Lehner R, Balog S, et al. Fluorescent plastic nanoparticles to track their interaction and fate in physiological environments. Environ Sci Nano. (2021) 8(2):502–13. doi: 10.1039/D0EN00944J
106. Fotopoulou KN and Karapanagioti HK. Surface properties of beached plastic pellets. Mar Environ Res. (2012) 81:70–7. doi: 10.1016/j.marenvres.2012.08.010
107. Bagrov DV, Senkovenko AM, Nikishin II, Skryabin GO, Kopnin PB, and Tchevkina EM. Application of AFM, TEM, and NTA for characterization of exosomes produced by placenta-derived mesenchymal cells. J Physics: Conf Ser. (2021) 1942. doi: 10.1088/1742-6596/1942/1/012013
108. Li MI, Xu X, Xi N, Wang W, Xing X, and Liu L. Multiparametric atomic force microscopy imaging of single native exosomes. Acta Biochim Biophys Sin (Shanghai). (2021) 53(3):385–8. doi: 10.1093/abbs/gmaa172
109. Li W, Luo Y, and Pan X. Identification and characterization methods for microplastics basing on spatial imaging in micro-/nanoscales. In: Handbook of Environmental Chemistry. Berlin: Springer (2020).
110. Meyer E, Howald L, Overney R, Lüthi R, Gyalog T, Güntherodt HJ, et al. Structure and dynamics of solid surfaces observed by atomic force microscopy. Ultramicroscopy. (1992) 42–44. doi: 10.1016/0304-3991(92)90279-S
111. De Sousa KP, Rossi I, Abdullahi M, Ramirez MI, Stratton D, and Inal JM. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip Reviews: Nanomedicine Nanobiotechnology. (2023) 15(1):e1835. doi: 10.1002/wnan.v15.1
112. Whiteside TL. Biology of extracellular vesicles and the potential of tumor-derived vesicles for subverting immunotherapy of cancer. J Immunotherapy Cancer. (2025) 13:e010376. doi: 10.1136/jitc-2024-010376
113. Huang D, Rao D, Xi X, Zhang Z, and Zhong T. Application of extracellular vesicles proteins in cancer diagnosis. Front Cell Dev Biol. (2022) 10. doi: 10.3389/fcell.2022.1007360
114. Xue Y, Feng X, Fan X, Yu C, Liu Y, Wang J, et al. Extracellular vesicles for the diagnosis of cancers. Small Structures. (2022) 3:2100096. doi: 10.1002/sstr.202100096
115. Alavanda C, Dirimtekin E, Mortoglou M, Arslan Ates E, Guney AI, and Uysal-Onganer P. BRCA mutations and microRNA expression patterns in the peripheral blood of breast cancer patients. In: ACS Omega. Washington, DC: American Chemical Society (2023).
116. Nanou A, Zeune LL, Bidard FC, Pierga JY, and Terstappen LWMM. HER2 expression on tumor-derived extracellular vesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res. (2020) 22(1):86. doi: 10.1186/s13058-020-01323-5
117. Koutsaki M, Libra M, Spandidos DA, and Zaravinos A. The miR-200 family in ovarian cancer. Oncotarget. (2017) 8(41):68966–96. doi: 10.18632/oncotarget.18343
118. Huang J, Ding M, Lu Y, et al. MiR-1246b, a novel miRNA molecule of extracellular vesicles in bronchoalveolar lavage fluid, promotes nodule growth through FGF14 in patients with lung cancer. Cell Death Dis. (2023) 14(1):446. doi: 10.1038/s41419-023-06218-9
119. Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. (2013) 34(3):163–9. doi: 10.1155/2013/259454
120. Liu M, Mo F, Song X, et al. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ. (2021) 9:e12147. doi: 10.7717/peerj.12147
121. Wang F, Wang J, Zhang H, et al. Diagnostic value of circulating miR-155 for breast cancer: a meta-analysis. Front Oncol. (2024) 14. doi: 10.3389/fonc.2024.1374674
122. Luo J, Zhao Q, Zhang W, et al. A novel panel of microRNAs provides a sensitive and specific tool for the diagnosis of breast cancer. Mol Med Rep. (2014) 10:(2):785–91. doi: 10.3892/mmr.2014.2274
123. Li X, Tang X, Li K, and Lu L. Evaluation of Serum MicroRNAs (miR-9-5p, miR-17-5p, and miR-148a-3p) as Potential Biomarkers of Breast Cancer. BioMed Res Int. (2022) 2022. doi: 10.1155/2022/9961412
124. Sheedy P and Medarova Z. The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res. (2018) 8(9):1674–88. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6176190/.
125. Gao W, Pang D, and Yu S. Serum level of miR-142-3p predicts prognostic outcome for colorectal cancer following curative resection. J Int Med Res. (2019) 47(3):1176–83. doi: 10.1177/0300060519834815
126. Lin Z, Chen Y, Lin Y, Huang Y, Zhang L, Li J, et al. Potential miRNA biomarkers for the diagnosis and prognosis of esophageal cancer detected by a novel absolute quantitative RT-qPCR method. Sci Rep. (2020) 10(1):20065. doi: 10.1038/s41598-020-77119-6
127. Min L, Zhu S, Chen L, Liu X, Wei R, Wang J, et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J Extracell Vesicles. (2019) 8(1):1643670. doi: 10.1080/20013078.2019.1643670
128. Umwali Y, Yue CB, Zhang Y, Zhang X, and Gabriel ANA. Roles of exosomes in diagnosis and treatment of colorectal cancer. World J Clin cases. (2021) 9:4467–79. doi: 10.12998/wjcc.v9.i18.4467
129. Karimi N, Feizi MAH, Safaralizadeh R, Hasanzadeh M, Ghanbari R, Ghojazadeh M, et al. Serum overexpression of miR-301a and miR-23a in patients with colorectal cancer. J Chin Med Assoc. (2019) 82:215–20. doi: 10.1097/JCMA.0000000000000031
130. Min L, Chen L, Liu S, Zheng Z, Zhang J, Xu X, et al. Loss of circulating exosomal miR-92b is a novel biomarker of colorectal cancer at early stage. Int J Med Sci. (2019) 16:1231–7. doi: 10.7150/ijms.34540
131. Tang Y, Zhao Y, Song X, Song X, Niu L, and Xie L. Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer. J Clin Lab Anal. (2019) 33(9):e23004. doi: 10.1002/jcla.23004
132. Liu X, Pan B, Sun L, Chen X, Wang Y, Bai P, et al. Circulating exosomal miR-27a and miR-130a act as novel diagnostic and prognostic biomarkers of colorectal cancer. Cancer Epidemiol Biomarkers Prev. (2018) 27(7):746–54. doi: 10.1158/1055-9965.EPI-18-0067
133. Sun L, Liu X, Pan B, Hu X, Zhu Y, Su Y, et al. Serum exosomal miR-122 as a potential diagnostic and prognostic biomarker of colorectal cancer with liver metastasis. J Cancer. (2020) 11(3):630–7. doi: 10.7150/jca.33022
134. Zhao YJ, Song X, Niu L, Tang Y, Song X, and Xie L. Circulating exosomal mir-150-5p and mir-99b-5p as diagnostic biomarkers for colorectal cancer. Front Oncol. (2019) 9. doi: 10.3389/fonc.2019.01129
135. Li J, Yang P, Chen F, et al. Hypoxic colorectal cancer-derived extracellular vesicles deliver microRNA-361-3p to facilitate cell proliferation by targeting TRAF3 via the noncanonical NF-κB pathways. Clin Transl Med. (2021) 11(3):e349. doi: 10.1002/ctm2.v11.3
136. Xu YF, Hannafon BN, Zhao YD, Postier RG, and Ding WQ. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget. (2017) 8(44):77028–40. doi: 10.18632/oncotarget.20332
137. Goto T, Fujiya M, Konishi H, et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. (2018) 18(1):116. doi: 10.1186/s12885-018-4006-5
138. Machida T, Tomofuji T, Maruyama T, et al. MIR 1246 and MIR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol Rep. (2016) 36(4):2375–81. doi: 10.3892/or.2016.5021
139. Wang J, Chen J, Chang P, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res. (2009) 2(9):807–13. doi: 10.1158/1940-6207.CAPR-09-0094
140. Zhou X, Yan Y, Shen Y, Xu M, and Xu W. Exosomes: emerging insights into the progression of pancreatic cancer. Int J Biol Sci. (2024) 20:4098–113. doi: 10.7150/ijbs.97076
141. Yang J, Zhang Y, Gao X, et al. Plasma-derived exosomal ALIX as a novel biomarker for diagnosis and classification of pancreatic cancer. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.628346
142. Shao H, Zhang Y, Yan J, et al. Upregulated microRNA-483-3p is an early event in pancreatic ductal adenocarcinoma (PDAC) and as a powerful liquid biopsy biomarker in PDAC. Onco Targets Ther. (2021) 14:2163–75. doi: 10.2147/OTT.S288936
143. Yoshizawa N, Sugimoto K, Tameda M, et al. MiR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic biomarker for pancreatic ductal adenocarcinoma. Oncol Lett. (2020) 19(4):2677–84. doi: 10.3892/ol.2020.11357
144. Melo SA, Luecke LB, Kahlert C, et al. Author Correction: Glypican-1 identifies cancer exosomes and detects early pancreatic cancer (Nature, (2015), 523, 7559, (177-182), 10.1038/nature14581). Nature. (2015) 610:2022. doi: 10.1038/nature14581
145. Lux A, Kahlert C, Grützmann R, and Pilarsky C. c-Met and PD-l1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci. (2019) 20(13):3305. doi: 10.3390/ijms20133305
146. Park SJ, Park JY, Shin K, et al. Clinical significance of serum-derived exosomal PD-L1 expression in patients with advanced pancreatic cancer. BMC Cancer. (2023) 23(1):389. doi: 10.1186/s12885-023-10811-8
147. Sonar S. Decoding the mysteries of prostate cancer via cutting-edge liquid biopsy-based urine exosomes profiling. J Liquid Biopsy. (2024) 6:100162. doi: 10.1016/j.jlb.2024.100162
148. Chen L, Hu W, Li G, Guo Y, Wan Z, and Yu J. Inhibition of miR-9-5p suppresses prostate cancer progress by targeting StarD13. Cell Mol Biol Lett. (2019) 24(1):30. doi: 10.1186/s11658-019-0145-1
149. Rodríguez M, Bajo-Santos C, Hessvik NP, et al. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer. (2017) 16(1):156. doi: 10.1186/s12943-017-0726-4
150. Işin M, Uysaler E, Özgür E, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. (2015) 6. doi: 10.3389/fgene.2015.00168
151. Overbye A, Skotland T, Koehler CJ, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. (2015) 6(30):30357–76. doi: 10.18632/oncotarget.v6i30
152. Yuan L, Chen Y, Ke L, et al. Correction: Plasma extracellular vesicle phenotyping for the differentiation of early-stage lung cancer and benign lung diseases. Nanoscale Horiz. (2023) 8(4):578. doi: 10.1039/D3NH90028B
153. Jiang Yf, Wei S, Geng N, et al. Evaluation of circulating small extracellular vesicle-derived miRNAs as diagnostic biomarkers for differentiating between different pathological types of early lung cancer. Sci Rep. (2022) 12(1):6312. doi: 10.1038/s41598-022-22194-0
154. Fang H, Liu Y, He Y, et al. Extracellular vesicle-delivered miR-505-5p, as a diagnostic biomarker of early lung adenocarcinoma, inhibits cell apoptosis by targeting TP53AIP1. Int J Oncol. (2019) 54(5):1625–36. doi: 10.3892/ijo.2019.4738
155. Batool SM, Muralidharan K, Hsia T, et al. Highly sensitive EGFRvIII detection in circulating extracellular vesicle RNA of glioma patients. Clin Cancer Res. (2022) 28(13):2844–54. doi: 10.1158/1078-0432.CCR-22-0444
156. Li C, Zheng X, Li W, Bai F, Lyu J, and Meng QH. Serum miR-486-5p as a diagnostic marker in cervical cancer: With investigation of potential mechanisms. BMC Cancer. (2018) 18(1):70. doi: 10.1186/s12885-017-3753-z
157. Molika P, Leetanaporn K, Rungkamoltip P, Roytrakul S, Hanprasertpong J, and Navakanitworakul R. Proteomic analysis of small extracellular vesicles unique to cervical cancer. Transl Cancer Res. (2023) 12(4):1416–27. doi: 10.21037/tcr-23-517
158. Yang L, Yang Z, Liu Z, Qi N, and Tao L. Diagnostic value of plasma-derived exosomal miR-223 for epithelial ovarian cancer. BMC Womens Health. (2024) 24(1):45. doi: 10.1186/s12905-024-02976-6
159. Nimitrungtawee N, Inmutto N, Chattipakorn SC, and Chattipakorn N. Extracellular vesicles as a new hope for diagnosis and therapeutic intervention for hepatocellular carcinoma. Cancer Med. (2021) 10(5):1641–52. doi: 10.1002/cam4.v10.23
160. Pan JH, Zhou H, Zhao XX, et al. Role of exosomes and exosomal microRNAs in hepatocellular carcinoma: Potential in diagnosis and antitumour treatments (Review). Int J Mol Med. (2018) 41(3):1189–99. doi: 10.3892/ijmm.2018.3383
161. Mohankumar S and Patel T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Brief Funct Genomics. (2016) 15(2):121–8. doi: 10.1093/bfgp/elv058
162. Juratli MA, Pollmann NS, Oppermann E, et al. Extracellular vesicles as potential biomarkers for diagnosis and recurrence detection of hepatocellular carcinoma. Sci Rep. (2024) 14(1):2436. doi: 10.1038/s41598-024-55888-8
163. Li D, Jia S, Wang S, and Hu L. Glycoproteomic analysis of urinary extracellular vesicles for biomarkers of hepatocellular carcinoma. Molecules. (2023) 28(3):1293. doi: 10.3390/molecules28031293
164. Kim SS, Baek GO, Son JA, et al. Early detection of hepatocellular carcinoma via liquid biopsy: panel of small extracellular vesicle-derived long noncoding RNAs identified as markers. Mol Oncol. (2021) 15(7):1966–79. doi: 10.1002/1878-0261.13049
165. Ren R, Sun H, Ma C, Liu J, and Wang H. Colon cancer cells secrete exosomes to promote self-proliferation by shortening mitosis duration and activation of STAT3 in a hypoxic environment. Cell Biosci. (2019) 9(1):44. doi: 10.1186/s13578-019-0325-8
166. Liu H, Liu Y, Sun P, et al. Colorectal cancer-derived exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1 expression. Clin Sci. (2020) 134(10):1177–93. doi: 10.1042/CS20191087
167. Ballehaninna UK and Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. (2012) 3(3):105–19. doi: 10.3978/j.issn.2078-6891.2011.021
168. Li Z, Tao Y, Wang X, et al. Tumor-secreted exosomal miR-222 promotes tumor progression via regulating P27 expression and re-localization in pancreatic cancer. Cell Physiol Biochem. (2018) 51(2):786–99. doi: 10.1159/000495281
169. Herreros-Villanueva M, Zhang JS, Koenig A, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. (2013) 2:e61. doi: 10.1038/oncsis.2013.23
170. Qin C, Li T, Lin C, et al. The systematic role of pancreatic cancer exosomes: distant communication, liquid biopsy and future therapy. Cancer Cell Int. (2024) 24(1):56. doi: 10.1186/s12935-024-03456-5
171. Nogueira L, Corradi R, and Eastham JA. Other biomarkers for detecting prostate cancer. BJU Int. (2010) 105(8):1148–54. doi: 10.1111/j.1464-410X.2009.09088.x
172. Dhar R, Devi A, Gorai S, Jha SK, Alexiou A, and Papadakis M. Exosome and epithelial–mesenchymal transition: A complex secret of cancer progression. J Cell Mol Med. (2023) 27(11):3017–30. doi: 10.1111/jcmm.v27.11
173. Logozzi M, Angelini DF, Giuliani A, et al. Increased plasmatic levels of psa-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: A prospective study. Cancers (Basel). (2019) 11(10):1449. doi: 10.3390/cancers11101449
174. Tutrone R, Lowentritt B, Neuman B, et al. ExoDx prostate test as a predictor of outcomes of high-grade prostate cancer – an interim analysis. Prostate Cancer Prostatic Dis. (2023) 26(1):234–40. doi: 10.1038/s41391-023-00675-1
175. Exosomedx Bio-Techne Exosome. The ExoDxTM Prostate Test. Available online at: https://www.exosomedx.com/physicians/exodx-prostate-test
176. Medina JE, Annapragada AV, Lof P, et al. Early detection of ovarian cancer using cell-free DNA fragmentomes and protein biomarkers. Cancer Discovery. (2025) 15:105–18. doi: 10.1158/2159-8290.CD-24-0393
177. Meng Y, Yao Z, Ke X, Zhang Q, Wang Y, Wang X, et al. Extracellular vesicles-based vaccines: Emerging immunotherapies against cancer. J Control Release. (2024) 378:438–59. doi: 10.1016/j.jconrel.2024.12.010
178. Hsu C-Y, Pallathadka H, Jasim SA, Alnasser HA, Al-Kahtani A, Nazir S, et al. Innovations in cancer immunotherapy: A comprehensive overview of recent breakthroughs and future directions. Crit Rev Oncol Hematol. (2024) 206:104588. doi: 10.1016/j.critrevonc.2024.104588
179. Cho H, Jung I, Ju H, Baek MC, and Yea K. Engineered CD8+ T cell-derived extracellular vesicles induce enhanced anti-cancer efficacy and targeting to lung cancer cells. Cytokine. (2023) 169:156249. doi: 10.1016/j.cyto.2023.156249
180. Habibizadeh M, Lotfollahzadeh S, Mahdavi P, Mohammadi S, and Tavallaei O. Nanoparticle-mediated gene delivery of TRAIL to resistant cancer cells: A review. Heliyon. (2024) 10:e36057. doi: 10.1016/j.heliyon.2024.e36057
181. Jiang L, Gu Y, Du Y, Tang X, Wu X, and Liu J. Engineering exosomes endowed with targeted delivery of triptolide for Malignant melanoma therapy. ACS Appl Mater Interfaces. (2021) 13:42411–28. doi: 10.1021/acsami.1c10325
182. Qiu Y, Sun J, Qiu J, Chen G, Wang X, Mu Y, et al. Antitumor activity of cabazitaxel and MSC-TRAIL derived extracellular vesicles in drug-resistant oral squamous cell carcinoma. Cancer Manag Res. (2020) 12:10809–20. doi: 10.2147/CMAR.S277324
183. Chen F, Xu B, Li J, Yang X, Gu J, Yao X, et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. (2021) 40(1):38. doi: 10.1186/s13046-021-01834-9
184. Yuan Q, Su K, Li S, Long X, Liu L, Yang M, et al. Pulmonary delivery of extracellular vesicle-encapsulated dinaciclib as an effective lung cancer therapy. Cancers (Basel). (2022) 14:3550. doi: 10.3390/cancers14143550
185. Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol. (2021) 4(1):1175. doi: 10.1038/s42003-021-02004-5
186. McAndrews KM, Che SPY, LeBleu VS, and Kalluri R. Effective delivery of STING agonist using exosomes suppresses tumor growth and enhances antitumor immunity. J Biol Chem. (2021) 296:100523. doi: 10.1016/j.jbc.2021.100523
187. Zhang C, Qin C, Dewanjee S, Liu W, Li M, Chen X, et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Mol Cancer. (2024) 23(1):43. doi: 10.1186/s12943-024-01932-0
188. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. (2019) 177(2):414–427.e13. doi: 10.1016/j.cell.2019.02.016
189. Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. (2021) 268:120546. doi: 10.1016/j.biomaterials.2020.120546
190. Dan Y, Ma J, Long Y, Jiang Y, Fang L, and Bai J. Melanoma extracellular vesicles inhibit tumor growth and metastasis by stimulating CD8 T cells. Mol Immunol. (2024) 169:78–85. doi: 10.1016/j.molimm.2024.03.003
191. Ma J, Wei K, Zhang H, Tang K, Li F, Zhang T, et al. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8 þ T cells. Cancer Immunol Res. (2018) 6:1057–68. doi: 10.1158/2326-6066.CIR-17-0716
192. Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, and Duroux M. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. (2014) 1846:75–87. doi: 10.1016/j.bbcan.2014.04.005
193. Tibbitt MW, Dahlman JE, and Langer R. Emerging frontiers in drug delivery. J Am Chem Soc. (2016) 138:704–17. doi: 10.1021/jacs.5b09974
194. Haney MJ, Zhao Y, Jin YS, Wang Q, Klyachko NL, Kabanov AV, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol. (2020) 15:487–500. doi: 10.1007/s11481-019-09884-9
195. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. (2015) 12:655. doi: 10.1016/j.nano.2015.10.012
196. Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine. (2019) 14:8603. doi: 10.2147/IJN.S218988
197. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, and Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. (2020) 101:519–30. doi: 10.1016/j.actbio.2019.10.022
198. Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm Res. (2015) 32:2003. doi: 10.1007/s11095-014-1593-y
199. Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, and Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. (2020) 261:118369. doi: 10.1016/j.lfs.2020.118369
200. Hosseini NF, Amini R, Ramezani M, Saidijam M, Hashemi SM, and Najafi R. AS1411 aptamer-functionalized exosomes in the targeted delivery of doxorubicin in fighting colorectal cancer. Biomedicine Pharmacotherapy. (2022) 155:113690. doi: 10.1016/j.biopha.2022.113690
201. Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to Malignant tumors. ACS Nano. (2013) 7:7698–710. doi: 10.1021/nn402232g
202. Armstrong JPK, Holme MN, and Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. (2017) 11:69. doi: 10.1021/acsnano.6b07607
203. Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. (2020) 18:1–15. doi: 10.1186/s12951-019-0560-5
204. Wang T, Luo Y, Lv H, Wang J, Zhang Y, and Pei R. Aptamer-based erythrocyte-derived mimic vesicles loaded with siRNA and doxorubicin for the targeted treatment of multidrug-resistant tumors. ACS Appl Mater Interfaces. (2019) 11:45455–66. doi: 10.1021/acsami.9b16637
205. Li S, Wu Y, Ding F, Yang J, Li J, Gao X, et al. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. (2020) 12:10854–62. doi: 10.1039/D0NR00523A
206. Diehl CJ and Ciulli A. Discovery of small molecule ligands for the von Hippel-Lindau (VHL) E3 ligase and their use as inhibitors and PROTAC degraders. Chem Soc Rev. (2022) 51(1):261–94. doi: 10.1039/D2CS00387B
207. Chirnomas D, Hornberger KR, and Crews CM. Protein degraders enter the clinic - a new approach to cancer therapy. Nat Rev Clin Oncol. (2023) 20(2):85–100. doi: 10.1038/s41571-023-00736-3
208. Yim J, Park J, Kim G, Jo A, Koh M, and Park J. Conditional PROTAC: recent strategies for modulating targeted protein degradation. ChemMedChem. (2024) 20:265–78. doi: 10.1002/cmdc.202400326
209. Zhang NY, Hou DY, Hu XJ, Liang JX, Wang MD, Song ZZ, et al. Nano proteolysis targeting chimeras (PROTACs) with anti-hook effect for tumor therapy. Angewandte Chemie - Int Edition. (2023) 62(37):e202308049. doi: 10.1002/anie.202308049
210. Li D, Yu D, Li Y, and Yang R. A bibliometric analysis of PROTAC from 2001 to 2021. Eur J Medicinal Chem. (2022) 244. doi: 10.1016/j.ejmech.2022.114838
211. Li X, Zhang Z, Gao F, Ma Y, Wei D, Lu Z, et al. c-myc-targeting PROTAC based on a TNA-DNA bivalent binder for combination therapy of triple-negative breast cancer. J Am Chem Soc. (2023) 145. doi: 10.1021/jacs.3c02619
212. Huang J, Lin B, and Li B. Anti-androgen receptor therapies in prostate cancer: A brief update and perspective. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.865350
213. Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. (2019) 52(5):e12669. doi: 10.1111/cpr.12669
214. O’Brien CG, Ozen MO, Ikeda G, Vaskova E, Jung JH, Bayardo N, et al. Mitochondria-rich extracellular vesicles rescue patient-specific cardiomyocytes from doxorubicin injury: insights into the SENECA trial. JACC CardioOncol. (2021) 3(2):255–67. doi: 10.1016/j.jaccao.2021.05.006
215. Sun H, Bhandari K, Burrola S, Wu J, and Ding WQ. Pancreatic ductal cell-derived extracellular vesicles are effective drug carriers to enhance paclitaxel’s efficacy in pancreatic cancer cells through clathrin-mediated endocytosis. Int J Mol Sci. (2022) 23(9):4773. doi: 10.3390/ijms23094773
216. Fischer T, Winter I, Drumm R, and Schneider M. Cylindrical microparticles composed of mesoporous silica nanoparticles for the targeted delivery of a small molecule and a macromolecular drug to the lungs: Exemplified with curcumin and siRNA. Pharmaceutics. (2021) 13(6):844. doi: 10.3390/pharmaceutics13060844
217. Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Advanced Sci. (2019) 6(20):1801899. doi: 10.1002/advs.201801899
218. Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Controlled Release. (2013) 172(1):229–38. doi: 10.1016/j.jconrel.2013.08.014
219. Yerneni SS, Yalcintas EP, Smith JD, Averick S, Campbell PG, and Ozdoganlar OB. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. (2022) 149:241–53. doi: 10.1016/j.actbio.2022.06.046
220. Tang P, Song F, Chen Y, et al. Preparation and characterization of extracellular vesicles and their cutting-edge applications in regenerative medicine. Appl Materials Today. (2024) 37:102084. doi: 10.1016/j.apmt.2024.102084
221. Hettich BF, Bader JJ, and Leroux JC. Encapsulation of hydrophilic compounds in small extracellular vesicles: loading capacity and impact on vesicle functions. Adv Healthc Mater. (2022) 11(6):2100047. doi: 10.1002/adhm.202100047
222. Liu Y, Xia P, Yan F, Yuan M, Yuan H, Du Y, et al. Engineered extracellular vesicles for delivery of an IL-1 receptor antagonist promote targeted repair of retinal degeneration. Small. (2023) 19(16):2302962. doi: 10.1002/smll.202302962
223. Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, et al. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl Mater Interfaces. (2016) 8(9):5852–61. doi: 10.1021/acsami.6b01315
224. Du J, Wan Z, Wang C, Lu F, Wei M, Wang D, et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. (2021) 11(18):8768–84. doi: 10.7150/thno.59121
225. Semeradtova A, Liegertova M, Herma R, Capkova M, Brignole C, and Del Zotto G. Extracellular vesicles in cancer´s communication: messages we can read and how to answer. Mol Cancer. (2025) 24. doi: 10.1186/s12943-025-02282-1
226. Budgude P, Kale V, and Vaidya A. Cryopreservation of mesenchymal stromal cell-derived extracellular vesicles using trehalose maintains their ability to expand hematopoietic stem cells in vitro. Cryobiology. (2021) 98:103–109. doi: 10.1016/j.cryobiol.2020.11.009
227. Ahmadian S, Jafari N, Tamadon A, Ghaffarzadeh A, Rahbarghazi R, and Mahdipour M. Different storage and freezing protocols for extracellular vesicles: a systematic review. Stem Cell Res Ther. (2024) 15(1):95. doi: 10.1186/s13287-024-04005-7
228. Akers JC, Ramakrishnan V, Yang I, Hua W, Mao Y, Carter BS, et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomarkers. (2016) 17(4):357–66. doi: 10.3233/CBM-160609
229. Saenz-de-Juano MD, Silvestrelli G, and Ulbrich SE. One-week storage of refrigerated bovine milk does not affect the size, concentration, or molecular properties of extracellular vesicles. J Dairy Sci. (2024) 107(2):1164–74. doi: 10.3168/jds.2023-23726
230. Bosch S, De Beaurepaire L, Allard M, Mosser M, Heichette C, Chretien D, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. (2016) 6:36162. doi: 10.1038/srep36162
231. Guarro M, Suñer F, Lecina M, Borrós S, and Fornaguera C. Efficient extracellular vesicles freeze-dry method for direct formulations preparation and use. Colloids Surf B Biointerfaces. (2022) 218:112745. doi: 10.1016/j.colsurfb.2022.112745
232. Görgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U, et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. (2022) 11(2):e12238. doi: 10.1002/jev2.12238
233. Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. (2013) 13(22):3354–64. doi: 10.1002/pmic.201300282
234. Susa F, Limongi T, Borgione F, Peiretti S, Vallino M, Cauda V, et al. Comparative studies of different preservation methods and relative freeze-drying formulations for extracellular vesicle pharmaceutical applications. ACS Biomater Sci Eng. (2023) 9(1):123–34. doi: 10.1021/acsbiomaterials.3c00678
235. Gelibter S, Marostica G, Mandelli A, Siciliani S, Podini P, Finardi A, et al. The impact of storage on extracellular vesicles: A systematic study. J Extracell Vesicles. (2022) 11(1):e12162. doi: 10.1002/jev2.12162
236. Lara P, Chan AB, Cruz LJ, Quest AFG, and Kogan MJ. Exploiting the natural properties of extracellular vesicles in targeted delivery towards specific cells and tissues. Pharmaceutics. (2020) 12(11):1022. doi: 10.3390/pharmaceutics12111022
237. Uddin MJ, Mohite P, Munde S, Ade N, Oladosu TA, Chidrawar VR, et al. Extracellular vesicles: The future of therapeutics and drug delivery systems. Intelligent Pharm. (2024) 2:100004. doi: 10.1016/j.ipha.2024.02.004
238. Rakshit T and Pal S. Extracellular vesicles for drug delivery and theranostics in vivo. JACS Au. (2024) 4(2):318–27. doi: 10.1021/jacsau.3c00611
239. Payandeh Z, Tangruksa B, Synnergren J, Heydarkhan-Hagvall S, Nordin JZ, El Andaloussi SE, et al. Extracellular vesicles transport RNA between cells: Unraveling their dual role in diagnostics and therapeutics. Mol Aspects Med. (2024) 99:101302. doi: 10.1016/j.mam.2024.101302
240. Rana S, Yue S, Stadel D, and Zöller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. (2012) 44(9):1574–84. doi: 10.1016/j.biocel.2012.06.018
241. Song H, Chen X, Hao Y, Wang J, Xie Q, and Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. (2022) 20:431. doi: 10.1186/s12951-022-01638-9
242. Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y, et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. (2019) 9(25):7680–96. doi: 10.7150/thno.37220
243. Näslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, and Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. (2014) 28(2):171–80. doi: 10.1097/QAD.0000000000000159
244. Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J Pharm Sci. (2017) 106(1):168–75. doi: 10.1016/j.xphs.2016.07.022
245. Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B, et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles. (2018) 7(1):1446660. doi: 10.1080/20013078.2018.1446660
246. Lara P, Palma-Florez S, Salas-Huenuleo E, Polakovicova I, Guerrero S, Lobos-Gonzalez L, et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J Nanobiotechnology. (2020) 18:20. doi: 10.1186/s12951-020-0573-0
247. Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. (2014) 33(5):1055–63. doi: 10.3892/ijmm.2014.1663
248. Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, et al. Urinary exosomes-based Engineered Nanovectors for Homologously Targeted Chemo-Chemodynamic Prostate Cancer Therapy via abrogating IGFR/AKT/NF-kB/IkB signaling. Biomaterials. (2021) 275:120867. doi: 10.1016/j.biomaterials.2021.120946
249. Buschow SI, Van Balkom BWM, Aalberts M, Heck AJR, Wauben M, and Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. (2010) 88(8):851–6. doi: 10.1038/icb.2010.64
250. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. (2018) 197(1):104–16. doi: 10.1164/rccm.201705-0925OC
251. Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, et al. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. (2019) 19(5):3040–8. doi: 10.1021/acs.nanolett.9b00287
252. Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PloS One. (2013) 8(12):e84256. doi: 10.1371/journal.pone.0084256
253. Petővári G, Tóth G, Turiák L, Kiss AL, Pálóczi K, Sebestyén A, et al. Dynamic interplay in tumor ecosystems: communication between hepatoma cells and fibroblasts. Int J Mol Sci. (2023) 24(18):13996. doi: 10.3390/ijms241813996
254. Xia W, Tan Y, Liu Y, Xie N, and Zhu H. Prospect of extracellular vesicles in tumor immunotherapy. Front Immunol. (2025) 16. doi: 10.3389/fimmu.2025.1525052
255. Ji R, Lin J, Gu H, Ma J, Fu M, and Zhang X. Gastric cancer derived mesenchymal stem cells promote the migration of gastric cancer cells through miR-374a-5p. Curr Stem Cell Res Ther. (2022) 18(6):853–63. doi: 10.2174/1574888x18666221124145847
256. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, and Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.02278
257. Aarsund M, Segers FM, Wu Y, and Inngjerdingen M. Comparison of characteristics and tumor targeting properties of extracellular vesicles derived from primary NK cells or NK-cell lines stimulated with IL-15 or IL-12/15/18. Cancer Immunology Immunotherapy. (2022) 71:231. doi: 10.1007/s00262-022-03161-0
258. Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. (2019) 10:3838. doi: 10.1038/s41467-019-11718-4
259. Montecalvo A, Shufesky WJ, Beer Stolz D, Sullivan MG, Wang Z, Divito S, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. (2008) 180(5):3081–90. doi: 10.4049/jimmunol.180.5.3081
260. Lee KM, An JH, Yang SJ, Park S-M, Lee J-H, Chae H-K, et al. Influence of canine macrophage-derived extracellular vesicles on apoptosis in canine melanoma and osteosarcoma cell lines. Anticancer Res. (2021) 41(11):5409–17. doi: 10.21873/anticanres.14823
262. Kim J and De Jesus O. Medication Routes of Administration. St. Petersburg, Florida, USA: StatPearls [Internet] (2023).
263. Wiklander OPB, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. (2015) 4:26316. doi: 10.3402/jev.v4.26316
264. Zhou X, Li Z, Sun W, Yang G, Xing C, and Yuan L. Delivery efficacy differences of intravenous and intraperitoneal injection of exosomes: perspectives from tracking dye labeled and miRNA encapsulated exosomes. Curr Drug Delivery. (2020) 17(3):206–14. doi: 10.2174/1567201817666200122163251
265. Brossa A, Fonsato V, Grange C, Tritta S, Tapparo M, Calvetti R, et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer. (2020) 147(6):1585–96. doi: 10.1002/ijc.v147.6
266. Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. (2023) 9(9):e17488. doi: 10.1016/j.heliyon.2023.e17488
267. Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. (2021) 236(8):5530–43. doi: 10.1002/jcp.v236.8
268. Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, and Hashemi SM. Immunomodulatory effects of mesenchymal stem cell–derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. (2018) 119(11):9433–43. doi: 10.1002/jcb.v119.11
269. Al Shoyaib A, Archie SR, and Karamyan VT. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res. (2020) 37:12. doi: 10.1007/s11095-019-2745-x
270. Gupta D, Wiklander OPB, Wood MJA, and El-Andaloussi S. Biodistribution of therapeutic extracellular vesicles. Extracellular Vesicles Circulating Nucleic Acids. (2023) 4(1):40–8. doi: 10.20517/evcna.2023.12
271. Azman M, Sabri AH, Anjani QK, Mustaffa MF, and Hamid KA. Intestinal absorption study: challenges and absorption enhancement strategies in improving oral drug delivery. Pharmaceuticals. (2022) 15(8):975. doi: 10.3390/ph15080975
272. Arntz OJ, Pieters BCH, Oliveira MC, Broeren MGA, Bennink MB, de Vries M, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. (2015) 59(12):2412–26. doi: 10.1002/mnfr.201500222
273. Kim KU, Kim J, Jang H, Dan KB, Kim BK, Ji YW, et al. Protective effects of human breast milk-derived exosomes on inflammatory bowel disease through modulation of immune cells. NPJ Sci Food. (2025) 9:14. doi: 10.1038/s41538-025-00400-3
274. Jiang Z, Fu Y, and Shen H. Development of intratumoral drug delivery based strategies for antitumor therapy. Drug Design Dev Ther. (2024) 18:2189–202. doi: 10.2147/DDDT.S467835
275. Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Ovazza E, Bassi C, et al. TNF-related apoptosis-inducing ligand (trail)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. (2016) 22(14):3499–512. doi: 10.1158/1078-0432.CCR-15-2170
276. Cassano R, Mellace S, and Trombino S. Responsive polymer-biomacromolecule conjugates for drug delivery. In: Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers. Amsterdam: Elsevier (2018).
277. Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. (2017) 11(11):10883–93. doi: 10.1021/acsnano.7b04495
278. Takov K, Yellon DM, and Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles. (2017) 6(1):1388731. doi: 10.1080/20013078.2017.1388731
279. Matsumoto A, Takahashi Y, Chang HY, Saito K, Niwa H, Ochiya T, et al. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J Extracell Vesicles. (2020) 9(1):1696517. doi: 10.1080/20013078.2019.1696517
280. Takakura Y, Hanayama R, Akiyoshi K, Abe M, and Takahashi K. Quality and safety considerations for therapeutic products based on extracellular vesicles. Pharm Res. (2024) 41:1573–94. doi: 10.1007/s11095-024-03757-4
281. Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu C-C, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. (2018) 3(8):e99263. doi: 10.1172/jci.insight.99263
282. Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, and Romberger DJ. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J Nutr Biochem. (2019) 64:123–31. doi: 10.1016/j.jnutbio.2018.10.017
283. Aslan C, Kiaie SH, Zolbanin NM, Mohammadi M, Avan A, and Hassanian SM. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. (2021) 21(1):34. doi: 10.1186/s12896-021-00683-w
284. Maji S, Yan IK, Parasramka M, Mohankumar S, Matsuda A, and Patel T. In vitro toxicology studies of extracellular vesicles. J Appl Toxicol. (2017) 37(8):981–90. doi: 10.1002/jat.3362
285. Huang F, Yao Y, Wu J, Yang G, Chen Y, and Li L. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κb/VEGF signaling. Am J Transl Res. (2017) 9(6):2813–23.
286. Wang L, He M, Fu L, and Jin Y. Exosomal release of microRNA-454 by breast cancer cells sustains biological properties of cancer stem cells via the PRRT2/Wnt axis in ovarian cancer. Life Sci. (2020) 257:118024. doi: 10.1016/j.lfs.2020.118024
287. Gyukity-Sebestyén E, Harmati M, Dobra G, Kubik J, Szerafin T, Bodó K, et al. Melanoma-derived exosomes induce PD-1 overexpression and tumor progression via mesenchymal stem cell oncogenic reprogramming. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.02459
288. Wang Z, Wang Q, Qin F, and Chen J. Exosomes: a promising avenue for cancer diagnosis beyond treatment. Front Cell Dev Biol. (2024) 12. doi: 10.3389/fcell.2024.1344705
289. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: An update. CA Cancer J Clin. (2011) 61(4):250–81. doi: 10.3322/caac.20114
290. Kwon N, Weng H, Rajora MA, and Zheng G. Activatable photosensitizers: from fundamental principles to advanced designs. Angewandte Chemie - Int Edition. (2025) 64. doi: 10.1002/anie.202423348
291. Li G, Wang C, Jin B, Luo J, and Chen X. Advances in smart nanotechnology-supported photodynamic therapy for cancer. Cell Death Discovery. (2024) 10(1):466. doi: 10.1038/s41420-024-02236-4
292. Ailioaie LM, Ailioaie C, and Litscher G. Fighting cancer with photodynamic therapy and nanotechnologies: current challenges and future directions. Int J Mol Sci. (2025) 26:2969. doi: 10.3390/ijms26072969
293. Patolsky F, Weizmann Y, Katz E, and Willner I. Magnetically amplified DNA assays (MADA): Sensing of viral DNA and single-base mis-matches by using nucleic acid modified magnetic particles. Angewandte Chemie - Int Edition. (2003) 42:2372–6. doi: 10.1002/anie.200250379
294. Sen S, Xavier J, Kumar N, Ahmad MZ, and Ranjan OP. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech. (2023) 13:100. doi: 10.1007/s13205-023-03521-2
295. Stickney Z, Losacco J, McDevitt S, Zhang Z, and Lu B. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. (2016) 472:53–59. doi: 10.1016/j.bbrc.2016.02.058
296. Zou J, Shi M, Liu X, Wang Y, Wang X, Wang Y, et al. Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem. (2019) 91:11565–73. doi: 10.1021/acs.analchem.8b05204
297. Wang Z, Du X, Lian W, Li Z, Zhang Z, and Chen X. A novel disulfidptosis-associated expression pattern in breast cancer based on machine learning. Front Genet. (2023) 14. doi: 10.3389/fgene.2023.1193944
Keywords: extracellular vesicles, cancer therapy, biomarkers, exosomes, tumor microenvironment, precision medicine
Citation: Almasry Y, Mustafa F, Alfuwais M, AlNachef S, Mohamed H, Gaber NS, Khan MI, Saadeldin IM and Yaqinuddin A (2025) Current trends in theranostic applications of extracellular vesicles in cancer. Front. Oncol. 15:1592006. doi: 10.3389/fonc.2025.1592006
Received: 11 March 2025; Accepted: 05 May 2025;
Published: 03 June 2025.
Edited by:
Jie Li, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Francesco De Francesco, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyTao Yang, Soochow University, China
Emna Fehri, Pasteur Institute of Tunis, Tunisia
Copyright © 2025 Almasry, Mustafa, Alfuwais, AlNachef, Mohamed, Gaber, Khan, Saadeldin and Yaqinuddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Yaqinuddin, YXlhcWludWRkaW5AYWxmYWlzYWwuZWR1
†These authors have contributed equally to this work
 Yazan Almasry
Yazan Almasry Fayrouz Mustafa
Fayrouz Mustafa Mohammed Alfuwais
Mohammed Alfuwais Sara AlNachef
Sara AlNachef Hager Mohamed
Hager Mohamed Nusaibah S. Gaber
Nusaibah S. Gaber Mohammed Imran Khan
Mohammed Imran Khan Islam M. Saadeldin
Islam M. Saadeldin Ahmed Yaqinuddin
Ahmed Yaqinuddin