Abstract
Background:
Epithelioid trophoblastic tumor (ETT) is a rare variant of gestational trophoblastic neoplasia. This article presents a case of vaginal ETT, initially misdiagnosed as vaginal carcinoma, in a patient with no history of gestational trophoblastic disease. The aim is to explore the clinical characteristics and diagnostic features of this condition.
Case presentation:
A 50-year-old woman presented with a 3-year history of vaginal pain. Following a vaginal fistula repair at an external hospital, a biopsy unexpectedly revealed vaginal carcinoma, prompting referral to our institution for further management. Pathological examination confirmed a diagnosis of extremely rare vaginal ETT, with immunohistochemistry showing characteristic marker positivity. Notably, the patient had no history of gestational trophoblastic disease, and serum Human Chorionic Gonadotropin (HCG) levels remained normal throughout. After diagnosis, the patient underwent total hysterectomy, bilateral salpingo-oophorectomy, and partial vaginectomy. Postoperative pathology confirmed the primary site to be the vagina, an unusual location for ETT. To further control the disease, the patient received 6 cycles of EMA-CO chemotherapy. Follow-up at 1 year showed no recurrence or metastasis, with stable disease.
Conclusion:
ETT often present with nonspecific symptoms, which can lead to misdiagnosis. Vaginal delivery and induced abortion may be potential risk factors. Clinically, in patients presenting with vaginal pain, masses, or genital tract fistulas, the possibility of a trophoblastic tumor should be considered and thoroughly evaluated.
Introduction
Epithelioid trophoblastic tumor (ETT) is a rare and distinct form of gestational trophoblastic neoplasia originating from intermediate trophoblasts of chorionic origin. First described in 1998, ETT arises from these intermediate trophoblasts (1). Retrospective studies indicate that vaginal bleeding is the most common clinical manifestation of ETT (2), with the lungs being the most frequent site of distant metastasis (3). ETT typically exhibits slow tumor growth, and most cases are confined to the uterus in the early stages, with metastasis being relatively uncommon. Although the precise pathogenesis of ETT remains unclear, existing research suggests a strong association with prior gestational events, particularly normal vaginal delivery, hydatidiform mole, and a history of abortion (4). Additionally, ETT shares histological features with cervical squamous cell carcinoma (5), and its nonspecific clinical presentation often leads to misdiagnosis (6). Due to the limited understanding of ETT, preoperative diagnosis is challenging, and definitive diagnosis is usually made only through postoperative pathological examination. This article presents the first reported case of ETT primarily manifesting as a genital tract fistula. The patient was initially misdiagnosed with vaginal carcinoma and had no history of gestational trophoblastic disease. This case aims to further explore the clinical characteristics and diagnostic challenges associated with this rare condition.
Case presentation
A 50-year-old perimenopausal woman presented with a 3-year history of vaginal pain. She had previously undergone vaginal fistula repair at an external hospital, where a postoperative biopsy suggested vaginal carcinoma, leading to her referral to our institution for further management. Colposcopy revealed no significant pathological changes in the cervix, but sutures were noted near the vaginal orifice. Acetic acid testing showed no obvious abnormalities at the fistula site. The posterior perineal commissure appeared thickened with thin acetowhite epithelium, raising suspicion for possible lesions (Figure 1). To further clarify the diagnosis, a pathological consultation was conducted at our hospital. Immunohistochemical staining was negative for CK5/6 and positive for α-Inhibin, P16, and P63 (Figure 2), which are consistent with epithelioid trophoblastic differentiation. Tumor markers (CEA, CA125, SCC) are normal and serum hCG levels were within normal limits at <2 mIU/mL (reference range: <10 mIU/mL), in contrast to typical gestational trophoblastic neoplasms. Based on the patient’s pathological findings, the diagnosis was consistent with ETT.
Figure 1

Colposcopy results. (A) Normal colposcopy image: A small opening and sutures are visible. (B) Colposcopy image after acetic acid test: A thin aceto-white area is visible in the posterior fornix.
Figure 2
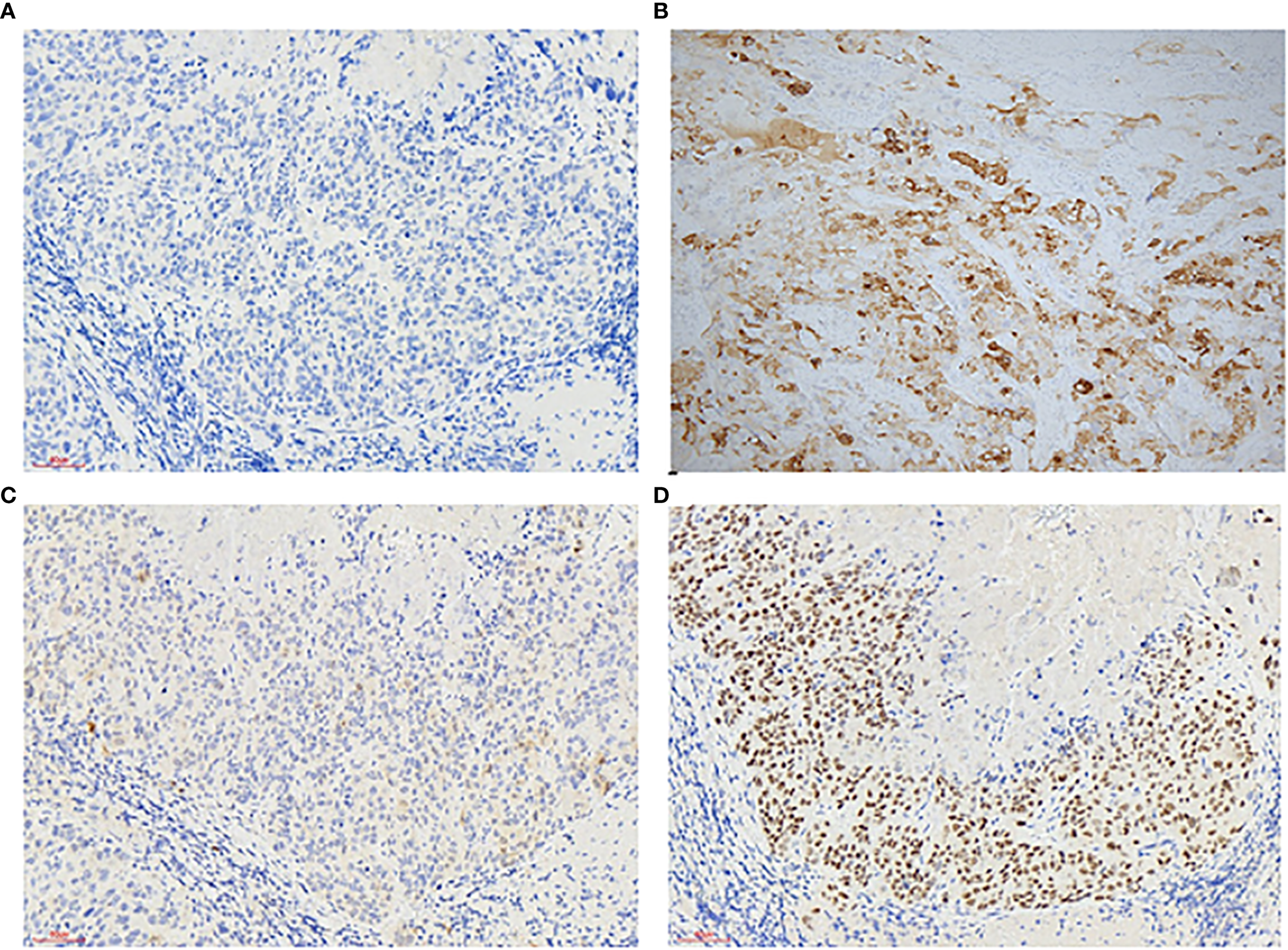
Immunohistochemistry of vaginal epithelial trophoblastic tumors: Immunohistochemical stains (200×) are positive for CK5/6 - (A) α-Inhibin + (B) P16 + (C) P63 + (D).
The patient’s obstetric history included two vaginal deliveries of healthy infants in 1999 and 2000, with no history of hydatidiform mole or other gestational trophoblastic diseases. The patient opted to undergo total hysterectomy, bilateral salpingo-oophorectomy, partial vaginectomy, and resection of the posterior perineal commissure. Pathological examination of the uterus and adnexa revealed no lesions, confirming the diagnosis of a primary vaginal ETT, a rare entity with an unclear pathogenesis. Pathological findings from the partial vaginal and posterior perineal commissure tissue further supported the diagnosis of ETT. Notably, serum HCG levels remained normal throughout the course of the disease, and PET-CT showed no evidence of distant metastasis. Given the potential aggressiveness of ETT, the patient received 6 cycles of EMA-CO chemotherapy to reduce the risk of recurrence. The treatment plan is as follows:
Day 1 (EMA regimen):
-
Actinomycin D 500 μg intravenous infusion over 1 hour;
-
Etoposide 100 mg/m² intravenous infusion over 1 hour;
-
Methotrexate 100 mg/m² intravenous bolus;
-
Methotrexate 200 mg/m² intravenous infusion over 12 hours.
Day 2:
-
Actinomycin D 500 μg intravenous infusion over 1 hour;
-
Etoposide 100 mg/m² intravenous infusion over 1 hour;
-
Calcium folinate (leucovorin) 15 mg intramuscular injection, administered every 12 hours for 4 doses, starting 24 hours after the methotrexate intravenous bolus.
Day 8 (CO regimen):
-
Vincristine 2 mg intravenous bolus, administered 3 hours prior to cyclophosphamide;
-
Cyclophosphamide 600 mg/m² intravenous infusion over 2 hours.
Day 15: Initiate the next cycle starting with Day 1.
The patient tolerated chemotherapy well, with no severe adverse effects. At the 1-year follow-up, there was no evidence of distant recurrence or metastasis, and the patient remained in stable condition. The patient is also currently being followed up.
Discussion
We herein present a clinically and academically critical case of primary vaginal ETT, which, to our knowledge, represents the first reported instance masquerading as a vaginal fistula. This unusual presentation underscores a notable diagnostic challenge, as a highly specialized malignant entity mimicked a benign gynecological condition—emphasizing the imperative for inclusion of ETT in the differential diagnosis of atypical vaginal lesions. ETT typically occurs in women of reproductive age, with an average onset at 36 years and peak incidence between 30 and 50 years. However, it can occasionally present in postmenopausal or perimenopausal women (7). The most common symptoms include abnormal vaginal bleeding and amenorrhea, though asymptomatic cases are rare. In this case, the patient was 50 years old and had experienced vaginal pain for 3 years. Her gynecological examination revealed only a small fistula at the inferior portion of the vagina is adjacent to the posterior perineal commissure. Given her clinical presentation, diagnosing ETT was particularly challenging. The differential diagnosis of ETT primarily includes cervical squamous cell carcinoma, placental site trophoblastic tumor (PSTT), choriocarcinoma, and epithelioid leiomyosarcoma. These conditions are typically distinguished based on histopathological features and immunohistochemical findings. The immunophenotype of ETT is characterized by both epithelial and trophoblastic markers (8, 9). Epithelial markers, such as P6CKpan, CK7, CK18, and EGFR, typically show strong and diffuse positivity, while EMA demonstrates moderate expression. In contrast, markers like CK5/6, CK34βE12, Cam5.2, and CEA exhibit weak or focal expression, with P16 typically being negative. Squamous cell carcinoma often shows strong expression of P16 and CK5/6. Trophoblastic markers, including HLA-G, P4-OHase, and CD10, generally show strong expression, with moderate positivity for α-Inhibin and weak or focal expression of HCG, hPL, and CD146. Notably, P63 is a key marker for distinguishing PSTT from ETT (10, 11). In this case, the patient exhibited positive expression of P63 and α-Inhibin, focal expression of P16, and negative expression of CK5/6, supporting the diagnosis of ETT.
To the best of our knowledge, this is the first reported case of primary vaginal ETT simulating a vaginal fistula. This conclusion is supported by a comprehensive literature review conducted using Boolean operators with the key terms “Epithelioid trophoblastic tumor” or “ETT” combined with “vagina” or “vaginal” across major databases including PubMed, Web of Science, and Embase, which identified only two previously reported cases of primary vaginal ETT, none of which were described with fistula-like features (Table 1). As indicated in Table 1, the typical manifestations of vaginal ETT include vaginal obstruction and pain. However, in this case, the patient primarily presented with vaginal pain accompanied by a vaginal fistula, without any signs of abnormal vaginal bleeding or obstruction. Based on current literature and the findings in this case, a direct diagnosis of ETT remains challenging due to its nonspecific symptoms and normal HCG levels. Nonetheless, vaginal delivery or induced abortion may be significant predisposing factors for the development of vaginal ETT. Ohira (12) suggested that episiotomy sites could also serve as a potential origin for primary vaginal ETT. Furthermore, Taliento, C reported that atypical epithelioid trophoblastic lesions, often accompanied by cysts and fistulas, may develop following cesarean section (14). Given the patient’s presentation of a genital tract fistula, a high index of suspicion for ETT is warranted in such cases.
Table 1
| Study | Age | Symptoms | Childbearing history | Mode of delivery | HCG, mIU/mL | Immunostaining | Treatment | Follow up |
|---|---|---|---|---|---|---|---|---|
| Ohira, S, 2000 (12) | 30 | Vaginal tumor and pain | G1P1 | Vaginal delivery (in 1999) | 2.8 | Positive for cytokeratin (CK22, CAM 5.2, CK18), inhibin-α, and Mel-CAM; focally positive for hPL, and negative for hCG, CD68, S-100, desmin, and vimentin; The Ki-67 index was approximately 15%. |
Surgery | 14 months |
| Jing Zhao, 2013 (13) | 43 | Vaginal tumor | G2P0 + 2 | Induced abortions (in June 2007 and another in March 2009) | 15.5 | Positive for AE1/AE3, hCG, hPL, PLAP, P63, and CAM5.2; Negative for CEA, actin, melan-A, inhibin-α, HMB45, E-cadherin, and cyclin D1. The Ki-67 labeling index was approximately 15% |
Chemotherapy and surgery. (Three courses of chemotherapy with a 3-week regimen of vincristine (VCR; 2 mg intravenously, day 1), floxuridine (FUDR; 800 mg/m2/day, intravenously, days 1–5), dactinomycin (Act-D, 200 kg/m2/day, intravenously, days 1–5), and etoposide (VP-16, 100 mg/m2/day, intravenously, days 1–5) |
8 months |
Summary of the clinicopathological characteristics of two cases of vaginal epithelioid trophoblastic tumor.
G, Gravida; P, Para; hPL, Human placental lactogen; PLAP, Placental alkaline phosphatase; P63, Tumor protein 63; CAM, Antibodies to cytokeratin; CEA, Carcinoembryonic antigen.
While choriocarcinoma is highly responsive to chemotherapy, ETT often exhibits significant chemoresistance, with surgical resection remaining the cornerstone of treatment (15). The management of primary vaginal ETT presents a significant challenge due to its extreme rarity and the consequent lack of evidence-based guidelines. The treatment strategy for our patient, comprising surgery followed by adjuvant EMA-CO chemotherapy, was formulated based on the established principles for high-risk gestational trophoblastic neoplasia (GTN) and ETT. It is important to acknowledge that other multi-agent regimens, such as FAEV (floxuridine, dactinomycin, etoposide, and vincristine) or EP-EMA (etoposide-cisplatin/etoposide-methotrexate-dactinomycin), are also employed in the treatment of high-risk GTN and could be considered valid alternatives for ETT. However, the superior efficacy and manageable toxicity profile of EMA-CO, coupled with our institution’s extensive experience with this protocol, guided our selection (16). Pelvic lymphadenectomy is typically not required for ETT; however, concurrent removal of uterine lesions is recommended if present. The efficacy of radiotherapy and chemotherapy in treating ETT remains uncertain. Studies suggest that the success rate of conservative treatment is approximately 20% (17). For cases of ETT not originating in the uterus, whether hysterectomy should be performed remains controversial. While the patient has achieved a 1-year disease-free interval—an encouraging early outcome—it is crucial to acknowledge that ETT is associated with a risk of recurrence beyond this period. Therefore, stringent long-term follow-up, including serial serum HCG monitoring and imaging, is mandated for this patient.
This report presents several strengths. Primarily, it constitutes, to the best of our knowledge, the first detailed documentation of a primary vaginal ETT masquerading clinically as a benign vaginal fistula. This unique presentation serves as a critical alert for clinicians, expanding the known clinical spectrum of ETT and potentially preventing diagnostic delay. Furthermore, the comprehensive description of the radiological and histopathological findings, including a complete immunohistochemical profile, provides a valuable reference for the diagnostic workup of similar rare cases. The successful application of a multimodal treatment strategy—combining surgery with adjuvant EMA-CO chemotherapy—also offers practical insights into the management of this elusive condition. However, our study is not without limitations. The inherent constraints of a single-case report limit the generalizability of our findings. Although the diagnosis of ETT is well-supported by histomorphology and immunohistochemistry (positive for α-Inhibin, p16, and p63; negative for CK5/6), additional staining for HLA-G and hPL was not performed to avoid imposing extra financial burden on the patient. While not essential for diagnosis in this case, inclusion of these markers could have provided further diagnostic confirmation. Finally, the relatively short follow-up period remains a constraint; given the documented potential for late recurrence in ETT, ongoing long-term surveillance of the patient is imperative.
Conclusion
Vaginal ETT often presents with nonspecific clinical manifestations, which can lead to misdiagnosis. Symptoms such as vaginal discomfort, mild bleeding, or masses may closely resemble those of other gynecological conditions, complicating early identification. Vaginal delivery and induced abortion are recognized as potential risk factors. Clinically, in patients presenting with vaginal pain, masses, or genital tract fistulas, especially those with these risk factors, the possibility of a trophoblastic tumor should be strongly suspected. Timely evaluation and accurate diagnosis are crucial, as early detection plays a key role in improving prognosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
D-ML: Investigation, Writing – original draft, Methodology. X-ZY: Writing – original draft, Software, Investigation. M-RQ: Methodology, Writing – review & editing, Investigation. R-QD: Writing – review & editing, Supervision, Investigation, Methodology.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We would like to express our gratitude to the Department of Obstetrics and Gynecology at West China Second Hospital for providing the platform and to the Departments of Pathology for their support in analyzing this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Shih IM Kurman RJ . Epithelioid trophoblastic tumor: A neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. (1998) 22:1393–403. doi: 10.1097/00000478-199811000-00010
2
Gorun F Tomescu L Motoc A Citu C Sas I Serban DM et al . Clinical features and management of trophoblastic epithelioid tumors: A systematic review. Med (Baltimore). (2022) 101:e29934. doi: 10.1097/MD.0000000000029934
3
Almarzooqi S Ahmad Al-Safi R Fahad Al-Jassar W Akhter SM Chiab-Rassou Y Albawardi A . Epithelioid trophoblastic tumor: report of two cases in postmenopausal women with literature review and emphasis on cytological findings. Acta Cytol. (2014) 58:198–210. doi: 10.1159/000357966
4
Horowitz NS Goldstein DP Berkowitz RS . Placental site trophoblastic tumors and epithelioid trophoblastic tumors: biology, natural history, and treatment modalities. Gynecol Oncol. (2017) 144:208–14. doi: 10.1016/j.ygyno.2016.10.024
5
Lewin SN Aghajanian C Moreira AL Soslow RA . Extrauterine epithelioid trophoblastic tumors presenting as primary lung carcinomas: morphologic and immunohistochemical features to resolve a diagnostic dilemma. Am J Surg Pathol. (2009) 33:1809–14. doi: 10.1097/PAS.0b013e3181b9cd67
6
Zhang X Lu W Lu B . Epithelioid trophoblastic tumor: an outcome-based literature review of 78 reported cases. Int J Gynecol Cancer. (2013) 23:1334–8. doi: 10.1097/IGC.0b013e31829ea023
7
Phippen NT Lowery WJ Leath CA 3rd Kost ER . Epithelioid trophoblastic tumor masquerading as invasive squamous cell carcinoma of the cervix after an ectopic pregnancy. Gynecol Oncol. (2010) 117:387–8. doi: 10.1016/j.ygyno.2010.02.013
8
Mao TL Kurman RJ Huang CC Lin MC Shih Ie M . Immunohistochemistry of choriocarcinoma: an aid in differential diagnosis and in elucidating pathogenesis. Am J Surg Pathol. (2007) 31:1726–32. doi: 10.1097/PAS.0b013e318058a529
9
Singer G Kurman RJ McMaster MT Shih Ie M . Hla-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol. (2002) 26:914–20. doi: 10.1097/00000478-200207000-00010
10
Shih IM Kurman RJ . P63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol. (2004) 28:1177–83. doi: 10.1097/01.pas.0000130325.66448.a1
11
Sung WJ Shin HC Kim MK Kim MJ . Epithelioid trophoblastic tumor: clinicopathologic and immunohistochemical analysis of three cases. Korean J Pathol. (2013) 47:67–73. doi: 10.4132/KoreanJPathol.2013.47.1.67
12
Ohira S Yamazaki T Hatano H Harada O Toki T Konishi I . Epithelioid trophoblastic tumor metastatic to the vagina: an immunohistochemical and ultrastructural study. Int J Gynecol Pathol. (2000) 19:381–6. doi: 10.1097/00004347-200010000-00015
13
Zhao J Xiang Y Zhao D Ren T Feng F Wan X . Isolated epithelioid trophoblastic tumor of the vagina: A case report and review of the literature. Onco Targets Ther. (2013) 6:1523–6. doi: 10.2147/OTT.S50553
14
Taliento C Loomans H Dewilde K Rompuy AV Van den Bosch T Froyman W . Atypical epithelioid trophoblastic lesion presenting as pseudocyst from the niche in the cesarean scar: A case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. (2025) 304:90–6. doi: 10.1016/j.ejogrb.2024.11.020
15
Lo C Low I Tan AL Baranyai J . Epithelioid trophoblastic tumor: A case report. Int J Gynecol Cancer. (2006) 16:1473–6. doi: 10.1111/j.1525-1438.2006.00609.x
16
Ji M Jiang S Zhao J Wan X Feng F Ren T et al . Efficacies of faev and ema/co regimens as primary treatment for gestational trophoblastic neoplasia. Br J Cancer. (2022) 127:524–30. doi: 10.1038/s41416-022-01809-3
17
Saso S Haddad J Ellis P Lindsay I Sebire NJ McIndoe A et al . Placental site trophoblastic tumours and the concept of fertility preservation. BJOG. (2012) 119:369–74; discussion 74. doi: 10.1111/j.1471-0528.2011.03230.x
Summary
Keywords
ETT, vaginal fistula, diagnosis, treatment, case report
Citation
Li D-m, Yu X-z, Qie M-r and Duan R-q (2025) Vaginal epithelioid trophoblastic tumor mimicking vaginal fistula: a case report and literature review. Front. Oncol. 15:1593126. doi: 10.3389/fonc.2025.1593126
Received
13 March 2025
Accepted
29 August 2025
Published
12 September 2025
Volume
15 - 2025
Edited by
Tullio Golia D’Augè, Sapienza University of Rome, Italy
Reviewed by
Indra Adi Susianto, Soegijapranata Catholic University, Indonesia
Matteo Terrinoni, University of Perugia, Italy
Updates
Copyright
© 2025 Li, Yu, Qie and Duan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-qi Duan, duanxiaoyi2016@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.